Abstract
Genetic code expansion allows unnatural (non-canonical) amino acid incorporation into proteins of interest by repurposing the cellular translation machinery. The development of this technique has enabled site-specific incorporation of many structurally and chemically diverse amino acids, facilitating a plethora of applications, including protein imaging, engineering, mechanistic and structural investigations, and functional regulation. Particularly, genetic code expansion provides great tools to study mammalian proteins, of which dysregulations often have important implications in health. In recent years, a series of methods has been developed to modulate protein function through genetically incorporated unnatural amino acids. In this review, we will first discuss the basic concept of genetic code expansion and give an up-to-date list of amino acids that can be incorporated into proteins in mammalian cells. We then focus on the use of unnatural amino acids to activate, inhibit, or reversibly modulate protein function by translational, optical or chemical control. The features of each approach will also be highlighted.
Keywords: chemical biology, genetic code expansion, protein chemistry, protein engineering, unnatural amino acid
Introduction
Knowledge of protein function is of pivotal importance to life science research. It can guide conventional drug development programmes and lead to novel strategies to address currently non-targetable systems [1–3]. In order to understand the precise role and interacting network of a protein, it is essential to analyse it within its native environment. For a mammalian protein, its function often also depends on its host cell (e.g. cell type and cell cycle stage), specific subcellular location and post-translational modifications. In addition, a protein of interest often exists in the presence of other closely related homologues (e.g. proteins within the same family), making it difficult to decipher the precise function of a specific protein in cells. Targeting the protein by small-molecule inhibition is often not possible in these cases, as protein homologues will also be affected. To tackle this problem, over the last two decades there has been a drive to develop and refine the technique of genetic code expansion which allows researchers to exploit the cellular translation machinery for site-specific incorporation of unnatural (non-canonical) amino acids into target proteins [4–14]. Consequently, this enables the use of building blocks beyond the 20 canonical amino acids and incorporation of unnatural amino acids with unprecedented functionality into target proteins in live cells. The repurposing of the translational machinery by this approach has paved the way for revealing the functions of proteins under physiological conditions [15–19]. For example, the technique can be used to site-specifically introduce an unnatural amino acid into the homologue of interest, whereby unique functionality (on the unnatural amino acid) can be used for selective activation, inhibition, or reversible regulation of the target homologue [7].
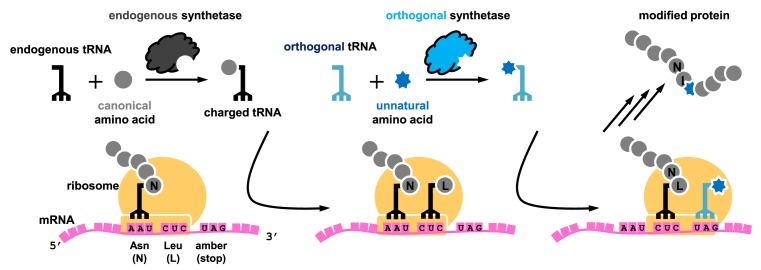
At the molecular level, the mechanism of protein translation is highly conserved in all organisms, where the cellular machinery ‘translates’ every nucleotide triplet as a codon consecutively on the mRNA into the corresponding amino acid. In nature, the endogenous aminoacyl-tRNA synthetase (aaRS)/tRNA pairs within the cell decode 61 of the total 64 codons to 20 canonical amino acids. The remaining three codons (UAG, UGA and UAA) are used for translation termination, and hence they are also known as ‘stop’ codons. In order to achieve site-specific incorporation of an unnatural amino acid, an orthogonal aaRS/tRNA pair is needed, which must decode a codon that does not correspond to any canonical amino acid, a so-called blank codon (Figure 1). Stop codons are most commonly used as a blank codon in genetic code expansion, and decoding of a stop codon is known as ‘suppression’ because it suppresses the translation termination. The amber stop codon (UAG) is often used as the blank codon due to its minimal occurrence in most organisms.
Figure 1. Mechanism of genetic code expansion for site-specific incorporation of an unnatural amino acid by amber suppression.
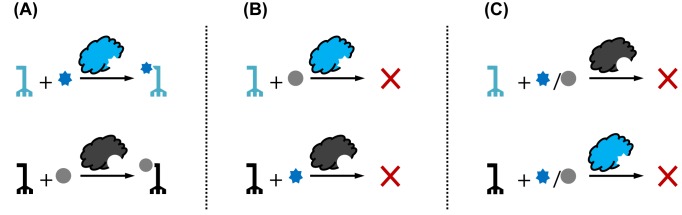
Within the concept of genetic code expansion, ‘orthogonality’ refers to the non-reactivity of the orthogonal aaRS/tRNA pair with the endogenous pair and canonical amino acids in the host cell. The orthogonal synthetase must only acylate the orthogonal tRNA with the designated unnatural amino acid; neither canonical amino acids nor endogenous tRNAs are substrates of the orthogonal synthetase; similarly, neither the unnatural amino acid nor orthogonal tRNA is a substrate of the endogenous synthetases (Figure 2).
Figure 2. Allowed and not allowed reactivities between the orthogonal and endogenous aaRS/tRNA pairs.
(A) Matching amino acid and aaRS/tRNA pairs; (B) mismatched amino acids; (C) mismatched aaRS/tRNA pairs.
Besides the amber codon, other stop codons [20–26] and different four-nucleotide codons [27,28] have been used as a blank codon. The use of four-nucleotide codons expands the theoretical codon numbers from 43 (64) to 44 (256) so that multiple different unnatural amino acids can be incorporated at the same time. However, decoding a four-nucleotide codon by the ribosome is less efficient than decoding the normal three-nucleotide codons. Although this issue has been addressed in Escherichia coli through ribosome engineering [29–31], the lower efficiency in decoding four-nucleotide codons remains an issue in mammalian systems [27,28].
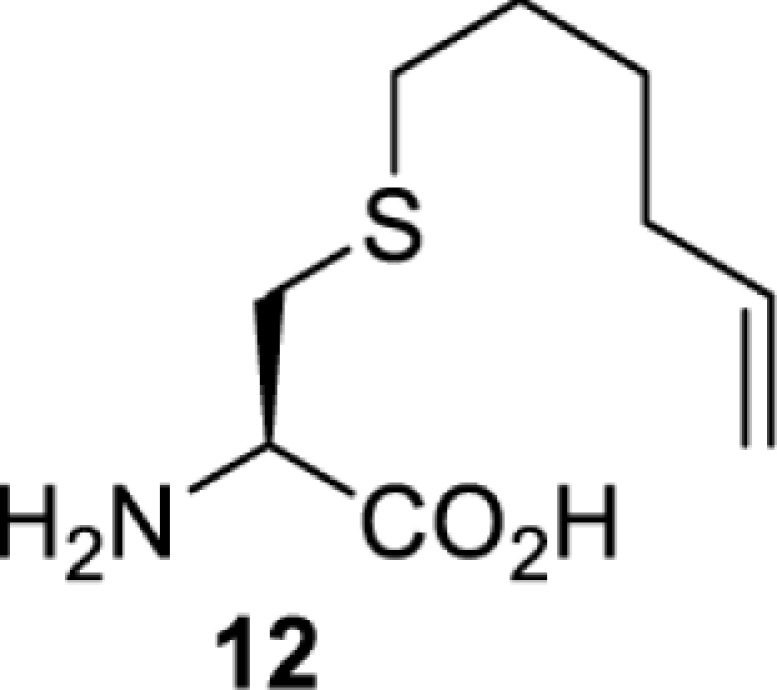
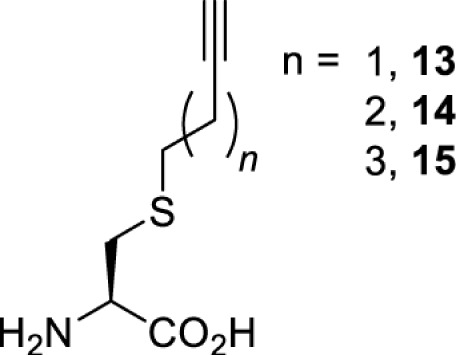
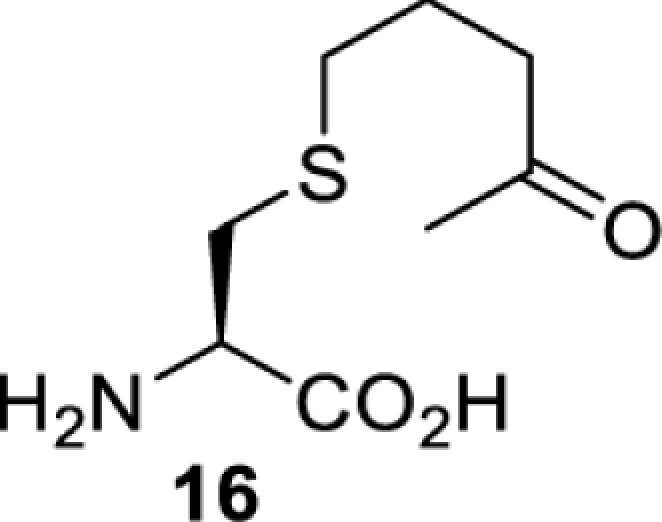
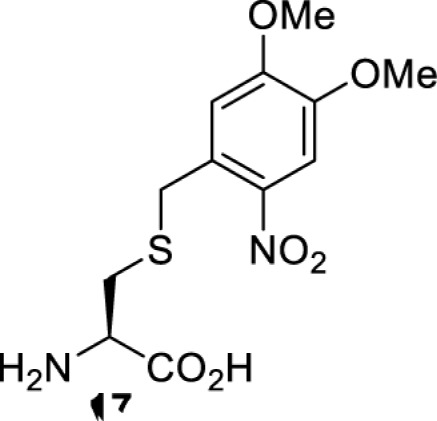
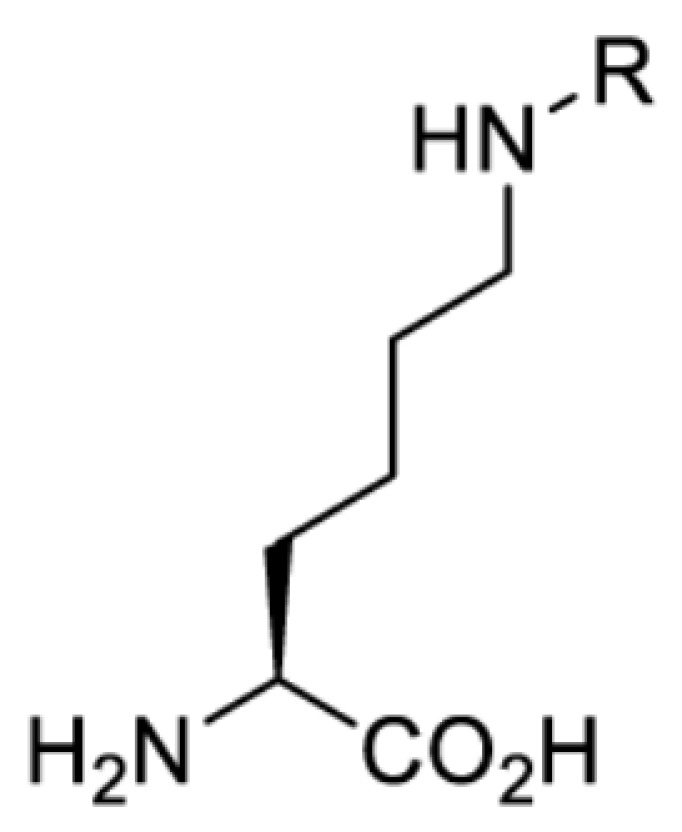
To date, many unnatural amino acids (1–110, Table 1) can be site-specifically incorporated into proteins produced by mammalian cells using genetic code expansion [5,32]. While the amino acids are structurally diverse, the majority of them can be incorporated through only a few orthogonal synthetases and their mutants. The Pyrrolysyl-tRNA synthetase (PylRS)/tRNAPyl pairs from archaea species Methanosarcina barkeri (Mb) and Methanosarcina mazei (Mm) have proven to be extraordinarily useful pairs [4]. The tRNAPyl naturally recognises the UAG codon and thus engineering of this tRNA is not needed. In addition, this pair is orthogonal in both E. coli and mammalian cells; hence, it facilitates the engineering of PylRS in E. coli and subsequently using the engineered PylRS mutant for incorporation of the designated unnatural amino acid in mammalian systems. As shown in Table 1, a wide range of amino acids has been incorporated into proteins in mammalian cells through only a few point mutations on the PylRS gene.
Table 1. Overview of unnatural amino acids that have been successfully incorporated into proteins in mammalian cells and used for a variety of applications to date.
| Amino acid | aaRS | Mutations | tRNA | Application |
|---|---|---|---|---|
| Cysteine and selenocysteine derivatives | ||||
 |
EcLeuRS [24] | M40I T252A Y499I Y527A H529G | Method development | |
 |
EcLeuRS [24] | M40I T252A Y499I Y527A H529G | Method development | |
 |
EcLeuRS [24] | M40I T252A Y499I Y527A H529G | Method development | |
 |
EcLeuRS [24] | M40I T252A Y499I Y527A H529G | Method development | |
 |
EcLeuRS [24] | M40I T252A Y499I Y527A H529G | Method development | |
 |
EcLeuRS [24] | M40I T252A Y499I Y527A H529G | Method development | |
 |
EcLeuRS [71] | M40G L41Q T252A Y499L Y527G H537F | Photoactivation | |
 |
EcLeuRS [72] | M40G L41Q Y499L Y527G H537F | Photoactivation | |
 |
MbPylRS [73,74] | N311Q C313A V366M | Photoactivation | |
 |
MbPylRS [75] | M241F A267S Y271C L274M | Photoactivation | |
 |
MbPylRS [75] | M241F A267S Y271C L274M | Photoactivation | |
 |
MbPylRS [76] | C313W W382T | MbPyltRNA | Method development |
 |
MbPylRS [40] | L274A C313S Y349F | Photocrosslinking | |
 |
MbPylRS [40] | L274A C313S Y349F | Photocrosslinking | |
| Phenylalanine derivatives | ||||
 |
EcTyrRS [77] | Y37V D182S F183M D265R | Method development | |
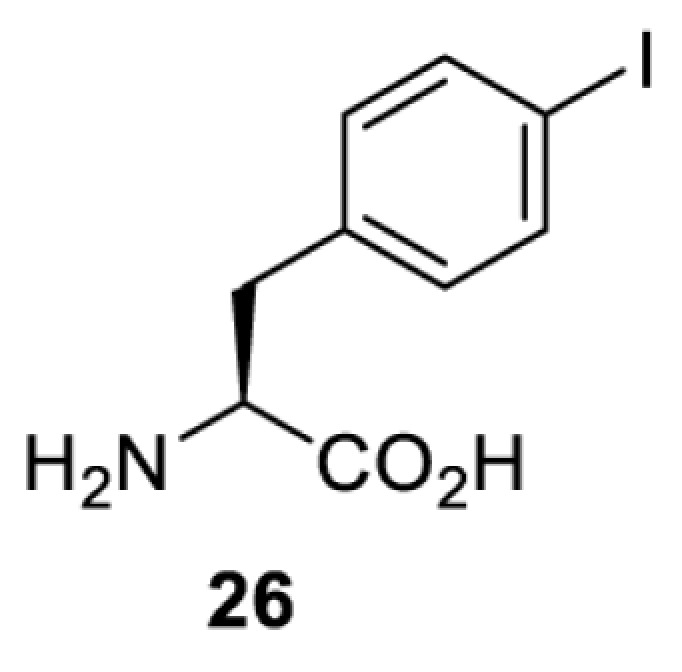 |
EcTyrRS [35,37,43,77] | Y37V D182S F183M D265R [77] Y37I D182S F183M [37,43] Y37V D165G D182S F183M L186A D265R [35] |
[35,77] | Method development [35,37,77] Protein engineering [43] |
| [37,43,77] | ||||
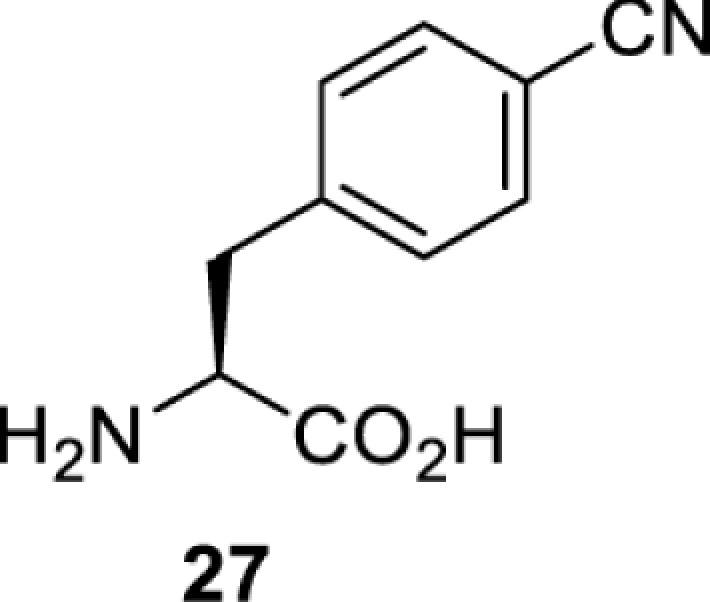 |
EcTyrRS [77] | Y37V D182S F183M D265R | Method development | |
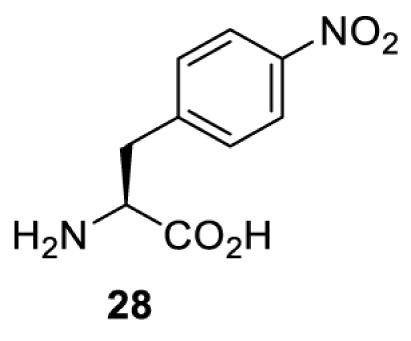 |
EcTyrRS [77] | Y37V D182S F183M D265R | Method development | |
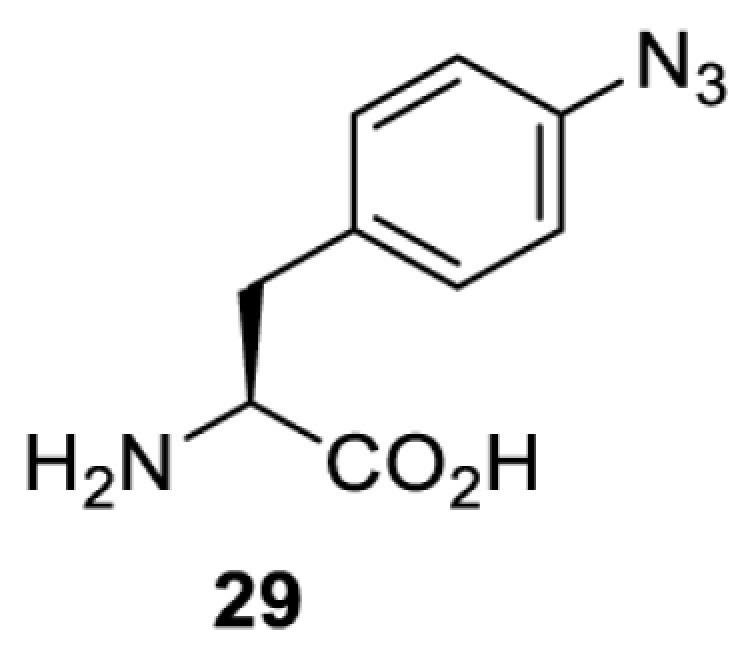 |
EcTyrRSCUA [16,17,35,37,38,43,49,55,68,77–96] | Y37L D182S F183A L186A D265R [78,81,84,85] Y37V D182S F183M D265R [77,90] Y37L D182S F183M L186A [16,17,37,38,43,49,55,68,79,80,82,83,86–89,91–96] Y37V D165G D182S F183M L186A D265R [35] |
[35,77,78,81,86,90] | Bioorthogonal labelling [38,79,83,87–89,96] Method development [17,25,35,37,77,78,90] Photocrosslinking [38,68,81,84,85,91–95] Protein engineering [43,49,55,83] Spectroscopic probe [16,80,82,86] |
| [16,17,37,38,43,49,55,68,77,79,80,82–85,87–96] | ||||
| EcTyrRSUCA [25] | Y37V D182S F183M | |||
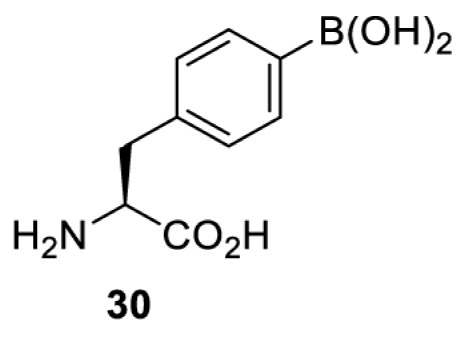 |
EcTyrRS [35,97] | Y37I D182S F183M D265R [97] Y37S D182S F183A L186E D265R [35] Y37G D182S F183I L186E D265R [35] Y37S D182S F183I L186E D265R [35] |
[35,97] | Method development [35] Spectroscopic probe [97] |
| [97] | ||||
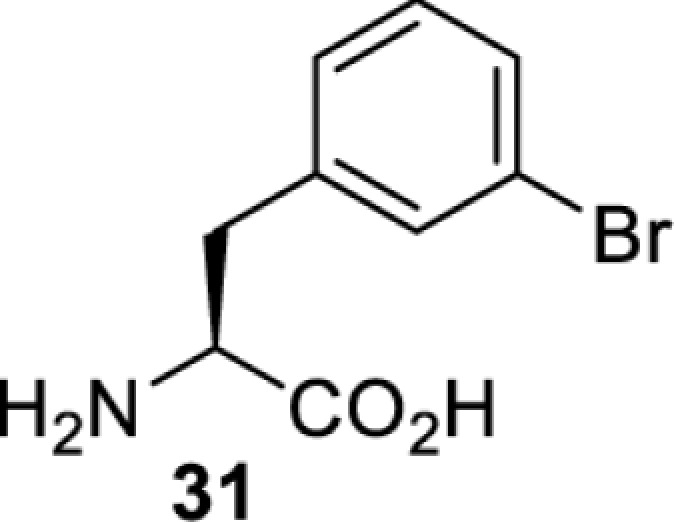 |
MmPylRS [50] | L301M Y306L L309A C348F | Method development | |
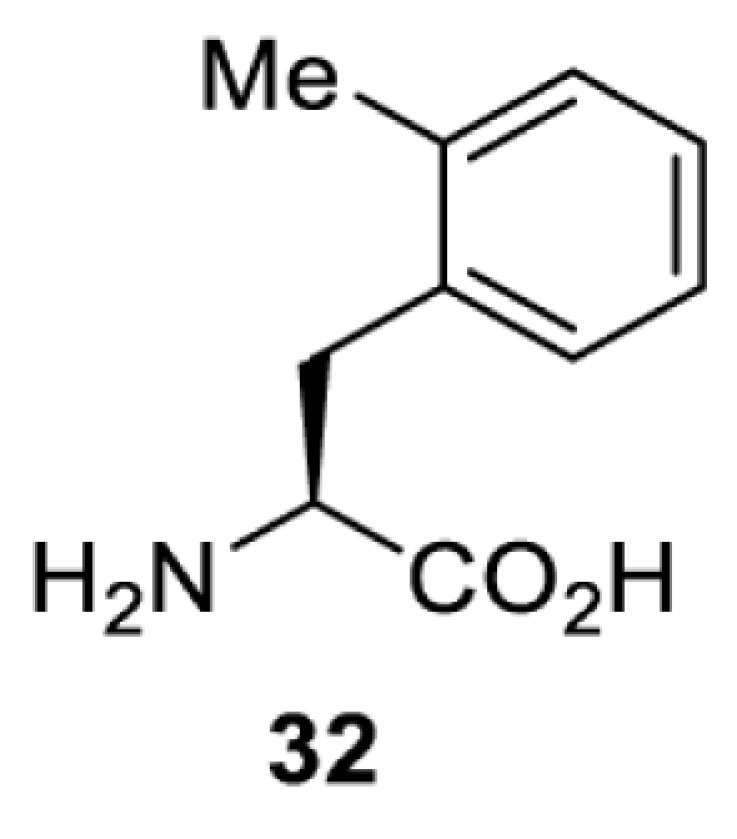 |
MmPylRS [98] | N346A C348A | Method development | |
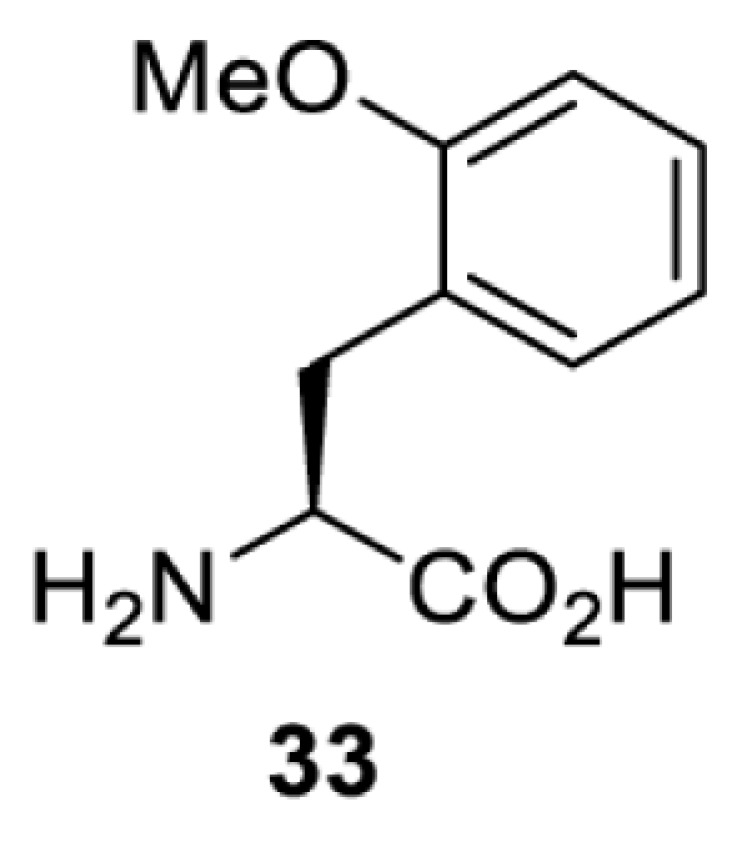 |
MmPylRS [98] | N346A C348A | Method development | |
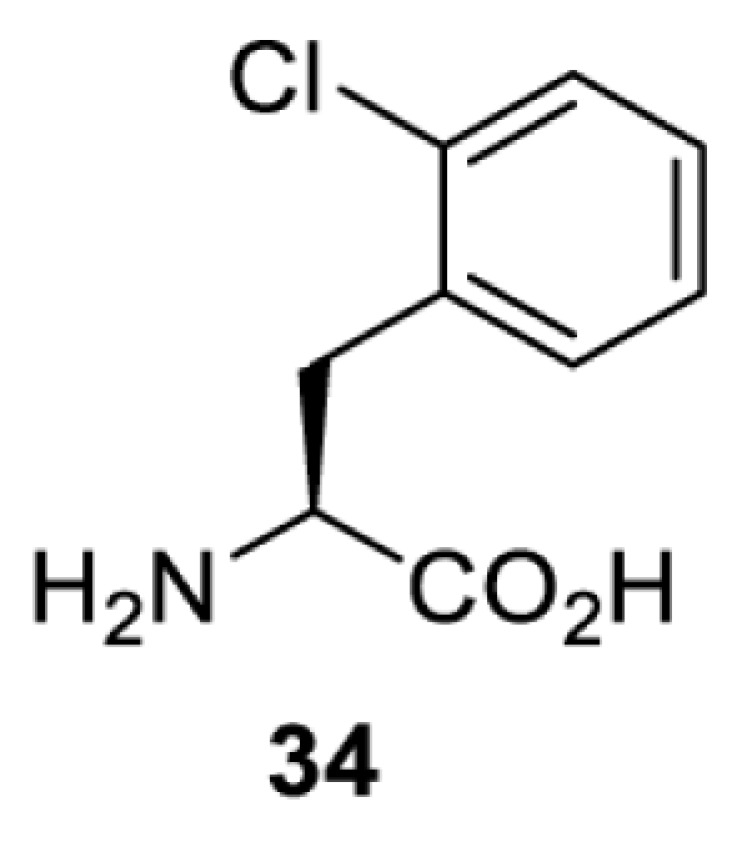 |
MmPylRS [98] | N346A C348A | Method development | |
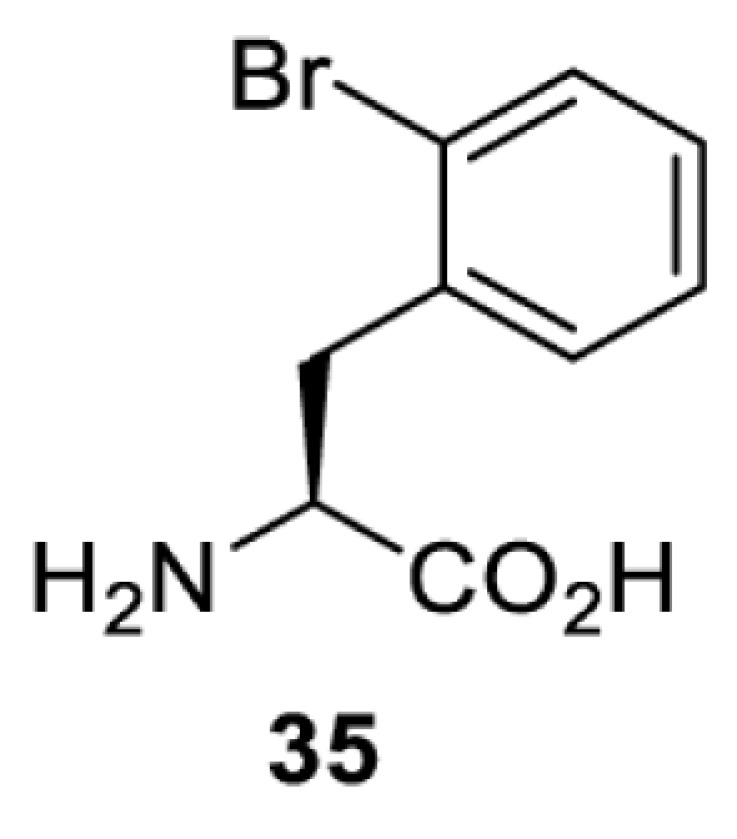 |
MmPylRS [98] | N346A C348A | Method development | |
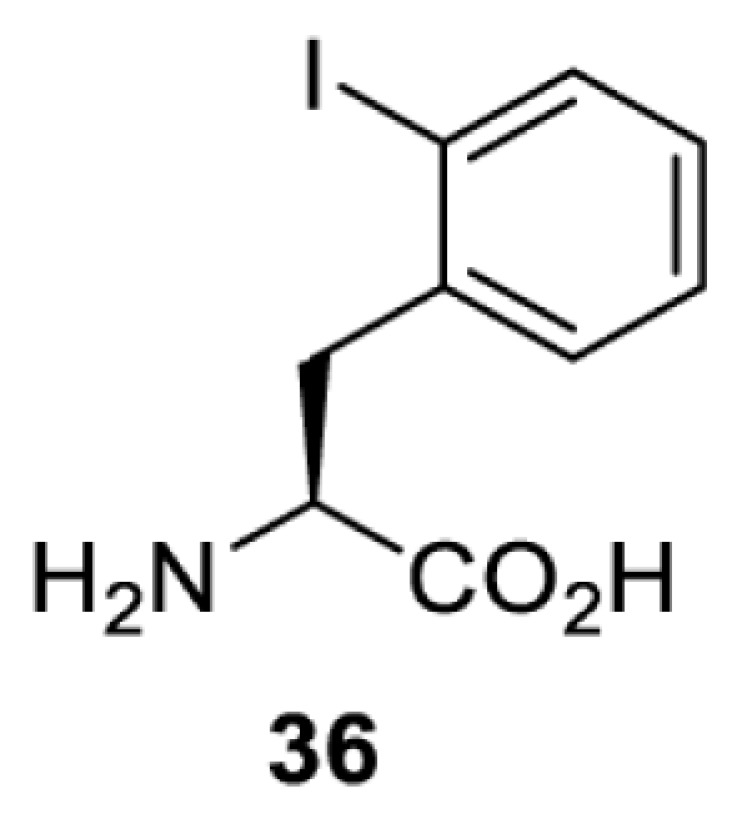 |
MmPylRS [98] | N346A C348A | Method development | |
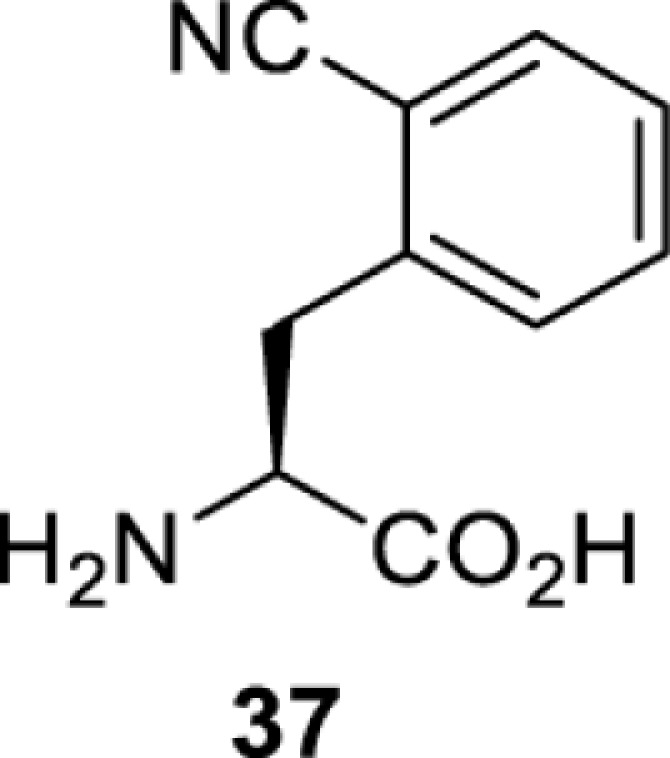 |
MmPylRS [98] | N346A C348A | Method development | |
 |
MmPylRS [98] | N346A C348A | Method development | |
 |
EcTyrRSCUA [21,35,37,43,77,83,87,90,99,100] | Y37I N165G D182G F183M L186A [83,99] Y37I D182G F183M L186A [37,43,87,100] Y37V D182S F183M D265R [21,77,90] Y37V D165G D182S F183M L186A D265R [35] |
[21,35,77,90] | Bioorthogonal labelling [83,87,100] Method development [25,35,37,77,83,90,99] Protein engineering [21,43] |
| [37,43,77,83,87,99,100] | ||||
| EcTyrRSUCA [25] | Y37V D182S F183M | |||
 |
EcTyrRS [84,101] | Y37I D182G F183M L186A D265R | Chemical crosslinking [84,101] Method development [101] |
|
 |
EcTyrRS [15,37,55,78,85,92,94,95,99,102–105] | Y37G D182G L186A D265R [78,85,103] Y37G D182G L186A [15,55,92,94,95,99,102,104,105] Y37G D182G F183Y L186M [37] |
[15,78,103] | Mechanistic studies [15] Method development [37,78,95,99,103,106] Photocrosslinking [85,92,94,102,104,105] Photoinhibition [55] |
| [37,55,85,92,94,95,99,102,104,105] | ||||
| MmPylRS [106] | A302T N346T C348T W417C [106] | [106] | ||
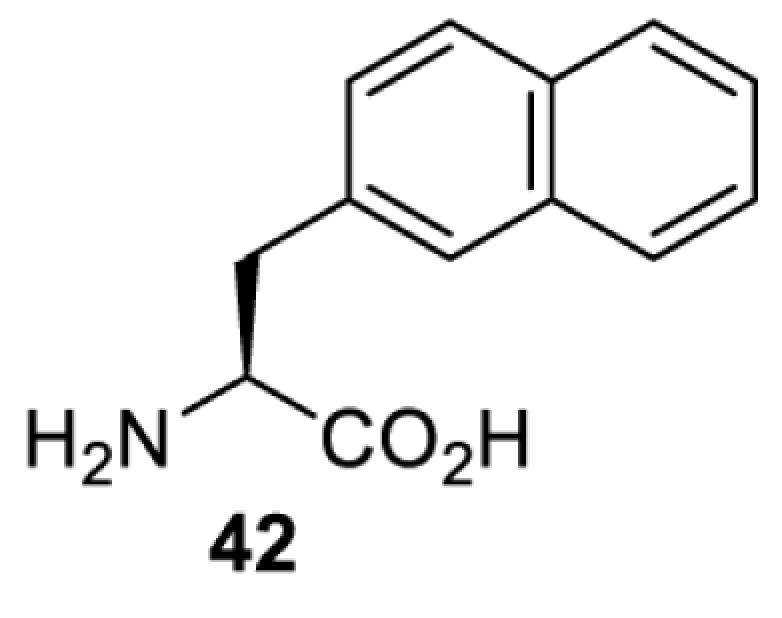 |
MmPylRS [106] | A302T N346G C348T V401I W417Y | Method development | |
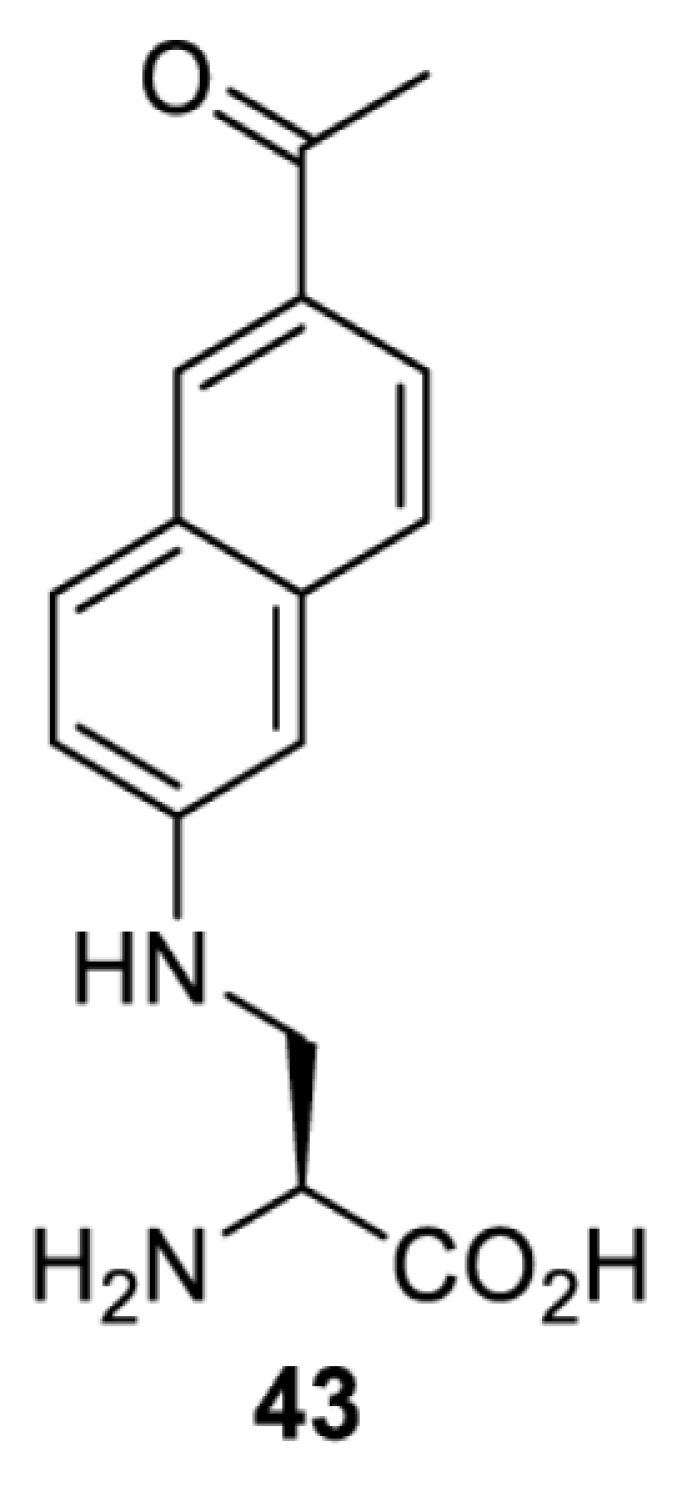 |
EcLeuRS [107–109] | L38F M40G L41P Y499V Y500L Y527A H537E L538S F541C A560V | Method development [107,108] Spectroscopic probe [109] |
|
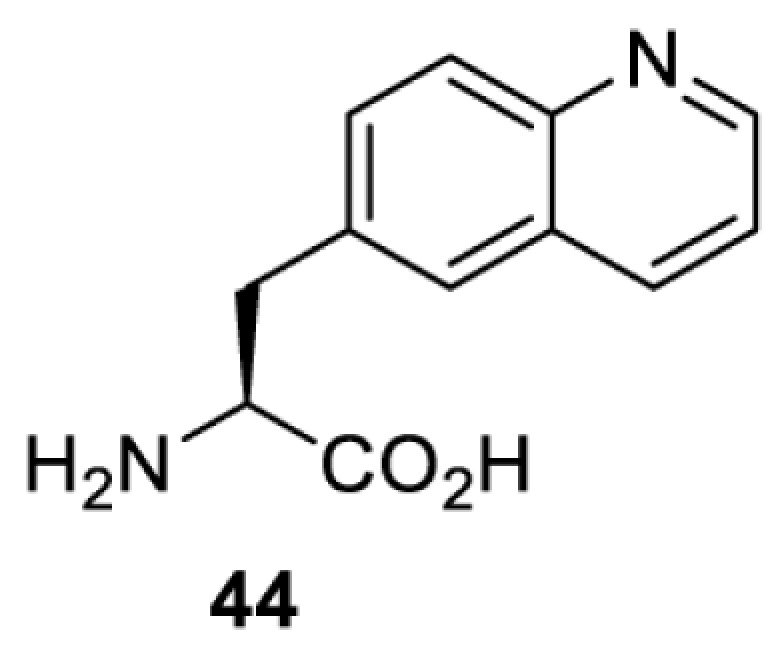 |
MmPylRS [110] | N346Q C348S V401G W417T | Spectroscopic Probe | |
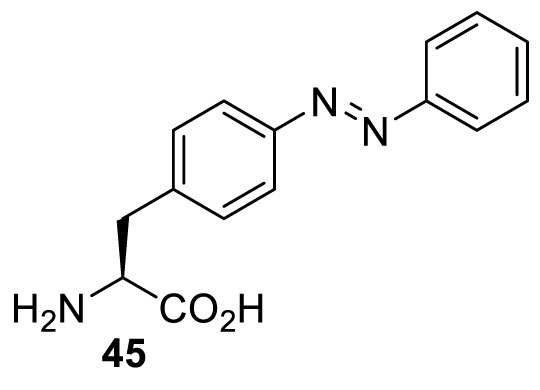 |
MbPylRS [111] | L270F L274M N311G C313G Y349F | Photoswitching | |
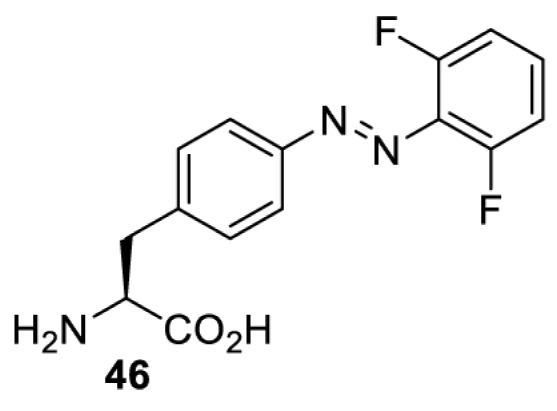 |
MbPylRS [111] | L270F L274M N311G C313G Y349F | Photoswitching | |
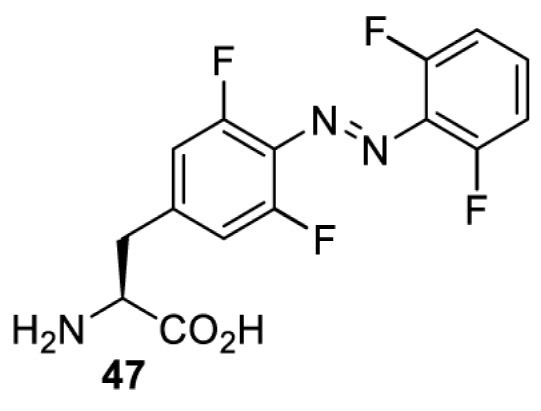 |
MbPylRS [111] | L270F L274M N311G C313G Y349F | Photoswitching | |
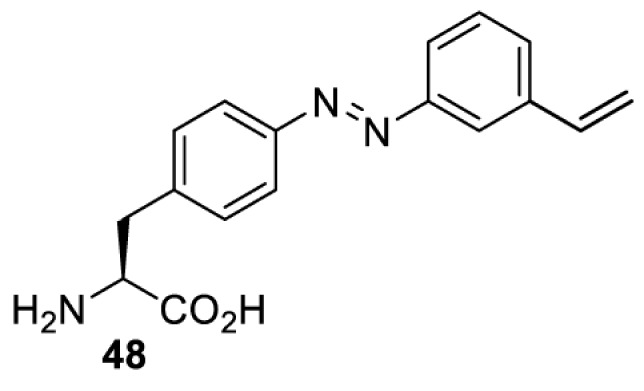 |
MmPylRS [56,112] | A302T L309S N346V C348G | Method development [112] Photoswitching [56] |
|
| Histidine derivatives | ||||
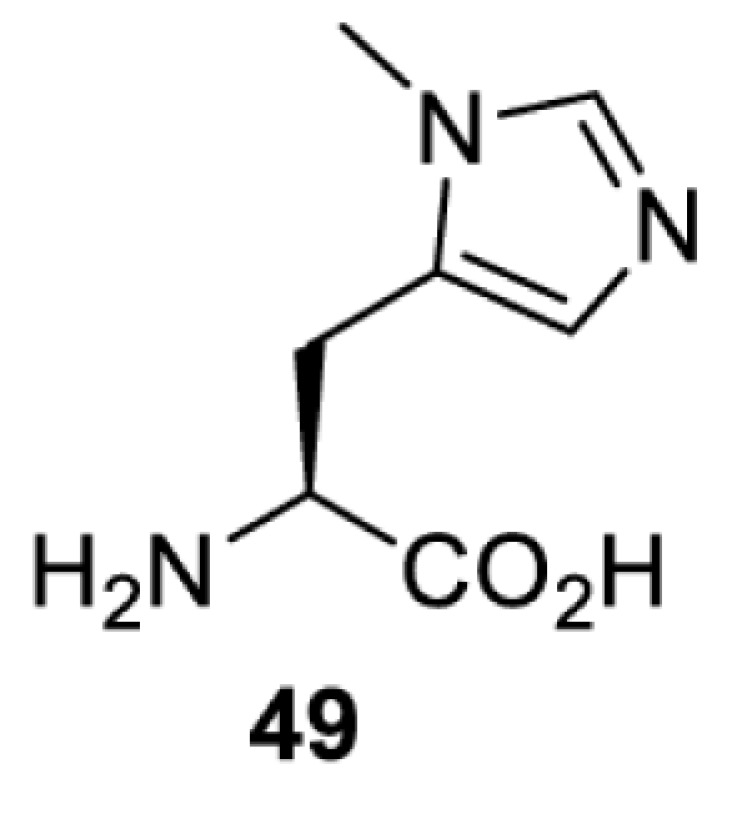 |
MaPylRS [26] | L121M L125I Y126F M129A V168F | Method development | |
| MbPylRS [113] | L270I Y271F L274G C313F Y349F | |||
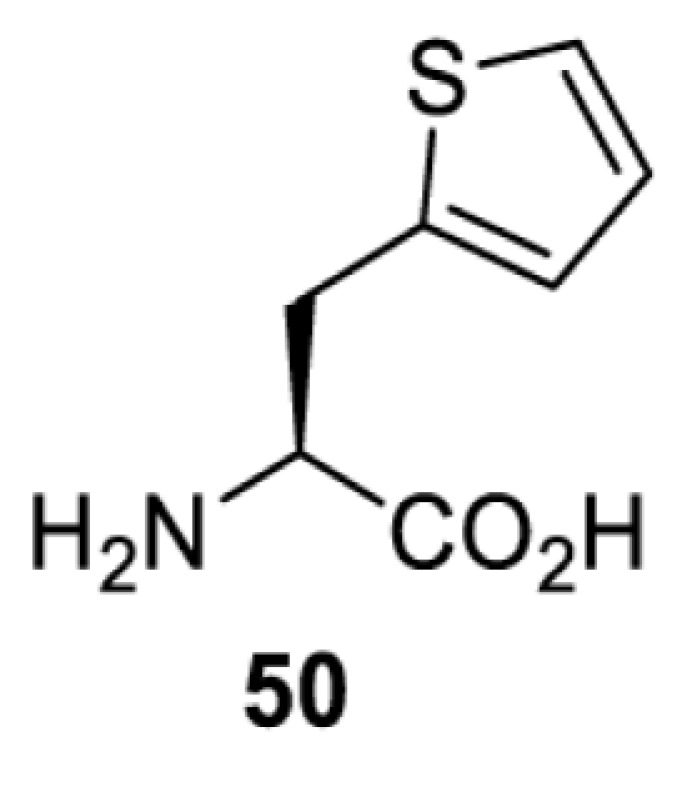 |
MbPylRS [113] | L270I Y271F L274G C313F Y349F | Method development | |
| Lysine derivatives | ||||
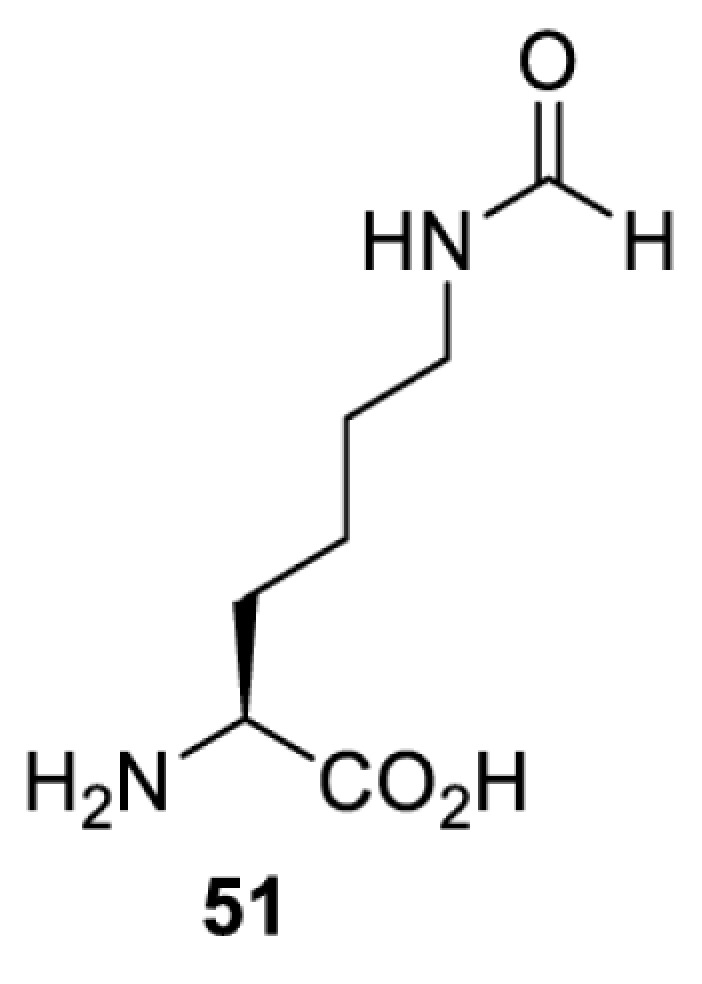 |
MbPylRS [114] | L266M L270I Y271F L274A C313F | Method development | |
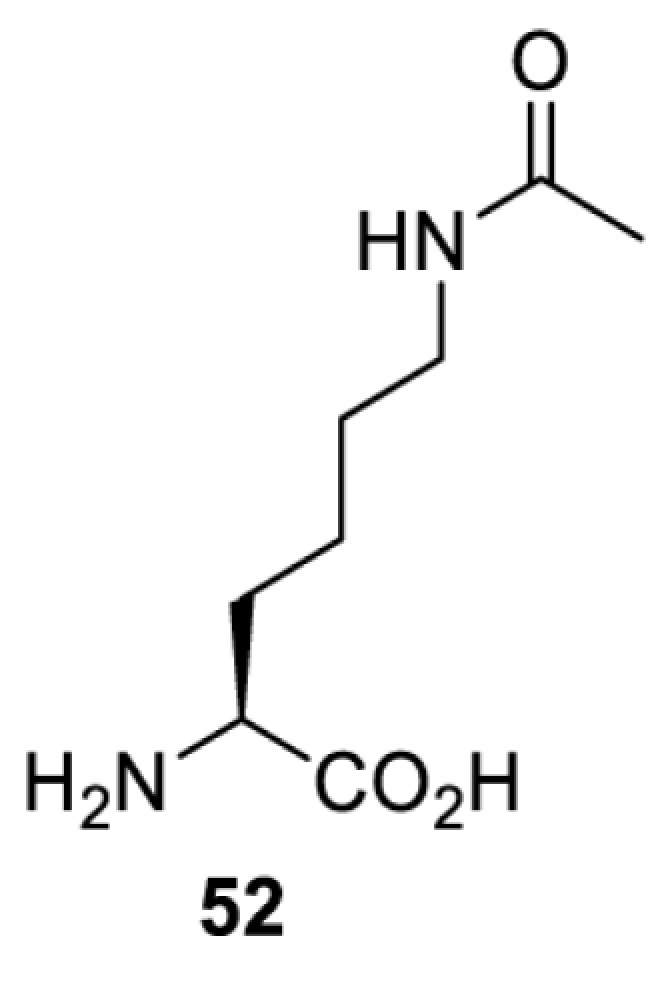 |
MbPylRS [115–117] | D76G L266V L270I Y271F L274A C313F [115] D76G L266M L270I Y271F L274A C313F [116,117] |
Method development [44,50,115,117,118] Spectroscopic probe [116] |
|
| MmPylRS [44,50,118] | L305I Y306F L309A C348F [118] L301M Y306L L309A C348F [44,50] |
[50,118] [44] |
||
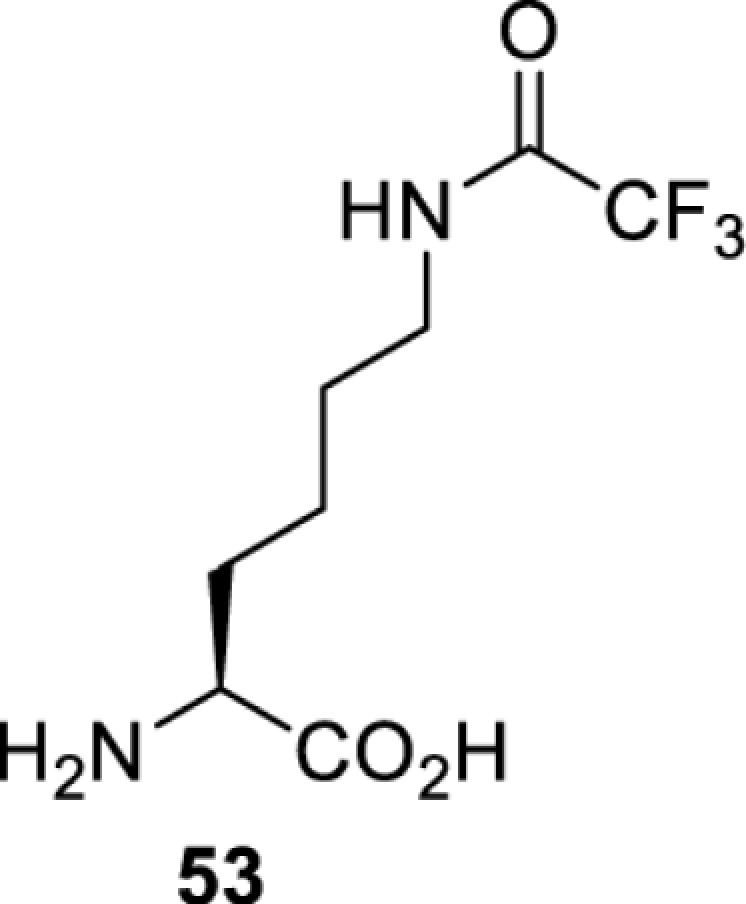 |
MmPylRS [50] | L301M Y306L L309A C348F | Method development | |
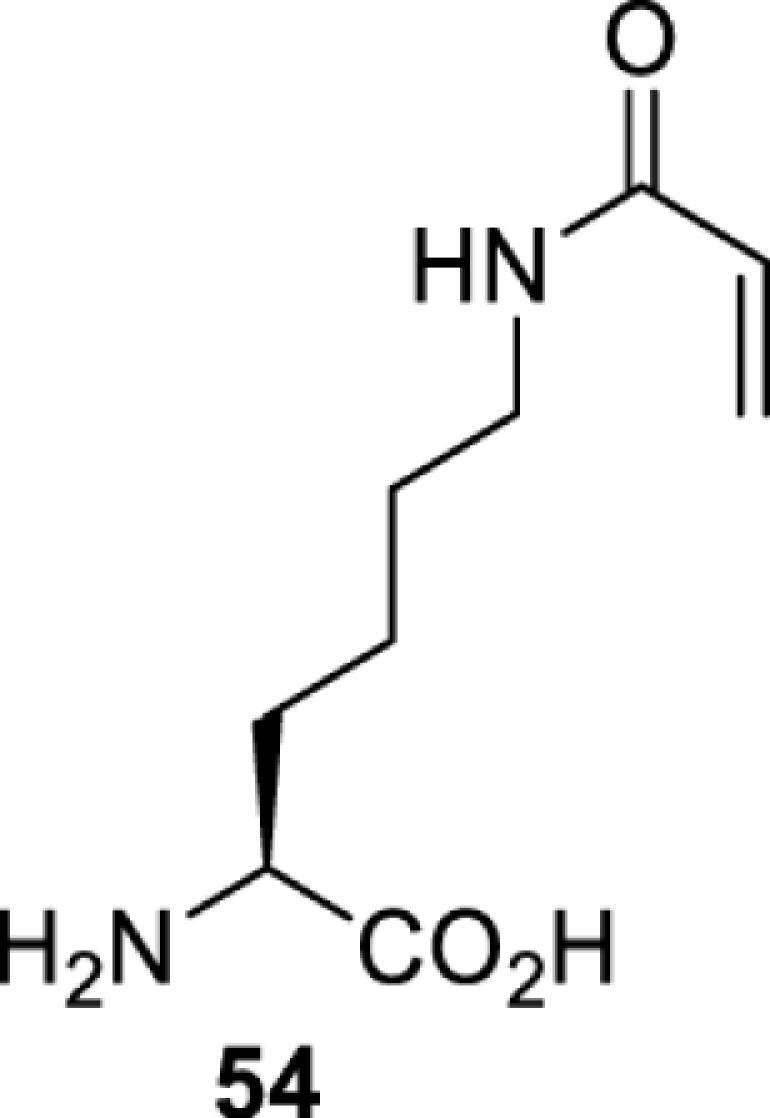 |
MbPylRS [119] | D76G L266M L270I Y271F L274A C313F | Method development | |
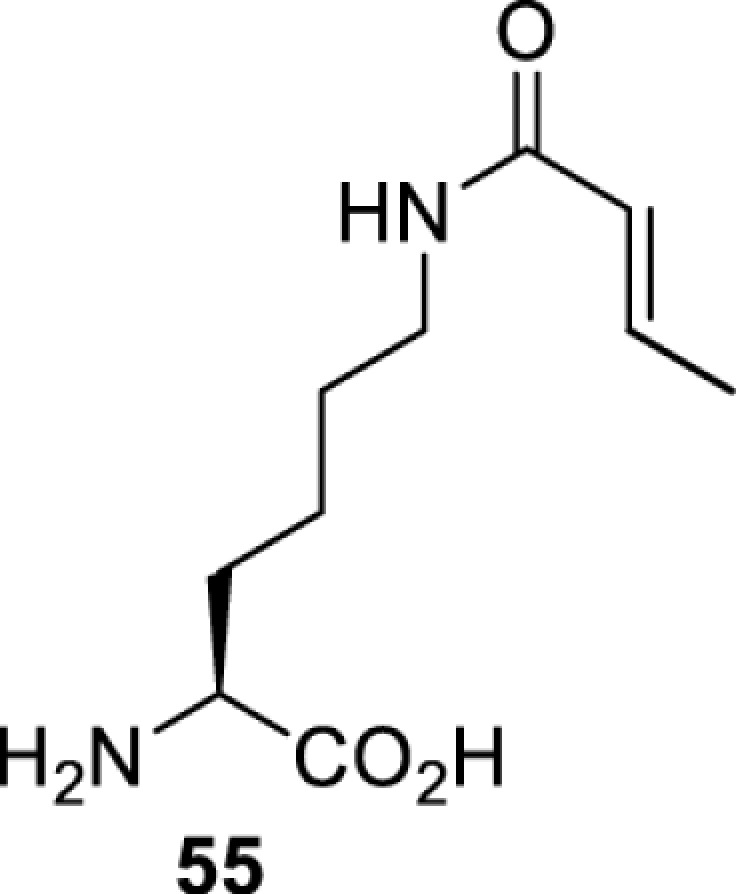 |
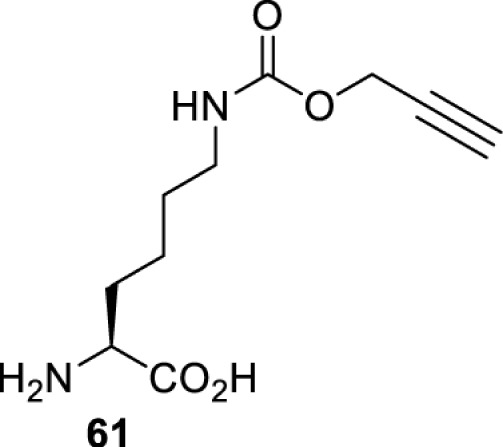
MbPylRS [77,120] | L274A C313F Y349F [120] wt [77] |
Method development | |
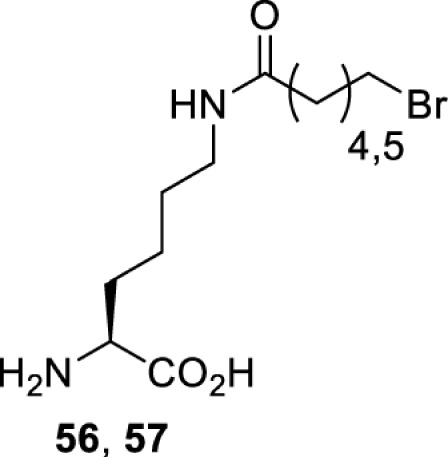 |
MbPylRS [121] | Y271M L274A C313A | Photocrosslinking | |
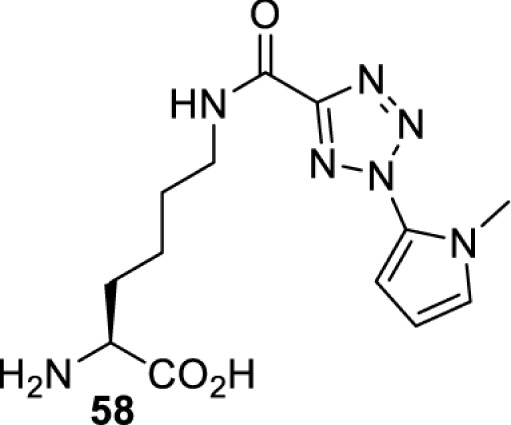 |
MmPylRS [122] | Y306V L309A C348F Y384F | Photocrosslinking | |
 |
MaPylRS [26] | wt | Method development [21,22,25,26,44,47,69,73,77,106,115,117,118,123–130] | |
| MbPylRS [21,25,44,125,129,130] | wt |
[25,44,125,129,130] [44] [25,44] |
||
| MmPylRS [22,26,44,47,69,73,77,106,115,117,118,123,124,126–128] | wt |
[21,22,26,47,69,73,77,106,115,118,123,124,126,127] [22,117,128] [21,44] [21] |
||
 |
MbPylRS [21,24,25,48,77,131–137] | Wt [21,24,25,48,77,132–136] L274A C313S Y349F [131] Y349F [137] |
[25,77,131] [24,25,48,132–136] |
Bioorthogonal labelling [127,131,137] Imaging [136] Method development [24,25,77,134,137] Protein engineering [21,48,132,133,135–137] |
| MmPylRS [127] | wt |
[127,137] [21] |
||
 |
MbPylRS [57,69,77,138,139] | wt | Bioorthogonal labelling [131,139] Chemical decaging [57] Imaging [69,129,138] Method development [69,77] |
|
| MmPylRS [69,129,131] | wt | |||
 |
MbPylRS [25,130] | wt | [25] | Bioorthogonal labelling [130] Method development [25] |
| MmPylRS [130] | wt | |||
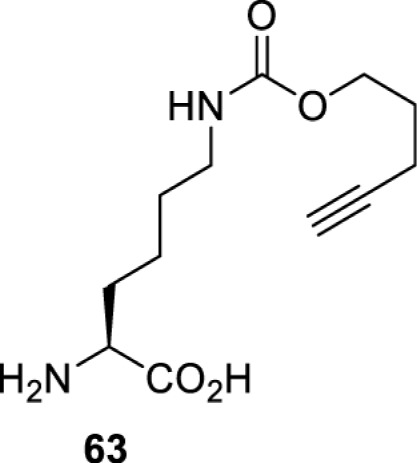 |
MbPylRS [131,140] | L274A C313S Y349F | Bioorthogonal labelling | |
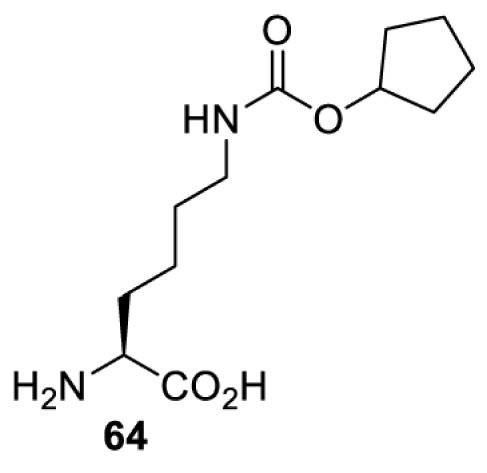 |
MmPylRS [141] | wt | Method development | |
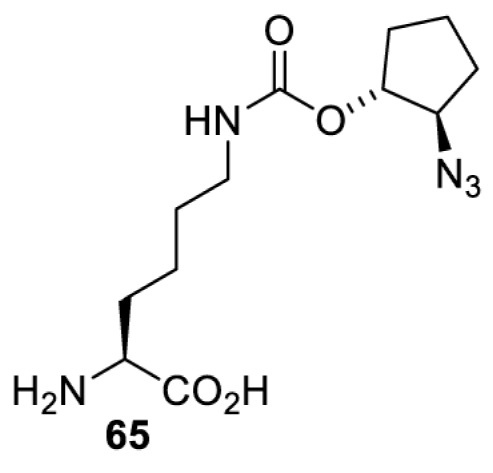 |
MbPylRS [140] | wt | Method development | |
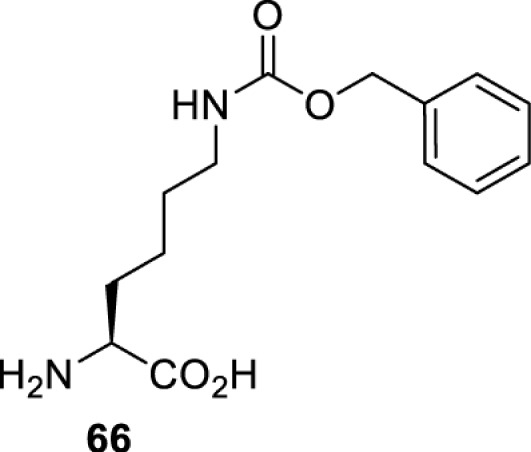 |
MmPylRS [118,142,143] | R61K G131E L309A C348V Y384F [118] Y306A Y384F [142] R61K G131E Y306A Y384F [143] |
[118,142] [143] |
Method development [118,142,143] |
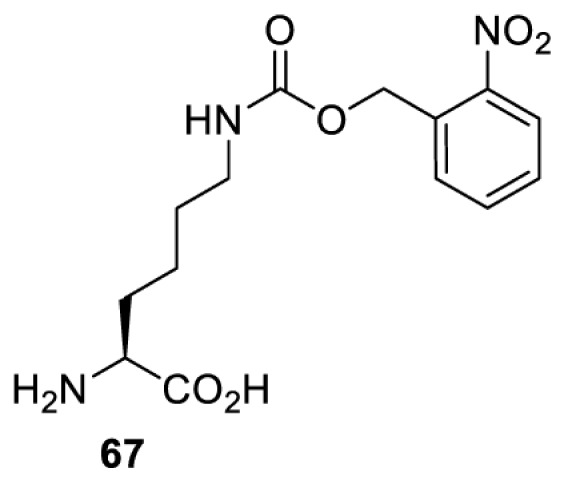 |
MbPylRS [140] | Y271I L274A C313A Y349F | Method development [140,141] Photoactivation [61,144] |
|
| MmPylRS [61,141,144] | Y306M L309A C348A Y384F | |||
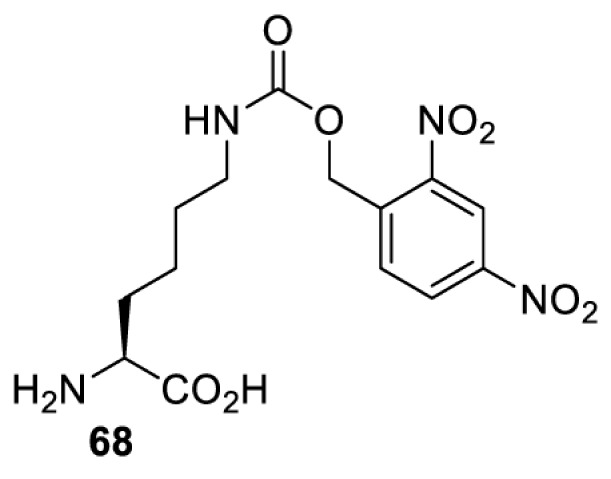 |
MbPylRS [145] | Y271M L274T C313A Y349F | Method development | |
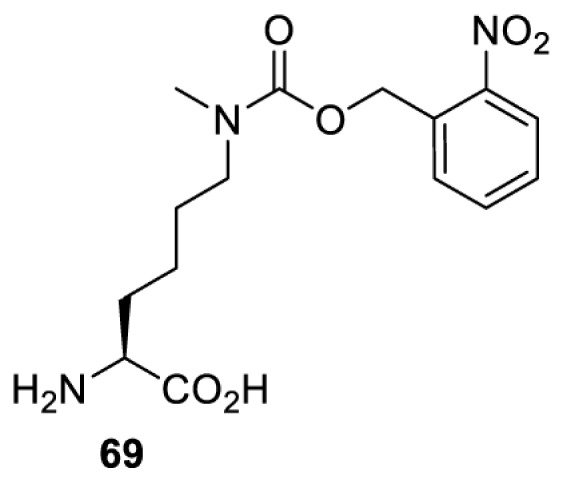 |
MbPylRS [146] | Y271I 274M C313A | Method development | |
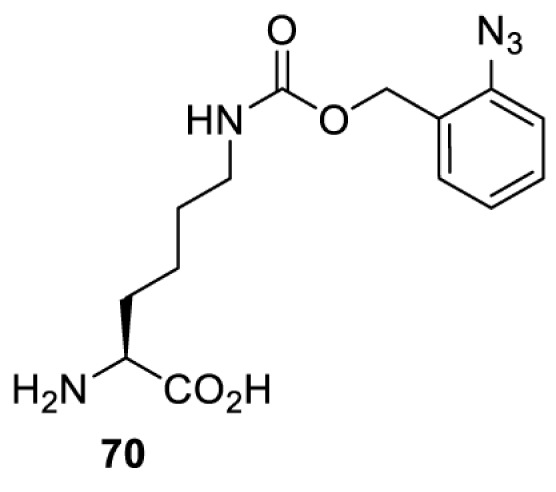 |
MbPylRS [63] | Y271A Y349F | Chemical decaging | |
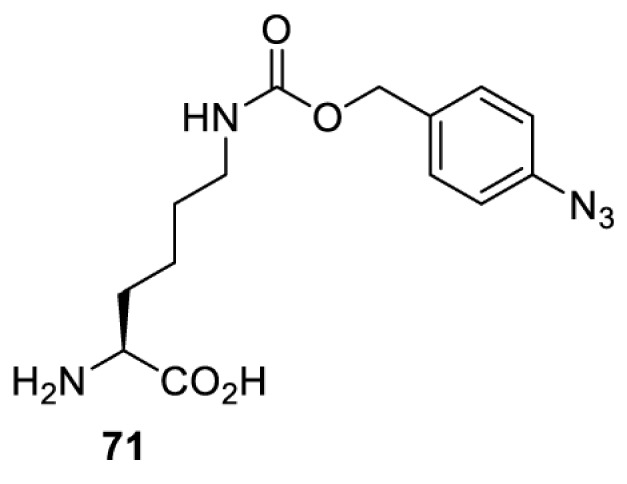 |
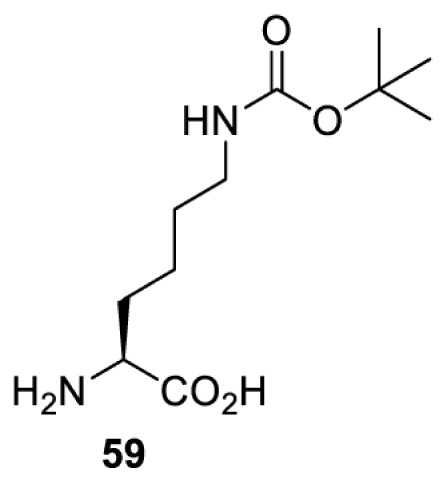
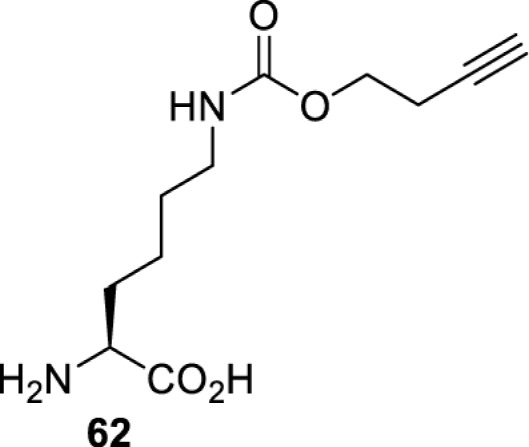
MbPylRS [62] | L274A C313S Y349F | Bioorthogonal labelling Chemical decaging |
|
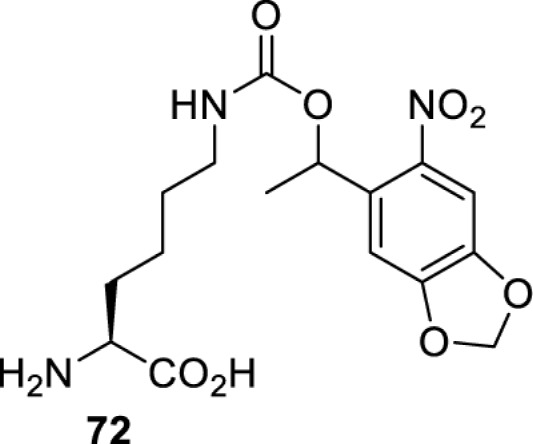 |
MbPylRS [66,67,69,75,125,147–152] | M241F A267S Y271C L274M [66,67,69,75,125,147–152] |
[66,67,69,75,125,147–152] [66] |
Method development [69] Photoactivation [66,67,75,125,147–152] |
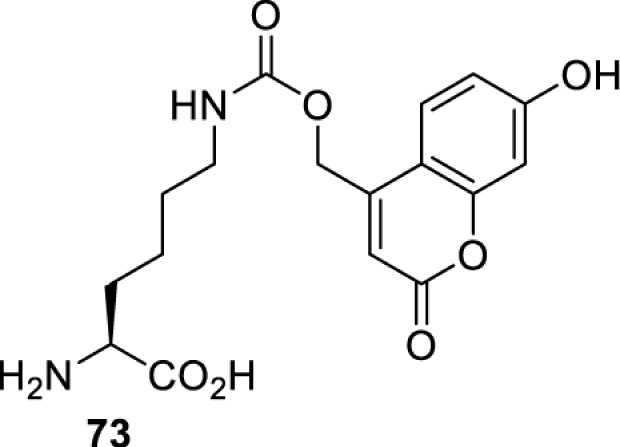 |
MbPylRS [153] | Y271A L274M | Photoactivation | |
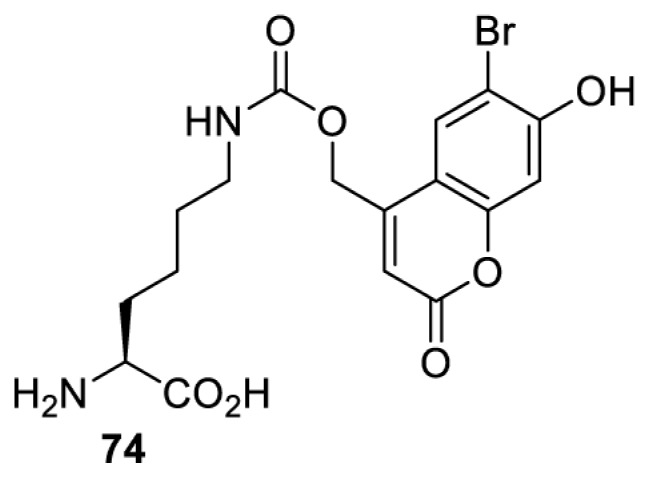 |
MbPylRS [153] | Y271A L274M | Photoactivation | |
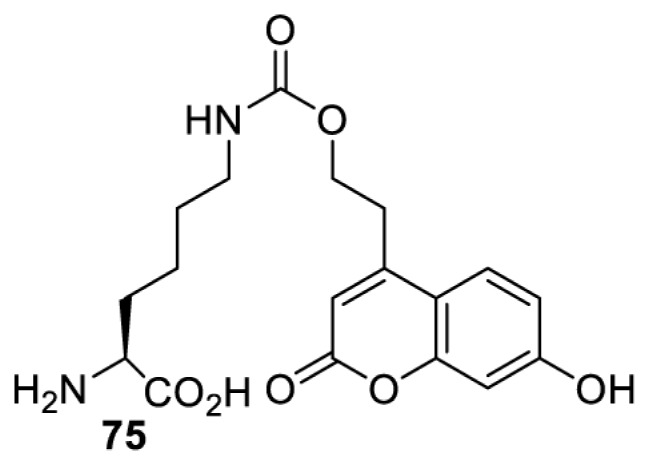 |
MbPylRS [153] | Y271A L274M | Method development | |
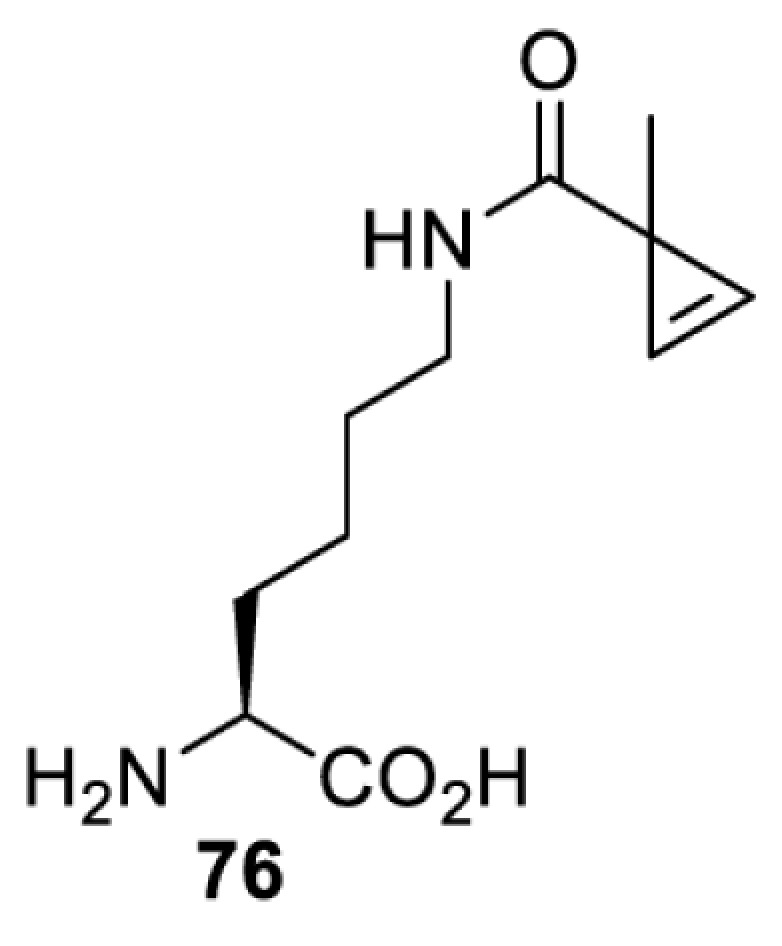 |
MbPylRS [154] | L266M L270I Y271L L274A C313 | Method development | |
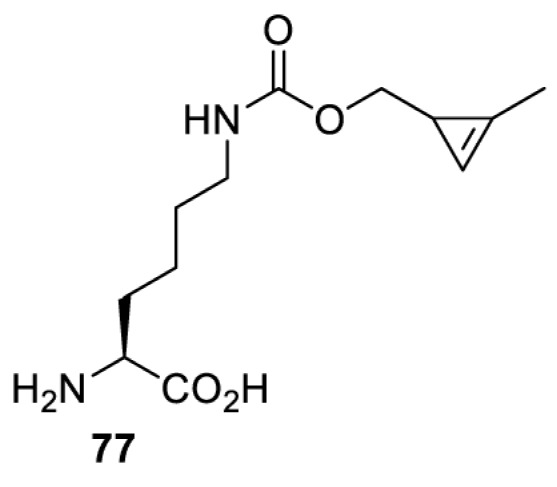 |
MbPylRS [24,25] | wt |
[25] [24] |
Imaging [123] Method development [22,24,25,69,115,155,156] |
| MmPylRS [22,69,115,123,155,156] | Wt [22,69,115,123,155,156] Y306A Y384F [155] |
[22,155] [69,115,123,156] |
||
| Mx1201PylRS [155] | wt | C41CA | ||
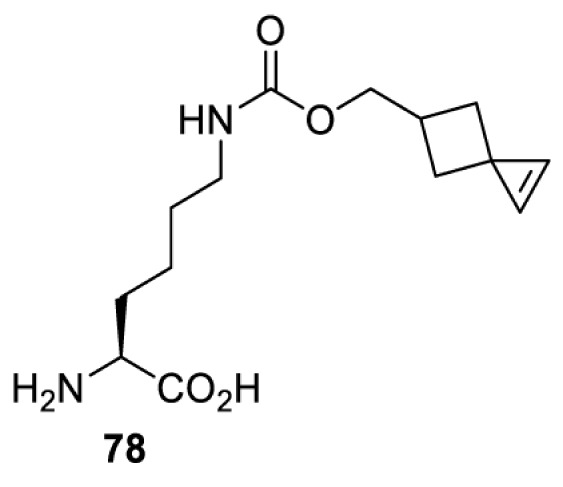 |
MmPylRS [124] | wt | Bioorthogonal labelling | |
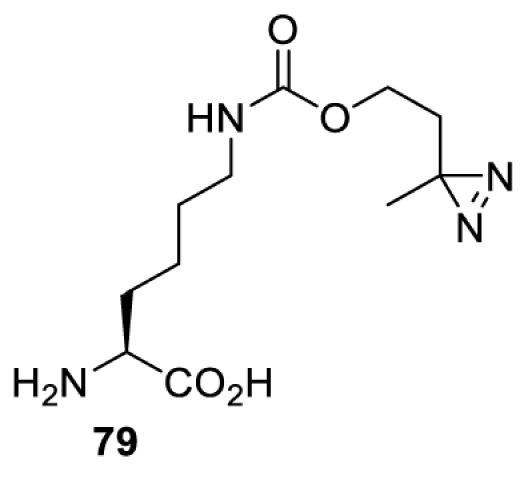 |
MbPylRS [77,157,158] | Wt [77,158] L274M 313A Y349F [157] |
Method development [77,155,159] Photocrosslinking [157,158] |
|
| MmPylRS [155,159] | Y306A Y384F |
[159] U25C [155] |
||
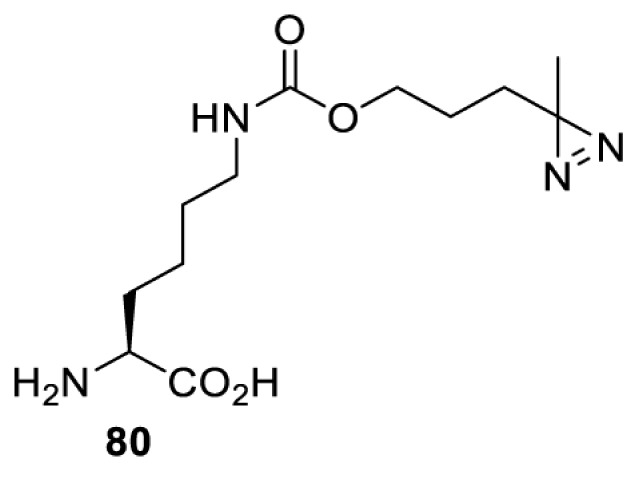 |
MmPylRS [159] | Y306A Y384F | Method development | |
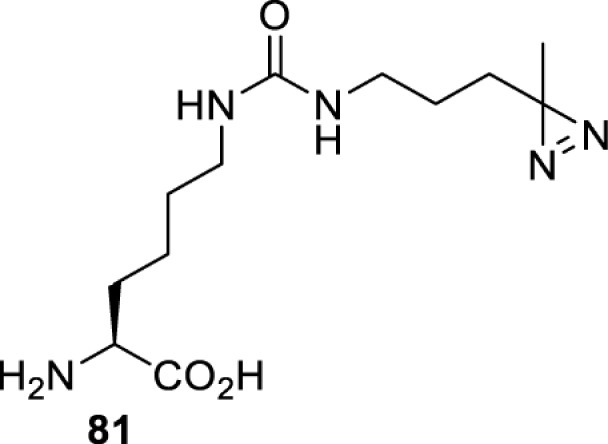 |
MbPylRS [132,140,160] | L274A C313S Y349F | Method development [140] Photocrosslinking [132,160] Protein engineering [132] |
|
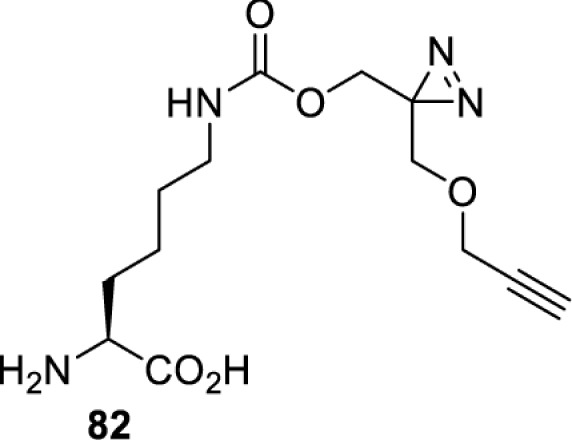 |
MmPylRS [159] | Y306A Y384F | Method development | |
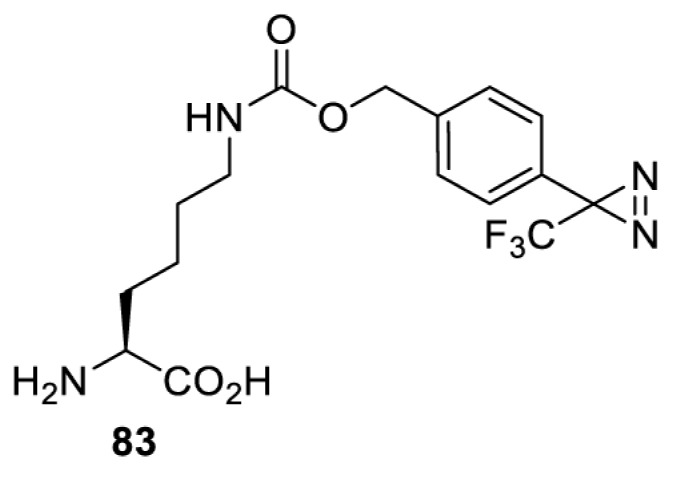 |
MmPylRS [142] | Y306A Y384F | Photocrosslinking | |
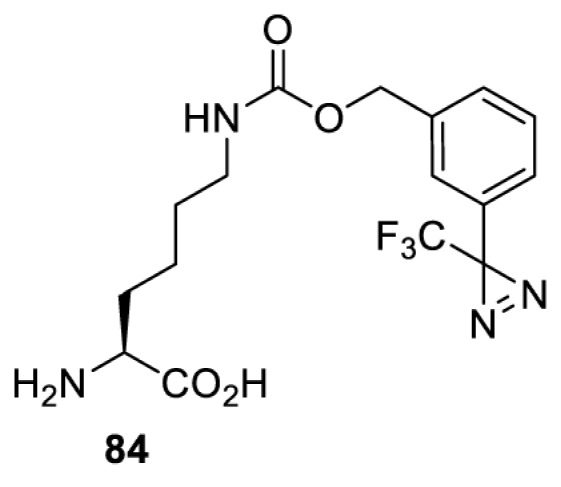 |
MmPylRS [143] | R61K G131E Y306A Y384F | Photocrosslinking | |
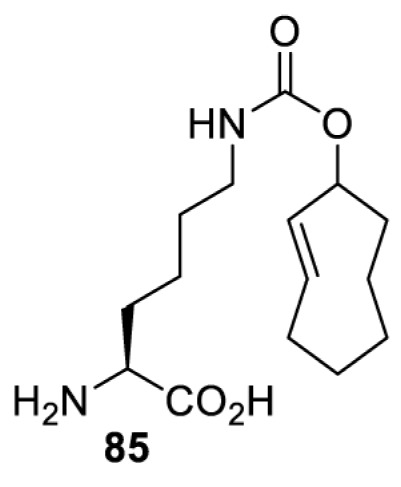 |
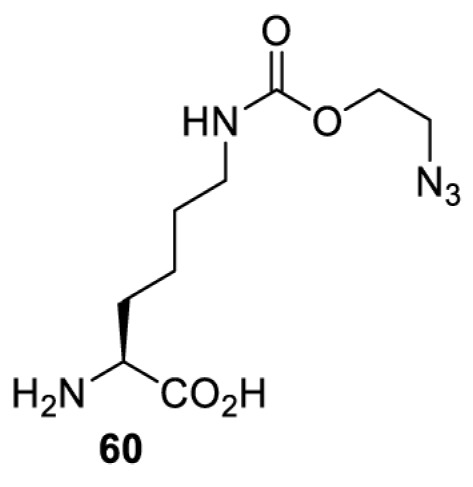
MmPylRS [18,39,59–61,123,155,161–166] | Y306A Y384F [18,39,59–61,123,155,161–166] |
[18,39,59–61,123,161–166] [155] |
Imaging [123,161,162,164,166] Chemical decaging [18,59–61] Chemical crosslinking [163] Method development [155,165] Protein labelling [39] |
| Mx1201PylRS [155] | Y126A | |||
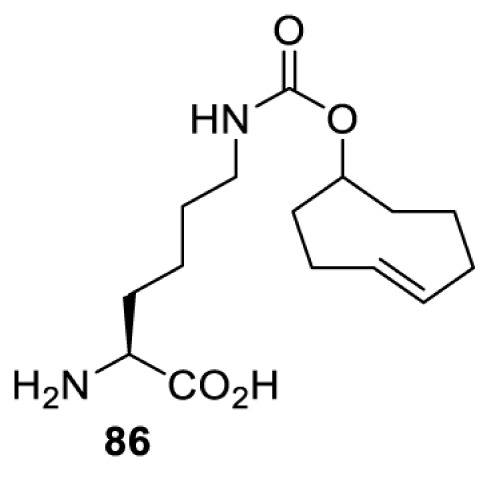 |
MbPylRS [123,167,168] | Y271A L274M C313A | Bioorthogonal labelling [124,167] Imaging [123,168] Method development [169] |
|
| MmPylRS [124,169] | Y306A Y384F [169] Y306A L309M C348A [124] |
|||
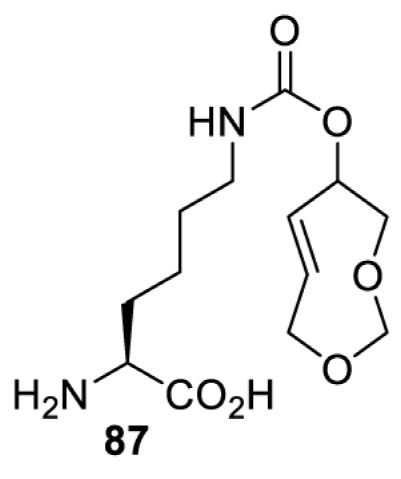 |
MmPylRS [165] | Y306A Y384F | Method development | |
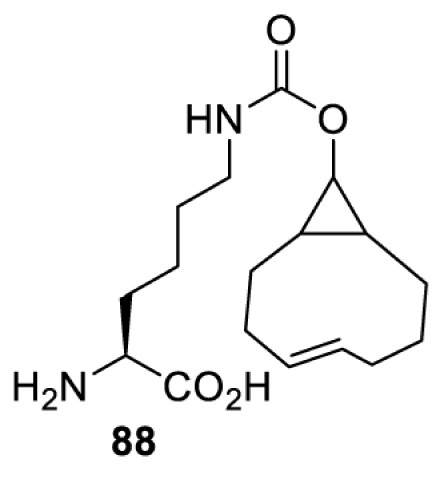 |
MbPylRS [167,168] | Y271A L274M C313A | Bioorthogonal labelling [167] Method development [167,169] |
|
| MmPylRS [169] | Y306A Y384F | |||
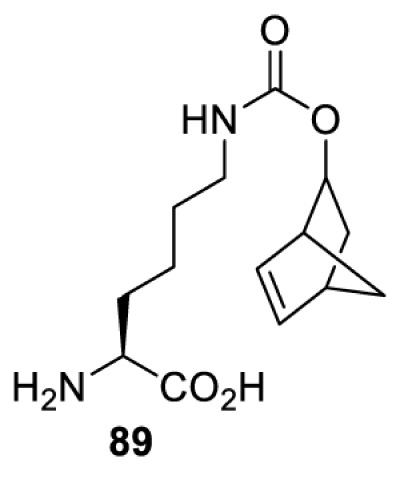 |
MbPylRS [24] | wt | Bioorthogonal labelling [39,127,131] Method development [24,169] |
|
| MmPylRS [39,127,131,169] | Wt [127,131] Y306A Y384F [39,169] |
|||
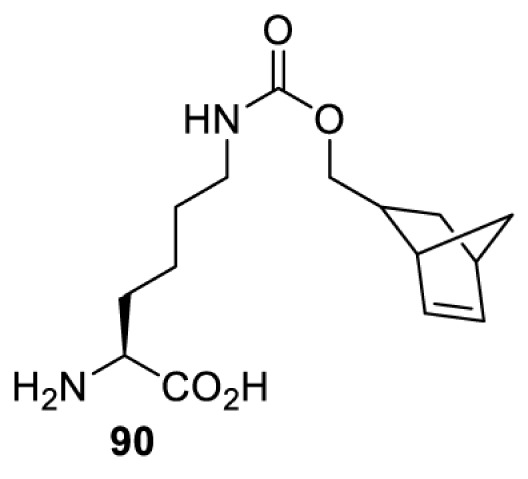 |
MmPylRS [169] | Y306A Y384F | Method development | |
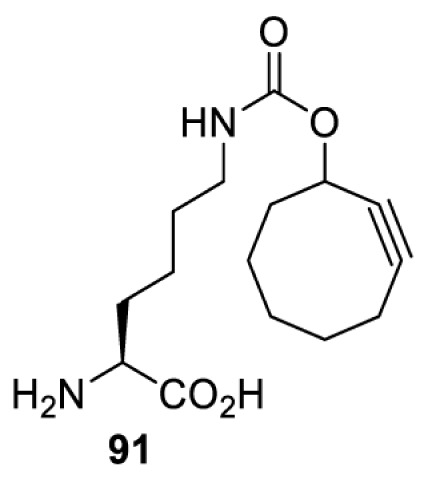 |
MmPylRS [39,161,166,169,170] | Y306A Y384F | Bioorthogonal labelling [39] Imaging [161,166,170] Method development [169] |
|
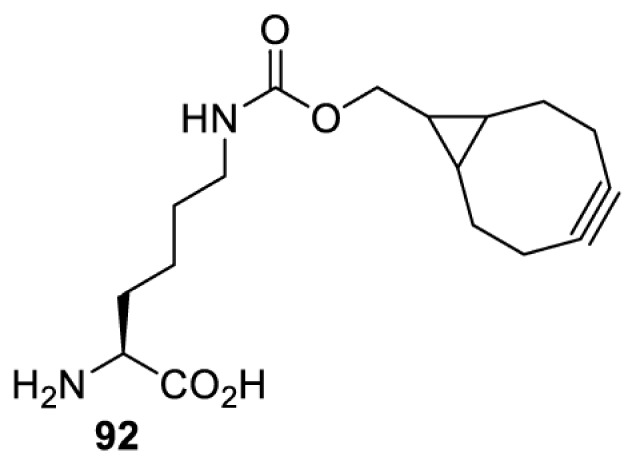 |
MbPylRS [19,64,140,168,171,172] | Y271M L274G C313A [19,64,168,171,172] M241F A267S Y271C L274M [140] |
[64,140,168,171,172] [19] |
Chemical inhibition [64] Bioorthogonal labelling [39,131,167] Imaging [123,128,161,166,168,171,172] Method development [155,159,165] Protein engineering [140] Spectroscopic probe [19] |
| MmPylRS [39,123,128,131,155,159,161,165–167] | Y306A 384F [39,123,128,131,155,159,161, 165–167] |
[39,123,128,131,159,161, 165–167] [155] |
||
| Tryptophan derivatives | ||||
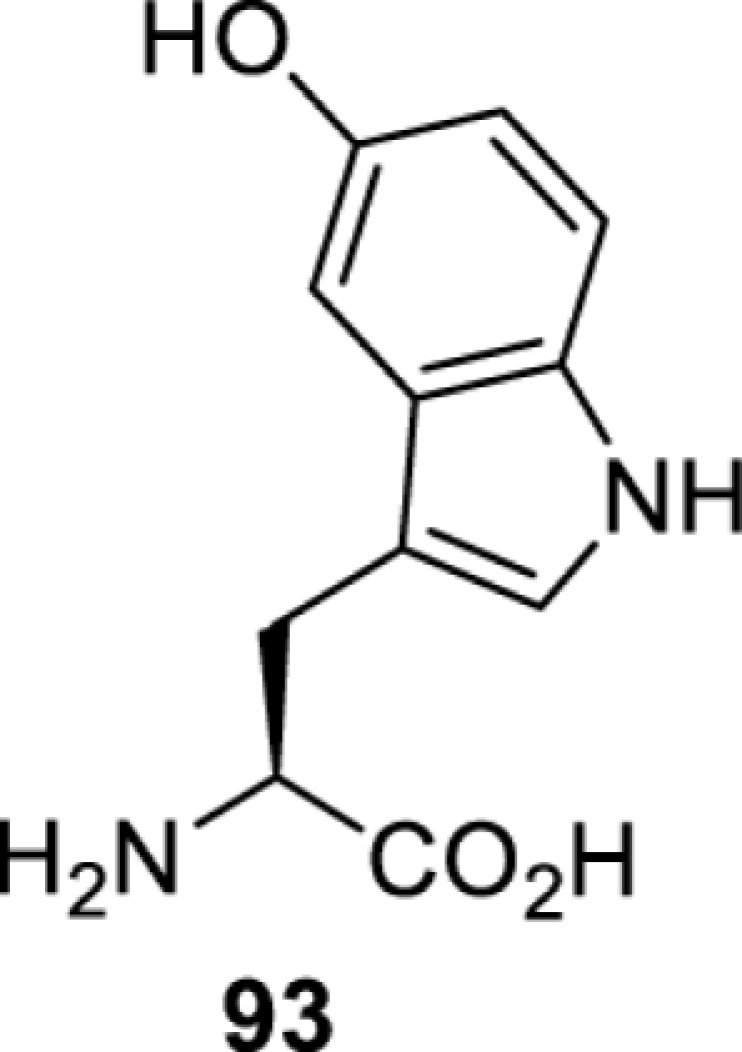 |
EcTrpRS [34] | S8A V144S V146A S8A V144G V146C |
Method development | |
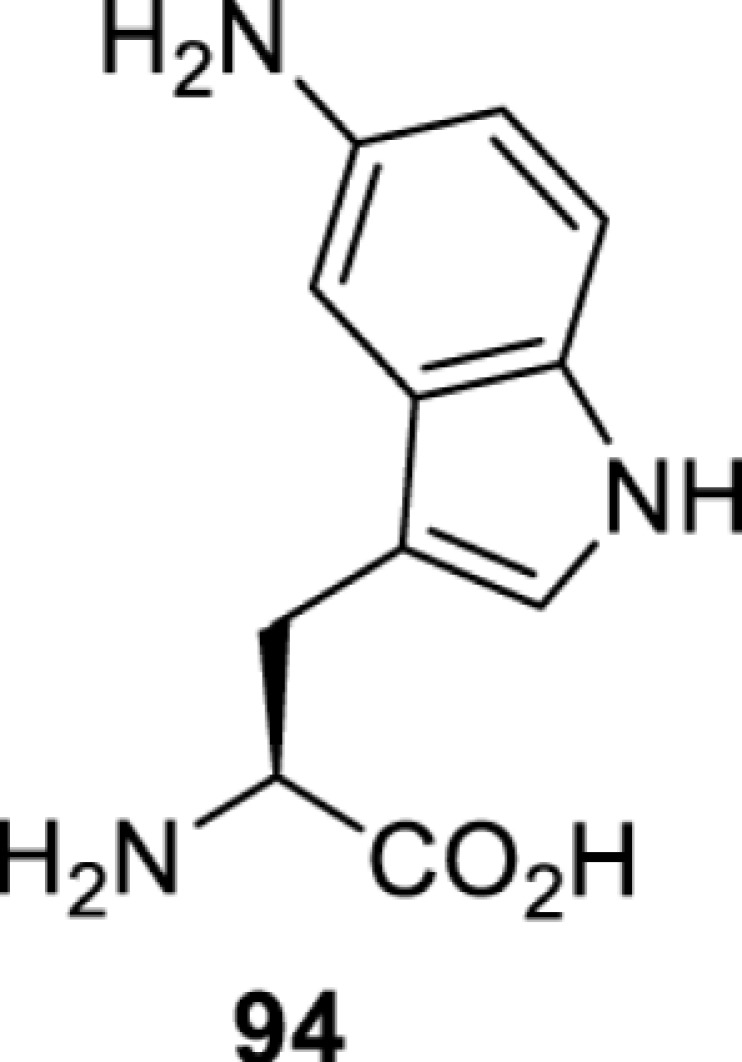 |
EcTrpRS [34] | S8A V144S V146A | Method development | |
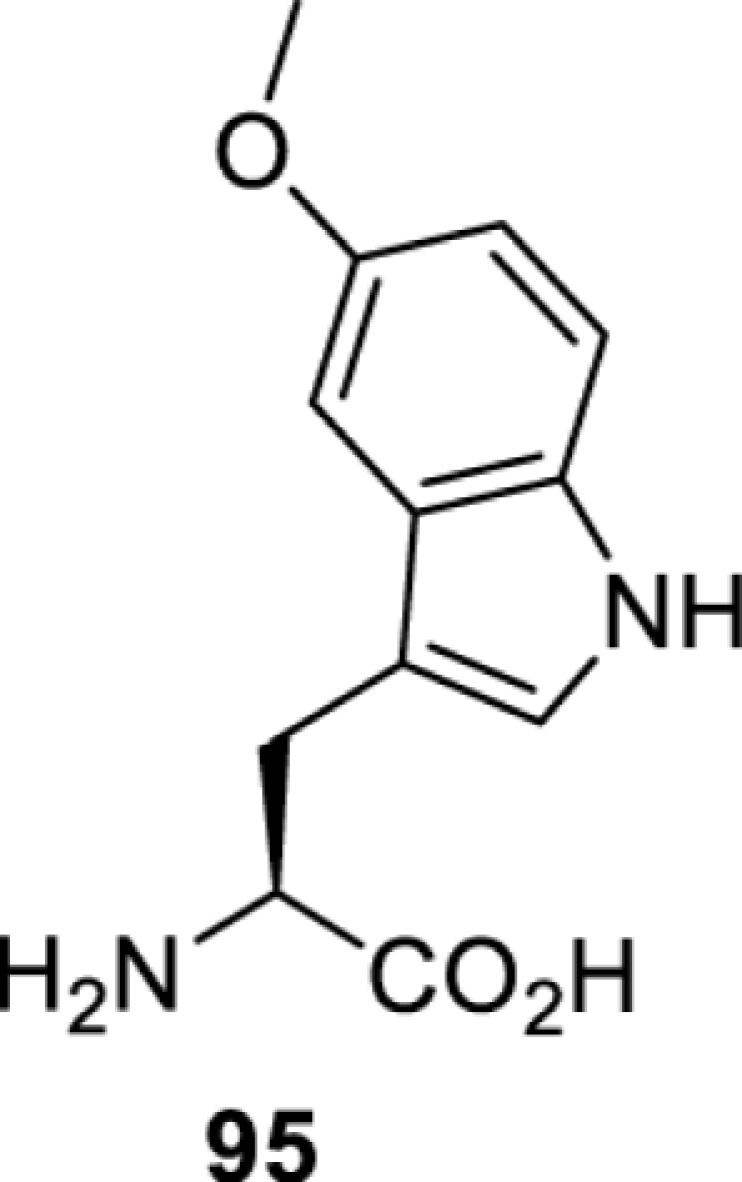 |
EcTrpRS [34] | S8A V144G V146C | Method development | |
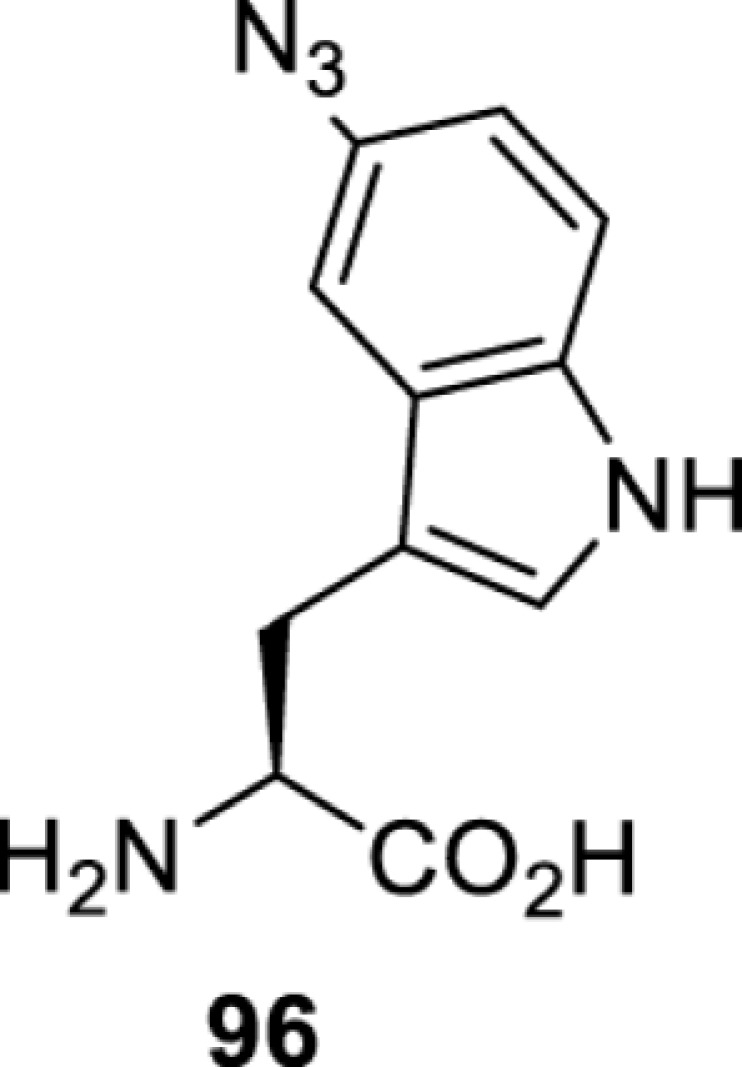 |
EcTrpRS [34] | S8A V144G V146C | Method development | |
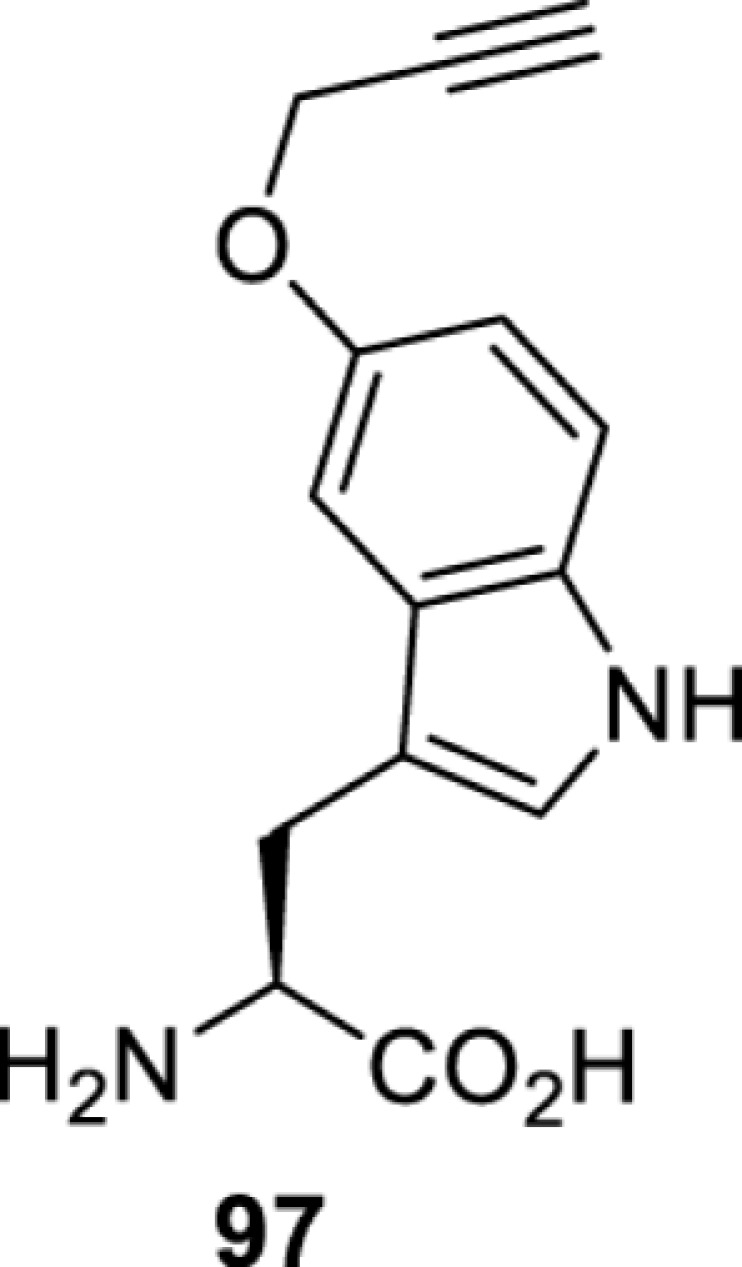 |
EcTrpRS [34] | S8A V144G V146C | Method development | |
| Tyrosine derivatives | ||||
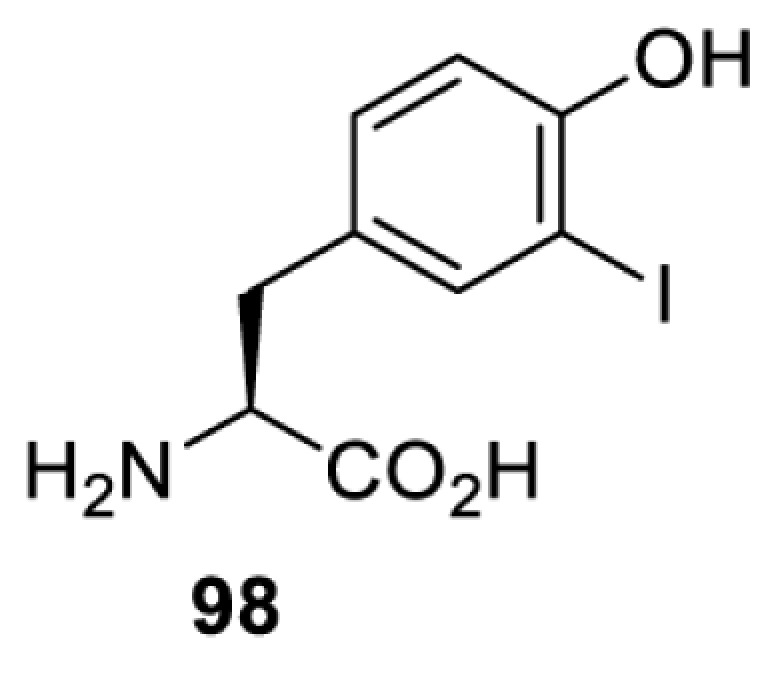 |
EcTyrRS [46] | Y37V Q195C | Method development | |
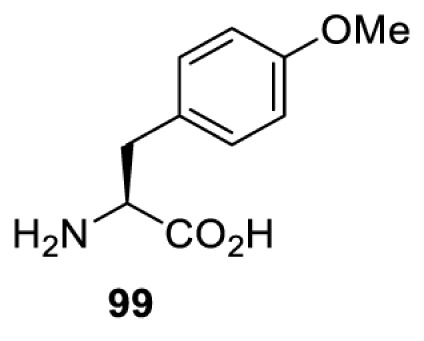 |
EcTyrRSCUA [15,21,35,37,77,78,90] | Y37T D182T F183M D265R [78] Y37V D182S F183M [37] Y37V D182S F183M D265R [21,77,90] Y37T D182T F183M [15] Y37V D165G D182S F183M L186A D265R [35] |
[15,21,35,77,78,90] | Mechanistic studies [15] Method development [21,25,35,37,77,78,90] |
| [21,37,77,90] | ||||
| EcTyrRSUCA [25] | Y37V D182S F183M | |||
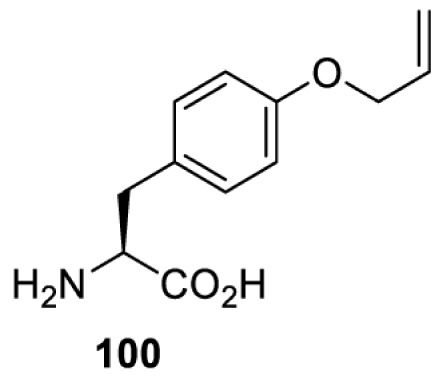 |
EcTyrRS [77] | Y37V D182S F183M D265R | Method development | |
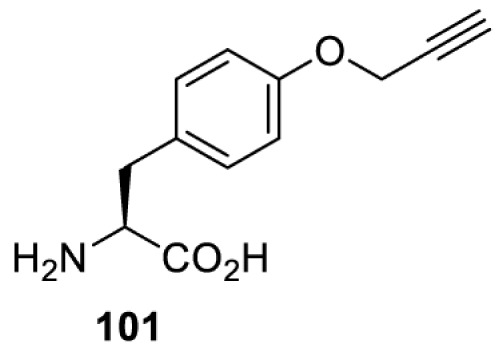 |
EcTyrRS [25,35,37,77] | Y37V D182S F183M D265R [77] Y37S D182T F183M L186V [37] Y37V D182S F183M [25] Y37V D165G D182S F183M L186A D265R [35] |
[25,35,77] | Method development |
| [37,77] | ||||
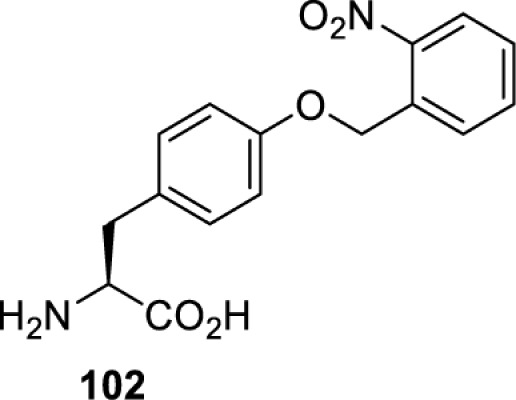 |
MbPylRS [126,148,173] | L270F L274M N311G C313G Y349F [173] L270F L274M N311G C313G [126,148] |
Photoactivation | |
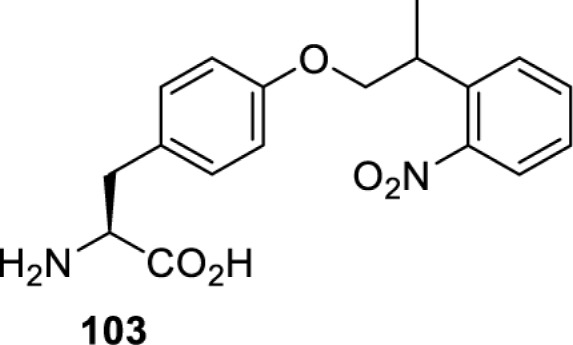 |
MbPylRS [173] | L270F L274M N311G C313G Y349F | Photoactivation | |
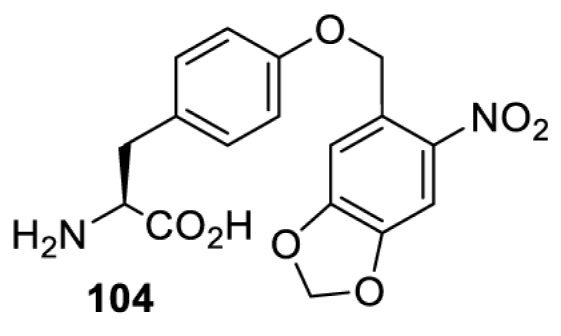 |
MbPylRS [173] | L270F L274M N311G C313G Y349F | Photoactivation | |
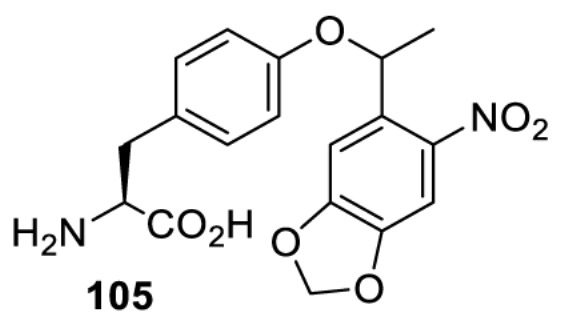 |
MbPylRS [173] | L270F L274M N311G C313G Y349F | Photoactivation | |
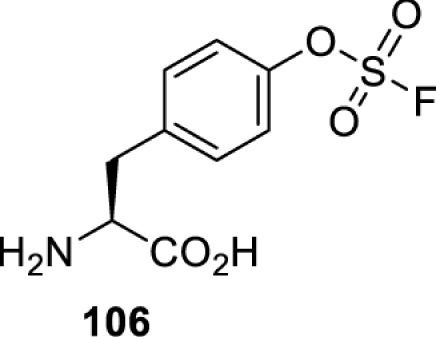 |
MmPylRS [174] | N346T C348I Y384L W417K | Bioorthogonal labelling | |
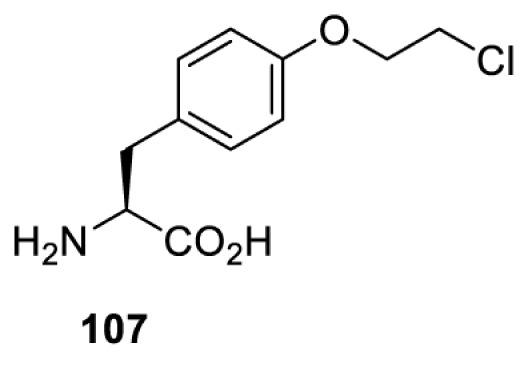 |
EcTyrRS [35] | Y37V D182S F183M D265R Y37V D165G D182S F183M L186A D265R |
Method development | |
| Miscellaneous unnatural amino acids | ||||
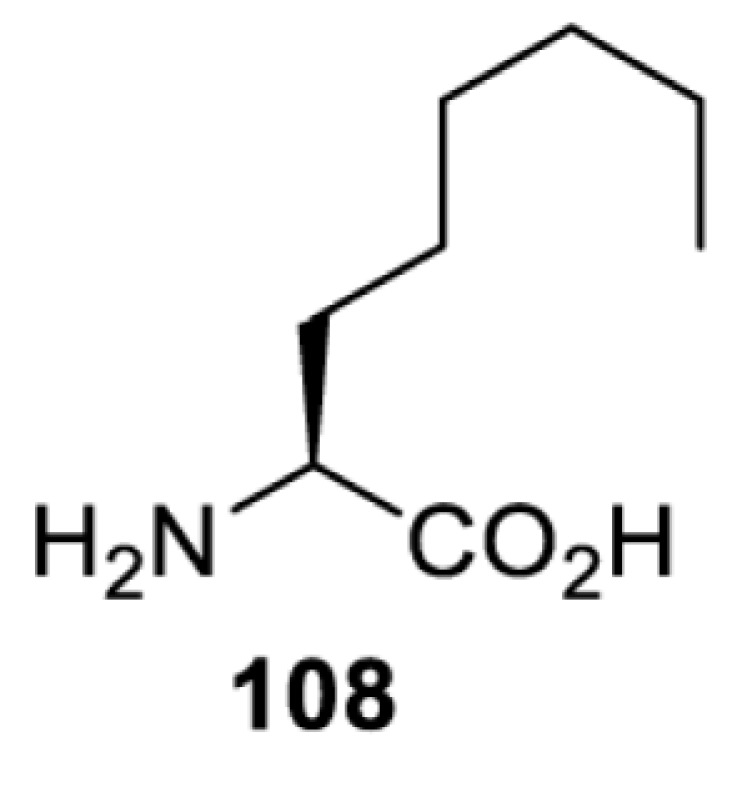 |
EcLeuRS [24,25] | M40I T252A Y499I Y527A H529G [24] E20K M40V L41S T252R Y499S Y527L H529G H537G [25] |
Method development | |
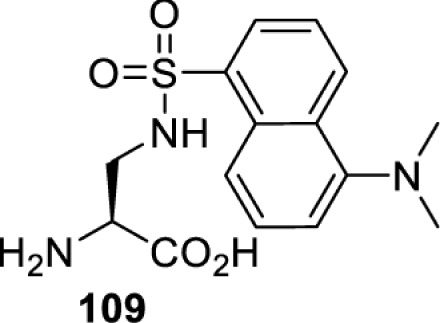 |
EcLeuRS [15,103,175] | M40A L41N T252A Y499I Y527G H537T | Mechanistic studies [15,103] Method development [175] |
|
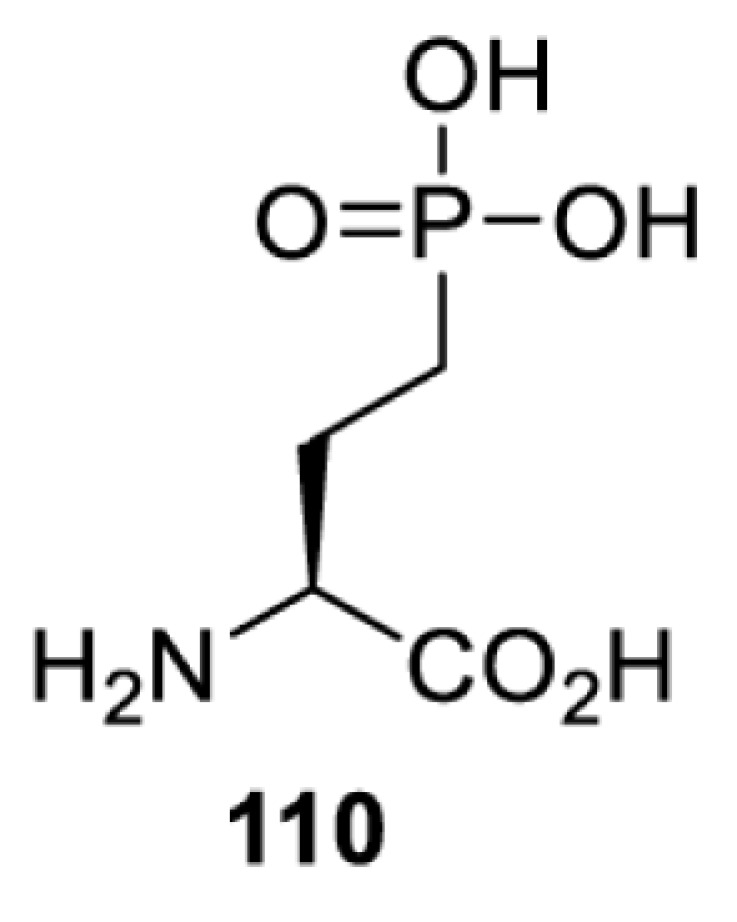 |
MmSepRS [176] | wt | Method development | |
Method development includes demonstration of incorporation, optimisation of incorporation efficiency, application as a control substrate, proof-of-principle of a technique for subsequent studies etc. Abbreviations: Ma, Methanomethylophilus alvus; wt, wild type.
Many engineered E. coli aaRS/tRNA pairs have also been used as orthogonal pairs in mammalian cells. The most successful ones are the E. coli tyrosine, leucine and tryptophan pairs [33]. However, as all these synthetases naturally recognise a canonical amino acid, it is necessary to abolish their natural activity towards the canonical amino acid and to recognise only the designated unnatural amino acid. As it is technically difficult to perform directed evolution in mammalian cells due to low efficiency in transfection and screening, synthetase engineering is normally carried out in E. coli [34,35] or yeast [15,36,37] so that large mutant libraries can be easily screened. It is also necessary to modify the E. coli tRNA so that it decodes a blank codon instead of a codon corresponding to a canonical amino acid.
Based on the simplicity of the established methodology [38–40] and the promiscuity of many orthogonal synthetases towards different unnatural amino acids (vide infra) [41], the number of genetically incorporable unnatural amino acids has steadily increased. In addition, some orthogonal aaRS/tRNA pairs are mutually orthogonal [21,24–26,42] and can be used at the same time to incorporate multiple different unnatural amino acids into a protein of interest.
As recent reviews cover fundamental aspects of genetic code expansion [4,6,9,13], the engineering of new orthogonal synthetases [8], and general [5,10,11,14] or specific [7,12] applications of genetic code expansion in eukaryotic systems, we will focus on recent advances and applications of genetic code expansion for controlling protein function in mammalian cells through translational, optical or chemical means.
Translational control by amber suppression
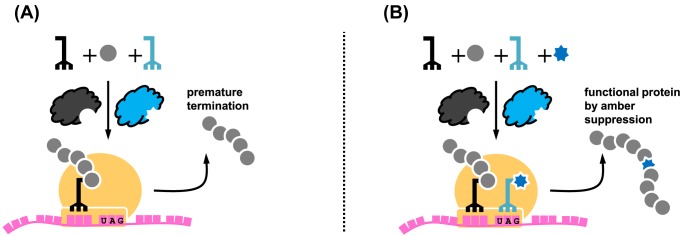
Genetic code expansion by unnatural amino acid incorporation in response to an amber stop codon provides the simplest way to ‘switch on’ protein production. In this case, an amber stop codon is placed into the gene of interest (Figure 3) for incorporation of the unnatural amino acid into a permissive site of the target protein [43]. In the absence of the designated unnatural amino acid, protein translation stops prematurely at the amber stop codon, generating truncated and non-functional protein product and thus giving an effect as a nonsense mutation (Figure 3A). In the presence of the unnatural amino acid, the orthogonal tRNA is acylated and decodes the amber codon, leading to amber suppression and generation of full-length, functional protein product (Figure 3B). Thus, simple addition of the unnatural amino acid into the growth medium ‘switches on’ the protein production and function [44]. In contrast with commonly used systems for inducible mammalian protein expression (e.g. the tetracycline transcriptional transactivation) [45], the lag time is shorter in the translational control by amber suppression (i.e. time for translation and folding) than the gene activation approaches (i.e. time for transcription, mRNA processing, translation and folding). Amber suppression also allows a more stringent control, as the background activity (if any) can be further minimised by including multiple amber codons into the gene of interest. The translational control by amber suppression approach is fully complementary to conventional genetic approaches (e.g. knockout, knockdown) that deplete a protein in cells to ‘switch off’ its function. In addition, the unnatural amino acid approach is reversible, as removing the unnatural amino acid in the growth medium will ‘switch off’ the translation of the protein of interest.
Figure 3. Use of amber suppression to switch on protein production.
(A) Absence of the unnatural amino acid leads to recognition of the UAG codon for translation termination. (B) Addition of the unnatural amino acid leads to amber suppression and successful production of the full-length and functional protein.
The translational switch-on process has been widely employed as a reporter system to test incorporation of new unnatural amino acids by using luminescent proteins like green fluorescent protein [46] or luciferase [20]. Upon successful incorporation, cells can emit light, whose intensity directly correlates to the unnatural amino acid incorporation efficiency. Apart from the reporter strategy, this approach has also been used to regulate function of other proteins, such as Cas9 for controllable gene editing in mouse embryos [47].
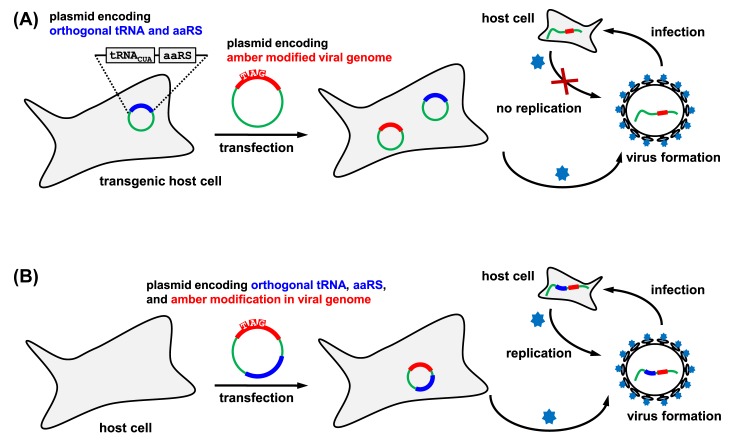
Besides the general use of the ‘translational activation’ approach to study protein function, this principle has been proven to be powerful in controlling virus replication [43,48,49]. By introducing TAG codons within the virus genes, viruses can only be generated using cell lines containing an orthogonal synthetase/tRNACUA pair, and the resulting viruses are replication-incompetent in normal cells due to the lack of amber suppressor tRNA (Figure 4A) [43,48]. Such replication-incompetent viruses offer an additional tier of control for live-attenuated vaccines and significantly increase their safety. This concept has been further developed by including the genes encoding the orthogonal aaRS/tRNA pair into the viral genome (Figure 4B) [49]. In this case, viruses can be replicated in wild-type cells and the native hosts, as long as the unnatural amino acid is supplemented. Here, spatial control can also be achieved by local administration of the unnatural amino acid as demonstrated in examples of mice with an expanded genetic code [50,51]. Thus, the approach can be used for controlling viral vectors in gene therapy, where spatiotemporal virus replication and gene editing are highly desirable.
Figure 4. Translational activation approaches to control virus replication.
(A) Use of genetic code expansion to control replication of an amber codon tagged virus within transgenic host cells containing the orthogonal tRNA/synthetase pairs [43,48]. (B) Use of genetic code expansion to control replication of an amber codon tagged virus within normal host cells with the orthogonal tRNA/synthetase pair gene encoded by the viral genome [49].
While the translational control approach is quite simple, the response is not instantaneous. There is always a lag time from when the unnatural amino acid is administered into the culture medium until the full-length protein is produced and folded. Similarly, depleting the unnatural amino acid in the growth medium will stop production of new proteins, but the protein function will only be completely switched off when all previously produced proteins are degraded in the cells. Thus, the kinetics of the switching off process largely depend on the half-life of the protein, so the response rate is the same as with genetic knockdown.
Light-induced activation or inhibition
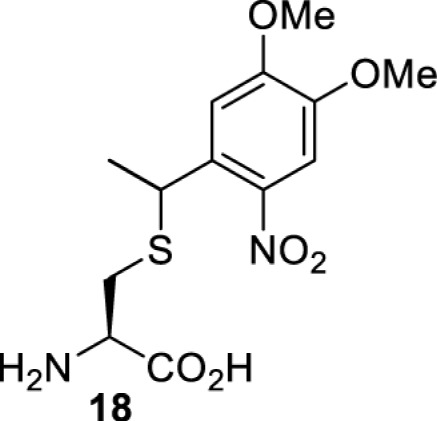
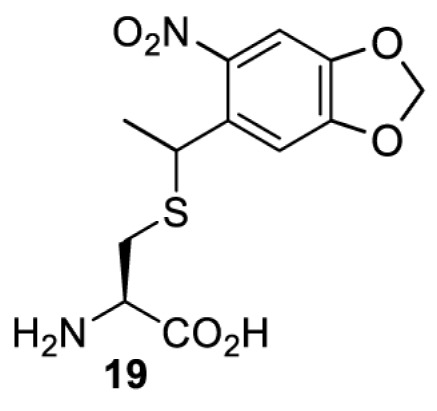
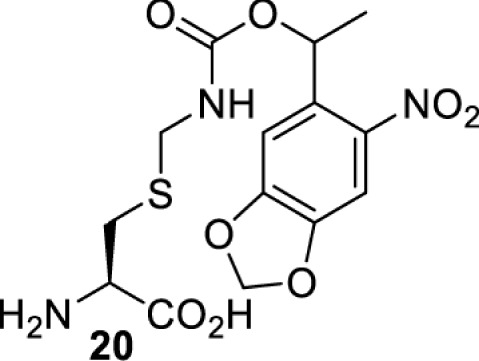
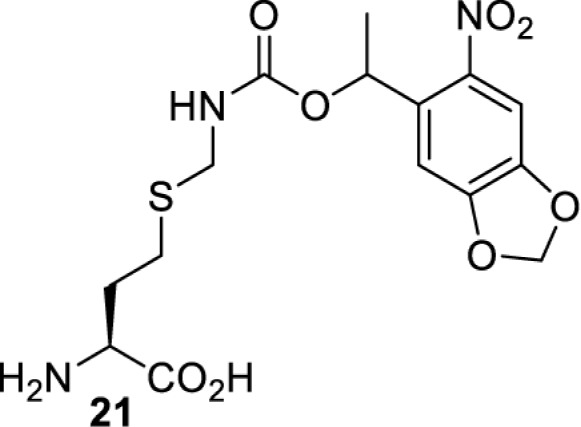
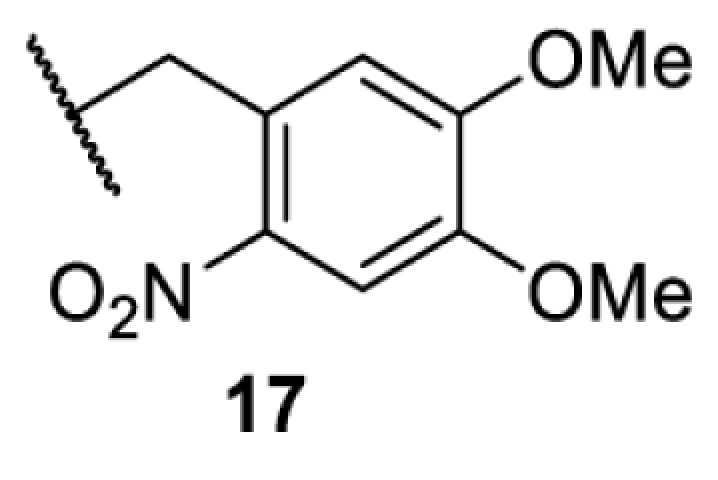
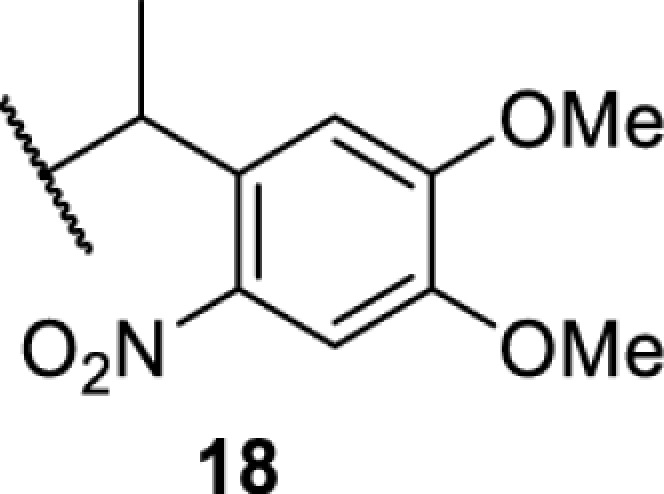
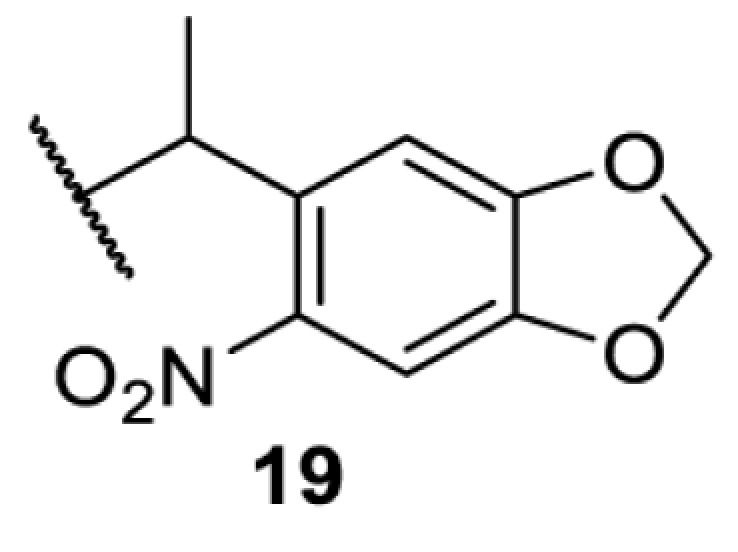
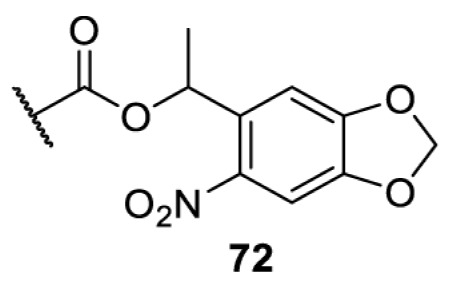
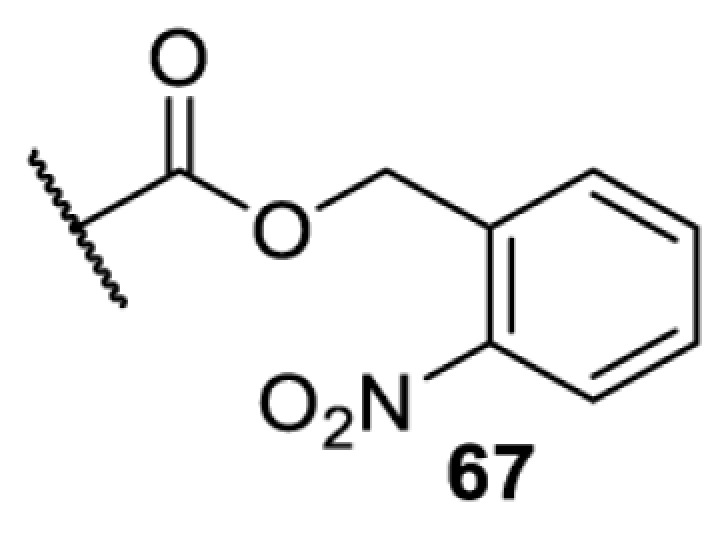
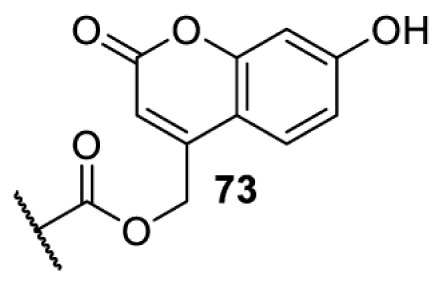
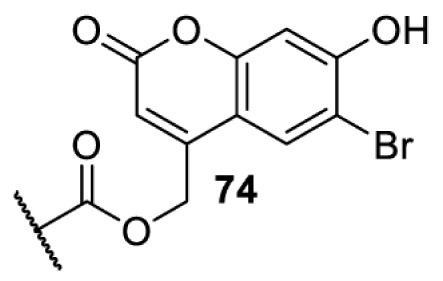
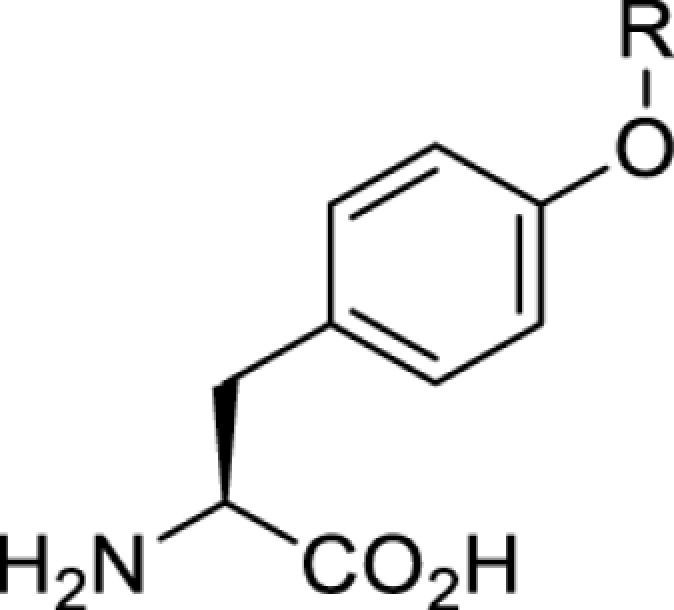
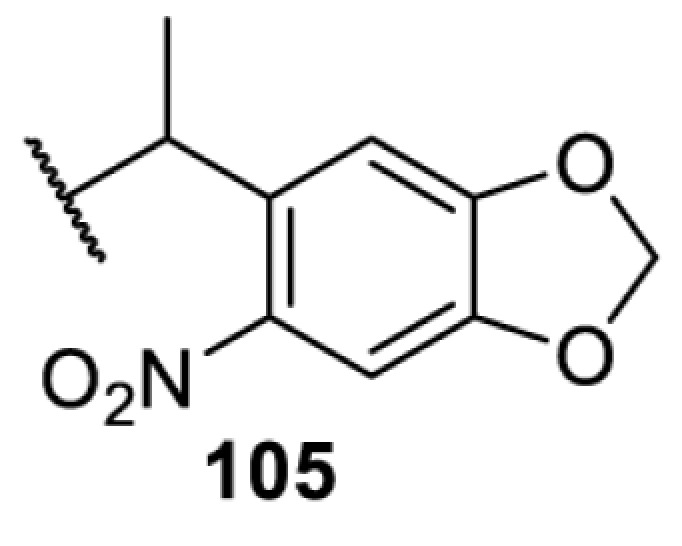
The slower kinetics of the translational control approach limit its applicability to study biological processes where fast response is needed. This can be addressed by using light to unmask or modify unnatural amino acids and subsequently regulate protein function. Depending on the nature of the light-responsive group, it is possible to either activate, inhibit, or reversibly switch on/off protein function (Table 2). Unnatural amino acids containing a photocage (i.e. a photolabile protecting group) [52] have been widely used for protein activation. When replacing a functionally critical amino acid residue with the corresponding photocaged amino acid, the target protein becomes inactive; upon light irradiation, the photocage is removed, thereby restoring the protein’s function. To date, photocaged cysteines (17–21), lysines (67, 72–74) and tyrosines (102–105) have been used to control enzyme function, intein splicing, protein subcellular localisation, virus–host interactions, and cell signalling cascades [13]. The light-activation approach is particularly useful for kinetic studies as it provides extreme spatiotemporal resolution. Spatial control can be achieved to even subcellular locations using focused light beams, which is virtually impossible when using the translational control approach. Theoretically, it is also possible to incorporate photocaged serine in mammalian cells as it has been demonstrated in yeast [53]. Therefore, the light-activation approach is applicable to regulate any protein that has a functionally critical cysteine, lysine, tyrosine, or serine residue in mammalian cells, including but not limited to kinases, DNA- and RNA-binding proteins, proteases, phosphatases, oxidoreductases, isomerases, and ubiquitin-modifying enzymes [54].
Table 2. Overview of photocaged unnatural amino acids that have been incorporated in mammalian cell systems for activating protein function upon irradiation with light of specific wavelength λ.
| Amino acid | Photocaging group (R) | System | Proteins | λdecag (nm) |
|---|---|---|---|---|
 |
 |
HEK293T | eGFP, potassium channel Kir2.1 [71] | 385 |
| eGFP [72] | Long wavelength UV | |||
 |
eGFP, mCherry, Npu DnaE intein, Src kinase [72] | |||
 |
TEV protease [73,74], Npu DnaE intein [74] | 365 | ||
 |
sfGFP, luciferase [75] | |||
 |
 |
HEK 293T | sfGFP, luciferase [75] | 365 |
 |
 |
HEK293 [67,125] HEK293T [66,75,147–150,152] HEK293ET [151] HeLa [150,152] |
Nuclear localisation peptide for subcellular localisation of SATB1 and FOXO3 transcription factors, and TEV protease [149] | 350 |
| sfGFP, luciferase [75] | 365 | |||
| p53 transcription factor [125] | ||||
| Isocitrate dehydrogenase [67] | ||||
| Cas9 endonuclease [150] | ||||
| Cre recombinase [148] | ||||
| Capsid of adeno-associated virus 2 [147] | ||||
| T7RNA polymerase [152] | ||||
| MEK1 kinase [151] | ||||
| LCK kinase [66] | 405 | |||
 |
HEK293T | Luciferase [144] | 365 | |
 |
HEK293T or CHO K1 | eGFP and luciferase [153] | 365 405 |
|
 |
365 405 7601 |
|||
 |
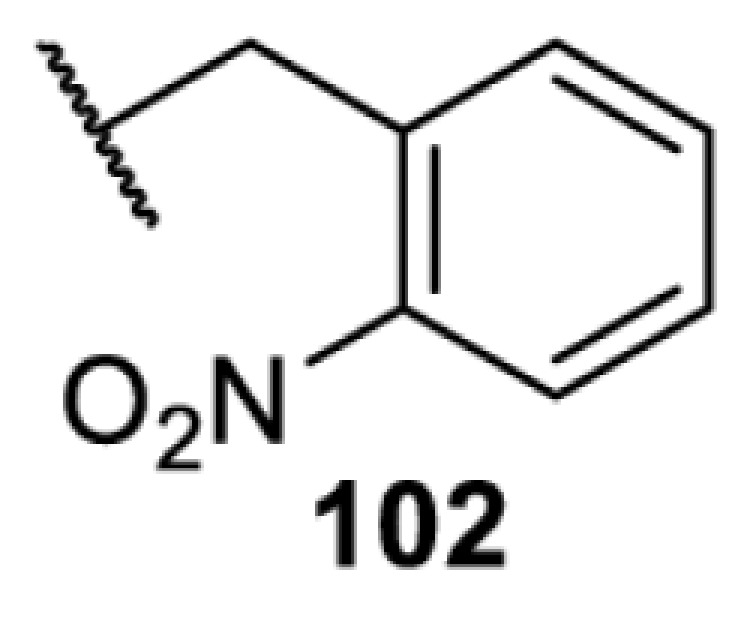 |
HEK293 [126] HEK293T [148,173] |
Cre recombinase [148]STAT1transcription factor [126]Luciferase [173] | 365 |
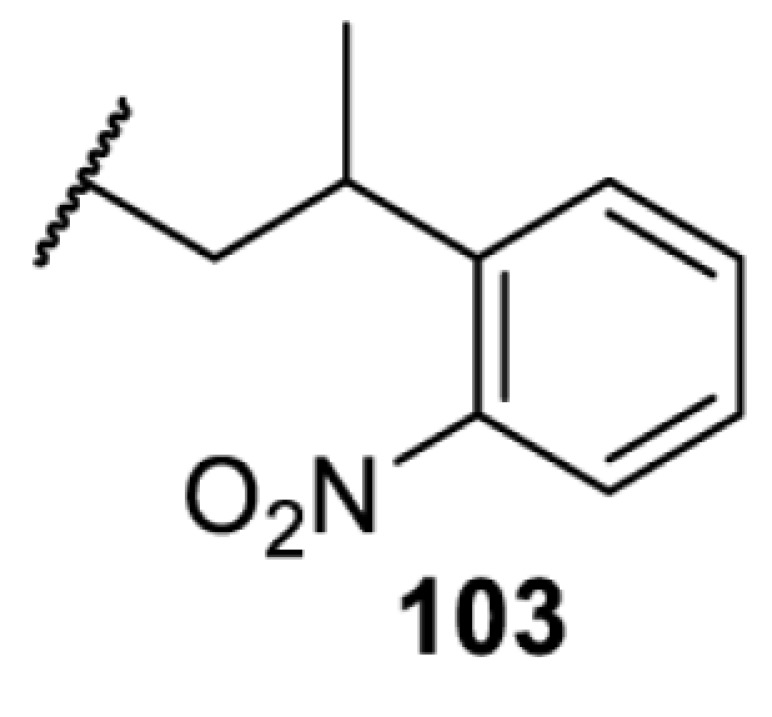 |
HEK293T | luciferase, TEV protease [173] | ||
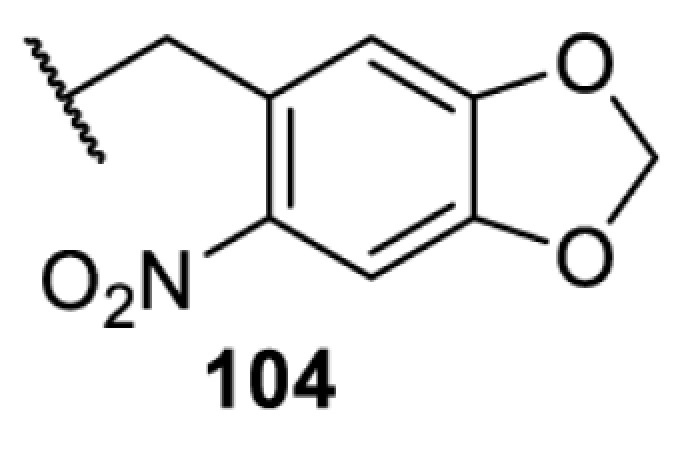 | ||||
 |
Decaging is only achieved at this wavelength via a two-photon activation using a specialised multiphoton laser setup. Abbreviation: Npu, Nostoc punctiforme.
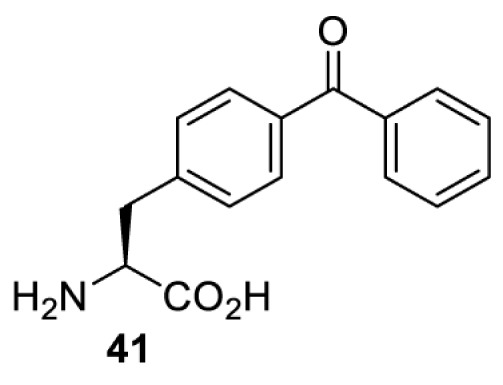
In contrast, the incorporation of a photocrosslinking amino acid can be used to inhibit protein function upon light irradiation [55]. In this case, a photocrosslinking amino acid is placed in the interior of the target protein. Upon light irradiation, a highly reactive functionality (e.g. radical, nitrene, carbene) is generated and reacted non-specifically with a nearby amino acid residue, causing cross-linking of the protein and subsequent abolishment of the protein’s activity. The feasibility of this approach has been demonstrated with the use of p-benzoylphenylalanine (41) in the study of glutamate receptors, GluA1 and GluA2 [55]. When compared with the use of a photocaged amino acid, inhibiting a protein by photocrosslinking does not rely on the existence of a functionally critical residue, and thus theoretically, it can be used to investigate any protein in mammalian cells. Nevertheless, for each protein target it is necessary to screen a suitable site for placing the photocrosslinking amino acid. Protein variants containing the photocrosslinking amino acid must (i) behave in the same way as the wild-type protein (i.e. phenotypically silent) before light irradiation; and (ii) be fully inhibited after light irradiation causing the photocrosslinking. Due to these criteria, the screening process can be laborious and time-consuming.
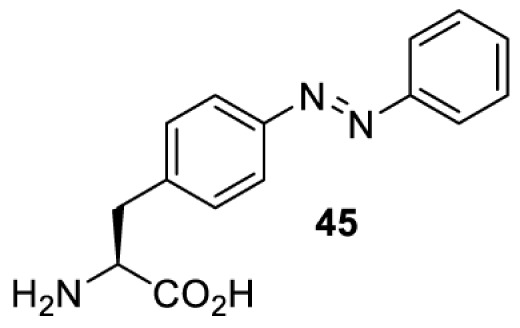
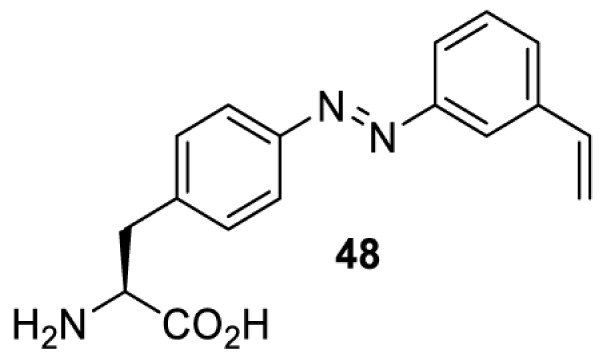
In addition to light-induced activation and inhibition, reversible regulation of a protein function can be achieved through incorporation of a photoswitchable amino acid (Table 3). For example, 48, containing an azobenzene functionality which undergoes reversible cis-trans isomerisation upon irradiation with blue and UV light, has been used to control the activity of a glutamate receptor [56]. However, the general applicability of this approach suffers from similar constraints as inhibition by photocrosslinking. Extensive screening is often needed to identify a suitable site for incorporation, such that the resulting protein variant is fully active or inactive upon irradiation with light of a specific wavelength. At the current state of the art, there is no guarantee that such a site can be found in the target protein.
Table 3. Overview of photoswitchable unnatural amino acids that have been incorporated in mammalian cell systems used to modulate protein function upon irradiation with the given wavelengths λ.
Overall, the use of light-responsive amino acids offers superior temporal control of protein function as the response is significantly faster (seconds) than the translational control by amber suppression (minutes to hours). Additionally, spatial control can be achieved at subcellular level, which is not possible with the translational approach. Generally, UV light at approximately 360 nm (i.e. UVA) is required (Tables 2 and 3) to induce the change (i.e. decaging, cross-linking, or isomerisation). However, UVA light has been shown to alter cellular signalling processes [57] or influence proper cellular function, if high intensity irradiation is applied (i.e. 50 J.cm−2) [58]. Though not necessarily problematic, this has to be considered when planning to apply light-responsive unnatural amino acids. Thus, there is a trend to develop new functionalities that can be modulated by light of higher wavelengths [52]. In particular, red and near-infrared light (650–750 nm) are appealing because they cause no harm to cells even under excessive exposure, and they can penetrate tissues for in vivo applications. To date, coumarin-caged lysines (73 and 74) are the only genetically incorporable unnatural amino acids that can be decaged within these wavelengths, although by two-photon approach that requires a specialised multiphoton laser setup [52]. Nevertheless, with the continuous advances in light-responsive chemical functionalities and orthogonal aaRS engineering, it is expected that more unnatural amino acids with the desired photophysical properties can be incorporated through genetic code expansion.
Small-molecule induced activation or inhibition
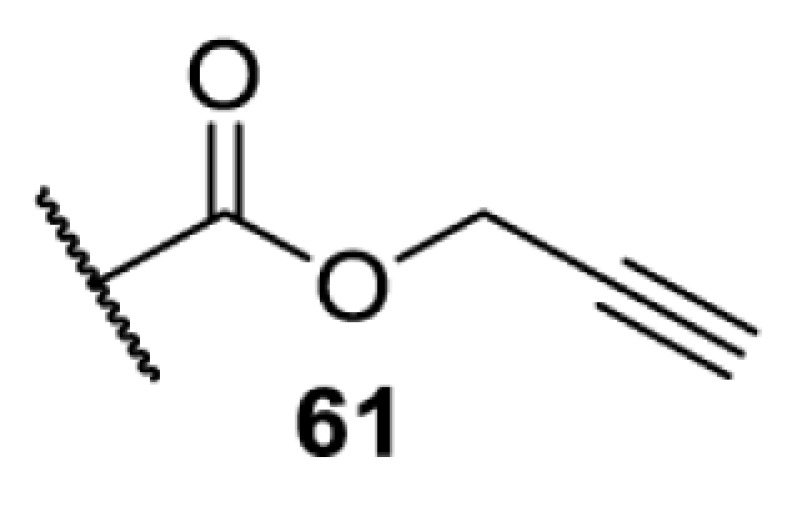
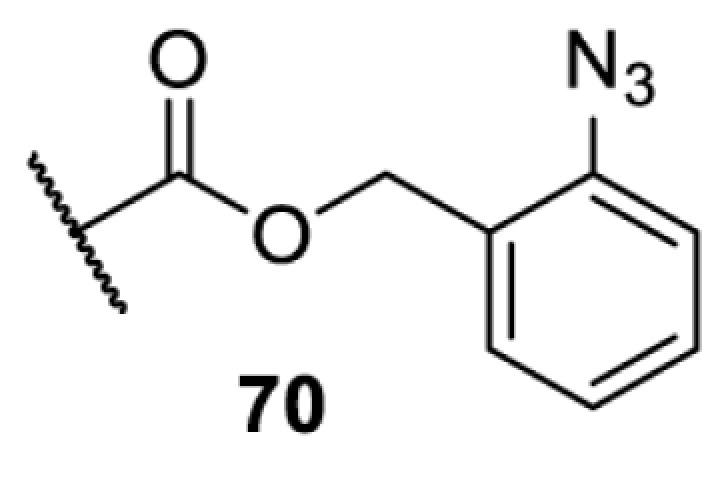
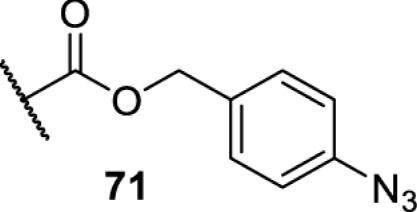
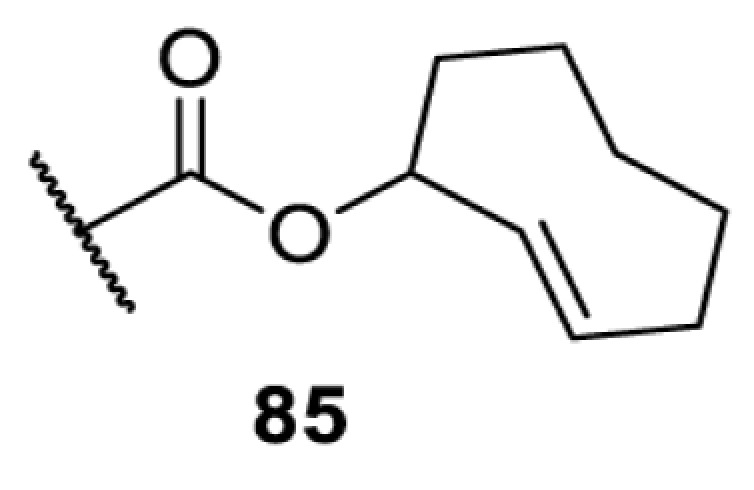
In addition to light, small molecules can also be used to unmask or modify unnatural amino acids and subsequently regulate protein function with prompt response. For example, several protecting groups can be removed bioorthogonally inside live mammalian cells, and these chemistries have been used to switch on protein function by genetic code expansion. Intracellular bioorthogonal reactions that have been used in this purpose include inverse electron demand Diels–Alder reactions [18,59–61], 1,3-dipolar cycloadditions [62], Staudinger reactions [63], and palladium-catalysed propargyl removal (Table 4) [57]. Currently, all of these have only been demonstrated in caged lysine molecules (61, 70, 71, 85) through a number of examples, including activation of luciferases, kinases, nucleases etc. Theoretically, all these protecting groups can be applied to other nucleophilic amino acids (e.g. cysteine, serine, threonine, tyrosine) subjected to successful engineering of the corresponding orthogonal synthetases.
Table 4. Overview of bioorthogonally protected unnatural amino acids tested in mammalian cell systems and their deprotection conditions.
| Amino acid | System | Proteins | Reaction | Reagent | |
|---|---|---|---|---|---|
 |
 |
HeLa, CHO, HEK293T, IH3T3, Caco-2, A549, HeLa | GFP, OspF phosphothreonine lyase | Pd-catalysed Tsuji–Trost-like reaction | Pd(II) complexes [57] |
 |
HEK293T | eGFP, SATB1 transcription factor, Cre recombinase, Cas9 endonuclease | Staudinger | Various phosphines [63] | |
 |
HEK293T | eGFR, luciferase, OspF phosphothreonine lyase, Src kinase | 1,3-dipolar cycloaddition | trans-cyclooctenes [62] | |
 |
HEK293T | GFP [61], Luciferase [59,61], MEK1 [18,60] and MEK2 [18] and FAK [60] and Src [60] kinases | Inverse electron demand Diels–Alder reactions | Tetrazines [18,59–61] | |
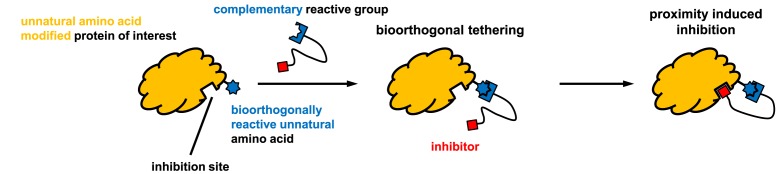
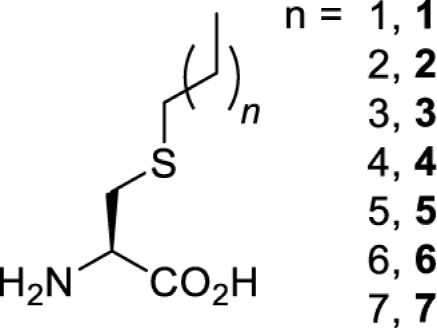
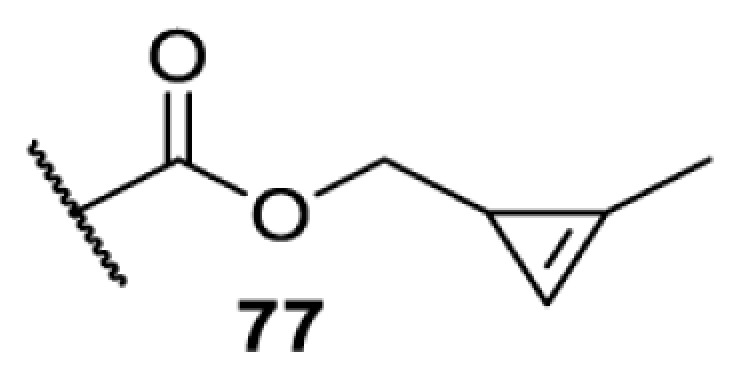
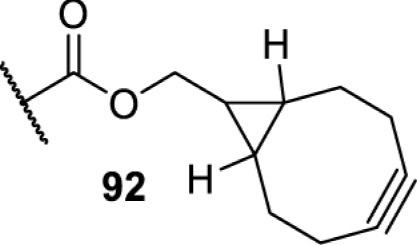
On the other hand, bioorthogonal amino acids (e.g. 77 and 92, Table 5) have been used for rapid and selective inhibition of a specific enzyme in live mammalian cells [64]. In this case, a bioorthogonal amino acid is placed into the target enzyme without affecting the enzyme function. Upon contact with an inhibitor conjugate bearing the complementary bioorthogonal group, the enzyme variant is tethered to the conjugate and thus the enzyme activity is inhibited (Figure 5). The inhibition is exquisitely selective and can even discriminate between isoforms that differ by a single amino acid residue. Using this approach, selective inhibition of an intracellular kinase for which no selective small-molecule inhibitor exists was achieved. In addition, placing a photoswitchable moiety (i.e. azobenzene) into the inhibitor conjugate enables reversible modulation of enzyme activity by light.
Table 5. Overview of bioorthogonally reactive unnatural amino acids that have been incorporated in mammalian cell systems used to deactivate protein function upon reaction with the specified reagents.
| Amino acid | System | Proteins | Reaction | Reagent | |
|---|---|---|---|---|---|
 |
 |
HEK293T | MEK1 and LCK kinases | inverse electron demand Diels–Alder reactions | Inhibitor–tetrazine conjugates [64] |
 |
MEK1 and MEK2 kinases |
||||
Figure 5. Example of the small-molecule approach to protein inhibition.
Use of unnatural amino acid incorporation for selective inhibition of protein function by bioorthogonal tethering [64].
In comparison with light-induced activation or inhibition, small molecules can be used to activate or inhibit the target protein in deep animal tissue or intact animals which are not easily accessible by light. However, as mentioned above, only a few reactions have so far been shown to be bioorthogonal with high reaction rates to allow fast response [65]. The extension of this methodology is therefore tied to the development of novel bioorthogonal reactions. In contrast to the use of light for activation or inhibition, the small-molecule approach, similar to the translational approach, only allows spatial control by using cell-compartment selective compounds or reactions, or local injections.
Conclusion
Genetic code expansion has matured into a technique that can be routinely used in mammalian systems. Controlling protein function is currently mostly achieved by translation, light, and small molecules. These methods have been summarised and discussed, and their features have been compared (Table 6). Translational control is arguably the easiest to perform but suffers from the longer response time (up to several hours). On the other hand, both light and small-molecule induced methods have a faster response (seconds to minutes). The light approach is particularly appealing where subcellular spatial resolution is needed. While all three approaches have shown promise, most of the reviewed applications are so far proof-of-principle studies. The dissemination of this technique could be enhanced by the community simplifying access to plasmids (e.g. through plasmid repository), standardising the reporting format of aaRS mutants with full sequencing information, and providing protocols with extensive details. Only general implementation of protein control by genetic code expansion in the wider scientific community to unravel new biological insights will truly demonstrate the power of these techniques [55,56,66–68]. Since genetic code expansion has also been recently demonstrated in mice [47,50,51,60,69,70], we foresee that optimisation will be tailored to target cells, tissues, and mammalian models and many of the aforementioned approaches will be applied in vivo, providing the complete native environment to study function of mammalian proteins. With further promotion and adaptation of genetic code expansion, this is to be expected.
Table 6. Comparison of protein function control approaches currently enabled by genetic code expansion.
| Approach | Temporal Control | Spatial control | Reversibility |
|---|---|---|---|
| Translational control | Yes, slow | Only by local administration | Yes, but high lag time |
| Optical control | Yes, fast | Yes, very high (to subcellular levels) | Yes, for photoswitchable amino acids |
| Chemical control | Yes, medium | Only by local administration | Yet to be established |
Summary
Genetic code expansion can now be routinely used for incorporation of more than 100 different unnatural amino acids in mammalian cells using only four orthogonal aaRS/tRNA pairs and their mutants as shown in Table 1.
Protein function can be temporally regulated (activation or inhibition) by simple translational control, i.e. supplementation of the desired unnatural amino acid to allow full-length, functional protein production.
More rapid control can be achieved by incorporating stimuli responsive amino acids which allow activation, inhibition, or reversible regulation of protein function by light or small molecules.
Most of the approaches have been demonstrated in proof-of-principle studies, but are ready for adaptation by the broader scientific community.
Abbreviations
- aaRS
aminoacyl-tRNA synthetase
- Ec
Escherichia coli
- Mx
Methanomethylophilus alvus Mx1201
- PylRS
Pyrrolysyl-tRNA synthetase
Funding
This work was supported by the BBSRC [grant number BB/P009506/1 (to Y.-H.T.)]; and the Wellcome Trust [grant numbers 202056/Z/16/Z (to L.Y.P.L.), 200730/Z/16/Z (to Y.-H.T.)]. The funders play no role in design, decision to publish, or preparation of this manuscript.
Competing interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.McDonnell D.P., Wardell S.E. and Norris J.D. (2015) Oral selective estrogen receptor downregulators (SERDs), a breakthrough endocrine therapy for breast cancer. J. Med. Chem. 58, 4883–4887 10.1021/acs.jmedchem.5b00760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petta I., Lievens S., Libert C., Tavernier J. and De Bosscher K. (2016) Modulation of protein-protein interactions for the development of novel therapeutics. Mol. Ther. 24, 707–718 10.1038/mt.2015.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Montfort R.L.M. and Workman P. (2017) Structure-based drug design: aiming for a perfect fit. Essays Biochem 61, 431–437 10.1042/EBC20170052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wan W., Tharp J.M. and Liu W.R. (2014) Pyrrolysyl-tRNA synthetase: an ordinary enzyme but an outstanding genetic code expansion tool. Biochim. Biophys. Acta 1844, 1059–1070 10.1016/j.bbapap.2014.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dumas A., Lercher L., Spicer C.D. and Davis B.G. (2015) Designing logical codon reassignment - Expanding the chemistry in biology. Chem. Sci. 6, 50–69 10.1039/C4SC01534G [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leisle L., Valiyaveetil F., Mehl R.A. and Ahern C.A. (2015) Incorporation of non-canonical amino acids. Adv. Exp. Med. Biol. 869, 119–151 10.1007/978-1-4939-2845-3_7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neumann-Staubitz P. and Neumann H. (2016) The use of unnatural amino acids to study and engineer protein function. Curr. Opin. Chem. Biol. 38, 119–128 [DOI] [PubMed] [Google Scholar]
- 8.Wang L. (2017) Engineering the genetic code in cells and animals: biological considerations and impacts. Acc. Chem. Res. 50, 2767–2775 10.1021/acs.accounts.7b00376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Italia J.S., Zheng Y., Kelemen R.E., Erickson S.B., Addy P.S. and Chatterjee A. (2017) Expanding the genetic code of mammalian cells. Biochem. Soc. Trans. 45, 555–562 10.1042/BST20160336 [DOI] [PubMed] [Google Scholar]
- 10.Debelouchina G.T. and Muir T.W. (2017) A molecular engineering toolbox for the structural biologist. Q. Rev. Biophys. 50, e7. 10.1017/S0033583517000051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chin J.W. (2017) Expanding and reprogramming the genetic code. Nature 550, 53–60 10.1038/nature24031 [DOI] [PubMed] [Google Scholar]
- 12.Zhang S., He D., Lin Z., Yang Y., Song H. and Chen P.R. (2017) Conditional chaperone-client interactions revealed by genetically encoded photo-cross-linkers. Acc. Chem. Res. 50, 1184–1192 10.1021/acs.accounts.6b00647 [DOI] [PubMed] [Google Scholar]
- 13.Young D.D. and Schultz P.G. (2018) Playing with the molecules of life. ACS Chem. Biol. 13, 854–870 10.1021/acschembio.7b00974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown W., Liu J. and Deiters A. (2018) Genetic code expansion in animals. ACS Chem. Biol. 13, 2375–2386 10.1021/acschembio.8b00520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang W., Takimoto J.K., Louie G.V., Baiga T.J., Noel J.P., Lee K.F.. et al. (2007) Genetically encoding unnatural amino acids for cellular and neuronal studies. Nat. Neurosci. 10, 1063–1072 10.1038/nn1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye S., Zaitseva E., Caltabiano G., Schertler G.F.X., Sakmar T.P., Deupi X.. et al. (2010) Tracking G-protein-coupled receptor activation using genetically encoded infrared probes. Nature 464, 1386–1390 10.1038/nature08948 [DOI] [PubMed] [Google Scholar]
- 17.Zhu S., Riou M., Yao C.A., Carvalho S., Rodriguez P.C., Bensaude O.. et al. (2014) Genetically encoding a light switch in an ionotropic glutamate receptor reveals subunit-specific interfaces. Proc. Natl. Acad. Sci. U.S.A. 111, 6081–6086 10.1073/pnas.1318808111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng S., Fan X., Wang J., Zhao J. and Chen P.R. (2017) Dissection of kinase isoforms through orthogonal and chemical inducible signaling cascades. ChemBioChem 18, 1593–1598 10.1002/cbic.201700255 [DOI] [PubMed] [Google Scholar]
- 19.Baumdick M., Gelleri M., Uttamapinant C., Beránek V., Chin J.W. and Bastiaens P.I.H. (2018) A conformational sensor based on genetic code expansion reveals an autocatalytic component in EGFR activation. Nat. Commun. 9, 3847. 10.1038/s41467-018-06299-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Köhrer C., Sullivan E.L. and RajBhandary U.L. (2004) Complete set of orthogonal 21st aminoacyl-tRNA synthetase-amber, ochre and opal suppressor tRNA pairs: concomitant suppression of three different termination codons in an mRNA in mammalian cells. Nucleic Acids Res. 32, 6200–6211 10.1093/nar/gkh959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao H., Chatterjee A., Choi S.H., Bajjuri K.M., Sinha S.C. and Schultz P.G. (2013) Genetic incorporation of multiple unnatural amino acids into proteins in mammalian cells. Angew. Chem. Int. Ed. 52, 14080–14083 10.1002/anie.201308137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmied W.H., Elsässer S.J., Uttamapinant C. and Chin J.W. (2014) Efficient multisite unnatural amino acid incorporation in mammalian cells via optimized pyrrolysyl tRNA synthetase/tRNA expression and engineered eRF1. J. Am. Chem. Soc. 136, 15577–15583 10.1021/ja5069728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Serfling R., Lorenz C., Etzel M., Schicht G., Böttke T., Mörl M.. et al. (2018) Designer tRNAs for efficient incorporation of non-canonical amino acids by the pyrrolysine system in mammalian cells. Nucleic Acids Res. 46, 1–10 10.1093/nar/gkx1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng Y., Mukherjee R., Chin M.A., Igo P., Gilgenast M.J. and Chatterjee A. (2018) Expanding the scope of single- and double-noncanonical amino acid mutagenesis in mammalian cells using orthogonal polyspecific leucyl-tRNA synthetases. Biochemistry 57, 441–445 10.1021/acs.biochem.7b00952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng Y., Addy P.S., Mukherjee R. and Chatterjee A. (2017) Defining the current scope and limitations of dual noncanonical amino acid mutagenesis in mammalian cells. Chem. Sci. 8, 7211–7217 10.1039/C7SC02560B [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beranek V., Willis J. C.W. and Chin J.W. (2019) An evolved Methanomethylophilus alvus pyrrolysyl-tRNA Synthetase/tRNA pair is highly active and orthogonal in mammalian cells. Biochemistry 58, 387–390 10.1021/acs.biochem.8b00808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niu W., Schultz P.G. and Guo J. (2013) An expanded genetic code in mammalian cells with a functional quadruplet codon. ACS Chem. Biol. 8, 1640–1645 10.1021/cb4001662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taki M., Matsushita J. and Sisido M. (2006) Expanding the genetic code in a mammalian cell line by the introduction of four-base codon/anticodon pairs. ChemBioChem 7, 425–428 10.1002/cbic.200500360 [DOI] [PubMed] [Google Scholar]
- 29.Neumann H., Wang K., Davis L., Garcia-Alai M. and Chin J.W. (2010) Encoding multiple unnatural amino acids via evolution of a quadruplet-decoding ribosome. Nature 464, 441–444 10.1038/nature08817 [DOI] [PubMed] [Google Scholar]
- 30.Wang K., Sachdeva A., Cox D.J., Wilf N.M., Lang K., Wallace S.. et al. (2014) Optimized orthogonal translation of unnatural amino acids enables spontaneous protein double-labelling and FRET. Nat. Chem. 6, 393–403 10.1038/nchem.1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmied W.H., Tnimov Z., Uttamapinant C., Rae C.D., Fried S.D. and Chin J.W. (2018) Controlling orthogonal ribosome subunit interactions enables evolution of new function. Nature 564, 444–448 10.1038/s41586-018-0773-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiao H. and Schultz P.G. (2016) At the interface of chemical and biological synthesis: an expanded genetic code. Cold Spring Harbor Perspect. Biol. 8, a023945. 10.1101/cshperspect.a023945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vargas-Rodriguez O., Sevostyanova A., Söll D. and Crnković A. (2018) Upgrading aminoacyl-tRNA synthetases for genetic code expansion. Curr. Opin. Chem. Biol. 46, 115–122 10.1016/j.cbpa.2018.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Italia J.S., Addy P.S., Wrobel C.J., Crawford L.A., Lajoie M.J., Zheng Y.. et al. (2017) An orthogonalized platform for genetic code expansion in both bacteria and eukaryotes. Nat. Chem. Biol. 13, 446–450 10.1038/nchembio.2312 [DOI] [PubMed] [Google Scholar]
- 35.Italia J.S., Latour C., Wrobel C.J.J. and Chatterjee A. (2018) Resurrecting the bacterial tyrosyl-tRNA Synthetase/tRNA pair for expanding the genetic code of both E. coli and eukaryotes. Cell Chem. Biol. 25, 1304–1312 10.1016/j.chembiol.2018.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chin J.W., Cropp T.A., Anderson J.C., Mukherji M., Zhang Z. and Schultz P.G. (2003) An expanded eukaryotic genetic code. Science 301, 964–967 10.1126/science.1084772 [DOI] [PubMed] [Google Scholar]
- 37.Liu W., Brock A., Chen S., Chen S. and Schultz P.G. (2007) Genetic incorporation of unnatural amino acids into proteins in mammalian cells. Nat. Methods 4, 239–244 10.1038/nmeth1016 [DOI] [PubMed] [Google Scholar]
- 38.Naganathan S., Grunbeck A., Tian H., Huber T. and Sakmar T.P. (2013) Genetically-encoded molecular probes to study G protein-coupled receptors. J. Vis. Exp. 10.3791/50588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nikić I., Kang J.H., Girona G.E., Aramburu I.V. and Lemke E.A. (2015) Labeling proteins on live mammalian cells using click chemistry. Nat. Protoc. 10, 780–791 10.1038/nprot.2015.045 [DOI] [PubMed] [Google Scholar]
- 40.Yang Y., Song H., He D., Zhang S., Dai S., Xie X.. et al. (2017) Genetically encoded releasable photo-cross-linking strategies for studying protein-protein interactions in living cells. Nat. Protoc. 12, 2147–2168 10.1038/nprot.2017.090 [DOI] [PubMed] [Google Scholar]
- 41.Young D.D., Young T.S., Jahnz M., Ahmad I., Spraggon G. and Schultz P.G. (2011) An evolved aminoacyl-tRNA synthetase with atypical polysubstrate specificity. Biochemistry 50, 1894–1900 10.1021/bi101929e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Willis J.C.W. and Chin J.W. (2018) Mutually orthogonal pyrrolysyl-tRNA synthetase/tRNA pairs. Nat. Chem. 10, 831–837 10.1038/s41557-018-0052-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang N., Li Y., Niu W., Sun M., Cerny R., Li Q.. et al. (2014) Construction of a live-attenuated HIV-1 vaccine through genetic code expansion. Angew. Chem. Int. Ed. 53, 4867–4871 10.1002/anie.201402092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mali P. and Katrekar D. (2017) In vivo RNA targeting of point mutations via suppressor tRNAs and adenosine deaminases. bioRxiv [Google Scholar]
- 45.Gossen M., Freundlieb S., Bender G., Muller G., Hillen W. and Bujard H. (1995) Transcriptional activation by tetracyclines in mammalian cells. Science 268, 1766–1769 10.1126/science.7792603 [DOI] [PubMed] [Google Scholar]
- 46.Sakamoto K., Hayashi A., Sakamoto A., Kiga D., Nakayama H., Soma A.. et al. (2002) Site-specific incorporation of an unnatural amino acid into proteins in mammalian cells. Nucleic Acids Res. 30, 4692–4699 10.1093/nar/gkf589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suzuki T., Asami M., Patel S.G., Luk L.Y.P., Tsai Y.-H. and Perry A.C.F. (2018) Switchable genome editing via genetic code expansion. Sci. Rep. 8, 10051. 10.1038/s41598-018-28178-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Si L., Xu H., Zhou X., Zhang Z., Tian Z., Wang Y.. et al. (2016) Generation of influenza A viruses as live but replication-incompetent virus vaccines. Science 354, 1170–1173 10.1126/science.aah5869 [DOI] [PubMed] [Google Scholar]
- 49.Yuan Z., Wang N., Kang G., Niu W., Li Q. and Guo J. (2017) Controlling multicycle replication of live-attenuated HIV-1 using an unnatural genetic switch. ACS Synth. Biol. 6, 721–731 10.1021/acssynbio.6b00373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Han S., Yang A., Lee S., Lee H.W., Park C.B. and Park H.S. (2017) Expanding the genetic code of Mus musculus. Nat. Commun. 8, 14568. 10.1038/ncomms14568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krogager T.P., Ernst R.J., Elliott T.S., Calo L., Beránek V., Ciabatti E.. et al. (2018) Labeling and identifying cell-specific proteomes in the mouse brain. Nat. Biotechnol. 36, 156–159 10.1038/nbt.4056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klán P., Šolomek T., Bochet C.G., Blanc A., Givens R., Rubina M.. et al. (2013) Photoremovable protecting groups in chemistry and biology: reaction mechanisms and efficacy. Chem. Rev. 113, 119–191 10.1021/cr300177k [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lemke E.A., Summerer D., Geierstanger B.H., Brittain S.M. and Schultz P.G. (2007) Control of protein phosphorylation with a genetically encoded photocaged amino acid. Nat. Chem. Biol. 3, 769–772 10.1038/nchembio.2007.44 [DOI] [PubMed] [Google Scholar]
- 54.The UniProt Consortium, 2019, UniProt: a worldwide hub of protein knowledge, Nucleic Acids Res., 47, D506–D515, 10.1093/nar/gky1049, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Klippenstein V., Ghisi V., Wietstruk M. and Plested A.J. (2014) Photoinactivation of glutamate receptors by genetically encoded unnatural amino acids. J. Neurosci. 34, 980–991 10.1523/JNEUROSCI.3725-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klippenstein V., Hoppmann C., Ye S., Wang L. and Paoletti P. (2017) Optocontrol of glutamate receptor activity by single side-chain photoisomerization. Elife 6, e25808. 10.7554/eLife.25808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li J., Yu J., Zhao J., Wang J., Zheng S., Lin S.. et al. (2014) Palladium-triggered deprotection chemistry for protein activation in living cells. Nat. Chem. 6, 352–361 10.1038/nchem.1887 [DOI] [PubMed] [Google Scholar]
- 58.Wäldchen S., Lehmann J., Klein T., van de Linde S. and Sauer M. (2015) Light-induced cell damage in live-cell super-resolution microscopy. Sci. Rep. 5, 15348. 10.1038/srep15348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li J., Jia S. and Chen P.R. (2014) Diels-Alder reaction-triggered bioorthogonal protein decaging in living cells. Nat. Chem. Biol. 10, 1003–1005 10.1038/nchembio.1656 [DOI] [PubMed] [Google Scholar]
- 60.Zhang G., Li J., Xie R., Fan X., Liu Y., Zheng S.. et al. (2016) Bioorthogonal chemical activation of kinases in living systems. ACS Cent. Sci. 2, 325–331 10.1021/acscentsci.6b00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fan X., Ge Y., Lin F., Yang Y., Zhang G., Ngai W.S.. et al. (2016) Optimized tetrazine derivatives for rapid bioorthogonal decaging in living cells. Angew. Chem. Int. Ed. 55, 14046–14050 10.1002/anie.201608009 [DOI] [PubMed] [Google Scholar]
- 62.Ge Y., Fan X. and Chen P.R. (2016) A genetically encoded multifunctional unnatural amino acid for versatile protein manipulations in living cells. Chem. Sci. 7, 7055–7060 10.1039/C6SC02615J [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Luo J., Liu Q., Morihiro K. and Deiters A. (2016) Small-molecule control of protein function through Staudinger reduction. Nat. Chem. 8, 1027–1034 10.1038/nchem.2573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsai Y.-H., Essig S., James J.R., Lang K. and Chin J.W. (2015) Selective, rapid and optically switchable regulation of protein function in live mammalian cells. Nat. Chem. 7, 554–561 10.1038/nchem.2253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lang K. and Chin J.W. (2014) Bioorthogonal reactions for labeling proteins. ACS Chem. Biol. 9, 16–20 10.1021/cb4009292 [DOI] [PubMed] [Google Scholar]
- 66.Liaunardy-Jopeace A., Murton B.L., Mahesh M., Chin J.W. and James J.R. (2017) Encoding optical control in LCK kinase to quantitatively investigate its activity in live cells. Nat. Struct. Mol. Biol. 24, 1155–1163 10.1038/nsmb.3492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Walker O.S., Elsässer S.J., Mahesh M., Bachman M., Balasubramanian S. and Chin J.W. (2016) Photoactivation of mutant isocitrate dehydrogenase 2 reveals rapid cancer-associated metabolic and epigenetic changes. J. Am. Chem. Soc. 138, 718–721 10.1021/jacs.5b07627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Simms J., Uddin R., Sakmar T.P., Gingell J.J., Garelja M.L., Hay D.L.. et al. (2018) Photoaffinity cross-linking and unnatural amino acid mutagenesis reveal insights into calcitonin gene-related peptide binding to the calcitonin receptor-like receptor/receptor activity-modifying protein 1 (CLR/RAMP1) complex. Biochemistry 57, 4915–4922 10.1021/acs.biochem.8b00502 [DOI] [PubMed] [Google Scholar]
- 69.Ernst R.J., Krogager T.P., Maywood E.S., Zanchi R., Beránek V., Elliott T.S.. et al. (2016) Genetic code expansion in the mouse brain. Nat. Chem. Biol. 12, 776–778 10.1038/nchembio.2160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen Y., Ma J., Lu W., Tian M., Thauvin M., Yuan C.. et al. (2017) Heritable expansion of the genetic code in mouse and zebrafish. Cell Res 27, 294–297 10.1038/cr.2016.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kang J.Y., Kawaguchi D., Coin I., Xiang Z., O’Leary D.D., Slesinger P.A.. et al. (2013) In vivo expression of a light-activatable potassium channel using unnatural amino acids. Neuron 80, 358–370 10.1016/j.neuron.2013.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ren W., Ji A. and Ai H.-W. (2015) Light activation of protein splicing with a photocaged fast intein. J. Am. Chem. Soc. 137, 2155–2158 10.1021/ja508597d [DOI] [PubMed] [Google Scholar]
- 73.Nguyen D.P., Mahesh M., Elsässer S.J., Hancock S.M., Uttamapinant C. and Chin J.W. (2014) Genetic encoding of photocaged cysteine allows photoactivation of TEV protease in live mammalian cells. J. Am. Chem. Soc. 136, 2240–2243 10.1021/ja412191m [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gramespacher J.A., Stevens A.J., Nguyen D.P., Chin J.W. and Muir T.W. (2017) Intein zymogens: conditional assembly and splicing of split inteins via targeted proteolysis. J. Am. Chem. Soc. 139, 8074–8077 10.1021/jacs.7b02618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Uprety R., Luo J., Liu J., Naro Y., Samanta S. and Deiters A. (2014) Genetic encoding of caged cysteine and caged homocysteine in bacterial and mammalian cells. ChemBioChem 15, 1793–1799 10.1002/cbic.201400073 [DOI] [PubMed] [Google Scholar]
- 76.Liu J., Zheng F., Cheng R., Li S., Rozovsky S., Wang Q.. et al. (2018) Site-specific incorporation of selenocysteine using an expanded genetic code and palladium-mediated chemical deprotection. J. Am. Chem. Soc. 140, 8807–8816 10.1021/jacs.8b04603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chatterjee A., Xiao H., Bollong M., Ai H.-W. and Schultz P.G. (2013) Efficient viral delivery system for unnatural amino acid mutagenesis in mammalian cells. Proc. Natl. Acad. Sci. U.S.A. 110, 11803–11808 10.1073/pnas.1309584110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Takimoto J.K., Adams K.L., Xiang Z. and Wang L. (2009) Improving orthogonal tRNA-synthetase recognition for efficient unnatural amino acid incorporation and application in mammalian cells. Mol. BioSyst. 5, 931–934 10.1039/b904228h [DOI] [PubMed] [Google Scholar]
- 79.Smits A.H., Borrmann A., Roosjen M., van Hest J.C. and Vermeulen M. (2016) Click-MS: tagless protein enrichment using bioorthogonal chemistry for quantitative proteomics. ACS Chem. Biol. 11, 3245–3250 10.1021/acschembio.6b00520 [DOI] [PubMed] [Google Scholar]
- 80.Ye S., Huber T., Vogel R. and Sakmar T.P. (2009) FTIR analysis of GPCR activation using azido probes. Nat. Chem. Biol. 5, 397–399 10.1038/nchembio.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Coin I., Perrin M.H., Vale W.W. and Wang L. (2011) Photo-cross-linkers incorporated into G-protein-coupled receptors in mammalian cells: a ligand comparison. Angew. Chem. Int. Ed. 50, 8077–8081 10.1002/anie.201102646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen S., Chen Z.-j., Ren W. and Ai H.-W. (2012) Reaction-based genetically encoded fluorescent hydrogen sulfide sensors. J. Am. Chem. Soc. 134, 9589–9592 10.1021/ja303261d [DOI] [PubMed] [Google Scholar]
- 83.Naganathan S., Ye S., Sakmar T.P. and Huber T. (2013) Site-specific epitope tagging of G protein-coupled receptors by bioorthogonal modification of a genetically encoded unnatural amino acid. Biochemistry 52, 1028–1036 10.1021/bi301292h [DOI] [PubMed] [Google Scholar]
- 84.Coin I., Katritch V., Sun T., Xiang Z., Siu F.Y., Beyermann M.. et al. (2013) Genetically encoded chemical probes in cells reveal the binding path of urocortin-I to CRF class B GPCR. Cell 155, 1258–1269 10.1016/j.cell.2013.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang W., Li T., Felsovalyi K., Chen C., Cardozo T. and Krogsgaard M. (2014) Quantitative analysis of T cell receptor complex interaction sites using genetically encoded photo-cross-linkers. ACS Chem. Biol. 9, 2165–2172 10.1021/cb500351s [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen Z.J. and Ai H.-W. (2014) A highly responsive and selective fluorescent probe for imaging physiological hydrogen sulfide. Biochemistry 53, 5966–5974 10.1021/bi500830d [DOI] [PubMed] [Google Scholar]
- 87.Tian H., Naganathan S., Kazmi M.A., Schwartz T.W., Sakmar T.P. and Huber T. (2014) Bioorthogonal fluorescent labeling of functional G-protein-coupled receptors. ChemBioChem 15, 1820–1829 10.1002/cbic.201402193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Naganathan S., Ray-Saha S., Park M., Tian H., Sakmar T.P. and Huber T. (2015) Multiplex detection of functional G protein-coupled receptors harboring site-specifically modified unnatural amino acids. Biochemistry 54, 776–786 10.1021/bi501267x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tian H., Sakmar T.P. and Huber T. (2015) Micelle-enhanced bioorthogonal labeling of genetically encoded azido groups on the lipid-embedded surface of a GPCR. ChemBioChem 16, 1314–1322 10.1002/cbic.201500030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zheng Y., Lewis T.L. Jr, Igo P., Polleux F. and Chatterjee A. (2017) Virus-enabled optimization and delivery of the genetic machinery for efficient unnatural amino acid mutagenesis in mammalian cells and tissues. ACS Synth. Biol. 6, 13–18 10.1021/acssynbio.6b00092 [DOI] [PubMed] [Google Scholar]
- 91.Koole C., Reynolds C.A., Mobarec J.C., Hick C., Sexton P.M. and Sakmar T.P. (2017) Genetically encoded photocross-linkers determine the biological binding site of exendin-4 peptide in the N-terminal domain of the intact human glucagon-like peptide-1 receptor (GLP-1R). J. Biol. Chem. 292, 7131–7144 10.1074/jbc.M117.779496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Grunbeck A., Huber T., Sachdev P. and Sakmar T.P. (2011) Mapping the ligand-binding site on a G protein-coupled receptor (GPCR) using genetically encoded photocrosslinkers. Biochemistry 50, 3411–3413 10.1021/bi200214r [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ray-Saha S., Huber T. and Sakmar T.P. (2014) Antibody epitopes on g protein-coupled receptors mapped with genetically encoded photoactivatable cross-linkers. Biochemistry 53, 1302–1310 10.1021/bi401289p [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Grunbeck A., Huber T., Abrol R., Trzaskowski B., Goddard W.A. III and Sakmar T.P. (2012) Genetically encoded photo-cross-linkers map the binding site of an allosteric drug on a G protein-coupled receptor. ACS Chem. Biol. 7, 967–972 10.1021/cb300059z [DOI] [PubMed] [Google Scholar]
- 95.Rannversson H., Andersen J., Sorensen L., Bang-Andersen B., Park M., Huber T.. et al. (2016) Genetically encoded photocrosslinkers locate the high-affinity binding site of antidepressant drugs in the human serotonin transporter. Nat. Commun. 7, 11261. 10.1038/ncomms11261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Park M., Sivertsen B.B., Els-Heindl S., Huber T., Holst B., Beck-Sickinger A.G.. et al. (2015) Bioorthogonal labeling of ghrelin receptor to facilitate studies of ligand-dependent conformational dynamics. Chem. Biol. 22, 1431–1436 10.1016/j.chembiol.2015.09.014 [DOI] [PubMed] [Google Scholar]
- 97.Chen Z.-j., Ren W., Wright Q.E. and Ai H.-W. (2013) Genetically encoded fluorescent probe for the selective detection of peroxynitrite. J. Am. Chem. Soc 135, 14940–14943 10.1021/ja408011q [DOI] [PubMed] [Google Scholar]
- 98.Tharp J.M., Wang Y.S., Lee Y.J., Yang Y. and Liu W.R. (2014) Genetic incorporation of seven ortho-substituted phenylalanine derivatives. ACS Chem. Biol. 9, 884–890 10.1021/cb400917a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ye S., Köhrer C., Huber T., Kazmi M., Sachdev P., Yan E.C.. et al. (2008) Site-specific incorporation of keto amino acids into functional G protein-coupled receptors using unnatural amino acid mutagenesis. J. Biol. Chem. 283, 1525–1533 10.1074/jbc.M707355200 [DOI] [PubMed] [Google Scholar]
- 100.Axup J.Y., Bajjuri K.M., Ritland M., Hutchins B.M., Kim C.H., Kazane S.A.. et al. (2012) Synthesis of site-specific antibody-drug conjugates using unnatural amino acids. Proc. Natl. Acad. Sci. U.S.A. 109, 16101–16106 10.1073/pnas.1211023109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xiang Z., Ren H., Hu Y.S., Coin I., Wei J., Cang H.. et al. (2013) Adding an unnatural covalent bond to proteins through proximity-enhanced bioreactivity. Nat. Methods 10, 885–888 10.1038/nmeth.2595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hino N., Okazaki Y., Kobayashi T., Hayashi A., Sakamoto K. and Yokoyama S. (2005) Protein photo-cross-linking in mammalian cells by site-specific incorporation of a photoreactive amino acid. Nat. Methods 2, 201–206 10.1038/nmeth739 [DOI] [PubMed] [Google Scholar]
- 103.Shen B., Xiang Z., Miller B., Louie G., Wang W., Noel J.P.. et al. (2011) Genetically encoding unnatural amino acids in neural stem cells and optically reporting voltage-sensitive domain changes in differentiated neurons. Stem Cells 29, 1231–1240 10.1002/stem.679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Murray C.I., Westhoff M., Eldstrom J., Thompson E., Emes R. and Fedida D. (2016) Unnatural amino acid photo-crosslinking of the IKs channel complex demonstrates a KCNE1:KCNQ1 stoichiometry of up to 4:4. Elife 5, e11815. 10.7554/eLife.11815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Valentin-Hansen L., Park M., Huber T., Grunbeck A., Naganathan S., Schwartz T.W.. et al. (2014) Mapping substance P binding sites on the neurokinin-1 receptor using genetic incorporation of a photoreactive amino acid. J. Biol. Chem. 289, 18045–18054 10.1074/jbc.M113.527085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lacey V.K., Louie G.V., Noel J.P. and Wang L. (2013) Expanding the library and substrate diversity of the pyrrolysyl-tRNA synthetase to incorporate unnatural amino acids containing conjugated rings. ChemBioChem 14, 2100–2105 10.1002/cbic.201300400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chatterjee A., Guo J., Lee H.S. and Schultz P.G. (2013) A genetically encoded fluorescent probe in mammalian cells. J. Am. Chem. Soc. 135, 12540–12543 10.1021/ja4059553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mitchell A.L., Addy P.S., Chin M.A. and Chatterjee A. (2017) A unique genetically encoded FRET pair in mammalian cells. ChemBioChem 18, 511–514 10.1002/cbic.201600668 [DOI] [PubMed] [Google Scholar]
- 109.Park S.-H., Ko W., Lee H.S. and Shin I. (2019) Analysis of protein–protein interaction in a single live cell by using a FRET system based on genetic code expansion technology. J. Am. Chem. Soc. 141, 4273–4281 10.1021/jacs.8b10098, [DOI] [PubMed] [Google Scholar]
- 110.Fu C., Kobayashi T., Wang N., Hoppmann C., Yang B., Irannejad R.. et al. (2018) Genetically encoding quinoline reverses chromophore charge and enables fluorescent protein brightening in acidic vesicles. J. Am. Chem. Soc. 140, 11058–11066 10.1021/jacs.8b05814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Luo J., Samanta S., Convertino M., Dokholyan N.V. and Deiters A. (2018) Reversible and tunable photoswitching of protein function through genetic encoding of azobenzene amino acids in mammalian cells. ChemBioChem 19, 2178–2185 10.1002/cbic.201800226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hoppmann C., Lacey V.K., Louie G.V., Wei J., Noel J.P. and Wang L. (2014) Genetically encoding photoswitchable click amino acids in Escherichia coli and mammalian cells. Angew. Chem. Int. Ed. 53, 3932–3936 10.1002/anie.201400001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xiao H., Peters F.B., Yang P.Y., Reed S., Chittuluru J.R. and Schultz P.G. (2014) Genetic incorporation of histidine derivatives using an engineered pyrrolysyl-tRNA synthetase. ACS Chem. Biol. 9, 1092–1096 10.1021/cb500032c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang T., Zhou Q., Li F., Yu Y., Yin X. and Wang J. (2015) Genetic incorporation of N(epsilon)-Formyllysine, a new histone post-translational modification. ChemBioChem 16, 1440–1442 10.1002/cbic.201500170 [DOI] [PubMed] [Google Scholar]
- 115.Elsässer S.J., Ernst R.J., Walker O.S. and Chin J.W. (2016) Genetic code expansion in stable cell lines enables encoded chromatin modification. Nat. Methods 13, 158–164 10.1038/nmeth.3701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xuan W., Yao A. and Schultz P.G. (2017) Genetically encoded fluorescent probe for detecting sirtuins in living cells. J. Am. Chem. Soc. 139, 12350–12353 10.1021/jacs.7b05725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cohen S. and Arbely E. (2016) Single-plasmid-based system for efficient noncanonical amino acid mutagenesis in cultured mammalian cells. ChemBioChem 17, 1008–1011 10.1002/cbic.201500681 [DOI] [PubMed] [Google Scholar]
- 118.Mukai T., Kobayashi T., Hino N., Yanagisawa T., Sakamoto K. and Yokoyama S. (2008) Adding l-lysine derivatives to the genetic code of mammalian cells with engineered pyrrolysyl-tRNA synthetases. Biochem. Biophys. Res. Commun. 371, 818–822 10.1016/j.bbrc.2008.04.164 [DOI] [PubMed] [Google Scholar]
- 119.Li F., Zhang H., Sun Y., Pan Y., Zhou J. and Wang J. (2013) Expanding the genetic code for photoclick chemistry in E. coli, mammalian cells, and A. thaliana. Angew. Chem. Int. Ed. 52, 9700–9704 10.1002/anie.201303477 [DOI] [PubMed] [Google Scholar]
- 120.Kim C.H., Kang M., Kim H.J., Chatterjee A. and Schultz P.G. (2012) Site-specific incorporation of epsilon-N-crotonyllysine into histones. Angew. Chem. Int. Ed. 51, 7246–7249 10.1002/anie.201203349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cigler M., Müller T.G., Horn-Ghetko D., von Wrisberg M.K., Fottner M., Goody R.S.. et al. (2017) Proximity-triggered covalent stabilization of low-affinity protein complexes in vitro and in vivo. Angew. Chem. Int. Ed. 56, 15737–15741 10.1002/anie.201706927 [DOI] [PubMed] [Google Scholar]
- 122.Tian Y., Jacinto M.P., Zeng Y., Yu Z., Qu J., Liu W.R.. et al. (2017) Genetically encoded 2-aryl-5-carboxytetrazoles for site-selective protein photo-cross-linking. J. Am. Chem. Soc. 139, 6078–6081 10.1021/jacs.7b02615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Peng T. and Hang H.C. (2016) Site-specific bioorthogonal labeling for fluorescence imaging of intracellular proteins in living cells. J. Am. Chem. Soc. 138, 14423–14433 10.1021/jacs.6b08733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ramil C.P., Dong M., An P., Lewandowski T.M., Yu Z., Miller L.J.. et al. (2017) Spirohexene-tetrazine ligation enables bioorthogonal labeling of class B G protein-coupled receptors in live cells. J. Am. Chem. Soc. 139, 13376–13386 10.1021/jacs.7b05674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gautier A., Nguyen D.P., Lusic H., An W., Deiters A. and Chin J.W. (2010) Genetically encoded photocontrol of protein localization in mammalian cells. J. Am. Chem. Soc. 132, 4086–4088 10.1021/ja910688s [DOI] [PubMed] [Google Scholar]
- 126.Arbely E., Torres-Kolbus J., Deiters A. and Chin J.W. (2012) Photocontrol of tyrosine phosphorylation in mammalian cells via genetic encoding of photocaged tyrosine. J. Am. Chem. Soc. 134, 11912–11915 10.1021/ja3046958 [DOI] [PubMed] [Google Scholar]
- 127.Lang K., Davis L., Torres-Kolbus J., Chou C., Deiters A. and Chin J.W. (2012) Genetically encoded norbornene directs site-specific cellular protein labelling via a rapid bioorthogonal reaction. Nat. Chem. 4, 298–304 10.1038/nchem.1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Aloush N., Schvartz T., König A.I., Cohen S., Brozgol E., Tam B.. et al. (2018) Live cell imaging of bioorthogonally labelled proteins generated with a single pyrrolysine tRNA gene. Sci. Rep. 8, 14527. 10.1038/s41598-018-32824-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Schneider N., Gabelein C., Wiener J., Georgiev T., Gobet N., Weber W.. et al. (2018) Genetic code expansion method for temporal labeling of endogenously expressed proteins. ACS Chem. Biol. 13, 3049–3053 10.1021/acschembio.8b00594 [DOI] [PubMed] [Google Scholar]
- 130.Li N., Ramil C.P., Lim R.K. and Lin Q. (2015) A genetically encoded alkyne directs palladium-mediated protein labeling on live mammalian cell surface. ACS Chem. Biol. 10, 379–384 10.1021/cb500649q [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yang Y., Lin S., Lin W. and Chen P.R. (2014) Ligand-assisted dual-site click labeling of EGFR on living cells. ChemBioChem 15, 1738–1743 10.1002/cbic.201400057 [DOI] [PubMed] [Google Scholar]
- 132.Zheng Y., Yu F., Wu Y., Si L., Xu H., Zhang C.. et al. (2015) Broadening the versatility of lentiviral vectors as a tool in nucleic acid research via genetic code expansion. Nucleic Acids Res. 43, e73. 10.1093/nar/gkv202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang C., Yao T., Zheng Y., Li Z., Zhang Q., Zhang L.. et al. (2016) Development of next generation adeno-associated viral vectors capable of selective tropism and efficient gene delivery. Biomaterials 80, 134–145 10.1016/j.biomaterials.2015.11.066 [DOI] [PubMed] [Google Scholar]
- 134.Zhang Z., Xu H., Si L., Chen Y., Zhang B., Wang Y.. et al. (2017) Construction of an inducible stable cell line for efficient incorporation of unnatural amino acids in mammalian cells. Biochem. Biophys. Res. Commun. 489, 490–496 10.1016/j.bbrc.2017.05.178 [DOI] [PubMed] [Google Scholar]
- 135.Yao T., Zhou X., Zhang C., Yu X., Tian Z., Zhang L.. et al. (2017) Site-specific PEGylated adeno-associated viruses with increased serum stability and reduced immunogenicity. Molecules 22, 1155. 10.3390/molecules22071155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Katrekar D., Moreno A.M., Chen G., Worlikar A. and Mali P. (2018) Oligonucleotide conjugated multi-functional adeno-associated viruses. Sci. Rep. 8, 3589. 10.1038/s41598-018-21742-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kelemen R.E., Mukherjee R., Cao X., Erickson S.B., Zheng Y. and Chatterjee A. (2016) A precise chemical strategy to alter the receptor specificity of the adeno-associated virus. Angew. Chem. Int. Ed. 55, 10645–10649 10.1002/anie.201604067 [DOI] [PubMed] [Google Scholar]
- 138.Zhang J., Yan S., He Z., Ding C., Zhai T., Chen Y.. et al. (2018) Small unnatural amino acid carried raman tag for molecular imaging of genetically targeted proteins. J. Phys. Chem. Lett. 9, 4679–4685 10.1021/acs.jpclett.8b01991 [DOI] [PubMed] [Google Scholar]
- 139.Lühmann T., Jones G., Gutmann M., Rybak J.-C., Nickel J., Rubini M.. et al. (2015) Bio-orthogonal immobilization of fibroblast growth factor 2 for spatial controlled cell proliferation. ACS Biomater. Sci. Eng. 1, 740–746 10.1021/acsbiomaterials.5b00236 [DOI] [PubMed] [Google Scholar]
- 140.Lin S., Yan H., Li L., Yang M., Peng B., Chen S.. et al. (2013) Site-specific engineering of chemical functionalities on the surface of live hepatitis D virus. Angew. Chem. Int. Ed. 52, 13970–13974 10.1002/anie.201305787 [DOI] [PubMed] [Google Scholar]
- 141.Chen P.R., Groff D., Guo J., Ou W., Cellitti S., Geierstanger B.H.. et al. (2009) A facile system for encoding unnatural amino acids in mammalian cells. Angew. Chem. Int. Ed. 48, 4052–4055 10.1002/anie.200900683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yanagisawa T., Hino N., Iraha F., Mukai T., Sakamoto K. and Yokoyama S. (2012) Wide-range protein photo-crosslinking achieved by a genetically encoded N(epsilon)-(benzyloxycarbonyl)lysine derivative with a diazirinyl moiety. Mol. Biosyst. 8, 1131–1135 10.1039/c2mb05321g [DOI] [PubMed] [Google Scholar]
- 143.Kita A., Hino N., Higashi S., Hirota K., Narumi R., Adachi J.. et al. (2016) Adenovirus vector-based incorporation of a photo-cross-linkable amino acid into proteins in human primary cells and cancerous cell lines. Sci. Rep. 6, 36946. 10.1038/srep36946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhao J., Lin S., Huang Y., Zhao J. and Chen P.R. (2013) Mechanism-based design of a photoactivatable firefly luciferase. J. Am. Chem. Soc. 135, 7410–7413 10.1021/ja4013535 [DOI] [PubMed] [Google Scholar]
- 145.Ren W., Ji A., Wang M.X. and Ai H.-W. (2015) Expanding the Genetic Code for a Dinitrophenyl Hapten. ChemBioChem 16, 2007–2010 10.1002/cbic.201500204 [DOI] [PubMed] [Google Scholar]
- 146.Groff D., Chen P.R., Peters F.B. and Schultz P.G. (2010) A genetically encoded epsilon-N-methyl lysine in mammalian cells. ChemBioChem 11, 1066–1068 10.1002/cbic.200900690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Erickson S.B., Mukherjee R., Kelemen R.E., Wrobel C.J.J., Cao X. and Chatterjee A. (2017) Precise photoremovable perturbation of a virus–host interaction. Angew. Chem. Int. Ed. 56, 4234–4237 10.1002/anie.201700683 [DOI] [PubMed] [Google Scholar]
- 148.Luo J., Arbely E., Zhang J., Chou C., Uprety R., Chin J.W.. et al. (2016) Genetically encoded optical activation of DNA recombination in human cells. Chem. Commun. 52, 8529–8532 10.1039/C6CC03934K [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Engelke H., Chou C., Uprety R., Jess P. and Deiters A. (2014) Control of protein function through optochemical translocation. ACS Synth. Biol. 3, 731–736 10.1021/sb400192a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hemphill J., Borchardt E.K., Brown K., Asokan A. and Deiters A. (2015) Optical control of CRISPR/Cas9 gene editing. J. Am. Chem. Soc. 137, 5642–5645 10.1021/ja512664v [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gautier A., Deiters A. and Chin J.W. (2011) Light-activated kinases enable temporal dissection of signaling networks in living cells. J. Am. Chem. Soc. 133, 2124–2127 10.1021/ja1109979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hemphill J., Chou C., Chin J.W. and Deiters A. (2013) Genetically encoded light-activated transcription for spatiotemporal control of gene expression and gene silencing in mammalian cells. J. Am. Chem. Soc. 135, 13433–13439 10.1021/ja4051026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Luo J., Uprety R., Naro Y., Chou C., Nguyen D.P., Chin J.W.. et al. (2014) Genetically encoded optochemical probes for simultaneous fluorescence reporting and light activation of protein function with two-photon excitation. J. Am. Chem. Soc. 136, 15551–15558 10.1021/ja5055862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yu Z., Pan Y., Wang Z., Wang J. and Lin Q. (2012) Genetically encoded cyclopropene directs rapid, photoclick-chemistry-mediated protein labeling in mammalian cells. Angew. Chem. Int. Ed. 51, 10600–10604 10.1002/anie.201205352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Meineke B., Heimgartner J., Lafranchi L. and Elsässer S.J. (2018) Methanomethylophilus alvus Mx1201 provides basis for mutual orthogonal pyrrolysyl tRNA/Aminoacyl-tRNA synthetase pairs in mammalian cells. ACS Chem. Biol. 13, 3087–3096 10.1021/acschembio.8b00571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Elliott T.S., Townsley F.M., Bianco A., Ernst R.J., Sachdeva A., Elsässer S.J.. et al. (2014) Proteome labeling and protein identification in specific tissues and at specific developmental stages in an animal. Nat. Biotechnol. 32, 465–472 10.1038/nbt.2860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ai H.-W., Shen W., Sagi A., Chen P.R. and Schultz P.G. (2011) Probing protein-protein interactions with a genetically encoded photo-crosslinking amino acid. ChemBioChem 12, 1854–1857 10.1002/cbic.201100194 [DOI] [PubMed] [Google Scholar]
- 158.Chou C., Uprety R., Davis L., Chin J.W. and Deiters A. (2011) Genetically encoding an aliphatic diazirine for protein photocrosslinking. Chem. Sci. 2, 480–483 10.1039/C0SC00373E [DOI] [Google Scholar]
- 159.Hoffmann J.E., Dziuba D., Stein F. and Schultz C. (2018) A bifunctional noncanonical amino acid: synthesis, expression, and residue-specific proteome-wide incorporation. Biochemistry 57, 4747–4752 10.1021/acs.biochem.8b00397 [DOI] [PubMed] [Google Scholar]
- 160.Zhang C., Lu J., Su H., Yang J. and Zhou D. (2017) Fatty acid synthase cooperates with protrudin to facilitate membrane outgrowth of cellular protrusions. Sci. Rep. 7, 46569. 10.1038/srep46569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nikić I., Plass T., Schraidt O., Szymański J., Briggs J.A., Schultz C.. et al. (2014) Minimal tags for rapid dual-color live-cell labeling and super-resolution microscopy. Angew. Chem. Int. Ed. 53, 2245–2249 10.1002/anie.201309847 [DOI] [PubMed] [Google Scholar]
- 162.Nikić I., Estrada Girona G., Kang J.H., Paci G., Mikhaleva S., Koehler C., Shymanska N.V.. et al. (2016) Debugging eukaryotic genetic code expansion for site-specific Click-PAINT super-resolution microscopy. Angew. Chem. Int. Ed. 55, 16172–16176 10.1002/anie.201608284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rutkowska A., Plass T., Hoffmann J.E., Yushchenko D.A., Feng S. and Schultz C. (2014) T-CrAsH: a heterologous chemical crosslinker. ChemBioChem 15, 1765–1768 10.1002/cbic.201402189 [DOI] [PubMed] [Google Scholar]
- 164.Hoffmann J.E., Plass T., Nikić I., Aramburu I.V., Koehler C., Gillandt H.. et al. (2015) Highly stable trans-cyclooctene amino acids for live-cell labeling. Chem. Eur. J. 21, 12266–12270 10.1002/chem.201501647 [DOI] [PubMed] [Google Scholar]
- 165.Kozma E., Nikić I., Varga B.R., Aramburu I.V., Kang J.H., Fackler O.T.. et al. (2016) Hydrophilic trans-cyclooctenylated noncanonical amino acids for fast intracellular protein labeling. ChemBioChem 17, 1518–1524 10.1002/cbic.201600284 [DOI] [PubMed] [Google Scholar]
- 166.Sakin V., Hanne J., Dunder J., Anders-Össwein M., Laketa V., Nikic I.. et al. (2017) A versatile tool for live-cell imaging and super-resolution nanoscopy studies of hiv-1 env distribution and mobility. Cell Chem. Biol. 24, 635–645 10.1016/j.chembiol.2017.04.007 [DOI] [PubMed] [Google Scholar]
- 167.Lang K., Davis L., Wallace S., Mahesh M., Cox D.J., Blackman M.L.. et al. (2012) Genetic encoding of bicyclononynes and trans-cyclooctenes for site-specific protein labeling in vitro and in live mammalian cells via rapid fluorogenic Diels-Alder reactions. J. Am. Chem. Soc. 134, 10317–10320 10.1021/ja302832g [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Uttamapinant C., Howe J.D., Lang K., Beránek V., Davis L., Mahesh M.. et al. (2015) Genetic code expansion enables live-cell and super-resolution imaging of site-specifically labeled cellular proteins. J. Am. Chem. Soc. 137, 4602–4605 10.1021/ja512838z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Plass T., Milles S., Koehler C., Szymański J., Mueller R., Wießler M.. et al. (2012) Amino acids for Diels-Alder reactions in living cells. Angew. Chem. Int. Ed. 51, 4166–4170 10.1002/anie.201108231 [DOI] [PubMed] [Google Scholar]
- 170.Xue L., Prifti E. and Johnsson K. (2016) A general strategy for the semisynthesis of ratiometric fluorescent sensor proteins with increased dynamic range. J. Am. Chem. Soc. 138, 5258–5261 10.1021/jacs.6b03034 [DOI] [PubMed] [Google Scholar]
- 171.Borrmann A., Milles S., Plass T., Dommerholt J., Verkade J.M., Wiessler M.. et al. (2012) Genetic encoding of a bicyclo[6.1.0]nonyne-charged amino acid enables fast cellular protein imaging by metal-free ligation. ChemBioChem 13, 2094–2099 10.1002/cbic.201200407 [DOI] [PubMed] [Google Scholar]
- 172.Schvartz T., Aloush N., Goliand I., Segal I., Nachmias D., Arbely E.. et al. (2017) Direct fluorescent-dye labeling of alpha-tubulin in mammalian cells for live cell and superresolution imaging. Mol. Biol. Cell 28, 2747–2756 10.1091/mbc.e17-03-0161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Luo J., Torres-Kolbus J., Liu J. and Deiters A. (2017) Genetic encoding of photocaged tyrosines with improved light-activation properties for the optical control of protease function. ChemBioChem 18, 1442–1447 10.1002/cbic.201700147 [DOI] [PubMed] [Google Scholar]
- 174.Wang N., Yang B., Fu C., Zhu H., Zheng F., Kobayashi T.. et al. (2018) Genetically encoding fluorosulfate-l-tyrosine to react with lysine, histidine, and tyrosine via SuFEx in proteins in vivo. J. Am. Chem. Soc. 140, 4995–4999 10.1021/jacs.8b01087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Takimoto J.K., Xiang Z., Kang J.Y. and Wang L. (2010) Esterification of an unnatural amino acid structurally deviating from canonical amino acids promotes its uptake and incorporation into proteins in mammalian cells. ChemBioChem 11, 2268–2272 10.1002/cbic.201000436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Beránek V., Reinkemeier C.D., Zhang M.S., Liang A.D., Kym G. and Chin J.W. (2018) Genetically encoded protein phosphorylation in mammalian cells. Cell Chem. Biol. 25, 1067–1074 10.1016/j.chembiol.2018.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]