Abstract
Background
Nonhuman primate models of developmental programming by maternal mismatch between pregnancy and lactation diets are needed for translation to human programming outcomes. We present baboon offspring morphometry from birth-3 years, and blood cortisol and adrenocorticotropin (ACTH) from 2–24 months.
Methods
Control mothers ate chow; mismatch mothers ate 30% less than controls during pregnancy and high-fat high-energy diet through lactation.
Results
Mismatch mothers lost weight during pregnancy. At birth, there were trends toward lower weight in Mismatch offspring of both sexes (p=0.06). From 0–3 years, catch-up growth occurred. Mismatch offspring male and female body weight increased faster than controls (p<0.001). Mismatch female offspring showed greater increase in BMI (p<0.001) and abdominal circumference (p=0.008) vs controls. ACTH and cortisol slopes from 2–24 months of age were similar between groups in both sexes. Cortisol and ACTH increased after weaning in all groups.
Conclusions
Mismatch produces sexually dimorphic postnatal growth phenotypes.
Keywords: mismatch, nonhuman primates, developmental programming, maternal nutrition, high fat diet, undernutrition, baboon, cortisol, ACTH, catch-up growth
Introduction
Sub-optimal maternal nutrition during pregnancy and lactation has lifelong consequences for offspring development. Fetal nutrition is often limited in utero -- by decreased availability of nutrients, placental insufficiency, maternal disease (e.g., hypertension and renal disease), and maternal tobacco, alcohol, and recreational drug use1 -- followed by overnutrition after birth. After weaning, nutrition may be available in great excess since the typical Western diet is obesogenic. The Barker hypothesis predicts that offspring exposed to this mismatch of pre- and post-natal nutrition will be predisposed to obesity and metabolic dysfunction.2,3 When a fetus is exposed to low nutrition, the organism undergoes adaptive changes such as enhancing gluconeogenesis and developing methods to conserve glucose and amino acids4 that increase its survival chances in utero in the face of a reduced nutrient supply. These changes give rise to a so-called “thrifty phenotype.” If food becomes plentiful after birth, these programmed alterations in metabolism can predispose individuals to obesity, diabetes, and cardiovascular disease.5–8
We have developed a baboon model of pre- and post-natal nutritional mismatch. Mothers in the mismatch group received 30% less global food than control mothers during pregnancy. Mismatch mothers then received an obesogenic diet ad libitum during lactation while control mothers ate normal primate center baboon chow ad libitum. We present here offspring body weight and morphometrics for the first three years of life. Since the fetal hypothalamo-pituitary-adrenal (HPA) axis plays a fundamental role in balancing perinatal growth and differentiation during gestation,9 we also measured plasma adrenocorticotropin (ACTH) and cortisol levels. The equivalent age in humans would be birth through an approximate age of 10 years.10
Materials and methods
Humane care guidelines
All procedures were approved by the Texas Biomedical Research Institute (TBRI) Institutional Animal Care and Use Committee and conducted in AAALAC approved facilities.11
Animal management and housing
Female baboons were housed with a proven breeder male in custom-built group cages containing up to 16 females per cage and allowing normal physical and social interaction and environmental enrichment in the Southwest National Primate Research Center (SNPRC) at TBRI. Details of housing and breeding have been described previously.11,12 Animal health was supervised by SNPRC veterinarians. Females of similar morphometric characteristics were randomly assigned prior to breeding to the control group or the maternal mismatch group.
Control mothers ate ad libitum SNPRC biscuits (Purina Monkey Diet and Monkey Diet Jumbo, Purina LabDiets, St Louis, MO) containing 12% energy from fat, 0.29% from glucose, 0.32% from fructose, and a metabolizable energy content of 3.07 kcal/g. During pregnancy, mismatch mothers received 70% of the average daily ad lib amount of global feed eaten on a weight-adjusted basis by control animals at the same gestational stage. Precise control of dietary intake was accomplished with an individual feeding system previously described in detail.13,14 The use of the individual feeding system allowed mothers to live in group housing connected to a chute leading to individual cages in which they were housed at feeding time. Mothers were trained to run into the individual cages for feeding time only, then released back to social cages after feeding.
Throughout lactation, mismatch mothers received ad lib control diet + ad lib Purina 5045–6 (Purina LabDiets, St Louis, MO, USA), a high fat and high energy diet containing 45% energy from fat, 4.6% from glucose, 5.6% from fructose, and a metabolizable energy content of 4.03 kcal/g. Mismatch mothers also had continuous access to a high fructose beverage during lactation. They were provided with both diets because animals ate more when both diets were available.15
Lactating mothers and offspring remained on their assigned diets and continued to be housed in the same group cages until all offspring were separated from their mothers and weaned onto SNPRC biscuits (Purina Monkey Diet, Purina LabDiets, St Louis, MO) at approximately 9 months of age. After weaning at 9 months of age, offspring were separated into male (N = 8 mismatch, 20 control) and female (N = 6 mismatch, 20 control) juvenile groups. Since dietary group was assigned before sex of offspring was known, we were unable to control the exact number of males and females in each group.
Morphometrics
Offspring of control and mismatch mothers were weighed and body dimensions measured at birth and at 2, 3, 6, 9, 12, 18, 24, 30, and 36 months of age. Additional timepoints for offspring body weight were available from periodic veterinary health checks. Body weight was measured with a digital scale. Body length, head circumference, abdominal circumference, chest circumference, and hip circumference were assessed with a tape measure by trained and experienced personnel according to details in Table 1. All measurements were taken three times and the means used as final values. We have demonstrated inter-observer reliability of these measurements.13 Body mass index (BMI) was calculated as body weight (kg) / body length (cm)2. Sample sizes varied at different timepoints (Table 2) due to animal availability (e.g., location and involvement in other studies).
Table 1.
Morphometric measurement methods.
| Measurement | Method |
|---|---|
| Body length (cm) | measured from the back of the head at the intersection of the parietal and lambdoidal sutures to the rump at the largest protrusion of the coccyx to the heel of the right foot |
| Chest circumference (cm) | measured all the way around the chest at the level of the nipples |
| Head circumference (cm) | measured over the most prominent part on the back of the head (occiput) and just above the eyebrows (supraorbital ridges), i.e., the largest circumference of the head |
| Hips circumference (cm) | measured all the way around the hips at the level of the greatest protrusion of the innominate/pelvic bone (anterior superior iliac spine) |
| Abdominal circumference (cm) | measured all the way around the abdomen at the level of the umbilicus |
Table 2.
Sample sizes for F1 morphometrics taken at each timepoint.
| Age (mo) | Group | Females | Males | Age (mo) | Group | Females | Males |
|---|---|---|---|---|---|---|---|
| 0 | CTR | 19 | 17‒19 | 9 | CTR | 19 | 19‒20 |
| MM | 15* | 16‒17* | MM | 5‒6 | 8 | ||
| 0.5 | CTR | 17‒18 | 19‒20 | 12 | CTR | 14‒17 | 18‒20 |
| MM | 6 | 8 | MM | 6 | 8 | ||
| 1 | CTR | 18–20 | 19–20 | 18 | CTR | 15‒17 | 20 |
| MM | 6 | 8 | MM | 6 | 7‒8 | ||
| 2 | CTR | 16‒19 | 19‒20 | 24 | CTR | 14‒15 | 18‒20 |
| MM | 6 | 8 | MM | 5‒6 | 5 | ||
| 3 | CTR | 19‒20 | 19‒20 | 30 | CTR | 14‒16 | 14‒15 |
| MM | 6 | 8 | MM | 5 | 4‒5 | ||
| 6 | CTR | 19 | 20 | 36 | CTR | 13‒15 | 14‒16 |
| MM | 5‒6 | 8 | MM | 4‒5 | 4 | ||
Sample size of MM group was higher at birth than at other ages because some animals continued to eat a reduced diet after birth for a separate study,12 while MM animals were switched to the high-fat high-energy diet after birth.
Blood sampling
Offspring blood samples were taken at 2, 3, 6, 9, 12, 18, and 24 months of age. Sample sizes varied at different timepoints (Table 3) due to animal availability (e.g., location and involvement in other studies). Blood samples were taken in the group cage area within 5 minutes of isolation and tranquilization with 10 mg/kg ketamine IM. We have shown that ketamine administration does not affect ACTH and cortisol levels within 10 minutes of administration.13 Serum ACTH1–39 was measured by a two-site ELISA and cortisol by chemiluminescent immunoassay on an Immulite® 1000 Immunoassay System (Siemens Healthcare Diagnostics). The intra-/inter-assay coefficients of variation for ACTH were 4.8 /7.2. The intra-/inter-assay coefficients of variation for cortisol were 5.6/8.4.16
Table 3.
Sample sizes for F1 ACTH and cortisol at each timepoint.
| Age (mo) | Group | Females | Males |
|---|---|---|---|
| 2 | CTR | 5 | 9 |
| MM | 6 | 8 | |
| 3 | CTR | 3 | 3 |
| MM | 4 | 4 | |
| 6 | CTR | 4 | 8 |
| MM | 6 | 8 | |
| 9 | CTR | 4 | 10 |
| MM | 6 | 8 | |
| 12 | CTR | 4 | 10 |
| MM | 6 | 8 | |
| 18 | CTR | 3 | 10 |
| MM | 6 | 6 | |
| 24 | CTR | 2 | 5 |
| MM | 5 | 5 | |
Statistical analyses
Maternal single timepoint variables pre-pregnancy weight, delivery weight, % weight gain, and BMI were compared between controls and mismatch with two-tailed Student’s t-tests. Maternal weight gain was divided by offspring birth weight to test whether mothers who gained more weight had heavier neonates (offspring birth weight / Wt gain % (kg)), then compared between groups with t-test. Birth weight and birth morphometrics were compared between groups with one-tailed t-tests; the one-tailed approach was justified because offspring were born under circumstances we have previously shown lead to a significantly reduced body size.13 Postnatal morphometric variables, ACTH, and cortisol were regressed against age, then control and mismatch slopes compared using ANCOVA. All ACTH and cortisol data were ln-transformed to attain normal distribution. Mean ACTH and cortisol were also analyzed between mismatch and controls before and after weaning at age 9 months with two-way t-tests. Data are presented as mean ± standard error of the mean (SEM). Significance was set at p ≤ 0.05.
Results
Maternal morphometrics at delivery
There were no morphometric differences between control mothers that gave birth to females versus control mothers that gave birth to males. Similarly, there were no morphometric differences between mismatch mothers that gave birth to females versus mismatch mothers that gave birth to males.
Mothers of males.
Mismatch and control mothers had similar pre-pregnancy weight, but mismatch mothers lost weight during pregnancy, leading to lower weight gain and weight at delivery in mismatch compared to controls (Table 4). BMI at delivery was similar among mismatch and control mothers of males, as was the relationship of offspring birth weight to maternal weight gain.
Table 4.
Maternal variables in CTR and MM mothers, with separate analyses by F1 sex. Among both MM and CTR mothers, for all variables, mothers who gave birth to females were similar to mothers who gave birth to males.
| Pre-pregnancy weight (kg) | Weight at delivery (kg) | Weight gain (%) | BMI at delivery (kg/cm2) | Birth weight/weight gain | |
|---|---|---|---|---|---|
| MALES | |||||
| CTR | 15.1 ± 0.45 | 16.0 ± 0.43 | 4.0 ± 1.67 | 15.5 ± 0.41 | 0.51 ± 0.61 |
| N | 16 | 19 | 16 | 18 | 16 |
| MM | 14.9 ± 0.44 | 14.6 ± 0.51* | ‒2.1 ± 2.51* | 14.6 ± 0.59 | 0.23 ± 0.71 |
| N | 16 | 17 | 16 | 17 | 16 |
| FEMALES | |||||
| CTR | 14.4 ± 0.47 | 15.1 ± 0.46 | 4.1 ± 2.94 | 14.8 ± 0.40 | 0.59 ± 0.39 |
| N | 17 | 19 | 16 | 18 | 16 |
| MM | 16.0 ± 0.64* | 15.0 ± 0.62 | ‒7.4 ± 2.38* | 14.3 ± 0.50 | 2.6 ± 1.94 |
| N | 14 | 15 | 14 | 15 | 14 |
Mean ± SEM.
p ≤ 0.05 vs CTR of same sex.
Mothers of females.
Prior to pregnancy mismatch mothers weighed more than control mothers (Table 4). Weight and BMI at delivery were similar between mothers of females, but as with mothers of males, mismatch mothers lost weight during pregnancy such that % weight gain was lower in mismatch than controls. The relationship of offspring birth weight to maternal weight gain was similar between maternal groups.
Offspring morphometrics at birth
Male offspring.
At birth, male mismatch tended to weigh less (p = 0.06) than male controls (Table 5). Male mismatch had larger abdominal circumference (p = 0.05) and tended to have lower BMI (p = 0.08) than male controls, with borderline lower ratio of hip:abdominal circumference between groups (p = 0.11). Body length and circumferences of the head, chest, and hip were similar between male mismatch and controls.
Table 5.
F1 morphometrics at birth in males and females.
| Weight (kg) | Length (cm) | BMI (kg/cm2) | Head circum. (cm) | Chest circum. (cm) | Abdominal circum. (cm) | Hip circum. (cm) | Hip/abdomen circum. | |
|---|---|---|---|---|---|---|---|---|
| Males | ||||||||
| CTR | 0.91 ± 0.03 | 37.2 ± 0.58 | 6.4 ± 0.18 | 22.7 ± 0.28 | 19.3 ± 0.35 | 16.5 ± 0.52 | 15.0 ± 0.46 | 0.92 ± 0.03 |
| N | 19 | 17 | 17 | 18 | 19 | 19 | 19 | 19 |
| MM | 0.84 ± 0.02+ | 37.6 ± 0.67 | 6.0 ± 0.24+ | 22.8 ± 0.29 | 19.7 ± 0.28 | 17.7 ± 0.48* | 15.3 ± 0.36 | 0.87 ± 0.02+ |
| N | 17 | 16 | 16 | 16 | 16 | 16 | 16 | 16 |
| Females | ||||||||
| CTR | 0.79 ± 0.03 | 36.6 ± 0.71 | 6.1 ± 0.35 | 22.1 ± 0.28 | 19.3 ± 0.36 | 16.9 ± 0.45 | 15.1 ± 0.50 | 0.90 ± 0.02 |
| N | 19 | 19 | 19 | 19 | 19 | 19 | 19 | 19 |
| MM | 0.72 ± 0.04+ | 36.7 ± 0.88 | 5.4 ± 0.33+ | 21.8 ± 0.28 | 18.7 ± 0.44 | 15.4 ± 0.64* | 14.1 ± 0.32+ | 0.93 ± 0.02 |
| N | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 |
Mean ± SEM.
p ≤ 0.05; +0.05 < p ≤ 0.10.
Female offspring.
At birth, female mismatch tended to weigh less (p = 0.06) than female controls (Table 5). Female mismatch showed lower abdominal circumference (p = 0.03) and trends toward lower hip circumference (p = 0.06) and BMI (p = 0.10) than female controls. Body length, ratio of hip:abdominal circumference, and circumferences of the head and chest were similar between female mismatch and controls.
Morphometrics from birth through 3 years of age
Male offspring.
Mismatch male body weight increased more steeply than controls (Fig 1; p < 0.0001). Mismatch males showed a trend toward steeper increase in BMI (p < 0.07) than controls (Fig 2). At age 3 years, mismatch males weighed 18.3% more and had 14.2% higher BMI than control males. Growth slopes of body length and circumferences of the head, chest, abdomen, and hips were similar between groups.
Figure 1.
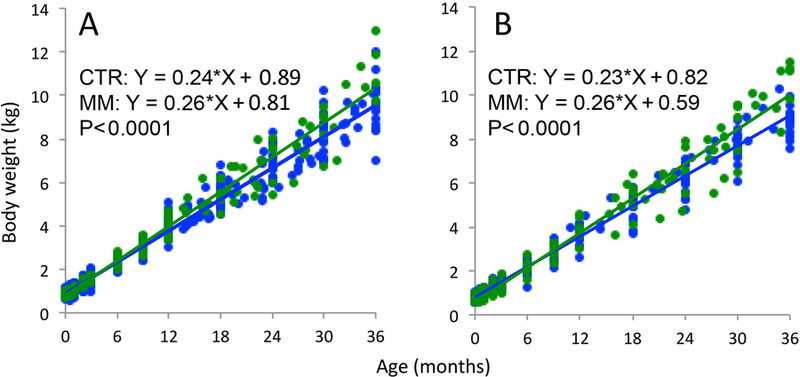
Body weight in control (CTR- blue) and mismatch (MM- green) offspring from birth through age 3 years. (A) MM males (N = 4–17) had steeper growth slopes than CTR males (N = 14–20). (B) MM females (N = 4–15) had steeper growth slopes than CTR females (N = 13–20). Sample sizes details for each timepoint in Table 2.
Figure 2.
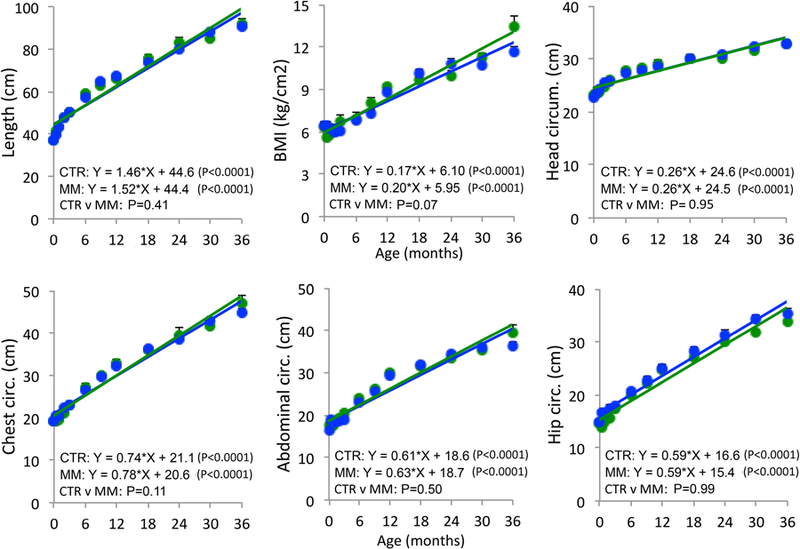
Male offspring morphometrics from birth through age 3 years in controls (CTR- blue; N = 14–20) and mismatch (MM- green; N = 4–17). Sample sizes details for each timepoint given in Table 2. Mean ± SEM.
Female offspring.
Mismatch female body weight grew more steeply than controls (Fig 1; p < 0.0001). Mismatch females showed steeper increase in BMI (p < 0.0001) and abdominal circumference (p = 0.008) compared to control females (Fig 3). At age 3 years, mismatch females weighed 16.5% more than control females, had 19% higher BMI, and had 10.3% higher abdominal circumference. Growth slopes of body length and circumferences of the head, chest, and hips were similar between groups.
Figure 3.
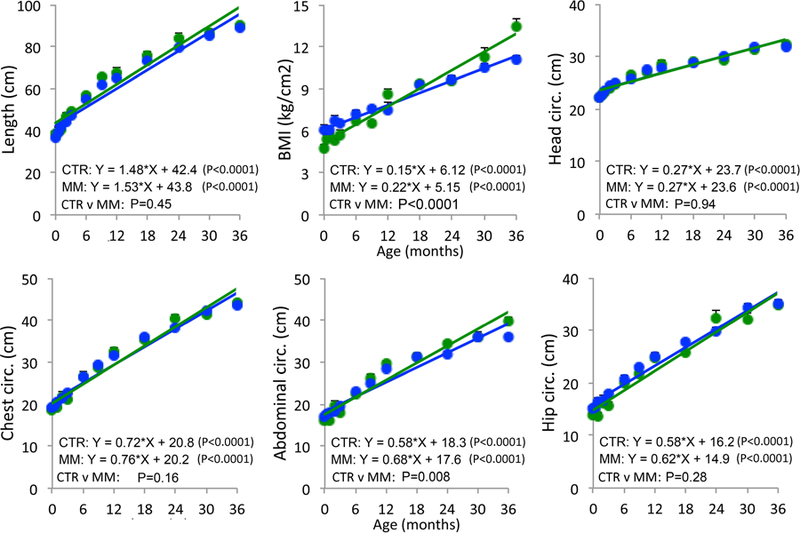
Female offspring morphometrics from birth through age 3 years in controls (CTR- blue; N = 13–20) and mismatch (MM- green; N = 4–15). Sample sizes details for each timepoint given in Table 2. Mean ± SEM.
ACTH and cortisol
ACTH and cortisol slopes from 2–24 months of age were similar between groups in both sexes (Fig 4). Groups of both sexes were also similar in mean cortisol and ACTH before and after weaning at 9 months of age (Fig 5). Cortisol and ACTH increased after weaning in males (p < 0.001) and females (p < 0.001) of both groups.
Figure 4.
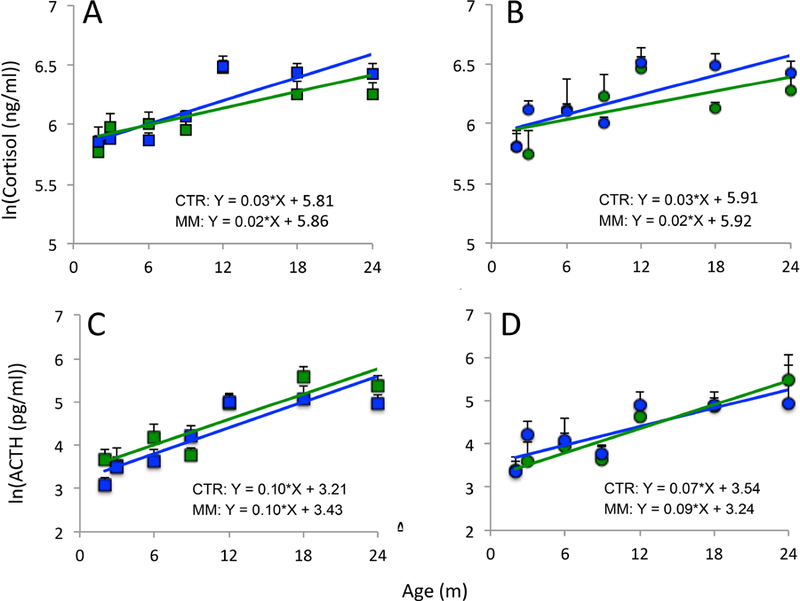
Cortisol in males (A) and females (B) and ACTH in males (C) and females (D) from 2–24 months of age. There were no differences in slopes between controls (CTR- blue; N = 3–10 males, 2–5 females) and mismatch (MM- green; N = 4–8 males, 4–6 females). Sample size details for each timepoint given in Table 3. Mean ± SEM.
Figure 5.
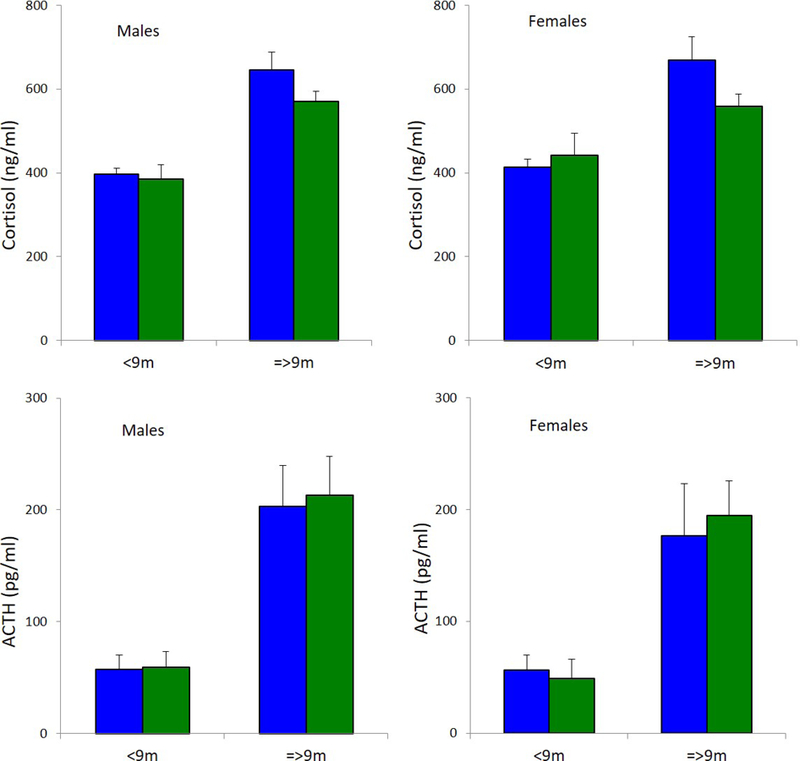
When compared before and after weaning at 9 months of age, mean cortisol and ACTH were similar between controls (CTR- blue; N = 3–10 males, 2–5 females) and mismatch (MM- green; N = 4–8 males, 4–6 females) of both sexes. In males and females of both groups, cortisol (p < 0.001) and ACTH (p < 0.001) increased after weaning. Sample size details for each timepoint given in Table 3. Mean ± SEM.
Discussion
Both under-nutrition in utero17,18 and over-nutrition in early postnatal life19,20 are known to have long-lasting developmental consequences. The combination of these two challenges during development has the potential for life-long functional adverse impacts on health. Fetuses exposed to under-nutrition tend to develop a thrifty phenotype, preparing for life in an environment of potentially reduced food availability by adjusting metabolism.2,3,5–7 If the prediction of reduced nutritional access is incorrect, and postnatally the individual experiences nutritional abundance, the adjustments made, such as increased glucose production and conservation, can be detrimental by predisposing to post-natal obesity and insulin resistance with increased risk of development of cardiometabolic syndrome.5–7 The occurrence of a mismatch in nutrition pre- and post-natally in human offspring is not uncommon. Poor nutrition in the womb can be caused not just by low maternal intake, but also by placental insufficiency, young or old maternal age, substance abuse, and maternal disease.1 Once the organism begins extra-uterine life, nutritional access may be greatly improved or even excessive as a result of unlimited nutrient availability, often in excess in western society.
In this study in our baboon model of mismatch between pre- and post-natal nutrition, we provided mothers with an 30% reduced diet during pregnancy and an obesogenic diet after delivery. Mismatch mothers lost weight during pregnancy while control mothers gained. Mismatch offspring were born with a trend toward reduced BMI and body weight. The profile of weight difference between groups changed direction and increased over the first three years of life, with mismatch of both sexes showing steeper growth slopes of body weight and BMI such that mismatch were eventually heavier with larger BMI. Interestingly, at birth mismatch males had larger abdominal circumference than control males, while in mismatch females abdominal circumference was smaller than in controls. The smaller abdominal circumference in mismatch females fits with changes seen in intrauterine growth restriction (IUGR) in humans,21–23 but the increase in mismatch males is of interest. It should be noted that the extent of IUGR, although statistically significant and thus meriting the term growth restricted, is relatively small at ~11% reduced from controls. This would correspond to about the 30th percentile in human newborns.24 We interpret this finding as showing that in the presence of moderate growth restriction males and females respond differently in their growth response and males show a resistance to a decrease in abdominal circumference and asymmetric growth retardation. The situation will undoubtedly change in the presence of a greater degree of growth restriction. At birth, mismatch males showed borderline lower ratio of hip:abdominal circumference than controls, while mismatch females showed smaller hip circumferences than controls with similar ratio of hip:abdominal circumference between groups. Postnatally, male growth slope of chest circumference was borderline steeper in mismatch compared to controls, while slopes of body length and circumferences of the head, hips, and abdomen were similar between groups over the first three years of life. In postnatal females, abdominal circumference of mismatch increased more steeply than controls, with mismatch females eventually having larger abdominal circumference despite starting out smaller at birth. Female growth slopes of body length and circumferences of the head, chest, and hips were similar between groups. Strikingly, by age 3 years, MM of both sexes had surpassed controls in nearly every measure of body size. Human and animal studies have shown that rapid postnatal catch-up growth following poor intrauterine growth increases risk of age-associated diseases.25 In humans, studies have documented that catch-up growth is associated with early indicators of type 2 diabetes and obesity,26,27 endothelial dysfunction,28 hypertension,29 cardiovascular disease,30 and nonalcoholic fatty liver disease.31 Similarly, experimental studies with rodents that were protein or calorie restricted in utero and then received obesogenic diets postnatally have shown reduced longevity,32,33 obesity,34,35 fatty livers,36 insulin resistance,37 hyperleptinaemia,35 and hepatic fibrosis and inflammation.38 Given the neonatal catch-up growth trajectory documented in the current study, we hypothesize these mismatch baboons will be predisposed to the same age-related diseases seen in humans and other animal models developmentally programmed by nutritional mismatch.
In addition to catch-up growth, cortisol and ACTH changes are of considerable interest in the mismatch model. The fetal hypothalamo-pituitary-adrenal axis plays a fundamental role in driving late fetal differentiation and slowing growth as delivery approaches, thereby balancing perinatal growth and differentiation during late gestation9 in preparation for an independent extra-uterine existence. In the baboons studied here, ACTH and cortisol concentrations in mismatch and controls were similar. It is noteworthy that in both groups cortisol increased after weaning at 9 months of age, confirming that weaning activates the hypothalamo-pituitary-adrenal axis in young baboons regardless of maternal diet.13,39 In another model of baboon maternal 30% nutrient reduction continuing through lactation, leading to offspring IUGR, we showed increased ACTH and cortisol compared to CTR in the second half of gestation, with similar ACTH and cortisol post-natally to those observed here in the mismatch model.13 For practical reasons, we were unable to measure fetal hormones in the current study, in which mothers were subjected to the same 30% nutrient reduction protocol during pregnancy, but we suggest based on prior findings there still could have been hormonal differences between mismatch and controls during fetal life that would have affected growth and development, even though post-natal hormonal levels were similar. During late gestation, cortisol plays a pivotal role in preparing the fetus for birth and adaptations needed for extra-uterine life, with maturational effects on the thyroid, lungs, intestines, liver, kidney, and brain favoring cellular differentiation over proliferation.40 Given that our model of IUGR has produced life-long changes to neurodevelopment,41 behavior,42–44 lipid metabolism,17 and cardiovascular outcomes,45–50 it is important to investigate these same life course functions in mismatch offspring, who may also have been exposed to glucocorticoid levels inappropriate for the current stage of development. Comparison with the extensive developmental programming in later life in IUGR offspring exposed to continuing nutrient deprivation during lactation will provide useful insights into the relative effects of pre- and postnatal challenges.
The baboon model of mismatch between pre- and post-natal nutrition sheds light on a cause of human age-related disease predisposition. The influence of programming on health span and life span have been well covered in a comprehensive review by Preston et al.8
Further study of the model will unveil mechanisms underlying common human diseases, such as obesity, diabetes, hypertension, and cardiovascular disease. This translational research is essential to forming the basic understanding required to develop therapeutic interventions. For example, innovative studies with rats have demonstrated the effectiveness of a simple intervention of postnatal supplementation of the antioxidant coenzyme Q10 in attenuating or even preventing the negative effects of pre- and post-natal nutritional mismatch.37,38 Animal models are uniquely situated to reveal ways to combat suboptimal development, leading to improvement of human health. Baboons are particularly strong models for human developmental outcomes because of their genetic similarity to humans, precocial stage at birth, long gestational period, singleton births, large brains, hormonal profile during gestation, and existence of wide-ranging data on maternal, fetal, juvenile, and adult baboon physiology.
Acknowledgements
The baboon cohorts were funded by NIH R24OD011183 and R24RR031459. We thank Karen Moore for administrative support. We are grateful to Martha Avila, Steve Rios, McKenna Considine, and Sam Vega for their work in animal husbandry and management. Ken Gerow and Geoff Clarke are greatly appreciated for valuable consultation on statistical approaches.
Footnotes
Institution at Which Work Was Performed: Southwest National Primate Research Center, San Antonio, Texas, USA
References
- 1.Resnik R, Creasy RK. Intrauterine growth restriction. In: Creasy RK, Resnik R, Iams JD, Lockwood CJ, Moore TR, eds. Creasy and Resnik’s Maternal-Fetal Medicine: Principles and Practice 6th ed. Philadelphia, PA: Saunders Elsevier; 2009:635–650. [Google Scholar]
- 2.Barker DJP. Mothers, Babies and Health in Later Life 2nd ed. Edinburgh: Churchill Livingstone; 1998. [Google Scholar]
- 3.Nathanielsz PW. Life in the Womb: The Origin of Health and Disease Promethean Press; 1999. [Google Scholar]
- 4.Nijland MJ, Mitsuya K, Li C, Ford S, McDonald TJ, Nathanielsz PW, Cox LA. Epigenetic modification of fetal baboon hepatic phosphoenolpyruvate carboxykinase following exposure to moderately reduced nutrient availability. J Physiol 2010;588(8):1349–1359. doi: 10.1113/jphysiol.2009.184168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hales CN, Barker DJP. The thrifty phenotype hypothesis: Type 2 diabetes. Br Med Bull 2001;60(1):5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- 6.Bateson P, Barker D, Clutton-Brock T, Deb D, D’Udine B, Foley RA, Gluckman P, Godfrey K, Kirkwood T, Lahr MM, McNamara J, Metcalfe NB, Monaghan P, Spencer HG, Sultan SE. Developmental plasticity and human health. Nature 2004;430(6998):419–421. doi: 10.1038/nature02725. [DOI] [PubMed] [Google Scholar]
- 7.Ravelli A, van der Meulen J, Michels R, Osmond C, Barker D, Hales C, Bleker O. Glucose tolerance in adults after prenatal exposure to famine. The Lancet 1998;351(9097):173–177. doi: 10.1016/S0140-6736(97)07244-9. [DOI] [PubMed] [Google Scholar]
- 8.Preston JD, Reynolds LJ, Pearson KJ. Developmental origins of health span and life span: A mini-review. Gerontology 2018;64(3):237–245. doi: 10.1159/000485506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fowden AL, Giussani DA, Forhead AJ. Intrauterine programming of physiological systems: causes and consequences. Physiology (Bethesda) 2006;21:29–37. doi: 10.1152/physiol.00050.2005. [DOI] [PubMed] [Google Scholar]
- 10.Bronikowski AM, Alberts SC, Altmann J, Packer C, Carey KD, Tatar M. The aging baboon: Comparative demography in a non-human primate. PNAS 2002;99(14):9591–9595. doi: 10.1073/pnas.142675599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schlabritz-Loutsevitch NE, Howell K, Rice K, Glover EJ, Nevill CH, Jenkins SL, Bill Cummins L, Frost PA, McDonald TJ, Nathanielsz PW. Development of a system for individual feeding of baboons maintained in an outdoor group social environment. J Med Primatol 2004;33(3):117–126. doi: 10.1111/j.1600-0684.2004.00067.x. [DOI] [PubMed] [Google Scholar]
- 12.Li C, Levitz M, Hubbard GB, Jenkins SL, Han V, Ferry RJ, McDonald TJ, Nathanielsz PW, Schlabritz-Loutsevitch NE. The IGF axis in baboon pregnancy: placental and systemic responses to feeding 70% global ad libitum diet. Placenta 2007;28(11–12):1200–1210. doi: 10.1016/j.placenta.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li C, Jenkins S, Mattern V, Comuzzie AG, Cox LA, Huber HF, Nathanielsz PW. Effect of moderate, 30 percent global maternal nutrient reduction on fetal and postnatal baboon phenotype. J Med Primatol 2017. doi: 10.1111/jmp.12290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schlabritz-Loutsevitch NE, Howell K, Rice K, Glover EJ, Nevill CH, Jenkins SL, Cummins LB, Frost PA, McDonald TJ, Nathanielsz PW. Development of a system for individual feeding of baboons maintained in an outdoor group social environment. J Med Primatol 2004;33(3):117–126. doi: 10.1111/j.1600-0684.2004.00067.x. [DOI] [PubMed] [Google Scholar]
- 15.Nathanielsz PW, Yan J, Green R, Nijland M, Miller JW, Wu G, McDonald TJ, Caudill MA. Maternal obesity disrupts the methionine cycle in baboon pregnancy. Physiological reports 2015;3(11). doi: 10.14814/phy2.12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li C, Ramahi E, Nijland MJ, Choi J, Myers DA, Nathanielsz PW, McDonald TJ. Up-regulation of the fetal baboon hypothalamo-pituitary-adrenal axis in intrauterine growth restriction: coincidence with hypothalamic glucocorticoid receptor insensitivity and leptin receptor down-regulation. Endocrinology 2013;154(7):2365–2373. doi: 10.1210/en.2012-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuo AH, Li C, Mattern V, Huber HF, Comuzzie A, Cox L, Schwab M, Nathanielsz PW, Clarke GD. Sex-dimorphic acceleration of pericardial, subcutaneous, and plasma lipid increase in offspring of poorly nourished baboons. Int J Obes 2018;42(5):1092–1096. doi: 10.1038/s41366-018-0008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zambrano E, Reyes-Castro LA, Nathanielsz PW. Aging, glucocorticoids and developmental programming. Age (Dordr) 2015;37(3):9774. doi: 10.1007/s11357-015-9774-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lomas-Soria C, Reyes-Castro LA, Rodríguez-González GL, Ibáñez CA, Bautista CJ, Cox LA, Nathanielsz PW, Zambrano E. Maternal obesity has sex dependent effects on insulin, glucose and lipid metabolism and the liver transcriptome in young adult rat offspring. J Physiol (Lond) July 2018. doi: 10.1113/JP276372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor PD, Matthews PA, Khan IY, Rees D, Itani N, Poston L. Generation of maternal obesity models in studies of developmental programming in rodents. Methods Mol Biol 2018;1735:167–199. doi: 10.1007/978-1-4939-7614-0_9. [DOI] [PubMed] [Google Scholar]
- 21.Platz E, Newman R. Diagnosis of IUGR: traditional biometry. Semin Perinatol 2008;32(3):140–147. doi: 10.1053/j.semperi.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Chauhan SP, Cole J, Sanderson M, Magann EF, Scardo JA. Suspicion of intrauterine growth restriction: Use of abdominal circumference alone or estimated fetal weight below 10%. J Matern Fetal Neonatal Med 2006;19(9):557–562. doi: 10.1080/14767050600798267. [DOI] [PubMed] [Google Scholar]
- 23.Suhag A, Berghella V. Intrauterine Growth Restriction (IUGR): Etiology and diagnosis. Curr Obstet Gynecol Rep 2013;2(2):102–111. doi: 10.1007/s13669-013-0041-z. [DOI] [Google Scholar]
- 24.Peleg D, Kennedy CM, Hunter SK. Intrauterine growth restriction: identification and management. Am Fam Physician 1998;58(2):453–460, 466–467. [PubMed] [Google Scholar]
- 25.Tarry-Adkins JL, Ozanne SE. Nutrition in early life and age-associated diseases. Ageing Res Rev 2017;39:96–105. doi: 10.1016/j.arr.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Crowther NJ, Cameron N, Trusler J, Gray IP. Association between poor glucose tolerance and rapid post natal weight gain in seven-year-old children. Diabetologia 1998;41(10):1163–1167. doi: 10.1007/s001250051046. [DOI] [PubMed] [Google Scholar]
- 27.Ong KK, Petry CJ, Emmett PM, Sandhu MS, Kiess W, Hales CN, Ness AR, Dunger DB, ALSPAC study team. Insulin sensitivity and secretion in normal children related to size at birth, postnatal growth, and plasma insulin-like growth factor-I levels. Diabetologia 2004;47(6):1064–1070. doi: 10.1007/s00125-004-1405-8. [DOI] [PubMed] [Google Scholar]
- 28.Touwslager RNH, Houben AJHM, Tan FES, Gielen M, Zeegers MP, Stehouwer CDA, Gerver W-JM, Westerterp KR, Wouters L, Blanco CE, Zimmermann LJ, Mulder ALM. Growth and endothelial function in the first 2 years of life. J Pediatr 2015;166(3):666–671.e1. doi: 10.1016/j.jpeds.2014.11.059. [DOI] [PubMed] [Google Scholar]
- 29.Law CM, Shiell AW, Newsome CA, Syddall HE, Shinebourne EA, Fayers PM, Martyn CN, de Swiet M. Fetal, infant, and childhood growth and adult blood pressure: a longitudinal study from birth to 22 years of age. Circulation 2002;105(9):1088–1092. [DOI] [PubMed] [Google Scholar]
- 30.Eriksson JG, Forsén T, Tuomilehto J, Winter PD, Osmond C, Barker DJ. Catch-up growth in childhood and death from coronary heart disease: longitudinal study. BMJ 1999;318(7181):427–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Faienza MF, Brunetti G, Ventura A, D’Aniello M, Pepe T, Giordano P, Monteduro M, Cavallo L. Nonalcoholic fatty liver disease in prepubertal children born small for gestational age: influence of rapid weight catch-up growth. Horm Res Paediatr 2013;79(2):103–109. doi: 10.1159/000347217. [DOI] [PubMed] [Google Scholar]
- 32.Ozanne SE, Hales CN. Lifespan: catch-up growth and obesity in male mice. Nature 2004;427(6973):411–412. doi: 10.1038/427411b. [DOI] [PubMed] [Google Scholar]
- 33.Jennings BJ, Ozanne SE, Dorling MW, Hales CN. Early growth determines longevity in male rats and may be related to telomere shortening in the kidney. FEBS Lett 1999;448(1):4–8. [DOI] [PubMed] [Google Scholar]
- 34.Bieswal F, Ahn M-T, Reusens B, Holvoet P, Raes M, Rees WD, Remacle C. The importance of catch-up growth after early malnutrition for the programming of obesity in male rat. Obesity (Silver Spring) 2006;14(8):1330–1343. doi: 10.1038/oby.2006.151. [DOI] [PubMed] [Google Scholar]
- 35.Cervantes-Rodríguez M, Martínez-Gómez M, Cuevas E, Nicolás L, Castelán F, Nathanielsz PW, Zambrano E, Rodríguez-Antolín J. Sugared water consumption by adult offspring of mothers fed a protein-restricted diet during pregnancy results in increased offspring adiposity: the second hit effect. Br J Nutr 2014;111(4):616–624. doi: 10.1017/S0007114513003000. [DOI] [PubMed] [Google Scholar]
- 36.Carr SK, Chen J-H, Cooper WN, Constância M, Yeo GSH, Ozanne SE. Maternal diet amplifies the hepatic aging trajectory of Cidea in male mice and leads to the development of fatty liver. FASEB J 2014;28(5):2191–2201. doi: 10.1096/fj.13-242727. [DOI] [PubMed] [Google Scholar]
- 37.Tarry-Adkins JL, Fernandez-Twinn DS, Madsen R, Chen J-H, Carpenter A, Hargreaves IP, McConnell JM, Ozanne SE. Coenzyme Q10 prevents insulin signaling dysregulation and inflammation prior to development of insulin resistance in male offspring of a rat model of poor maternal nutrition and accelerated postnatal growth. Endocrinology 2015;156(10):3528–3537. doi: 10.1210/en.2015-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tarry-Adkins JL, Fernandez-Twinn DS, Hargreaves IP, Neergheen V, Aiken CE, Martin-Gronert MS, McConnell JM, Ozanne SE. Coenzyme Q10 prevents hepatic fibrosis, inflammation, and oxidative stress in a male rat model of poor maternal nutrition and accelerated postnatal growth. Am J Clin Nutr 2016;103(2):579–588. doi: 10.3945/ajcn.115.119834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mandalaywala TM, Higham JP, Heistermann M, Parker KJ, Maestripieri D. Physiological and behavioural responses to weaning conflict in free-ranging primate infants. Anim Behav 2014;97:241–247. doi: 10.1016/j.anbehav.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liggins GC. The role of cortisol in preparing the fetus for birth. Reprod Fertil Dev 1994;6(2):141–150. [DOI] [PubMed] [Google Scholar]
- 41.Franke K, Clarke GD, Dahnke R, Gaser C, Kuo AH, Li C, Schwab M, Nathanielsz PW. Premature brain aging in baboons resulting from moderate fetal undernutrition. Front Aging Neurosci 2017;9:92. doi: 10.3389/fnagi.2017.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huber HF, Ford SM, Bartlett TQ, Nathanielsz PW. Increased aggressive and affiliative display behavior in intrauterine growth restricted baboons. J Med Primatol 2015;44(3):143–157. doi: 10.1111/jmp.12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keenan K, Bartlett TQ, Nijland M, Rodriguez JS, Nathanielsz PW, Zürcher NR. Poor nutrition during pregnancy and lactation negatively affects neurodevelopment of the offspring: Evidence from a translational primate model. Am J Clin Nutr 2013;98(2):396–402. doi: 10.3945/ajcn.112.040352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodriguez JS, Bartlett TQ, Keenan KE, Nathanielsz PW, Nijland MJ. Sex-dependent cognitive performance in baboon offspring following maternal caloric restriction in pregnancy and lactation. Reprod Sci 2012;19(5):493–504. doi: 10.1177/1933719111424439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuo AH, Li C, Li J, Huber HF, Nathanielsz PW, Clarke GD. Cardiac remodelling in a baboon model of intrauterine growth restriction mimics accelerated ageing. J Physiol (Lond) 2017;595(4):1093–1110. doi: 10.1113/JP272908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuo AH, Li C, Huber HF, Schwab M, Nathanielsz PW, Clarke GD. Maternal nutrient restriction during pregnancy and lactation leads to impaired right ventricular function in young adult baboons. J Physiol 2017;595(13):4245–4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuo AH, Li J, Li C, Huber HF, Nathanielsz PW, Clarke GD. Poor perinatal growth impairs baboon aortic windkessel function. J Dev Orig Health Dis 2018;9(2):137–142. doi: 10.1017/S2040174417000770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuo AH, Li C, Huber HF, Clarke GD, Nathanielsz PW. Intrauterine growth restriction results in persistent vascular mismatch in adulthood. J Physiol (Lond) 2018;596(23):5777–5790. doi: 10.1113/JP275139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clarke GD, Li J, Kuo AH, Nathanielsz PW. TU-F-CAMPUS-I-03: Quantitative cardiac MRI reveals functional abnormalities in intrauterine growth restricted (IUGR) baboons. Med Phys 2015;42(6):3646–3647. doi: 10.1118/1.4925828. [DOI] [Google Scholar]
- 50.Kuo AH, Li C, Huber HF, Nathanielsz PW, Clarke GD. Ageing changes in biventricular cardiac function in male and female baboons (Papio Sp.). J Physiol 596(21):5083–5098. [DOI] [PMC free article] [PubMed] [Google Scholar]


