Abstract
Background:
The optimal time to initiate parenteral nutrition (PN) in critically ill adults in whom enteral nutrition is not feasible is controversial.
Objective:
The objectives were to compare in-hospital mortality and hospital length of stay in patients initiated on PN within 7 days or after 7 days of poor nutrient intake.
Methods:
This single-center, retrospective study included critically ill adult patients who received at least 2 consecutive days of PN during hospitalization from May 2014 to July 2016.
Results:
The median duration of PN (interquartile range) was 8 (5-13) days. In total, 110 patients received PN within 7 days of poor nutrient intake while 49 patients received PN after 7 days of poor nutrient intake. There was no statistically significant difference in in-hospital mortality between groups (29.09% vs 18.37%, P = .1535). Patients initiated within 7 days had a significantly shorter median hospital length of stay than patients initiated after 7 days (20 days vs 27 days, P = .0013). There were 69 patients who were classified as obese. Obese patients initiated within 7 days had a significantly shorter median hospital length of stay than obese patients initiated after 7 days (17 days vs 33 days, P = .0007).
Conclusions:
Time to initiation of PN did not impact in-hospital mortality. However, there was an association between early initiation of PN and a shorter hospital length of stay that was most pronounced among obese patients.
Keywords: Nutritional support, nutrition therapy, parenteral nutrition, critical care
Introduction
Nutritional deficits are a complication of critical illness. Enteral nutrition (EN) is associated with fewer adverse effects but is not feasible to correct nutritional deficits in all cases.1 Consequences of not meeting nutritional targets include weakness, infection, an increased duration of mechanical ventilation and death.2 Furthermore, the optimal time to initiate parenteral nutrition (PN) in critically ill adults in whom EN is not feasible is controversial. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient published by the American Society for Parenteral and Enteral Nutrition (ASPEN) and the Society of Critical Care Medicine (SCCM) recommend withholding PN over the first 7 days if the critically ill patient is at low nutrition risk (eg, NRS 2002 [Nutrition Risk Screening] ⩽ 3 or NUTRIC [NUTrition Risk in Critically Ill] score ⩽ 5), but initiating as soon as possible if at a high nutrition risk (eg, NRS 2002 ⩾ 5 or NUTRIC score ⩾ 5) or severely malnourished and early EN is not feasible.3 Conversely, the European Society for Clinical Nutrition and Metabolism guidelines recommend that clinicians initiate PN in all patients within 24 to 48 h after the patient is admitted to the intensive care unit (ICU) if EN is contraindicated and the patient is not expected to receive normal nutrition within 3 days.1 The When Is PN Appropriate? Consensus Recommendations suggest initiating PN after 7 days for well-nourished stable patients, within 3 to 5 days in those who are nutritionally at-risk, and as soon as feasible in those with baseline moderate or severe malnutrition if oral intake or EN is not possible or sufficient.4 Investigation is warranted in light of the inconsistency among guideline recommendations and other literature. The purpose of this study was to investigate in-hospital mortality and hospital length of stay based on initiation of PN within 7 days or after 7 days of poor nutrient intake in critically ill adult patients.
Methods
This study was conducted at Cooper University Hospital, a 600-bed urban academic medical center. Adult patients admitted to this institution and initiated on PN for at least 2 consecutive days from May 2014 to July 2016 were retrospectively evaluated for study inclusion. Patients were excluded if they were <18 years of age, pregnant, received concomitant EN, were initiated on PN prior to admission or were not admitted to an ICU.
At Cooper University Hospital, a dietitian consult service in conjunction with the multidisciplinary support of physicians, pharmacists, and nurses manages PN without a formal nutrition support team. For individualized PN orders, dietitians advise appropriate macronutrient provision using a published predictive equation or a simplistic weight-based estimate before PN is initiated.3 Indirect calorimetry is unavailable at this institution. Generally, on day 1 of PN, 50% of goal intake is provided and if tolerated patients are advanced to goal intake on day 2 of PN. At this institution, lipid injectable emulsion (ILE) is provided from the time of PN initiation unless contraindicated. During the study period, exclusively soy-oil based ILE was used at this institution. Suggested default electrolytes in the PN order set and pharmacist recommendations support physicians in ordering appropriate micronutrients. The institution’s guideline suggests weaning PN when enteral intake achieves 50% to 75% of requirements for energy, protein, and micronutrients.
The objectives of this study were to compare in-hospital mortality and hospital length of stay in critically ill adult patients initiated on PN within 7 days of poor nutrient intake and after 7 days of poor nutrient intake. Seven days was used for categorization in this study since guideline authors incorporated that timeframe into current recommendations.3 Poor nutrient intake was defined as less than 50% of daily nutritional requirement. A pre-determined subgroup analysis stratified patients based on nutritional classification at the time of PN initiation. This study described nutritional status at presentation based on percentage of ideal body weight (IBW). Actual body weight (ABW) represented the admission weight or pre-hospitalization usual weight if documented from the dietitian’s interview with the patient or their caregiver. Patients were identified for the underweight subgroup if their ABW was less than 89% of their IBW. Patients were identified for the normal weight subgroup if their ABW was within the range of 90% to 129% of their IBW. Patients in the obese subgroup had an ABW greater than 130% of their IBW. This subgroup analysis compared in-hospital mortality and hospital length of stay in critically ill patients initiated on PN within 7 days of poor nutrient intake and after 7 days of poor nutrient intake.
Baseline patient demographics and PN characteristics were collected retrospectively using the institution’s Electronic Medical Record (EMR). Baseline demographics were determined at the time of PN initiation. Investigators of this study used baseline demographics documented in the EMR to calculate morbidity and mortality scores, which include the Charlson Comorbidity Index (CCI), Acute Physiology and Chronic Health Evaluation II (APACHE II) score, and the Sequential Organ Failure Assessment (SOFA) score.5-7 Investigators also retrospectively calculated a modified NUTRIC (mNUTRIC) score, excluding interleukin-6.8 The number of co-morbidities for the mNUTRIC score was determined from the CCI.
All statistical analyses were conducted using SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA). Quantitative variables were compared using Student t-test if normally distributed or Wilcoxon Rank-Sum, otherwise. In addition, hospital length of stay was graphically represented using Kaplan-Meier curves and analyzed using forward selection Cox Regression model to control for any significant confounder. Categorical variables were analyzed using χ2 test or Fisher’s exact test. A multivariate logistic regression with forward selection was completed to determine the effect of the days of poor nutrient intake on in-hospital mortality, controlling for any significant confounders identified through the bivariate analyses. Statistical significance was defined as a P < .05.
Results
There were 546 patients who were screened for inclusion. Of these patients, 375 were excluded as they were not critically ill at the time of PN initiation and 12 patients were excluded given the unclear duration of poor nutrient intake. A total of 159 patients were included in this analysis. In the total study population, the mean age was 61.6 years and the mean body mass index (BMI) was 29 kg/m2. The median duration (interquartile range [IQR]) of PN was 8 (5-13) days and 98% received central PN. The median number of days of poor nutrition intake prior to PN initiation in those who received PN within 7 days and those who received PN after 7 days was 4.00 (3.00-6.00) and 10.00 (9.00-14.00), respectively. The mNUTRIC, SOFA score, and APACHE II score were similar between groups (Table 1).
Table 1.
Patient demographics and PN characteristics.
| Characteristic | ⩽7 days of poor nutrient intake (n = 110) | >7 days of poor nutrient intake (n = 49) | PP-value |
|---|---|---|---|
| Age (years), mean ± SD | 60.19 ± 16.97 | 64.78 ± 19.10 | .1324a |
| Male, n (%) | 61 (55.45%) | 28 (57.14%) | .8430b |
| Race, n (%) | |||
| White | 71 (64.55%) | 32 (65.31%) | 9776d |
| African American | 25 (22.73%) | 10 (20.41%) | |
| Hispanic/Latino | 8 (7.27%) | 4 (8.16%) | |
| Other | 6 (5.45%) | 3 (6.12%) | |
| Nutrition classification, n (%) | |||
| Underweight, <89% IBW | 9 (8.18%) | 3 (6.12%) | .3948b |
| Normal, 90%–130% IBW | 50 (45.45%) | 28 (57.14%) | |
| Obese, >130% IBW | 51 (46.36%) | 18 (36.73%) | |
| mNUTRIC Score, median (IQR) | 5.00 (4.00–6.00) | 5.00 (4.00–6.00) | .7439 c |
| BMI, median (IQR) | 28.58 (22.92–34.28) | 26.05 (23.13–30.92) | .3787c |
| Location at PN initiation, n (%) | |||
| Surgical and trauma ICU | 33 (30.00%) | 22 (44.90%) | .1441b |
| Medical ICU | 67 (60.91%) | 25 (51.02%) | |
| Cardiac ICU | 10 (9.09%) | 2 (4.08%) | |
| CCI score with age factored, median (IQR) | 5.00 (2.00–7.00) | 6.00 (3.00–8.00) | .1028c |
| APACHE II score, median (IQR) | 24.5 (19.00–30.00) | 21.00 (18.00–27.00) | .1759c |
| SOFA score, median (IQR) | 7.00 (4.00–11.00) | 6.00 (3.00–9.00) | .1781c |
| CrCl, median (IQR) | 64.61 (37–121) | 60.69 (23–113) | .4023c |
| Albumin, median (IQR) (n = 141) | 2.30 (1.80–2.75) | 2.20 (2.00–2.50) | .7632c |
| WBC count within 24 h prior to PN initiation, median (IQR) (n = 158) | 13.38 (8.39–18.38) | 14.61 (10.30–21.40) | .4277c |
| Liver disease, n (%) | 17 (15.45%) | 5 (10.20%) | .3760b |
| Chronic kidney disease/end–stage renal disease, n (%) | 12 (10.91%) | 10 (20.41%) | .1092b |
| Acute kidney injury, n (%) | 40 (36.36%) | 23 (46.94%) | .2081b |
| Organ system with major diagnosis in discharge summary, n (%) | |||
| Cardiovascular | 16 (14.55%) | 4 (8.16%) | |
| Digestive | 63 (57.27%) | 28 (57.14%) | |
| Endocrine | 5 (4.55%) | 2 (4.08%) | |
| Lymphatic and immune | 2 (1.82%) | 1 (2.04%) | |
| Musculoskeletal | 3 (2.73%) | 2 (4.08%) | |
| Reproductive | 5 (4.55%) | 2 (4.08%) | |
| Respiratory | 12 (10.91%) | 8 (16.33%) | |
| Skin | 0 (0.00%) | 1 (2.04%) | |
| Urinary | 4 (3.64% | 1 (2.04%) | |
| Central PN, n (%) | 109 (99.09%) | 47 (95.92%) | .2248d |
| Consecutive days of PN, median (IQR) | 8 (5–12) | 8 (5–13) | .6590c |
| Protein at goal (g/kg/day), median (IQR) | 1.61 (1.44–1.89) | 1.54 (1.34–1.80) | .2468c |
| Energy provision (non-protein and protein calories) at goal, kcal/kg/day, median (IQR) | 23.22 (19.73–26.61) | 24.79 (21.55–26.78) | .5272c |
| Time to advance to goal, days, median (IQR) | 2 (2–3) | 2 (2–3) | .8230c |
| Hyperglycemia (>300 mg/dL), n (%) | 2 (1.82%) | 1 (2.04%) | 1.0000d |
| Intermittent hemodialysis at initiation, n (%) | 7 (6.36%) | 2 (4.08%) | .7224b |
| Continuous veno–venous hemofiltration at initiation, n (%) | 17 (15.45%) | 5 (10.20%) | .3760b |
Abbreviations: APACHE II, Acute Physiology and Chronic Health Evaluation II; BMI, body mass index; CCI, Charlson Comorbidity Index; CrCl, creatinine clearance estimated using Cockcroft-Gault; IBW, ideal body weight; ICU, intensive care unit; IQR, interquartile range; mNUTRIC, Modified NUTrition Risk in the Critically Ill; PN, parenteral nutrition; SD, standard deviation; SOFA, Sequential Organ Failure Assessment; WBC, white blood cell.
t-test.
Chi-squared test.
Wilcoxon Rank-Sum test.
Fisher’s exact test.
Results comparing in-hospital mortality and hospital length of stay for patients who were initiated on PN within 7 days of poor nutrient intake (n = 110) vs patients who were initiated on PN after 7 days of poor nutrient intake (n = 49) are reported in Table 2. While there was no statistically significant difference in in-hospital mortality rates based on PN initiation within 7 days or after 7 days (29.09% vs 18.37%, P = .1535), patients who were initiated on PN within 7 days had a significantly shorter median hospital length of stay compared with those initiated on PN after 7 days (20 days vs 27 days, P = .0013). Patients were more likely to have a shorter duration of hospitalization if they were initiated on PN within 7 days of poor nutrient intake (hazard ratio [HR] = 1.65, 95% CI [1.17-2.33], P-value = .0042; controlling for age, location at initiation, and total APACHE II score) (Figure 1).
Table 2.
Bivariate analysis of outcomes for patients initiated on PN within 7 days or after 7 days of poor nutrient intake.
| Outcome | ⩽7 days of poor nutrient intake (n = 110) | >7 days of poor nutrient intake (n = 49) | P-value |
|---|---|---|---|
| In-hospital mortality, n (%) | 32 (29.09%) | 9 (18.37%) | .1535a |
| Hospital length of stay, days, median (IQR) | 20 (14–30) | 27 (20–44) | .0013b |
Abbreviations: IQR, interquartile range; PN, parenteral nutrition.
Chi-squared test.
Wilcoxon Rank-Sum test.
Figure 1.
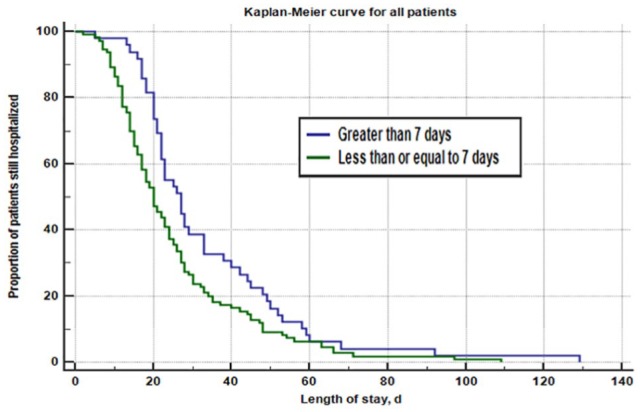
Patients who were initiated on PN within 7 days had a significantly shorter median hospital length of stay compared with those initiated on PN after 7 days (20 days vs 27 days, P = .0013). Patients were more likely to have a shorter duration of hospitalization if they were initiated on PN within 7 days of poor nutrient intake (HR = 1.47, 95% CI [1.07-2.01], P-value = .0206). After controlling for age, location at initiation, and total APACHE II score, patients were more likely to have a shorter duration of hospitalization if they were initiated on PN within 7 days of poor nutrient intake (HR = 1.65, 95% CI [1.17-2.33], P-value = .0042). APACHE II indicates Acute Physiology and Chronic Health Evaluation II; CI, confidence interval; HR, hazard ratio; PN, parenteral nutrition.
A subgroup analysis consisting of underweight patients (n = 12), normal weight patients (n = 78), and obese patients (n = 69) compared in-hospital mortality and hospital length of stay in patients initiated on PN within 7 days of poor nutrient intake and after 7 days of poor nutrient intake (Table 3). Underweight, normal weight, and obese patients initiated on PN within 7 days had a median baseline mNUTRIC score of 5 (5.0-8.0), 5 (4.0-6.0), and 5 (3.5-6.5), respectively. Underweight, normal weight and obese patients initiated on PN after 7 days had a median baseline mNUTRIC score of 7 (7.0-7.0), 4 (3.0-6.0), and 5 (5.0-6.75), respectively. Obese patients initiated on PN within 7 days (n = 51) had a shorter median hospital length of stay compared with obese patients initiated on PN after 7 days (n = 18) (17 days vs 33 days, P = .0007). Obese patients were more likely to have a shorter duration of hospitalization if initiated on PN within 7 days of poor nutrient intake (HR = 3.43, 95% CI [1.81-6.50], P-value = .0002; controlling for age, BMI, acute kidney injury (AKI) at initiation, continuous veno-venous hemodialysis (CVVHD) at initiation and moderate/severe renal disease) (Figure 2). No significant differences in hospital length of stay were found for underweight and normal weight patients. No statistically significant difference in in-hospital mortality rates based on PN initiation within 7 days or after 7 days was found for obese (37.25% vs 22.22%, P = .2248), normal (24.00% vs 14.29%, P = .3081), or underweight patients (11.11% vs 33.33%, P = .4545).
Table 3.
Subgroup analysis of outcomes and baseline modified NUTRIC score stratified by nutrition classification for patients initiated on PN within 7 days or after 7 days of poor nutrient intake.
| Nutrition classification | ⩽7 days of poor nutrient intake (n = 110) | >7 days of poor nutrient intake (n = 49) | P-value |
|---|---|---|---|
| Underweight (n = 12) | (n = 9) | (n = 3) | |
| Protein at goal (g/kg/day), median (IQR) | 1.59 (1.42–1.92) | 1.79 (1.52–1.85) | .8636a |
| Energy provision (non-protein and protein calories) at goal, kcal/kg/day, median (IQR) | 29.07 (25.56–29.75) | 28.48 (26.10–30.40) | 1.000a |
| mNUTRIC score, median (IQR) | 5.0 (5.0–8.0) | 7.00 (7.0–7.0) | .072a |
| In-hospital mortality, n (%) | 1 (11.11%) | 1 (33.33%) | .4545b |
| Hospital length of stay, days, median (IQR) | 24 (18–27) | 22 (18–26) | .7101a |
| Normal (n = 78) | (n = 50) | (n = 28) | |
| Protein at goal (g/kg/day), median (IQR) | 1.53 (1.28–1.71) | 1.51 (1.37–1.76) | .787a |
| Energy provision (non-protein and protein calories) at goal, kcal/kg/day, median (IQR) | 24.07 (21.31–26.41) | 24.91 (22.67–25.43) | .534a |
| mNUTRIC score, median (IQR) | 5.0 (4.0–6.0) | 4.0 (3.0–6.0) | .2986a |
| In-hospital mortality, n (%) | 12 (24.00%) | 4 (14.29%) | .3081b |
| Hospital length of stay, days, median (IQR) | 22 (15–37) | 23 (20–46) | .2876a |
| Obese (n = 69) | (n = 51) | (n = 18) | |
| Protein at goal (g/kg/day), median (IQR) | 1.77 (1.55–2.09) | 1.62 (1.35–2.20) | .6179a |
| Energy provision (non-protein and protein calories) at goal, kcal/kg/day, median (IQR) | 20.80 (19.05–26.07) | 23.24 (17.12–26.68) | .9727a |
| mNUTRIC score, median (IQR) | 5 (3.5–6.5) | 5 (5.0–6.75) | .2476a |
| In-hospital mortality, n (%) | 19 (37.25%) | 4 (22.22%) | .2448b |
| Hospital length of stay, days, median (IQR) | 17 (11–28) | 33 (22–45) | .0007a |
Abbreviations: IQR, interquartile range; mNUTRIC, modified NUTrition Risk in the Critically Ill; PN, parenteral nutrition; SD, standard deviation.
Wilcoxon Rank-Sum test.
Fisher’s exact test.
Figure 2.
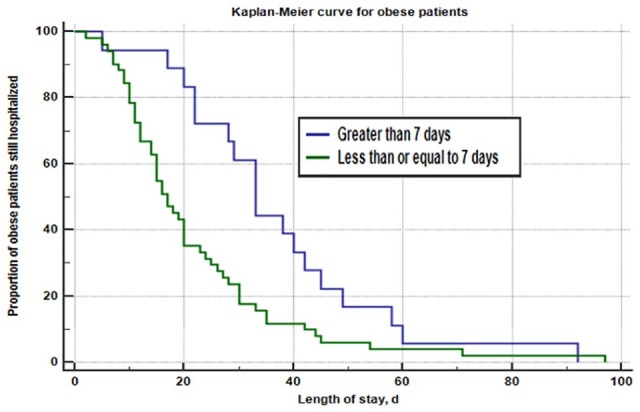
Obese patients initiated on PN within 7 days (n = 51) had a shorter median hospital length of stay compared with obese patients initiated on PN after 7 days (n = 18) (17 days vs 33 days, P = .0007). A pre-determined subgroup analysis found that obese patients were more likely to have a shorter duration of hospitalization if initiated on PN within 7 days of poor nutrient intake (HR = 1.94, 95% CI [1.20-3.13], P-value = .0099). After controlling for age, BMI, AKI at initiation, CVVHD at initiation and moderate/severe renal disease, obese patients were still more likely to have a shorter duration of hospitalization if initiated on PN within 7 days of poor nutrient intake (HR = 3.43, 95% CI [1.81-6.50], P-value = .0002). AKI indicates acute kidney injury; BMI, body mass index; CI, confidence interval; HR, hazard ratio; CVVHD, continuous veno-venous hemodialysis; PN, parenteral nutrition.
A multivariate logistic regression with forward selection found no statistically significant effect of number of days of poor nutrient intake on in-hospital mortality after controlling for the following statistically significant confounders: baseline liver disease, CCI, and the SOFA score (Table 4). Although there was a non-statistically significant trend toward improved mortality in patients initiated on PN after 7 days, there was a shorter length of stay among patients discharged live from the hospital who were initiated on PN within 7 days (Table 5).
Table 4.
Multivariate logistic regression with forward selection on the effect of number days of poor nutrient intake prior to PN initiation on chance of hospital mortality controlling for potential confounders.
| Effect | OR estimate | 95% Wald confidence limits | P-value | |
|---|---|---|---|---|
| Days of poor nutrient (⩽7 days vs >7 days) prior to PN initiation | 2.023 | 0.769 | 5.324 | .1534 |
| Liver disease (yes vs no) | 2.860 | 0.968 | 8.448 | .0573 |
| CCI score with age factored | 1.213 | 1.115 | 1.358 | .0051 |
| SOFA score | 1.231 | 1.115 | 1.358 | <.0001 |
Abbreviations: CCI, Charlson Comorbidity Index; PN, parenteral nutrition; SOFA, Sequential Organ Failure Assessment.
Table 5.
Bivariate subgroup analysis of length of stay for patients initiated on PN within 7 days or after 7 days of poor nutrient intake who survived.
| Outcome | ⩽7 days of poor nutrient intake | >7 days of poor nutrient intake | P-value |
|---|---|---|---|
| All patients discharged live (n = 118) | (n = 78) | (n = 40) | |
| Hospital length of stay, days, median (IQR) | 23 (15–32.75) | 26 (20.75–45.75) | .0338a |
| Obese patients discharged live (n = 46) | (n = 32) | (n = 14) | |
| Hospital length of stay, days, median (IQR) | 18.5 (14–28.5) | 35.5 (24.75–48) | .0020a |
Abbreviations: IQR, interquartile range; PN, parenteral nutrition.
Wilcoxon Rank-Sum test.
Discussion
To date, there were no studies identified comparing outcomes of critically ill patients initiated on PN within 7 days and after 7 days of poor nutrient intake. This study found no statistically significant difference on in-hospital mortality rates between critically ill patients who were initiated on PN within 7 days and after 7 days of poor nutrient intake, but patients initiated on PN within 7 days had a significantly shorter median hospital length of stay. A key methodologic strength is that results of this study are not subject to immortal time bias. This is evident since investigators of this study accounted for how long patients were without adequate nutritional provision before starting PN both during and prior to hospital admission. Given the median mNUTRIC score for both study groups reflecting high nutrition risk, these findings in the overall patient population in this study support the less restricted use of PN that emerged in recent guidelines and consensus recommendations for patients at high nutrition risk.3,4 Previously, 2009 guidelines recommended PN not be given to any patients unable to receive EN within the first 7 days regardless of nutrition status and disease severity.9 Historically, data were limited for PN use in high nutrition risk or malnourished patients, and suboptimal PN management practices may have contributed to unfavorable outcomes in early studies.4
Patient outcomes in studies comparing early and late initiation of PN in critically ill patients have been variable, but generally favor delaying PN initiation in patients who are not at high nutrition risk or malnourished. Of note, these studies have often used predictive equations to determine patients’ nutritional requirements. While the use of predictive equations is common in clinical practice and recommended by the guidelines in the absence of indirect calorimetry, studies have demonstrated that nutritional goals derived from this approach are often incorrect.10,11 An early, randomized trial assigned patients to receive either PN or prolonged glucose administration (250-300 g/day) for up to 15 days after surgery.12 Providing no nutrition after 14 days of hospitalization resulted in higher mortality and longer hospital length of stay; however, withholding PN in the initial postoperative period did not negatively impact outcomes for most patients.12 An unblinded, multicenter, randomized study compared the outcomes of critically ill patients initiated on PN within 48 h of ICU admission and on day 8 of ICU admission.13 In the subset of patients who had an absolute contraindication to EN and exclusively received PN, patients initiated early had a higher rate of infections and were less likely to be discharged alive than patients initiated on PN after 8 days of ICU admission. However, the median ICU length of stay for patients in that study was only 3 to 4 days and many were admitted for elective procedures indicating the severity of illness was questionable.13
Conversely, other studies have reported benefits of early initiation of PN in critically ill patients. A multicenter, randomized, single-blind study evaluated early PN in critically ill patients with short-term relative contraindications to early EN.14 In total, 686 patients were randomized to receive early PN while 686 patients were randomized to receive standard care. Standard care patients remained unfed for an average of 2.8 days before starting PN or EN. Patients in the early PN group started PN within an average of 44 min. There was no statistically significant difference in mortality rates between groups. However, patients in the early initiation group had a decreased duration of mechanical ventilation and experienced less muscle wasting and loss of body fat compared with the standard care group.14 A smaller, prospective study of patients undergoing gastrointestinal surgery found that patients who received adequate nutrition within 7 days were less likely to have postoperative complications.15 Heidegger and colleagues conducted a randomized controlled trial at two centers in Switzerland to assess whether delivery of 100% of the energy target from days 4 to 8 in the ICU with supplemental PN could optimize clinical outcomes.16 Supplemental PN 4 days after ICU admission reduced nosocomial infections.16 In a follow-up study, investigators determined that providing supplemental PN from days 4 to 8 in critically ill patients is associated with improved immunity and less systemic inflammation.17 In alignment with these findings from Switzerland, it has been reported that surgical ICU patients with appropriate energy and protein provision are more likely to be discharged home.18
Since findings in heterogeneous critically ill patients have been variable and current guideline and consensus recommendations provide recommendations based on nutritional risk,3,4 the investigators conducted a pre-determined subgroup analysis stratifying patients based on nutritional classification at the time of PN initiation. In this study, the time to initiation of PN did not impact in-hospital mortality within each nutritional classification. This finding may be due to high mNUTRIC scores in underweight, normal weight, and obese groups. However, this study suggests that critically ill obese patients initiated on PN within 7 days of poor nutrient intake will have a shorter duration of hospitalization compared to obese patients initiated on PN after 7 days of poor nutrient intake. Since mNUTRIC scores were high in underweight, normal weight, and obese groups, these results may indicate that obesity adds an additional element of nutritional risk that should be considered and studied further for potential earlier initiation of PN. In fact, protein turnover and catabolism rate are higher for patients with obesity suggesting that lack of nutrition therapy may impact outcomes.19 Current consensus recommendations suggest initiating PN within 3 to 5 days in adult patients who are at nutritionally-at-risk based on factors such as weight loss, BMI < 18.5 kg/m2, and altered or inadequate intake for more than 7 days, but obesity is not incorporated into this definition.4 Data regarding the impact of obesity on morbidity and mortality are conflicting.19-23 Meta-analyses have associated obesity during critical illness with an increased ICU length of stay without an increase in mortality.20,21 As a consequence of our results and other literature, obesity should be studied further as a potential indicator of patients who are nutritionally-at-risk warranting earlier PN initiation.
As another area for future study, the provision of energy and protein within 7 days of poor nutrient intake and after 7 days of poor nutrient intake should be considered. In this study, patients were advanced to goal in a median of 2 days and energy and protein provision at goal were similar for groups initiated on PN within or after 7 days of poor nutrient intake for underweight, normal weight, and obese patients. Of note, caloric provision was not as conservative as cited in the literature, especially for obese patients. Based on a low quality of evidence, guidelines suggest that feeding with ⩽20 kcal/kg/day or 80% of estimated energy needs, but adequate protein (⩾1.2 g/kg/day) may be appropriate in high risk or severely malnourished patients requiring PN in the first week of hospitalization to reduce infectious complications, duration of mechanical ventilation, and hospital length of stay.3,24 In addition, a significant percentage of this study’s patient population was obese, but the hypocaloric feeding approach that is recommended for critically ill obese patients to improve nitrogen balance and shorten length of stay in the ICU had not been consistently implemented at the institution in the time period of retrospective review.3,25
Beyond patient outcomes, shortening the median hospital length of stay by 7 days for critically ill patients initiated on PN within 7 days has remarkable cost savings implications. This effect was more pronounced for critically ill, obese patients in which the median hospital length of stay was 16 days shorter for those initiated on PN within 7 days. Hospital length of stay, the development of subsequent infectious complications, and costs are often interrelated. Pradelli and colleagues demonstrated that optimizing energy provision with supplemental PN on days 4 to 8 if EN is insufficient decreases the cumulative energy deficit, reduces the risk of nosocomial infection by 10%, and results in lower costs.26 Doig and colleagues conducted an economic analysis of cost implications of early PN to critically ill patients with short-term relative contraindications to EN.27 Early PN reduced the need for mechanical ventilation and decreased the ICU length of stay resulting in a significant reduction in hospital costs per patient.27
Several limitations were identified in this study. First, as a retrospective, observational single-center study, the results of this study may not be generalizable to other institutions or patient populations. The investigators relied on available documentation to determine the number of days of poor nutrient intake prior to PN initiation. Also, the nutritional classification in this study was based on ABW relative to IBW as opposed to the recommended NUTRIC or NRS scores. The institution at which this study was conducted had not implemented a scoring system for nutritional risk. Investigators found ABW relative to IBW to be appropriate for the subgroup analysis as it can be easily applied in clinical practice. The NRS score classifies nutritional risk based on several factors including the amount of time to develop a >5% weight loss, a decrease in nutrient intake, BMI, age, and co-morbidities. While the NRS score has demonstrated a relation between positive outcomes and nutrition support in patients with a score greater than or equal to 3, it is impossible to accurately calculate retrospectively. However, investigators found it feasible to capture a baseline mNUTRIC score that does not include interleukin-6 and uses the CCI as a substitute for the standard co-morbidities included in the NUTRIC score.15 Finally, an a priori power analysis calculation was not conducted and the small sample size may have resulted in a type II error for evaluation of in-hospital mortality. Furthermore, the small sample size of underweight patients may have limited our ability to find a statistically significant difference in patient outcomes for this high nutrition risk patient population; however, previous meta-analyses have suggested benefits of PN in malnourished critically ill patients.28,29
Conclusions
Time to initiation of PN did not have a significant impact on in-hospital mortality in this study, but warrants further investigation. However, patients who received PN within 7 days of poor nutrient intake had a shorter hospital length of stay compared with patients who received PN after 7 days of poor nutrient intake. A subgroup analysis found that obese patients who received PN within 7 days of poor nutrient intake had a shorter hospital length of stay compared with obese patients who received PN after 7 days of poor nutrient intake. Future studies should confirm these results that suggest critically ill obese patients may be a nutritionally at-risk population warranting earlier PN initiation and investigate optimal provision of energy and protein based on days of poor nutrient intake and time in the ICU.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: S. Shah and A. L. Bingham contributed to the conception of the research; all authors contributed to the design of the research; S. Shah contributed to the acquisition of data; S. Shah and L. Pontiggia contributed to the analysis of data; all authors contributed to the interpretation of data; and S. Shah and A. L. Bingham drafted the manuscript. All authors critically revised the manuscript, agree to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final manuscript.
References
- 1. Singer P, Berger MM, Van den Berghe G, et al. ESPEN guidelines on parenteral nutrition: intensive care. Clin Nutr. 2009;28:387–400. [DOI] [PubMed] [Google Scholar]
- 2. Alberda C, Gramlich L, Jones N, et al. The relationship between nutritional intake and clinical outcomes in critically ill patients: results of an international multicenter observational study. Intensive Care Med. 2009;35:1728–1737. [DOI] [PubMed] [Google Scholar]
- 3. Taylor BE, McClave SA, Martindale RG, et al. ; Society of Critical Care Medicine and the American Society for Parenteral and Enteral Nutrition. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). Crit Care Med. 2016;44:390–438. [DOI] [PubMed] [Google Scholar]
- 4. Worthington P, Balint J, Bechtold M, et al. When is parenteral nutrition appropriate? JPEN J Parenter Enteral Nutr. 2017;41:324–327. [DOI] [PubMed] [Google Scholar]
- 5. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 6. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 7. Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-Related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. [DOI] [PubMed] [Google Scholar]
- 8. Heyland DK, Dhaliwal R, Jiang X, Day AG. Identifying critically ill patients who benefit the most from nutrition therapy: the development and initial validation of a novel risk assessment tool. Crit Care. 2011;15:R268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McClave SA, Martindale RG, Vanek VW, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). J Parenter Enteral Nutr. 2009;33:277–316. [DOI] [PubMed] [Google Scholar]
- 10. De Waele E, Opsomer T, Honore PM, et al. Measured versus calculated resting energy expenditure in critically ill adult patients. Do mathematics match the gold standard? Minerva Anestesiol. 2015;81:272–282. [PubMed] [Google Scholar]
- 11. Frankenfield DC, Coleman A, Alam S, Cooney RN. Analysis of estimation methods for resting metabolic rate in critically ill adults. JPEN J Parenter Enteral Nutr. 2009;33:27–36. [DOI] [PubMed] [Google Scholar]
- 12. Sandstrom R, Drott C, Hyltander A, et al. The effect of postoperative intravenous feeding (TPN) on outcome following major surgery evaluated in a randomized controlled study. Ann Surg. 1993;217:185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Casaer MP, Mesotten D, Hermans G, et al. Early versus late parenteral nutrition in critically ill adults. N Engl J Med. 2011;365:506–517. [DOI] [PubMed] [Google Scholar]
- 14. Doig GS, Simpson F, Sweetman EA, et al. Early PN Investigators of the ANZICS Clinical Trials Group. Early parenteral nutrition in critically ill patients with short-term relative contraindications to early enteral nutrition: a randomized controlled trial. JAMA. 2013;309:2130–2138. [DOI] [PubMed] [Google Scholar]
- 15. Rahman A, Hasan RM, Agarwala R, Martin C, Day AG, Heyland DK. Identifying critically ill patients who will benefit most from nutritional therapy: further validation of the “modified NUTRIC” nutritional risk assessment tool. Clin Nutr. 2016;35:158–162. [DOI] [PubMed] [Google Scholar]
- 16. Heidegger CP, Berger MM, Graf S, et al. Optimisation of energy provision with supplemental parenteral nutrition in critically ill patients: a randomised controlled clinical trial. Lancet. 2013;381:385–393. [DOI] [PubMed] [Google Scholar]
- 17. Berger MM, Pantet O, Jacquelin-Ravel N, et al. Supplemental parenteral nutrition improves immunity with unchanged carbohydrate and protein metabolism in critically ill patients: the SPN2 randomized tracer study [published online ahead of print November 5, 2018]. Clin Nutr. doi: 10.1016/j.clnu.2018.10.023. [DOI] [PubMed] [Google Scholar]
- 18. Yeh DD, Fuentes E, Quraishi SA, et al. Adequate nutrition may get you home: effect of caloric/protein deficits on the discharge destination of critically ill surgical patients. JPEN J Parenter Enteral Nutr. 2016;40:37–44. [DOI] [PubMed] [Google Scholar]
- 19. Dickerson RN, Patel JJ, McClain CJ. Protein and calorie requirements associated with the presence of obesity. Nutr Clin Pract. 2017;32:86S–93S. [DOI] [PubMed] [Google Scholar]
- 20. Akinnusi ME, Pineda LA, El Solh AA. Effect of obesity on intensive care morbidity and mortality: a meta-analysis. Crit Care Med. 2008;36:151–158. [DOI] [PubMed] [Google Scholar]
- 21. Oliveros H, Villamor E. Obesity and mortality in critically ill adults: a systematic review and meta-analysis. Obesity (Silver Spring). 2008;16:515–521. [DOI] [PubMed] [Google Scholar]
- 22. Neville AL, Brown CV, Weng J, Demetriades D, Velmahos GC. Obesity is an independent risk factor of mortality in severely injured blunt trauma patients. Arch Surg. 2004;139:983–987. [DOI] [PubMed] [Google Scholar]
- 23. Yaegashi M, Jean R, Zuriqat M, Noack S, Homel P. Outcome of morbid obesity in the intensive care unit. J Intensive Care Med. 2005;20:147–154. [DOI] [PubMed] [Google Scholar]
- 24. McCowen KC, Friel C, Sternberg J, et al. Hypocaloric total parenteral nutrition: effectiveness in prevention of hyperglycemia and infectious complications- a randomized controlled trial. Crit Care Med. 2000;28:e3606–e3611. [DOI] [PubMed] [Google Scholar]
- 25. Dickerson RN, Boschert KJ, Kudsk KA, Brown RO. Hypocaloric enteral tube feeding in critically ill obese patients. Nutrition. 2002;18:241–246. [DOI] [PubMed] [Google Scholar]
- 26. Pradelli L, Graf S, Pichard C, Berger MM. Supplemental parenteral nutrition in intensive care patients: a cost saving strategy. Clin Nutr. 2018;37:573–579. [DOI] [PubMed] [Google Scholar]
- 27. Doig GS, Simpson F; Group Investigators of the Anzics Clinical Trials. Early parenteral nutrition in critically ill patients with short-term relative contraindications to early enteral nutrition: a randomized controlled trial. Clinicoecon Outcome Res. 2013;5:369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Braunschweig CL, Levy P, Sheean PM, Wang X. Enteral compared with parenteral nutrition: a meta-analysis. Am J Clin Nutr. 2001;74:534–542. [DOI] [PubMed] [Google Scholar]
- 29. Heyland DK, MacDonald S, Keefe L, Drover JW. Total parenteral nutrition in critically ill patients: a meta-analysis. JAMA. 1998;280:2013–2019. [DOI] [PubMed] [Google Scholar]


