Introduction
Imiquimod (IMQ) is a topical immune-response modifier with antitumor and antiviral properties and is used as a treatment for actinic keratosis, basal cell carcinoma, and human papillomavirus–associated genital and anal warts. When applied, IMQ leads to an inflammatory reaction that induces regression of skin neoplasms and virus-induced papillomas. The most common local side effects include erythema, edema, oozing and erosions. Flu-like symptoms such as headache, dizziness, fatigue, apathy, and sinusitis are known systemic side effects.1, 2 We present 2 patients who had telogen effluvium after vaginal IMQ treatment, which is an as-yet unreported side effect of IMQ treatment.
Case report
Patient 1
A 31-year old woman of Korean descent and normal weight was referred to our outpatient clinic by the department of gynecology and obstetrics because of diffuse hair loss of recent onset. Sudden and severe hair shedding had started 4 weeks prior but meanwhile almost completely resolved. A detailed medical history revealed that she had cervical intraepithelial neoplasia (CIN) grade III diagnosed previously and was therefore enrolled in a clinical trial assessing the efficacy of locally applied IMQ for the treatment of CIN II and III. The study medication consisted of self-applied vaginal suppositories containing 6.25 mg IMQ in adeps solidus. The treatment protocol required 1 suppository per week during weeks 1 and 2, 2 suppositories per week during weeks 3 and 4, and 3 suppositories per week from weeks 5 to 16 (planned cumulative IMQ dose: 262.5 mg).3 During the trial period, the patient used a cumulative IMQ dose of 71.875 mg. She suffered from multiple treatment-induced adverse effects such as high fever (up to 38.9°C), fatigue, flu-like symptoms, myalgia, headache, dizziness, and weight loss. Besides the study medication, the patient had been taking ibuprofen on demand against IMQ-related symptoms and an oral contraceptive pill containing ethinylestradiol and gestodene that she had already been using for several years. The last IMQ application was made 1 week before the onset of the hair loss.
When the patient was finally seen at our department, her hair density appeared normal, and the trichoscopic examination was unrevealing, only the hair pull test was slightly positive. Besides a melanonychia striata, no other dermatologic findings were present. Our main differential diagnoses included drug-induced hair loss or symptomatic hair loss. An extensive laboratory analysis to rule out an underlying autoimmune disease, chronic infection, hormonal imbalance, or nutritional deficiencies was without pathologic findings. When the patient returned to our clinic 7 months later, the hair loss and all systemic signs and symptoms had completely subsided.
Patient 2
Another participant in the above-mentioned trial, a 32-year old white woman with CIN III, also attended our outpatient clinic because of diffuse hair loss that started 15 weeks after initiation of IMQ treatment. During the trial period, the patient used a cumulative IMQ dose of 250 mg. Under IMQ treatment, mild-to-moderate fatigue, flu-like symptoms, headache, and local reactions in terms of increased vaginal discharge and genital itching occurred frequently. Clinical examination found a slightly decreased hair density in the frontoparietal and occipital area of the head (Fig 1). In these regions, the hair pull test was positive. Trichoscopy, scalp, nails, and the remainder of the skin were completely normal. A trichogram showed a decreased ratio of anagen to telogen hairs in both the frontoparietal (79:21) and occipital (73:27) region from the patient's scalp (Fig 2). Laboratory analysis was negative for acute or chronic infection, endocrinologic disease, autoimmunity, and iron or vitamin deficiency. Two months after cessation of IMQ treatment, the increased hair loss reverted to normal.
Fig 1.
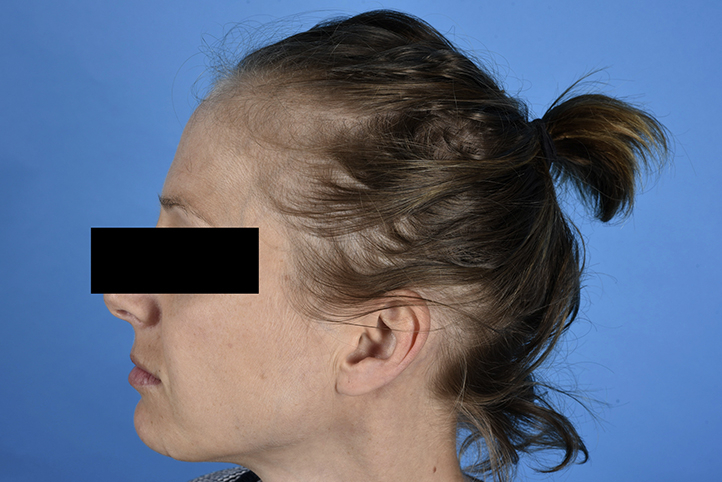
Decreased hair density in the frontoparietal and occipital region of the scalp after a 16 week course of vaginal IMQ application.
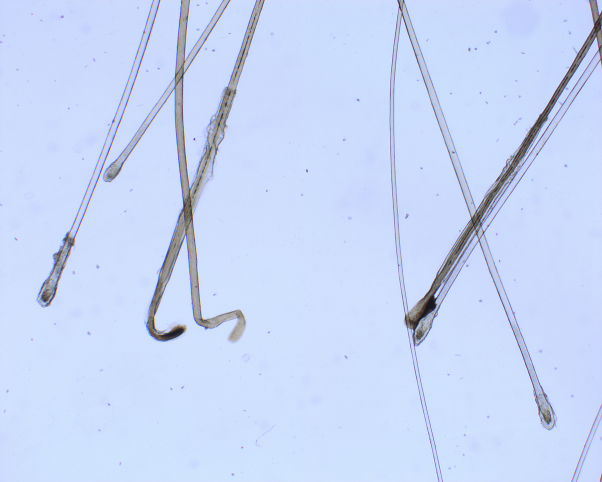
Fig 2.
Trichogram of plucked hairs from the occipital area of the scalp (patient 2) shows an increased number of telogen hairs.
Discussion
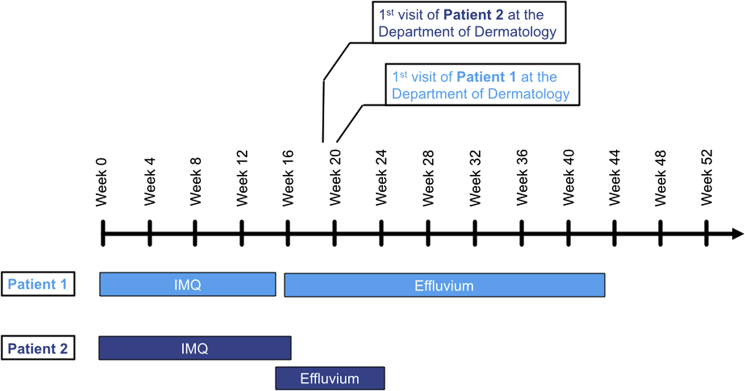
A wide range of cytotoxic insults to the hair follicle may induce premature transition of the hair from anagen to telogen phase resulting in diffuse telogen effluvium.4, 5 Potential causes of telogen effluvium include drugs, chronic infection, autoimmune disease, surgery, chronic systemic disorders, endocrine imbalance, iron or vitamin deficiencies, and intoxication.6 In our patients, there was a clear chronologic sequence between the start of the intravaginal IMQ treatment and diffuse hair loss—starting in both cases toward the end of the 16-week treatment course and resolving 7 months (patient 1) and 2 months (patient 2) after discontinuation of IMQ (Fig 3).
Fig 3.
Schematic representation of IMQ treatment and effluvium in both patients.
In both patients, the onset of hair loss was preceded by severe systemic symptoms. Results of a trichogram performed in patient 2 were in agreement with a diagnosis of diffuse telogen effluvium and excluded patterned hair loss, as the rate of telogen hairs was equally increased in the androgen-sensitive parietal and androgen-insensitive occipital area of the scalp.
The first randomized controlled phase II trial on vaginal IMQ treatment for patients with CIN II-III had included 59 patients (IMQ, n = 30; placebo, n = 29). IMQ was significantly more effective compared with placebo, inducing complete histologic remission in 47% of the patients compared with 14% in the placebo group. Local and systemic side effects were common in the IMQ group, including vulvar pain or pruritus (93%), headache (83%), myalgia (77%), and flu-like symptoms (97%). No cutaneous side effects were reported.3
A systematic literature research found only 1 case of localized hair loss in the crown region of the scalp after topical IMQ treatment for actinic keratosis, presumably caused by a local rather than a systemic side effect.7 We were unable to identify any other report on dermatologic or gynecologic patients with IMQ-induced hair loss. However, compared with the widespread use in dermatology, IMQ use in gynecology as an intravaginal application is still limited.
Interestingly, an investigation in mice found that topical IMQ application during the mid and late telogen stage may promote hair regrowth by rapid anagen induction.8 Whether and to which extent this effect might also apply to human hair follicles is currently unknown.
Our observation draws attention to IMQ therapy as a potential cause for reversible diffuse telogen effluvium, particularly in patients experiencing severe systemic adverse reactions. It is likely that the incidence of such cases will increase in the future. As the use of IMQ is expanding and new routes of topical application (such as intravaginal suppositories for treating CIN) are being explored that are associated with increased bioavailability, it is important to recognize this as a yet unreported side effect of IMQ therapy. This recognition will allow for early identification and proper counselling of patients affected by IMQ-induced hair loss and will help avoid unnecessary diagnostic workup.
Footnotes
Funding sources: None.
Conflicts of interest: Christoph Grimm, MD, has received a research grant and honoraria from MEDA pharma.
This report was a poster presentation at the annual meeting of the Austrian Society of Dermatology and Venereology (Oesterreichische Gesellschaft für Dermatologie und Venerologie, OEGDV), December 1, 2018.
References
- 1.Graceway Pharmaceuticals Imiquimod (Aldara) Product Insert. 2010. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020723s022lbl.pdf
- 2.van Seters M., van Beurden M., ten Kate F.J.W. Treatment of vulvar intraepithelial neoplasia with topical imiquimod. N Engl J Med. 2008;358(14):1465–1473. doi: 10.1056/NEJMoa072685. [DOI] [PubMed] [Google Scholar]
- 3.Grimm C., Polterauer S., Natter C. Treatment of cervical intraepithelial neoplasia with topical imiquimod: a randomized controlled trial. Obstet Gynecol. 2012;120(1):152–159. doi: 10.1097/AOG.0b013e31825bc6e8. [DOI] [PubMed] [Google Scholar]
- 4.Tosi A., Misciali C., Piraccini B.M., Peluso A.M., Bardazzi F. Drug-induced hair loss and hair growth. Incidence, management and avoidance. Drug Saf. 1994;10(4):310–317. doi: 10.2165/00002018-199410040-00005. [DOI] [PubMed] [Google Scholar]
- 5.Headington J.T. Telogen effluvium. New concepts and review. Arch Dermatol. 1993;129(3):356–363. doi: 10.1001/archderm.129.3.356. [DOI] [PubMed] [Google Scholar]
- 6.Yu V., Juhász M., Chiang A., Atanaskova Mesinkovska N. Alopecia and associated toxic agents: a systematic review. Skin Appendage Disord. 2018;4(4):245–260. doi: 10.1159/000485749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conde J., Davis K., Ntuen E., Balmer N., Jones D., McMichael A. A case of imiquimod-induced alopecia. J Dermatolog Treat. 2010;21(2):122–124. doi: 10.1080/09546630902991484. [DOI] [PubMed] [Google Scholar]
- 8.Amberg N., Holcmann M., Stulnig G., Sibila M. Effects of imiquimod on hair follicle stem cells and hair cycle progression. J Invest Dermatol. 2016;136(11):2140–2149. doi: 10.1016/j.jid.2016.06.613. [DOI] [PubMed] [Google Scholar]