Abstract
Hypoglycemia remains the limiting factor of near-normal glucose control in people with diabetes using insulin therapy. Continuous glucose monitoring (CGM) may be able to avoid hypoglycemia and support the management of hypoglycemia problems in clinical care. Real-time continuous glucose monitoring (rtCGM) systems provide alerts if certain predetermined hypo- or hyperglycemic thresholds are exceeded. The combination of rtCGM systems with insulin pumps allows insulin delivery to be suspended if glucose falls below certain predefined thresholds. This might also support avoidance of hypoglycemia. More sophisticated closed-loop systems allow a semiautomatic insulin dosage, which also have the potential for the prevention of hypoglycemia. In this overview, we discuss and illustrate (1) the efficacy of CGM for intervention in people with hypoglycemia problems and for the avoidance of biochemical as well as clinical hypoglycemia; (2) the potential of CGM technology for the identification of people with diabetes who are at risk for hypoglycemia problems; and (3) the implications of the current state of the art for future research regarding CGM and hypoglycemia. As an example, how rtCGM data can facilitate identification of people with diabetes and an elevated risk of hypoglycemia, a secondary analysis of the HypoDE data is presented. We conclude that CGM technology can assist in the reliable identification of people with diabetes who are at risk for hypoglycemia problems, is a powerful intervention for the avoidance of mild as well as severe hypoglycemia, and can also stimulate research on the course of hypoglycemia problems.
Keywords: continuous glucose monitoring, hypoglycemia, flash glucose monitoring, intervention
The Scope of the Problem
For the prevention of diabetic complications, diabetes therapies, especially insulin therapy, are striving for near-normoglycemia. The Diabetes Control and Complications Trial (DCCT) could clearly demonstrate that short-term1 as well as long-term prevention2 of micro- as well as macrovascular complications was associated with near-normoglycemia. However, they also found that tighter glycemic control was associated with a tripling of the incidence of severe hypoglycemia (third party assistance required for treatment).3,4 Severe hypoglycemia and its associated neuroglycopenia interrupts daily life and has a high risk potential especially during certain activities like driving, swimming or challenging tasks.5,6 Hypoglycemia problems may also cause employment restrictions or insurance problems.7 Hypoglycemia-induced emotional and behavioral changes can also negatively affect social life of people with diabetes and can result in interpersonal conflicts and arguments.8,9 The international DAWN-2 study showed that family members are also frequently affected by hypoglycemia problems of the person with diabetes.10,11 More severe forms of hypoglycemia can result in coma, seizure, stroke, or cardiac arrhythmias.7,12 In people with type 1 and type 2 diabetes, severe hypoglycemia is also a risk factor for early mortality.13-15 Hypoglycemia-related fears and worries are rather common in people with diabetes.16,17 In his excellent review, Brian Frier introduced hypoglycemia as the most common and feared adverse effect of insulin therapy.7
The epidemiological evidence on the prevalence and incidence of hypoglycemia suggests a nonnormal distribution of hypoglycemic episodes amongst people with diabetes.7 In the DCCT, 22% of the study participants had at least five hypoglycemic events resulting in coma or seizure during this period. In addition, this minority accounted for more than 55% of all these severe hypoglycemic events. At the same time, 30% of the participants experienced no episode of severe hypoglycemia during the 9-year follow-up.3 A multinational and multicenter study including more than 1000 people with type 1 diabetes corroborated the unequal distribution of severe hypoglycemia. In this study from Pedersen-Bjergaard,18 severe hypoglycemia was reported by a minority of 36.7% of participants. The distribution was highly skewed with 5% of subjects accounting for 54% of all episodes. A recent register study investigating the prevalence of severe hypoglycemia confirmed these findings again, showing that 10% of individuals registered with type 1 diabetes accounted for all of the observed hypoglycemic events.19 Interestingly, a similar accumulation of severe hypoglycemia also appears to exist in type 2 diabetes. Henderson et al20 reported that severe hypoglycemia occurred in only 15% of insulin-treated people with type 2 diabetes. There was also a subgroup of nearly 3% with two or more severe hypoglycemic events.
In conclusion, the risk of severe hypoglycemia is not the same for all people with diabetes. There is clear epidemiological evidence for the existence of certain groups that have a particularly high risk of hypoglycemia. Several risk factors for hypoglycemia problems, for example, previous hypoglycemic events, long diabetes duration respectively duration of insulin therapy have been identified. A more detailed overview about the risk factors for severe hypoglycemia in people with type 1 and type 2 diabetes is provided in the review by Frier.7
A key risk factor for the occurrence of severe hypoglycemia is impaired hypoglycemia awareness. In published studies, the pathophysiological basis for the development of impaired hypoglycemia awareness is referred to as hypoglycemia-associated autonomic failure (HAAF).21,22 In addition to a diminished glucagon response to low glucose values, glycemic thresholds for blood glucose counterregulation and hypoglycemia warning symptoms are at a lower glucose level in people with impaired hypoglycemia awareness.21 Etiologically important for the genesis of HAAF is a frequent exposure to biochemically mild hypoglycemia.23 Frequent and/or long-lasting mild hypoglycemic episodes seem to be associated with an adaptation process toward lower glucose values, leading to a shift of glycemic thresholds to lower glucose levels for hypoglycemia warning symptoms and glucose counterregulation. Nocturnal hypoglycemia during sleep poses a special risk for this adaptation process because it is frequently slept through and therefore not recognized. With the shift of glycemic threshold toward lower glucose levels, people with impaired hypoglycemia awareness are alerted at lower glucose levels of impending hypoglycemia, thus increasing the risk for severe hypoglycemia.24 Strict avoidance of hypoglycemia can ameliorate HAAF syndrome by shifting the threshold for hypoglycemia warning symptoms and protective glucose counterregulation to near-normal glucose levels, resulting in an improvement of impaired hypoglycemia awareness.25-27
Avoidance of low glucose values and the identification of people with diabetes and impaired hypoglycemia awareness is important for an improved management of hypoglycemia problems. Continuous glucose monitoring (CGM) is an increasingly important diabetes technology that has the potential to facilitate avoidance of low glucose values and the identification of people with diabetes and impaired hypoglycemia awareness. This technology is currently available as real-time continuous glucose monitoring (rtCGM) or as flash sensor-based glucose monitoring. The latter requires scanning of the current glucose values with a reader or smartphone to get access to current glucose values, the history of glucose course, and trend information. This system is therefore named intermittent scanning CGM (iscCGM).
RtCGM and iscCGM provide a more comprehensive overview of glycemic control than traditional indicators, such as A1c or the results of the self-monitored blood glucose (SMBG). Whereas A1c is primarily an indicator of hyperglycemia and the risks of diabetes-specific complications, it is less able to reveal exposure to low glucose values and thus is not best suited to indicate the risk of severe hypoglycemia. Spot blood glucose measurements can show exposure to low glucose values, but gaps of several hours between these measurements may leave many exposures to low glucose values undetected, especially during the night. In contrast to these methods, rtCGM or iscCGM provide continuously measured glucose levels and is therefore optimally suited to assess and reliably quantify exposure to low glucose values. Since rtCGM devices can alert people with diabetes of low glucose values, rtCGM is also a valuable interventional tool for avoiding low glucose values and ameliorating HAAF and impaired hypoglycemia awareness.
In this overview, we discuss the potential of CGM technology for diagnostic and interventional purposes in people experiencing problems with hypoglycemia as well as the implications of CGM on future hypoglycemia research. We believe that CGM technology (1) is a powerful intervention tool for the avoidance of mild as well as severe hypoglycemia, (2) can reliably assist in the identification of people with diabetes who are at risk for hypoglycemia problems, and (3) can facilitate research on the course of problematic hypoglycemia.
Is rtCGM an Effective Intervention to Reduce Hypoglycemia Problems?
Table 1 gives an overview about randomized controlled trials testing the efficacy of rtCGM systems in people with hypoglycemia problems or testing the efficacy of rtCGM on a hypoglycemia-related primary endpoint. In addition, Table 1 comprises studies investigating the efficacy of iscCGM. Currently, there are only four published randomized controlled studies that specifically included people with diabetes and hypoglycemia problems. These four studies were the studies by Ly et al,28 IN CONTROL,29 HypoCOMPaSS,30 and HypoDE.31 The study of Battelino et al32 was powered for a hypoglycemia-specific primary endpoint but did not specifically include people with hypoglycemia problems.
Table 1.
Comparison of Hypoglycemia-Related rtCGM/iscCGM Randomized Controlled Trials.
| Study | Inclusion criterion hypoglycemia problems | Hypoglycemia-related primary endpoint | Primary endpoint | Hypoglycemia-related secondary endpoint | Insulin application |
|---|---|---|---|---|---|
| Battelino et al32 | No | Yes | Time spent in hypoglycemia | Yes | CSII & MDI |
| Ly et al28 | Yes | Yes | Episode of severe hypoglycemia | Yes | CSII |
| Little et al30
HypoCOMPaSS |
Yes | Yes | Hypoglycemia awareness | Yes | CSII & ICT |
| van Beers et al29
IN CONTROL |
Yes | No | Time in range | Yes | CSII & ICT |
| Heinemann et al31,33
HypoDE |
Yes | Yes | Low glucose event | Yes | MDI |
| Bolinder et al35
IMPACT |
No | No | Time in range | Yes | CSII & MDI |
| Haak et al36
REPLACE |
No | No | HbA1c | Yes | MDI |
Bold: significant effect in favor for rtCGM respectively iscCGM.
All studies examining the effects of rtCGM technology in people with diabetes and hypoglycemia problems yielded positive results on hypoglycemia-specific primary and secondary outcomes, except the HypoCOMPaSS study.30 While this study included only people with impaired hypoglycemia awareness, it was powered to test the impact of one insulin application method (multiple daily insulin injections [MDI] vs another [insulin pumps]). Surprisingly, it was not powered for detecting a difference between rtCGM and SMBG. In addition, adherence to rtCGM in the HypoCOMPaSS30 study (only 57% wearing time in the rtCGM arm during the study period) was relatively low compared to the three other studies (Ly et al; IN CONTROL; HypoDE), which recoded a wearing time greater than 90%.28,29,31 The results of the HypoCOMPaSS study regarding the efficacy of rtCGM on hypoglycemia should therefore be interpreted with caution.
The other three randomized controlled trials, IN CONTROL,29 HypoDE,33 as well as the study published by Ly et al,28 showed an improvement of biochemical as well as severe hypoglycemic episodes.28,29,33 All three studies included only people with type 1 diabetes and hypoglycemia problems, which was novel since people with hypoglycemia problems were frequently excluded from other rtCGM studies. However, these three studies differed regarding insulin treatment. In the study by Ly et al,28 all participants were treated with insulin pump therapy. In the IN CONTROL study, 42% of the participants were treated with an insulin pump, whereas in the HypoDE study, only participants with MDI treatment were included. The study by Ly et al and the HypoDE study had a hypoglycemia-specific endpoint, whereas the IN CONTROL study used “time in range” as the primary outcome.
Interestingly, all three studies were able to reduce exposure to low glucose values. Participants of the study conducted by Ly et al were able to reduce the time spent in a hypoglycemic glucose range (<70 mg/dl, <3.9 mmol/L) by 1.4 percentage points or by 20 minutes per day.28 The IN CONTROL study reduced exposure to glucose values ≤70 mg/dl (≤3.9 mmol/L) by 4.7 percentage points,29 similar to the reduction observed in the HypoDE study (4.8% percentage points).33 Since the exposure to biochemical hypoglycemia is a key factor for the development of HAAF,21 these reductions can be considered to be clinically meaningful.
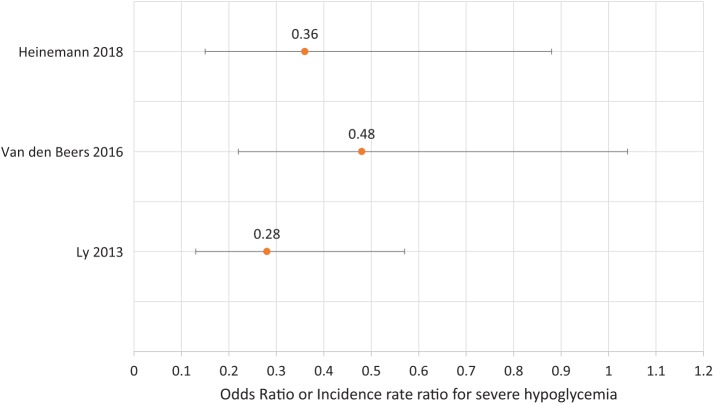
All three studies were also able to show a significant or marginally significant reduction in the rate of clinical hypoglycemia. Figure 1 shows the incidence rate ratios and odds ratios for a reduction in severe hypoglycemia. Use of sensor-augmented insulin pump therapy could reduce the incidence of the combined endpoint of moderate (third-party assistance) and severe hypoglycemia (coma or seizures) by 72%.28 The reduction of hypoglycemia-induced coma and seizure could also be significantly reduced by 34%. However, these results were disputed by the German IQWIG (Institute for Quality and Efficiency in Health Care) due to the small number of people affected.34 In the HypoDE study, a reduction in the incidence of clinical hypoglycemia of 64% was achieved.33 The IN CONTROL study showed a halved risk for clinical hypoglycemia during rtCGM use compared to SMBG use.29 In summary, it could be demonstrated that the use of rtCGM was able to reduce biochemical as well as clinical hypoglycemia to a clinically meaningful extent. Of note is that these beneficial effects were demonstrated in populations with different insulin regimens.
Figure 1.
Efficacy of rtCGM for the reduction of severe hypoglycemia (odds ratio or incidence rate ratio).
The mechanism of hypoglycemia prevention by CGM is currently not well understood. All of the CGM studies described above used devices that alert study participants if glucose levels fall below a predefined threshold. In addition to the low glucose alerts, the constant availability of glucose information (level of glucose, previous course, and trend arrows), which allows immediate response to low or lowering glucose values, might be an explanation for the preventive effect of CGM on hypoglycemia in people with type 1 diabetes.
A rather new CGM technology is iscCGM. This technology can provide current glucose information, the previous course of the glucose, and expected glucose trends as often as the user actively scans the sensor. Currently, there are two randomized controlled trials that evaluated the efficacy of an iscCGM system that was not able to provide low glucose alerts in people with type 1 diabetes35 and type 2 diabetes.36 In the IMPACT study, a group of people with well-controlled type 1 diabetes (HbA1c < 7.5%) was included.35 However, participants with impaired awareness of hypoglycemia were excluded from the IMPACT study. Nevertheless, the IMPACT study demonstrated a reduction in the number of low glucose values (<70 mg/dl, <3.9 mmol/L) by 1.24 hours per day or by 5.1 percentage points, which might indicate that CGM data is beneficial for the avoidance of low glucose values, even in the absence of low glucose alerts. At the end of the study, however, still 8.6% of glucose values (2.03 h) were recorded as lower than 70 mg/dl (3.9 mmol/L). A subsequent analysis of the IMPACT sample demonstrated that a reduction in the exposure to such low glucose values was present in participants with MDI as well as with insulin pumps.37 However, clinical hypoglycemia was not positively influenced using iscCGM, possibly due to too few events that has limited the statistical power to demonstrate such effects. The same iscCGM technology as used in the IMPACT study was also tested in people with type 2 diabetes in the REPLACE study.36 In this study, participants were selected based on an elevated A1c. The REPLACE study could demonstrate a significant reduction of biochemically defined hypoglycemia according to different thresholds. However, the prevalence of hypoglycemia problems was very low in this sample, probably due to the sample selection, which might be responsible for the lack of a significant effect of iscCGM on clinical hypoglycemia in this study.
In summary, there is well-founded evidence from methodologically sound studies that CGM can reduce exposure to low glucose values as well as clinical hypoglycemia problems. Interestingly, the preventive effect of CGM on hypoglycemia seems to be stable across different insulin application regimens (insulin pumps, sensor-augmented insulin pump therapy or MDI) and different CGM technologies. Studies evaluating the use of iscCGM could also demonstrate a reduced exposure to low glucose values while a reduction of clinical hypoglycemia was not observed using iscCGM until now. This might be due to the sample selection of the IMPACT and REPLACE study (exclusion of participants with hypoglycemia problems or poorly controlled individuals with type 2 diabetes) or to the missing of alerts in the iscCGM-systems used in these studies.
Can CGM Facilitate Diagnosis of Hypoglycemia Problems?
CGM technology provides glucose data every 5 to 15 minutes. Thus, caregivers and people with diabetes receive a complete overview of daily and nocturnal glucose values, allowing previously unrecognized asymptomatic mild episodes of hypoglycemia to be easily detected.
The consensus paper by Danne et al38 recommends the use of CGM data to evaluate exposure to low glucose values by assessing the percentage or duration of low glucose values and the number of low glucose events. It is recommended that the percentage or duration of low glucose readings lower than 70 mg/dl (3.9 mmol/L) and lower than 54 mg/dl (3.0 mmol/L) be assessed. In addition, the number of hypoglycemic events (for at least 15 minutes) with glucose values <70 mg/dl (3.9 mmol/l) and <54 mg/dl (3.0 mmol/L) should be evaluated. A more severe low glucose event is indicated when the glucose values are <54 mg/dl (3.0 mmol/L) for at least 120 minutes. The low blood glucose index (LBGI) should also be calculated as an indicator of hypoglycemia risk.
From a diagnostic perspective it is important to understand to what extent the CGM-derived parameters of the consensus statement can assist in identifying people who are at risk for hypoglycemia problems. The identification of people who are at increased risk for severe hypoglycemia or impaired awareness of hypoglycemia opens the possibility of undertaking proactive actions to prevent hypoglycemia problems.
To provide an example of using these parameters for identifying people with diabetes and problems with hypoglycemia, we reanalyzed data from the HypoDE study, a randomized controlled rtCGM study targeting the reduction of low glucose events by rtCGM. In this study, only people with type 1 diabetes and impaired awareness of hypoglycemia or severe hypoglycemia in the last 12 months were included. The details of the study have been previously published.33,39 For these specific prospective analyses, we used the baseline rtCGM data of the control group that received masked CGM for 28 days during the baseline phase. The control group underwent SMBG during the 26-week intervention and outcome phase. For the control group, we prospectively analyzed the utility of the CGM-derived parameters at baseline to predict future occurrences of clinical hypoglycemia during the following intervention and outcome phase. Since nearly all participants were classified as hypoglycemia unaware due to the inclusion criteria, we further analyzed the utility of the CGM-derived parameters to predict the persistence of impaired awareness of hypoglycemia (defined by a questionnaire40) at the end of the intervention and outcome phase.
The area under the receiver operating characteristic (ROC) curves is a good measure of the screening performance of the CGM parameters to predict future severe hypoglycemia or the persistence of impaired awareness of hypoglycemia. Supplementary Figure S1 shows the area under the ROC curves for the ability of CGM parameters to predict future severe hypoglycemia. All six parameters—% <70 mg/dl (3.9 mmol/L), % <54 mg/dl (3.0 mmol/L), LBGI, number of <70 mg/dl-events (3.9 mmol/l), number of <54 mg/dl-events (3.0 mmol/L), number of prolonged <54 mg/dl-events (3.0 mmol/L)—showed areas under the ROC curves between 0.69 and 0.73, indicating a significantly better ability of all six CGM parameters to predict future severe hypoglycemia than identification by chance, which would correspond to an area under the ROC curve of 0.50. Since all six parameters had a similar area under the ROC curve and overlapping confidence intervals (Table 2), there is no clear advantage of a specific parameter out of these six parameters recommended by the consensus statement for this particular sample from the HypoDE study.
Table 2.
Screening Performance of Different CGM-Derived Parameters to Predict Severe Hypoglycemia or Persistence of Impaired Awareness of Hypoglycemia.
| CGM parameter | Area under ROC (95% CI) | Cutoff values | Sensitivity | Specificity |
|---|---|---|---|---|
| Prediction of severe hypoglycemia | ||||
| % <70 mg/dl (3.9 mmol/L) | 0.69 (0.54-0.85) | 7.0% | 71.4% | 66.6% |
| % <54 mg/dl (3.0 mmol/L) | 0.69 (0.52-0.85) | 2.4% | 71.4% | 59.6% |
| LBGI | 0,70 (0.55-0.86) | 1.6 | 71.4% | 57.7% |
| Number of <70 mg/dl (3.9 mmol/L) glucose events per 28 days | 0.72 (0.54-0.87) | 28 | 71.4% | 69.6% |
| Number of <54 mg/dl (3.0 mmol/L) glucose events per 28 days | 0.73 (0.55-0.86) | 10.5 | 71.4% | 59.6% |
| Number of prolonged <54 mg/dl (3.0 mmol/L) glucose events per 28 days | 0.69 (0.53-0.85) | 1 | 79.0% | 56.0% |
| Persistence of hypoglycemia unawareness | ||||
| % <70 mg/dl (3.9 mmol/L) | 0.78 (0.67-0.89) | 6.7% | 75.7% | 75.4% |
| % <54 mg/dl (3.0 mmol/L) | 0.79 (0.68-0.90) | 2.4% | 75.7% | 79.9% |
| LBGI | 0.79 (0.67-0.86 | 2.0 | 83.4% | 62.1% |
| Number of <70 mg/dl (3.9 mmol/L) glucose events per 28 days | 0.73 (0.60-0.85) | 31 | 75.7% | 62.1% |
| Number of <54 mg/dl (3.0 mmol/L) glucose events per 28 days | 0.79 (0.67-0.90) | 12.5 | 78.8% | 62.1% |
| Number of prolonged <54 mg/dl (3.0 mmol/L) glucose events per 28 days | 0.79 (0.68-0.90) | 1.5 | 73.0% | 83.0% |
Prospective analysis of the persistence of impaired awareness of hypoglycemia yielded similar results (Supplementary Figure S2). The areas under the ROC curves for the persistence of impaired hypoglycemia awareness were slightly higher than for the prediction of future severe hypoglycemia. All six CGM parameters significantly better predicted the persistence of impaired awareness of hypoglycemia than identification by chance, but no specific parameter showed a clear superiority for this prediction.
Based on the results of the ROC-analyses, the cutoff values to predict an increased risk for future severe hypoglycemia or the persistence of impaired awareness of hypoglycemia are shown in Table 2. For example, having 7% respectively 6.7% or more glucose values <70 mg/dl (3.9 mmol/L) can predict future risk for severe hypoglycemia or persistence of impaired hypoglycemia awareness with the best sensitivity and specificity. A cutoff value of 2.5% or more is suggested for glucose values <54 mg/dl (3.0 mmol/L). An LBGI of 1.6 or more predicts severe hypoglycemia. A total of 28 low glucose events <70 mg/dl (3.9 mmol/L) (one per day in the baseline phase) and 10.5 low glucose events <54 mg/dl (3.0 mmol/L; one in every three days) are indicative of future severe hypoglycemia. One prolonged event <54 mg/dl (3.0 mmol/L) indicates an increased risk of severe hypoglycemia. The corresponding sensitivity and specificity values also indicate that the screening performance of these cutoff values has room for improvement. However, these cutoff values might help to assist in the clinical evaluation of the recommended hypoglycemia-specific CGM parameters. The lower part of Table 2 provides the corresponding cutoff values for the prediction of the persistence of impaired awareness of hypoglycemia. Interestingly, similar cutoff values as for the prediction of severe hypoglycemia are suggested.
Table 3 provides the hazard ratios for the future occurrence of severe hypoglycemia. When the percentage of glucose values <70 mg/dl (3.9 mmol/L) increases by one percentage point, the risk for future severe hypoglycemia increases by 14%. An increase of one percentage point of glucose values <54 mg/dl (3.0 mmol/L) was statically associated with a 1.27-fold elevated risk. An increase of the three different low glucose events was associated with a risk-gain for future severe hypoglycemia between 4% and 33% per event. The right part of Table 3 provides the respective hazard ratios for the persistence of impaired awareness of hypoglycemia. These hazard ratios may provide clinically meaningful information to caregivers and people with diabetes about the relevance of the CGM parameters regarding the hypoglycemia risk.
Table 3.
Hazard Ratios for Future Severe Hypoglycemia or the Persistence of Hypoglycemia Unawareness Based on CGM-Derived Parameters.
| CGM parameter | Hazard ratio (95% CI) | Hazard ratio (95% CI) |
|---|---|---|
| Severe hypoglycemia | Persistence of hypoglycemia unawareness | |
| % <70 mg/dl (3.9 mmol/L) | 1.14 (1.02-1.26) | 1.23 (1.09-1.39) |
| % <54 mg/dl (3.0 mmol/L) | 1.27 (1.05-1.53) | 1.53 (1.18-1.98) |
| LBGI | 1.67 (1.11-2.53) | 2.34 (1.43-3.96) |
| Number of <70 mg/dl (3.9 mmol/L) glucose events per 28 days | 1.04 (1.01-1.07) | 1.05 (1.01-1.07) |
| Number of <54 mg/dl (3.0 mmol/L) glucose events per 28 days | 1.10 (1.03-1.17) | 1.13 (1.06-1.22) |
| Number of prolonged <54 mg/dl (3.0 mmol/L) glucose events per 28 days | 1.33 (1.03-1.72) | 1.95 (1.30-2.90) |
These analyses show that CGM data may be a valuable diagnostic tool to identify people with diabetes who are at an increased risk for future severe hypoglycemia or reduced hypoglycemia awareness. Furthermore, the hazard ratios may assist in explaining the relevance of CGM data for patients’ own risk assessment regarding the future occurrence of severe hypoglycemia or impaired awareness of hypoglycemia. Identification of people with diabetes who are at an increased risk for severe hypoglycemia may also help to prevent problems with hypoglycemia by adjusting insulin therapy, lifestyle and/or glycemic targets in a timely fashion. However, it must be kept in mind that the suggested cutoff values and hazard ratios are derived from a post hoc analysis of the HypoDE study, which included only people with hypoglycemia problems. Therefore, the generalizability to people with diabetes but without a history of hypoglycemia problems is clearly limited. Thus, we are still in need of prospective studies with different study populations to corroborate the suggested cutoff values and hazard ratios.
Implications for Future Research
It has been demonstrated that use of rtCGM in people with type 1 diabetes on different treatment regimens can reduce biochemical as well as clinical hypoglycemia. However, future research needs to address the question of which mediating mechanisms are responsible for the observed effect. It remains unclear whether avoidance of mild hypoglycemia by the use of CGM technology contributes to a restoration of normal hypoglycemia awareness due to a shift of glycemic thresholds toward higher glucose levels for glucose counterregulation and symptoms of low blood glucose.25,27,41,42 The use of CGM technology for endocrine glucose counterregulation remains controversial. Ly et al43 found an improvement of epinephrine response in people with type 1 diabetes and impaired awareness of hypoglycemia after rtCGM use. However, in the randomized controlled trial, a recovery of the epinephrine response in response to low glucose values was not observed.28 In this regard it is interesting that all intervention studies in people with hypoglycemia problems28,29,33 used the unawareness questionnaire by Clarke et al,40 an established measure of reduced awareness. In none of these studies, however, an impact of CGM use on the unawareness score could be observed. A possible explanation could be that CGM prevents frequent low glucose values as well as clinical hypoglycemia primarily by low glucose alerts or proactive avoidance of low glucose values, but not by reverting impaired awareness of hypoglycemia and changing glycemic thresholds for endocrine or symptomatic counterregulation. An alternative view on these findings is that the unawareness questionnaire is an assessment of the future risk of hypoglycemia in an epidemiological context but does not depict pathophysiological mechanisms contributing to HAAF syndrome. Clearly, more research is needed to illuminate mechanisms of how CGM affects the reduction and prevention of biochemical or clinical hypoglycemia.
The role of glucose alerts on the prevention of low glucose values is also not fully understood since the IMPACT study using an iscCGM system without an alert function was also able to demonstrate a reduction of biochemical but not clinical severe hypoglycemia.35 The role of alerts might be different for the prevention of biochemical and severe hypoglycemia, especially during the night.
A new area of interest is also the share function, allowing wearers of CGM systems to share their glucose data with significant others. Anecdotical evidence suggests lower exposure to hypoglycemic glucose values in children.44
In clinical care, it is also worth understanding how much reduction of low glucose values is necessary to result in better hypoglycemia awareness or what amount of exposure to mild biochemical hypoglycemia is tolerable without compromising hypoglycemia awareness.
The reanalysis of the HypoDE study showed that CGM-derived parameters have diagnostic value with regard to identifying people at risk for hypoglycemia problems since their diagnostic performance is better than chance. However, the data also indicate that the screening performance of the CGM-derived parameters used had room for improvement in the HypoDE sample. Since this sample was specifically selected for hypoglycemia problems, the results regarding cutoff scores and screening performance require corroboration by prospective data from other samples or from samples consisting of people with and without problems with hypoglycemia.
In addition to using CGM as a standalone system, more advanced technologies, such as closed-loop or artificial pancreas systems, are being developed that allow for an automatic or semiautomatic glucose control via integrating CGM and insulin pumps.45,46 Studies on these closed-loop systems in adults also showed that the proportion of low glucose values or time spent in a low glucose range could be significantly reduced by 0.8 percentage points. However, given the low exposure to low glucose values in the CGM-alone condition (only 3%), it seems unlikely that the study population had hypoglycemia problems.46 The level of percentage of low glucose values under the closed-loop condition in adults (2.9%) is remarkable lower than the level observed in the study by Ly et al28 or the IN CONTROL study,29 but equivalent to the HypoDE study.31 Thus, more studies are needed to show the impact of closed-loop systems in people with diabetes and hypoglycemia problems. Currently, there are no published randomized controlled trials with closed-loop systems or artificial pancreas systems in people with diabetes and hypoglycemia problems that have a reasonable duration to observe an impact on clinical hypoglycemia problems.47,48 Bi-hormonal pumps delivering insulin and glucagon are a further promising area of research. The effect of bi-hormonal pumps for prevention of hypoglycemia and the restoration of HAAF is of special interest in promising future research.
Overall Conclusion
In summary, CGM is a valuable tool for the identification of people at risk for hypoglycemia problems and for the prevention of mild and clinical hypoglycemia without compromising glycemic control or increasing the risk of complications. More information on the interpretation of CGM results and a better understanding of the mechanisms of hypoglycemia prevention by CGM are needed.
Supplemental Material
Supplemental material, DST831695_Supplemental_Material_CLN for Impact of CGM on the Management of Hypoglycemia Problems: Overview and Secondary Analysis of the HypoDE Study by Norbert Hermanns, Lutz Heinemann, Guido Freckmann, Delia Waldenmaier and Dominic Ehrmann in Journal of Diabetes Science and Technology
Footnotes
Abbreviations: CGM, continuous glucose monitoring; DAWN, Diabetes Attitudes Wishes Needs; DCCT, Diabetes Control and Complications Trial; HAAF, hypoglycemia-associated autonomic failure; iscCGM, intermittent scanning continuous glucose monitoring; IQWIG, Institute for Quality and Efficiency in Health Care (Institut für Qualität und Wirtschaftlichkeit); LBGI, low blood glucose index; MDI, multiple daily insulin injections; ROC, receiver operating characteristic; rtCGM, real-time continuous glucose monitoring; SMBG, self-monitored blood glucose.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: NH reports grants from Dexcom Inc, during the conduct of the study; personal fees from Novo Nordisk; grants and personal fees from Abbott; grants and personal fees from Berlin Chemie; grants and personal fees from Ypsomed; grants and personal fees from Roche, outside the submitted work. LH reports grants from Dexcom, during the conduct of the study; personal fees from Roche Diagnostics, Integrity Ltd, Medtronic, and Sanofi, outside the submitted work. LH owns shares of Profil Institut für Stoffwechselforschung GmbH, Neuss, Germany and ProSciento, San Diego, CA, USA. GF reports grants from Dexcom Inc, during the conduct of the study; personal fees from Abbott, grants and personal fees from Ascensia, personal fees from Berlin-Chemie, personal fees from Becton-Dickinson, personal fees from Dexcom, personal fees from LifeScan, personal fees from Menarini, personal fees from Novo Nordisk, grants and personal fees from Roche, personal fees from Sanofi, personal fees from Sensile, personal fees from Ypsomed, outside the submitted work. DW reports grants from Dexcom Inc, during the conduct of the study. DE reports grants from Dexcom Inc, during the conduct of the study; personal fees from Berlin-Chemie AG, outside the submitted work.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs: Norbert Hermanns  https://orcid.org/0000-0002-2903-2677
https://orcid.org/0000-0002-2903-2677
Guido Freckmann  https://orcid.org/0000-0002-0406-9529
https://orcid.org/0000-0002-0406-9529
References
- 1. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986. [DOI] [PubMed] [Google Scholar]
- 2. Nathan DM, McGee P, Steffes MW, Lachin JM. Relationship of glycated albumin to blood glucose and HbA1c values and to retinopathy, nephropathy, and cardiovascular outcomes in the DCCT/EDIC study. Diabetes. 2014;63:282-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. The Diabetes Control and Complications Trial Research Group. Hypoglycemia in the Diabetes Control and Complications Trial. Diabetes. 1997;46:271-286. [PubMed] [Google Scholar]
- 4. Gubitosi-Klug RA, Braffett BH, White NH, et al. Risk of severe hypoglycemia in type 1 diabetes over 30 years of follow-up in the DCCT/EDIC study. Diabetes Care. 2017;40:1010-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clarke WL, Cox DJ, Gonder-Frederick LA, Kovatchev B. Hypoglycemia and the decision to drive a motor vehicle by persons with diabetes. JAMA. 1999;282:750-754. [DOI] [PubMed] [Google Scholar]
- 6. Cox DJ, Penberthy JK, Zrebiec J, et al. Diabetes and driving mishaps: frequency and correlations from a multinational survey. Diabetes Care. 2003;26:2329-2334. [DOI] [PubMed] [Google Scholar]
- 7. Frier BM. Hypoglycaemia in diabetes mellitus: epidemiology and clinical implications. Nat Rev Endocrinol. 2014;10:711-722. [DOI] [PubMed] [Google Scholar]
- 8. Hermanns N, Kubiak T, Kulzer B, Haak T. Emotional changes during experimentally induced hypoglycaemia in type 1 diabetes. Biol Psychol. 2003;63:15-44. [DOI] [PubMed] [Google Scholar]
- 9. McCrimmon RJ, Deary IJ, Frier BM. Appraisal of mood and personality during hypoglycaemia. Physiol Behav. 1999;67:27-33. [DOI] [PubMed] [Google Scholar]
- 10. Kovacs BK, Nicolucci A, Holt RI, et al. Diabetes attitudes, wishes and needs second study (DAWN2): cross-national benchmarking indicators for family members living with people with diabetes. Diabet Med. 2013;30:778-788. [DOI] [PubMed] [Google Scholar]
- 11. Nefs G, Pouwer F, Holt RI, et al. Correlates and outcomes of worries about hypoglycemia in family members of adults with diabetes: the second Diabetes Attitudes, Wishes and Needs (DAWN2) study. J Psychosom Res. 2016;89:69-77. [DOI] [PubMed] [Google Scholar]
- 12. Fisher M, Heller SR. Mortality, cardiovascular morbidity and possible effects of hypoglycaemia on diabetes complications. In: Frier BM, Fisher M, eds. Hypoglycaemia in Clinical Diabetes. Vol. 2 Chichester, UK: John Wiley; 2007:265-283. [Google Scholar]
- 13. Cryer PE. Death during intensive glycemic therapy of diabetes: mechanisms and implications. Am J Med. 2011;124:993-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cryer PE. Severe hypoglycemia predicts mortality in diabetes. Diabetes Care. 2012;35:1814-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ. 2010;340:b4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gonder-Frederick LA, Clarke WL, Cox DJ. The emotional, social and behavioral implications of insulin-induced hypoglycemia. Semin Clin Neuropsychiatry. 1997;2:57-65. [DOI] [PubMed] [Google Scholar]
- 17. Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care. 2005;28:626-631. [DOI] [PubMed] [Google Scholar]
- 18. Pedersen-Bjergaard U, Pramming S, Heller SR, et al. Severe hypoglycaemia in 1076 adult patients with type 1 diabetes: influence of risk markers and selection. Diabetes Metab Res Rev. 2004;20:479-486. [DOI] [PubMed] [Google Scholar]
- 19. Ishtiak-Ahmed K, Carstensen B, Pedersen-Bjergaard U, Jorgensen ME. Incidence trends and predictors of hospitalization for hypoglycemia in 17,230 adult patients with type 1 diabetes: a Danish register linkage cohort study. Diabetes Care. 2017;40:226-232. [DOI] [PubMed] [Google Scholar]
- 20. Henderson JN, Allen KV, Deary IJ, Frier BM. Hypoglycaemia in insulin-treated type 2 diabetes: frequency, symptoms and impaired awareness. Diabet Med. 2003;20:1016-1021. [DOI] [PubMed] [Google Scholar]
- 21. Cryer PE. Mechanisms of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med. 2013;369:362-372. [DOI] [PubMed] [Google Scholar]
- 22. Cryer PE. Hypoglycemia in type 1 diabetes mellitus. Endocrinol Metab Clin North Am. 2010;39:641-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cryer PE. Iatrogenic hypoglycemia as a cause of hypoglycemia-associated autonomic failure in IDDM. Diabetes. 1992;6:255-260. [DOI] [PubMed] [Google Scholar]
- 24. Frier BM. Impaired awareness of hypoglycaemia. In: Frier BM, Fisher M, eds. Hypoglycaemia in Clinical Diabetes. Vol. 2 Chichester, UK: John Wiley; 2007:141-170. [Google Scholar]
- 25. Cranston I, Lomas J, Maran A, Macdonald I, Amiel SA. Restoration of hypoglycaemia awareness in patients with long-duration insulin-dependent diabetes. Lancet. 1994;344:283-287. [DOI] [PubMed] [Google Scholar]
- 26. Fanelli C, Pampanelli S, Epifano L, et al. Long-term recovery from unawareness, deficient counterregulation and lack of cognitive dysfunction during hypoglycaemia, following institution of rational, intensive insulin therapy in IDDM. Diabetologia. 1994;37:1265-1276. [DOI] [PubMed] [Google Scholar]
- 27. Dagogo-Jack S, Rattarasarn C, Cryer PE. Reversal of hypoglycemia unawareness, but not defective glucose counterregulation, in IDDM. Diabetes. 1994;43:1426-1434. [DOI] [PubMed] [Google Scholar]
- 28. Ly TT, Nicholas JA, Retterath A, Lim EM, Davis EA, Jones TW. Effect of sensor-augmented insulin pump therapy and automated insulin suspension vs standard insulin pump therapy on hypoglycemia in patients with type 1 diabetes: a randomized clinical trial. JAMA. 2013;310:1240-1247. [DOI] [PubMed] [Google Scholar]
- 29. van Beers CA, DeVries JH, Kleijer SJ, et al. Continuous glucose monitoring for patients with type 1 diabetes and impaired awareness of hypoglycaemia (IN CONTROL): a randomised, open-label, crossover trial. Lancet Diabetes Endocrinol. 2016;4:893-902. [DOI] [PubMed] [Google Scholar]
- 30. Little SA, Leelarathna L, Walkinshaw E, et al. Recovery of hypoglycemia awareness in long-standing type 1 diabetes: a multicenter 2 x 2 factorial randomized controlled trial comparing insulin pump with multiple daily injections and continuous with conventional glucose self-monitoring (HypoCOMPaSS). Diabetes Care. 2014;37:2114-2122. [DOI] [PubMed] [Google Scholar]
- 31. Heinemann L, Freckmann G, Ehrmann D, et al. Real-time continuous glucose monitoring in adults with type 1 diabetes and impaired hypoglycaemia awareness or severe hypoglycaemia treated with multiple daily insulin injections (HypoDE): a multicentre, randomised controlled trial. Lancet. 2018;391:1367-1377. [DOI] [PubMed] [Google Scholar]
- 32. Battelino T, Phillip M, Bratina N, Nimri R, Oskarsson P, Bolinder J. Effect of continuous glucose monitoring on hypoglycemia in type 1 diabetes. Diabetes Care. 2011;34:795-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Heinemann L, Freckmann G, Ehrmann D, et al. Real-time continuous glucose monitoring in adults with type 1 diabetes and impaired hypoglycaemia awareness or severe hypoglycaemia treated with multiple daily insulin injections (HypoDE): a multicentre, randomised controlled trial. Lancet. 2018;391:1367-1377. [DOI] [PubMed] [Google Scholar]
- 34. Heinemann L, Hermanns N. IQWiG reanalyzes and raises questions about an article by Ly et al which concluded low glucose suspend is very beneficial. J Diabetes Sci Technol. 2015;10:185-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bolinder J, Antuna R, Geelhoed-Duijvestijn P, Kroger J, Weitgasser R. Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. Lancet. 2016;388:2254-2263. [DOI] [PubMed] [Google Scholar]
- 36. Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline JP, Rayman G. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017;8:55-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Oskarsson P, Antuna R, Geelhoed-Duijvestijn P, Krger J, Weitgasser R, Bolinder J. Impact of flash glucose monitoring on hypoglycaemia in adults with type 1 diabetes managed with multiple daily injection therapy: a pre-specified subgroup analysis of the IMPACT randomised controlled trial. Diabetologia. 2018;61:539-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40:1631-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Heinemann L, Deiss D, Hermanns N, et al. HypoDE: research design and methods of a randomized controlled study evaluating the impact of real-time CGM usage on the frequency of CGM glucose values <55 mg/dl in patients with type 1 diabetes and problematic hypoglycemia treated with multiple daily injections. J Diabetes Sci Technol. 2015;9:651-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Clarke WL, Cox DJ, Gonder-Frederick LA, Julian D, Schlundt D, Polonsky W. Reduced awareness of hypoglycemia in adults with IDDM. A prospective study of hypoglycemic frequency and associated symptoms. Diabetes Care. 1995;18:517-522. [DOI] [PubMed] [Google Scholar]
- 41. Fanelli CG, Epifano L, Rambotti AM, et al. Meticulous prevention of hypoglycemia normalizes the glycemic thresholds and magnitude of most of neuroendocrine responses to, symptoms of, and cognitive function during hypoglycemia in intensively treated patients with short-term IDDM. Diabetes. 1993;42:1683-1689. [DOI] [PubMed] [Google Scholar]
- 42. Fanelli CG, Epiphano L, Rambotti AM, et al. Meticulos prevention of hypoglycemia normalizes the glycemic thresholds and magnitude of most neuroendocrine responses to symptoms of, and cognitive function during hypoglycemia in intensively treated patients with short term IDDM. Diabetes. 1992;42:1683-1689. [DOI] [PubMed] [Google Scholar]
- 43. Ly TT, Hewitt J, Davey RJ, Lim EM, Davis EA, Jones TW. Improving epinephrine responses in hypoglycemia unawareness with real-time continuous glucose monitoring in adolescents with type 1 diabetes. Diabetes Care. 2011;34:50-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Parker AIW, Jimenez J, A., Graham C. Effect of Sharing Continuous Glucose Monitoring Data from Young Children With Diabetes on CGM Usage and Hypoglycemic Exposure. New York, NY: Wiley; 2017. [Google Scholar]
- 45. Leelarathna L, Dellweg S, Mader JK, et al. Assessing the effectiveness of 3 months day and night home closed-loop insulin delivery in adults with suboptimally controlled type 1 diabetes: a randomised crossover study protocol. BMJ Open. 2014;4:e006075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Thabit H, Tauschmann M, Allen JM, et al. Home use of an artificial beta cell in type 1 diabetes. N Engl J Med. 2015;373:2129-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Abitbol A, Rabasa-Lhoret R, Messier V, et al. Overnight glucose control with dual-and single-hormone artificial pancreas in type 1 diabetes with hypoglycemia unawareness: a randomized controlled trial. Diabetes Technol Ther. 2018;20:189-196. [DOI] [PubMed] [Google Scholar]
- 48. Kropff J, DeVries JH. Continuous glucose monitoring, future products, and update on worldwide artificial pancreas projects. Diabetes Technol Ther. 2016;18:S2-53-S52-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DST831695_Supplemental_Material_CLN for Impact of CGM on the Management of Hypoglycemia Problems: Overview and Secondary Analysis of the HypoDE Study by Norbert Hermanns, Lutz Heinemann, Guido Freckmann, Delia Waldenmaier and Dominic Ehrmann in Journal of Diabetes Science and Technology