Abstract
Conditional Knockout of mGluR5 From Astrocytes During Epilepsy Development Impairs High-Frequency Glutamate Uptake
Umpierre AD, West PJ, White JA, et al. J Neurosci. 2019;39(4):727-742. doi:10.1523/JNEUROSCI.1148-18.2018
Astrocyte expression of metabotropic glutamate receptor 5 (mGluR5) is consistently observed in resected tissue from patients with epilepsy and is equally prevalent in animal models of epilepsy. However, little is known about the functional signaling properties or downstream consequences of astrocyte mGluR5 activation during epilepsy development. In the rodent brain, astrocyte mGluR5 expression is developmentally regulated and confined in expression/function to the first weeks of life, with similar observations made in human control tissue. Herein, we demonstrate that mGluR5 expression and function dramatically increase in a mouse model of temporal lobe epilepsy. Interestingly, in both male and female mice, mGluR5 function persists in the astrocyte throughout the process of epileptogenesis following status epilepticus. However, mGluR5 expression and function are transient in animals that do not develop epilepsy over an equivalent time period, suggesting that patterns of mGluR5expression may signify continuing epilepsy development or its resolution. We demonstrate that, during epileptogenesis, astrocytes reacquire mGluR5-dependent calcium transients following agonist application or synaptic glutamate release, a feature of astrocyte–neuron communication absent since early development. Finally, we find that the selective and conditional knockout of mGluR5 signaling from astrocytes during epilepsy development slows the rate of glutamate clearance through astrocyte glutamate transporters under high-frequency stimulation conditions, a feature that suggests astrocyte mGluR5 expression during epileptogenesis may recapitulate earlier developmental roles in regulating glutamate transporter function.
Commentary
Temporal lobe epilepsy (TLE) is a crippling disorder characterized by spontaneously recurring seizures, cognitive deficits, and structural remodeling of the limbic system. A common neurological insult preceding TLE is status epilepticus (SE), an abnormally prolonged seizure that can result in neuronal injury and alteration of neuronal networks.1 Following SE, the brain enters a period of cellular and molecular transformation that often develops into TLE.2,3 Herein, we summarize a recent investigation into this transformation.
A signature feature associated with TLE is abnormal astrocyte morphology. Specifically, astrocytes enlarge and extend multiple processes around synapses during an event known as astrogliosis.4 Curiously, astrocytes in TLE brains also reexpress metabotropic glutamate receptor 5 (mGluR5), a group I, Gq-coupled, metabotropic glutamate receptor whose expression is normally restricted to early brain development5 (Figure 1). Activation of mGluR5 in developing astrocytes increases intracellular calcium levels in an Inositol 1,4,5-triphosphate (IP3)-dependent manner.5-8 Several important functions have been ascribed to astrocytic calcium signaling events during development, including (a) astrocytic ensheathment of synapses9 and (b) astrocytic release of transmitters.10 Collectively, these astrocytic calcium signals are proposed to remodel glutamatergic synapses during development. However, the role of mGluR5 calcium signaling during epileptogenesis is not well delineated.
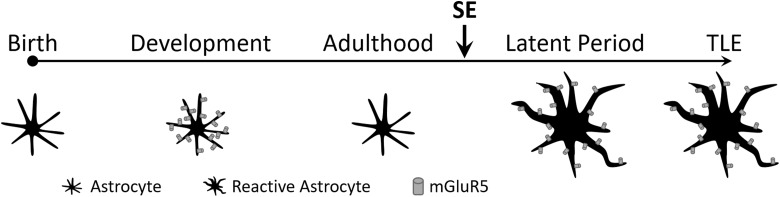
Figure 1.
Reexpression of metabotropic glutamate receptor 5 (mGluR5) during the latent period. During development, glia cells known as astrocytes express the metabotropic glutamate receptor mGluR5. Expression of the receptor is absent in adulthood. However, following status epilepticus (SE) astrocytes undergo morphological transformation that includes reexpression of mGluR5. In those animals that eventually become epileptic (ie, exhibit spontaneously recurring seizures), mGluR5 expression is also present.
Umpierre et al11 now provide new insights into astrocytic mGluR5 function in epilepsy using a mouse model of TLE. The authors set out to identify a potential mechanism to explain the faster glutamate transport kinetics previously observed in TLE rats.12 Umpierre and colleagues show that mGluR5 reemergence triggers astrocytes to resume enhanced regulatory control over glutamate transport at the synapse during epileptogenesis, as in development. The authors hypothesize that renewed mGluR5 signaling during epileptogenesis serves to limit excitotoxic glutamate spillover by enabling more efficient glutamate uptake at the synapse.
To model epileptogenesis, the authors used repeated, low-dose kainic acid injections to induce SE (KA-SE).13 Following SE induction, hippocampal brain slices were collected at specific time points to determine the progression of astrogliosis and mGluR5 reexpression. Epileptogenesis was divided into 3 epochs: acute (24-48 hours), latent (7-9 days), and chronic (28-35 days). Immunohistochemical approaches were used to show that astrocyte enrichment of mGluR5 is most prominent 72 hours after KA-SE and during the latent period, two epochs that correspond to peak astrogliosis. Presumably, animals that eventually develop TLE also exhibit elevated astrocytic mGluR5 expression. Interestingly and for unknown reasons, mGluR5 expression subsides in animals that do not progress to the epileptic state, providing fodder for the speculation that transient mGluR5 expression represents a resolution to the original SE insult.
Once establishing the timeline of mGluR5 expression during epileptogenesis, the authors used several transgenic mouse lines to evaluate functional roles of reexpressed mGluR5. Using acute brain slices from mice that selectively express the GCaMP5G in astrocytes, the authors developed an unbiased “dye-ROI” approach to measure calcium signals in response to mGluR5 agonists. Briefly, a fluorescent dye was loaded along with an agonist-containing pipette to enable dye/agonist spread measurements. The authors then only measured gross calcium changes within the area of spread, thereby avoiding biases associated with user-defined region of interests (ROIs).
Using the dye-ROI approach, the authors provide strong evidence that mGluR5 agonist-evoked calcium responses emerge at 72 hours post KA-SE and become more robust in chronically epileptic mice, an observation that is consistent with the observed, anatomical expression of mGluR5. Such responses were absent in latent period mice in which mGluR5 activity was pharmacologically blocked. Moreover, calcium responses were also absent in mice engineered to transiently suppress mGluR5 expression via tamoxifen, providing strong support for the hypothesis that calcium signals are mGluR5 dependent.
In a final set of experiments, the authors evaluated astrocytic calcium transients evoked by synaptically released glutamate in brain slices harvested during the latent period. Similar to aforementioned results, mGluR5+/+ astrocytes exhibited exaggerated calcium signals in response to extracellular Schaffer collateral stimulation relative to mGluR5−/− astrocytes; the larger, evoked calcium signals observed in mGluR5+/+ astrocytes were attenuated by pharmacological blockade of mGluR5. By directly recording glutamate transporter currents in astrocytes, the authors also demonstrate that glutamate is more efficiently taken up in mGluR5+/+ astrocytes versus mGluR5−/− astrocytes (ie, transporter currents are faster and are associated with a smaller residual component). Collectively, these data indicate that astrocyte mGluR5-dependent calcium signaling is likely an important feature of synapse remodeling in epileptogenesis.
While imaging glutamate-evoked calcium signals in astrocytes, electrophysiological recordings were simultaneously performed to assess the dependence of field excitatory postsynaptic potentials (fEPSPs) on mGluR5 expression. No difference in fEPSP amplitude was observed between mGluR5+/+ and mGluR5−/− hippocampal slices stimulated at 40 Hz, consistent with previous observations.12 Considering that mGluR5+/+ astrocytes generate larger calcium signals and can clear glutamate faster, this lack of fEPSP amplitude difference between mGluR5+/+ and mGluR5−/− hippocampal slices is somewhat surprising. One might expect mGluR5−/− slices to exhibit larger fEPSPs due to slower glutamate clearance. Alternatively, perhaps, fEPSP kinetics might be slower in mGluR5−/− hippocampal slices. Future studies will determine exactly how mGluR5-dependent glutamate clearance regulates neuronal signaling.
Several follow-up questions to the current study await future experiments. First, why does mGluR5 reemergence wane in nonepileptic KA-SE mice but is maintained in chronically epileptic mice? One interpretation of this observation is that the SE-inducing injury resolves in those mice that do not develop epilepsy, and therefore elevated mGluR5 expression is not necessary to offset potential excitotoxicity. However, is it possible that persistent mGluR5 reexpression actually aggravates the latent period brain such that it is more likely to produce spontaneous seizures? In other words, could mGluR5 reexpression be bad? Indeed, support for this alternative hypothesis comes from a prior study showing that mGluR5-dependent calcium signaling after SE causes neuronal death.14 A more recent study also indicates that astrocytic calcium signals may facilitate seizure propagation.15 Thus, the precise role of mGluR5-mediated calcium signals during epileptogenesis remains unclear. With the development of many new transgenic tools, the field is well poised to more firmly define any protective or proconvulsive functions of mGluR5 reexpression in the epileptogenic brain. For example, one could determine whether tamoxifen-induced suppression of mGluR5 expression during the latent period alters the proportion of animals that ultimately become epileptic.
Overall, this study emphasizes the important function astrocytes serve in remodeling the hippocampal network during epileptogenesis, which recapitulates similar processes that occur during development. Whether reemerging mGluR5 expression during epileptogenesis is advantageous remains to be seen. Nevertheless, the authors identify the latent period as a potential critical node that likely defines the extent of epilepsy progression. Attention to astrocytic involvement during this important period may help isolate a specific moment for clinical intervention. Future investigations spotlighting how neuron–astrocyte communication is altered in TLE will lead to the pursuit of better therapeutic avenues to stop epilepsy progression in patients.
By Kathryn Salvati and Mark Beenhakker
References
- 1. Trinka E, Cock H, Hesdorffer D, et al. A definition and classification of status epilepticus—report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia. 2015;56(10):1515–1523. [DOI] [PubMed] [Google Scholar]
- 2. Dudek FE, Staley KJ. The Time Course and Circuit Mechanisms of Acquired Epileptogenesis. Bethesda, MD: National Center for Biotechnology Information; 2012. https://www.ncbi.nlm.nih.gov/books/NBK98152/. Accessed February 28, 2019. [PubMed] [Google Scholar]
- 3. Pitkänen A, Lukasiuk K, Dudek FE, Staley KJ. Epileptogenesis. Cold Spring Harb Perspect Med. 2015;5(10):a022822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sofroniew MV. Astrogliosis. Cold Spring Harb Perspect Biol. 2014;7(2):a020420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sun W, McConnell E, Pare JF, et al. Glutamate-dependent neuroglial calcium signaling differs between young and adult brain. Science. 2013;339(6116):197–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pasti L, Volterra A, Pozzan T, Carmignoto G. Intracellular calcium oscillations in astrocytes: a highly plastic, bidirectional form of communication between neurons and astrocytes in situ. J Neurosci. 1997;17(20):7817–7830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Petravicz J, Fiacco TA, McCarthy KD. Loss of IP3 receptor-dependent Ca2+ increases in hippocampal astrocytes does not affect baseline CA1 pyramidal neuron synaptic activity. J Neurosci. 2008;28(19):4967–4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bazargani N, Attwell D. Astrocyte calcium signaling: the third wave. Nat Neurosci. 2016;19(2):182–189. [DOI] [PubMed] [Google Scholar]
- 9. Tanaka M, Shih PY, Gomi H, et al. Astrocytic Ca2+ signals are required for the functional integrity of tripartite synapses. Mol Brain. 2013;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang F, Smith NA, Xu Q, et al. Photolysis of caged Ca2+ but not receptor-mediated Ca2+ signaling triggers astrocytic glutamate release. J Neurosci. 2013;33(44):17404–17412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Umpierre AD, West PJ, White JA, Wilcox KS. Conditional knock-out of mGluR5 from astrocytes during epilepsy development impairs high-frequency glutamate uptake. J Neurosci. 2019;39(4):727–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Takahashi DK, Vargas JR, Wilcox KS. Increased coupling and altered glutamate transport currents in astrocytes following kainic-acid-induced status epilepticus. Neurobiol Dis. 2010;40(3):573–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Umpierre AD, Bennett IV, Nebeker LD, et al. Repeated low-dose kainate administration in C57BL/6J mice produces temporal lobe epilepsy pathology but infrequent spontaneous seizures. Exp Neurol. 2016;279:116–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ding S, Fellin T, Zhu Y, et al. Enhanced astrocytic Ca2+ signals contribute to neuronal excitotoxicity after status epilepticus. J Neurosci. 2007;27(40):10674–10684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Heuser K, Nome CG, Pettersen KH, et al. Ca2+ signals in astrocytes facilitate spread of epileptiform activity. Cereb Cortex. 2018;28(11):4036–4048. [DOI] [PMC free article] [PubMed] [Google Scholar]