Abstract
Background:
The advent of continuous glucose monitoring (CGM) technology has transformed the approach to diabetes care. Multiple CGM systems are commercially available and increased accuracy has allowed development of hybrid and automated insulin delivery systems. Evidence of CGM clinical benefits has also increased exponentially in the last decade.
Methods:
Literature search, review of professional guidelines, and consensus statements were used to guide the preparation of this article. The clinical benefits of both professional and personal CGM in clinical practice as well as barriers to wider adotpion were explored. A stepped approach to review and interpretation of CGM data is suggested for use in the clinician’s office regardless of the software used.
Results:
Although increasing, the use of CGM in patients with diabetes is still not widespread; multiple barriers are still in place, despite the approval of CGM systems for patients above the age of 2 years old, the extension of coverage for Medicare beneficiaries and the integration of CGM with multiple insulin pump systems. Integration of CGM technology in clinical practice presents various challenges, from concerns relative to time constraints during office visits to lack of systematic approach to interpretation of the data.
Conclusions:
Understanding the usefulness of personal and professional CGM, appropriate patient selection as well as patient and provider training are crucial for the expansion of CGM therapy use in clinical practice. Utilizing the proposed stepped approach to CGM review and interpretation may allow wider adoption of CGM with more effective and efficient office visits.
Keywords: continuous glucose monitoring, diabetes, insulin pump, multiple daily injections, ambulatory glucose profile
CGM technology has advanced at a fast pace since the first systems were introduced in 1999; its use has transformed the approach to diabetes care in the last decade.1 Presently, eight CGM systems are FDA- approved (see Table 1), and more systems will likely become commercially available in the next 2-3 years. With improved accuracy and reduced mean absolute relative difference (MARD) to 10% or less for most systems, sensor glucose (SG) values can be used safely to make therapeutic dose decisions.2-4 With factory-calibrated sensors, CGM systems are becoming increasingly appealing tools to replace self-monitoring of blood glucose (SMBG) by fingerstick in the future.5,6 It is well appreciated that while SMBG gives single snapshots of glucose levels, CGM data give users and clinicians dynamic information of glucose levels overtime, displaying data continuously and providing alerts and alarms for current and impending hypo- or hyperglycemia. The duration of sensor life has also been extended, and commercially available sensors in the United States can be used for 7, 10, 14, and 90 days.7-12
Table 1.
Available Professional and Personal CGM Systems (FDA Approved).
| CGM system | MARD (%) |
Warm-up time | Sensor wear duration (days) | Calibrations | Audible alarm alerts | Pump integration, compatible devices software | Acetaminophen interference |
|---|---|---|---|---|---|---|---|
| Professional CGM | |||||||
| Medtronic iPro2 | 13.6 | 2 hours | Up to 6 | N/A | N/A | N/A | Yes |
| Dexcom G4 Platinum Professional | 9 | 2 hours | Up to 7 | 2/day | None if blinded; yes if unblinded | N/A | Yes |
| FreeStyle Libre Pro | 12.3 | 2 hours | Up to 14 | N/A | None | N/A | No |
|
Personal CGM |
|||||||
| Medtronic Enlite 2 | 13.6 | 2 hours | Up to 6 | 2/day | Yes Predictive alerts |
MiniMed Medtronic Revel MiniMed Medtronic 530G MiniMed Medtronic 630G |
Yes |
| Medtronic Guardian 3 Abdomen Arm |
10.6*−9.6** 9.1*−8.7** |
2 hours |
Up to 7 |
2-4/day |
Yes Predictive alerts |
MiniMed Medtronic 670G Medtronic Guardian Connect |
Yes |
| Dexcom G4 Platinum | 9 | 2 hours | Up to 7 | 2/day | Yes | Animas Vibe Tandem t:slim |
Yes |
| Dexcom G5 | 9 | 2 hours | Up to 7 | 2/day | Yes | Tandem t:slim X2 | Yes |
| Dexcom G6 | 9 | 2 hours | Up to 10 | None | Yes Hypoglycemia Predictive alerts |
Tandem t:slim X2 with Basal IQ | No |
| FreeStyle Libre 10 days 14 days |
9.7 9.4 |
12 hours 1 hour |
Up to 10 Up to 14 |
None§ None§ |
None None |
None None |
No No |
| Senseonics Eversense | 8.5 | 24 hours | Up to 90 | 2/day | Yes Hypoglycemia Predictive alerts |
None | No |
A finger prick test using a blood glucose meter is required during times of rapidly changing glucose levels when interstitial fluid glucose levels may not accurately reflect blood glucose levels or if hypoglycemia or impending hypoglycemia is reported by the system or when symptoms do not match the system readings (reference).
2 calibrations/day. **4 calibrations/day.
With increased CGM system accuracy and insulin pump systems integration, the path toward automated insulin delivery has also made incredible progress, allowing the development and study of multiple platforms and algorithms for closed loop systems.13-15 Presently, various integrated CGM/insulin pump systems are commercially available.16,17 Similarly, several automated insulin delivery models are being investigated.18-21 It is reasonable to predict that the transition to fully automated insulin delivery systems could become a reality in the next 5 years.
However, despite these significant advances in diabetes technology, persistent barriers to widespread CGM adoption continue to exist. In the clinical setting, routine integration of personal or professional CGM has not been optimized. This article aims at reviewing the clinical benefits of professional and personal CGM and its application to daily practice; it also aims to analyze the role of the patient and practitioner as barriers to wider adoption. Finally, it suggests a simple, stepped approach to review and interpretation of CGM data, a potentially useful tool for clinicians regardless of CGM system brand or software used.
Methods
Search and review of the medical literature, societies’ guidelines, consensus statements, professional experience, and endocrinology fellows training guided the preparation of this article. Detailed analysis of available CGM categories, their application in patients with diabetes, and the benefits and challenges of CGM integration in clinical practice were evaluated.
Results
Benefits of CGM Therapy
The clinical benefits of personal CGM use in patients with type 1 diabetes (T1DM) have been demonstrated in multiple clinical trials over the last decade. Reduction of HbA1c and hypoglycemia as well as glycemic variability is well established, regardless of the method of insulin therapy.22 The 2016 CGM Consensus Conference publication from the American Association of Clinical Endocrinologists (AACE) states that CGM is likely to benefits any patient on intensive insulin therapy, regardless of the type of diabetes.23 In 2016, the Endocrine Society Clinical Practice Guidelines on diabetes and technology recommended intermittent use of CGM for adult patients with type 2 diabetes (T2DM) (not on prandial insulin) with HbA1c ⩾7%, willing and able to use the device.24 The reason for intermittent use was dictated by the fact that, at the time of publication, data for evidence using CGM in patients on MDI was not yet available. More recently, evidence of personal CGM benefits in patients with T2DM on intensive insulin therapy has been published, and reports of positive effects on glycemic control for T2DM patients not on insulin therapy are also increasing.25-28
CGM use improves diabetes-specific quality of life markers such as diabetes distress and hypoglycemic confidence,29 and lowers adolescent and parent distress.30 Similarly, economic benefits of CGM therapy have been reported with increased frequency, with decrease in total health care costs and hospital admissions.31,32
Role of Personal and Professional CGM in Clinical Practice
CGM use in clinical practice falls in two main categories, personal and professional. The characteristics of available CGM systems are outlined in Table 1. Personal CGM systems, known as real-time (rt-CGM) or intermittently scanned (is-CGM) systems, record glucose every 5-15 minutes via a disposable or implantable sensor with life duration from 6 to 90 days, depending on manufacturer. The information is sent via a transmitter to a display device, either a dedicated reader or a smart phone App. While Medtronic (Northridge, CA), Dexcom (San Diego, CA) and Abbott (Alameda, CA) CGM systems use an enzymatic reaction with glucose oxidase as substrate, Senseonics (Germantown, MA) Eversense® CGM system uses a fluorescence sensing technology. Some CGM systems are used nonadjunctively and patients can make insulin dose decisions based on SG readings. This particular feature has decreased the frequency of SMBG and has been shown to be safe and effective3 without worsening of glucose control.33 CGM coverage is now available for Medicare beneficiaries34,35 and the most recent factory-calibrated CGM systems approval has virtually eliminated the need for confirmatory blood glucose levels by fingerstick.9,12
Professional CGM systems (diagnostic or retrospective CGM) are usually owned by practices and worn intermittently by patients in either a “blinded or “unblinded” fashion. Their features are outlined in Table 1. Even though these tools are usually recommended mostly for patients with T1DM,24,36 in reality, any patient with diabetes presenting to the endocrinologist office could greatly benefit from wearing blinded or unblinded professional CGM.37 Often the professional CGM sessions can be used as a “catalyst” for patients to move toward starting personal CGM therapy. In T2DM patients, professional CGM could have a great added value for the ability to “uncover” the presence of hypo- and hyperglycemia or hypoglycemia unawareness. It has been recently reported that patients with T2DM have significant fear of hypoglycemia or hypoglycemia unawareness, which may limit intensification of therapy to achieve glycemic goals.38 For these patients, mostly on intensive insulin regimen, professional CGM could provide not only direct information of patients’ “glycemic status,” but also actionable interventions, whether pharmacological or educational, and facilitate personalized regimen changes. Evidence from randomized-controlled trials as well as observational studies indicate that professional CGM is beneficial for T2DM patients on various therapies.26
Standards of care for diabetes and guidelines from professional societies recommend that all patients with T1DM and those with T2DM on intensive insulin therapy should be placed on therapeutic CGM (personal CGM) or use professional CGM intermittently.23,24,39
As previously reported,40 to maximize the benefits of professional CGM sessions in clinical practice, patients should be advised to keep a blood glucose log, as well as medication, food and activity diary. Documenting events that may contribute to changes in glucose levels such as physical activity, stressors, illness, menses, special events, is also advised.
In addition, scheduling the CGM procedure to include weekdays as well as weekends is very useful, so that information relative to variable schedule and its effects on glucose profiles can be captured. When setting up a professional CGM session, targeted therapeutic interventions can be made or new medications can be started; patients can use them for a portion of the procedure. This enables patients to promptly evaluate the effects of the new regimen on their glucose profiles upon review of CGM data with the clinician. If hypoglycemia unawareness is strongly suspected, unblinded professional CGM should be recommended, setting up hypoglycemia alerts accordingly and instructing patients on how to adequately treat hypoglycemic episodes should they occur during the procedure. Clear instructions should be given to the patient if frequent hypoglycemia below 55 mg/dL is noted during the procedure, so that immediate action can be taken to modify medication regimen prior to the end of the procedure.
While some argue that blinded CMG should no longer be used,41 blinded CGM can still be used successfully in clinical practice. For example, in patients with T2DM with language or cognitive barriers who may be overwhelmed by the large amount of data generated by unblinded CGM, using FreeStyle Libre Pro system could provide great advantage in view of its simplicity of use and minimal training.
On the other hand, many patients are able to recognize overtime their glucose trends and patterns; for them, real-time CGM or unblinded professional CGM is undoubtedly a great tool. This process however takes time, would require pattern recognition training, and may or may not be accomplished within the limited time allowed for a professional unblinded CGM procedure.
For this reason, clinicians should carefully consider the most appropriate type of professional CGM on an individual basis. The assistance of a certified diabetes educator within a Diabetes Education Program, if available, can be invaluable.
Benefits of Patient and Provider CGM Training in Clinical Practice
The use of personal CGM has been gradually increasing. However, despite the substantial evidence of clinical, patient-related outcomes and cost -effectiveness benefits of CGM, the widespread adoption of CGM is still lacking. The T1D Exchange Registry Clinic Network has over 30,000 individuals with T1DM from over 80 clinics across the United States. In 2010-2012 they reported that only 7% of participants were using CGM.42 In 2014 Wong et al reported that of 1662 CGM users for at least 1 year, 41% had discontinued using CGM. The three main reported reasons were discomfort (42%), problems with insertion (33%), or adhesion (30%).43 Since 2012, the use of CGM in the T1D Exchange Registry has increased, with the largest uptake in the pediatric population. The most recent T1D Exchange Registry statistics report 30% CGM use in enrolled participants (2016-2018).44 In addition, Abbott has recently reported that 800,000 people in 43 countries worldwide are using FreeStyle Flash Libre system.12
Historically, known barriers have included perceived poor accuracy, cost, alarm fatigue and short duration of sensors.43,44 Although many of these barriers have been overcome with improved accuracy, increase in sensor duration, comfort of insertion and decreased need or elimination of calibration, challenges due to cost and reimbursement continue to remain a reality. Notably, there is increasing evidence suggesting that health care professionals’ attitude toward CGM constitutes a barrier to CGM use expansion.45 In addition, Tanenbaum et al46 have reported a readiness chart for clinicians (MDs and CDEs) who are likely to prescribe diabetes technology tools. Three “clinician personas” were described, based on their readiness to promote CGM technology and comfort in keeping up with technology advances. Of these (n = 209) only 20% were “ready,” while 41% fell in the category of “cautious” and 40% were “not yet ready.” These data emphasize how clinicians may benefit from tailored training, as well as additional resources to help expanding the use of CGM therapy in clinical practice.
Therefore, provider and patient education is crucial. To achieve the full benefits of CGM therapy, patients need adequate training, such as understanding insertion techniques, and calibration techniques for the systems that still require it, reviewing frequency and optimal timing of calibrations. CGM use on a nearly daily basis and active participation with data review or reports notifications should be encouraged. Setting alerts and alarms should be done in stages. To avoid alarm fatigue, it may be prudent to set-up only hypoglycemia alerts upon training and postpone hyperglycemia alerts set-up to a later time, based on individual patient needs. Alternatively, wide thresholds should be considered (70-250 mg/dL) as suggested previously.47 For patients without history of hypoglycemia unawareness, who are concerned about alarm fatigue, Abbott’s FreeStyle Flash Libre offers a viable solution in that it only alerts the users when they scan the reader over the sensor/transmitter unit. Advanced training should also be offered to patients after several weeks of CGM use to include predictive alerts, recognition of patterns and trends as well as setting up trend arrows-based insulin dose adjustments. Of note, available CGM systems have different rate of change trend arrows meaning (see Table 2). To avoid confusion and dosing errors, education should be focused to the specific system patients are using.48-50
Table 2.
Rate of Change Trend Arrows in FDA-Approved CGM Systems and Integrated Insulin Pump Systems.
| Medtronic MiniMed Enlite 2 (530G) |
Medtronic MiniMed Guardian 3 (670G) Enlite 2 (630G) Guardian Connect |
Dexcom G4 Platinum G5, t:slim X2 G6, t:slim X2-Basal IQ |
FreeStyle Libre | Senseonics Eversense | |
|---|---|---|---|---|---|
|
N/A | Glucose is rising at a rate of ⩾3 mg/dL per minute | N/A | N/A | N/A |

|
Glucose is rising at a rate of 2 mg/dL or more per minute | Glucose is rising at a rate of ⩾2 but <3 mg/dL per minute | Glucose is rapidly rising >3 mg/dL per minute | N/A | N/A |

|
Glucose is rising at a rate of 1 to 2 mg/dL per minute | Glucose is rising at a rate of ⩾1 but <2 mg/dL per minute | Glucose is rising 2-3 mg/dL per minute | Glucose is rising quickly (>2 mg/dL per minute) | Very rapidly rising glucose levels, rising at a rate more than 2 mg/dL per minute |

|
N/A | N/A | Glucose is slowly rising 1-2 mg/dL per minute | Glucose is rising (1-2 mg/dL per minute) | Moderately rising glucose level, rising at a rate between 1 mg/dL and 2 mg/dL per minute |

|
N/A | N/A | Steady; glucose is not increasing/decreasing >1 mg/dL per minute | Glucose is changing slowly (<1 mg/dL per minute) | Gradually rising or falling glucose levels, falling or rising at a rate between 0 and 1 mg/dL per minute |

|
N/A | N/A | Glucose is slowly falling 1-2 mg/dL per minute | Glucose is falling (1-2 mg/dL per minute) | Moderately falling glucose levels, falling at a rate between 1 mg/dL and 2 mg/dL per minute |
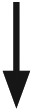
|
Glucose is falling at a rate of 1 to 2 mg/dL per minute | Glucose is falling at a rate of ⩾1 but <2 mg/dL per minute | Glucose is falling 2-3 mg/dL per minute | Glucose is falling quickly (>2 mg/dL per minute) | Very rapidly falling glucose levels, falling at a rate more than 2 mg/dL per minute |
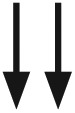
|
Glucose is falling at a rate of 2 mg/dL or more per minute | Glucose is falling at a rate of ⩾2 but <3 mg/dL per minute | Glucose is rapidly falling >3 mg/dL per minute | N/A | N/A |
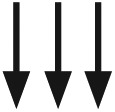
|
N/A | Glucose is falling at a rate of ⩾3 mg/dL per minute | N/A | N/A | N/A |
Discussion
Optimal integration of CGM technology in clinical practice presents multiple challenges for providers. Whether dealing with professional or personal CGM systems, clinicians are faced with the need to become familiar with several brand-specific software programs and reports. Although there are efforts toward standardization of CGM review and interpretation,51 there are no systematic, standardized approaches, nor formal “CGM education courses” available to providers. Further challenges include the need for ancillary staff to upload CGM and/or pump systems, and the time to sift through multiple reports from which to gather meaningful information and implement regimen changes for patients within the often-restricted time of an office visit.
The development of the ambulatory glucose profile (AGP) has been a fundamental initial step to standardize CGM data reporting tools. The AGP combines input from multiple days displaying CGM data into a single 24-hour period of time. It is one of the most helpful reports for the provider and a great place to start the CGM review. Initially developed by Mazze et al,52 this report has been adopted by various platforms, including Dexcom CLARITY®, Diasend®-Glooko, Tidepool®, LibreView® and Medtronic CareLink™ and is recommended as the standard for visualization of CGM data.51 The use of AGP in clinical practice, with modification of work flow to include printing of AGP report before rooming the patient, has been shown to reduce visits time in two centers.53 Ideally, at least 14 days of data should be reviewed, as this has been shown to provide a good estimate of glycemic values for a 3-month period.54 The AGP has multiple components, each of them contributing to gather information on the patient glycemic status at the time of the visit. Review of 14 days with CGM use of at least 70% are the first steps to ensure adequacy of data. SG average, coefficient of variation (CV), and standard deviation (SD) should follow. The CV is a measure of glycemic variability (GV), and an important element to review, as increased variability is associated with increased risk of hypoglycemic events.55,56 GV has also been the focus of numerous studies and has been associated to increased risk for diabetes complications, cognitive function, and quality of life.51
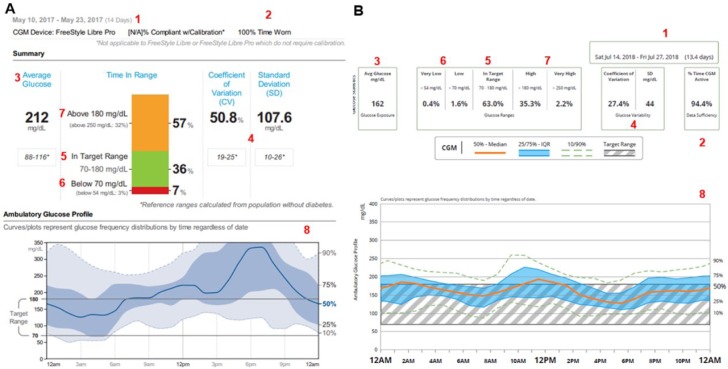
When reviewing CGM data, it is critical to encourage the patient to aim for the highest percentage of glucose levels in target range (or Time in Range, TIR) of 70-180 mg/dL with very low percentage of time spent in hypoglycemia (Figure 1). Achievement of high percentage spent in target range should be a process, and individual targets should be established with patients. Hypoglycemia definition has been revised57 and in the AGP particular attention should be given to the percentage of time spent in level 1 or level 2 hypoglycemia (<70 mg/dL and <54 mg/dL, respectively). Hyperglycemia has also been redefined to includes level 1 and level 2 (>180 mg/dL and >250 mg/dL, respectively). Identification of episodes of level 2 hypoglycemia of any duration require immediate action during CGM review. Although there is no available clinical trial assessing the optimal percentage of time to spend in target range, hypo or hyperglycemia, it is reasonable to consider using the targets achieved with the most advanced insulin delivery/CGM technology, the hybrid-closed loop.58 In the pivotal study participants achieved on average 72% TIR, with <3% in hypoglycemia and <25% in hyperglycemia. TIR and HbA1c are also highly correlated, and TIR of ~70% corresponds to an HbA1c of ~7%.59
Figure 1.
Ambulatory glucose profile (AGP) information and time in ranges glucometrics with Libre View (A) and Dexcom Clarity (B) software. AGP profile and time in ranges glucometrics can be interpreted similarly regardless of the software. Basic information tools include:
1. Number of days analyzed. Ideally at least 10-14 days are needed.52
2. Time of sensor wear. Ideally as close to 100%, but at least ⩾70%.56
3. Glucose average or glucose exposure to have a sense of the glucose management indicator (GMI).60
4. Standard deviation and coefficient of variation to determine glycemic variability and increase risk for hypoglycemia.53,54
5. Time spent in target range. Ideally close to 70%.56
6. Time spent in hypoglycemia. Ideally <3%.49
7. Time spent in hyperglycemia.
8. AGP profile with median, 25-75% percentile, and 10-90% percentile to identify areas of concern with recurrent trends and patterns.
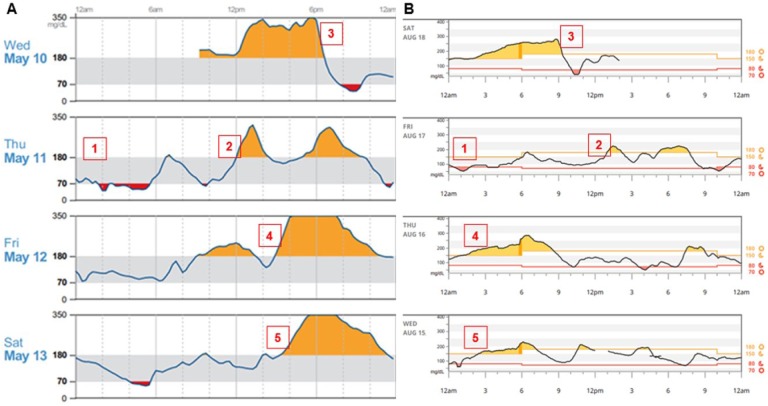
Review of daily views reports, whether for CGM alone or in combination with insulin pump data, is paramount (see Figures 2 and 3), as they allow for more granular information, so that glycemic fluctuations can be correlated to specific causes. Recurring patterns of hypo/hyperglycemia should be reviewed, and focus should be directed to the duration of each episode, especially hypoglycemia. Time spent in hyperglycemia, fasting, as well as from postprandial glycemic excursions should be reviewed next, and interventions made to adjust therapy as needed, whether related to basal insulin (or basal rates if using insulin pump therapy), mealtime insulin doses, or insulin sensitivity factor. Lifestyle effects on glucose levels such as physical activity, meal quality, and alcohol use should be reviewed and discussed. Also, effects of excessive insulin pump suspension time or insulin pump site failure should be identified and discussed to optimize insulin pump use. Finally, CGM alerts settings should be reviewed and individualized, so that alarm fatigue is avoided or minimized. A proposed stepped method to facilitate meaningful interpretation of CGM data is described in Table 3.
Figure 2.
Daily CGM view using Libre View (A) or Dexcom Clarity (B). Daily view allows the clinician the opportunity to identify the following:
1. Nocturnal hypoglycemia, whether this is isolated or recurrent.
2. Postprandial hyperglycemia.
3. Postprandial hypoglycemia, likely induced by multiple correction doses of insulin, causing insulin stacking and hypoglycemia.
4-5. Recurrent wide glycemic fluctuations at dinnertime (A) or overnight (B).
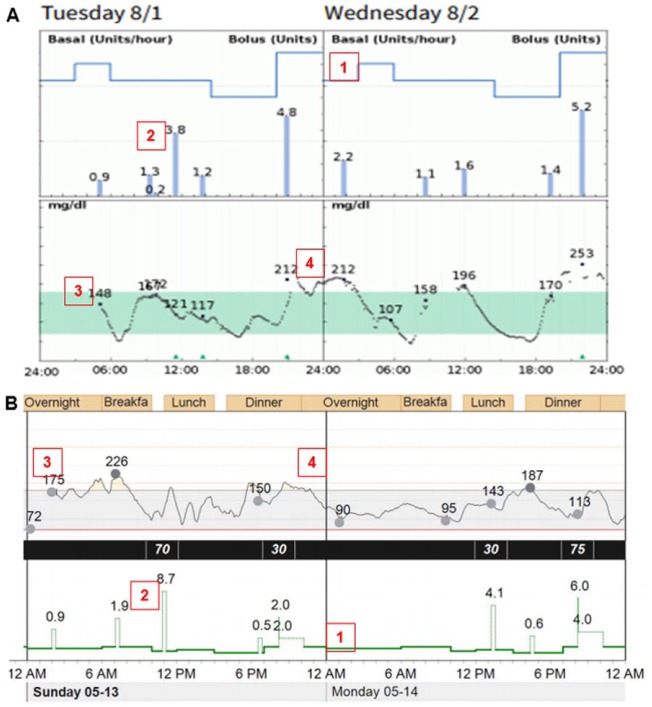
Figure 3.
Usefulness of integrated insulin pump and CGM visualization.
A. Diasend-Glooko, data from Tandem t:slim X2 with Dexcom G5 CGM and OneTouch Verio glucose meter.
B. Medtronic CareLink, data from MiniMed 530G insulin pump with Enlite 2 CGM and Ascensia Contour Next USB Link glucose meter.
Basic information tools include:
1. Basal rate.
2. Bolus.
3. Glucose meter values.
4. CGM tracing.
Table 3.
Stepped Approach to CGM Data Review and Interpretation.
| Whenever Possible Compare with Previous Data to Assess Glucometrics Changes | |
|---|---|
| CGM key metric for data analysis | Targets, action |
| Data sufficiency | 10-14 days54 |
| CGM use | >70%58 |
| Sensor glucose average—glucose exposure | Mean glucose values over the preceding 2 weeks |
| Glycemic variability (GV) • Coefficient of variation (CV) • Standard deviation (SD) |
<36%55
<33% of mean sensor glucose value56 |
| Percentage of time in target range (70-180 mg/dL) | >70%59 |
| Percentage of time in hypoglycemia • Level 1 (<70-54 mg/dL) • Level 2 (<54 mg/dL) |
<3%51
- Alert, monitor - Clinically significant, immediate action required |
| Percentage of time in hyperglycemia • Level 1 (>180 mg/dL) • Level 2 (>250 mg/dL) |
<25%58
- Alert, monitor - Clinically significant, immediate action required |
| AGP graphs | - Identify hypoglycemia patterns time of day/night (10th
percentile <70 mg/dL or <54 mg/dL) - Identify hyperglycemia patterns time of day/night (90th percentile >250 mg/dL) - Identify areas of greater GV (wider “cloud” or “ribbon”—25th-75th percentile) - Identify time of day with recurrent patterns (smaller width of the “cloud” or “ribbon”) - Determine if hypo/hyperglycemia is caused by basal rates/basal insulin dose - Identify mealtime patterns and glycemic excursions |
| CGM daily view—modal day view and/or integrated insulin pump/CGM daily views | Hypoglycemia - Identify nocturnal hypoglycemia and assess whether this is isolated or recurrent - Monitor for weekdays vs weekends hypoglycemia - Verify or obtain information from patient to assess contributors of nocturnal hypoglycemia - Basal rates or long acting insulin dose - Physical activity, type, duration, and time of day - Alcohol intake - Other evening activities - Identify daytime pre- or postprandial hypoglycemia - Verify or obtain information from patient to assess contributors of daytime hypoglycemia - Physical activity, type, duration, and time of day - Meal times or missed meal after insulin dosing - Meal quality (high or low glycemic index) - Insulin dosing times - Insulin to carbohydrate ratio - Insulin sensitivity factor (or correction factor) - Insulin stacking from aggressive use of correction dose Hyperglycemia - Identify daytime pre-, postprandial, and nocturnal hyperglycemia - Monitor for weekdays vs weekends hyperglycemia - Obtain information from patient to assess contributors - Meal times - Missed mealtime insulin dose - Meal quality (high or low glycemic index) - Insulin dosing times - Insulin to carbohydrate ratio - Insulin sensitivity factor (or correction factor) - Basal rates or long acting insulin dose - Prolonged use of temporary basal or suspension of insulin delivery - Possible insulin pump site failure |
Conclusion
CGM therapy has transformed the care of diabetes in the last decade, and its benefits could extend to any patient with diabetes regardless of the type of pharmacological therapy. With factory-calibrated sensors, CGM may replace the need for SMBG in the near future. However, patient and provider CGM uptake is still limited, and standardized CGM interpretation protocols are lacking.
Tailored education for patients and clinicians for their respective needs may be the key to expanded use of CGM in clinical practice. This article proposes suggestions for streamlined patient education and a stepped approach to CGM interpretation for the clinicians. Formal studies and validation of CGM interpretation protocols are needed to develop “best practices” for CGM integration in clinical practice.
Footnotes
Abbreviations: AACE, American Association of Clinical Endocrinologists; ADA, American Diabetes Association; AGP, ambulatory glucose profile; CGM, continuous glucose monitoring; FDA, Food and Drug Administration; HbA1c, glycated hemoglobin; MARD, mean absolute relative difference; MDI, multiple daily injections; SG, sensor glucose; SMBG, self-monitoring of blood glucose; T1DM, type 1 diabetes; T2DM, type 2 diabetes.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: GA has served as a consultant for Dexcom and Medtronic; also as steering committee member for Dexcom and on an advisory board for Novo Nordisk; her institution has received research grant support from AstraZeneca, Novo Nordisk, and Dexcom. KW has no multiplicity interests to disclose.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Rodbard D. Continuous glucose monitoring: a review of recent studies demonstrating improved glycemic outcomes. Diabetes Technol Ther. 2017;19:S25-S37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kovatchev BP, Patek SD, Ortiz EA, Breton MD. Assessing sensor accuracy for non-adjunct use of continuous glucose monitoring. Diabetes Technol Ther. 2015;17:177-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aleppo G, Ruedy KJ, Riddlesworth TD, et al. REPLACE-BG: a randomized trial comparing continuous glucose monitoring with and without routine blood glucose monitoring in adults with well-controlled type 1 diabetes. Diabetes Care. 2017;40:538-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. US Food and Drug Administration. FDA expands indication for continuous glucose monitoring system, first to replace fingerstick testing for diabetes treatment decisions. Available at: https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm534056.htm. Accessed August 18, 2018.
- 5. Bailey T, Bode BW, Christiansen MP, Klaff LJ, Alva S. The performance and usability of a factory-calibrated flash glucose monitoring system. Diabetes Technol Ther. 2015;17:787-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shah VN, Laffel LM, Wadwa RP, Garg SK. Performance of a factory-calibrated real-time continuous glucose monitoring system utilizing an automated sensor applicator. Diabetes Technol Ther. 2018;20:428-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Christiansen MP, Garg SK, Brazg R, et al. Accuracy of a fourth-generation subcutaneous continuous glucose sensor. Diabetes Technol Ther. 2017;19:446-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. FreeStyle Libre. No more routine finger sticks for Americans with diabetes: Abbott’s Freestyle® Libre approved in the US [news release]. Abbott Park, IL: Abbott Laboratories; Available at: http://abbott.mediaroom.com/2017-09-27-No-More-Routine-Finger-Sticks-1-for-Americans-with-Diabetes-Abbott-s-FreeStyle-R-Libre-Approved-in-the-U-S. Accessed August 20, 2018. [Google Scholar]
- 9. Dexcom G6. Available at: https://www.dexcom.com/news/fda-authorizes-dexcom-g6. Accessed August 20, 2018.
- 10. Senseonics Eversense. Available at: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm611454.htm. Accessed July 28, 2018.
- 11. Guardian Connect. Available at: https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm604253.htm. Accessed July 28, 2018.
- 12. Abbott’s Freestyle® Libre. Abbott FreeStyle Libre 14 days. Available at: http://abbott.mediaroom.com/2018-07-27-Abbotts-FreeStyle-R-Libre-14-Day-Flash-Glucose-Monitoring-System-Now-Approved-in-U-S. Accessed August 20, 2018.
- 13. Beta Bionics. Introducing the iLet™. A Massachusetts Public Benefit Corporation and a Certified B Corporation®. Available at: https://www.betabionics.org/. Accessed August 20, 2018.
- 14. TypeZero® Technologies, Inc.Available at: https://typezero.com/. Accessed August 20, 2018.
- 15. Bigfoot™ Biomedical Available at: https://www.bigfootbiomedical.com/. Accessed August 20, 2018.
- 16. US Food and Drug Administration. Center for Devices and Radiological Health: MiniMed 670G System approval letter. Food and Drug Administration. September 28, 2016. Available at: https://www.fda.gov/NewsEvents/Newsroom/%20PressAnnouncements/ucm522974.htm. Accessed August 20, 2018.
- 17. BusinessWire® Available at: https://www.businesswire.com/news/home/20180621006260/en/Tandem-Diabetes-Care-Announces-FDA-Approval-tslim. Accessed July 8, 2018.
- 18. Kovatchev B, Cheng P, Anderson SM, et al. Feasibility of long term closed-loop control: a multicenter 6-month trial of 24/7 automated insulin delivery. Diabetes Technol Ther. 2017;19:18-24. [DOI] [PubMed] [Google Scholar]
- 19. El-Khatib FH, Balliro C, Hillard MA, et al. Home use of a bihormonal bionic pancreas versus insulin pump therapy in adults with type 1 diabetes: a multicentre randomised crossover trial. Lancet. 2017;389:369-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thabit H, Hovorka R. Coming of age: the artificial pancreas for type 1 diabetes. Diabetologia. 2016;59:1795-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Buckingham BA, Sherr JL, Forlenza GP, et al. Safety and performance of the Omnipod® hybrid closed-loop system in adults with type 1 diabetes over five days under free-living conditions. Paper presented at: 78th American Diabetes Association Scientific Sessions; June 22-26, 2018; Orlando, FL. [Google Scholar]
- 22. Peters A. The evidence base for continuous glucose monitoring. In: Hirsch IB, Battelino T, Peters AL, Chamberlain JJ, Aleppo G, Bergenstal RM, eds. Role of Continuous Glucose Monitoring in Diabetes Treatment. Arlington, VA: American Diabetes Association; 2018:3-7. [Google Scholar]
- 23. Fonseca VA, Grunberger G, Anhalt H, et al. Continuous glucose monitoring: a consensus conference of the American Association of Clinical Endocrinologists and American College of Endocrinology. Endocr Pract. 2016;22:1008-1021. [DOI] [PubMed] [Google Scholar]
- 24. Peters AL, Ahmann AJ, Battelino T, et al. Diabetes technology-continuous subcutaneous insulin infusion therapy and continuous glucose monitoring in adults: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101:3922-3937. [DOI] [PubMed] [Google Scholar]
- 25. Carlson AL, Mullen DM, Bergenstal RM. Clinical use of continuous glucose monitoring in adults with type 2 diabetes. Diabetes Technol Ther. 2017;19(suppl 2):s4-s11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vigersky R, Shrivastav M. Role of continuous glucose monitoring for type 2 in diabetes management and research. J Diabetes Complications. 2017;31:280-287. [DOI] [PubMed] [Google Scholar]
- 27. Beck RW, Riddlesworth TD, Ruedy K, et al. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Intern Med. 2017;167:365-374. [DOI] [PubMed] [Google Scholar]
- 28. Gómez AM, Umpierrez GE, Muñoz OM, et al. Continuous glucose monitoring versus capillary point-of-care testing for inpatient glycemic control in type 2 diabetes patients hospitalized in the general ward and treated with a basal bolus insulin regimen. J Diabetes Sci Technol. 2015;10:325-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Polonsky WH Hessler D Ruedy KJ Beck RW DIAMOND Study Group. The impact of continuous glucose monitoring on markers of quality of life in adults with type 1 diabetes: further findings from the DIAMOND randomized clinical trial. Diabetes Care. 2017;40:736-741. [DOI] [PubMed] [Google Scholar]
- 30. Vesco AT, Jedrasko AM, Garza KP, Weissberg-Benchell J. Continuous glucose monitoring associated with less diabetes specific emotional distress and lower A1c among adolescents with Type 1 diabetes. J Diabetes Sci Technol. 2018;12:792-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Parkin CG, Graham C, Smolskis J. Continuous glucose monitoring use in type 1 diabetes: longitudinal analysis demonstrates meaningful improvements in HbA1c and reductions in health care utilization. J Diabetes Sci Technol. 2017;11:522-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gill M, Zhu C, Shah M, Chhabra H. Health care costs, hospital admissions, and glycemic control using a standalone, real-time, continuous glucose monitoring system in commercially insured patients with type 1 diabetes. J Diabetes Sci Technol. 2018;12:800-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Puhr S, Calhoun P, Welsh JB, Walker TC. The effect of reduced self-monitored blood glucose testing after adoption of continuous glucose monitoring on hemoglobin A1c and time in range. Diabetes Technol Ther. 2018;20:557-560. [DOI] [PubMed] [Google Scholar]
- 34. Dexcom®, Inc https://www.dexcom.com/news/medicare-announces-criteria-covering-dexcom-g5-mobile-cgm-for-all-people-with-diabetes-on-intensive-insulin. Accessed August 20, 2018.
- 35. Tucker ME. Medicare to cover FreeStyle Libre glucose monitoring system. Available at: www.medscape.com/viewarticle/890879. Accessed August 20, 2018.
- 36. Slattery D, Choudhary P. Clinical use of continuous glucose monitoring in adults with type 1 diabetes. Diabetes Technol Ther. 2017;19(suppl 2):s55-s61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wright EE, Gavin JR. Clinical use of professional continuous glucose monitoring. Diabetes Technol Ther. 2017;19:(suppl 2):s12-s15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Polonsky WH, Fisher L, Hessler D, Edelman SV. Identifying the worries and concerns about hypoglycemia in adults with type 2 diabetes. J Diabetes Complications. 2015;29:1171-1176. [DOI] [PubMed] [Google Scholar]
- 39. American Diabetes Association. Glycemic targets: standards of medical care 2018. Diabetes Care. 2018;41:(suppl 1):s55-s64. [DOI] [PubMed] [Google Scholar]
- 40. Hirsch IB, Verderese CA. Professional flash continuous glucose monitoring with ambulatory glucose profile reporting to supplement A1c: rationale and practical implementation. Endocr Pract. 2017;23:1333-1344. [DOI] [PubMed] [Google Scholar]
- 41. Ahn D, Pettus J, Edelman S. Unblinded CGM should replace blinded CGM in the clinical management of diabetes. J Diabetes Sci Technol. 2016;10:793-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Beck RW, Tamborlane WV, Bergenstal RM, et al. The T1D Exchange clinic registry. J Clin Endocrinol Metab. 2012;97:4383-4389. [DOI] [PubMed] [Google Scholar]
- 43. Wong JC, Foster NC, Maahs DM, et al. Real-time continuous glucose monitoring among participants in the T1D Exchange clinic registry. Diabetes Care. 2014;37:2702-2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Foster NC, Miller K, DiMeglio L, et al. Marked increases in CGM use has not prevented increases in HbA1c levels in participants in the T1D Exchange (T1DX) Clinic Network. Paper presented at: 78th American Diabetes Association Scientific Sessions; June 22-26, 2018; Orlando, FL. [Google Scholar]
- 45. Pickup JC, Ford Holloway M, Samsi K. Real-time continuous glucose monitoring in type 1 diabetes: a qualitative framework analysis of patient narratives. Diabetes Care. 2015;38:544-550. [DOI] [PubMed] [Google Scholar]
- 46. Tanenbaum ML, Adams RN, Lanning MS, et al. Using cluster analysis to understand clinician readiness to promote continuous glucose monitoring adoption. J Diabetes Sci Technol. 2018;12:1108-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hirsch IB. Clinical review: realistic expectations and practical use of continuous glucose monitoring for the endocrinologist. J Clin Endocrinol Metab. 2009;94:2232-2238. [DOI] [PubMed] [Google Scholar]
- 48. Aleppo G, Laffel LM, Ahmann AJ, et al. A practical approach to using trend arrows on the Dexcom G5 CGM system for the management of adults with diabetes. J Endocr Soc. 2017;1:1445-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Klonoff DC, Kerr D. A simplified approach using rate of change arrows to adjust insulin with real-time continuous glucose monitoring. J Diabetes Sci Technol. 2017;11:1063-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Aleppo G. Approaches for successful outcomes with continuous glucose monitoring. In: Hirsch IB, Battelino T, Peters AL, Chamberlain JJ, Aleppo G, Bergenstal RM, eds. Role of Continuous Glucose Monitoring in Diabetes Treatment. Arlington, VA: American Diabetes Association; 2018:13-18. [PubMed] [Google Scholar]
- 51. Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40:1631-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mazze RS, Lucido D, Langer O, Hartmann K, Rodbard D. Ambulatory glucose profile: representation of verified self-monitored blood glucose data. Diabetes Care. 1987;10:111-117. [DOI] [PubMed] [Google Scholar]
- 53. Mullen DM, Bergenstal R, Criego A, Arnold KC, Goland R, Richter S. Time savings using a standardized glucose reporting system and ambulatory glucose profile. J Diabetes Sci Technol. 2018;12:614-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Riddlesworth TD, Beck RW, Gal RL, et al. Optimal sampling duration for continuous glucose monitoring to determine long-term glycemic control. Diabetes Technol Ther. 2018;20:314-316. [DOI] [PubMed] [Google Scholar]
- 55. Monnier L, Colette C, Wojtusciszyn A, et al. Defining the threshold between low and high glucose variability in diabetes. Diabetes Care. 2017;40:832-883. [DOI] [PubMed] [Google Scholar]
- 56. Hirsch IB. Glycemic variability: it’s not just about A1C anymore! Diabetes Technol Ther. 2005;7:780-783. [DOI] [PubMed] [Google Scholar]
- 57. Agiostratidou G, Anhalt H, Ball D, et al. Standardizing clinically meaningful outcome measures beyond HbA1c for Type 1 diabetes: a consensus report of the American Association of Clinical Endocrinologists, the American Association of Diabetes Educators, the American Diabetes Association, the Endocrine Society, JDRF International, the Leona M. and Harry B. Helmsley Charitable Trust, the Pediatric Endocrine Society, and the T1D Exchange. Diabetes Care. 2017;40:1622-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bergenstal RM, Garg S, Weinzimer SA, et al. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA. 2016;316:1407-1408. [DOI] [PubMed] [Google Scholar]
- 59. Bergenstal R. Continuous glucose monitoring data as an adjunct to A1C. In: Hirsch IB, Battelino T, Peters AL, Chamberlain JJ, Aleppo G, Bergenstal RM, eds. Role of Continuous Glucose Monitoring in Diabetes Treatment. Arlington, VA: American Diabetes Association; 2018:19-20. [PubMed] [Google Scholar]
- 60. Bergenstal RM, Beck RW, Close KL, Grunberger G, Sacks DB, Kowalski A, et al. Glucose Management Indicator (GMI): A New Term for Estimating A1C From Continuous Glucose Monitoring. Diabetes Care. 2018:41(11):2275-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]