Abstract
The biodegradability and clearance of metal-based nanomaterials have been questioned worldwide, which have greatly limited their clinical translation. Herein, ultrathin manganese dioxide (MnO2) nanosheets with broad near-infrared (NIR) absorption and pH-dependent degradation properties were prepared. After being modified with polyethylene glycol-cyclic arginine-glycineaspartic acid tripeptide (PEG-cRGD), the MnO2 nanosheets were then used as photothermal agent and nanocarrier to encapsulate chlorin e6 (Ce6) for targeted photothermal (PTT) and photodynamic (PDT) of cancer. As expected, the MnO2-PEG-cRGD nanosheets show high Ce6 loading capacity (351 mg/g), superb photothermal conversion performance (37.2%) and excellent colloidal stability. These nanosheets also exhibit pH-dependent and NIR-induced Ce6 release. Furthermore, the MnO2 nanosheets can be degraded by reacting with hydrogen peroxide in the acidic microenvironment, which are able to elevate the oxygen concentration in situ and thus reverses the tumor hypoxia. Thanks to these favorable properties and the cRGD-mediated tumor-targeted ability, the fabricated MnO2-PEG-cRGD/Ce6 nanocomposites can be effectively up taken by alpha-v beta-3 (αvβ3) integrin over-expressed prostatic carcinoma PC3 cells and achieve favorable therapeutic outcomes under a single 660 nm NIR laser, which is also verified by in vitro studies. The biodegradable MnO2-PEG-cRGD/Ce6 nanosheets developed in this work can be a promising nanoplatform for synergetic PTT/PDT cancer therapy.
Keywords: MnO2 nanosheet, targeted delivery, photothermal therapy, photodynamic therapy, Ce6
Introduction
Cancer is a major worldwide menace to human health, which has become one of the main focuses of the biomedical world. The conventional cancer treatment methods, such as surgery, chemotherapy, and radiotherapy are often associated with lethal side effects, multidrug resistance, and low therapy efficiency (Huo et al., 2017; Shi et al., 2017; Bray et al., 2018). Great progress of nanoscience and nanobiotechnology has led to the generation of abundant antitumor therapy strategies (Fan et al., 2017; Tang et al., 2017). Among of various treatment strategies, phototherapy, including photothermal therapy (PTT; Jaque et al., 2014) and photodynamic therapy (PDT; Lucky et al., 2015) have drawn considerable attention due to the low systemic toxicity, high minimal invasiveness, and high selectivity. However, the conventional sole-modal therapy, either PTT or PDT, generally cannot restrain the tumors completely owing to the intrinsic drawbacks of phototherapy. Such as the insufficient penetration depth of near-infrared (NIR) light and inevitable injury to nearby normal tissues induced by high temperature in PTT, as well as the local hypoxia of tumors severely hampers the application of PDT (Ocsoy et al., 2016; Yang et al., 2016).
Synergistic therapy is one of promising ways that integrated two or more therapeutic modes into a single nanoplatform, which can enhance the treatment efficacy. Based on this, many researches have gradually shifted from PTT or PDT toward the combined PTT/PDT therapy, which results in remarkable super additive therapeutic effects (Dai et al., 2017; Fan et al., 2017). To fabricate this kind of combined therapy nanoplatform, well-designed nanoscale structures with multiple integrated functionalities, including photothermal conversion agent and photosensitizers are indispensable (Wu et al., 2018a; Zhang et al., 2011). However, different wavelengths of lasers were employed in most of strategies to activate the photothermal conversion agent and photosensitizer for PTT and PDT (Liu et al., 2014; 2017a), which is inconvenient due to the equipment/time cost and synergistic interactions evaluation. Thus, it is highly desirable to develop a photo-activatable nanoplatform to realize PTT and PDT simultaneously upon a single laser irradiation to avoid the time interval between PDT and PTT.
Recently, various types of nanocarriers, including graphene (Chen et al., 2016b), transition metal dichalcogenides (Li et al., 2015), black phosphorus (BP; Chen et al., 2017b), and other types (Song et al., 2015; Liu et al., 2018a; Zhen et al., 2018) were used for the combination of photothermal and photodynamic therapy to acquire the satisfactory synergistic therapeutic output. Two dimensional (2 D) materials with a nanoscale thickness have been widely investigated in many fields (Liu et al., 2017b; Zhang et al., 2019). As a promising 2D nanomaterial, manganese dioxide (MnO2) nanosheets have been preliminarily demonstrated in nanomedicine, especially in drug delivery because of its large surface areas, high NIR absorbance and good biocompatibility (Chen et al., 2016a). Compared with spherical nanoparticles, 2D MnO2 nanosheets possess peculiar photothermal-conversion capability due to their ultrathin thickness intrinsically (Liu et al., 2018b), which can be served as an excellent photothermal agent for highly efficient PTT against tumor. Similar with other reported 2 D nanomaterial, the large surface-area-to-volume ratio make the MnO2 nanosheet can be used as drug delivery platforms to load various drug molecules via π–π stacking and hydrophobic interactions (Ji et al., 2018; Peng et al., 2018). Meanwhile, either in the form of spherical nanoparticles or 2 D nanosheets, MnO2 has been explored to overcome hypoxia because of the performance of acidic hydrogen peroxide (H2O2) response, thus results in improving the efficacy of PDT (Chen et al., 2019). What is more, manganese is a vital element in the human body and its metabolism will not cause serious immune responses. For instance, Chen et al. (2014) reported an intelligent theranostic platform based on 2 D MnO2 nanosheets for concurrent ultrasensitive pH-responsive MRI and drug release/delivery. Recently, ultrathin MnO2 nanosheets-based theranostics nanoplatform has been designed by Liu et al. (2018b) for PTT of cancer. Despite these progresses of MnO2-based materials in nanomedicine applications, as yet, no synergetic PTT/PDT nanoplatform based on targeted modification MnO2 nanosheets and photosensitizers has been reported. It is supposed that combining targeted modification MnO2 nanosheets with photosensitizers should be a promising strategy for cancer treatment.
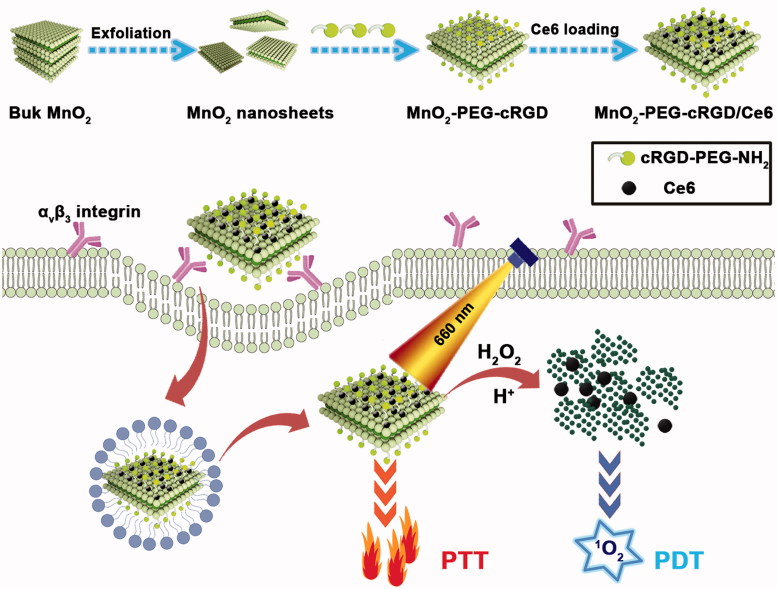
Herein, we have designed a nanoplatform by immobilizing chlorin e6 (Ce6) on polyethylene glycol-cyclic arginine-glycineaspartic acid tripeptide (PEG-cRGD) functionalized MnO2 nanosheets (MnO2-PEG-cRGD/Ce6), which can be used for overcoming the hypoxia of cancer cells and synergistic PTT/PDT (Scheme 1). MnO2 nanosheets were prepared by ultrasonicating exfoliation of bulk MnO2, which was initially synthesized based on the oxidation of manganese chloride by H2O2 in the presence of tetramethylammonium hydroxide and then functionalized with PEG-cRGD to enhance their biocompatibility. The extraordinary surface area of MnO2 nanosheets enables it highly efficient loading of small molecules to achieve different functions. Ce6, a widely used commercial photosensitizer was loaded on the surface of MnO2-PEG-cRGD. As expected, MnO2-PEG-cRGD nanosheets shows a high Ce6 loading capacity (351 mg/g) and exhibits pH/NIR responsive DOX release behavior, as well as significantly increase of large singlet oxygen (SO) generation. Interestingly, the fabricated MnO2-PEG-cRGD/Ce6 exhibits superior photothermal conversion efficiency (37.2%), compared with MnO2-PEG-cRGD (21.4%). Owing to the modification of cRGD peptide, MnO2-PEG-cRGD/Ce6 could be specifically taken by alpha-v beta-3 (αvβ3) integrin receptors over-expressed prostatic carcinoma PC3 cells (Tian et al., 2019). This nanoplatform demonstrates a combination therapy including PTT and PDT based on in vitro tests, which exhibits an excellent synergetic anticancer activity under a single 660 nm NIR laser. Overall, our MnO2-PEG-cRGD/Ce6 is a promising biodegradable nanoplatform for synergistic PTT/PDT under a single laser and this work may explore the application of MnO2-based nanomaterials in combinatorial cancer therapy.
Scheme 1.
Schematic illustration for the preparation of MnO2-PEG-cRGD/Ce6 composite as a biodegradable nanoplatform for synergistic PTT/PDT targeted therapy.
Materials and methods
Materials
Manganese chloride (MnCl2), tetrabutylammonium hydroxide ((C4H9)4NOH), calcein acetoxymethyl ester (calcein AM), and propidium iodide (PI) were purchased from Aladdin Reagent (Shanghai, China). Ce6 was obtained from J&K Scientific Ltd., Shanghai, China. 1,3-diphenylisobenzofuran (DPBF), [Ru(dpp)3]Cl2 (RDPP), and 2,7-dichlorofluorescin diacetate (DCFH-DA) were obtained from Sigma-Aldrich (St. Louis, MO). cRGD-PEG2000-NH2 was bought from Xi’an Ruixi Biological Technology Co. Ltd (Xi’an, China). Phosphate buffered solution (PBS), fetal bovine serum (FBS), penicillin-streptomycin, and dimethyl sulfoxide (DMSO) solution were purchased from Gibco Laboratories (Carlsbad, CA). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), Annexin V-FITC/PI apoptosis and necrosis detection kit, and 4-6-diamidino-2-phenylindole (DAPI) were purchased from Yunnan Chenglv Biological Technology Co. Ltd (Kunming, Yunnan). The PC3 prostatic carcinoma cell line and L929 cell line maintained in Dulbecco's modified eagle medium (DMEM) medium was purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). Deionized water (H2O) was purified by a Millipore system (Milli-Q, 18.2 MΩ cm). All chemicals were used throughout.
Synthesis of MnO2-PEG-cRGD nanosheets
The MnO2 nanosheets were synthesized according to the previous report with some modification (Zhao et al., 2014). In brief, a mixture of tetramethylammonium hydroxide (0.6 M, 15 mL) and 3 wt % H2O2 was added into MnCl2 solution (0.3 M, 10 mL) within 15 s. The resulting dark brown suspension was stirred vigorously for 12 h at room temperature and the bulk manganese dioxide was obtained by centrifugation (2000 rpm, 10 min), washed thoroughly with water and ethanol for three times, and finally dried under vacuum at 55 °C. Then, MnO2 nanosheets were prepared by ultrasonicating exfoliation of the above bulk MnO2. Briefly, 10 mg of bulk manganese dioxide was dispersed in 20 mL water and ultrasonicated for 15 h (500 W). Then, the dispersion was centrifuged at 2000 rpm for 20 min, and the supernatant was collected for further use. For cRGD-PEG2000-NH2 modification, the cRGD-PEG2000-NH2 (0.45 mg/mL, 15 mL) was added into a concentration of 4 mg mL−1 MnO2 nanosheets aqueous solution. After that, the mixture was ultrasonicated for 2 h and then centrifuged at 8000 rpm for 10 min. The precipitate was suspended in water for further use.
Synthesis of MnO2-PEG-cRGD/Ce6 nanoparticles
For Ce6 loading, 0.5 mL of Ce6 (5 mg mL−1 in DMSO) was dropwise added into 5 mL MnO2-PEG-cRGD phosphate buffer solution (0.4 mg mL−1, pH 7.4) and dispersed with the assistance of an ultrasonic bath. After stirring for 24 h in the dark, the resulting MnO2-PEG-cRGD/Ce6 were collected by centrifugation and washed for three times with PBS to remove excess unbounded Ce6. The whole process was carried out in the dark to avoid the quenching of Ce6. Meanwhile, the loading capacity can be calculated by using ultraviolet-visible (UV-Vis) spectrometer at a wavelength of 404 nm according to the previous report (Zhang et al., 2019).
Characterization
Transmission electron microscopy (TEM) was carried out by JEOL 2010 F electron microscope (Tokyo, Japan) at an operating voltage of 200 kV. X-ray diffraction (XRD) measurements were carried out using a D/max 2550 VB+/PC X-ray diffractometer (Rigaku Cop., Tokyo, Japan) with Cu Kg radiation (λ = 0.154056 nm) at 40 kV. Dynamic light scattering (DLS) and zeta potential measurements were determined on Malvern Zetasizer (Nano ZS model ZEN3600, Worcestershire, UK). X-ray photoelectron spectroscopy (XPS) analysis was taken on an ESCALAB 250 spectrometer (Thermo-VG Scientific, Waltham, MA). Fourier-transform infrared spectroscopy (FTIR) spectra were obtained from a Nicolet Nexus 870 spectrometer (Nicolet Instruments Inc, USA). UV-Vis spectra were recorded on a UV3600 instrument (Shimadzu Corporation, Japan). Mn concentrations of samples were determined by inductively coupled plasma atomic emission spectrometer (ICP-AES, Agilent Technologies, Palo Alto, CA). NIR laser was acquired on a 660 nm laser device (Shanghai Connect Fiber Optics Company) and the temperature was recorded on a DT-8891E thermocouple linked to a digital thermometer (Shenzhen Everbest Machinery Industry, Shenzhen, China). Confocal laser florescence scanning images were obtained by Leica TCS SP2 microscope (CLSM, Leica Microsystems, Mannheim, Germany).
Photothermal property of the MnO2-PEG-cRGD/Ce6
To study the photothermal conversion property of the MnO2-PEG-cRGD/Ce6, different concentrations of dispersion (20, 50, 100, 150, and 200 μg mL−1) were placed in a quartz cuvette and irradiated with a 660 nm NIR laser (0.6 W/cm2) for 10 min and the temperature was recorded. Then, the MnO2-PEG-cRGD/Ce6 solution at a concentration of 100 μg/mL was irradiated with different power densities (0.2, 0.4, 0.6, 0.8, and 1.0 W/cm−2) over a period of 10 min. Meanwhile, the sample of water, MnO2-PEG-cRGD and Ce6 were also tested for comparison. The photothermal stability of the MnO2-PEG-cRGD/Ce6 was evaluated via monitoring the temperature change with or without 660 nm NIR laser irradiation (0.6 W/cm2) for five cycles. Finally, the photothermal conversion efficiency (η) of the MnO2-PEG-cRGD/Ce6 and MnO2-PEG-cRGD was calculated by the following equation that was reported by Wu et al. (2018b).
| (1) |
Where, h is the heat transfer coefficient, S is the surface area of the container, Tmax is equilibrium temperature, Tam is surrounding ambient temperature, Qs is the heat associated with the light absorbance of the solvent, I is the laser power, and A is the absorbance of the MnO2-PEG-cRGD/Ce6 or MnO2-PEG-cRGD at 660 nm.
In vitro pH/NIR responsive Ce6 release
In vitro Ce6 release from MnO2-PEG-cRGD/Ce6 was measured at varied pH of 7.4 and 5.5 with or without 660 nm laser irradiation. In brief, 10 mg of MnO2-PEG-cRGD/Ce6 were dispersed in 5 mL of PBS solution (pH 7.4 or 5.0). Then, 1 mL of solution was taken and encapsulated into a dialysis bag (MWCO: 5000 Da) and dialyzed against PBS. Afterward, the sample was directly irradiated by 660 nm NIR laser (0.6 W/cm2) for 10 min. At designated time intervals, 1.5 mL of solution outside the dialysis bag was collected and the amount of released Ce6 was determined with UV-Vis spectrometer at a wavelength of 404 nm.
Photodynamic properties of the MnO2-PEG-cRGD/Ce6
The production of oxygen (O2) was detected with RDPP, whose fluorescence can be quenched by O2 (Ma et al., 2017). In brief, 100 µL of ethanol solution of RDPP (10 × 10−3 M) was added to MnO2-PEG-cRGD/Ce6 (1 mL, 50 µg mL−1). Then 300 μL of H2O2 (100 mM) was added and the fluorescence intensity of RDPP (λex = 455 nm) at 615 nm was recorded every 2 min. Meanwhile, the H2O2 or MnO2-PEG-cRGD/Ce6 without H2O2 addition was also tested as control.
Next, the generation of SO (1O2) was evaluated by the chemical probe DPBF. Typically, DPBF ethanol solution (20 μL, 100 mM) were added to 1 mL of free Ce6 or MnO2-PEG-cRGD/Ce6 solution. Then, 100 mM of H2O2 was added into the above system, following by laser irradiation (660 nm, 0.6 W/cm2) for 10 min. The absorption intensity of DPBF at 410 nm was monitored. Meanwhile, the sample without laser irradiation was used as control.
Degradation behavior of MnO2-PEG-cRGD
The degradation behavior of as-prepared MnO2-PEG-cRGD was investigated according a previous report (Chen et al., 2017a,b). MnO2-PEG-cRGD (1.5 mg) was dispersed in 30 mL of PBS solution (pH 5.0) with addition of H2O2 (10 mM) and maintained at 37 °C shaker with shaking speed of 100 rpm. At different time points, 1 mL of solution was taken out and centrifuged, then were re-dispersed by ethanol and dropped on copper grid for TEM observation.
Cell culture and biocompatibility of MnO2-PEG-cRGD in vitro
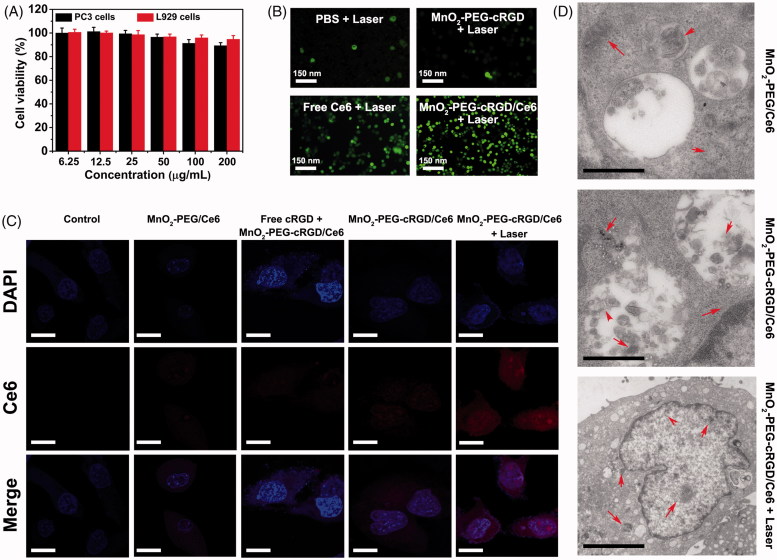
PC3 cells and L929 cells were cultured in DMEM supplemented with 10% FBS and penicillin (100 units/mL)/streptomycin (100 μg/mL) under standard conditions (37 °C, 5% carbon-di-oxide). To estimate the biocompatibility of MnO2-PEG-cRGD, PC3 cells and L929 cells (2 × 104 cells/well) were seeded in 96-well plates and then incubated for 24 h, respectively. After fully adhesion and spread of cells, the fresh medium containing different concentrations (200, 100, 50, 25, 12.5, and 6.25 μg/mL) of MnO2-PEG-cRGD was added and cultured for another 24 h. Finally, the cells were repeatedly rinsed with PBS and the cell viability was evaluated by standard MTT assay.
Generation of intracellular SO
First, the intracellular generation of O2 was investigated also with RDPP. HeLa cells (2.0 × 105 cells per dish) were seeded into culture dishes and incubated with RDPP (0.5 μM) for 4 h. After that, the cells were further incubated with MnO2-PEG/Ce6, MnO2-PEG-cRGD/Ce6 and free Ce6 with an equivalent Ce6 concentration of 10 µg mL−1 for 24 h. The cells were washed with PBS for three times and then exposed to 1 mM H2O2 in DMEM medium for 45 min at 37 °C. Finally, the cells were washed with PBS and fluorescence images were captured by the CLSM. The RDPP were excited at 488 nm, and the emission was collected between 600 and 700 nm. The cells without any treatment served as control. Next, the intracellular 1O2 generation was detected by using the DCFH-DA probe. Briefly, PC3 cells (2 × 105 cells per dish) were treated with MnO2-PEG-cRGD/Ce6 and free Ce6 with an equivalent Ce6 concentration of 10 µg mL−1 overnight. After that, the cells were rinsed with PBS, followed by the addition of DCFH-DA (1 mL, 10 µM in DMEM medium) and incubated for 20 min. Then, the cells were irradiated with 660 nm light (0.6 W cm−2) for 10 min. The cells without any treatment were served as controls. The fluorescence images were captured by CLSM at an excitation of 488 nm.
In vitro cellular uptake
The targeted cell uptake of the MnO2-PEG-cRGD/Ce6 was also investigated. PC3 cells (αvβ3 integrin receptor positive) were seeded on a glass-bottomed culture dish (2 × 105 cells/well) for 24 h. All the cells were treated with MnO2-PEG/Ce6 and MnO2-PEG-cRGD/Ce6 with or without 660 nm laser irradiation for 10 min at an equivalent Ce6 concentration of 5 μg mL−1 for 4 h. After that, the cells in all the groups were washed three times with PBS, fixed with 4% formaldehyde for 20 min. The cell nuclei were stained with DAPI for 10 min, the cells were washed three times with PBS (5 mL) and then imaged by a CLSM (Jena, Germany). For competitive inhibition experiments, PC3 cells were pretreated for 4 h with free cRGD (10 μM) prior to incubation with MnO2-PEG-cRGD/Ce6. For bio-transmission electron microscope (Bio-TEM, Hitachi HT7700, Tokyo, Japan) analysis, a similar protocol was performed as the one used in CLSM experiment. After washing with PBS three times, PC3 cells were fixed with 2% glutaraldehyde for 2 h at room temperature, followed by 2 h in 1% OsO4, dehydrated through a graded ethanol series, embedded in EPOM812 and examined by bio-TEM imaging.
To further confirm the cellular uptake of the MnO2-PEG-cRGD/Ce6, ICP-AES analysis was performed. Similarly, PC3 and L929 cells were treated with free Ce6 and MnO2-PEG-cRGD/Ce6 with or without 660 nm laser irradiation for 10 min at an equivalent Ce6 concentration of 5 μg mL−1 for 4 h. Afterwards, the cells were washed with PBS for three times, trypsinized with 0.05% trypsin-EDTA, centrifuged, and resuspended in 1 mL of PBS. Subsequently, the cells were digested by aqua regia solution (1 mL) overnight, and diluted with 1 mL of water, followed by ICP-AES assay to determine the Mn content in the cell samples.
In vitro antitumor efficiency of MnO2-PEG-cRGD/Ce6
To study the antitumor performance in vitro, PC3 cells were cultured in 96-well plates at a density of 1 × 104 cells per well and incubated for 24 h. Then, the medium was replaced with 100 μL of fresh medium containing different concentrations of MnO2-PEG-cRGD, free Ce6, and MnO2-PEG-cRGD/Ce6 at the equivalent Ce6 concentrations and the cells were incubated for 6 h. After that, the cells were irradiated by a 660 nm light (0.6 W cm−2) for 10 min. After incubation for another 10 h, the cell viability was evaluated by MTT assay. Meanwhile, the cytotoxicity of MnO2-PEG/Ce6 at the equivalent Ce6 concentrations against PC3 cells was also measured for comparison.
To further prove the PTT/PDT therapy efficacy, live/dead cell staining assay was conducted. PC3 cells were seeded in six-well plates at a density of 5 × 105 cells per well for 24 h incubation, followed by incubation with MnO2-PEG-cRGD, free Ce6, and MnO2-PEG-cRGD/Ce6 at equivalent Ce6 concentrations of 5 μg mL−1, respectively. After incubation for 6 h, the cells were exposed to 660 nm light (0.6 W cm−2) for 10 min. After that, the cells were stained with Calcein-AM (5 μg mL−1) and PI (10 μg mL−1) for 30 min at 37 °C. Then, the cells after different treatments were washed with PBS three times and monitored with an inverted fluorescence microscope. The untreated cells were used as the control. The synergistic effect of PTT/PDT was evaluated by combination index (CI) analysis (Xu et al., 2018). Consequently, CI > 1 denotes antagonism; CI = 1 additivity, and CI < 1 synergism.
Cell apoptosis assay
The apoptosis and necrosis assay of PC3 cells treated with MnO2-PEG-cRGD under laser irradiation was evaluated by flow cytometry. The PC3 cells were seeded in six-well plate at a density of 5 × 105 cells/well and cultured for 24 h. Then, the cells were treated separately with MnO2-PEG-cRGD, free Ce6 and MnO2-PEG-cRGD/Ce6 (the equivalent Ce6 concentrations of 5 μg mL−1). After 6 h incubation, the cells were irradiated with 10 min laser irradiation (0.6 W/cm2). Cells untreated and subjected to only laser irradiation was tested as the control. After further incubation for 18 h, all the cells were digested, collected, and re-suspended in the annexin-binding buffer. After that, the cells were stained with recombinant human anti-Annexin-V-FITC (5 μL) and PI (5 μL) for 15 min in the dark, and then examined by flow cytometry (BD FACS Aria TM III, BD Biosciences, Franklin Lakes, NJ).
Statistical analysis
One-way analysis of variance (ANOVA) statistical method was performed to evaluate the experimental data. A value of 0.05 was selected as the significance level and the data were indicated with (*) for p < .05, (**) for p < .01, and (***) for p < .001, respectively.
Results and discussion
Preparation and characterization of MnO2-PEG-cRGD/Ce6 nanoparticles
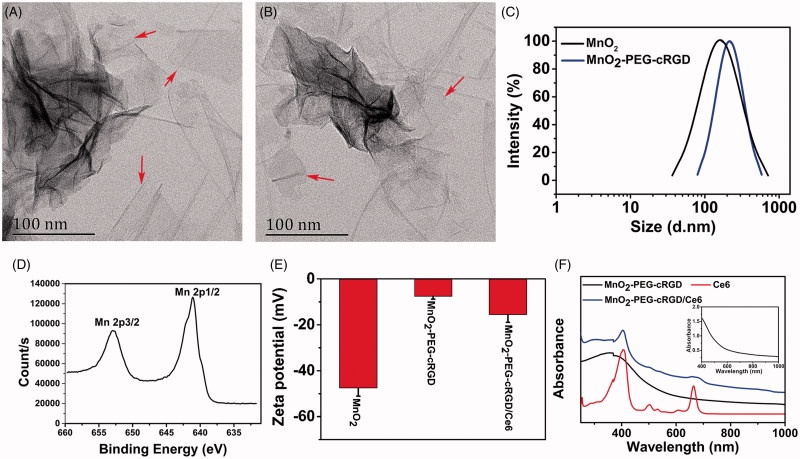
MnO2 nanosheets were prepared from bulk MnO2, which is synthesized by oxidation of MnCl2 in the presence of H2O2 and tetramethylammonium hydroxide (Zhao et al., 2014). Then, as-prepared MnO2 nanosheets were modified with cRGD-PEG2000-NH2 under vigorous sonication for compatible with biological application as well as introduce the targeting ligands (Scheme 1). As revealed by TEM imaging, the as-synthesized MnO2-PEG-cRGD nanosheets exhibited the obvious planar morphology with the thickness of about 1 nm, indicating the few layers structure (Figure 1(B)). Compared to MnO2 nanosheets (Figure 1(A)), the PEG and cRGD modification showed negligible change of morphology and uniform layer structure. DLS results show that the average diameter of MnO2 and MnO2-PEG-cRGD are 217 ± 14.6 and 271 ± 26.7 nm, respectively (Figure 1(C)). XRD spectra of MnO2-PEG-cRGD (Supplementary Figure S1) showed the obvious characteristic peaks at 2θ = 36.6 and 65.9°, which are corresponded to (221) and (002) planes of α(m)-MnO2 (Devaraj & Munichandraiah, 2008), indicated the formation of 2 D-layered MnO2 nanosheets. Also, the valence states of manganese component were determined by XPS analysis (Supplementary Figure S2). It can be observed that two peaks at 653.5 and 642.7 eV from high-resolution XPS spectra (Figure 1(D)) of Mn 2p associated with the Mn 2p1/2 and Mn 2p3/2 peaks of MnO2, respectively (Wu et al., 2018c). The FTIR spectrum of the MnO2-PEG-cRGD shows the strong bands of amide I and amide II around 1652 and 1524 cm−1 and the characteristic peaks of PEG (944, 1358, and 2972 cm−1), confirming the successful modification of PEG-cRGD on the surface of MnO2 nanosheets (Supplementary Figure S3). Also, the zeta potentials of MnO2 nanosheets are around −47.5 mV but turned into −7.6 mV after the formation of MnO2-PEG-cRGD (Figure 2(E)). Meanwhile, MnO2-PEG-cRGD exhibits good dispersity in PBS, but the MnO2 nanosheets showed lower colloidal stability (Supplementary Figure S4), thus confirming the PEGylation could improve the physiological stability of nanomaterials.
Figure 1.
TEM images of (A) MnO2 and (B) MnO2-PEG-cRGD nanosheets. (C) DLS size distribution of MnO2 and MnO2-PEG-cRGD nanosheets. (D) Mn 2p XPS spectra for MnO2 nanosheets. (E) Zeta potential of MnO2, MnO2-PEG-cRGD, and MnO2-PEG-cRGD/Ce6 (n = 3). (F) UV-Vis spectra of Ce6, MnO2-PEG-cRGD, and MnO2PEG-cRGD/Ce6. Inset: UV-Vis spectra of MnO2-PEG-cRGD nanosheets between 400 and 1000 nm.
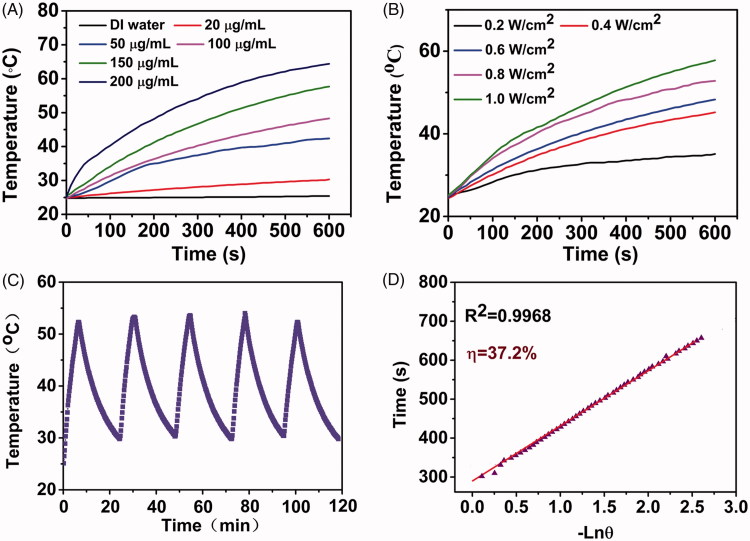
Figure 2.
(A) Photothermal conversion curves of water and MnO2-PEG-cRGD/Ce6 nanoparticles at different concentrations upon irradiation with an 660 nm laser (0.6 W cm − 2). (B) Photothermal conversion curves of MnO2-PEG-cRGD/Ce6 nanoparticles (100 µg mL − 1) at different laser power densities. (C) Photothermal conversion stability of MnO2-PEG-cRGD/Ce6 nanoparticles. The laser was turned on for 10 min and then turned off for each cycle. (D) Linear relationship between time and − ln θ calculated from cooling period after the laser was turned off.
Similar with some other 2 D materials, the two-dimensional structure of MnO2-PEG-cRGD nanosheets could be used as nanocarrier to load some small molecules with excellent loading capacity. Ce6 is a widely used photosensitizer with NIR light absorption, which is able to generate cytotoxic singlet oxygen for PDT (Dong et al., 2016). Herein, Ce6 was loaded on the surface of MnO2-PEG-cRGD nanosheet to form MnO2-PEG-cRGD/Ce6 nanosystem via physical adsorption. The decreased zeta potential of MnO2-PEG-cRGD/Ce6 also suggested that the Ce6 was successfully loaded onto MnO2-PEG-cRGD (Figure 1(E)). Compared to MnO2-PEG-cRGD, two new absorption peaks at 407 and 665 nm was appeared in the UV-Vis-NIR spectra of MnO2-PEG-cRGD/Ce6 (Figure 1(F)), which was assigned to characteristic Soret-band and Q-band of Ce6, thus indicating the successful loading of Ce6. At the studied conditions, about 351 mg of Ce6 was loaded into 1 g of MnO2-PEG-cRGD. The high drug loading capacity may be due to the Mn atoms in the sheets could form special coordinate bonds with the Mn atoms in Ce6 molecules (Ma et al., 2018).
Photothermal and photodynamic properties of MnO2-PEG-cRGD/Ce6
Inspired by the strong NIR absorbance of MnO2-PEG-cRGD/Ce6, the photothermal conversion property of the nanosheets was explored. It should be noted that 660 nm laser was employed as the light source to realize PTT and PDT simultaneously upon a single laser irradiation to avoid the time interval in biomedical application. As shown in Figure 2(A,B), the temperature of the MnO2-PEG-cRGD/Ce6 nanoparticles increased sharply under 660 nm NIR irradiation. The temperature of the MnO2-PEG-cRGD/Ce6 solution (100 μg/mL) increased to 64.4 °C after 10 min of laser (1 W/cm2) irradiation, while the temperature of deionized water only increased by 0.7 °C. Meanwhile, both concentration and laser-power-dependent photothermal effect was observed, indicated the heat generation could be neatly tuned. Meanwhile, MnO2-PEG-cRGD/Ce6 showed an excellent yet stable light-to-heat conversion property within five cycles of laser irradiation (Figure 2(C)). On the basis of the data obtained from the time constant of heat transfer and the maximum steady-state temperature (Figure 2(D) and Supplementary Figure S5), the photothermal conversion efficiency (η) of MnO2-PEG-cRGD/Ce6 was assessed to be 37.2%, which is higher than that of MnO2-PEG-cRGD (21.4%) as well as higher than some literature reported 2 D PTT agents (Yang et al., 2017b). The enhanced photothermal conversion efficiency confirmed the excellent photothermal synergistic effect of MnO2-PEG-cRGD and Ce6, which provides a potential for photothermal therapy.
It is known that MnO2 is reduced into Mn(ιι) ions in presence of acidic H2O2 by oxidation of H2O2 into O2, which could be explained by the following reactions (Chen et al., 2016a):
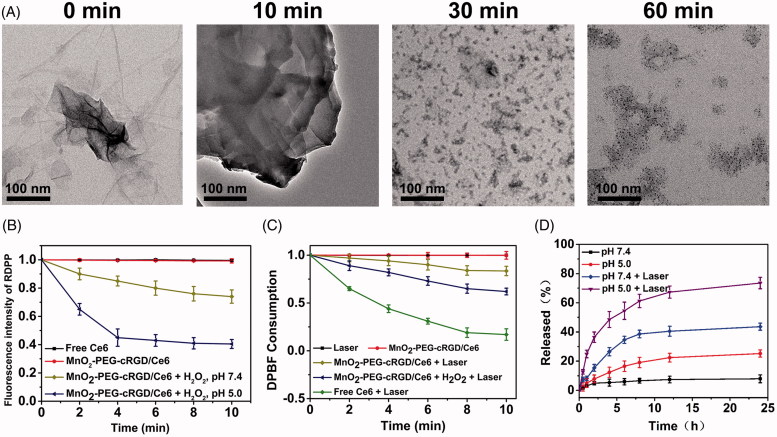
Considering about this reactivity, we wondered whether the reduction of MnO2 would induce the dissociation of MnO2-PEG-cRGD/Ce6. Thus, the degradation behavior of MnO2-PEG-cRGD/Ce6 nanoparticles in acidic H2O2 was carefully studied. The morphology evolution of MnO2-PEG-cRGD/Ce6 in the process of degradation was directly observed using TEM (Figure 3(A)). After only 10 min of immersion in PBS (pH 5) containing 1 mM of H2O2, degradation is evident as certified by the change of shape and more distinct structure collapse. After 30 min, the MnO2-PEG-cRGD/Ce6 with undergone destructive change was observed and eventually become ultra-small and irregularly ruptured clusters after 1 h incubation. Due to the cancer cell is often featured with high H2O2 levels and low pH values, the biodegradability of this nanosheets would favor its application for cancer therapy.
Figure 3.
(A) TEM observation of MnO2-PEG-cRGD/Ce6 after degradation in pH 5 containing 1 mM of H2O2 for 10, 30, and 60 min, respectively. (B) Generation of O2 under the reduction of MnO2-PEG-cRGD/Ce6 (50 µg mL − 1) by 10 × 10 − 3 M H2O2 in acidic PBS (pH 5). (C) Consumption of DPBF over time due to 1O2 generation: (a) laser only, (b) MnO2-PEG-cRGD/Ce6 without laser, free Ce6, and (c) MnO2-PEG-cRGD/Ce6 with laser in the absence (d) or presence (e) of 100 mM H2O2 (pH 7.4). (D) Release profiles of Ce6 at different pHs with or without 660 nm NIR laser (0.6 W/cm2, 10 min).
Then, the oxygen generation of MnO2-PEG-cRGD/Ce6 was then quantified by using an RDPP O2 probe. Obviously, the fluorescence intensity of RDPP is quenched rapidly after addition of H2O2 (100 μM) to MnO2-PEG-cRGD/Ce6 PBS suspension (pH 5) within 4 min (Figure 3(B)), whereas much slower quenching of RDPP was occurred in pH 7.4. By contrast, there is no fluorescence intensity decreased was observed in the group of Ce6 or MnO2-PEG-cRGD/Ce6 without H2O2. These results confirmed that the generation of O2 is resulted from the oxidation of H2O2 by MnO2 nanosheets. Next, we wondered whether MnO2-PEG-cRGD/Ce6 in the presence of H2O2 could be used for PDT or not. Thus, the production of 1O2 by MnO2-PEG-cRGD/Ce6 under laser irradiation (660 nm, 0.6 W/cm2) was measured by a DPBF probe. As expected, the absorption of DPBF was decreased in MnO2-PEG-cRGD/Ce6 suspension, and more obvious decrease of the absorbance of DPBF was detected in the presence of H2O2, which is due to the H2O2-triggered O2 production (Figure 3(C)). As compared to the free Ce6, the absorbance intensity of DPBF gradually decreases with a relative slow rate in MnO2-PEG-cRGD/Ce6, indicating the photodynamic effect of MnO2-PEG-cRGD/Ce6 deriving from the slow release of Ce6. These results demonstrated the MnO2-PEG-cRGD/Ce6 nanoparticles could be served as an efficient PDT agent to reverse the tumor hypoxia for enhancing the therapeutic efficiency under specific laser irradiation.
In vitro pH/NIR responsive Ce6 release
Previous studies have been reported that most of 2 D materials-based nanoplatform can release the loaded cargos in response to acidic and thermal stimuli (Karimi et al., 2016). Thus, the feasibility of pH and light irradiation-triggered release of Ce6 from the nanosystem was further investigated under different treatments. As illustrated in Figure 3(D), less than 8% of Ce6 was released from the MnO2-PEG-cRGD/Ce6 at pH 7.4 after 24 h, while for pH 5, the amount of Ce6 released reached to around 25.2% within 24 h. The pH-responsive release of Ce6 may be due to the protonation of amino groups in Ce6 under acidic conditions, resulting in increasing the hydrophilicity of Ce6 and decreasing binding with nanosheets. In addition, the release performance triggered by NIR laser irradiation was further investigated. As shown in Figure 3(D), a significantly increased Ce6 release was obtained: 43.6% at pH = 7.4 and 73.5% at pH = 5 under 660 nm laser irradiation for 5 min time interval. The NIR-induced drug release may be due to the MnO2-PEG-cRGD/Ce6 could absorb the NIR light and convert into local heating, the heat dissociates the strong interactions between Ce6 and nanosheets, thus more Ce6 molecules are released (Lu et al., 2017).
In vitro biocompatibility and generation of intracellular singlet oxygen
Encouraged by the above-mentioned results, we further evaluated the in vitro biocompatibility and 1O2 generation efficiency of the nanoparticles. Biocompatibility of MnO2-PEG-cRGD is the prerequisite for their biomedical application. Hence, the cytotoxicity of MnO2-PEG-cRGD nanosheets was examined in PC3 cells and L929 cells by MTT assay (Figure 4(A)). No obvious cytotoxicity of MnO2-PEG-cRGD in different cell lines was observed, even at concentrations up to 200 μg mL−1, indicating their good biocompatibility for further biological applications.
Figure 4.
(A) Relative viability of PC3 and L929 cells after incubation with MnO2-PEG-cRGD (25, 50, 100, 200, 300, and 500 µg mL − 1) for 24 h. (B) CLSM of intracellular 1O2 generation after PC3 cells were treated with PBS, MnO2-PEG-cRGD, free Ce6, and MnO2-PEG-cRGD/Ce6 under laser illumination. (C) CLSM of PC3 cells treated with MnO2-PEG/Ce6 and MnO2-PEG-cRGD/Ce6 (relative Ce6 = 5.0 μg/mL) for 4 h with or without laser illumination (Blue fluorescence is associated with DAPI; red fluorescence is expressed by released Ce6). Scale bars = 50 μm. (D) Bio-TEM images of PC3 cells incubated with MnO2-PEG/Ce6 and MnO2-PEGcRGD/Ce6 with or without laser irradiation. Scale bar: 1 μm.
Then, the intracellular O2 generation ability of MnO2-PEG-cRGD/Ce6 was examined by CLSM with O2 probe RDPP. It can be observed that the green fluorescence gradually quenched in the cells after incubated with MnO2-PEG/Ce6 or MnO2-PEG-cRGD/Ce6, while strong RDPP fluorescence was still detected in the PC3 cells that treated with free Ce6 (Supplementary Figure S6). These results indicated the increase of intracellular O2 resulted from the cleavage of intracellular H2O2 by MnO2-PEG/Ce6 or MnO2-PEG-cRGD/Ce6, which overcomes the hypoxia of cancer cells. Furthermore, according to the intensity of green fluorescence, O2 generation was significantly high in the cells treated by MnO2-PEG-cRGD/Ce6, which may be due to the high uptake of MnO2-PEG-cRGD/Ce6.Next, the intracellular 1O2 production by MnO2-PEG-cRGD/Ce6 within PC3 cells was examined by using DCFH-DA probe. After treated with MnO2-PEG-cRGD/Ce6 with 660 nm laser irradiation (0.6 W cm−2), strong fluorescence intensity of DCFH-DA was observed (Figure 4(B)), while much weaker DCFH-DA fluorescence in the cells treated with Ce6 and MnO2-PEG-cRGD. These results confirmed MnO2-PEG-cRGD/Ce6 can improve the production of 1O2 under laser irradiation, which can greatly improve the therapeutic effect of PDT.
In vitro cellular uptake
It has been recognized that cRGD-decorated nanoparticles could be more efficiently internalized by cells via αvβ3 integrin receptor mediated endocytosis (Fang et al., 2017; Zhong et al., 2014). Therefore, the cellular uptake behavior of our MnO2-PEG-cRGD/Ce6 nanoparticles was investigated by using PC3 cells (αvβ3 integrin receptor positive). As shown in Figure 4(C), the PC3 cells group treated with MnO2-PEG-cRGD/Ce6 showed greater Ce6 fluorescence than those treated with MnO2-PEG/Ce6, which reflected the active targeting effect of MnO2-PEG-cRGD/Ce6 toward PC3 cells. Also, the competitive inhibition experiments revealed that 4 h pretreatment of PC3 cells with free cRGD before incubation with MnO2-PEG-cRGD/Ce6 under NIR laser irradiation displayed weak Ce6 fluorescence, further proved active targeting effect of MnO2-PEG-cRGD/Ce6 toward PC3 cells. In addition, the Ce6 signal was much stronger both in cytoplasm and nuclei after the NIR light irradiation (660 nm, 10 min), which is due to the local mild hyperthermia not only caused minor disruptions to cell membrane for improving intracellular delivery of nanoparticles (Lu et al., 2017), but also significantly promoted the Ce6 release.
To check the targeting ability of MnO2-PEG-cRGD/Ce6, the Mn uptake within PC3 cells or L929 cells that treated with the nanoparticles was further investigated by quantitative ICP-AES analysis (Supplementary Figure S7). Obviously, the uptake Mn in the PC3 cells is significantly higher than that in L929 cells owing to the targeting effect of cRGD. Also, the uptake of Mn in laser irradiation group was as higher as nearly three-fold than that for MnO2-PEG-cRGD/Ce6 without laser irradiation. Meanwhile, the localization of the NPs has been evaluated by bio-TEM analysis. PC3 cancer cells were incubated with MnO2-PEG-cRGD/Ce6 and MnO2-PEG-cRGD/Ce6 and corresponding bio-TEM image is respectively displayed in Figure 4(D). Under TEM observation, a greater number MnO2-PEG-cRGD/Ce6 nanoparticle were found in lysosomes in MnO2-PEG-cRGD/Ce6 after NIR light irradiation. By contrast, fewer nanoparticles were endocytosed in the group that treated with MnO2-PEG-cRGD/Ce6, as well as MnO2-PEG/Ce6 nanoparticles. These results verified that MnO2-PEG-cRGD/Ce6 mediate cellular uptake mostly due to the combination of receptor-mediated endocytosis and NIR-induce local mild hyperthermia.
In vitro synergistic therapy
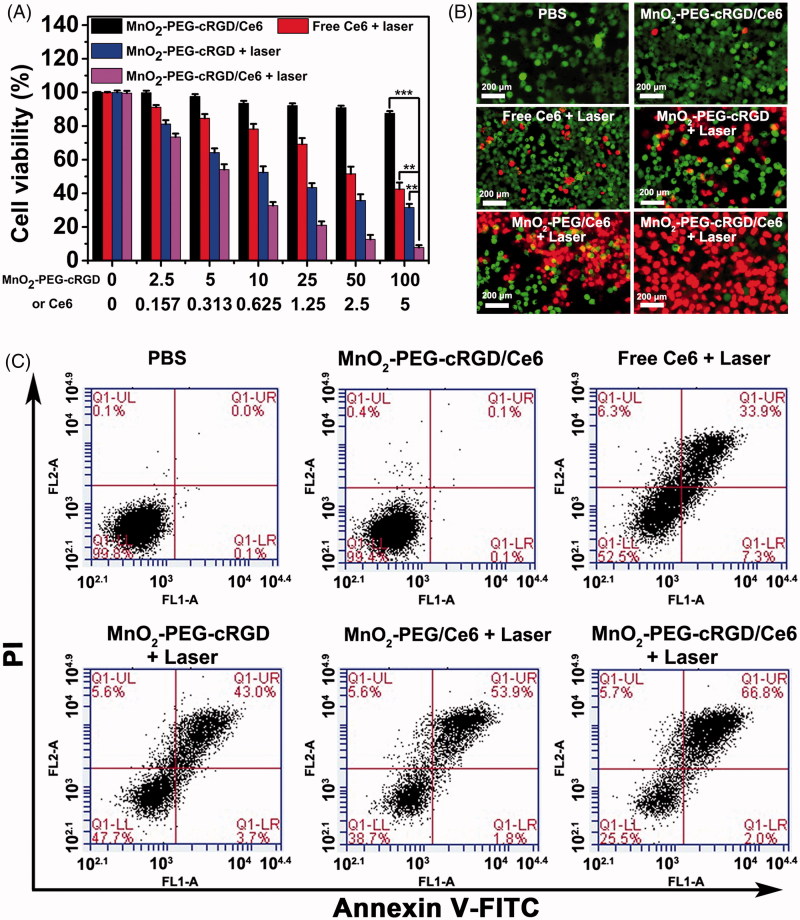
Next, we detected the cytotoxicity of combinatorial PTT and PDT of MnO2-PEG-cRGD/Ce6 nanoplatform for cancer cells in vitro. First, cells were incubated with free Ce6, MnO2-PEG-cRGD, or MnO2-PEG-cRGD/Ce6 in presence of NIR irradiation (660 nm, 0.6 W cm−2) for 10 min at increasing concentrations. Clearly, a MnO2-PEG-cRGD or Ce6 dose-dependent cytotoxicity was observed in both treatments (Figure 5(A)). Ultimately, about 90% of the PC3 cells were killed by the MnO2-PEG-cRGD/Ce6-induced synergistic effect (MnO2-PEG-cRGD: 100 μg/mL, Ce6: 5 μg/mL), which is much higher than that of MnO2-PEG-cRGD with 30% of cells death and free Ce6 with 34% of cells death. Meanwhile, cells treated with MnO2-PEG/Ce6 also exhibited a dosage-dependent cell death (Figure S8), but a bit lower than that of treated with MnO2-PEG-cRGD/Ce6 at the same concentration of Ce6, owing to the absence of cRGD modification. The half maximal inhibitory concentration (IC50) of MnO2-PEG-cRGD/Ce6 is 0.36 µg mL−1, which is smaller than that of Ce6 (2.74 µg mL−1) and MnO2-PEG-cRGD/Ce6 (0.61 µg mL−1). Overall, MnO2-PEG-cRGD/Ce6 show the best therapeutic output compared with MnO2-PEG-cRGD and MnO2-PEG/Ce6, indicating the advantage of synergistic PTT/PDT cancer therapy. Also, the combination index (CI) of PTT and PDT is calculated to be 0.36, further verifying the synergistic effect. For deeper insight of the synergistic effect, live/dead cell staining assay was conducted (Figure 5(B)). Compared to the control group and any other treatment group, almost all of the cells were death after treated with MnO2-PEG-cRGD/Ce6 with laser irradiation. These results are consistent with the results of MTT experiments.
Figure 5.
(A) Relative viabilities of PC3 cells after incubation with free Ce6, MnO2-PEG-cRGD with 660 nm light irradiation or MnO2-PEG-cRGD/Ce6 with or without 660 nm light irradiation (0.6 W cm − 2, 10 min). ***p < .001, **p < .01. (B) Fluorescence images of calcein-AM (green)/PI (red) double stained cells after different treatments. (C) Flow cytometric analysis of cell apoptosis for 24 h caused by different treatments, using the Annexin V-FITC/PI staining. The four areas represent different phases of the cells: necrotic (Q1), late-stage apoptotic (Q2), early apoptotic (Q3), and live (Q4).
Cell apoptosis assay
To further evaluate the mechanism of synergistic PTT/PDT anticancer effect of PC3 cells, the cells after different treatments were double-labeled with Annexin V-FITC/PI for flow cytometry analysis (Figure 5(C)). As expected, the percentage of apoptosis/necrotic cells was determined to be 68.8% (early apoptosis + later apoptosis) in group of MnO2-PEG-cRGD/Ce6 with laser irradiation. However, few cells were in apoptosis/necrotic status by the treatment of MnO2-PEG/Ce6 under laser irradiation (55.7%), as well as compared to the cells after other treatment groups, further indicating the powerful apoptosis-inducing capacity of MnO2-PEG-cRGD/Ce6 against PC3 tumor cells. These results, together with the MTT result and live/dead staining assay evidenced that the as-fabricated MnO2-PEG-cRGD/Ce6 simultaneously possess optimal synergistic activity of PDT and PTT.
Compared with previously reported inorganic photothermal agents, the developed MnO2-PEG-cRGD/Ce6 are especially attractive due to the unique multicomponent functional elements. The photothermal-conversion efficiency of the prepared nanosheets in this work was calculated to be to be 37.2%, which is comparable to MnO2 nanosheets (Liu et al., 2018b; Wu et al., 2018c), Au nanorods (21%; Zeng et al., 2013), Ag2S (22%; Yang et al., 2017a) and MoS2-PEG nanoflakes (25.7%) (Feng et al., 2015). On the other hand, compared with previously reported 2 D nanosheets-based nanosystems (Ma et al., 2017; Liu et al., 2018b; Ji et al., 2018), the cRGD modification make it can be recognized and taken up by PC3 cells via the receptor-mediated endocytosis mechanism, resulting in enhanced tumor penetration and potent antitumor effect. Also, our work presents a novel multifunctional drug delivery system that is purely composed by biocompatible (PEG) and biodegradable (MnO2) components, with substantial potential for future clinical translation.
Conclusion
In summary, MnO2-PEG-cRGD nanosheets loaded with a Ce6 photosensitizer have been successfully prepared and used as a biocompatible nanoplatform for PTT/PDT synergistic cancer therapy. MnO2-PEG-cRGD/Ce6 exhibited strong NIR absorption and efficient generation of hyperthermia upon laser irradiation with a high photothermal conversion efficiency of 39%, which is compared to MnO2-PEG-cRGD nanosheets (28.7%) due to the synergetic effect of MnO2-PEG-cRGD and Ce6. The introduced cRGD renders nanoplatform with the passive targeting capability. The MnO2-PEG-cRGD/Ce6 exhibited pH-controlled and NIR-induced Ce6 release. Furthermore, the MnO2 sheets will be reduced by overexpressed acidic H2O2, which could efficiently generate O2 and further enhancing the therapeutic efficiency of PDT. With the cRGD-mediated targeting ability, MnO2-PEG-cRGD/Ce6 shows satisfactory antitumor efficacy, which mainly attribute to the combined photothermal and photodynamic outcomes. Moreover, our MnO2-PEG-cRGD/Ce6 nanosheets may indeed be a promising nanoplatform with great potential for future clinical translation. Also, the photosensitizer loaded strategy in this work also shows general potential for controlled delivery of other biomolecules or drugs to achieve different functions.
Supplementary Material
Funding Statement
This work was supported by the Science and Technology Planning Project of Guangdong Province (2014A020212519) and the Key Medical Discipline Construction Project of Guangdong Province.
Disclosure statement
The author reports no conflicts of interest in this work.
References
- Bray F, Ferlay J, Siegel RL, et al. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424. [DOI] [PubMed] [Google Scholar]
- Chen Q, Feng L, Liu J, et al. (2016a). Intelligent albumin-MnO2 nanoparticles as pH-/H2O2-responsive dissociable nanocarriers to modulate tumor hypoxia for effective combination therapy. Adv Mater 28:7129–36. [DOI] [PubMed] [Google Scholar]
- Chen L, Feng Y, Zhou X, et al. (2017a). One-pot synthesis of MoS2 nanoflakes with desirable degradability for photothermal cancer therapy. ACS Appl Mater Interfaces 9:17347–58. [DOI] [PubMed] [Google Scholar]
- Chen J, Meng H, Yang R, et al. (2019). Recent advances in functionalized MnO2 nanosheets for biosensing and biomedicine applications. Nanoscale Horiz 4:321–38. [DOI] [PubMed] [Google Scholar]
- Chen W, Ouyang J, Liu H, et al. (2017b). Black phosphorus nanosheet-based drug delivery system for synergistic photodynamic/photothermal/chemotherapy of cancer. Adv Mater 29:1603864. [DOI] [PubMed] [Google Scholar]
- Chen Y, Su Y, Hu S, Chen S (2016b). Functionalized graphene nanocomposites for enhancing photothermal therapy in tumor treatment. Adv Drug Deliv Rev 105:190–204. [DOI] [PubMed] [Google Scholar]
- Chen Y, Ye D, Wu M, et al. (2014). Break-up of two-dimensional MnO2 nanosheets promotes ultrasensitive pH-triggered theranostics of cancer. Adv Mater Weinheim 26:7019. [DOI] [PubMed] [Google Scholar]
- Dai Y, Xu C, Sun X, Chen X (2017). Nanoparticle design strategies for enhanced anticancer therapy by exploiting the tumour microenvironment. Chem Soc Rev 46:3830–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaraj S, Munichandraiah N (2008). Effect of crystallographic structure of MnO2 on its electrochemical capacitance properties. J Phys Chem C 112:4406–17. [Google Scholar]
- Dong Z, Feng L, Zhu W, et al. (2016). CaCO3 nanoparticles as an ultra-sensitive tumor-pH-responsive nanoplatform enabling real-time drug release monitoring and cancer combination therapy. Biomaterials 110:60–70. [DOI] [PubMed] [Google Scholar]
- Fan W, Yung B, Huang P, Chen X (2017). Nanotechnology for multimodal synergistic cancer therapy. Chem Rev 117:13566–638. [DOI] [PubMed] [Google Scholar]
- Fang Y, Jiang Y, Zou Y, et al. (2017). Targeted glioma chemotherapy by cyclic RGD peptide-functionalized reversibly core-crosslinked multifunctional poly(ethylene glycol)-b-poly(ε-caprolactone) micelles. Acta Biomater 50:396–406. [DOI] [PubMed] [Google Scholar]
- Feng W, Chen L, Qin M, et al. (2015). Flower-like PEGylated MoS2 nanoflakes for near-infrared photothermal cancer therapy. Sci Rep 5:17422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo M, Wang L, Chen Y, Shi J (2017). Tumor-selective catalytic nanomedicine by nanocatalyst delivery. Nat Commun 8:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaque D, Maestro LM, Rosal BD, et al. (2014). Nanoparticles for photothermal therapies. Nanoscale 6:9494–530. [DOI] [PubMed] [Google Scholar]
- Ji X, Kong N, Wang J, et al. (2018). A novel top-down synthesis of ultrathin 2D boron nanosheets for multimodal imaging-guided cancer therapy. Adv Mater e1803031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi M, Ghasemi A, Zangabad PS, et al. (2016). Smart micro/nanoparticles in stimulus-responsive drug/gene delivery systems. Chem Soc Rev 45:1457–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Zhao A, Dong K, et al. (2015). Chemically exfoliated WS2 nanosheets efficiently inhibit amyloid β-peptide aggregation and can be used for photothermal treatment of Alzheimer’s disease. Nano Res 8:3216–27. [Google Scholar]
- Liu B, Li C, Chen G, et al. (2017a). Synthesis and optimization of MoS2@Fe3O4-ICG/Pt(IV) nanoflowers for MR/IR/PA bioimaging and combined PTT/PDT/chemotherapy triggered by 808 nm laser. Adv Sci (Weinh) 4:1600540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Ji X, Liu J, et al. (2017b). Tantalum sulfide nanosheets as a theranostic nanoplatform for computed tomography imaging-guided combinatorial chemo-photothermal therapy. Adv Funct Mater 27:1703261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Wang C, Cui W, et al. (2014). Combined photothermal and photodynamic therapy delivered by PEGylated MoS2 nanosheets. Nanoscale 6:11219–25. [DOI] [PubMed] [Google Scholar]
- Liu Z, Zhang S, Lin H, et al. (2018b). Theranostic 2D ultrathin MnO2 nanosheets with fast responsibility to endogenous tumor microenvironment and exogenous NIR irradiation. Biomaterials 155:54–63. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhen W, Jin L, et al. (2018a). All-in-one theranostic nano agent with enhanced reactive oxygen species generation and modulating tumor microenvironment ability for effective tumor eradication. ACS Nano 12:4886–93. [DOI] [PubMed] [Google Scholar]
- Lu N, Huang P, Fan W, et al. (2017). Tri-stimuli-responsive biodegradable theranostics for mild hyperthermia enhanced chemotherapy. Biomaterials 126:39–48. [DOI] [PubMed] [Google Scholar]
- Lucky SS, Soo KC, Zhang Y (2015). Nanoparticles in photodynamic therapy. Chem Rev 115:1990–2042. [DOI] [PubMed] [Google Scholar]
- Ma Z, Jia X, Bai J, et al. (2017). MnO2 gatekeeper: An intelligent and O2-evolving shell for preventing premature release of high cargo payload core, overcoming tumor hypoxia, and acidic H2O2-sensitive MRI. Adv Funct Mater 27:1604258. [Google Scholar]
- Ma N, Zhang MK, Wang XS, et al. (2018). NIR light-triggered degradable MoTe2 nanosheets for combined photothermal and chemotherapy of cancer. Adv Funct Mater 28:1801139. [Google Scholar]
- Ocsoy I, Isiklan N, Cansiz S, et al. (2016). Combined photothermal and photodynamic therapy delivered by PEGylated MoS2 nanosheets. Rsc Adv 6:30285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L, Mei X, He J, et al. (2018). Monolayer nanosheets with an extremely high drug loading toward controlled delivery and cancer theranostics. Adv Mater 30:1707389. [DOI] [PubMed] [Google Scholar]
- Shi J, Kantoff PW, Wooster R, Farokhzad OC (2017). Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer 17:20–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Liang C, Gong H, et al. (2015). Photosensitizer-conjugated albumin-polypyrrole nanoparticles for imaging-guided in vivo photodynamic/photothermal therapy. Small 11:3932–41. [DOI] [PubMed] [Google Scholar]
- Tang H, Zheng Y, Chen Y (2017). Materials chemistry of nanoultrasonic biomedicine. Adv Mater 29:1604105. [DOI] [PubMed] [Google Scholar]
- Tian L, Tao L, Li H, et al. (2019). Hollow mesoporous carbon modified with cRGD peptide nanoplatform for targeted drug delivery and chemo-photothermal therapy of prostatic carcinoma. Colloid Surface A 570:386–95. [Google Scholar]
- Wu J, Bremner DH, Niu S, et al. (2018a). Chemodrug-gated biodegradable hollow mesoporous organosilica nanotheranostics for multimodal imaging-guided low-temperature photothermal therapy/chemotherapy of cancer. ACS Appl Mater Interfaces 10:42115–26. [DOI] [PubMed] [Google Scholar]
- Wu J, Bremner DH, Niu S, et al. (2018b). Functionalized MoS2 nanosheet-capped periodic mesoporous organosilicas as a multifunctional platform for synergistic targeted chemo-photothermal therapy. Chem Eng J 342:90–102. [Google Scholar]
- Wu Y, Li D, Zhou F, et al. (2018c). Versatile in situ synthesis of MnO2 nanolayers on upconversion nanoparticles and their application in activatable fluorescence and MRI imaging. Chem Sci 9:5427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Teng Z, Zhang Y, et al. (2018). Flexible MoS2-embedded human serum albumin hollow nanocapsules with long circulation times and high targeting ability for efficient tumor ablation. Adv Funct Mater 28:1804081. [Google Scholar]
- Yang Y, Aw J, Xing B (2017b). Nanostructures for NIR light-controlled therapies. Nanoscale 9:3698–718. [DOI] [PubMed] [Google Scholar]
- Yang G, Sun X, Liu J, et al. (2016). Light-responsive, singlet-oxygen-triggered on-demand drug release from photosensitizer-doped mesoporous silica nanorods for cancer combination therapy. Adv Funct Mater 26:4722–32. [Google Scholar]
- Yang T, Tang Y, Liu L, et al. (2017a). Size-dependent Ag2S nanodots for second near-infrared fluorescence/photoacoustics imaging and simultaneous photothermal therapy. ACS Nano 11:1848–57. [DOI] [PubMed] [Google Scholar]
- Zeng J, Goldfeld D, Xia Y (2013). A plasmon-assisted optofluidic (PAOF) system for measuring the photothermal conversion efficiencies of gold nanostructures and controlling an electrical switch. Angew Chem Int Ed 52:4169–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Guo Z, Huang D, et al. (2011). Synergistic effect of chemo-photothermal therapy using PEGylated graphene oxide. Biomaterials 32:8555–61. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wu J, Williams GR, et al. (2019). Functionalized MoS2-nanosheets for targeted drug delivery and chemo-photothermal therapy. Colloids Surf B Biointerfaces 173:101–8. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Fan H, Zhou G, et al. (2014). Activatable fluorescence/MRI bimodal platform for tumor cell imaging via MnO2 nanosheet-aptamer nanoprobe. J Am Chem Soc 136:11220–3. [DOI] [PubMed] [Google Scholar]
- Zhen W, Liu Y, Lin L, et al. (2018). BSA-IrO2: catalase-like nanoparticles with high photothermal conversion efficiency and a high x-ray absorption coefficient for anti-inflammation and antitumor theranostics. Angew Chem Int Ed 57:10309–13. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Wang C, Cheng R, et al. (2014). cRGD-directed, NIR-responsive and robust AuNR/PEG-PCL hybrid nanoparticles for targeted chemotherapy of glioblastoma in vivo. J. Control Release 195:63–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.