Abstract
Glycemic variability (GV) a well-established risk factor for hypoglycemia and a suspected risk factor for diabetes complications. GV is also a marker of the instability of a person’s metabolic system, expressed as frequent high and low glucose excursions and overall volatile glycemic control. In this review, the author discusses topics related to the assessment, quantification, and optimal control of diabetes, including (1) the notion that optimal control of diabetes, that is, lowering of HbA1c—the commonly accepted gold-standard outcome—can be achieved only if accompanied by simultaneous reduction of GV; (2) assessment and visualization of the two principal dimensions of GV, amplitude and time, which is now possible via continuous glucose monitoring (CGM) and various metrics quantifying GV and the risks associated with hypo- and hyperglycemic excursions; and (3) the evolution of diabetes science and technology beyond quantifying GV and into the realm of GV control via pharmacological agents, for example, GLP-1 receptor agonists and DPP-4 inhibitors, which have pronounced variability-reducing effect, or real-time automated closed-loop systems commonly referred to as the “artificial pancreas.” The author concludes that CGM allows close tracking over time, and therefore precise quantification, of glycemic variability in diabetes. The next step—optimal control of glucose fluctuations—is also taken by medications with pronounced GV-lowering effect primarily in type 2 diabetes, and by automated insulin delivery in type 1 diabetes. Contemporary CGM-based artificial pancreas systems use specific GV representations as input signals, and thus their main objective is to minimize GV and, from there, optimize glycemic control.
Keywords: glycemic variability, hypoglycemia, hyperglycemia, continuous glucose monitoring, closed-loop control, artificial pancreas
Decades ago, glycated hemoglobin A1c was identified as the primary marker of long-term average glycemic control,1,2 and still remains the gold-standard assay reflecting average glycaemia, accepted as a standard marker for average glycemic control, and proposed as a diagnostic criterion for diabetes.3,4 The utility of HbA1c as a predictor of diabetes complications in type 1 diabetes has been established by the landmark Diabetes Control and Complications Trial (DCCT)5-7 and by the Stockholm Diabetes Intervention study;8,9 in 1998, the UK Prospective Diabetes Study confirmed that intensive treatment with insulin or with oral medications reduced markedly the chronic complications of type 2 diabetes.10 The Epidemiology of Diabetes Interventions and Complications (EDIC) study11 continued the work of the DCCT and in 2016 confirmed that “overall mortality in the combined DCCT/EDIC cohort was similar to that of the general population, but was higher in the DCCT conventional therapy group. Mortality increased significantly with increasing mean HbA1c.”12
However, glycemic variability (GV) in type 1 and type 2 diabetes remained, and still remains, at the root of clinicians’ inability to safely achieve near-normal average glycemia, as reflected by HbA1c. While reducing hyperglycemia and targeting HbA1c values of 7% or less result in decreased risk of micro- and macro-vascular complications,5-10 the risk for hypoglycemia increases with tightening glycemic control.13-17 Consequently, hypoglycemia has been implicated as the primary barrier to tight control.18,19 Therefore, people with diabetes face a lifelong optimization problem: to maintain strict glycemic control without increasing their risk for hypoglycemia. In clinical terms, the optimization problem of diabetes was formulated as a “trade-off between glycemic control and iatrogenic hypoglycemia,”20 meaning that lowering of HbA1c must be accompanied by concurrent mitigation of the risk for hypoglycemia. This postulate, however, was strictly focused on desired glycemic outcomes and did not prescribe means for quantifying the trade-off between HbA1c lowering and the occurrence of hypoglycemia that should be achieved with optimal control of diabetes. A step toward quantitative understanding of this clinical paradigm was taken by the recent International Consensus on use of continuous glucose monitoring (CGM), which relied on the output generated by contemporary CGM devices to recommend metrics and interpretation of the voluminous and complex CGM data streams.21
In this review, we discuss topics related to the assessment, quantification, and optimization of diabetes control, including GV, the amplitude and timing of blood glucose (BG) fluctuations, and various metrics of GV and the risks of hypo- and hyperglycemia. We argue that diabetes is one of the best-quantified human conditions—a progress that takes diabetes science and technology beyond quantifying GV and into the realm of active optimal control using not only pharmacological agents, but also engineering solutions such as real-time automated closed-loop control commonly referred to as the “artificial pancreas.”
Risk Factors for Glycemic Variability
To begin deciphering and quantifying the clinical optimization paradigm of diabetes, we refer to a figure published in Nature Reviews Endocrinology 13:428, 2017,22 which illustrates that a strategy for achieving such an optimization can be successful only if it reduced GV. This is because bringing average glycemia down is possible only if GV is constrained—otherwise BG fluctuations would inevitably enter the range of hypoglycemia:22
The message of Figure 1 is that BG levels are a dynamical process in time, which exhibits quantifiable differences between health and diabetes reflected by average glucose levels, and by the magnitude and timing of glycemic variation. In health, glucose metabolism is controlled by a hormonal network including the pancreas, the liver, the gut, and the brain, reacting rapidly to food intake and physical activity to attenuate postprandial hyperglycemic excursions, minimize exposure to hypoglycemia, and ensure stable fasting BG. The latter reflects a natural steady state to which the metabolic system will converge, if left undisturbed. In type 2 diabetes, the fasting steady-state BG levels are abnormally elevated due to a combination of insulin resistance and inadequate beta cell response; in type 1 diabetes, a steady state to which the system would converge on its own, does not exist.
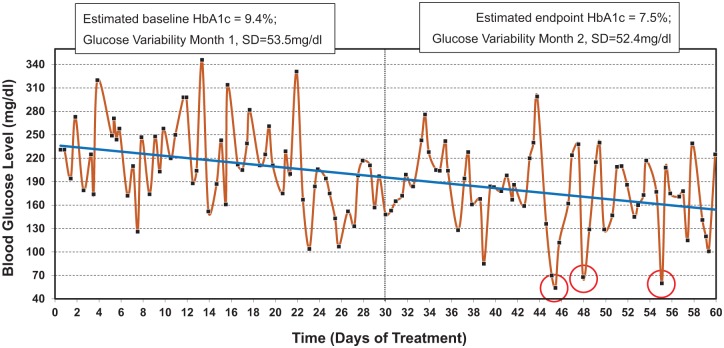
Figure 1.
Self-monitoring data recorded over the course of 60 days. Downward trend in blood glucose level is evident and the estimated HbA1 decreases from 9.4% at baseline to 7.5% at the end of observation. However, glycemic variability remains relatively unchanged from the first to the second month of observation, which results in 3 hypoglycemic episodes (below 70 mg/dl) registered by SMBG at days 45, 48, and 55.
Thus, in pathophysiology, the metabolic control network is degraded. In type 2 diabetes, the network structure is largely preserved, but insulin secretion is deficient relative to hepatic and peripheral insulin resistance. The incretin response is deficient,23,24 and this landmark finding triggered the introduction of new classes of medications known as GLP-1 receptor agonists (incretin mimetics), and DPP-4 inhibitors (incretin enhancers).25 In type 1 diabetes, insulin secretion is virtually absent, while glucagon secretion from the alpha-cell is still preserved; thus, external insulin replacement is mandatory via injections, continuous subcutaneous insulin infusion (CSII) using insulin pumps, or closed-loop control (CLC) using a combination of CGM and CSII driven by a control algorithm. These pharmacological and engineering means for diabetes control have two elements in common: (1) both target lowering of GV and (2) both are imperfect, thus achieving glycemic control that is still inferior to the BG stability in health. It follows that, although the long-term effect of pharmacological or engineering therapies of diabetes is assessed by HbA1c, the real-time goals of these treatments are guided by reduced GV. For example, a number of GV measures were predictive of glycemic outcomes during treatment intensification in type 2 diabetes,26 and major medication effects in type 2 diabetes manifested as significant reduction of GV.27-29
The limitations of HbA1c as the sole marker of glycemic control have been discussed extensively,22,30-33 and the clinical utility of GV has been debated at length.34-38 The DCCT data showed that only 8% of severe hypoglycemic episodes could be predicted from known variables, including HbA1c.17 In other studies, HbA1c has never been substantially associated with severe hypoglycemia.39-42 The ACCORD trial concluded that targeting HbA1c lower than 6.0% did not lead to improved outcomes for people with long-standing type 2 diabetes; on the contrary, aggressive treatment was associated with increase in all-cause mortality, which led to the termination of the intensive regimen of this trial 17 months before its scheduled end.43 In the ADVANCE Collaborative trial, intensive glucose control lowered HbA1c to 6.5% and resulted in significant reduction in the incidence of major microvascular events but not in the incidence of major macrovascular events or overall mortality.44 These effects were accompanied by increased incidence of severe hypoglycemia in the experimental group, which can be associated with the message presented in Figure 1: safe and clinically meaningful reduction of HbA1c can be achieved only if combined with concurrent reduction in GV.
Assessment of Glycemic Variability
While the pros and cons of GV as a marker of glycemic control will inevitably continue to be debated, it is quite clear that one of the reasons that these debates have not been settled, is the lack of an universally accepted marker of GV.45 Over the years, various metrics quantifying GV have been introduced, including but not limited to standard deviation (SD) and coefficient of variation (CV), as well as diabetes-specific metrics, such as the M-Value based on a logarithmic transformation of the BG deviation from a preset value;46 the mean amplitude of glycemic excursions (MAGE), which by definition is “devoid of time component,” that is, focuses solely on the minimum-to-maximum BG span, regardless of the time it takes for BG to transition from one extreme to the next;47 the Lability Index;48 and the mean absolute glucose change (MAG).49 Risk-based metrics of GV were introduced in 1997 to account for the fact that the BG measurement scale is quite asymmetric: the hypoglycemic range (below 70 mg/dl) is much narrower numerically than the hyperglycemic range (BG>180 mg/dl). This asymmetry creates a number of computational problems and challenges.50 For example, a BG excursion from 180 to 240 mg/dl is much larger numerically than a BG excursion from 70 to 50 mg/dl, yet the latter carries a much greater imminent risk to the patient. The asymmetry was resolved by a scale transformation with analytical form and parameters that have remained unchanged in the past 20 years.22 The contemporary use of this scale transformation includes: quantifying GV via the low BG index (LBGI)—a metric specifically designed to be sensitive to the frequency and extent of hypoglycemic excursions;41 the high BG index (HBGI) which is orthogonal to the LBGI and is designed to be sensitive to the frequency and extent of hyperglycemia;51 the average daily risk range (ADRR), which is a risk-based metric of overall GV;52 as well as the use of risk-based cost function in the design of artificial pancreas algorithms, which is illustrated in the next section.53-55
In February 2017, the Advanced Technologies & Treatments for Diabetes (ATTD) Congress convened an international panel of physicians, researchers, and individuals with diabetes to address the issue of using CGM data to quantify glycemic outcomes, including GV. The ATTD consensus recognized that “measurement of HbA1c has been the traditional method for assessing glycemic control. However, it does not reflect intra- and interday glycemic excursions that may lead to acute events (such as hypoglycemia) or postprandial hyperglycemia, which have been linked to both microvascular and macrovascular complications. CGM, either from real-time use or intermittently viewed, addresses many of the limitations inherent in HbA1c testing and self-monitoring of blood glucose.”21 In an attempt to prioritize the multitude of existing GV measures in their relevance to CGM, the ATTD Consensus recommended 14 key metrics to be documented and utilized to assess glycemic control, including mean glucose, CV, times in several BG ranges (eg, below 70 mg/dl, 70-180 mg/dl, above 180 mg/dl), estimated A1c (eA1c), and risks for hypo- and hyperglycemia as quantified by the LBGI and HBGI21 (Table 1). CV was recommended as a primary indicator of GV due to its relative sensitivity to hypoglycemia (as compared to SD), and easy computation. Based on literature results, the Consensus suggested that “stable glucose levels are defined as a CV <36%, and unstable glucose levels are defined as CV ≥36%.”
Table 1.
Calculation of Times in Range and the LBGI/HBGI Illustrating the Similarities of the Computational Process.
| Metrics based on a dicontinuous stepwise cost function | |
| Time in range cost function r(x) |
, where
for several BG readings x1, . . . , xn measured in mg/dl. |
| Time below 70 mg/dl is calculated as the average of the cost function values within a certain range |
, whereif xi < 70 and 0 otherwise |
| Time within 70-180 mg/dl is calculated as the average of the cost function values within the target range |
, whereif 70 ≤ xi ≤ 180 and 0 otherwise |
| Time above 180 mg/dl is calculated as the average of the cost function values within a certain range |
, whereif xi >180 and 0 otherwise |
| Metrics based on a continuous quadratic cost (risk) function | |
| LBGI and HBGI cost (risk) function r(x) |
, where
for several BG readings x1, . . . , xn measured in mg/dl |
| LBGI is calculated as the average of the cost function values within a certain range |
, whereif xi ≤ 112.5 and 0 otherwise |
| HBGI is calculated as the average of the cost function values within a certain range |
, whereif xi >112.5 and 0 otherwise |
The specific emphasis placed on times in glucose ranges reaffirmed the notion that glycemic fluctuation is a process in time, which has two principal dimensions: amplitude associated with the extent of BG extremes, and time, which identifies the rate of event progression.56 The ATTD consensus recognized that, using CGM, it is now possible to assess both of these dimensions in real time and retrospectively. Figure 2 (originally published in Kovatchev and Cobelli56) presents these two key dimensions:
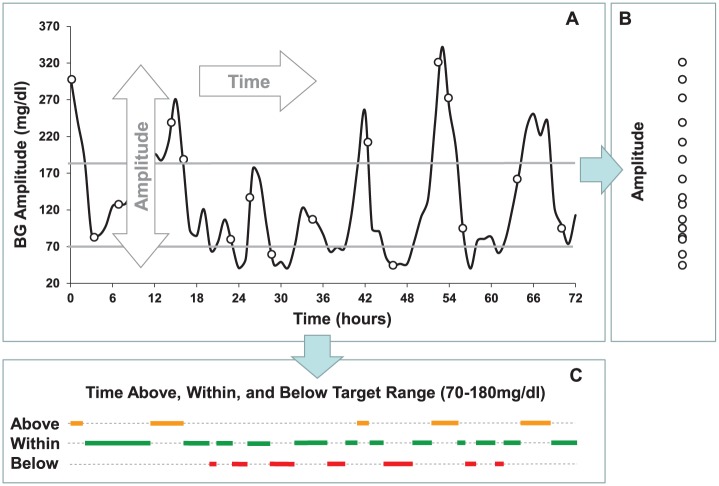
Figure 2.
Principal components of glycemic variability. Glucose fluctuations are a process in time which has two dimensions—amplitude and time. Projected along its amplitude axis, this process is measured by metrics such as SD or MAGE (Panel B). Projected along its time axis, this process is assessed by temporal characteristics, such as time within target range and time spent in hypo- or hyperglycemia (Panel C).
As seen in Figure 2, all GV metrics reviewed above fall in two general categories: The first category is metrics of amplitude, which are quantified from the projection of the CGM data along the y-axis, for example, from Panel B. These metrics account for the extent of BG fluctuations, without placing them in time—most traditional metrics of GV fall in this category. The second category is time-dependent metrics, which are quantified from the projection of the data along the x-axis, for example, Panel C. These metrics are representative of the duration of various events—in this example the duration of time spent below, within, and above a predetermined target range of 70-180 mg/dl. To clarify this distinction further, let’s conduct the following mental experiment: If the x-axis in Figure 2 were compressed to 24 hours (instead of the current 72 hours) keeping the magnitude of the BG fluctuations intact, amplitude metrics such as CV or SD would not change—their values would be preserved because they are computed solely from the projection of the data on the y-axis. However, the absolute time spent in normoglycemia would be reduced 3-fold. We should note that the frequently computed “percent time” in range is a metric that does not depend on the pace of events because its time component cancels out when the data are converted from absolute to relative time scale. To describe the real pace of events, more appropriate would be metrics that reflect absolute timing, such as time in range for 24 hours, for example, 12 out of 24 hours within 70-180 mg/dl.
The formulas for computing SD, CV, MAGE, MAG, LBGI, HBGI, ADRR, and a number of other metrics of GV have been presented in the original publications introducing these metrics and are summarized in several reviews, for example, Table 1 in Kovatchev,22 which lists traditional, risk-based, and CGM-based metrics of glucose variability. Thus, we will not include these formulas in this presentation. However, to streamline further the understanding and classification of the various GV metrics and to link their utility to optimal control of diabetes, we would offer analytical and graphical representations of the computing process of time in range (TIR) and the LBGI/HBGI. As seen in Table 1, the similarities in the calculations are substantial and the only difference is in the “penalty” imposed by these two types of metrics on BG deviations from a predetermined target, that is, in the cost function used for their calculation which is discontinuous-stepwise for TIR and continuous-quadratic for the LBGI and HBGI. The latter is also called risk function, hence the LBGI and the HBGI are referred to as risk indices increasing proportionally to the risk for hypo- and hyperglycemia, respectively, carried by BG deviations toward hypo- and hyperglycemia.
Control of Glycemic Variability
To elucidate further the link between GV and optimal control, Figure 3 presents graphically the calculation of TIRs (eg, time below 70 mg/dl or time above 180 mg/dl, or time within the target range), which are computed using a stepwise cost function (blue line) that is equal to 100 in the desired range and is 0 otherwise. Similarly, the LBGI and the HBGI are computed using a quadratic cost function (red line, also called risk function) that increases smoothly from 0 to 100 when BG excursions venture into hypo- and hyperglycemia. As noted above, the only difference between the computation of TIRs and the LBGI/HBGI is in the cost function; everything else is a summation across relevant glucose values (see Table 1).
Figure 3.
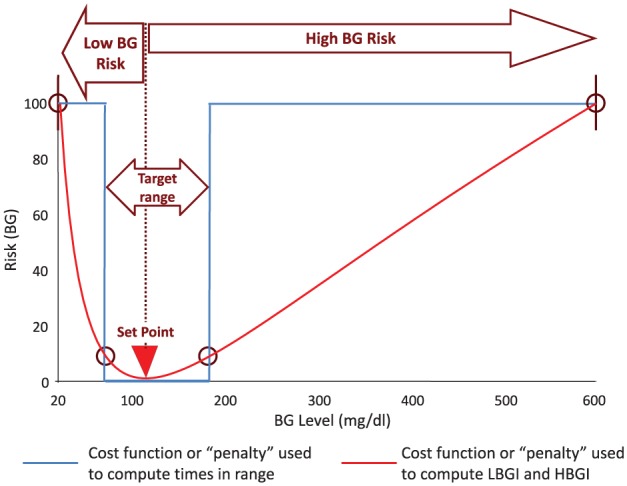
The blood glucose risk function (red line) which increases steeply when BG levels descend into the hypoglycemic range, and more gradually with the onset of hyperglycemia, used to compute the low and high blood glucose indices and as a cost function in closed-loop control algorithms. The cost function used to compute various times in range, for example, below 70 mg/dl or above 180 mg/dl (blue line).
The notion of cost function, or penalty imposed to BG deviations from a desired value (typically called set point), is directly relevant to both the assessment of outcomes from clinical trials, and to the design and the functioning of CLC algorithms. The choice of a GV metric, or an algorithm cost function, can determine the outcomes of a clinical trial, or the algorithm behavior. For example, a study evaluating the improvement in GV and the correlation between baseline GV, HbA1c, and hypoglycemic events in 1699 patients with type 2 diabetes undergoing treatment intensification with basal insulin or comparators for 24 weeks, found that several GV metrics improved significantly from baseline to week 24, but not to the same extent.26 The largest proportional reduction was achieved by the HBGI (65.5%). In addition, pretreatment GV was associated with glycemic outcomes at 24 weeks, and the HBGI was the metric most predictive of HbA1c improvement.26
Similarly, a pooled analysis of patient-level data from three 24-week, randomized, phase III clinical trials (N = 1198 patients with type 2 diabetes) evaluated the impact on GV of the GLP-1 receptor agonist Lixisenatide as add-on to basal insulin (N = 665 patients) versus placebo (N = 533 patients). While there were statistically significant differences in the improvement of HbA1c on Lixisenative versus placebo, the most pronounced effect of this GLP-1 receptor agonist was reduction in GV. The values of SD, MAG, MAGE, and HBGI significantly decreased on Lixisenatide versus placebo, while LBGI values remained unchanged, indicating that the improvement in average glycemia and GV was achieved without increase in the risk for hypoglycemia.27 This result is consistent with Figure 1, and with the findings of the FLAT-SUGAR study—reduction of GV associated with the use of GLP-1 receptor agonists while maintaining equivalent HbA1c levels.29
We should also note that, because HbA1c reflects only a slow-moving average, the utility of therapies specifically targeting reduction of GV and mitigation of fast glucose fluctuations, cannot be discerned from analysis of HbA1c data alone. Thus, while by tradition virtually all studies evaluating new pharmaceutical agents have been focused on HbA1c reduction, a choice of a different outcome metric can emphasize the pronounced variability-reducing effect of some classes of medications, such as GLP-1 receptor agonists or DPP-4 inhibitors, and obtain these findings at a fraction of the sample size needed to demonstrate HbA1c improvement. This means that, if the expected outcome of a treatment intervention is reduction in GV and the sample size of a study is calculated to achieve a significant HbA1c effect, the study is likely over-powered for its expected outcome. Indeed, studies using CGM and sample sizes substantially smaller than the large investigations discussed above, were able to achieve statistically significant results when focusing primarily on the variability-lowering effects of medications such as Pramlintide (synthetic amylin),57 Liraglutide (GLP1 receptor agonist),58 and Vildagliptin versus Sitagliptin (DPP4 inhibitors).59
In the setting of automated insulin delivery most, if not all, contemporary CLC algorithms aim to maximize the time spent within a desired target range, minimize hypoglycemic events and prevent postprandial hyperglycemia, all of which require real-time reduction of GV.55 Papers reflecting the progress of the artificial pancreas field have been published regularly.60-65 Thus, our objective here is different—to highlight a little known aspect of the design of many CLC algorithms—their relationship to quantifying glucose variability to optimize real-time automated control. This is typically achieved by using a cost function similar to the ones presented in Figure 3, which penalizes BG deviations away from a certain set point.53 In some CLC designs, the cost function is asymmetric, placing rapidly increasing “penalties” on BG levels that are approaching hypoglycemia, and more gradually increasing penalties on BGs approaching hyperglycemia.54 It was therefore suggested that “in order to address the asymmetry of the control problem, future MPC (model-predictive control) formulations could use output feedback in the risk domain, instead of the glucose domain, thus adding a clinical weighting to the controller cost function.”53 This suggestion refers to the risk function presented in Figure 3 (red line) and in essence links the calculation of risk-based metrics of GV with the design of automated closed-loop algorithms. We should note, however, that the use of a TIR-based cost function for the design of CLC algorithms is highly inconvenient computationally, due to its discontinuity (blue line in Figure 3).
Discussion
Because intensive treatment to lower HbA1c characteristically results in increased risk for hypoglycemia, patients with diabetes face a lifelong optimization problem to reduce their average glycemic levels and postprandial hyperglycemia, while simultaneously avoiding hypoglycemia. As visualized in Figure 1, this optimization can be achieved only in the context of lowering GV, that is, stabilization of the metabolic system. GV is a reflection of an underlying biobehavioral process of BG fluctuation that has two principal dimensions: amplitude reflecting the extent of BG excursion, and time reflecting the frequency of BG variation and the rate of event progression. CGM enables advanced means for observation of this process: CGM data are detailed time series that track BG fluctuations over time in short increments (eg, every 5 minutes).66 Time series reflect the dynamics of the metabolic system in real time (with RT-CGM) or retrospectively (eg, with Flash glucose monitoring), and therefore present unique opportunities for analysis and optimization.67,68 In particular, both of these approaches produce dense data that allow the computation of various metrics of GV.69,70 In addition, RT-CGM enables automated actions, such as low glucose suspend (LGS), predictive LGS, and CLC.
When choosing study outcomes, one should be mindful whether the desired outcome needs to emphasize only the average glycemia of the participants, or should venture in more advanced characteristics reflecting the dynamics of BG fluctuations. If the latter is of interest, a second question is whether to focus on the amplitude or on the timing of BG fluctuations, or whether the outcomes should reflect the risks associated with hypo- or hyperglycemia. If only the amplitude of BG fluctuations is of interest, metrics such as SD or CV can be used—all are computed from the y-axis projection of the data in Panel B, Figure 2. If the timing of the BG fluctuation process is of prime interest, then metrics such as TIR would be relevant, as computed from the x-axis projection of the BG trace (Panel C in Figure 2). If both the amplitude and the timing of BG fluctuations are of importance, metrics such as the LBGI and HBGI can be used to combine these two principal dimensions of the BG fluctuation process, and to also provide a sense of the risks associated with the outcome: rapidly increasing with the extent and frequency of hypoglycemia and more gradually increasing with the extent and frequency of hyperglycemia (Figure 3).
Conclusions
For decades, HbA1c has been the sole metric of glycemic control used by physicians and patients to optimize diabetes therapy, and by clinical studies as their primary outcome. However, HbA1c has certain shortcomings, the most prominent of which is its limited responsiveness to hypoglycemia. Thus, along with HbA1c, GV is increasingly regarded as a risk factor for hypo- and hyperglycemia, and as a primary marker reflecting treatment optimization. Various GV metrics exist reflecting the amplitude and timing of BG fluctuation. Certain classes of medications, for example, GLP-1 receptor agonists and DPP-4 inhibitors, have pronounced variability-reducing effect; thus GV analyses will help evaluate better their utility in the treatment of diabetes. CGM technology exists for the direct observation of BG fluctuations; thus the assessment of diabetes treatment efficacy can move beyond the HbA1c assay as the sole marker of glycemic control. The next step—real-time control of BG fluctuations—is also taken by RT-CGM based automated insulin delivery systems that use specific GV representations as input signals and formulate their main objective—to reduce GV as a precursor to optimal glycemic control.
Footnotes
Abbreviations: ADRR, average daily risk range; ATTD, Advanced Technologies & Treatments for Diabetes; BG, blood glucose; CGM, continuous glucose monitoring; CLC, closed-loop control; CSII, continuous subcutaneous insulin infusion; CV, coefficient of variation; DCCT, Diabetes Control and Complications Trial; DPP-4, dipeptidyl peptidase 4; EDIC, Epidemiology of Diabetes Interventions and Complications; GLP-1, glucagon-like peptide-1; GV, glycemic variability; HBGI, high blood glucose index; LBGI, low blood glucose index; LGS, low glucose suspend; MAG, mean absolute glucose change; MAGE, mean amplitude of glycemic excursions; MPC, model-predictive control; RT-CGM, real-time continuous glucose monitoring; SD, standard deviation; TIR, time in range.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The author reports: Patents and patent applications related to diabetes technology managed by the University of Virginia Licensing and Ventures group; research support managed by the University of Virginia from Dexcom, Roche Diagnostics, Sanofi, Tandem Diabetes Care; speaking engagement/advisory panel/consultant: Dexcom, Sanofi, Tandem Diabetes Care.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The University of Virginia Precision Individualized Medicine for Diabetes (PrIMeD) Project supported the writing of this review. The presented concepts and technologies have been developed under NIH/NIDDK grants RO1 DK051562, RO1 DK 085623, and UC4 DK108483.
ORCID iD: Boris Kovatchev  https://orcid.org/0000-0003-0495-3901
https://orcid.org/0000-0003-0495-3901
References
- 1. Aaby Svendsen P, Lauritzen T, Soegard U, Nerup J. Glycosylated haemoglobin and steady-state mean blood glucose concentration in type 1 (insulin-dependent) diabetes. Diabetologia. 1982;23:403-405. [DOI] [PubMed] [Google Scholar]
- 2. Santiago JV. Lessons from the diabetes control and complications trial. Diabetes. 1993;42:1549-1554. [DOI] [PubMed] [Google Scholar]
- 3. International Expert Committee. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. American Diabetes Association. Standards of medical care in diabetes-2010. Diabetes Care. 2010;33:S11-S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications of insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986. [DOI] [PubMed] [Google Scholar]
- 6. Diabetes Control and Complications Trial Research Group. The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the Diabetes Control and Complications Trial. Diabetes. 1995;44:968-983. [PubMed] [Google Scholar]
- 7. Lachin JM, Genuth S, Nathan DM, Zinman B, Rutledge BN, DCCT/EDIC Research Group. Effect of glycemic exposure on the risk of microvascular complications in the diabetes control and complications trial revisited. Diabetes. 2008;57:995-1001. [DOI] [PubMed] [Google Scholar]
- 8. Reichard P, Pihl M. Mortality and treatment side effects during long-term intensified conventional insulin treatment in the Stockholm Diabetes Intervention Study. Diabetes. 1994;43:313-317. [DOI] [PubMed] [Google Scholar]
- 9. Reichard P, Pihl M, Rosenqvist U, Sule J. Complications in IDDM are caused by elevated blood glucose level: The Stockholm Diabetes Intervention Study (SDIS) at 10-year follow up. Diabetologia. 1996;39:1483. [DOI] [PubMed] [Google Scholar]
- 10. UK Prospective Diabetes Study Group (UKPDS). Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes. Lancet. 1998;352:837-853. [PubMed] [Google Scholar]
- 11. Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group Epidemiology of Diabetes Interventions and Complications (EDIC). Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care. 1999;22:99-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group. Mortality in type 1 diabetes in the DCCT/EDIC versus the general population. Diabetes Care. 2016;39:1378-1383.27411699 [Google Scholar]
- 13. White NH, Skor DA, Cryer PE, Levandoski L, Santiago JV. Identification of type I diabetic patients at increased risk for hypoglycemia during intensive therapy. N Engl J Med. 1983;308:485-491. [DOI] [PubMed] [Google Scholar]
- 14. Cryer PE, Gerich JE. Glucose counterregulation, hypoglycemia, and intensive therapy of diabetes mellitus. N Engl J Med. 1985;313:232-241. [DOI] [PubMed] [Google Scholar]
- 15. Amiel SA, Tamborlane WV, Simonson DC, Sherwin RS. Defective glucose counterregulation after strict glycemic control of insulin-dependent diabetes mellitus. N Engl J Med. 1987;316:1376-1383. [DOI] [PubMed] [Google Scholar]
- 16. Amiel SA, Sherwin RS, Simonson DC, Tamborlane WV. Effect of intensive insulin therapy on glycemic thresholds for counterregulatory hormone release. Diabetes. 1988;37:901-907. [DOI] [PubMed] [Google Scholar]
- 17. Diabetes Control and Complications Trial Research Group. Hypoglycemia in the diabetes control and complications trial. Diabetes. 1997;46:271-286. [PubMed] [Google Scholar]
- 18. Cryer PE. Hypoglycemia: the limiting factor in the management of IDDM. Diabetes. 1994;43:1378-1389. [DOI] [PubMed] [Google Scholar]
- 19. Cryer PE. Hypoglycaemia: the limiting factor in the glycaemic management of type I and type II diabetes. Diabetologia. 2002;45:937-948. [DOI] [PubMed] [Google Scholar]
- 20. Cryer PE. Glycemic goals in diabetes: trade-off between glycemic control and iatrogenic hypoglycemia. Diabetes. 2014;63:2188-2195. [DOI] [PubMed] [Google Scholar]
- 21. Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40:1631-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kovatchev BP. Metrics for glycaemic control: from HbA1c to continuous glucose monitoring. Nat Rev Endocrinol. 2017;13:425-436. [DOI] [PubMed] [Google Scholar]
- 23. Nauck MA, Homberger E, Siegel EG, et al. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J Clin Endocrinol Metab. 1986;63:492-498. [DOI] [PubMed] [Google Scholar]
- 24. Nauck M, Stöckmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia. 1986;29:46-52. [DOI] [PubMed] [Google Scholar]
- 25. Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696-1705. [DOI] [PubMed] [Google Scholar]
- 26. Inzucchi SE, Umpierrez G, DiGenio A, Zhou R, Kovatchev BP. How well do glucose variability measures predict patient glycaemic outcomes during treatment intensification in type 2 diabetes? Diab Res Clin Pract. 2015;110:234-240. [DOI] [PubMed] [Google Scholar]
- 27. Umpierrez G, O’Neal D, DiGenio A, et al. Lixisenatide reduces glycaemic variability in insulin-treated patients with type 2 diabetes. Diabetes Obes Metab. 2017;19:1317-1321. [DOI] [PubMed] [Google Scholar]
- 28. FLAT-SUGAR Trial Investigators. Design of FLAT-SUGAR: randomized trial of prandial insulin versus prandial GLP-1 receptor agonist together with basal insulin and metformin for high-risk type 2 diabetes. Diabetes Care. 2015;38:1558-1566. [DOI] [PubMed] [Google Scholar]
- 29. FLAT-SUGAR Trial Investigators. Glucose variability in a 26-week randomized comparison of mealtime treatment with rapid-acting insulin versus GLP-1 agonist in participants with type 2 diabetes at high cardiovascular risk. Diabetes Care. 2016;39:973-981. [DOI] [PubMed] [Google Scholar]
- 30. Hirsch IB, Brownlee M. Should minimal blood glucose variability become the gold standard of glycemic control? J Diabetes Complications. 2005;19:178-181. [DOI] [PubMed] [Google Scholar]
- 31. Brownlee M, Hirsch IB. Glycemic variability: a hemoglobin A1c-independent risk factor for diabetic complication? JAMA. 2006;295:1707-1708. [DOI] [PubMed] [Google Scholar]
- 32. Monnier L, Colette C. Glycemic variability. Diabetes Care. 2008;31(suppl 2):S150-S154. [DOI] [PubMed] [Google Scholar]
- 33. McCall AL, Kovatchev BP. The median is not the only message: a clinician’s perspective on mathematical analysis of glycemic variability and modeling in diabetes mellitus. J Diabetes Sci Technol. 2009;3:3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Siegelaar SE, Holleman F, Hoekstra JBL, DeVries JH. Glucose variability; does it matter? Endocr Rev. 2010;31:171-182. [DOI] [PubMed] [Google Scholar]
- 35. DeVries JH. Glucose variability: where it is important and how to measure it. Diabetes. 2013;62:1405-1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hirsch IB. Glycemic variability and diabetes complications: does it matter? of course it does! Diabetes Care. 2015;38:1610-1614. [DOI] [PubMed] [Google Scholar]
- 37. Service FJ. Glucose variability. Diabetes. 2013;62:1398-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bergenstal RM. Glycemic variability and diabetes complications: does it matter? Simply put, there are better glycemic markers! Diabetes Care. 2015;38:1615-1621. [DOI] [PubMed] [Google Scholar]
- 39. Cox DJ, Kovatchev BP, Julian DM, et al. Frequency of severe hypoglycemia in IDDM can be predicted from self-monitoring blood glucose data. J Clin Endocrinol Metab. 1994;79:1659-1662. [DOI] [PubMed] [Google Scholar]
- 40. Gold AE, Frier BM, MacLeod KM, Deary IJ. A structural equation model for predictors of severe hypoglycaemia in patients with insulin-dependent diabetes mellitus. Diabet Med. 1997;14:309-315. [DOI] [PubMed] [Google Scholar]
- 41. Kovatchev BP, Cox DJ, Gonder-Frederick LA, Young-Hyman D, Schlundt D, Clarke WL. Assessment of risk for severe hypoglycemia among adults with IDDM: validation of the low blood glucose index. Diabetes Care. 1998;21:1870-1875. [DOI] [PubMed] [Google Scholar]
- 42. Cox DJ, Gonder-Frederick LA, Ritterband L, Clarke WL, Kovatchev BP. Prediction of severe hypoglycemia. Diabetes Care. 2007;30:1370-1373. [DOI] [PubMed] [Google Scholar]
- 43. Gerstein HC Miller ME Byington RP et al.;. Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545-2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Patel A MacMahon S Chalmers J et al.;. ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560-2572. [DOI] [PubMed] [Google Scholar]
- 45. Rodbard D. The challenges of measuring glycemic variability. J Diabetes Sci Technol. 2012;6:712-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schlichtkrull J, Munck O, Jersild M. The M-value, an index of blood-sugar control in diabetics. Acta Med Scand. 1965;177:95-102. [DOI] [PubMed] [Google Scholar]
- 47. Service FJ, Molnar GD, Rosevear JW, Ackerman E, Gatewood LC, Taylor WF. Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes. 1970;19:644-655. [DOI] [PubMed] [Google Scholar]
- 48. Ryan EA, Shandro T, Green K, et al. Assessment of the severity of hypoglycemia and glycemic lability in type 1 diabetic subjects undergoing islet transplantation. Diabetes. 2004;53:955-962. [DOI] [PubMed] [Google Scholar]
- 49. Hermanides J, Vriesendorp TM, Bosman RJ, Zandstra DF, Hoekstra JB, Devries JH. Glucose variability is associated with intensive care unit mortality. Crit Care Med. 2010;38:838-842. [DOI] [PubMed] [Google Scholar]
- 50. Kovatchev BP, Cox DJ, Gonder-Frederick LA, Clarke WL. Symmetrization of the blood glucose measurement scale and its applications. Diabetes Care. 1997;20:1655-1658. [DOI] [PubMed] [Google Scholar]
- 51. Kovatchev BP, Cox DJ, Gonder-Frederick LA, Clarke WL. Methods for quantifying self-monitoring blood glucose profiles exemplified by an examination of blood glucose patterns in patients with type 1 and type 2 diabetes. Diabetes Technol Ther. 2002;4:295-303. [DOI] [PubMed] [Google Scholar]
- 52. Kovatchev BP, Otto E, Cox D, Gonder-Frederick L, Clarke W. Evaluation of a new measure of blood glucose variability in diabetes. Diabetes Care. 2006;29:2433-2438. [DOI] [PubMed] [Google Scholar]
- 53. Percival MW, Zisser H, Jovanovič L, Doyle FJ., III Closed-loop control and advisory mode evaluation of an artificial pancreatic β cell: use of proportional-integral-derivative equivalent model-based controllers. J Diabetes Sci Technol. 2008;2:636-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hughes CS, Patek SD, Breton MD, Kovatchev BP. Hypoglycemia prevention via pump attenuation and red-yellow-green “traffic” lights using continuous glucose monitoring and insulin pump data. J Diabetes Sci Technol, 2010;4:1146-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Doyle FJ, III, Huyett LM, Lee JB, Zisser HC, Dassau E. Closed-loop artificial pancreas systems: engineering the algorithms. Diabetes Care. 2014;37:1191-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kovatchev BP, Cobelli C. Glucose variability: timing, risk analysis, and relationship to hypoglycemia in diabetes. Diabetes Care. 2016;39:502-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. McCall AL, Cox DJ, Crean J, Gloster M, Kovatchev BP. A novel analytical method for assessing glucose variability: using CGMS in type 1 diabetes mellitus. Diabetes Technol Ther. 2006;8:644-653. [DOI] [PubMed] [Google Scholar]
- 58. Mori Y, Taniguchi Y, Sezaki K, Yokoyama J, Utsunomiya K. Liraglutide narrows the range of circadian glycemic variations in Japanese type 2 diabetes patients and nearly flattens these variations in drug-naive type 2 diabetes patients: a continuous glucose monitoring-based study. Diabetes Technol Ther. 2011;13:1139-1144. [DOI] [PubMed] [Google Scholar]
- 59. Guercia B, Monnier L, Serusclat P, et al. Continuous glucose profiles with vildagliptin versus sitagliptin in add-on to metformin: results from the randomized Optima study. Diabetes Metab. 2012;38:359-366. [DOI] [PubMed] [Google Scholar]
- 60. Hovorka R. Closed-loop insulin delivery: from bench to clinical practice. Nat Rev Endocrinol. 2011;7:385-395. [DOI] [PubMed] [Google Scholar]
- 61. Cobelli C, Renard E, Kovatchev BP. Perspectives in diabetes: artificial pancreas: past, present, future. Diabetes. 2011;60;2672-2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Renard E, Cobelli C, Zisser H, Kovatchev BP. Artificial pancreas goes outpatient: a new diabetes ecosystem. J Diabetes Sci Technol. 2013;7:1411-1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cefalu WT, Tamborlane WV. The artificial pancreas: are we there yet? Diabetes Care. 2014;37:1182-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kovatchev BP, Tamborlane WV, Cefalu WT, Cobelli C. The artificial pancreas in 2016: a digital treatment ecosystem for diabetes. Diabetes Care. 2016;39:1123-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kovatchev BP. The artificial pancreas in 2017: the year of transition from research to clinical practice. Nat Rev Endocrinol. 2018;14:74-76. [DOI] [PubMed] [Google Scholar]
- 66. Kovatchev BP, Clarke WL. Peculiarities of the continuous glucose monitoring data stream and their impact on developing closed-loop control technology. J Diabetes Sci Technol. 2008;2:158-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rodbard D. New and improved methods to characterize glycemic variability using continuous glucose monitoring. Diabetes Technol Ther. 2009;11:551-565. [DOI] [PubMed] [Google Scholar]
- 68. Clarke W, Kovatchev B. Statistical tools to analyze continuous glucose monitor data. Diabetes Technol Ther. 2009;11:S45-S54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rodbard D. Interpretation of continuous glucose monitoring data: glycemic variability and quality of control. Diabetes Technol Ther. 2009:11:S55-S67. [DOI] [PubMed] [Google Scholar]
- 70. Maahs DM, Buckingham BA, Castle JR, et al. Outcome measures for artificial pancreas clinical trials: a consensus report. Diabetes Care. 2016;39:1175-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]