Abstract
Absence epilepsy is a disorder of thalamocortical networks. Animal models have provided detailed information regarding the core cellular, synaptic, and network features that contribute to the electroencephalogram spike and wave discharge characteristic of typical absence epilepsy. Understanding of seizure networks and dynamics is a critical step toward improving treatments, yet competing conceptual models have evolved to explain seizure initiation and propagation. Recent studies have questioned 2 key model concepts: (1) T-type Ca2+ channel-dependent burst firing in thalamic relay neurons may not be essential for seizure generation, bringing into question the proposed mechanism for the antiepileptic drug ethosuximide in reducing thalamic bursting and (2) widespread synchronized neural activity may not be a core feature of the seizures, indicating that reductions in synchrony would not be a productive therapeutic goal. In this review, I will discuss these current findings, highlight the innovative approaches that have enabled these insights, and provide a unified framework that incorporates these sometimes-conflicting ideas. Finally, I lay out future work that will be necessary to finally resolve the remaining issues.
Introduction
The general network features underlying absence seizures have been known for some time. Early work in felines documented that behavioral absences in naive animals could be triggered simply by periodic stimulation of midline thalamus, which produced a spike and wave discharge (SWD) electroencephalogram (EEG) signal indistinguishable from absence seizures in people.1 Beyond this, the feline penicillin model provided the understanding that not only do both cortical and thalamic structures participate in generation of SWD but also that both structures are required.2 More recently, rodent genetic and experimental models have provided additional insights and greater understanding of the specific cellular and synaptic features of absence epileptic networks.3–5
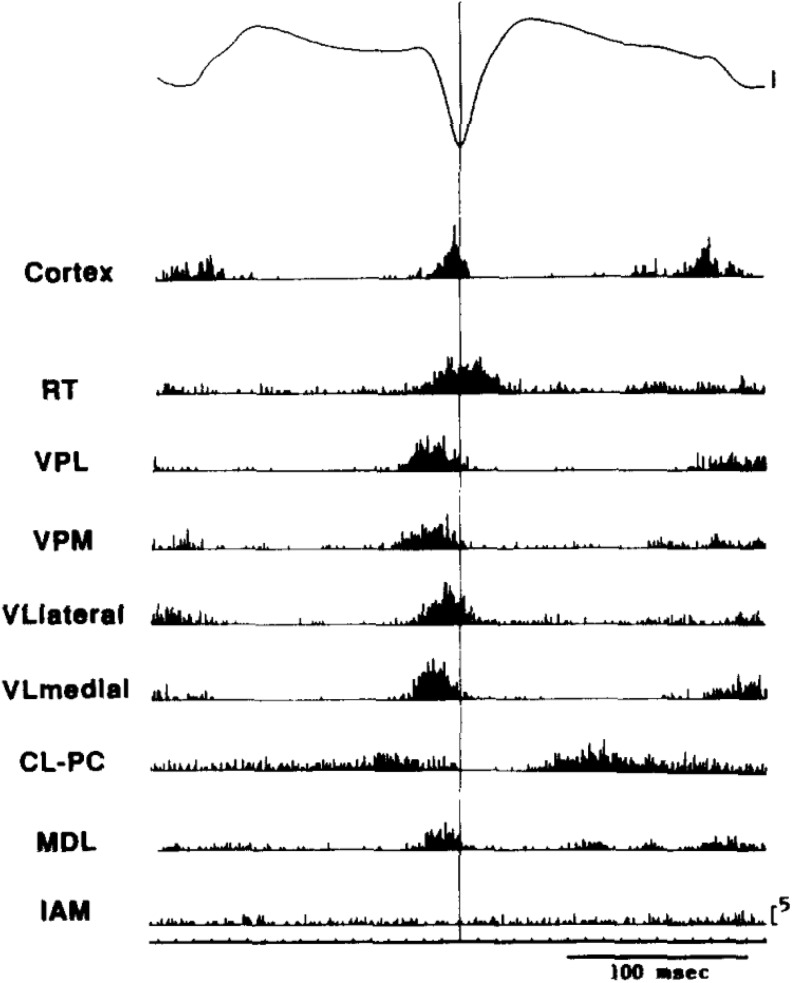
One key electrophysiological feature of SWD is coordinated, that is, synchronized, firing of the neural elements in the extended thalamocortical (TC) network. First observed in the feline model (reviewed in Gloor et al)2 and more recently in rodent models (Figure 1), action potential firing is largely synchronized to the spike of the spike and wave EEG signal in both cortical and thalamic portions of the TC loop, with little spiking during the wave itself. This suggests that activity becomes entrained into coordinated firing across widespread brain regions and does so quite rapidly as the SWD becomes bilaterally synchronous within just a few cycles of activity. This explains in part the large amplitude signal observed with the scalp EEG, arising from coordinated synaptic responses and/or firing in the underlying neural population. Hence, one key concept in absence networks is widespread synchrony. However, the precise nature of such synchrony remains obscure. Might it, for example, involve recruitment of the majority of neurons in each participating region? Or, at the other extreme, might just a small proportion of neurons (perhaps less than 5%) be sufficient for engagement of the epileptic network, if the firing is appropriately coordinated?
Figure 1.
Widespread synchrony and participation of thalamic and cortical neurons during spike wave discharges in the WAG/Rij genetic rat model of absence seizures. Signals were aligned and averaged across many individual spike wave cycles. Multiunit spikes were detected in multiple electrode locations throughout the thalamus. Note that coordinated spiking occurs in all cortical and thalamic locations (except IAM and perhaps CL-PC) and that peak spiking rate occurs very near the peak of the EEG spike, with very little spiking outside this time frame, suggesting synchronous firing and coordinated pauses across the thalamocortical axis. Adapted with permission from Inoue et al.6 CL-PC indicates centrolateral–paracentral; EEG, electroencephalogram; IAM, interanteromedial; MDL, mediodorsal, lateral portion; RT, reticular thalamus; VPL, ventroposteriolateral; VPM, ventroposterior medial; VLlateral, ventrolateral, lateral portion; VLlmedial, ventrolateral medial portion.
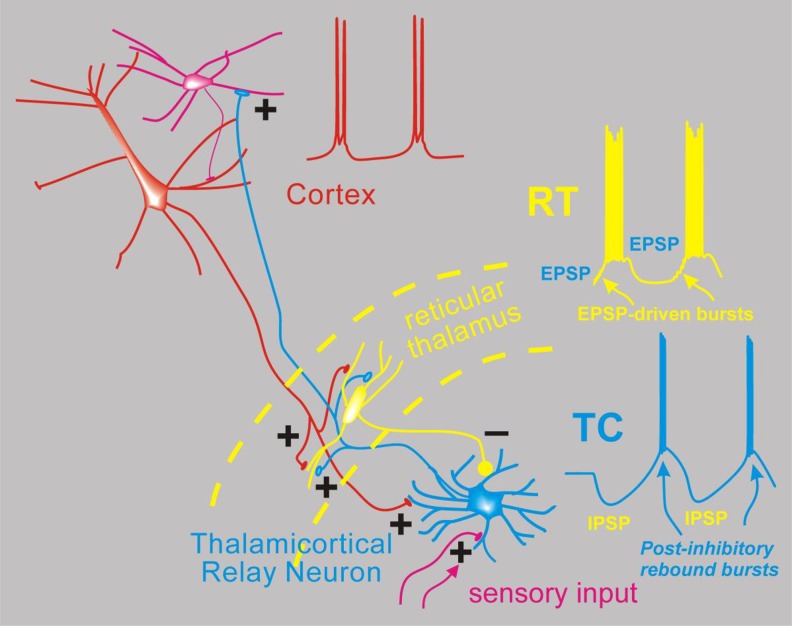
The second key concept, developed largely from in vitro models of epileptic subnetworks, is that seizure-related coordinated firing in the TC system7 is dependent on 2 cellular features (Figure 2). First, TC relay neurons are endowed with high levels of low-voltage-activated Ca2+ channels (CaV3.1, T-type, reviewed in Huguenard).8 This provides them with the relatively unique ability to fire paradoxical burst spiking responses after a membrane hyperpolarization,9 such as that produced by synaptic inhibition. Second, the thalamic subnetwork is composed mainly of reciprocally connected TC cells and reticular thalamic (RT) neurons. The latter, composed exclusively of GABA-containing inhibitory neurons,10 also expresses CaV3-family channels and generates burst responses, often without requiring membrane hyperpolarization. This intrathalamic excitatory/inhibitory loop (Figure 2), with powerful burst firing in each part, is proposed to provide the coordination of firing, at least in the thalamic portion of the network, that is characteristic of absence seizures (reviewed in Fogerson and Huguenard).3 As TC cells are reciprocally interconnected via long-range bidirectional excitation with corresponding cortical regions, the cortical activity might then coordinate, reinforce, and/or entrain the thalamic subnetwork (Figure 2). These cellular/synaptic features and their dynamics are critical for our understanding of current and future antiabsence medications. The current working model is that ethosuximide and related succinimides and oxazolidinediones block thalamic CaV3 channels to exert their seizure-blocking effects.11,12 The second key concept is then that CaV-dependent burst firing in thalamus coordinates neural activity and contributes to the generation of SWD.
Figure 2.
Simplified network model for absence seizures. Depicted are interconnected neurons of the thalamus and cortex and the spiking activity proposed to occur during spike wave discharge of an absence seizure. From the bottom, thalamocortical (TC) relay neurons (blue) normally receive sensory input in the form of excitatory synapses (+) and transform this into TC cell spikes that propagate the sensory signal to the cortex (red). Thalamocortical neurons emit axon collaterals into the reticular thalamus (RT, yellow), where excitatory synapses activate the resident inhibitory neurons. These then provide feedback inhibition (inhibitory post-synaptic potentials [IPSPs]) to TC cells. During absence seizures, the network presumably becomes synchronized. In this scenario, multiple RT cells fire together producing strong IPSPs onto TC cells, and since TC cells contain high levels of T type calcium channels,8 strong inhibition leads to robust postinhibitory rebound bursts that in turn reactivate both RT and cortex (excitatory blue axonal fibers). Cortical cell firing (red) is also synchronized so that its excitatory output can combine with TC output to strongly activate RT cells, which also contain T-type calcium channels and generate bursts of action potentials via direct synaptic excitation. In this model, cells from all 3 classes (Cortex, TC, RT) actively participate in a near synchronous manner, with the inhibitory output from RT delaying TC cell bursting and thus playing a major role in pacing the epileptic network.
Because of the high level of experimental control afforded by simplified brain slice microcircuit preparations, the nature of the interactions between cell types in the network have been rigorously evaluated, thus leading to a well-characterized model, at least in mice, rats, and ferrets.13–15 This oversimplified model, with basically just 3 cell types (TC, RT, and cortical neurons) and sufficient network convergence/divergence, can potentially explain the basic timing and coordination of TC networks during SWD. However, this type of model ignores other interacting networks, such as those in basal ganglia,16 cerebellum,17 and various neuromodulatory systems.18,19 Further, this simple model provides no predictions regarding the site of seizure initiation per se as presumably the large-scale hypersynchronized core network might be engaged by precursor activity in either cortical or thalamic subnetworks. Indeed, there is evidence for each (eg, Meeren et al and Sorokin et al).20,21 This leaves open the question of how well the simplified in vitro models fit with behavior of intact in vivo networks in which seizures are actually generated. Modern neuroscience has provided advanced electrophysiological and imaging approaches that now allow for the first time a detailed understanding of large-scale cellular activities in various mouse and rat models of SWD. These methods allow for manipulating and/or recording of the activity of multiple neurons simultaneously, and results are starting to become available to address this larger issue. The methods of optogenetics, silicon multielectrode probes, and activity-dependent imaging have been separately applied in 3 studies that will be summarized in this review. These studies all bear on the key questions outlined earlier regarding the conceptual model. First, what is the degree of synchrony in neural populations during SWD, and second what is the evidence for rebound burst responses in thalamic neurons during SWD?
Neuronal Quiescence During Seizures
In one of the first epilepsy studies utilizing genetically encoded Ca2+ indicators (GEGIs), Meyer et al22 used a viral approach to express sixth-generation GECIs in the GCaMP family in neurons of primary visual cortex of mice. Through iterative engineering, each successive generation of GCaMP sensors shows improvements in terms of temporal responsiveness and sensitivity. For example, GCaMP6m can show a nearly 100-fold change in fluorescence based on activity-dependent changes in intracellular Ca2+ 23 and so provides a readily detectable optical signal that can be well resolved, even in vivo, especially with increased spatial resolution afforded by 2 photon (2P) microscopy. In a highly technical and sensitive approach, Meyer et al utilized their expertise in 2P studies of the visual system to address this question using epileptic stargazer mice24 that have a loss of function of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-receptor targeting protein γ2.25 They trained mice to adopt normal locomotive behavior on a rotary treadmill while head-fixed and implanted for EEG recordings, and with 2P microscopy, could record individual GCaMP fluorescent signals in neocortical neurons both during normal behavior and during seizures, all in unanesthetized animals. Most experiments focused on superficial neurons in layers II and III, but all cortical layers were sampled in at least some animals. Other analysis and experiments were performed but here we will focus on the GCaMP imaging data that provide a means to record simultaneous activity from many neurons within a microscopic field. The large-scale imaging approach is powerful in that it allows for real-time measurement of coordinated neural activity.
In contrast to the expected finding of enhanced synchrony and increased neural firing of neocortical neurons during SWD, these authors found the opposite. In V1, primary visual cortex, neural activity was largely decreased, with 80% of neurons showing reductions in GCaMP signals during SWD seizures compared to baseline. Both somatic and neuropil (regions outside of clear soma boundaries) signals were routinely lower during seizures and often showed rebound responses following each seizure that could be greater than baseline. These general findings in layer II/III were reproduced in all cortical lamina (II/III, IV, V, and VI) of V1.
The GCaMP signal reflects increases in not only intracellular calcium but also the properties of the sensor itself. For example, GCaMP6m has a rise time of ∼200 milliseconds and a decay time of ∼1 second23; both of these values are orders of magnitude larger than the time of an individual action potential (∼1 millisecond). Thus, while the signals can be quite bright and reliable, they do not provide information on precise timing of neural firing. Nevertheless, mathematical approaches have been developed to estimate the timing and number of action potentials that might have driven the changes in Ca2+ signal. For example, once the GCaMP signal associated with validated action potential response is obtained, then individual action potential events can be estimated from the GCaMP time series by the method of deconvolution.26 Using this approach, the authors estimated synchrony of activity within the microscopic field by correlation analysis and found by this approach that pairwise correlations were decreased during the seizures.
The authors conclude then that neural activity decreases in visual cortical neurons during absence seizures in stargazer mice and found little or no evidence of synchronized cortical firing. These conclusions are striking and indicate that many assumptions about absence networks need to be validated. In interpreting these results from mouse, there are several technical considerations to be considered. First, might neural activity in primary visual cortex be either indirectly related, or even unrelated to absence seizures? In animal models of absence, the appearance of SWD is primarily frontoparietal,27 with reduced power in posterior regions, including visual cortex.17,20,28,29 This general EEG localization pattern is consistent with clinical findings in absence patients.30–32 How might these findings of decreased firing and synchrony of mouse V1 neurons during SWD relate to brain-wide activity in typical absence epilepsy in people? Most clinical studies utilizing Blood Oxygen Level-Dependent imaging (BOLD) signals have documented BOLD increases in thalamus, consistent with increased neural activity, and decreases in cortical areas largely centered in the default mode network, but with little evidence of change in primary cortices, such as visual cortex.33–37 The finding of rebound activity in V1 following each seizure in stargazer mice might suggest, for example, that a general suppression of other cortical regions outside the primary epileptic regions is a feature of absence epilepsy and that there is a natural rebound in neural response following such periods of silencing. A further consideration for interpreting the results is the nature of cross-correlation analysis, which will have limited temporal resolution due to the slow nature of GCaMP signaling noted earlier.
Manipulations of Thalamic Burst Firing in the Context of Absence Seizures
Sorokin et al21 used optogenetic methods to manipulate the activity of thalamic neurons in 2 rodent seizure models of absence epilepsy and methods to modify neurons to probe their function during seizures. Similar to the Meyer et al’s study, they used stargazer mice, and also used WAG/Rij rats, which are an inbred strain with high incidence of SWD.38 The approach was to modify the ability of thalamic neurons to generate burst responses to determine whether there was a relationship between thalamic bursting and the seizures themselves. Using again a viral approach, they engineered thalamic relay neurons to express 1 of 2 optogenetic effectors, halorhodopsin (eNpHR3.0)39 which activates a Cl− pump, causes membrane hyperpolarization and inhibits neurons, or stabilized step function opsin (SSFO; Yizhar et al40), which produces a long-lasting, subthreshold membrane depolarization that can increase the probability of firing without directly evoking action potentials. These opsins were expressed in relay neurons of the somatosensory thalamus, which is one of the most active thalamic regions during absences.20,41 Fiber optic cannula/electrodes were then implanted above the thalamus to (1) deliver yellow (594 nm) or blue (488 nm) light to the thalamic region where opsins were expressed and (2) record electrical activity in the form of extracellular multiunit responses in the same region to which light was delivered.
Sorokin et al found that a pulse of 594-nm light, as brief as 50 milliseconds, applied unilaterally to eNpHR-expressing TC cells silenced neurons during the pulse, presumably a result of membrane hyperpolarization.21 The fiber-attached multiunit electrode recorded a brief post-light stimulation rebound burst of action potentials in a group of nearby TC neurons. This was as expected according to the postinhibitory rebound model (Figure 2). These pulses, either singly or in trains, could initiate bilateral SWD in both epilepsy models, which suggests that coordinated rebound burst firing in a group of TC cells is sufficient to initiate an absence seizure.
Next, they used SSFO to test whether membrane depolarization in TC cells, which would prevent burst firing, would prevent or abort seizures. For the latter, they adopted a real-time detection algorithm that could rapidly identify seizures within 0.5 second of their onset and then used the detection signal to trigger TC depolarization with blue light. Using this approach, they were able to block burst firing, as measured with the multiunit electrodes, and replace it with regular, tonic firing. This change in firing mode was rapid and associated with a nearly immediate (<< 1s) blockade of the seizure both electrically and behaviorally.
While optogenetics provides a powerful tool to modify neural activity in specific types of neurons, there are several technical limitations to this study as well. First of all, the recorded activity was from coarse metal electrodes that could not resolve single neural activities such that the recorded “burst” responses likely resulted from an unknown mixture of single-cell bursting and nearby simultaneous cell firing. Thus, the incidence of true, single neuron postinhibitory burst responses (as in Figure 2) remains unknown. Second, the triggering of SWD and associated seizures by eNpHR triggered hyperpolarization, and presumed postinhibitory rebound was only proven in animal models with known absence seizures, so it remains to be determined whether coordinated postinhibitory rebound is sufficient to evoke seizures in naive nonepileptic animals. Third, the blockade of burst firing with SSFO-mediated depolarization was associated with an increase in overall firing rate, and it remains to be determined which of these 2 factors (reduced bursting or increased firing) is primarily responsible for seizure termination. Fourth, optogenetic stimulation can result in simultaneous activation and/or inhibition of large portions of a local network. This likely induces an artificial network response that is far more synchronous from that occurring normally.
Reduced Thalamic Activity and Bursting During SWD
McCafferty et al42 used a third approach, high-density silicon probe recording, to determine neural responses in thalamus and cortex in 2 rat models of absence epilepsy, first in a well-established inbred rat strain, Genetic Absence Epilepsy Rats from Strasbourgh,43 and also in naive rats treated with γ-butyrolactone.44 The development of high-density multielectrode silicon probes along with multichannel amplifiers is revolutionizing neuroscience, as now it is possible to record simultaneously from hundreds, if not thousands, of individual neurons with high temporal precision on the order of milliseconds. Although the individual electrodes pick up multiunit activity from several nearby neurons, the density of individual electrode pads on the silicon probes is sufficient that individual neural action potentials can be recorded simultaneously from multiple locations. This dramatically improves the ability to detect well-resolved individual “units,” presumed neurons, because firing of that unit will provide synchronized and stereotyped signals on all electrode pads receptive to that neuron. Thus, with this approach, it becomes possible to get closer to the questions of both pattern of spiking (bursts vs regular and tonic spikes) as well as coordinated activity between neurons, now with millisecond resolution.
McCafferty et al exploited this power to record extracellular, well-resolved units from cortex and thalamic TC and RT neurons during experimental absences in rats. They find that a subset of RT neurons increase burst firing during the seizures, and consistent with the loss of firing (in cortex) during seizure reported by Meyer et al, found a simultaneous 52% reduction in TC cell firing, including apparent silence of many TC neurons during the seizures, suggesting a requirement for RT cell but not TC cell participation in the seizures. While RT cells showed clear increases in burst firing, the effects on TC burst firing were less clear. By some measures, seizure-related bursting was low, for example, incidence of bursts within seizures was only 16%. Yet by others, the overall participation in bursts shows seizure-related increases. For example, TC tonic firing rate went down during seizures by about 50%, while burst rate actually rose about 3-fold, starting even before the seizure is detected and persisting at a level of about double the background throughout the entire seizure duration. Thus, the ratio of bursting to tonic firing dramatically increased from about 1:20 (5%) before the seizures to 6:10 (60%), a greater than 10-fold increase in relative burst rate.
To further test for a role of CaV3 channel–dependent burst firing during the seizures, the authors utilized reverse microdialysis, a method to deliver reliable concentrations of drugs into the brain parenchyma. They delivered various concentrations of TTA-P2, a potent and specific antagonist of CaV3 family members45 into sensory cortex or various locations in dorsal thalamus while monitoring seizure incidence and unit firing activity. They found that TTA-P2 was effective in all cortical and thalamic locations but at different concentrations. For example, cortical or centro-lateral thalamic locations required 1 mmol/L of the compound to reduce seizure incidence, but lower concentrations (0.3 mmol/L) were effective at more lateral thalamic locations near RT. This suggested that absence seizures were relatively insensitive to CaV3 channel blockade in TC cells, and the authors propose that effects seen were a result of diffusion of the channel blocker to RT and that reduction in RT bursting was primarily responsible for the seizure reduction.
As with previous studies, these powerful and advanced methods provide new and important information regarding seizure networks. The multiple electrode sampling does provide increased confidence that single neuron responses were captured, and so for the first time, this study provided very detailed information on the precise firing timing of many neurons extended across many brain regions during absence seizures. Like the other studies, the methods and approach have technical limitations. For example, an alternate explanation to the increased sensitivity of RT to CaV3 channel blockade is that there is a critical mass effect, and RT, due to its smaller volume, is more uniformly influenced by a local microdialysis probe. Thus, the overall proportion of RT neurons that can burst following CaV3 channel blockade becomes very low. By contrast, TC cells that participate in SWD seizures extend across multiple thalamic nuclei,46 such that a point-source microdialysis probe may be less likely to broadly influence their function without using higher drug concentrations. On this point, the authors have previously reported using different experimental conditions (eg, with anesthesia) that the approaches they applied here abolished burst firing broadly across many thalamic regions, yet this was not directly shown for the current study.
An additional concern that applies broadly to unit isolation in epileptic networks is that the ability to isolate units is strongly compromised during periods of local synchrony, such as occurs with the macro-EEG “spikes” of the spike and wave complexes. These are associated with intense neuronal firing at much higher frequencies than the background activity, and individual action potential waveforms can overlap and interfere with each other, potentially leading to underreporting of spike activity, especially during the seizures themselves. Our TC cell spike isolation experience with mouse and rat absence models is similar to that reported by McAfferty (their Figure 1B, mid-seizure). Typically, we observe 10 to 20 action potentials on individual electrodes per epileptic spike, of which only a few can be unambiguously isolated, leading to underreporting of spike counts by ∼80% during such epochs. Both the reported decreased spiking of TC cells and low incidence of TC bursting during seizures would be influenced at some level by this technical consideration.
Conclusions
While each of these reports has utilized powerful, modern neuroscience methods to address the question of neural participation during seizures, they have arrived at different conclusions. McCafferty et al and Meyers et al conclude that neural activity, at least in some thalamic or cortical regions, decreases during seizures. By comparison, recall that BOLD studies have documented SWD-related decreases in cortical activity and increases in thalamic activity in patients with typical absence seizures (reviewed in Moeller et al, 2013).36 These results in animal models are interesting and, at first glance, difficult to reconcile with the large amplitude scalp EEG activities of seizures. Electroencephalogram SWD must represent coordinated neural responses in neocortex that summates to produce a large scalp signal, but this may largely represent feedforward synaptic inhibition, along with a reduction in overall neural firing, which would lead to decreased cortical BOLD signals during SWD. This decreased cortical activity presumably underlies the loss of consciousness associated with behavioral absences.
It will be interesting to see whether the lack of activity in V1 during SWD in stargazer mice is also seen in primary somatosensory cortex, where the seizures presumably originate. To fully resolve whether thalamic neurons decrease their overall firing and proportion of burst responses will require more direct measures of single-cell activities than that afforded by either multiunit recording (Sorokin) or multi-electrode unit isolation (McCafferty et al).42 This could be obtained with intracellular recordings that provide unambiguous signals from individual cells. While technically quite challenging to perform in vivo, this has recently been accomplished in felines47 and songbirds,48 suggesting it may be feasible in rodents.
Finally, the issue of the primary timing element responsible for the pacing of SWD remains unresolved. McCafferty et al demonstrate that RT activity seems to cause temporary cessation of TC cell firing, suggesting that the duration of RT inhibitory responses do indeed shape the overall rhythm. Their isolated unit results provide limited support for the concept of synchronized postinhibitory burst responses in TC cells. Yet functionally, even if the postinhibitory responses are not strong, single neuron multiple action potential bursts, they still could result in synchronized firing of multiple TC cells, as observed by Sorokin, resulting in a “population” burst dependent in part on thalamic CaV3 channels which boost firing of TC cells, even in the absence of clear single cell burst responses.49 Overall, the evidence continues to suggest that CaV3 channels in thalamus are critical for generation of SWD, especially those in RT nucleus. Resolution of the issue of requirement for TC cell burst firing during seizures will require more refined methods than currently available.
What have we learned from these 3 papers? First, that synchronous firing of neurons during seizures might be much lower than previously suspected, yet it remains a reliable feature, at least in portions of the network. Single and multiunit action potentials are well timed to the spike of the spike wave discharge in somatosensory TC cells21,42 and cortical cells,42 . Further, there is decreased activity, approaching silence, in the waves between spikes. By contrast, cortical regions outside somatosensory areas can show much lower level activity and synchrony.22 This indicates that the cortical mantle does not uniformly engage in a globally coordinated seizure discharge in absences. Second, that burst firing is a reliably detected feature in RT neurons, as is postinhibitory rebound action potential firing (bursts or single spikes),21,42 in TC cells even if the individual neuron burst responses might be intermittent or variable. Finally, these results continue to support the model in which RT cell output, dominated by burst firing during SWD, provides powerful inhibition of TC cells (as that is their only major target) that mandate periods of neuronal silence corresponding to SWD waves and thus plays a major role in pacing the epileptic network. Overall, these recent findings require a revision of the original model to add that blockade of CaV3 channels in either RT or TC cells will suppress absences, with the effect on RT cells potentially more powerful at the network level than the originally proposed effect on TC cells, and that the level of network synchrony during seizures may be much lower than previously thought.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Hunter J, Jasper H. Reactions of unanaesthetised animals to thalamic stimulation. Trans Am Neurol Assoc. 1948;73(73 Annual Meet):171. [PubMed] [Google Scholar]
- 2. Gloor P, Avoli M, Kostopoulos G. Thalamo-cortical relationships in generalized epilepsy with bilaterally synchronous spike-and-wave discharge In Generalized Epilepsy: Neurobiological Approaches, Boston, MA: Birkhäuser; 1990;190–212. [Google Scholar]
- 3. Fogerson PM, Huguenard JR. Tapping the brakes: cellular and synaptic mechanisms that regulate thalamic oscillations. Neuron. 2016;92(4):687–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maheshwari A, Noebels JL. Monogenic models of absence epilepsy: windows into the complex balance between inhibition and excitation in thalamocortical microcircuits. Prog Brain Res. 2014;213:223–252. [DOI] [PubMed] [Google Scholar]
- 5. McCormick DA, Contreras D. On the cellular and network bases of epileptic seizures. Annu Rev Physiol. 2001;63:815–846. [DOI] [PubMed] [Google Scholar]
- 6. Inoue M, Duysens J, Vossen JM, Coenen AM. Thalamic multiple-unit activity underlying spike-wave discharges in anesthetized rats. Brain Res. 1993;612(1-2):35–40. [DOI] [PubMed] [Google Scholar]
- 7. Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262(5134):679–685. [DOI] [PubMed] [Google Scholar]
- 8. Huguenard JR. Low-threshold calcium currents in central neurons. Annu Rev Physiol. 1996;58:329–348. [DOI] [PubMed] [Google Scholar]
- 9. Llinas R, Jahnsen H. Electrophysiology of mammalian thalamic neurones in vitro. Nature. 1982;297(5865):406–408. [DOI] [PubMed] [Google Scholar]
- 10. Houser CR, Vaughn JE, Barber RP, Roberts E. GABA neurons are the major cell type of the nucleus reticularis thalami. Brain Res. 1980;200(2):341–354. [DOI] [PubMed] [Google Scholar]
- 11. Coulter DA, Huguenard JR, Prince DA. Characterization of ethosuximide reduction of low-threshold calcium current in thalamic neurons. Ann Neurol. 1989;25(6):582–593. [DOI] [PubMed] [Google Scholar]
- 12. Coulter DA, Huguenard JR, Prince DA. Differential effects of petit mal anticonvulsants and convulsants on thalamic neurones: calcium current reduction. Br J Pharmacol. 1990;100(4):800–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huguenard JR, Prince DA. Intrathalamic rhythmicity studied in vitro: nominal T current modulation causes robust anti-oscillatory effects. J Neurosci. 1994;14(9):5485–5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. von Krosigk M, Bal T, McCormick DA. Cellular mechanisms of a synchronized oscillation in the thalamus. Science. 1993;261(5119):361–364. [DOI] [PubMed] [Google Scholar]
- 15. Warren RA, Agmon A, Jones EG. Oscillatory synaptic interactions between ventroposterior and reticular neurons in mouse thalamus in vitro. J Neurophysiol. 1994;72(4):1993–2003. [DOI] [PubMed] [Google Scholar]
- 16. Slaght SJ, Paz T, Chavez M, Deniau J-M, Mahon S, Charpier S. On the activity of the corticostriatal networks during spike-and-wave discharges in a genetic model of absence epilepsy. J Neurosci. 2004;24(30):6816–6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dow RS, Fernandez-Guardiola A, Manni E. The influence of the cerebellum on experimental epilepsy. Electroencephalogr Clin Neurophysiol. 1962;14:383–398. [DOI] [PubMed] [Google Scholar]
- 18. Lannes B, Vergnes M, Marescaux C, et al. Lesions of noradrenergic neurons in rats with spontaneous generalized non-convulsive epilepsy. Epilepsy Res. 1991;9(2):79–85. [DOI] [PubMed] [Google Scholar]
- 19. Warter JM, Vergnes M, Depaulis A, et al. Effects of drugs affecting dopaminergic neurotransmission in rats with spontaneous petit mal-like seizures. Neuropharmacology. 1988;27(3):269–274. [DOI] [PubMed] [Google Scholar]
- 20. Meeren HK, Pijn JP, Van Luijtelaar EL, Coenen AM, Lopes da Silva FH. Cortical focus drives widespread corticothalamic networks during spontaneous absence seizures in rats. J Neurosci. 2002;22(4):1480–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sorokin JM, Davidson TJ, Frechette E, et al. Bidirectional control of generalized epilepsy networks via rapid real-time switching of firing mode. Neuron. 2017;93(1):194–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Meyer J, Maheshwari A, Noebels J, Smirnakis S. Asynchronous suppression of visual cortex during absence seizures in stargazer mice. Nat Commun. 2018;9(1):1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen T-W, Wardill TJ, Sun Y, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499(7458):295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Noebels JL, Qiao X, Bronson RT, Spencer C, Davisson MT. Stargazer: a new neurological mutant on chromosome 15 in the mouse with prolonged cortical seizures. Epilepsy Res. 1990;7(2):129–135. [DOI] [PubMed] [Google Scholar]
- 25. Chen L, Chetkovich DM, Petralia RS, et al. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature. 2000;408(6815):936–943. [DOI] [PubMed] [Google Scholar]
- 26. Yaksi E, Friedrich RW. Reconstruction of firing rate changes across neuronal populations by temporally deconvolved Ca2+ imaging. Nat Methods. 2006;3(5):377–383. [DOI] [PubMed] [Google Scholar]
- 27. Studer F, Laghouati E, Jarre G, David O, Pouyatos B, Depaulis A. Sensory coding is impaired in rat absence epilepsy. J Physiol. 2019;597(3):951–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Letts VA, Beyer BJ, Frankel WN. Hidden in plain sight – spike-wave discharges in mouse inbred strains. Genes Brain Behav. 2014;13(6):519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nersesyan H, Hyder F, Rothman DL, Blumenfeld H. Dynamic fMRI and EEG recordings during spike-wave seizures and generalized tonic-clonic seizures in WAG/Rij rats. J Cereb Blood Flow Metab. 2004;24(6):589–599. [DOI] [PubMed] [Google Scholar]
- 30. Koutroumanidis M, Smith S. Use and abuse of EEG in the diagnosis of idiopathic generalized epilepsies. Epilepsia. 2005;46(Suppl 9):96–107. [DOI] [PubMed] [Google Scholar]
- 31. Rodin E, Ancheta O. Cerebral electrical fields during petit mal absences. Electroencephalogr Clin Neurophysiol. 1987;66(6):457–466. [DOI] [PubMed] [Google Scholar]
- 32. Szaflarski JP, DiFrancesco M, Hirschauer T, et al. Cortical and subcortical contributions to absence seizure onset examined with EEG/fMRI. Epilepsy Behav. 2010;18(4):404–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Benuzzi F, Ballotta D, Mirandola L, et al. An EEG-fMRI study on the termination of generalized spike-and-wave discharges in absence epilepsy. PLoS One. 2015;10(7):e0130943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Carney PW, Masterton RA, Harvey AS, Scheffer IE, Berkovic SF, Jackson GD. The core network in absence epilepsy. Differences in cortical and thalamic BOLD response. Neurology. 2010;75(10):904–911. [DOI] [PubMed] [Google Scholar]
- 35. Hamandi K, Salek-Haddadi A, Laufs H, et al. EEG-fMRI of idiopathic and secondarily generalized epilepsies. Neuroimage. 2006;31(4):1700–1710. [DOI] [PubMed] [Google Scholar]
- 36. Moeller F, Stephani U, Siniatchkin M. Simultaneous EEG and fMRI recordings (EEG-fMRI) in children with epilepsy. Epilepsia. 2013;54(6):971–982. [DOI] [PubMed] [Google Scholar]
- 37. Pugnaghi M, Carmichael DW, Vaudano AE, et al. Generalized spike and waves: effect of discharge duration on brain networks as revealed by BOLD fMRI. Brain Topogr. 2014;27(1):123–137. [DOI] [PubMed] [Google Scholar]
- 38. Coenen AM, Drinkenburg WH, Inoue M, Van Luijtelaar EL. Genetic models of absence epilepsy, with emphasis on the WAG/Rij strain of rats. Epilepsy Res. 1992;12(2):75–86. [DOI] [PubMed] [Google Scholar]
- 39. Gradinaru V, Zhang F, Ramakrishnan C, et al. Molecular and cellular approaches for diversifying and extending optogenetics. Cell. 2010;141(1):154–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yizhar O, Fenno LE, Prigge M, et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477(7363):171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vergnes M, Marescaux C, Depaulis A, Micheletti G, Warter JM. Spontaneous spike and wave discharges in thalamus and cortex in a rat model of genetic petit mal-like seizures. Exp Neurol. 1987;96(1):127–136. [DOI] [PubMed] [Google Scholar]
- 42. McCafferty C, David F, Venzi M, et al. Cortical drive and thalamic feed-forward inhibition control thalamic output synchrony during absence seizures. Nat Neurosci. 2018;21(5):744–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vergnes M, Marescaux C, Micheletti G, et al. Spontaneous paroxysmal electroclinical patterns in rat: a model of generalized non-convulsive epilepsy. Neurosci Lett. 1982;33(1):97–101. [DOI] [PubMed] [Google Scholar]
- 44. Snead OC. Pharmacological models of generalized absence seizures in rodents. J Neural Transm Suppl. 1992;35:7–19. [DOI] [PubMed] [Google Scholar]
- 45. Shipe WD, Barrow JC, Yang Z-Q, et al. Design, synthesis, and evaluation of a novel 4-aminomethyl-4-fluoropiperidine as a T-type Ca2+ channel antagonist. J Med Chem. 2008;51(13):3692–3695. [DOI] [PubMed] [Google Scholar]
- 46. Vergnes M, Marescaux C, Depaulis A. Mapping of spontaneous spike and wave discharges in Wistar rats with genetic generalized non-convulsive epilepsy. Brain Res. 1990;523(1):87–91. [DOI] [PubMed] [Google Scholar]
- 47. Chauvette S, Crochet S, Volgushev M, Timofeev I. Properties of slow oscillation during slow-wave sleep and anesthesia in cats. J Neurosci. 2011;31(42):14998–15008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vallentin D, Kosche G, Lipkind D, Long MA. Inhibition protects acquired song segments during vocal learning in zebra finches. Science. 2016;351(6270):267–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Deleuze C, David F, Béhuret S, et al. T-type calcium channels consolidate tonic action potential output of thalamic neurons to neocortex. J Neurosci. 2012;32(35):12228–12236. [DOI] [PMC free article] [PubMed] [Google Scholar]