Abstract
This study aims to study the potentials of sulforaphane (SFN) against retinal ischemia/reperfusion (I/R) injury. A rat retinal I/R injury method was established. Retinal thickness change and retinal ganglion cell (RGC) death were determined using hematoxylin and eosin (H&E) staining and Fluoro-Gold (FG) labeling. The inflammatory cytokines production and microglia activation were evaluated by using quantitative real-time polymerase chain reaction (qRT-PCR), Western blot, and enzyme-linked immunosorbent assay (ELISA). Knockdown NLRP3 was performed, and the according changes of retinal RGCs were assessed. SFN administration significantly inhibited I/R and caused retinal thickness change and prevented RGCs death in retinal I/R model. SFN suppressed inflammatory cytokines production, microglia activation, and inflammasome activation. In accordance, NLRP3 knockdown presented the similar inhibitory effect on I/R rats. This study demonstrates that SFN prevents RGCs death and acts as a potent neuroprotective modulator in retinal I/R injury, which may be associated with inhibition of the NLRP3 inflammasome activation.
Keywords: inflammasome, inflammation, retinal ganglion cell, sulforaphane
Introduction
Elevation of intraocular pressure (IOP) induces retinal ischemia/reperfusion (I/R) injury, which results in cellular damage and induces retinal ganglion cells (RGCs) apoptosis.1 Activation of nuclear factor-kappa B (NF-κB) triggers the secretion of some inflammatory cytokines, such as tumor necrosis factor alpha (TNF-α) and interleukin 1 beta (IL-1β), which cause more retinal RGCs death.1,2 Inflammasome is a multiple subunits complex, including NLRP3, pro-caspase-1, and apoptosis-associated speck-like protein containing a CARD (ASC), which activates Caspases family to process the production and maturation of inflammatory cytokines.1,3 NLRP3 inflammasome works as an injury sensor and then amplifies the injury signal, leading to the subsequent RGC death.3,4 Blocking the NLRP3 inflammasome activation can suppress the diabetic retinopathy, which suggests that targeting NLRP3 inflammasome is a potential therapeutic strategy to treat glaucoma.5 In addition, the excessive activation of retinal microglial cells has been reported to contribute to the retinal denegation, indicating prevention of microglia reactivity would be another candidate to treat I/R-induced RGCs death.6
Sulforaphane (SFN; 1-isothiocyanato-4-(methylsulfinyl) butane, C6H11NOS2) is a natural dietary isothiocyanate derivative, which has been reported to have lots of biological effects and health benefits, including anti-inflammatory, neuroprotection, anti-oxidant, anti-cancer, anti-microbial, and anti-diabetic.7 In this present study, we examined the potentials of SFN against retinal I/R injury.
Materials and methods
Animals
Female Sprague-Dawley rats were ordered from the Model Animal Research Center of Nanjing University. The rats were bred and maintained in the Binzhou Medical University Hospital animal care facility following the standard rearing conditions of 12 h light and 12 h dark. All rats’ studies were performed following the guidelines established by the Binzhou Medical University Hospital Animal Care and Use Committee.
I/R rat model
The right eye of each experimental rat was set for the experimental procedure and the left eye was performed (sham procedure) as a control. First, 8-week-old rats were anesthetized by xylazine (10 mg/kg) intraperitoneal injection. The corneas were topically anesthetized using tetracaine hydrochloride (0.5%), then dilated the pupils with tropicamide (1.0%). Elevate the IOP of 110 mm Hg (Tono-Lab XL Mentor, Norwell, MA, USA) for 1 h by cannulating the right eye anterior chamber (AC) with a normal saline reservoir connected to a 30-gauge infusion needle.2 The 1.4% viscoelastic solution, which improves better wound sealing after injection and reduces outflow of the suspension injected into the AC, was used here to result in better IOP values. Retinal ischemia and subsequent reperfusion were confirmed by the color of iris and red reflex. The contralateral left eye was served as the health control by the sham procedure without elevating the IOP. The treated rats were allowed to recover for 48 h before euthanasia.
NLRP3 knockdown analyses
The scrambled siRNA (sense: 5-UUC UCC GAACGUGUCACG UTT-3, anti-sense: 5-ACG UGA CACGUUCGGAGA ATT-3) and NLRP3-specific siRNA (sense: 5-GAUCAACCUCUCUACCAGA-3, anti-sense: 5-UCUGGUAGAGAGGUUGAUC-3) were constructed as scrambled shRNA and NLRP3 shRNA plasmids, respectively. The I/R-induced acute glaucoma rats were intravenously injected 8 mg/kg control and NLRP3 shRNA plasmids; 48 h later, the treated rats were euthanized for gene and protein analyses.
Gene expression analyses
Total RNA was isolated and extracted from frozen tissues using the TRIZol, and 1 µg total RNA was converted into complementary DNA (cDNA) using random hexamers and moloney murine leukemia virus (M-MLV, Invitrogen Life Technologies, Carlsbad, CA, USA). The quantitative real-time polymerase chain reaction (qRT-PCR) was performed in iCycler Multicolor RT-PCR system using SYBR Green (Bio-Rad, Hercules, CA, USA); target gene was normalized to internal control β-actin, and fold change was calculated by using the 2–ΔΔCT method. The qRT-PCR primers used in this study were listed below: TNF-α primers, F: 5′-CGTGGAACTGGCAGAAGAGG-3′, R: 5′-CTGCCACAAGCAGGAATGAG-3′; IL-1β primers, F: 5′-GTTCCCATTAGACAACTGCACTACAG-3′, R: 5′-GTC GTTGCTTGGTTCTCCTTGTA-3′; CD11b primers, F: 5′-CAGATCAACAATGTGACCGTATGGG-3′, R: 5′-CATCATGTCCTTGTACTGC-CGCTTG-3′; MHC-II primers, F: 5′-AGAGACCATCTGGAGACTTG-3′, R: 5′-CATCTGGGGTGTTGTTGGA-3′; NLRP3 primers, F: 5′-ATCAACAGGCGAGACCTCTG-3′, R: 5′-GTCCTCCTGGCATACCATAGA-3′; ASC primers, F: 5′-GACAGTGCAACTGCGAGAAG-3′, R: 5′-CGACTCCAGATAGTAGCTGACAA-3′; Caspase-1 primers, F: 5′-CGTGGAGAGAAACAAGGAGTG-3′, R: 5′-AATGAAAAGTGAGCCCCTGAC-3′; and β-actin primers, F: 5′-GGCGGACTATGACTTAGTTG-3′; R: 5′-AAACAACAA TGTGCAATCAA-3′.
SFN administration
SFN was purchased from Sigma–Aldrich (S4441, St. Louis, MO, USA), which was dissolved in dimethyl sulfoxide (DMSO; final dose 0.01% v/v) first and then diluted with phosphate buffered saline. The daily SFN oral administration started at 1-week before acute glaucoma surgery until the end of study. There were four groups (six rats/group) of Sprague-Dawley female rats: I/R group (vehicle gavage), I/R + SFN-5 (5 mg/kg SFN gavage), I/R + SFN-10 (10 mg/kg SFN gavage), and I/R + SFN-20 (20 mg/kg SFN gavage). Treated rats were euthanized on day 9, and tissues were harvested for the subsequent analysis.
Histological analyses
Rats were euthanized and both eyes were enucleated and fixed with 4% paraformaldehyde for 12 h prior to paraffin embedding. Four 4-mm-think sections were cut and 10 µm per slide were further cut for the hematoxylin and eosin (H&E) staining.8 For retinal thickness measurement, from inner limiting membrane to outer limiting membrane, four adjacent areas, which are in 1 mm distance to the optic nerve center, were selected and measured by using AxioVision program (Carl Zeiss AG, Jena, Germany).
Western blotting analyses
Frozen retina homogenates were lysed using the radioimmunoprecipitation buffer (Bio-Equip, Shanghai, China). The NARP3 (ab210491, 1:1000 dilution) primary antibodies were ordered from Abcam (Shanghai, China); the primary antibody of ASC (sc-514414, 1:500 dilution) and Caspase-1 (sc-56036, 1:500 dilution) were ordered from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA); and the internal control GAPDH (glyceraldehyde-3-phosphate dehydrogenase) antibody was ordered from Abcam (ab181602, 1:2000 dilution).
Enzyme-linked immunosorbent assay
The protein level of TNF-α and IL-1β in retinas was quantified by TNF alpha Rat ELISA Kit (Thermo Fisher Scientific, Waltham, MA, USA) and Rat IL-1 beta ELISA Kit (Abcam), respectively, following the instructions provided by the manufacturer.
Fluoro-Gold labeling and quantification
Fluoro-Gold™ (FG) was purchased from Biotium, Inc. (Fremont, CA, USA). The anesthetized rats were placed on a blanket for FG injection. Both superior colliculi of the experimental rat were injected 1 µL 4% FG (w/v). After 48 h of reperfusion, the retinal flat mount was made, and then the FG-positive RGCs were identified using AxioImager fluorescent microscope (Carl Zeiss AG). The gold dots are surviving cells, which were counted by using ImageJ (National Institutes of Health (NIH), Bethesda, MD, USA).
Statistical analysis
The statistical analyses were carried out by using SPSS 11.0 package. Differences between groups were analyzed using analysis of variance (ANOVA). All data came from at least three independent experiments and represented mean ± standard deviation (SD). Statistical significance thresholds were set at *P < 0.05.
Results
SFN administration inhibits I/R-induced retinal thickness
Retinal lesion happened right after the acute elevation of IOP, and retinal edema, condensation of nuclear chromatin, and vacuolar degeneration were observed in the rat model. H&E staining showed the five retina layers: ganglion cell layer (GCL), inner plexiform layer (IPL), inner nuclear layer (INL), outer plexiform layer (OPL), and outer nuclear layer (ONL). In comparison with control group, the retinal thickness was decreased in I/R group (Supplemental Figure S1a and S1c). In addition, the number of RGCs was decreased in I/R group too (Supplemental Figure S1b). Both indicated that the acute elevation of IOP caused retinal thickness change. In contrast, the decrease of I/R-induced retinal thickness was significantly inhibited after 1 week of 10 mg/kg (I/R + SFN-10) or 20 mg/kg (I/R + SFN-20) SFN gavage (Supplemental Figure S1a and S1c). The number of RGCs was increased in I/R + SFN-10 and I/R + SFN-20 groups compared with I/R group (Supplemental Figure S1b). There was no difference between I/R group and 5 mg/kg (I/R + SFN-5) SFN administration group, which indicated the dose-dependent inhibitory effect of SFN. These data suggested that SFN inhibited I/R-induced retinal thickness change.
SFN administration prevents I/R-induced RGCs death
We further investigated the I/R-induced RGCs death among these five groups of rats using FG labeling analysis. As shown in Supplemental Figure S2a, the surviving RGCs were dramatically reduced after I/R injury. Notably, upon 10 and 20 mg/kg SFN administration, the surviving RGCs were significantly increased compared to I/R group. The quantification of RGCs survival was shown in Supplemental Figure S2b. The above data indicated that SFN prevented I/R-induced cell death.
SFN administration inhibits the inflammatory cytokines production triggered by I/R
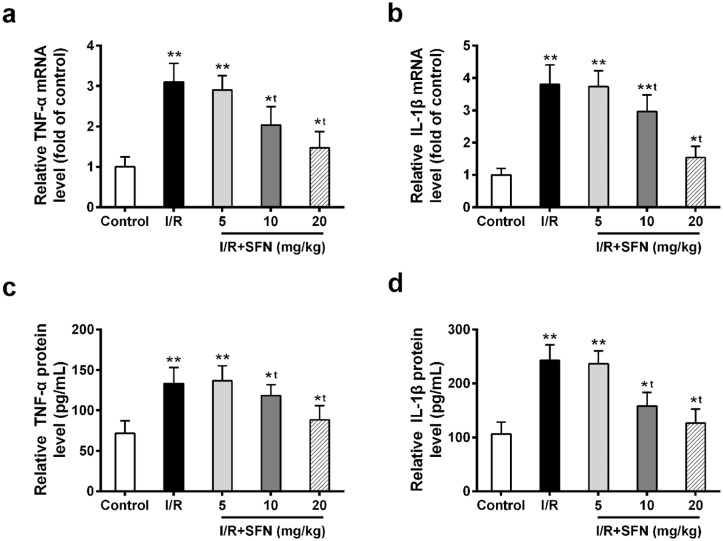
We evaluated the effect of SFN on inflammatory cytokine marker genes, TNF-α and IL-1β, in control, I/R injury, and I/R + SFN (5, 10, and 20 mg/kg) rat groups using qRT-PCR. Both of their mRNA levels were significantly induced in I/R rat retina (Figure 1(a) and (b)). In contrast, SFN administration (10 and 20 mg/kg) dramatically decreased the gene expression level of the two inflammatory cytokine markers (Figure 1(a) and (b)). In addition, the protein level of TNF-α and IL-1β in retinas was consistent with their mRNA result; both were suppressed after SFN treatment (Figure 1(c) and (d)). These data suggested that SFN administration inhibited the I/R-induced inflammatory cytokines production in retinas.
Figure 1.
SFN administration suppressed the I/R-induced inflammatory cytokines production. (a and b) Relative mRNA expressions of TNF-α (a) and IL-1β (b) were analyzed in control group, I/R injury group, and I/R + SFN (5, 10, and 20 mg/kg) group using qRT-PCR. (c and d) The protein level of (c) TNF-α and (d) IL-1β in retinas was quantified by ELISA. Data are presented as mean ± SD, n = 6 for each group, *P < 0.05, **P < 0.01 compared to control group, tP < 0.05 compared to I/R group.
SFN administration inhibits microglia activation
We investigated the effects of SFN on retina microglia activation after I/R injury. Two of microglial cell markers, CD11b and MHC-II, were analyzed in this study. As shown in Supplemental Figure S3, both of their mRNA levels were increased after I/R injury, which indicated that microglia was activated in our rat model. After 1 week of SFN administration (10 and 20 mg/kg) on these I/R injury rats, the microglial cell markers were significantly decreased compared to I/R rats (Supplemental Figure S3a and S3b). These data suggested that SFN administration inhibited microglia activation in retinal I/R rats.
SFN administration inhibits the NLRP3 inflammasome activation
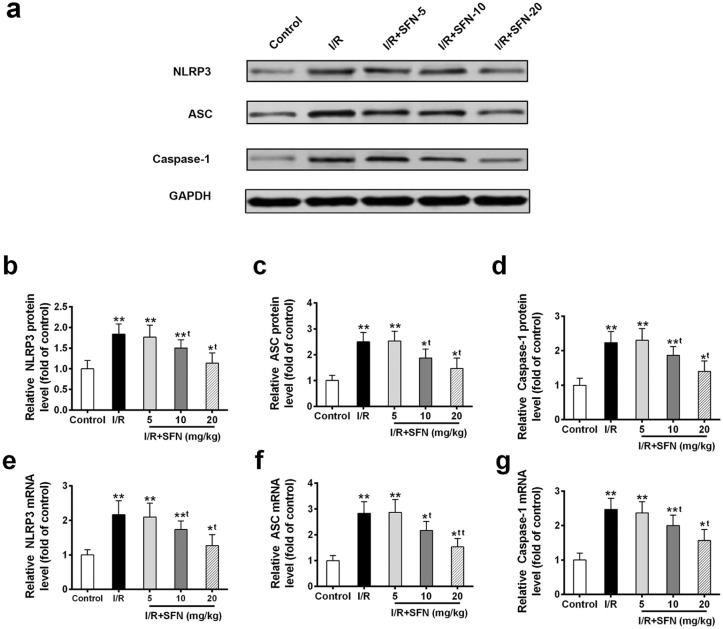
To evaluate whether NLRP3 inflammasome activation was altered after I/R injury and SFN administration, we conducted Western blotting and qRT-PCR to analyze the expression level of three important components of inflammasome complex. The retina protein levels of NLRP3, ASC, and Caspase-1 were upregulated after I/R injury compared to control group (Figure 2(a)–(d)). In addition, their mRNA levels were increased in I/R group too (Figure 2(e)–(g)). Notably, upon 1 week of SFN administration (10 and 20 mg/kg), all three inflammasome complex components were downregulated compared to I/R group on both protein and mRNA levels (Figure 2(a)–(g)).
Figure 2.
SFN administration suppresses the inflammasome activation. (a) Western blot analysis of inflammasome complex components (b) NLRP3, (c) ASC, and (d) Caspase-1 in control, I/R injury, and I/R + SFN (5, 10, and 20 mg/kg) treated rat retinas. qRT-PCR analysis of (e) NLRP3, (f) ASC, and (g) Caspase-1 in indicated treatment. Data are presented as mean ± SD, n = 6 for each group, *P < 0.05, **P < 0.01 compared to control group, tP < 0.05, ttP < 0.01 compared to I/R group.
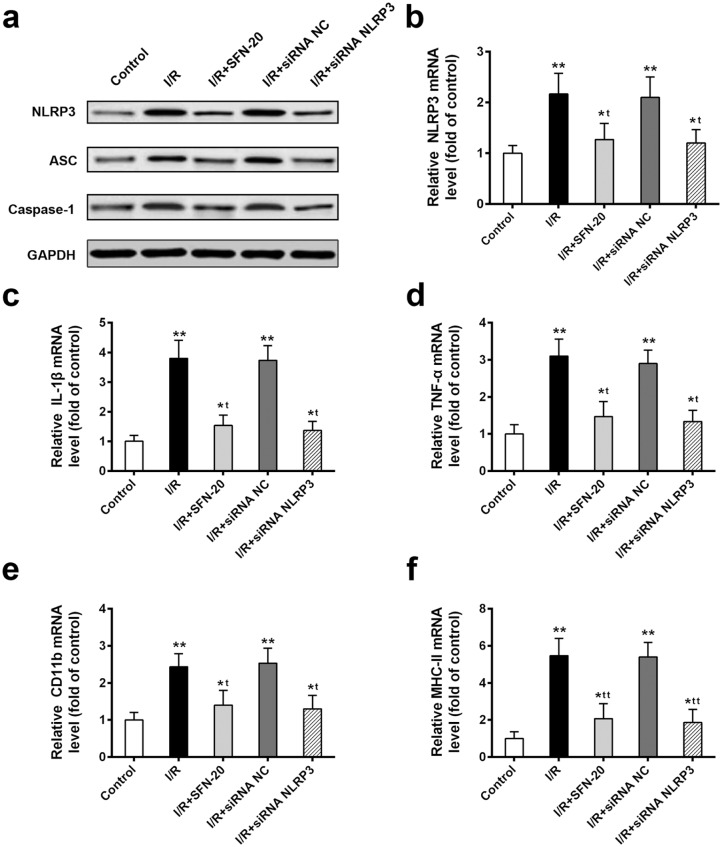
To further investigate the SFN-mediated neuroprotection associated with NLRP3 inflammasome inhibition, we performed NLRP3 knockdown using siRNA. The comparable inflammasome inhibition effect between I/R + SFN-20 (20 mg/kg) and I/R + siNLRP3 was observed on the protein level of inflammasome complex components (Figure 3(a)). Notably, similar to NLRP3 knockdown, I/R + SFN-20 group showed significant decrease of NLRP3 mRNA (Figure 3(b)). Moreover, similar to I/R + SFN-20 treatment, the inflammatory cytokines production (Figure 3(c) and (d)) and microglia activation (Figure 3(e) and (f)) were significantly decreased in I/R + siNLRP3 compared to I/R injury rats. These data indicated that SFN-induced neuroprotection may be through inactivating NLRP3.
Figure 3.
SFN-mediated neuroprotection may be through inactivating NLRP3 in retinal I/R injured rats. (a) Western blot analysis of inflammasome complex components in indicated treatment. (b) The NLRP3 mRNA level in indicated treatment. (c and d) The inflammatory cytokines IL-1β (c) and TNF-α (d) production in different treated groups. (e and f) The activation of microglial markers CD11b (e) and MHC-II (f) in different treated groups. Data are presented as mean ± SD, n = 6, for each group, *P < 0.05, **P < 0.01 compared to control group, tP < 0.05, ttP < 0.01 compared to I/R group.
Discussion
SFN has lots of biological activities and health benefits, such as protecting cardiomyocytes from hypoxia/reoxygenation injury9 and improving neurobehavioral deficits in the Alzheimer’s disease mouse model.10
The dysfunction of NLRP3 inflammasomes is highly linked with a series of auto-inflammatory syndromes. Chi et al.2 found that inhibiting NLRP3 inflammasome activity and IL-1β production could significantly attenuate retinal ischemic damage. In this study, we found that SFN can block both the mRNA and protein levels of NLRP3, subsequently decrease the production of inflammatory cytokines, and inhibit microglia activity. Several previous studies reveal that blocking NLRP3 inflammasome activation may provide a novel treatment strategy to preserve vision,2,3,5,8,11 which is similar with our results. Notably, SFN administration showed similar effect to NLRP3 knockdown on the inhibition of inflammatory cytokines production and microglia activation, which is in consistent with several previous studies and suggests that SFN-induced neuroprotection may inactivate through NLRP3 in acute glaucoma.2,3,5,11 Further investigations are warranted—such as “Does NLRP3 sufficiently mediate the neuroprotective effect of SFN?” and “Is there any other target of SFN?”—and the underlying signal pathways need to be clarified.
Collectively, our results provide new insight into the pathogenesis of retinal I/R injury and point to a treatment strategy.
Supplemental Material
Supplemental material, Supplementary_Materials_(1) for Sulforaphane alleviates retinal ganglion cell death and inflammation by suppressing NLRP3 inflammasome activation in a rat model of retinal ischemia/reperfusion injury by Yuerong Gong, Xiaoning Cao, Lei Gong and Weiguo Li in International Journal of Immunopathology and Pharmacology
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Weiguo Li  https://orcid.org/0000-0003-4151-9389
https://orcid.org/0000-0003-4151-9389
Supplemental material: Supplemental material for this article is available online.
References
- 1. Soto I, Howell GR. (2014) The complex role of neuroinflammation in glaucoma. Cold Spring Harbor Perspectives in Medicine 4(8): a017269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chi W, Li F, Chen H, et al. (2014) Caspase-8 promotes NLRP1/NLRP3 inflammasome activation and IL-1beta production in acute glaucoma. Proceedings of the National Academy of Sciences of the United States of America 111: 11181–11186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. He Y, Hara H, Nunez G. (2016) Mechanism and regulation of NLRP3 inflammasome activation. Trends in Biochemical Sciences 41(12): 1012–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ozaki T, Yamashita T, Tomita H, et al. (2016) The protection of rat retinal ganglion cells from ischemia/reperfusion injury by the inhibitory peptide of mitochondrial mu-calpain. Biochemical and Biophysical Research Communications 478(4): 1700–1705. [DOI] [PubMed] [Google Scholar]
- 5. Lin C, Wu F, Zheng T, et al. (2018) Kaempferol attenuates retinal ganglion cell death by suppressing NLRP1/NLRP3 inflammasomes and caspase-8 via JNK and NF-κB pathways in acute glaucoma. Eye 33: 777–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kierdorf K, Prinz M. (2013) Factors regulating microglia activation. Frontiers in Cellular Neuroscience 7: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vanduchova A, Anzenbacher P, Anzenbacherova E. (2019) Isothiocyanate from broccoli, sulforaphane, and its properties. Journal of Medicinal Food 22(2): 121–126. [DOI] [PubMed] [Google Scholar]
- 8. Lei C, Lin R, Wang J, et al. (2017) Amelioration of amyloid beta-induced retinal inflammatory responses by a LXR agonist TO901317 is associated with inhibition of the NF-kappaB signaling and NLRP3 inflammasome. Neuroscience 360: 48–60. [DOI] [PubMed] [Google Scholar]
- 9. Li YP, Wang SL, Liu B, et al. (2016) Sulforaphane prevents rat cardiomyocytes from hypoxia/reoxygenation injury in vitro via activating SIRT1 and subsequently inhibiting ER stress. Acta Pharmacologica Sinica 37(3): 344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang R, Zhang J, Fang L, et al. (2014) Neuroprotective effects of sulforaphane on cholinergic neurons in mice with Alzheimer’s disease-like lesions. International Journal of Molecular Sciences 15(8): 14396–14410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang G, Yeon SH, Lee HE, et al. (2018) Suppression of NLRP3 inflammasome by oral treatment with sulforaphane alleviates acute gouty inflammation. Rheumatology 57(4): 727–736. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_Materials_(1) for Sulforaphane alleviates retinal ganglion cell death and inflammation by suppressing NLRP3 inflammasome activation in a rat model of retinal ischemia/reperfusion injury by Yuerong Gong, Xiaoning Cao, Lei Gong and Weiguo Li in International Journal of Immunopathology and Pharmacology