Abstract
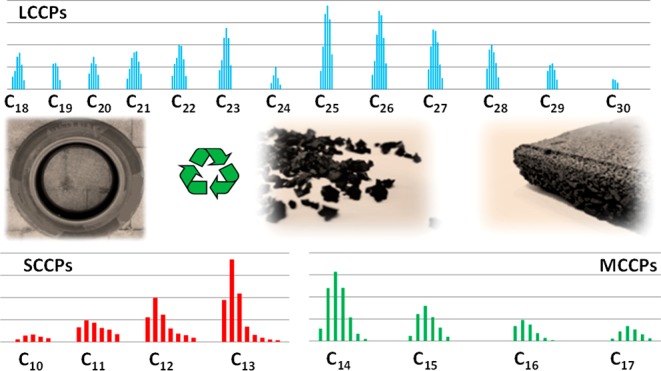
Chlorinated paraffins (CPs) are used in various products to improve their physicochemical characteristics. Due to recycling, CPs may end up in “new” recycled products. In this study we investigated CPs present in end-of-life car tires that are recycled to rubber granulates used on artificial soccer fields, and playground tiles. The ∑CP(C10-C30) concentrations ranged from 1.5 to 67 μg/g in car tires, 13–67 μg/g in rubber granulates, and 16–74 μg/g in playground tiles. MCCPs were the dominant CP group with an average contribution of 72%. LCCPs up to C30, were detected for the first time in car tires, rubber granulates, and playground tiles. The CPs application in tires is unclear, the low CP concentrations found in this study (<0.007%) could possibly indicate contamination during the manufacturing process. The presence of CPs in the granulates and tiles, in addition to the multiple chemicals already detected, emphasizes the need to further investigate the migration and leaching behavior, in order to assess potential risks of CPs for humans and the environment. The presence of CPs in car tires may be another source of CPs for the environment. The CP volume brought into the environment by tire wear particles (TWP) from car tires in the European Union, is estimated at 2.0–89 tons annually.
Introduction
Recycling polymers addresses the need for resource efficiency in a finite world.1 At the same time it raises questions about toxic residues being introduced into recycled products.2 Chemical additives, present in specific products, which are recycled after years of use, are not always considered in the new recycled or reused products. For example, in Europe, approximately half of the recycled end-of-life car tires are used on artificial soccer fields, sport flooring, or molded into rubber playground tiles.3 While children are not directly exposed to car tires, they will be exposed to the recycled products like rubber granulates and playground tiles. Exposure to the additives and residues in end-of-life car tires can then suddenly become a risk. In The Netherlands, a discussion has started regarding the exposure of soccer players to carcinogenic residues from recycled tire rubber granulates when playing on artificial-turf soccer fields.4 In total 120 tons of rubber granulate is used on one artificial-turf soccer field, and every year, another 300 kg is added to maintain the field.4 The recycling of rubber tires reduces the overall burden of rubber waste on landfills but by using these rubber granulates on artificial-turf soccer fields they are transferred and reintroduced to a new environment. Various studies revealed that rubber granulates contain carcinogenic polycyclic aromatic hydrocarbons (PAHs) in concentrations up to 20 mg/kg.4−6 In addition to PAHs, multiple chemical additives have been reported to be present in rubber granulates such as benzothiazoles, phthalates, metals, and bisphenol A.4−6 A recent literature review by Perkins et al.,7 identified 306 chemical constituents of rubber granulate used as infill material in synthetic turf athletic fields. No information was provided on the prevalence of chlorinated paraffins (CPs). In this study we will focus on the presence of CPs in end-of-life care tires, and the recycled products rubber granulates and playground tiles. CPs are complex mixture of chlorinated alkanes and are globally used in high volumes in high temperature lubricants, metal cutting fluids, sealants, and as a flame retardant and plasticizer.8 Based on their carbon chain length, the CPs are divided in three subgroups: short-chain CPs (SCCPs, ≤C13), medium-chain CPs (MCCPs, C13–C17), and long-chain CPs (LCCPs, ≥C18). Due to their worldwide presence in the environment and their persistency and bioaccumulative properties and toxicity to aquatic organisms, SCCPs are under restricted by European Union (EU), and the U.S. Environmental Protection Agency (EPA) proposed significant new use rule for certain SCCPs.9 In 2017, SCCPs were designated as persistent organic pollutants (POPs) and listed in annex A of the United Nations Stockholm Convention.10 These regulations have led to a shift in use and production to higher chlorinated paraffins.11 Beside the SCCPs, MCCPs are ubiquitously detected in different environmental compartments.8−12 Limited environmental data is available on LCCPs. Based on the relatively limited toxicity data available for LCCPs, they seem to be less toxic to organisms compared to SCCPs and MCCPs.13 LCCPs have recently been detected in human blood (up to 530 ng/g lipid weight)14 from China and in wildlife samples from China and Sweden with levels up to 10 μg/g lipid weight, whereby in one of the biota samples from Sweden LCCPs were dominant.15,16 These findings may highlight the importance to include LCCPs in measurement programs for CPs.
Data on the presence of CPs in car tires, rubber granulates, and recycled playground tiles are lacking. Wang et al.17 were the first to report the occurrence of CPs in 15 car tires purchased in China with average SCCP and MCCP concentrations of 106 and 442 μg/g, respectively. Cao et al.18 analyzed dust collected from synthetic turf sport courts and reported geometric mean concentrations for SCCPs and MCCPs of 101 and 241 μg/g, respectively. In both studies LCCPs were not analyzed. The presence of CPs in car tires, recycled rubber granulates, and playground tiles may imply a risk for potential human and environmental exposure. Therefore, we investigated the presence of SCCPs, MCCPs, and LCCPs in end of life car tires, recycled rubber granulates and playground tiles, to assess CP concentrations and congener patterns. Furthermore, we will address the challenges and importance of quantifying the wax-grade LCCPs (average carbon chain length of approximately C25).
Materials and Methods
Information about the standards, chemicals and suppliers is provided in the Supporting Information (SI) of this manuscript.
Sample Collection
In total 25 samples were included in this study. Ten end of life car tires were collected from a garage in Postbauer-Heng (Germany), which consist of five different brands, manufactured at 10 different countries between 2006 and 2014(see SI Table S1).Rubber granulates were collected from nine artificial-turf soccer fields in the area of Amsterdam, The Netherlands. Approximately 100 g of rubber granulates were randomly collected from three different spots on each soccer field. Six new recycled playground tiles, five purchased in The Netherlands and one from Spain were analyzed. For playground tiles and car tires a sample of 6 × 6 cm was taken and homogenized into small particles (<3 mm). Samples were collected in prerinsed (hexane and acetone) glass flasks and stored in the dark at room temperature prior analysis.
Sample Pretreatment
The samples were washed with Milli-Q water and dried with Kimwipes and nitrogen gas, prior to analysis, to remove dust and soil. For each of the three sample matrices (car tires, rubber granulates, and playground tiles) one duplicate samples was included. The CP extraction was based on the method recently used for the extraction of CPs from domestic rubber and polymeric products in China described by Wang et al.17 Approximately, 1 g precut rubber sample was extracted with 10 mL dichloromethane/hexane (1:1, v/v) for 20 min in an ultrasonic bath. This extraction step was repeated twice and extracts were combined in a new preweighed sample tube. The extract was 100 times diluted in hexane before cleanup. Cleanup was performed using multilayer columns fitted with a plug of silanized glass wool, and filled from the bottom with 0.5 g of activated silica, 3.0 g of 40% (v/v) sulfuric acid silica, and 0.5 g of sodium sulfate, and washed with 10 mL of hexane. The diluted extract was quantitatively transferred to the column and the first fraction eluted with 10 mL of hexane was discarded. The fraction containing the CPs was eluted with 20 mL of dichloromethane/hexane (3:7, v/v) and evaporated to a volume of 0.5 mL at 30 °C under nitrogen and quantitatively transferred to a 1.5 mL glass vial. The fraction was evaporated to dryness and reconstituted in 0.5 mL acetonitrile, 50 μL 13C10-dechlorane plus was added as injection standard (2000 ng/mL).
Measurement of the CPs
The CP analysis was performed using a slightly adopted analytical method previously developed by Bogdal et al.19 Briefly, 10 μL of the cleaned rubber extracts was directly injected into the quadrupole time-of-flight mass spectrometry (qTOF-HRMS) (Compact, Bruker, Bremen, Germany) operating in negative atmospheric pressure chemical ionization (APCI) mode. The injection was performed with an Agilent 1290 infinity HPLC system (Agilent Technologies, Amstelveen, The Netherlands) using acetonitrile with 10% dichloromethane (v/v) as eluent with an isocratic flow of 250 μL/min. The 10% dichloromethane solution was used as dopant to enhance the formation of [M+Cl]− fragments to increase the sensitivity of the CP detection in the negative APCI mode.19 Detailed settings of the QTOF-MS are given in SI Table S2.
The mass spectrometer was operated in negative ionization mode and full scan data was collected from m/z 200–1500 at a minimal resolution of 25 000 fwhm (full width at half-maximum). Internal mass calibration was performed for each injection using the Agilent APCI-L low concentration tuning mix (part no: G1969-85010). In total, 792 m/z ratios were extracted from the full scan mass spectra using Bruker TASQ client 1.4. The 792 m/z ratios are related to the two most abundant [M+Cl]− ions of the CP isotope cluster corresponding to the CP congener groups with chain lengths of C7Cl3 to C30Cl30. Because no chromatographic separation was performed, potential mass interferences from the matrices might occur. Therefore, only mass values with a signal above 3 times the signal-to-noise (S/N) with a mass error below 5 ppm and an ion ratio deviation below 10% between the two most dominant m/z signals of the CP isotope cluster, were extracted from the full scan spectra.20
Quantification and Deconvolution
Quantification of CPs was performed using the deconvolution algorithm developed by Bogdal et al.19 The CP pattern measured in each sample was reconstructed from the CP patterns of the eight technical CP mixtures using a deconvolution algorithm. The relative contribution of the technical CP mixture was then used to calculate the CP concentrations by using external calibration standards, considering the known concentration of the technical CP mixtures. An example of the deconvolution is given in SI Figure S1. Detailed information on the deconvolution procedure can be found in the Supporting Information of Brandsma et al.21 The reconstructed CP pattern (i.e., the pattern recomposed with the estimated contribution of each CP mixtures), was compared to the initial CP pattern of the analyzed sample to determine the goodness of fit (R2). A R2 > 0.5 indicate acceptable deconvolution (good agreement between reconstructed and initial CP pattern), whereby samples with an R2 < 0.5 will be reported as indicative value.19,21
Quality Assurance and Quality Control
To investigate the background levels in our laboratory, 10 blanks were included and randomly analyzed in each batch of samples. The highest blank level observed for the SCCPs was 0.4 ng absolute amount (abs), for the MCCPs 1.1 ng abs, and 0.2 ng abs for the LCCPs. The limit of quantification (LOQ) was calculated from the concentration of the lowest standard, when it was a least 10 times the signal-to-noise ratio, or by 3 times the blank level. Based on an average sample intake of 0.01 g (1 g, 100 times diluted) this resulted in a LOQ for the SCCPs of 200 ng/g, for the MCCPs of 330 ng/g and for the LCCPs of 60 ng/g. Three recovery standards with known concentration were included which contained all eight commercial SCCP, MCCP, and LCCP standards. The recovery standards underwent the same extraction, cleanup, and analysis procedures as the rubber samples. Acceptable recoveries were observed for the SCCPs, MCCPs, and LCCPs with mean levels of 107 ± 8%, 108 ± 21%, and 96 ± 7%, respectively. Throughout the cleanup the samples were evaporated to dryness and redissolved in acetonitrile. To investigate if this evaporation step would result in concentration losses of the CPs, we have evaporated three standard solutions containing known CP concentrations, to dryness and redissolved them in acetonitrile and measured their concentrations. The recoveries of the evaporation step for the SCCPs, MCCPs, and LCCPs were 112 ± 16%, 106 ± 27%, and 121 ± 3%, respectively, which indicated that no losses of CPs occur during the evaporation step. Furthermore, matrix interferences were investigated whereby four analyzed samples from each sample group (car tires, rubber granulates, and playground tiles) were spiked with known CP concentrations (SI Table S3). The average recovery for SCCPs (n = 9) was 129 ± 15%, for MCCPs (n = 9) 106 ± 18%, and for LCCPs (n = 9) 102 ± 27%, which indicates that the results were not influenced by matrix interferences. Elevated recoveries were observed for some of the SCCP spiked samples, possibly resulting in an overestimation.
Unfortunately, no certified reference sample is available for CPs in rubber. However, recently the presence of SCCPs and MCCPs in the NIST SRM2585 dust sample was investigated by Shang et al.22 Therefore, in our study the NIST SRM2585 dust sample was included and analyzed with the same method used for the rubber samples. The average SCCPs and MCCPs levels observed in SRM2585 (n = 12) by Shang et al.22 were 7.6 ± 0.4 μg/g and 16 ± 2.1 μg/g, respectively. This is comparable with the average CP levels (n = 3) observed in our study, 7.1 ± 0.2 μg/g (R2 = 0.93) for the SCCPs, 10 ± 0.2 μg/g (R2 = 0.71) for the MCCPs. Especially considering that the between lab coefficient of variation (CV) of the laboratory exercises organized between 2011 and 2017 were 23–137%23 and that two different analysis techniques are used, the GC–MS operated in electron capture negative ion (ECNI) chemical ionization mode by Shang et al.22 and the APCI-qTOF-MS method in our study. In addition to CPs various other chlorinated compounds have been detected in the NIST SRM2585 dust sample. The use of the low resolution (LR) GC-ECNI-MS method may not be able to resolve these chlorinated compounds interferes from CPs, which could result in an overestimation of CP concentrations. This may also explain the lower MCCP levels observed in our study compared with the study of Shang et al.22 Beside SCCPs and MCCPs, the LCCPs were also detected in the NIST SRM2585 with an average concentration of 16 ± 0.4 μg/g (R2 = 0.01) for the C18–C30 (SI Table S4). LCCPs have not been reported before in the NIST SRM2585 dust sample. Therefore, no comparison could be made with literature data.
Quantification Challenges of the Wax-Grade LCCPs
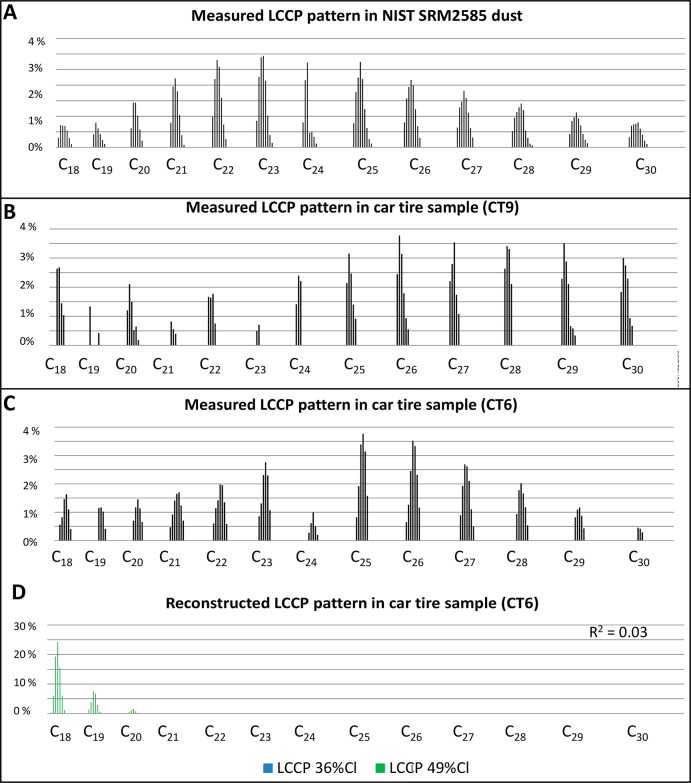
The carbon pattern of the LCCPs in the NIST SRM2585 was dominated by C23–C24-congeners, which indicated that this material contain wax-grade LCCPs (Figure 1A). Similar LCCP patterns, dominated by higher carbon congeners like C24, C25, or C26, have been observed in two car tire samples from Brazil (CT6) and Slovenia (CT9) in this study (Figure 1B and C). Standards containing the wax grade LCCPs are currently not available. Therefore, quantification based on the deconvolution method of Bogdal et al.19 where the CP patterns measured in the sample are reconstructed by the CP patterns in the technical mixtures resulted in a low R2. Examples of reconstructed patterns are given in Figures 1C and D for the car tire sample (CT6). The reported values for the LCCPs in the NIST SRM2585 and in the two car tires samples are therefore reported as indicative values. Overall, this highlights the demand for LCCP standards or technical mixtures that contain wax-grade LCCPs.
Figure 1.
Measured relative contribution of the LCCPs observed in, (A) NIST SRM2585 dust sample, (B) car tire from Slovenia (CT9), and (C) car tire from Brazil (CT6). The LCCP patterns in the samples are normalized to 100%. Figure 1D show the reconstructed pattern based on deconvolution of the technical CP mixtures LCCP 36%CL and 49% Cl. The goodness of fit (R2) of the deconvolution is also given.
Results and Discussion
CPs in Car Tires, Rubber Granulates, and Playground Tiles
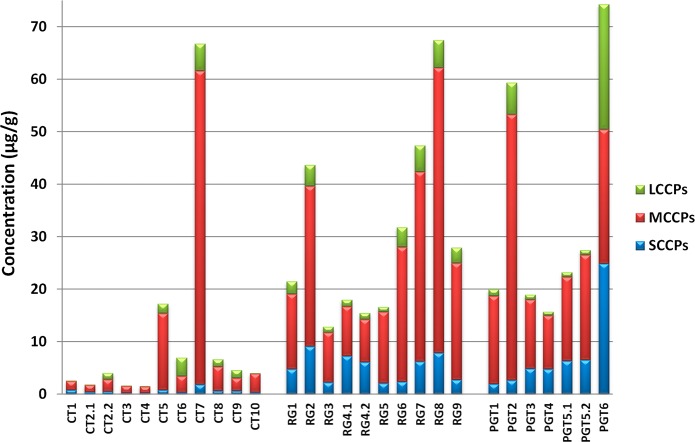
CPs were detected in all rubber samples analyzed, with ∑CP(C10–C30) concentrations ranging 1.5–67 μg/g (median 4.0 μg/g) in end-of-life car tires, 13–67 μg/g (median 24 μg/g) in the recycled rubber granulates and 16–74 μg/g (median 23 μg/g) in playground tiles (Figure 2, Table 1, SI Table S5). MCCPs were predominant with an average contribution of 72% followed by the SCCPs (16%) and LCCPs (12%). The MCCP levels range from 1.2 to 60 μg/g in the car tires, 8–54 μg/g in the rubber granulates, and 10–51 μg/g in playground tiles. The goodness of fit (R2) of all MCCPs levels in the 25 samples was >0.7. With the exception of the playground tile sample no. 6 (PGT6; 24 μg/g), the LCCP levels in the car tires (<0.1–5.2 μg/g), rubber granulates (0.9–5.2 μg/g) and playground tiles (0.7–6.1 μg/g) were all in the same range. LCCP levels for five of the 25 samples were reported as indicative values because of the low goodness of fit (R2 < 0.5). In the car tires samples, the SCCP levels (<0.2–1.8 μg/g) were significantly lower than in the rubber granulates (2.1–9.1 μg/g) and the playground tiles (1.9–25 μg/g) with P < 0.001 and P < 0.05 (ANOVA), respectively. The SCCP levels for four samples were reported as indicative value (R2 < 0.5). No explanation could be given for the significantly lower SCCP levels observed in the car tire samples. A relatively high variation of the CP concentrations in the car tires was observed compared to the recycled products (rubber granulates and playground tiles), probably because these products are composed of a mixture of multiple tires with high and low CP concentrations. CPs with carbon chain lengths lower than C10 were recently found for the first time in sediment samples from China24 and Sweden.25 Only in one of the rubber samples, playground tile (PGT6), a C9 CP was observed (SI Figure S2), which contributes 2% to the total SCCP concentration in this sample. This indicates that CPs with carbon chain lengths below C10 are present in products and should therefore be included in the measurements of CPs.
Figure 2.
Total SCCPs, MCCPs, and LCCPs concentration in μg/g measured in car tires (CT), rubber granulates (RG), and playground tiles (PGT). CT2.1 and CT2.2, RG4.1 and RG4.2, PG5.1 and PG5.2 are duplicate analysis.
Table 1. Total SCCPs, MCCPs, and LCCPs Concentration in μg/g Measured in Car Tires (CT), Rubber Granulates (RG), and Playground Tiles (PGT)a.
| SCCPs (μg/g) | R2 | %Cl | MCCPs (μg/g) | R2 | %Cl | LCCPs (μg/g) | R2 | %Cl | |
|---|---|---|---|---|---|---|---|---|---|
| CT1 | 0.8 | 0.8 | 60% | 1.7 | 0.9 | 52% | <0.1 | 0.9 | 51% |
| CT2.1 | 0.4 | 0.5 | 57% | 1.4 | 0.9 | 52% | <0.1 | 0.7 | 49% |
| CT2.2 | 0.5 | 0.6 | 59% | 2.3 | 0.8 | 53% | 1.2 | 0.2 | 48% |
| CT3 | <0.2 | 0.8 | 59% | 1.3 | 0.8 | 52% | <0.1 | 0.9 | 50% |
| CT4 | <0.2 | 0.8 | 58% | 1.2 | 0.9 | 53% | <0.1 | 0.8 | 48% |
| CT5 | 0.7 | 0.8 | 60% | 15 | 0.8 | 51% | 1.8 | 0.9 | 48% |
| CT6 | 0.3 | 0.6 | 58% | 3.1 | 0.8 | 50% | 3.5 | <0.1 | 53% |
| CT7 | 1.8 | 0.8 | 58% | 60 | 0.8 | 50% | 5.2 | 0.8 | 47% |
| CT8 | 0.6 | 0.8 | 60% | 4.6 | 0.8 | 53% | 1.4 | 0.1 | 48% |
| CT9 | 0.6 | 0.4 | 59% | 2.4 | 0.9 | 53% | 1.5 | <0.1 | 39% |
| CT10 | 0.3 | 0.9 | 57% | 3.6 | 0.8 | 52% | <0.1 | <0.1 | 52% |
| RG1 | 4.8 | 0.6 | 61% | 14 | 0.8 | 52% | 2.4 | 0.8 | 48% |
| RG2 | 9.1 | 0.2 | 64% | 31 | 0.8 | 52% | 4.1 | 0.8 | 50% |
| RG3 | 2.2 | 0.8 | 59% | 9.5 | 0.9 | 53% | 1.1 | 0.9 | 51% |
| RG4.1 | 7.2 | 0.1 | 65% | 9.4 | 0.9 | 53% | 1.3 | 0.6 | 51% |
| RG4.2 | 6.1 | 0.3 | 64% | 8.1 | 0.9 | 53% | 1.2 | 0.7 | 51% |
| RG5 | 2.1 | 0.8 | 58% | 14 | 0.8 | 51% | 0.9 | 1.0 | 50% |
| RG6 | 2.3 | 0.8 | 58% | 26 | 0.7 | 51% | 3.8 | 0.9 | 48% |
| RG7 | 6.2 | 0.8 | 59% | 36 | 0.8 | 52% | 5.0 | 1.0 | 49% |
| RG8 | 7.8 | 0.7 | 58% | 54 | 0.8 | 51% | 5.2 | 0.9 | 49% |
| RG9 | 2.7 | 0.8 | 57% | 22 | 0.7 | 51% | 2.9 | 1.0 | 48% |
| PGT1 | 1.9 | 0.8 | 59% | 17 | 0.8 | 51% | 1.3 | 1.0 | 48% |
| PGT2 | 2.6 | 0.6 | 59% | 51 | 0.9 | 51% | 6.1 | 0.5 | 47% |
| PGT3 | 4.8 | 0.7 | 56% | 13 | 1.0 | 52% | 1.0 | 0.8 | 50% |
| PGT4 | 4.7 | 0.8 | 58% | 10 | 0.9 | 52% | 0.7 | 0.8 | 49% |
| PGT5.1 | 6.3 | 0.5 | 59% | 16 | 1.0 | 51% | 0.9 | 0.9 | 50% |
| PGT5.2 | 6.5 | 0.4 | 57% | 20 | 1.0 | 53% | 0.9 | 0.9 | 51% |
| PGT6 | 25 | 0.8 | 58% | 26 | 0.9 | 55% | 24 | 0.5 | 50% |
Goodness of fit (R2) below 0.5 are given in italic, which indicates that the reported value is indicative. CT2.1 and CT2.2, RG4.1 and RG4.2, PG5.1 and PG5.2 are duplicate analysis.
The presence of CPs in the car tires, in comparable concentrations ranges to rubber granulates and playground tiles, indicates that CPs were already present in the car tires and not introduced during the shredder or molding process. CPs are used in many applications as plasticizers, lubricants, or flame retardants.8 Although, in the European environmental risk assessment report on LCCPs from 2009, it was stated that CPs are not used in car tires, CPs are typically used for their flame retarding properties in rubber.26 The amount of CPs added in rubber ranges in general from 1 to 4%, but increases up to 15% for specific applications.26,27 In our study, the CP levels in the car tires were orders of magnitude lower than the application levels (1–4%) needed to provide flame retardancy (Figure 2, Table 1). The application or source of CPs in the tires is currently unclear, and the addition at low levels as (secondary) plasticizer or softener cannot be excluded. CPs are used in high percentages (up to 15%) in industrial rollers covering and rubber conveyer belts and were proven to leach from blender components such as self-lubricating bearing, polymer washers, and polymer coatings.26,28 Therefore, it can be postulated that CP contamination of the car tires could take place during the manufacturing process.
Data on CPs in rubber car tires, rubber granulates, and playground tiles are scarce and no data exists on LCCPs. Wang et al.17 detected SCCPs and MCCPs in 15 car tires purchased in China with average concentrations of 106 μg/g and 442 μg/g, respectively. The average levels reported by Wang et al.17 for SCCPs and MCCPs are 100 times higher than the levels observed in car tires in our study, although still below the levels needed to provide flame retardancy or use as plasticizer. The concentrations reported for the CPs in car tires from China showed large variations between samples where the SCCPs ranged from <7.2 to 603 mg/kg and for MCCPs from <20 to 4850 mg/kg. The highest concentration in one of the car tires from China was 10 times higher than the second highest concentration, which influences the average CP value significantly. Even when excluding the highest concentration, the average CP value was still an order of magnitude higher than in our study. The high variation in CP concentrations observed in car tires could also be an indication that the CPs were not added to the car tires to improve the physical or chemical characteristics of the tires but might be a contamination during the manufacturing process.
Cao et al.18 measured CPs in dust collected from synthetic turf fields. The geometric mean (GM) of the SCCP and MCCP levels in the dust collected from the synthetic turf field are 101 μg/g and 241 μg/g, respectively. The synthetic turf blades (synthetic grass) were also analyzed and contained higher SCCP and MCCP levels with a GM of 260 and 632 μg/g, respectively. The rubber granulates used as filling on the synthetic turf field were not analyzed, and therefore, no comparison could be made with our study. However, the CPs levels in dust from the synthetic turf field reported by Cao et al.18 were an order of magnitude higher than those in the rubber granulates in our study.
Congener Group Patterns
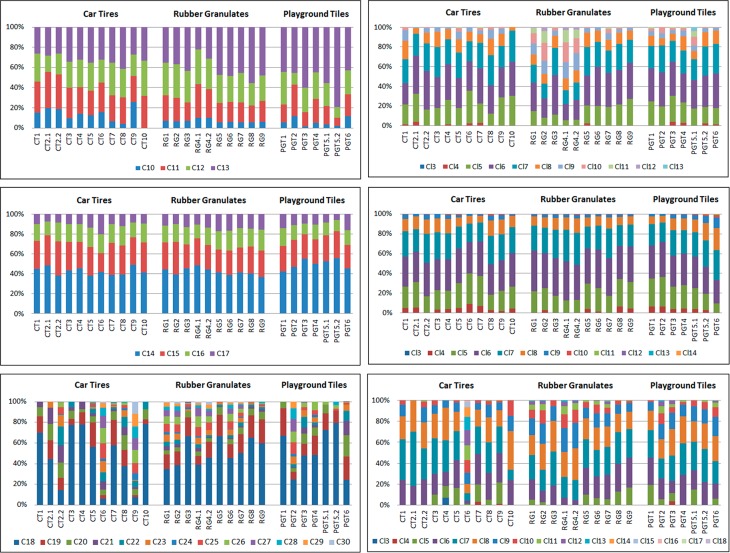
The SCCP, MCCP, and LCCP congener group patterns based on carbon length and chlorine atoms is shown in Figure 3. The calculated chlorination degree of the SCCPs, MCCPs, and LCCPs ranged from 57 to 65%, 50–55%, and 39–53%, respectively (Table 1). Comparable carbon and chlorine homologue patterns were observed for the MCCPs in all rubber samples, and these were dominated by C14 (44%), followed by C15 (27%), C16 (17%), and C17 (12%), with Cl6 (34%) and Cl7 (25%). These findings, whereby C14 is the predominant chain length, are comparable with the homologue pattern of the commercial technical mixture (MCCP, 52%Cl), which is dominated by C14 (67%) followed by C15 (26%), with Cl6 (34%) and Cl7 (33%) although the percentage of C14 is the rubber samples (44%) is lower. Data on MCCPs in rubber or other plastics are limited and only Wang et al.17 reported MCCPs in Chinese rubber samples (car tires, rubber tracks, and conveyor belts), which were also dominated by the C14-congeners, although with higher chlorine substitution (Cl7 and Cl8). This pattern was comparable with that of the technical CP product (CP-52), one of the common technical mixtures used in China.17 The individual chlorination degrees of the CP groups were not provided, although the combined values of SCCPs and MCCPs in the car tire samples ranged from 60.5 to 62.5%. In general, the MCCP homologue pattern observed in our study was not unique, and comparable carbon and chlorine homologue MCCP patterns have been shown in various samples all over the world such as in sediment,24,25 sewage sludge samples,21 house dust,22,29 wildlife,15,16 human blood,14 and in technical CP products used in Europe and China.
Figure 3.
SCCP, MCCP, and LCCP carbon and chlorine homologue pattern observed in the in car tires (CT), rubber granulates (RG) and playground tiles (PGT). CT2.1 and CT2.2, RG4.1 and RG4.2, PG5.1 and PG5.2 are duplicate analysis.
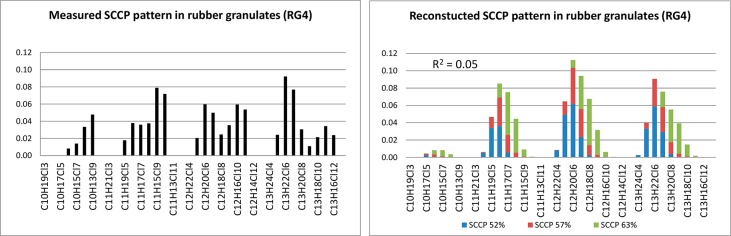
In contrast to the MCCPs, variation in the carbon and chlorine homologue patterns was observed for the SCCPs and LCCPs in the rubber samples analyzed in our study (Figure 3). In general, SCCPs were often dominated by C13 (40%) and C12 (26%) with Cl6 (32%) and Cl7 (24%). Exceptions were found in car tires from France (CT1) and China (CT2.1 and CT2.2), which were dominated by C11. In the car tire sample from Slovenia (CT9) the C10, C11, C12, and C13 were more or less equally distributed. The SCCP carbon chain length profile observed in the single car tire from China were comparable with those reported by Wang et al.,17 who observed that the average contribution of C11-congeners, in the 15 car tires analyzed, was higher than that of the C13-congeners. An irregular chlorine pattern for the SCCPs was observed in two rubber granulate samples (RG2 and RG4) as shown in Figure 4. These two samples (RG2 and RG4) contained not only the lower chlorinated congeners Cl5, Cl6 and Cl7, but also the higher chlorinated ones Cl8, Cl9, Cl10, and Cl11, resulting in a “double” chlorine pattern (Figure 4), which was in contrast with the chlorine homologue patterns observed in the other samples (all dominated by Cl6 followed by Cl7 and Cl5). This “double” chlorine pattern observed in the rubber granulates is probably caused by the worldwide use of various technical CP products with different SCCP patterns. Through recycling of the car tires after end-of-life, these different technical mixtures end up as a mixture in the “new” recycled products, such as rubber granulates. The pattern in these two rubber granulate samples (RG2 and RG4) could therefore not be reconstructed with the commercial available SCCPs standards, which resulted in R2 < 0.5.
Figure 4.
Measured and reconstructed SCCP pattern in rubber granulate sample RG4.
The LCCP patterns in the car tires, rubber granulates and playground tiles are shown in Figure 3 and were dominated by C18 and C19 with Cl7, Cl8, and Cl6. However, in some car tires and playground tiles, different patterns were observed. Especially in the car tire samples from Brazil (CT6) and Slovenia (CT9), where C25 and C26-congeners were dominant, which indicates the presence of wax-grade LCCPs (average carbon chain length of approximately C25) (Figure 1). The car tire sample from Brazil (CT6) contained higher chlorinated congeners (dominated by Cl12) compared to the car tire sample from Slovenia (CT9), which was dominated by Cl7. The car tire sample from China (CT2) and the playground tiles samples (PGT2 and PGT6) also contain longer carbon length congeners up to C29 which were dominated by C18 and C19. Overall, data on the presence of LCCPs is scarce and to our knowledge, no data exist on LCCPs in rubber. However, the presence of LCCPs has recently been reported in Scandinavian terrestrial birds and mammals16 with chain lengths up to C30. LCCPs up to C23 were also detected in human blood (up to 530 ng/g LW) from China.14 These results indicate that LCCPs have a bioaccumulative potential, which highlights the importance of including LCCPs in the measurement of CPs.
Human and Environmental Exposure
This study shows the presence of a new POP (SCCPs) in recycled products (rubber granulates and playground tiles). Although, CPs were observed in all rubber samples analyzed with levels up to 75 μg/g, the levels were far below the regulatory limit set by the European Commission of 0.15% for SCCPs.30 No regulatory limits are set for the MCCPs and LCCPs. Limited information is available on the exposure route of CPs from rubber and further research is needed to investigate the migration and leaching behavior of CPs from rubber materials to evaluate the potential human exposure to CPs In addition to CPs, multiple toxic additives have been detected in rubber granulates, for example benzothiazoles, phthalates, metals, bisphenol A, and PAHs.4−7 Evidence has been found that combined human exposure to PAHs and SCCPs results in an additive effect on the overall metabolism.31 Therefore, risk assessment should not only focus on the individual compounds present in the rubber, but should also address possible synergistic/additive effects.
The presence of CPs in car tires may be an additional source of CPs in the environment. Car tires are worn out after approximately 40 000 km and generate tire wear particles (TWP) during use.32 The amount of TWP generated in the European Union is estimated at 1 327 000 tons annually.33 Based on the ∑CPC10–C30 concentrations in car tires (ranging from 1.5 to 67 μg/g) and the estimated TWP generated in the European Union, a rough estimate of CP exposure to environment in the European Union is between 2.0 and 89 tons annually. It has also been suggested that based on the maximum estimate, TWP could be seen as an important source of contamination for CPs. Previous emission estimates for SCCPs included the use in rubber but did not include the use in car tires.34 To our knowledge, equivalent estimates for MCCPs and LCCPs are not available although the European Assessments of MCCPs and LCCPs estimated environmental concentrations for air and water.26,35 This new data may indicate that a reassessment of sources of CPs to the environment, particularly for MCCPs and LCCPs, should include use in car tires. The total registered manufacture and import of CPs in Europe is indicated between 10 000 and 100 000 tons annually.8 This indicates that a maximum of 1% of the total manufactured and imported CPs in Europe could enter the environment through TWP from car tires.
Acknowledgments
Funded by The Netherlands Organization for Scientific Research (VENI2017-722.017.009) in part by the National Research Foundation of South Africa (VU University Amsterdam - NRF Desmond Tutu doctoral scholarship grant number: 94075). We are thankful to Dr. Maria Llompart, Universidad de Santiago de Compostela, Spain, for providing the playground tile from Spain.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.est.9b01835.
Additional information on materials, standards, and extraction solvents used. Table S1. Information (brand, year, and country) of the car tires analyzed in this study. Table S2. Instrumental parameters LC-APCI-qTOF-MS. Table S3. Results of the spike experiment of the car tires (CT), rubber granulates (RG) and playground tiles (PGT). Table S4. CP levels in μg/g in the NIST SRM2585. Table S5. Concentrations of the individual carbon congeners in μg/g for the SCCPs, MCCPs, and LCCPs. Table S6. Calculated chlorination degrees for the eight technical CP mixtures. Figure S1. Measured and reconstructed CP pattern in rubber granulate (RG7) for the SCCPs, in the playground tile (PGT4) for the MCCPs and in the car tire (CT5) for the LCCPs Figure S2. Measured SCCP pattern in playground tile sample PGT6, including the presence of C9 (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Hoekstra A. Y.; Wiedmann T. O. Humanity’s unsustainable environmental footprint. Science 2014, 344, 1114–1117. 10.1126/science.1248365. [DOI] [PubMed] [Google Scholar]
- Leslie H. A.; Leonards P. E. G.; Brandsma S. H.; De Boer J.; Jonkers N. Propelling plastics into the circular economy—weeding out the toxics first. Environ. Int. 2016, 94, 230–234. 10.1016/j.envint.2016.05.012. [DOI] [PubMed] [Google Scholar]
- Diekman A.; Giese U.; Schaumann I. Polycyclic aromatic hydrocarbons in consumer goods made from recycled rubber material: A review. Chemosphere 2019, 220, 1163–1178. 10.1016/j.chemosphere.2018.12.111. [DOI] [PubMed] [Google Scholar]
- De Groot G. M., Oomen A. G., Mennen G. M., Evaluation of health risks of playing sports on synthetic turf pitches with rubber granulate Scientific background document. Report number: RIVM Rapport 10.21945/RIVM-2017-0017. p 250. [DOI]
- Li X.; Berger W.; Musante C.; Mattina M. I. Characterization of substances released from crumb rubber material used on artificial turf fields. Chemosphere 2010, 80, 279–285. 10.1016/j.chemosphere.2010.04.021. [DOI] [PubMed] [Google Scholar]
- Menichini E.; Abate V.; Attias L.; De Luca S.; Di Domenico A.; Fochi I.; Forte G.; Iacovella N.; Iamiceli A. L.; Izzo P.; Merli F.; Bocca B. Artificial-turf playing fields: Contents of metals, PAHs, PCBs, PCDDs and PCDFs, inhalation exposure to PAHs and related preliminary risk assessment. Sci. Total Environ. 2011, 409, 4950–4957. 10.1016/j.scitotenv.2011.07.042. [DOI] [PubMed] [Google Scholar]
- Perkins A. N.; Inayat-Hussain S. H.; Deziel N. C.; Johnson C. H.; Ferguson S. S.; Garcia-Milian R.; Thompson D. C.; Vasiliou V. Evaluation of potential carcinogenicity of organic chemicals in synthetic turf crumb rubber. Environ. Res. 2019, 169, 163–172. 10.1016/j.envres.2018.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Mourik L. M.; Gaus C.; Leonards P. E. G.; de Boer J. Chlorinated paraffins in the environment: A review on their production, fate, levels and trends between 2010 and 2015. Chemosphere 2016, 155, 415–428. 10.1016/j.chemosphere.2016.04.037. [DOI] [PubMed] [Google Scholar]
- Environmental Protection Agency (EPA). Risk Management for Short-Chain Chlorinated Paraffins. 2012. https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/risk-management-short-chain-chlorinated-paraffins (accessed 2 June 2019).
- POPRC (Persistent Organic Pollutants Review Committee). Recommendation by the Persistent Organic Pollutants Review Committee to List Short-Chain Chlorinated Paraffins in Annex A to the Convention. 2017, (UNEP/POPS/COP.8/14).
- Stockholm convention. Short-chain chlorinated paraffins (SCCPs) http://chm.pops.int/Implementation/Alternatives/AlternativestoPOPs/ChemicalslistedinAnnexA/Shortchainchlorinatedparaffins(SCCPs)/tabid/5986/Default.aspx (accessed 8 March 2019).
- Wei G. L.; Liang X. L.; Li D. Q.; Zhuo M. N.; Zhang S. Y.; Huang Q. X.; Liao Y. S.; Xie Z. Y.; Guo T. L.; Yuan Z. J. Occurrence, fate and ecological risk of chlorinated paraffins in Asia: A review. Environ. Int. 2016, 92–93, 373–387. 10.1016/j.envint.2016.04.002. [DOI] [PubMed] [Google Scholar]
- De Boer J. (Ed.). Handbook of Environmental Chemistry, Vol. on Chlorinated Paraffins; Springer: Heidelberg, Germany: 2010. [Google Scholar]
- Li T.; Wan Y.; Gao S.; Wang B.; Hu J. High-throughput determination and characterization of short-, median-, and long-chain chlorinated paraffins in human blood. Environ. Sci. Technol. 2017, 51, 3346–3354. 10.1021/acs.est.6b05149. [DOI] [PubMed] [Google Scholar]
- Du X.; Yuan B.; Zhou Y.; Benskin J. P.; Qiu Y.; Yin G.; Zhao J. Short-, medium- and long-chain chlorinated paraffins in wildlife from paddy fields in the Yangtze River Delta. Environ. Sci. Technol. 2018, 52, 1072–1080. 10.1021/acs.est.7b05595. [DOI] [PubMed] [Google Scholar]
- Yuan B.; Vorkamp K.; Roos A. M.; Faxneld S.; Sonne C.; Garbus S. E.; Lind Y.; Eulaers I.; Hellström P.; Dietz R.; Persson S.; Bossi R.; de Wit C. A. Accumulation of Short-, Medium-, and Long-Chain Chlorinated Paraffins in Marine and Terrestrial Animals from Scandinavia. Environ. Sci. Technol. 2019, 53, 3526–3537. 10.1021/acs.est.8b06518. [DOI] [PubMed] [Google Scholar]
- Wang C.; Gao W.; Liang Y.; Wang Y.; Jiang G. Concentration and congener profiles of chlorinated paraffins in domestic polymeric products in China. Environ. Pollut. 2018, 238, 326–335. 10.1016/j.envpol.2018.02.078. [DOI] [PubMed] [Google Scholar]
- Cao D.; Gao W.; Wu J.; Lv K.; Xin S.; Wang Y.; Jiang G. Occurrence and human exposure assessment of short- and medium-chain chlorinated paraffins in dusts from plastic sports courts and synthetic turf in Beijing, China. Environ. Sci. Technol. 2019, 53, 443–451. 10.1021/acs.est.8b04323. [DOI] [PubMed] [Google Scholar]
- Bogdal C.; Alsberg T.; Diefenbacher P. S.; MacLeod M.; Berger U. Fast quantification of chlorinated paraffins in environmental samples by direct injection high-resolution mass spectrometry with pattern deconvolution. Anal. Chem. 2015, 87, 2852–2860. 10.1021/ac504444d. [DOI] [PubMed] [Google Scholar]
- European Commission Directorate General for Health and Food Safety. Guidance document on analytical quality control and method validation procedures for pesticide residues and analysis in food and feed. SANTE/11813/2017.
- Brandsma S. H.; van Mourik L.; O’Brien J. K.; Eaglesham G.; Leonards P. E. G.; de Boer J.; Gallen J.; Gaus C.; Bogdal C. Medium-Chain Chlorinated Paraffins (CP’s) Dominate in Australian Sewage Sludge. Environ. Sci. Technol. 2017, 51, 3364–3372. 10.1021/acs.est.6b05318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang H.; Fan X.; Kubwabo C.; Rasmussen P. E. Short-chain and medium-chain chlorinated paraffins in Canadian house dust and NIST SRM 2585. Environ. Sci. Pollut. Res. 2019, 26, 1–10. 10.1007/s11356-018-04073-2. [DOI] [PubMed] [Google Scholar]
- van Mourik L. M.; van der Veen I.; Crum S.; de Boer J. Developments and interlaboratory study of the analysis of short-chain chlorinated paraffins. TrAC, Trends Anal. Chem. 2018, 102, 32–40. 10.1016/j.trac.2018.01.004. [DOI] [Google Scholar]
- Qiao L.; Gao L.; Xia D.; Huang H.; Zheng M. Short- and medium-chain chlorinated paraffins in sediments from the middle reaches of the Yangtze River: Spatial distributions, source apportionment and risk assessment. Sci. Total Environ. 2017, 575, 1177–1182. 10.1016/j.scitotenv.2016.09.193. [DOI] [PubMed] [Google Scholar]
- Yuan B.; Brüchert V.; Sobek A.; de Wit C. A. Temporal Trends of C8–C36 Chlorinated Paraffins in Swedish Coastal Sediment Cores over the Past 80 Years. Environ. Sci. Technol. 2017, 51, 14199–14208. 10.1021/acs.est.7b04523. [DOI] [PubMed] [Google Scholar]
- Brooke D. N., Crookes M. J., Merckel D.. Environmental Risk Assessment: Long-Chain Chlorinated Paraffins, Environment Agency January 2009. ISBN: 978-1-84432-977-9. [Google Scholar]
- Zitko Z.; Arsenault E.. Chlorinated Properties, Pollution Paraffins: Uses, and Potential, Environment Canada. Technical Report No. 491, 1974.
- Yuan B.; Strid A.; Darnerud P. O.; de Wit C. A.; Nyström J.; Bergman Å. Chlorinated paraffins leaking from hand blenders can lead to significant human exposures. Environ. Int. 2017, 109, 73–80. 10.1016/j.envint.2017.09.014. [DOI] [PubMed] [Google Scholar]
- Hilger B.; Fromme H.; Völkel W.; Coelhan M. Occurrence of chlorinated paraffins in house dust samples from Bavaria. Environ. Pollut. 2013, 175, 16–21. 10.1016/j.envpol.2012.12.011. [DOI] [PubMed] [Google Scholar]
- Commission Regulation (EU) 2015/2030 of 13 November 2015. http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32015R2030&qid=1492294902869&from=EN (accessed 8 March 2019).
- Wang F.; Zhang H.; Geng N.; Ren X.; Zhang B.; Gong Y.; Chen J. A metabolomics strategy to assess the combined toxicity of polycyclic aromatic hydrocarbons (PAHs) and short-chain chlorinated paraffins (SCCPs). Environ. Pollut. 2018, 234, 572–580. 10.1016/j.envpol.2017.11.073. [DOI] [PubMed] [Google Scholar]
- Wik A.; Dave G. Occurrence and effects of tire wear particles in the environment – A critical review and an initial risk assessment. Environ. Pollut. 2009, 157, 1–11. 10.1016/j.envpol.2008.09.028. [DOI] [PubMed] [Google Scholar]
- Wagner S.; Hüffer T.; Klöckner P.; Wehrhahn M.; Hofmann T.; Reemtsma T. Tire wear particles in the aquatic environment - A review on generation, analysis, occurrence, fate and effects. Water Res. 2018, 139, 83–100. 10.1016/j.watres.2018.03.051. [DOI] [PubMed] [Google Scholar]
- Glüge J.; Wang Z.; Bogdal C.; Scheringer M.; Hungerbühler H. Global production, use, and emission volumes of short-chain chlorinated paraffins – A minimum scenario. Sci. Total Environ. 2016, 573, 1132–1146. 10.1016/j.scitotenv.2016.08.105. [DOI] [PubMed] [Google Scholar]
- European Commission. Scientific Committee on Health and Environmental Risks (SCHER) Risk Assessment Report on Alkanes, C14–17, chloro MCCP. 15 January 2008.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.