Abstract
Accumulating evidence shows that curcumin exerts antitumor activities in a variety of malignancies. High mobility group box 1 (HMGB1) is associated with vascular endothelial growth factor D (VEGF-D)–induced lymphangiogenesis and tumor metastasis in gastric cancer. However, the molecular mechanisms by which curcumin regulates HMGB1-mediated lymphangiogenesis in gastric cancer remain unclear. In this study, the cytotoxic effects of curcumin were investigated in gastric cancer AGS and SGC-7901 cell lines by MTT assay, and curcumin-induced morphological changes and cell apoptosis were assessed by using flow cytometry analysis and caspase-3 activity. The effects of curcumin on HMGB1 and VEGF-D expression were examined by reverse transcription polymerase chain reaction (RT-PCR) and western blot analysis. As a result, we found that curcumin decreased cell viability and caused a dose-dependent cell apoptosis through the activation of caspase-3. The mRNA and protein expression levels of HMGB1 and VEGF-D were significantly eliminated by curcumin administration. Pre-treatment with the recombinant HMGB1 (rHMGB1) markedly abolished curcumin-reduced VEGF-D expression. Our findings suggested that curcumin might exert anti-lymphangiogenesis in gastric cancer by inhibition of HMGB1/VEGF-D signaling.
Keywords: curcumin, gastric cancer, HMGB1, lymphangiogenesis, VEGF-D
Introduction
Gastric cancer is the second leading cause of cancer-related death.1 The patients with gastric cancer usually have a poor prognosis due to the tumor metastasis and relapse. Thus, identification of the therapeutic strategy is urgendly needed.
Curcumin, a polyphenolic compound derived from turmeric (Curcuma longa), has been shown to have potent anti-metastatic effects in a variety of tumors.2 Curcumin has been shown to induce cell apoptosis and cell cycle arrest,3 and inhibit the proliferation and invasion of gastric cancer cells.4 Likewise, curcumin displays the anti-lymphangiogenic effects by inhibition of tube formation in rat lymphatic endothelial cells.5 However, how curcumin exerts anticancer effects in gastric cancer remains unknown.
High mobility group box 1 (HMGB1), a nuclear and extracellular protein, participates in a variety of physiologic and pathologic conditions, including immune response, inflammation, and cancer. HMGB1 is found as a useful biomarker for early diagnosis of gastric cancer.6 Curcumin downregulates the cell surface receptor of HMGB1 in human endothelial cells.7 HMGB1 is highly expressed in esophageal squamous cell carcinoma and regulates the expression of vascular endothelial growth factors (VEGF)-C to promote lymphangiogenesis.
Lymph node metastasis is a critical determinant of gastric cancer progression. Studies show that it is linked to tumor lymphangiogenesis and the formation of new lymphatic vessels.8 Curcumin has been reported to induce the downregulation of the lymphangiogenic VEGF-C and VEGF receptors VEGFR-2/3.9 HMGB1 and VEGF-D expressions are involved in tumor lymphangiogenesis, and VEGF-C and VEGF-D belong to the same family of VEGF. Herein, we hypothesize that curcumin could possess the anti-lymphangiogenesis by inhibition of HMGB1/VEGF-D signaling and provide a likely therapeutic strategy for the treatment of gastric cancer.
Material and methods
Cell culture
The human gastric cancer cell lines AGS and SGC-7901 were purchased from Chi Scientific, Inc. (China). SGC-7901 cells were cultured in RPMI-1640 (HyClone; GE Healthcare, Chicago, IL, USA) and AGS cells were in Dulbecco’s modified Eagle’s medium (HyClone; GE Healthcare) followed by 10% (v/v) heat-inactivated fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), penicillin-streptomycin (100 IU/mL to 100 μg/mL), 2 mM glutamine, and 10 mM HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) buffer at 37°C and 5% of the CO2 in an incubator. Cells were passaged by 0.25% trypsin–ethylenediaminetetraacetic acid (EDTA) when they reached confluence.
Drugs: preparation of curcumin stock solution
Curcumin (Sigma-Aldrich, Inc., St. Louis, MO, USA) was dissolved in dimethyl sulfoxide (DMSO) and the stock solution was stored at 50 mM. The dilute solutions of all reagents were prepared freshly before each experiment. Human recombinant HMGB1 (rHMGB1, 1690-HMB-050) was purchased from R&D systems, USA.
Morphological changes
Cells were cultured in six-well plates with 5 × 105 cells per well for 24 h. After 24 h incubation in standard conditions, the cells were treated with curcumin at different concentrations in triplicate. The cells that were treated with the medium containing DMSO were used as control. After 24 h, morphological changes in the cells treated with curcumin were investigated using an inverted microscope (MF52, Mshot Co., Guangzhou, China) and compared with control cells. With curcumin treatment, cell atrophy occurs and the cells appear round when observed under the microscope.
Apoptosis assay
AGS and SGC-7901 cells were plated in six-well plates (5 × 105 cells per well) and treated with a range of concentrations of curcumin for 24 h. The cells were then washed and stained according to the manufacturer’s instructions (Annexin V Apoptosis Detection Kit, Beyotime Inst, Roche, USA). The stained samples were then assessed by a FACScan flow cytometry (Becton Dickinson, NJ, USA) to identify apoptotic cells. The unstained cells included APC Annexin V (no propidium iodide (PI)) stained only, and PI (no APC Annexin V) stained only were used to set up for the compensation and quadrants in the flow cytometry analysis. The percentage of apoptotic cells (Annexin V positive cells) were recorded and calculated.
Quantitative real-time polymerase chain reaction
The quantitative real-time polymerase chain reaction (qRT-PCR) was used to detect the mRNA expression of HMGB1 and VEGF-D in GC (germinal center) cells. Total RNA was extracted using RNeasy Kit (Qiagen, #74104). The highest purity RNA (A260/A280 ratio of 1.8 or higher) was used to amplify PCR fragments. RNA concentration and purity for each sample was detected with Agilent 2100 Bioanalyzer (Agiletn Technlogies, Palo Alto, CA, USA). RNA reverse transcription was performed using the TaKaRa reverse transcriptase M-MLV kit (2641A; Takara) according to the manufacturer’s protocol. The qRT-PCR was performed in triplicate by using SYBR Premix Ex Tag™ II (RR820A; Takara) in 20 μL reaction volumes. Relative mRNA gene expression was calculated using the 2–∆∆Ct method using primers specific for human HMGB1: Primer ID (HQP008883) and human VEGFD: Primer ID (HQP005451) (GeneCopoeia Inc., Rockville, MD). The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was used as an internal control for normalization of RNA quantity and quality differences in all samples. We also used rHMGB1 to transfect into curcumin-treated gastric cancer cells.
Western blot analysis
AGS and SGC-7901 cells (5 × 105) were seeded in six-well plates. Cells were harvested and lysed after incubation with a range of concentrations of curcumin for 24 h. A total of 100 μg protein per lane was subjected to 10% SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis) and transferred to a membrane, and then was detected with primary antibodies: caspase-3 (9662, Cell Signaling), VEGF-D (sc-373866, Santa Cruz), and GAPDH (MBS2530174, MyBioSource). Appropriate horseradish peroxidase–conjugated secondary antibodies were added in tris buffered saline (TBS) containing 5% non-fat milk. The bound antibodies were visualized by using an enhanced chemiluminescence reagent (Millipore, USA) followed by exposure to X-ray film. Each sample was measured and normalized using a GAPDH run on the same gel. The data were expressed as relative expression ratio to GADPH. Triplicate experiments were performed.
Statistical analysis
Data were summarized as mean ± standard deviations (SD). Statistical analysis was performed using the one-way analysis of variance (ANOVA) and the post hoc test was Boferroni test. All statistical assessments were two-tailed. A P value of <0.05 represented statistical significance.
Results
The effects of curcumin on morphological changes of gastrc cancer cells
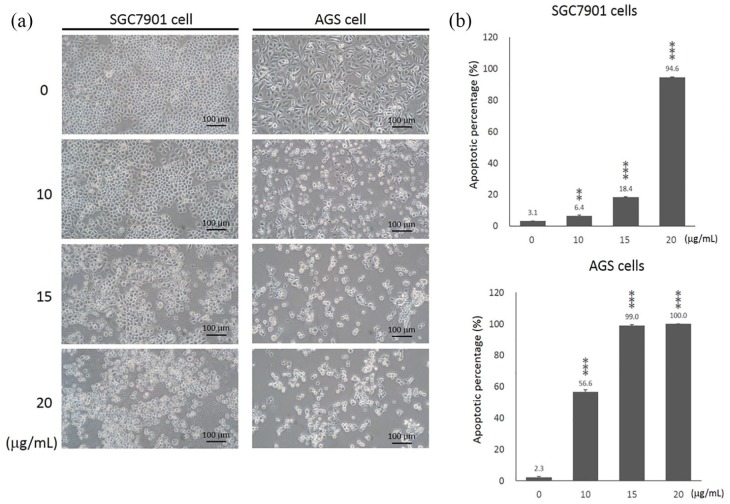
Study of control cells using an inverted microscope indicated that these cells had normal morphological specifications and maintained these specifications until the completion of treatment steps. Morphological study of the cells treated with curcumin for 24 h indicated that both AGS and SGC-7901 cells exhibited different degrees of morphological changes (Figure 1(a)). When treated with curcumin for 20 μg/mL, most of the cells were dead in these two cell lines after 24 h. At the dose of 15 μg/mL, curcumin reduced the number of viable cells and induced substantial morphological changes in both AGS and SGC-7901 cells after 24 h. However, the viable cells of AGS cells were not as much of SGC-7901 cells. This result showed that AGS cells may be more sensitive to the curcumin treatment than SGC-7901 cells (Figure 1(a)).
Figure 1.
The effects of curcumin on the morphology and cell apoptosis of gastric cancer cells. SGC-7901 and AGS cells were treated with curcumin at doses of 0, 10, 15, and 20 μg/mL for 24 h. (a) Cell morphological changes were studied using an inverted microscope (100× magnification). Scale bar: 100 μm. (b) The percentage of apoptosis cells (Annexin V positive cells) was determined using the fluorescence-activated cell sorting (FACS) analysis flow cytometry with APC Annexin V and PI staining. Data represent the mean ± SD.
**P < 0.01; ***P < 0.001.
The effects of curcumin on cell apoptosis
To determine the effects of curcumin on cell apoptosis of SGC-7901 and AGS cells, we performed a flow cytometry assay. Curcumin significantly induced cell apoptosis in SGC-7901 and AGS cells in a dose-dependent manner (Figure 1(b)). As shown in Figure 1(b), a significant amount of apoptosis cells was detected in SGC-7901 (94.63% ± 0.29, P < 0.001) cells by administration of 20 μg/mL curcumin. About 15 μg/mL curcumin significantly increased apoptotic cells of SGC-7901 from 3.10% (±0.10) to 18.39% (±0.38) (P < 0.001). At the dose of 10 μg/mL, curcumin significantly induced cell apoptosis of AGS cells (2.30% ± 0.46 vs 56.57% ± 1.35, P < 0.001). Most of AGS cells underwent cell apoptosis by curcumin at 15 μg/mL (99.03% ± 0.55, P < 0.001) and 20 μg/mL (100%, P < 0.001).
The effects of curcumin on the caspase-3 activation in gastric cancer cells
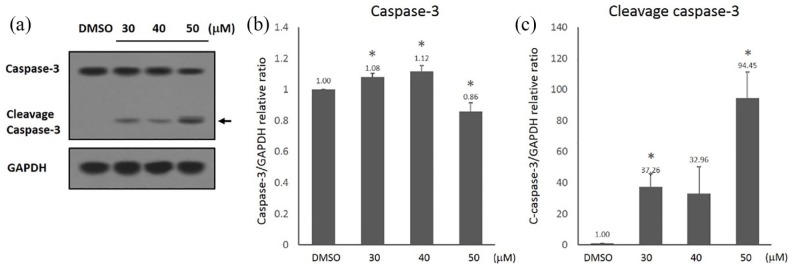
To investigate whether curcumin-induced cell apoptosis is dependent upon the caspase activation, we examined the molecular alteration of apoptosis-related proteins (caspase-3 and cleaved caspase-3) in SGC-7901 cells when treated with curcumin for 24 h. Caspase-3 and cleaved caspase-3 were measured by western blot analysis (Figure 2(a)). The expression levels of caspase-3 were significantly reduced in SGC-7901 cells when treated with 50 μM curcumin for 24 h, as compared with DMSO control group (Figure 2(b)). The activation of caspase-3 was studied by detecting the amount of cleaved caspase-3 protein. When the cells were treated with 10–50 μM curcumin for 24 h, the protein levels of cleaved caspase-3 in SGC-7901 cells were also significantly increased (Figure 2(c)). The data indicated that curcumin-induced cell apoptosis is associated with the caspase-3 activation.
Figure 2.
Curcumin increased the expression of apoptosis-related proteins. SGC-7901 cells were exposed to different concentrations of curcumin (0–50 μM). (a) Western blot analysis of the expressions of apoptosis-related proteins: caspase-3 and cleaved caspase-3; (b) quantitation of caspase-3 protein levels; and (c) quantitation of cleaved caspase-3 proteins levels. Protein expression levels were normalized by GAPDH. Data are represented as mean ± SD.
*P < 0.05.
Curcumin reduced the expression of HMGB1 and VEGF-D in gastric cancer cells
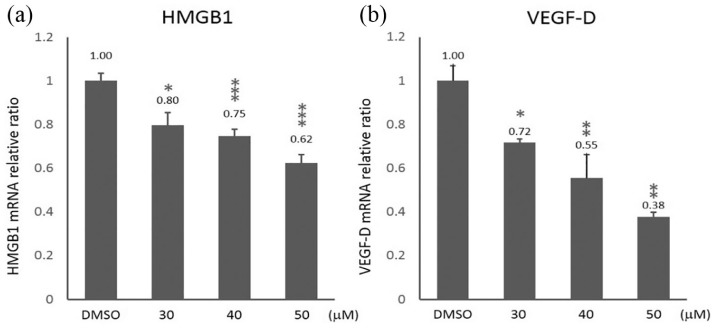
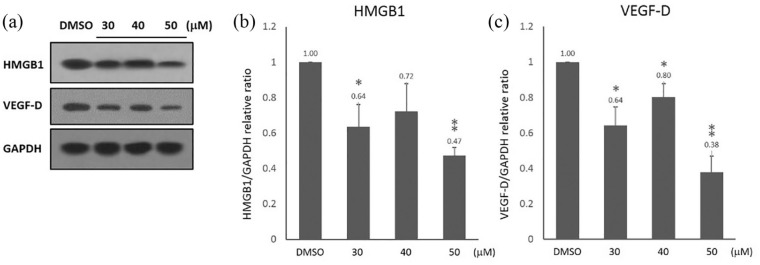
HMGB1 is highly expressed in gastric cancer and acts as a critical regulator of cell death and survival.10 VEGF-D is one of the key regulators of lymphangiogenesis which is associated with lymphatic metastasis in gastric cancer. To investigate the effects of curcumin on HMGB1 and VEGF-D expressions, we examined their expression levels in SGC-7901 cells treated with curcumin for 24 h. The qRT-PCR demonstrated that HMGB1 and VEGF-D mRNA levels were significantly decreased in SGC-7901 cells treated with curcumin in a dose-dependent manner (Figure 3(a) and (b)). Moreover, western blot was used to investigate the effects of curcumin on HMGB1 and VEGF-D protein levels in SGC-7901 cells (Figure 4(a)). We found that the protein levels of HMGB1 and VEGF-D were significantly reduced in SGC-7901 cells treated with 30–50 μM curcumin. As shown in Figure 4(b) and (c), the exposure to 50 µM curcumin for 24 h caused a more than 50% downregulation of HMGB1 and VEGF-D proteins in SGC-7901 cells.
Figure 3.
The effects of curcumin on the mRNA expression of HMGB1 and VEGF-D in SGC-7901 cells. SGC-7901 cells were treated with 30, 40, and 50 μM of curcumin for 24 h. The expression levels of (a) HMGB1 and (b) VEGF-D in curcumin-treated cells were analyzed by qRT-PCR. GAPDH served as internal marker. Data are represented as mean ± SD.
*P < 0.05; **P < 0.01; ***P < 0.001.
Figure 4.
The effects of curcumin on the protein expression of HMGB1 and VEGF-D in SGC-7901 cells. SGC-7901 cells were treated with 30, 40 and 50 μM of curcumin for 24 h. (a) Protein expression of HMGB1 and VEGF-D was analyzed by western blot. GAPDH served as a loading control. (b) Quantitation of HMGB1 protein levels. (c) Quantitation of VEGF-D protein levels. Protein expression levels were normalized by GAPDH. Data are represented as mean ± SD.
*P < 0.05; **P < 0.01; ***P < 0.001.
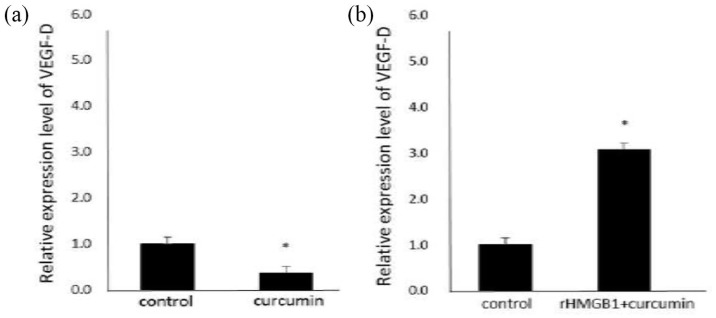
rHMGB1 eliminated the inhibitory effects of curcumin on VEGF-D mRNA expression
To address the role of HMGB1 in curcumin-induced downregulation of VEGF-D expression in gastric cancer cells, we used rHMGB1 to transfect into curcumin-treated gastric cancer cells. We found that rHMGB1 significantly induced VEGF-D expression in SGC-7901 cells and reversed the inhibitory effects of 50 µM curcumin on VEGF-D expression (Figure 5).
Figure 5.
The effects of rHMGB1 on VEGF-D expression in curcumin-treated SGC-7901 cells. SGC-7901 cells were treated with rHMGB1 (2 μg/mL) for 24 h, and then were exposed to 50 μM curcumin for 24 h. The mRNA expression of VEGF-D was evaluated by qRT-PCR.
*P < 0.05.
Discussion
The search for new antitumor agents that are more effective and less toxic is needed in the treatment of cancer. Curcumin possesses anticancer activities involved in regulating the mutagenesis, oncogene expression, tumorigenesis, and metastasis in multiple human carcinomas,2 such as prostate cancer, breast cancer, and colon cancer. The molecular mechanisms by which curcumin exerts anticancer effects are complicated and diverse. Curcumin inhibits the growth of gastric cancer cells4 and has the antiproliferative effects by inhibition of c-Myc/H19, PAK1/cyclin D1,11 and opphosphatidylinositol-3 kinase (PI3K) signaling pathways.12 Herein, we found that curcumin induces cell apoptosis of gastric cancer cell lines by the activation of caspase-3, and downregulated the expression of HMGB1 and VEGF-D.
HMGB1 and VEGF-D expressions are involved in tumor lymphangiogenesis. Curcumin can suppress HMGB1/VEGF-C and VEGFR-2/3 signaling to inhibit the lymphangiogenesis. Herein, curcumin was found to decrease the mRNA and protein expression of HMGB1 and VEGF-C in gastric cancer cells, and rHMGB1 could reverse the inhibitory effects of curcumin on VEGF-D expression. It was possible that curcumin inhibited the HMGB1/VEGF-D signaling to suppress lymphangiogenesis of gastric cancer cells.
As a natural product, curcumin exhibits the inhibitory effects on a multitude of pathways involved in carcinogenesis and tumor metastasis. These promising results for the use of curcumin in the treatment of gastric cancer were obtained, and the complexity of interaction between HMGB1/VEGF-D axis was needed to be elucidated. A systemic in vivo study of the potential mechanism of curcumin in the regulation of HMGB1/VEGF-D axis might provide an adjuvant therapy for gastric cancer.
In conclusion, curcumin might exert anti-lymphangiogenesis in gastric cancer cells by inhibition of the HMGB1/VEGF-D signaling, and no previous articles have been reported. Our research may provide the likely therapeutic strategy for the treatment of gastric cancer.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Jinshui Zhu  https://orcid.org/0000-0001-8537-8747
https://orcid.org/0000-0001-8537-8747
References
- 1. Chen W, Zheng R, Zhang S, et al. (2017) Cancer incidence and mortality in China, 2013. Cancer Letters 401: 63–71. [DOI] [PubMed] [Google Scholar]
- 2. Shanmugam MK, Rane G, Kanchi MM, et al. (2015) The multifaceted role of curcumin in cancer prevention and treatment. Molecules 20(2): 2728–2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xu XY, Meng X, Li S, et al. (2018) Bioactivity, health benefits, and related molecular mechanisms of curcumin: Current progress, challenges, and perspectives. Nutrients 10: E1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhou S, Yao D, Guo L, et al. (2017). Curcumin suppresses gastric cancer by inhibiting gastrin-mediated acid secretion. FEBS Open Bio 7: 1078–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Matsuo M, Sakurai H, Koizumi K, et al. (2007) Curcumin inhibits the formation of capillary-like tubes by rat lymphatic endothelial cells. Cancer Letters 251: 288–295. [DOI] [PubMed] [Google Scholar]
- 6. Chung HW, Lee SG, Kim H, et al. (2009) Serum high mobility group box-1 (HMGB1) is closely associated with the clinical and pathologic features of gastric cancer. Journal of Translational Medicine 7: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim DC, Lee W, Bae JS. (2011) Vascular anti-inflammatory effects of curcumin on HMGB1-mediated responses in vitro. Inflammation Research 60: 1161–1168. [DOI] [PubMed] [Google Scholar]
- 8. Tawada M, Hayashi S, Osada S, et al. (2012) Human gastric cancer organizes neighboring lymphatic vessels via recruitment of bone marrow-derived lymphatic endothelial progenitor cells. Journal of Gastroenterology 47: 1057–1060. [DOI] [PubMed] [Google Scholar]
- 9. Da W, Zhu J, Wang L, et al. (2015) Curcumin suppresses lymphatic vessel density in an in vivo human gastric cancer model. Tumor Biology 36: 5215–5223. [DOI] [PubMed] [Google Scholar]
- 10. Zhang QY, Wu LQ, Zhang T, et al. (2015) Autophagy-mediated HMGB1 release promotes gastric cancer cell survival via RAGE activation of extracellular signal-regulated kinases 1/2. Oncology Reports 33: 1630–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cai XZ, Wang J, Xiao-Dong L, et al. (2009) Curcumin suppresses proliferation and invasion in human gastric cancer cells by down-regulation of PAK1 activity and cyclin D1 expression. Cancer Biology & Therapy 8: 1360–1368. [DOI] [PubMed] [Google Scholar]
- 12. Fu H, Wang C, Yang D, et al. (2018) Curcumin regulates proliferation, autophagy, and apoptosis in gastric cancer cells by affecting PI3K and P53 signaling. Journal of Cellular Physiology 233: 4634–4642. [DOI] [PubMed] [Google Scholar]