Abstract
Insular seizures are great mimickers of seizures originating elsewhere in the brain. The insula is a highly connected brain structure. Seizures may only become clinically evident after ictal activity propagates out of the insula with semiology that reflects the propagation pattern. Insular seizures with perisylvian spread, for example, manifest first as throat constriction, followed next by perioral and hemisensory symptoms, and then by unilateral motor symptoms. On the other hand, insular seizures may spread instead to the temporal and frontal lobes and present like seizures originating from these regions. Due to the location of the insula deep in the brain, interictal and ictal scalp electroencephalogram (EEG) changes can be variable and misleading. Magnetic resonance imaging, magnetic resonance spectroscopy, magnetoencephalography, positron emission tomography, and single-photon computed tomography imaging may assist in establishing a diagnosis of insular epilepsy. Intracranial EEG recordings from within the insula, using stereo-EEG or depth electrode techniques, can prove insular seizure origin. Seizure onset, most commonly seen as low-voltage, fast gamma activity, however, can be highly localized and easily missed if the insula is only sparsely sampled. Moreover, seizure spread to the contralateral insula and other brain regions may occur rapidly. Extensive sampling of the insula with multiple electrode trajectories is necessary to avoid these pitfalls. Understanding the functional organization of the insula is helpful when interpreting the semiology produced by insular seizures. Electrical stimulation mapping around the central sulcus of the insula results in paresthesias, while stimulation of the posterior insula typically produces painful sensations. Visceral sensations are the next most common result of insular stimulation. Treatment of insular epilepsy is evolving, but poses challenges. Surgical resections of the insula are effective but risk significant morbidity if not carefully planned. Neurostimulation is an emerging option for treatment, especially for seizures with onset in the posterior insula. The close association of the insula with marked autonomic changes has led to interest in the role of the insula in sudden unexpected death in epilepsy and warrants additional study with larger patient cohorts.
Introduction
Barbara C. Jobst, MD, PhD, and Fred Lado, MD, PhD
This review is a report from the Epilepsy Specialist Symposium presented at the 2017 American Epilepsy Society Meeting in Washington DC. The faculty, through their amazing enthusiasm, delivered outstanding content so that nearly everyone in the room learned something new. The symposium was aimed at understanding the semiology of insular seizures, the invasive and noninvasive investigation of the insula, and surgical approaches. As the organizers, we would like to thank the faculty for their excellent contributions and for setting a high standard for future symposia.
Anatomy of the Insula
The insular lobe is a thin cortical structure located deep in the Sylvian fissure, covered by a rich vascular network and hidden by the fronto-parieto-temporal operculum. These anatomic constraints make its evaluation and surgical access difficult (Figure 1). The insula is a complex structure, with 7 cytoarchitectonic subdivisions that encompass 5 gyri1 and 4 different functional areas (cognitive, social-emotional, chemical-sensory, and sensory-motor) that overlap.2 Despite its name, the insula is not isolated.3 It is rather a highly connected brain region, and therefore seizures originating in the insula are great mimickers of seizures originating elsewhere. Insular epilepsy, although reported for a long time, is a form of epilepsy that remains difficult to recognize, evaluate, and treat surgically.
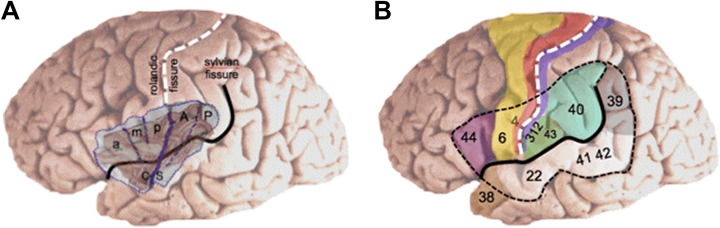
Figure 1.
A, The insula is anatomically subdivided in an anterior part that comprises 3 short gyri (a, anterior; m, middle; p, posterior) and a posterior part that comprises 2 long gyri (A, anterior; P, posterior). B, The insula is covered by the suprasylvian and infrasylvian opercular regions that are essential for motor, sensory, auditory, and language processing. Numbers refer to Brodmann’s area. CS indicates central sulcus of the insula.
Clinical Semiology of Insular Seizures
Barbara Jobst, MD, PhD, and Phillipe Kahane, MD, PhD
The insula is a multiconnected brain region that receives and sends information to frontal, temporal, and posterior cortical structures, which explains its strong involvement in cognitive, behavioral, and sensory processing.4 As such, seizure semiology of insular lobe seizures is far from being homogeneous, and a number of subjective and objective ictal clinical signs have been reported, including viscerosensory, somatosensory, olfactory, gustatory, and auditory auras; autonomic symptoms (vomiting, piloerection, heart rate changes); automotor and hypermotor behaviors; tonic and/or clonic motor manifestations; and language disturbances.5
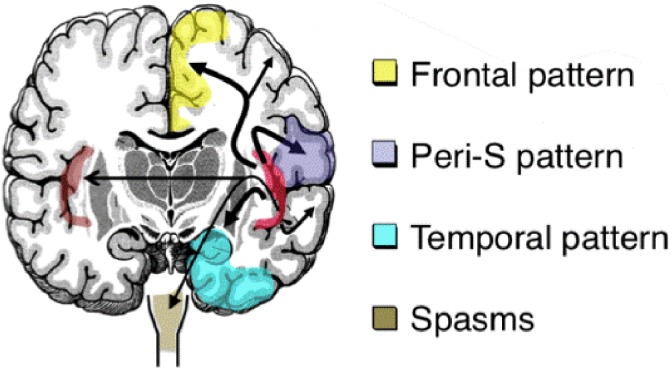
In 2004, Isnard et al6 elegantly brought attention to a clinical pattern highly suggestive of insular lobe seizures, which included laryngeal constriction, perioral unpleasant paresthesias, lateralized somatosensory sensations, dysarthria, and focal somatomotor signs. Further intracranial electroencephalogram (EEG) studies demonstrated that besides this “perisylvian” clinical pattern, insular seizures could also manifest with “temporal-like” symptoms (altered awareness with oroalimentary and manual automatisms),7-9 “frontal-like” symptoms (hyperkinetic behaviors or tonic motor signs),10 and even with epileptic spasms,11 therefore supporting the idea that insular epilepsy is a great mimicker, depending on the pattern of seizure spread (Figure 2).
Figure 2.
Various spread patterns of insula seizures to symptomatogenic zones. Peri-S indicates perisylvian.
Patients with insular epilepsy often undergo a long “odyssey” searching for help for their drug-resistant seizures until finally a diagnosis of insular epilepsy is made. Seizures can be misidentified as psychogenic nonepilepsy seizures for a lack of clear EEG correlates or misidentified as seizures originating in the frontal or temporal lobes. Patients may even have undergone previous unsuccessful epilepsy surgery until insular epilepsy is identified. Therefore, a careful analysis of seizure history, especially addressing patient-reported auras, is essential.
As insular seizures frequently begin with preserved awareness, a clear description of the aura may yield important information that points to an insular onset. A feeling of suffocation and breathlessness, painful sensations,12 or gustatory auras7 are highly suggestive of an insular or insulo-opercular ictal origin. Interestingly, and possibly because the insula is a multimodal area involved in the processing of various sensory stimuli, insulo-opercular seizures may also manifest as eating-, audiogenic-, and somatosensory-evoked reflex seizures.7,13 Additionally, ecstatic seizures, which have been proposed to involve the anterior insular cortex, can be triggered by thinking about specific memories or a pleasant emotional context.14
Noninvasive Investigation of Operculo-Insular Epilepsy
Dang Khoa Nguyen, MD, PhD
The heterogeneous clinical manifestations of insular seizures highlight the need for confirmation with noninvasive diagnostic tests.
Video EEG
Most auras in insular seizures cannot be appreciated on video, but certain clinical signs (eg, expression of pain, hand movement to the throat, long latency between electrical onset, and hypermotor manifestations) suggest an insular focus. On scalp EEG, because the insula is a deep structure, insular spikes are seen only if they project to the surface. Interictal epileptiform discharges are regularly found over frontopolar and frontotemporal regions with anterior operculo-insular foci, and over the midtemporal region extending to frontotemporal regions and/or central leads with posterior foci.15,16 During seizures, various nonspecific patterns can be seen when the discharge reaches surface electrodes. Interictal and ictal discharges generally allow lateralization of the epileptic focus.
Magnetic Resonance Imaging
Clinical diagnosis of insular epilepsy is greatly facilitated by the identification of an insular epileptogenic lesion, although nonlesional cases are frequent. Among 25 patients who underwent nontumoral epilepsy surgery involving operculo-insular resection, presurgical magnetic resonance imaging (MRI) of the operculo-insular area was normal or revealed questionable nonspecific findings in 18 (72%).17 Malformations of cortical development were commonly associated with medication resistant seizures.18
Magnetic Resonance Spectroscopy
Magnetic resonance spectroscopy (MRS) of the insula may be difficult compared with other regions due to its curved/pyramidal shape and the presence of cerebrospinal fluid in the Sylvian fissure. The value of proton MRS in identifying patients with insular epilepsy was assessed in 12 nonlesional cases with confirmed operculo-insular focus.19 Voxels were positioned to include bilateral anterior and posterior insular regions. Metabolite concentrations and ratios did not differ from those of noninsular epileptic patients and healthy controls, and asymmetry indices fared poorly in lateralizing the focus.
Magnetoencephalography
Magnetoencephalography (MEG) is one of the most useful tests to identify potential operculo-insular patients.20 Mohamed and colleagues21 reviewed MEG data of 14 patients with refractory insular seizures and identified the following 3 main patterns of spike sources: 7 (50%) had an anterior operculo-insular cluster, 2 (14%) had a posterior operculo-insular cluster, and 4 (29%) showed a diffuse perisylvian distribution. No spikes were detected in the remaining patients. Spike sources showed uniform orientation perpendicular to the Sylvian fissure. Nine patients underwent insular epilepsy surgery with favorable surgical outcome.
Single-Photon Computed Tomography and Positron Emission Tomography
Single-photon computed tomography (SPECT) can identify an operculo-insular epileptic focus. In a retrospective study of 17 patients with confirmed operculo-insular epilepsy, ictal SPECT correctly identified the focus in 65% and provided misleading information in 18%.22 Secondary activations in areas connected to the insula were common, but generally less intense. By contrast, interictal positron emission tomography (PET) yielded more equivocal findings, as it correctly identified the operculo-insular focus in 47% cases and was misleading in 24%.23
Genetic Testing
Genetic defects have been reported in operculo-insular epilepsy cases, including mutations in the CHRNB2 and CHRNA4 genes in 2 patients with sleep-related hypermotor seizures (functional testing under way).24 A subtle insular focal cortical dysplasia was reported in a patient with familial focal epilepsy associated with DEPDC5 mutation.25 Finally, Nguyen and colleagues26 described an epileptogenic network involving the temporo-insular region in a family with reflex bathing epilepsy associated with a Q555X mutation of synapsin 1 on chromosome Xp11-q21.
Invasive Recordings of Insular Lobe Seizures
Philippe Kahane, MD, PhD
The stereotactic intracerebral EEG (SEEG) method is especially well suited to evaluate insular epilepsy because it gives access directly to deep brain structures that cannot be recorded using subdural grids or strips. As for every SEEG study, a strong semiologically based hypothesis for seizure onset as well as for seizure propagation is a prerequisite for successful investigation of insular lobe epilepsy. Extensive knowledge of the structural, cytoarchitectonic, and functional anatomy of the insular lobe and its connections to the frontal, temporal, and posterior cortical structures are additional prerequisites.
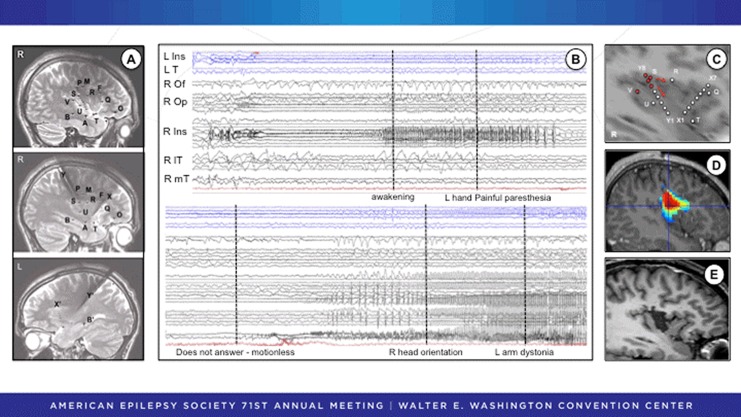
Seizure onset within the insula widely varies across patients. Seizure onset can be very restricted in space, and to differentiate insular from opercular seizure onset, extensive insulo-opercular coverage is necessary. The best approach is to combine a lateral orthogonal trajectory through the frontoparietal and temporal operculum27 with an oblique approach through the frontal or parietal cortices to allow a larger insular sampling28 (Figure 3A). Combined depth and subdural electrodes29,30 or hybrid operculo-insular electrodes31 can be also used to investigate the insulo-opercular complex. To better evaluate the extent of the future resection and to exclude any extrainsular onset, seizure spread must be examined, especially in MRI-negative cases. This includes appropriate sampling of extrainsular regions to which the insula is closely connected, taking into account the different patterns of connectivity that vary as a function of each insular gyrus32 with a rostrocaudal organization.33 The SEEG investigations of insular lobe seizures have shown the following: (1) Seizure onset patterns can be variable in the insula, but low-voltage fast discharge34 or high-frequency gamma activity7 are not uncommon (Figure 3B and C); (2) seizures often start very focally with a limited intrainsular spread before extrainsular propagation (Figure 3B-D), which therefore allows tailored limited resections (Figure 3E)35; (3) extrainsular spread explains clinical variability of insular lobe seizures; in particular, complex motor manifestations were shown to occur when the discharge spread over frontomesial and/or mesial and lateral temporal regions.16 This is in accordance with directed functional coupling analysis that reported a specific association between the insula and mesial frontal lobe during the propagation of insular seizures34; (4) corticocortical evoked potentials have showed that the 2 insulas are closely connected,36 with 8- to 24-milliseconds time to propagate from one to the other homotopic insular parcel37; therefore, insular seizures may propagate very quickly to the contralateral insula (Figure 3B) so that false lateralization may occur in insular or even temporo(-insular) epilepsy38; (5) typical insular signs can occur in seizures of extrainsular origin; in particular, insular spread is very common in seizures of temporal lobe origin22,39 and therefore may result in misleading seizure auras; and (6) the morbidity rate directly related to insular electrodes is low. In particular, none of the patients (including children) reported in 3 recent large studies experienced any hemorrhagic complications.40,41
Figure 3.
SEEG recording of a patient with very localized insular onset and subsequent insular resection. A, The SEEG study was focused on the right insulo-opercular region with additional electrodes sampling the right temporal and frontal lobes and the left insular and temporal regions. B, SEEG activity at seizure onset (upper panel) exhibits spikes and polyspike discharges quickly followed by a low-voltage fast activity in the superior part of the anterior long gyrus of the right insula (R Ins) that spreads to the right opercular cortex (R Op). Note the almost immediate involvement of the contralateral insula (L Ins). The patient describes a painful tingling sensation in the left hand and then (lower panel) loses contact and presents temporal-like symptoms when the seizure spread to the right mesiotemporal lobe (R mT), anteroinferior part of the insula (R Ins), and lateral temporal cortex (lT). The orbitofrontal cortex (Of) is spared. C, Schematic representation of the insular contacts involved in seizure onset before spreading to extrainsular regions. D, Epileptogenicity map indicating the highest value of activation in the 60- to 100-Hz frequency band at seizure onset. E, Tailored resection of the right anterior long insular gyrus. Postoperatively, there was transient dysgeusia that resolved completely. The patient has been seizure free without medication for 6 years. Pathological examination revealed a focal cortical dysplasia type IB. SEEG indicates stereo electroencephalogram.
Functional Mapping of the Insula: Contributions to Semiology of Insular Seizures
Jean Isnard, MD, PhD
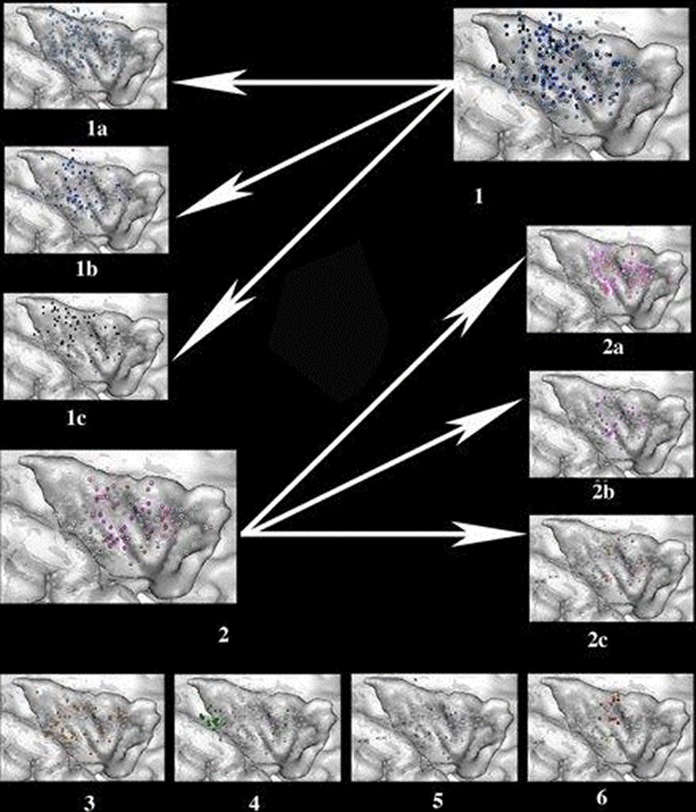
Electrical stimulation of cerebral cortex evokes clinical responses that mimic symptoms occurring at the onset or during the spread of the epileptic discharge. Thus, stimulation studies directly contribute to the localization of ictal symptoms, as shown for the insular cortex in the pioneering work of Penfield and Faulk.42 Since then, a number of insular stimulation studies have been performed during SEEG investigations,6 which all point to the great variety of clinical responses that can be observed. This is illustrated below by the results of the largest published series of insular stimulation in which 679 electrical stimulations were delivered in the insular cortex of 222 patients during SEEG procedures43 with 550 positive responses (Figure 4).
Figure 4.
Location and type of symptoms evoked by electrical stimulations of the insular cortex. (1) Somatosensory responses, including nonpainful, nonthermal sensations (light blue, 1A), thermal sensations (medium blue, 1B), and painful sensations (deep blue, 1C). (2) Visceral sensations, including constrictive sensations (light pink, 2A), viscero-vegetative sensations (deep pink, 2B), and viscero-psychic symptoms ( pink, 2C). (3) Vestibular sensations (orange). (4) Auditory sensations (green). (5) Speech disturbances (violet). (6) Olfactogustatory sensations (red for taste, yellow for smell).
Somatosensory Sensations
Somatosensory sensations represented the majority (n = 335, 61%) of all evoked symptoms.44 Paresthesias were most frequent (Figure 4.1A), followed by thermal sensations. Thermal responses were evoked by stimulation around the central sulcus of the insula (Figure 4.1B). Painful sensations were elicited mostly from the posterior third of the insula and described as burning, electric shock, painful pins, or cramps (Figure 4.1C). Painful responses to insular stimulation were first observed by Ostrowsky et al45 and then further confirmed by Mazzola et al.46 This later study showed that painful responses were rare (60 of >4000 stimulations) and were only elicited by insular and secondary somatosensory cortex (SII) stimulations. They were never observed when stimulating primary somatosensory cortex (SI) or any other cortical area. These results are in line with the SEEG study of Montavont et al,12 in which the insula or SII was systematically involved at seizure onset in all 5 patients suffering from painful seizures and in which ictal pain was reproduced by the stimulation of these 2 regions.
Visceral Symptoms
They accounted for 82 (15%) of insular lobe responses and thus represented the second largest group of electrically induced symptoms. Constrictive sensations located in the pharyngolaryngeal, retrosternal, or abdominal region were observed at 41 electrical stimulation sites; they ranged from a simple discomfort to a frightening sensation of strangulation. Viscero-vegetative signs, including nausea, salivation, facial blush, dyspnea, urge to urinate, and sweaty hands, were elicited from 27 sites (Figure 4.2B). Viscero-psychic symptoms, such as thoracic or abdominal heaviness associated with a feeling of fear, were elicited at 14 insular sites (Figure 4.2C).
Other Insular Responses
Less common responses were vestibular sensations, which were described as a feeling of body motion (Figure 4.3) and auditory sensations, which were evoked by stimulating the very posteroinferior part of the insula (Figure 4.4). Speech impairments, which consisted of speech arrest, slurred speech, or lowering of voice intensity (Figure 4.5), were evoked both in the nondominant and dominant hemispheres for language. Gustatory and olfactory sensations were very rare and represented, respectively, 2.7% and 1% of all responses (Figure 4.5).
Overall, none of the clinical signs evoked by stimulation is absolutely specific of insular onset. An exception are nociceptive symptoms, which are highly suggestive of an insular lobe origin.12
Pros and Cons of Insular Resection
Pro: Insular Resection Should Be the First-Line Intervention for Drug-Resistant Insular Epilepsy
Chengyuan Wu, MD
Surgical resection of the epileptogenic zone remains the first-line option for patients with drug-resistant epilepsy. Long-term seizure freedom rates are 66% in temporal lobe epilepsy, 46% in occipital and parietal lobe epilepsy, and 27% in frontal lobe epilepsy.47 With the increased adoption of invasive EEG, the diagnosis of epilepsy of insular onset has increased. The literature supports surgical resection for insular epilepsy. Across 5 separate case series involving 74 patients with an average follow-up of 3.5 years, 73% of patients were seizure free.48
As with all surgical interventions, we must weigh benefits with surgical risk. Unfortunately, early experience with open insular resections resulted in high morbidity and mortality.49 Resection of insular tumors continues to associate with morbidity rates from 20% to 45.5%.50 The insula certainly challenges surgeons with its deep-seated location, hidden by the frontal and temporal opercula, and its intimate relationship with the “candelabra” of the middle cerebral artery (MCA). Surgical risk, therefore, stems primarily from retraction injury and from damage of lenticulostriate arteries or MCA branches.51 An improved understanding of these concerns along with advances in surgical techniques has significantly reduced the risk of insular lobe surgery. In a more recent series, insular lesionectomy was associated with a permanent morbidity of 8% and no mortalities.50
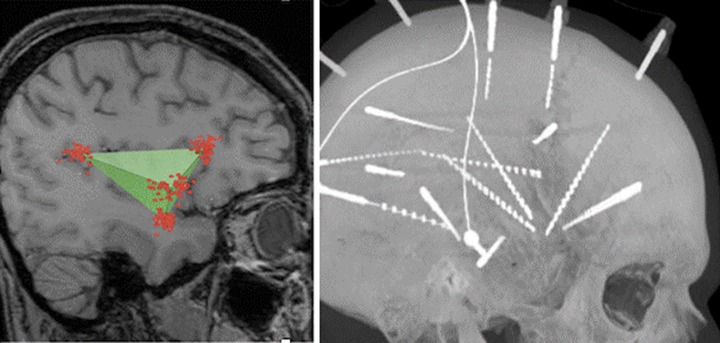
With invasive EEG serving as the means by which insular epilepsy can be properly diagnosed, we believe that the technique of electrode implantation should be informed by the method of surgical resection. Although the Talairach’s method of insular investigation involves orthogonal transopercular electrode trajectories,41 we favor an oblique approach as described by Afif et al.52 We have taken this approach one step further by implanting 3 or 4 electrodes in a manner that recreates the borders of the tetrahedron-shaped insula (Figure 5).
Figure 5.
The insula is shaped like a tetrahedron or triangular pyramid (left). By taking oblique approaches to the insula and implanting 4 electrodes, we are able to mimic this anatomy and define the borders of the insula (right).
In addition to improving our ability to localize the seizure onset zone to a particular region of the insula, this method of SEEG implantation allows us to take a “fence-post” approach to surgical resection of the insula.53 Because their entry points are distant from the craniotomy needed to access the insula, the electrodes remain in place during surgical resection. Consequently, the electrodes can then be used as internal landmarks to facilitate adequate, appropriate, and safe insular resection (Figure 6).
Figure 6.
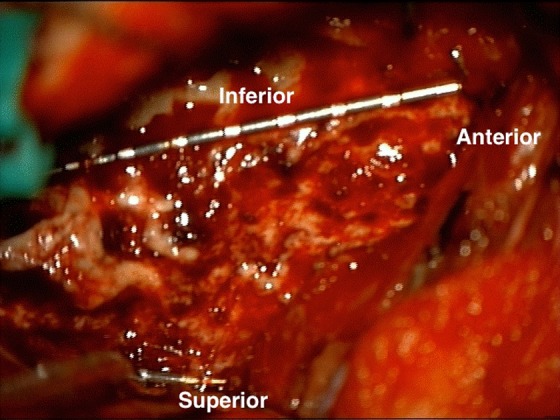
Intraoperative view of SEEG electrodes serving as internal landmarks during insular resection. A complete anterior insulectomy has been performed, as superior, inferior, and anterior insular electrodes can be seen at the borders of the resection. SEEG indicates stereo electroencephalogram.
In scenarios where the epileptogenic zone cannot be safely resected, neuromodulation is a viable option. When considering responsive neurostimulation (RNS), deep brain stimulation (DBS), or vagal nerve stimulation (VNS), however, we must understand that these modalities provide significant seizure reduction, but rarely seizure freedom. Specifically, although RNS, DBS, and VNS, in general, have reported seizure reduction rates of 70%, 40%, and 44%, respectively, they are associated with seizure freedom rates of 15% (for 1 year), 6%, and less than 10%.54-56 In comparison to the potential for seizure freedom with surgical resection, neuromodulation is definitively inferior and as such should only be considered when resection cannot be performed.
Overall, open resection of the insula is a safe and the most effective approach to drug-resistant insular epilepsy and should be considered as the first-line surgical option.
Con: Diagnosis and Treatment of Medically Refractory Insula Epilepsy: Challenges and Pitfalls
Jorge Gonzalez-Martinez, MD, PhD
Insular epilepsy is a particularly challenging topic in medically refractory epilepsies. The clinical and surgical challenges are intrinsically related to the heterogeneous semiological features of seizures arising from the insula and adjacent structures, the difficult access to the insular cortex, and the relative high morbidity associated with insula and perisylvian resections.
Regarding the technique of insular implantation, several series have addressed the technique and safety of insular exploration by the SEEG methodology.40, 41 The SEEG is arguably the most common and appropriate surgical method to explore the insula cortex among other invasive techniques. Since its inception by Talairach and Bancaud,57 the SEEG methodology, and in particular its technique, evolved over the years. Nevertheless, the common denominator among different techniques is accurate vascular imaging, particularly important for insula explorations. Even in the highly vascular insular cortex, SEEG can still be performed safely if planning and technique are performed carefully.
The most common SEEG depth electrode implantation technique is the transopercular approach,27 in which orientation of electrodes is perpendicular to the sagittal plane, as defined by the anterior commissure–posterior commissure line. The advantages of this approach include its common and widespread clinical application, its safety and efficacy for accessing the insula cortex and adjacent areas, and its ability to sample medial and lateral portions of the insula, as well as the adjacent frontal and temporal opercula.
Once localization methods confirm that seizures are in fact arising from the insula cortex, and the extent of the epileptogenic zone is defined, the surgical treatment strategies become the main challenge. Several published manuscripts reported the outcome and morbidity related to insula epilepsy surgeries.11,35, 48, 58 Alomar et al40 reported the results of 17 patients with nonlesional imaging who were surgically treated for medically intractable epilepsy (15 resection and 2 laser ablations). Overall, 11 of 15 patients with insular resection had favorable outcome (Engel I and Engel II), of whom 5 (33.3%) had an Engel I outcome and 6 (40%) an Engel II outcome. The remaining patients had either an Engel III (n = 3, 20%) or an Engel IV outcome (n = 1, 6.7%). In this cohort, 3 patients developed permanent neurological deficits related to hemiparesis (17.6%), with an additional 4 patients developing transient deficits and mild complications. Therefore, the total complication rate in this series was approximately 41%. Interestingly, all permanent motor deficits were related to the resection of the caudal dorsal insula and adjacent parietal operculum, possibly due to damage of the small caliber performant arteries originating from the MCA, which exclusively provide vascular supply to the more caudal aspect of the corona radiata, harboring motor, and sensory fibers (Figure 7).
Figure 7.
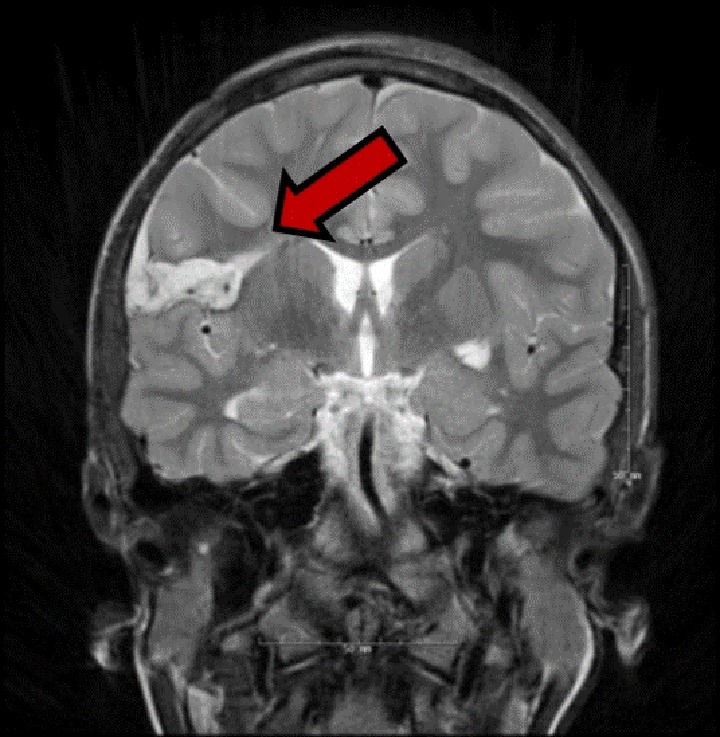
Postoperative T2 MRI image (coronal orientation) after right caudal rostral insula resection in a 22-year-old female, resulting in symptomatic infarct in the ipsilateral corona radiata (red arrow). The procedure resulted in seizure freedom, but with a only partially recovered left side hemiparesis. MRI indicates magnetic resonance imaging.
In conclusion, the authors highlighted the dangers related to the dorsal caudal insula resections. Because of the relatively high morbidity associated with open resections in the most caudal–rostral aspect of the insular cortex, preoperative discussion should include alternative treatment approaches including RNS and focal laser ablation. In order to overcome the potential high morbidity related to this area, 2 patients at our center underwent MRI-guided stereotactic laser ablation targeting the dorsal–caudal insula cortex, resulting in no complications and good control of focal seizures.
Is SUDEP Risk Increased in Insular Epilepsy?
Nuria Lacuey, MD, and Samden Lhatoo, MD, FRCP
The intimate role played by the insula in autonomic control and modulation renders it suspect in sudden unexpected death in epilepsy (SUDEP) pathophenomenology. There are 2 possible types of insular involvement in SUDEP: first, as the epileptogenic zone from where the fatal seizure arises, or second, as the structure, whether damaged or intact, critical to the genesis of autonomic, and/or respiratory seizure features, to which the seizure discharge secondarily spreads. The relative rarity of insular epilepsy accounts for the scant knowledge of SUDEP risk in this condition. Similarly, although the insula is part of the epileptogenic zone in some temporal, frontal, and opercular epilepsies, these too are relatively undercharacterized syndromes.
Among the few reported cases of insular epilepsy related to SUDEP is an SEEG proven case of left insular epilepsy with sleep-related seizures with hyperkinetic automatisms and anterosuperior insular seizure onset. The patient refused resective surgery and died of SUDEP 2 years after assessment.59 MORTality in Epilepsy Monitoring Unit Study (MORTEMUS), a study of SUDEP or near SUDEP in the epilepsy monitoring unit, reported 2 insular cases associated with near-SUDEP.60 Both patients experienced cardiac/cardiorespiratory compromise in the peri-ictal period of seizures with loss of awareness. One 10-year-old female patient had cardiorespiratory arrest in the postictal period and cardiopulmonary resuscitation (CPR) was instituted successfully within a minute of seizure end. The other was a 54-year-old female patient who suffered ictal asystole and underwent CPR. Given current knowledge of the relatively benign, self-limited nature of ictal asystole, the resuscitation instituted within a minute may have been superfluous, and the near-SUDEP label debatable.
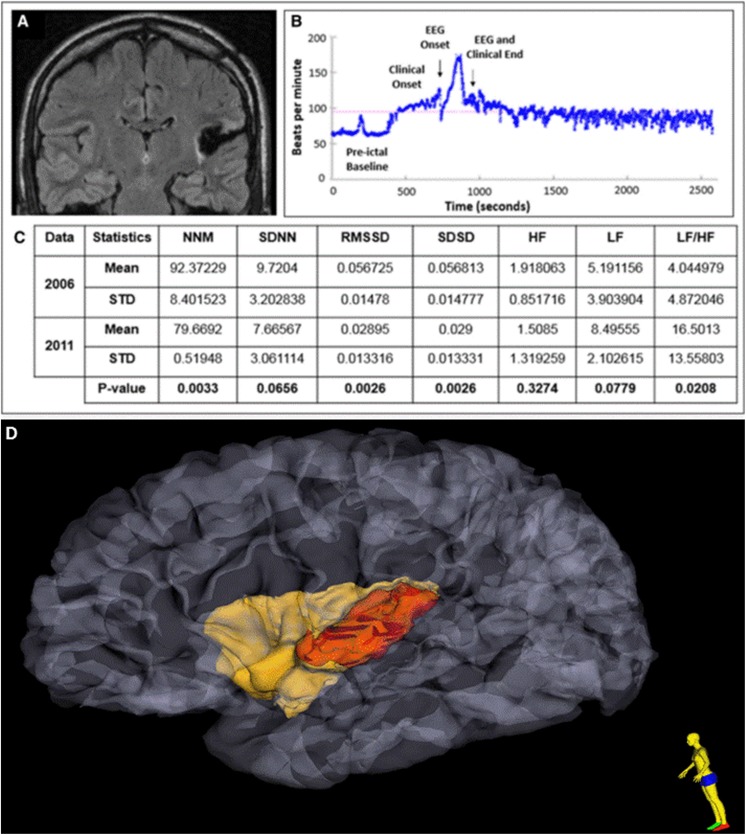
The contribution of the insula to seizure-related cardiorespiratory dysfunction and subsequent SUDEP is worthy of discussion. The insula is known to play a central role in the regulation of cardiac functions,61 and a few case reports have suggested that seizures of insular lobe origin, or seizures that spread to the insula, might provoke bradycardia,62,63 an atrioventricular block,64 or asystole.65 Both left and right insular damage are known to affect prognosis and mortality in stroke.66,67 Insular damage, in epilepsies emanating outside this structure, may lead to potentially fatal scenarios. Dysfunctional brain networking, with high insular connectivity, is known to exist in high SUDEP-risk patients.68 Direct structural damage to the insula, whether seizure induced or iatrogenic, has been associated with SUDEP in 2 intractable epilepsy cases.69 In a 33-year-old patient with left hemibody sensory and generalized convulsive seizures, PET hypometabolism in the left posterior parieto-opercular region, and depth electrodes demonstrating unequivocal fast frequency (gamma) discharges in the left posterior insula (Figure 8), posterior insular resection failed to produce seizure freedom. Serial assessments of interictal heart rate variability (HRV) demonstrated significant HRV decrease in the postsurgical period compared with the presurgical period; notably, he had marked sinus tachycardia in the postictal period that continued for more than 25 minutes. The insular resection may have contributed via changes in HRV to a tendency for ventricular arrhythmia and subsequent SUDEP, 2 years after surgery. In a second case, similarly intractable in the postsurgical period, milder left inferior insular damage was noted secondary to a temporal lobectomy. However, increasing HRV was noted over a 3-year period, indicating increased vagal tone and a confirmed tendency to postictal bradycardia. The SUDEP was confirmed 21 months after his last assessment with possible contribution of autonomic dysfunction as described.
Figure 8.
A, Postoperative MRI FLAIR sequence shows evidence of a left posterior temporo-insular resection cavity with surrounding gliosis in a patient with later SUDEP. B, Heart rate plots show ictal sinus tachycardia, followed by sustained postictal sinus tachycardia lasting at least 25 minutes after a nonfatal generalized convulsive seizure. C, Heart rate time and frequency domain parameters calculated during the presurgery (2006) and postsurgery (2011) epilepsy monitoring unit (EMU) evaluations and the results from generalized estimating equation (GEE) analysis. D Extent of insular resection and damage, after 3-dimensional reconstruction of pre- and postoperative MRI is delineated in red. MRI indicates magnetic resonance imaging; FLAIR, fluid-attenuated inversion recovery; SUDEP, sudden unexpected death in epilepsy; MNN, mean of normal to normal heart beats; SDNN, standard deviation of normal to normal heart beats.
Whereas it is easy to speculate on insular contributions to SUDEP, it is less easy to extrapolate anecdotal evidence to larger populations. Thus, cohort studies, with appropriate multimodal seizure assessments, are required to resolve these issues.
In conclusion, insular epilepsy can be difficult to recognize. Surgical treatment of insular epilepsy has it challenges but can be addressed with diligent clinical and electrophysiological investigations. Surgical approaches have to be weighted carefully but can be very successful in treating insular epilepsy.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Morel A, Gallay MN, Baechler A, Wyss M, Gallay DS. The human insula: architectonic organization and postmortem MRI registration. Neuroscience. 2013;236:117–135. [DOI] [PubMed] [Google Scholar]
- 2. Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct. 2010;214(4-5):519–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Collin G, Sporns O, Mandl RC, van den Heuvel MP. Structural and functional aspects relating to cost and benefit of rich club organization in the human cerebral cortex. Cerebral Cortex. 2014;24(9):2258–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Namkung H, Kim SH, Sawa A. The insula: an underestimated brain area in clinical neuroscience, psychiatry, and neurology. Trends Neurosci. 2017;40(4):200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dimova P. The insula: semiology In: Arzimanoglou A, Cross JH, Gaillard WD, et al. (Eds.), Pediatric Epilepsy Surgery. Montrouge, France: John Libbey Eurotext; 2016:121–129. [Google Scholar]
- 6. Isnard J, Guenot M, Sindou M, Mauguiere F. Clinical manifestations of insular lobe seizures: a stereo-electroencephalographic study. Epilepsia. 2004;45(9):1079–1090. [DOI] [PubMed] [Google Scholar]
- 7. Blauwblomme T, Kahane P, Minotti L, et al. Multimodal imaging reveals the role of gamma activity in eating-reflex seizures. J Neurol Neurosurg Psychiatry. 2011;82(10):1171–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kriegel MF, Roberts DW, Jobst BC. Orbitofrontal and insular epilepsy. J Clin Neurophysiol. 2012;29(5):385–391. [DOI] [PubMed] [Google Scholar]
- 9. Fei P, Soucy JP, Obaid S, Boucher O, Bouthillier A, Nguyen DK. The value of regional cerebral blood flow SPECT and FDG PET in operculoinsular epilepsy. Clin Nucl Med. 2018;43(3):e67–e73. [DOI] [PubMed] [Google Scholar]
- 10. Ryvlin P, Minotti L, Demarquay G, et al. Nocturnal hypermotor seizures, suggesting frontal lobe epilepsy, can originate in the insula. Epilepsia. 2006;47(4):755–765. [DOI] [PubMed] [Google Scholar]
- 11. Dylgjeri S, Taussig D, Chipaux M, et al. Insular and insulo-opercular epilepsy in childhood: an SEEG study. Seizure 2014;23(4):300–308. [DOI] [PubMed] [Google Scholar]
- 12. Montavont A, Mauguiere F, Mazzola L, et al. On the origin of painful somatosensory seizures. Neurology. 2015;84(6):594–601. [DOI] [PubMed] [Google Scholar]
- 13. Xiao H, Tran TP, Petrin M, et al. Reflex operculoinsular seizures. Epileptic Disord 2016;18(1):19–25. [DOI] [PubMed] [Google Scholar]
- 14. Gschwind M, Picard F. Ecstatic epileptic seizures: a glimpse into the multiple roles of the insula. Front Behav Neurosci. 2016;10:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Levy A, Yen Tran TP, Boucher O, Bouthillier A, Nguyen DK. Operculo-insular epilepsy: scalp and intracranial electroencephalographic findings. J Clin Neurophysiol. 2017;34(5):438–447. [DOI] [PubMed] [Google Scholar]
- 16. Proserpio P, Cossu M, Francione S, et al. Insular-opercular seizures manifesting with sleep-related paroxysmal motor behaviors: a stereo-EEG study. Epilepsia. 2011;52(10):1781–1791. [DOI] [PubMed] [Google Scholar]
- 17. Bouthillier A, Nguyen DK. Epilepsy surgeries requiring an operculoinsular cortectomy: operative technique and results. Neurosurgery. 2017;81(4):602–612. [DOI] [PubMed] [Google Scholar]
- 18. Chevrier MC, Bard C, Guilbert F, Nguyen DK. Structural abnormalities in patients with insular/peri-insular epilepsy: spectrum, frequency, and pharmacoresistance. AJNR Am J Neuroradiol. 2013;34(11):2152–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aitouche Y, Gibbs SA, Gilbert G, Boucher O, Bouthillier A, Nguyen DK. Proton MR spectroscopy in patients with nonlesional insular cortex epilepsy confirmed by invasive EEG recordings. J Neuroimaging. 2017;27(5):517–523. [DOI] [PubMed] [Google Scholar]
- 20. Heers M, Rampp S, Stefan H, et al. MEG-based identification of the epileptogenic zone in occult peri-insular epilepsy. Seizure 2012;21(2):128–133. [DOI] [PubMed] [Google Scholar]
- 21. Mohamed IS, Gibbs SA, Robert M, Bouthillier A, Leroux JM, Khoa Nguyen D. The utility of magnetoencephalography in the presurgical evaluation of refractory insular epilepsy. Epilepsia. 2013;54(11):1950–1959. [DOI] [PubMed] [Google Scholar]
- 22. Weil AG, Le NM, Jayakar P, et al. Medically resistant pediatric insular-opercular/perisylvian epilepsy. Part 2: outcome following resective surgery. J Neurosurg Pediatr. 2016;18(5):523–535. [DOI] [PubMed] [Google Scholar]
- 23. Herrero JL, Khuvis S, Yeagle E, Cerf M, Mehta AD. Breathing above the brain stem: volitional control and attentional modulation in humans. J Neurophysiol 2018;119(1):145–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Obaid S, Zerouali Y, Nguyen DK. Insular epilepsy: semiology and noninvasive investigations. J Clin Neurophysiol 2017;34(4):315–323. [DOI] [PubMed] [Google Scholar]
- 25. Baulac S, Ishida S, Marsan E, et al. Familial focal epilepsy with focal cortical dysplasia due to DEPDC5 mutations. Ann Neurol. 2015;77(4):675–683. [DOI] [PubMed] [Google Scholar]
- 26. Nguyen DK, Rouleau I, Senechal G, et al. X-linked focal epilepsy with reflex bathing seizures: characterization of a distinct epileptic syndrome. Epilepsia. 2015;56(7):1098–1108. [DOI] [PubMed] [Google Scholar]
- 27. Isnard J, Guenot M, Ostrowsky K, Sindou M, Mauguiere F. The role of the insular cortex in temporal lobe epilepsy. Ann Neurol. 2000;48(4):614–623. [PubMed] [Google Scholar]
- 28. Afif A, Chabardes S, Minotti L, Kahane P, Hoffmann D. Safety and usefulness of insular depth electrodes implanted via an oblique approach in patients with epilepsy. Neurosurgery. 2008;62(5 suppl 2):ONS471-ONS4719; discussion 479-480. [DOI] [PubMed] [Google Scholar]
- 29. Surbeck W, Bouthillier A, Weil AG, et al. The combination of subdural and depth electrodes for intracranial EEG investigation of suspected insular (perisylvian) epilepsy. Epilepsia. 2011;52(3):458–466. [DOI] [PubMed] [Google Scholar]
- 30. Weil AG, Fallah A, Lewis EC, Bhatia S. Medically resistant pediatric insular-opercular/perisylvian epilepsy. Part 1: invasive monitoring using the parasagittal transinsular apex depth electrode. J Neurosurg Pediatr. 2016;18(5):511–522. [DOI] [PubMed] [Google Scholar]
- 31. Bouthillier A, Surbeck W, Weil AG, Tayah T, Nguyen DK. The hybrid operculo-insular electrode: a new electrode for intracranial investigation of perisylvian/insular refractory epilepsy. Neurosurgery. 2012;70(6):1574–1580; discussion 1580. [DOI] [PubMed] [Google Scholar]
- 32. Almashaikhi T, Rheims S, Ostrowsky-Coste K, et al. Intrainsular functional connectivity in human. Hum Brain Mapp. 2014;35(6):2779–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ghaziri J, Tucholka A, Girard G, et al. The corticocortical structural connectivity of the human insula. Cereb Cortex. 2017;27(2):1216–1228. [DOI] [PubMed] [Google Scholar]
- 34. Hagiwara K, Jung J, Bouet R, et al. How can we explain the frontal presentation of insular lobe epilepsy? The impact of non-linear analysis of insular seizures. Clin Neurophysiol 2017;128(5):780–791. [DOI] [PubMed] [Google Scholar]
- 35. Gras-Combe G, Minotti L, Hoffmann D, Krainik A, Kahane P, Chabardes S. Surgery for nontumoral insular epilepsy explored by stereoelectroencephalography. Neurosurgery. 2016;79(4):578–588. [DOI] [PubMed] [Google Scholar]
- 36. Trebaul L, Deman P, Tuyisenge V, et al. Probabilistic functional tractography of the human cortex revisited. NeuroImage. 2018;181:414–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lacuey N, Zonjy B, Kahriman ES, et al. Homotopic reciprocal functional connectivity between anterior human insulae. Brain Struct Funct. 2016;221(5):2695–2701. [DOI] [PubMed] [Google Scholar]
- 38. Unnwongse K, Jehi L, Bulacio J, Gonzalez-Martinez J, Najm I. Contralateral insular involvement producing false lateralizing signs in bitemporal epilepsy: a stereo-encephalography case report. Seizure. 2012;21(10):816–819. [DOI] [PubMed] [Google Scholar]
- 39. Blauwblomme T, David O, Minotti L, et al. Prognostic value of insular lobe involvement in temporal lobe epilepsy: a stereoelectroencephalographic study. Epilepsia. 2013;54(9):1658–1667. [DOI] [PubMed] [Google Scholar]
- 40. Alomar S, Mullin JP, Smithason S, Gonzalez-Martinez J. Indications, technique, and safety profile of insular stereoelectroencephalography electrode implantation in medically intractable epilepsy. J Neurosurg. 2018;128(4):1147–1157. [DOI] [PubMed] [Google Scholar]
- 41. Salado AL, Koessler L, De Mijolla G, et al. SEEG is a safe procedure for a comprehensive anatomic exploration of the insula: a retrospective study of 108 procedures representing 254 transopercular insular electrodes. Oper Neurosurg (Hagerstown). 2018;14(1):1–8. [DOI] [PubMed] [Google Scholar]
- 42. Penfield W, Faulk ME., Jr The insula; further observations on its function. Brain 1955;78(4):445–470. [DOI] [PubMed] [Google Scholar]
- 43. Mazzola L, Mauguiere F, Isnard J. Electrical stimulations of the human insula: their contribution to the ictal semiology of insular seizures. J Clin Neurophysiol. 2017;34(4):307–314. [DOI] [PubMed] [Google Scholar]
- 44. Pugnaghi M, Meletti S, Castana L, et al. Features of somatosensory manifestations induced by intracranial electrical stimulations of the human insula. Clin Neurophysiol. 2011;122(10):2049–2058. [DOI] [PubMed] [Google Scholar]
- 45. Ostrowsky K, Magnin M, Ryvlin P, Isnard J, Guenot M, Mauguiere F. Representation of pain and somatic sensation in the human insula: a study of responses to direct electrical cortical stimulation. Cereb Cortex. 2002;12(4):376–385. [DOI] [PubMed] [Google Scholar]
- 46. Mazzola L, Isnard J, Peyron R, Mauguiere F. Stimulation of the human cortex and the experience of pain: Wilder Penfield’s observations revisited. Brain 2012;135(pt 2):631–640. [DOI] [PubMed] [Google Scholar]
- 47. Tellez-Zenteno JF, Dhar R, Wiebe S. Long-term seizure outcomes following epilepsy surgery: a systematic review and meta-analysis. Brain 2005;128(pt 5):1188–1198. [DOI] [PubMed] [Google Scholar]
- 48. von Lehe M, Parpaley Y. Insular cortex surgery for the treatment of refractory epilepsy. J Clin Neurophysiol. 2017;34(4):333–339. [DOI] [PubMed] [Google Scholar]
- 49. Silfvenius H, Gloor P, Rasmussen T. Evaluation of insular ablation in surgical treatment of temporal lobe epilepsy. Epilepsia. 1964;5:307–320. [DOI] [PubMed] [Google Scholar]
- 50. von Lehe M, Wellmer J, Urbach H, Schramm J, Elger CE, Clusmann H. Insular lesionectomy for refractory epilepsy: management and outcome. Brain 2009;132(pt 4):1048–1056. [DOI] [PubMed] [Google Scholar]
- 51. Rey-Dios R, Cohen-Gadol AA. Technical nuances for surgery of insular gliomas: lessons learned. Neurosurg Focus. 2013;34(2):E6. [DOI] [PubMed] [Google Scholar]
- 52. Afif A, Minotti L, Kahane P, Hoffmann D. Anatomofunctional organization of the insular cortex: a study using intracerebral electrical stimulation in epileptic patients. Epilepsia. 2010;51(11):2305–2315. [DOI] [PubMed] [Google Scholar]
- 53. Yoshikawa K, Kajiwara K, Morioka J, et al. Improvement of functional outcome after radical surgery in glioblastoma patients: the efficacy of a navigation-guided fence-post procedure and neurophysiological monitoring. J Neurooncol. 2006;78(1):91–97. [DOI] [PubMed] [Google Scholar]
- 54. Geller EB, Skarpaas TL, Gross RE, et al. Brain-responsive neurostimulation in patients with medically intractable mesial temporal lobe epilepsy. Epilepsia. 2017;58(6):994–1004. [DOI] [PubMed] [Google Scholar]
- 55. Wu C, Sharan AD. Neurostimulation for the treatment of epilepsy: a review of current surgical interventions. Neuromodulation. 2013;16(1):10–24; discussion 24. [DOI] [PubMed] [Google Scholar]
- 56. Jobst BC, Kapur R, Barkley GL, et al. Brain-responsive neurostimulation in patients with medically intractable seizures arising from eloquent and other neocortical areas. Epilepsia. 2017;58(6):1005–1014. [DOI] [PubMed] [Google Scholar]
- 57. Talairach J, Bancaud J, Bonis A, Szikla G, Tournoux P. Functional stereotaxic exploration of epilepsy. Confin Neurol. 1962;22:328–331. [DOI] [PubMed] [Google Scholar]
- 58. Desai A, Jobst BC, Thadani VM, et al. Stereotactic depth electrode investigation of the insula in the evaluation of medically intractable epilepsy. J Neurosurg. 2011;114(4):1176–1186. [DOI] [PubMed] [Google Scholar]
- 59. Ryvlin P. Avoid falling into the depths of the insular trap. Epileptic Disord. 2006;8(suppl 2):S37–S56. [PubMed] [Google Scholar]
- 60. Ryvlin P, Nashef L, Lhatoo SD, et al. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol. 2013;12(10):966–977. [DOI] [PubMed] [Google Scholar]
- 61. Oppenheimer S, Cechetto D. The insular cortex and the regulation of cardiac function. Compr Physiol. 2016;6(2):1081–1133. [DOI] [PubMed] [Google Scholar]
- 62. Seeck M, Zaim S, Chaves-Vischer V, et al. Ictal bradycardia in a young child with focal cortical dysplasia in the right insular cortex. Eur J Paediatr Neurol. 2003;7(4):177–181. [DOI] [PubMed] [Google Scholar]
- 63. Tayah T, Savard M, Desbiens R, Nguyen DK. Ictal bradycardia and asystole in an adult with a focal left insular lesion. Clin Neurol Neurosurg. 2013;115(9):1885–1887. [DOI] [PubMed] [Google Scholar]
- 64. Surges R, Scott CA, Walker MC. Peri-ictal atrioventricular conduction block in a patient with a lesion in the left insula: case report and review of the literature. Epilepsy Behav. 2009;16(2):347–349. [DOI] [PubMed] [Google Scholar]
- 65. Catenoix H, Mauguiere F, Guenot M, Isnard J, Ryvlin P. Recording the insula during ictal asystole. Int J Cardiol. 2013;169(2):e28–e30. [DOI] [PubMed] [Google Scholar]
- 66. Laowattana S, Zeger SL, Lima JA, Goodman SN, Wittstein IS, Oppenheimer SM. Left insular stroke is associated with adverse cardiac outcome. Neurology. 2006;66(4):477–483; discussion 463. [DOI] [PubMed] [Google Scholar]
- 67. Colivicchi F, Bassi A, Santini M, Caltagirone C. Prognostic implications of right-sided insular damage, cardiac autonomic derangement, and arrhythmias after acute ischemic stroke. Stroke. 2005;36(8):1710–1715. [DOI] [PubMed] [Google Scholar]
- 68. Allen LA, Harper RM, Kumar R, et al. Dysfunctional brain networking among autonomic regulatory structures in temporal lobe epilepsy patients at high risk of sudden unexpected death in epilepsy. Front Neurol. 2017;8:544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lacuey N, Zonjy B, Theerannaew W, et al. Left-insular damage, autonomic instability, and sudden unexpected death in epilepsy. Epilepsy Behav. 2016;55:170–173. [DOI] [PMC free article] [PubMed] [Google Scholar]