Case report
A 48-year-old woman with a 20-year history of ulcerative colitis (UC) presented to our autoimmune connective tissue disease clinic for evaluation of a 1-year history of hyperpigmented and atrophic patches. Her UC had been treated with multiple therapies, including antibiotics, tofacitinib, and mesalamine. Three years before presentation, the patient experienced a flare of her UC, which was accompanied by a pruritic, erythematous eruption on her scalp, ears, and hands. At this time, treatment with ustekinumab was initiated for both presumed psoriasis and UC flare. The patient responded well to this therapy, with improvement in both her skin condition and UC.
After approximately 1 year of ustekinumab treatment, however, the patient had a violaceous, atrophic patch on her back. This patch was biopsied at an outside institution and found to be histologically consistent with morphea. The patch was treated with methotrexate and was initially limited to the single lesion on her back. Despite 2 to 3 months of methotrexate, the morphea did not improve. In fact, as the patient continued to receive ustekinumab injections, the morphea rapidly progressed to include several areas on her trunk and bilateral upper and lower extremities.
When she presented to our clinic, the patient's physical examination was notable for several hyperpigmented and atrophic patches on her bilateral upper extremities, popliteal fossae, and dorsal feet (Fig 1). An indurated plaque was present on her lower back, along with several sclerotic plaques with surrounding erythema on the hips bilaterally. Activity index was noted to be 2 on the modified Localized Scleroderma Skin Severity Index.1 Laboratory workup, including heavy metals, Borrelia western blot, and RNA pol III IgG antibodies, was unremarkable. Given the concern that ustekinumab was promoting further progression of the patient's morphea, the treatment was discontinued. The methotrexate was also discontinued, and the patient received treatment with 200 mg of nonmodified cyclosporine daily.
Fig 1.
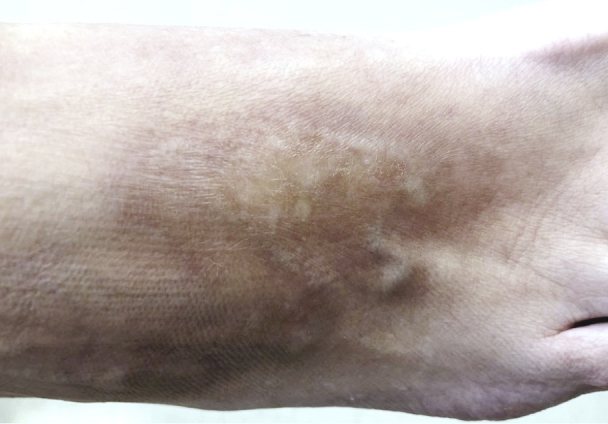
Morphea, dorsal foot.
At follow-up visit, less than 2 months off of ustekinumab, the patient's morphea improved and Localized Scleroderma Skin Severity Index was noted to be 1 (Fig 2). She had had no new or recurrence of her lesions and remains only with post-inflammatory hyperpigmentation 1 year into her follow-up.
Fig 2.
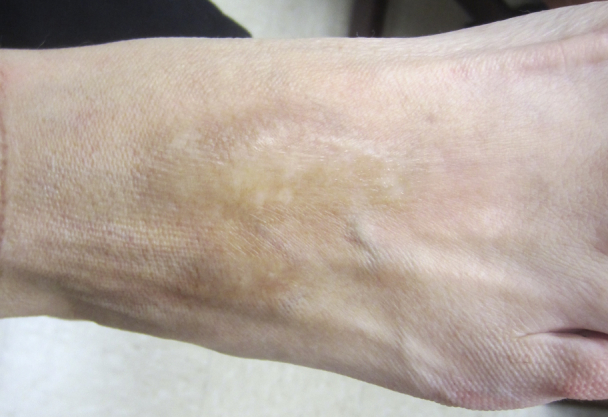
Morphea, dorsal foot, resolving.
Discussion
Ustekinumab is a fully human, monoclonal antibody that blocks interleukin (IL)-12 and IL-23 via their common p40 protein subunit.2 It is approved by the US Food and Drug Administration for the treatment of moderate-to-severe plaque psoriasis, psoriatic arthritis, and Crohn's disease. Common adverse effects of ustekinumab include headaches, fatigue, abdominal pain, and upper respiratory infections.2 Few cutaneous side effects have been reported.3 Although there was concern that the drug might increase the risk of nonmelanoma skin cancer because of blockade of IL-12 antitumor activity, clinical data have not shown this to be the case.2, 3 Multiple immunologically based dermatologic conditions, namely alopecia areata, linear IgA bullous dermatosis, and eczematous and lymphomatoid drug reaction, have been reported in conjunction with this drug.3
Morphea occurring in a patient undergoing treatment with ustekinumab, however, has only been reported once before.4 A rare fibrosing disorder of the skin and underlying tissues, morphea exists in various classifications (circumscribed, linear, generalized, pansclerotic, and mixed variant), which differ in their presentations.5 Lesions tend to have an initial inflammatory stage and over time become white and sclerotic, with borders that take on the characteristic violaceous ring.5
The pathophysiology of morphea is incompletely understood but sums to an imbalance between collagen synthesis and breakdown.5 It is thought that various etiologic factors induce endothelial injury, prompting the release of cytokines that increase the expression of vascular cell adhesion molecules. These molecules recruit T cells that produce profibrotic cytokines, such as IL-4 and IL-6 and transforming growth factor β.5 Profibrotic cytokines recruit antigen-presenting cells, allowing an opportunity for self-antigen presentation and formation of autoantibodies, and promote chemokines (CCL2, 5, 7, 17, 22, and 27 and CXCL8), which are thought to increase tissue fibrosis.5 This cascade, in addition to transforming growth factor β–mediated decreases in the production of proteases and increases in protease inhibitors, results in increased collagen and extracellular matrix deposition and decreased breakdown.5 Recent literature has also focused on the role of T-helper 17 (Th17) cells and their cytokines, such as IL-17, IL-23, and IL-21, in the pathogenesis of several inflammatory and autoimmune diseases, notably including systemic sclerosis.6
The ability of ustekinumab to alter the Th17 pathway of inflammation is critical to its utility in psoriasis and may also help explain the progression of morphea while under treatment with this drug. Briefly, in psoriasis, keratinocytes secrete antimicrobial peptides, activating an inflammatory cascade, involving IL-12 and IL-23. This action ultimately activates Th17 cells to release proinflammatory cytokines, such as IL-17A, that drive and sustain psoriatic disease.7 As in psoriasis, recent animal studies have shown increased levels of IL-17A and Th17 cells to contribute to inflammation and skin and lung fibrosis in systemic sclerosis by enhancing fibroblast proliferation and cytokine production.8 Although IL-17A levels are not increased in morphea, IL-17E+ cells have been found to be elevated in the dermis of patients with morphea and systemic sclerosis, alike.9 The exact role of Th17 cells and their cytokines in morphea has yet to be elucidated; however, they may cooperate or be misregulated to induce multiple effects on epithelial, endothelial, and fibroblastic cells.6
Various etiologies have been associated with sporadic cases of morphea.5 Medications, in particular, are rarely associated with morphea, but a recent literature review identified 15 reported cases.10 Given the patient's underlying inflammatory conditions, it is possible that the patient's morphea may have been brought on by other etiologies. However, as in the prior case of morphea under ustekinumab therapy,4 the progression of the disease and its regression after discontinuation of ustekinumab makes other possibilities less likely.
We report this rare event of medication-induced morphea likely caused by ustekinumab, to make physicians aware of this potential adverse cutaneous effect. The patient here saw great improvement in her initial dermatologic complaint under treatment with ustekinumab, likely through its intended and known action on the IL-12 and IL-23/Th17 pathway. Yet, she also had adverse cutaneous effects, perhaps through immunomodulation also attributable to ustekinumab that occurred unwittingly. As biologics and targeted therapies continue to see higher utilization in dermatology, it is important to remain abreast of the potential cutaneous effects of such manipulation of human biology.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Arkachaisri T., Vilaiyuk S., Li S. The localized scleroderma skin severity index and physician global assessment of disease activity: a work in progress toward development of localized scleroderma outcome measures. J Rheumatol. 2009;36(12):2819–2829. doi: 10.3899/jrheum.081284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cline A., Hill D., Lewallen R., Feldman S.R. Current status and future prospects for biologic treatments of psoriasis. Exp Rev Clin Immunol. 2016;12(12):1273–1287. doi: 10.1080/1744666X.2016.1202115. [DOI] [PubMed] [Google Scholar]
- 3.Otani I.M., Levin A.S., Banerji A. Cutaneous manifestations of reactions to biologics. Curr All Asthma Reps. 2018;18(2):12. doi: 10.1007/s11882-018-0764-z. [DOI] [PubMed] [Google Scholar]
- 4.Escalas J., Corral-Magana O., del Mar Escudero-Gongora M., Montis-palos M.C. onset of morphea in a psoriatic patient under ustekinumab: coexistence or adverse effect?: 4247. J Am Acad Dermatol. 2017;76(6):AB184. [Google Scholar]
- 5.Fett N., Werth V.P. Update on morphea: part I. Epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol. 2011;64(2):217–228. doi: 10.1016/j.jaad.2010.05.045. quiz 229-230. [DOI] [PubMed] [Google Scholar]
- 6.Shabgah A.G., Fattahi E., Shahneh F.Z. Interleukin-17 in human inflammatory diseases. Postepy dermatologii i alergologii. 2014;31(4):256–261. doi: 10.5114/pdia.2014.40954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tausend W., Downing C., Tyring S. Systematic review of interleukin-12, interleukin-17, and interleukin-23 pathway inhibitors for the treatment of moderate-to-severe chronic plaque psoriasis: ustekinumab, briakinumab, tildrakizumab, guselkumab, secukinumab, ixekizumab, and brodalumab. J Cutan Med Surg. 2014;18(3):156–169. doi: 10.2310/7750.2013.13125. [DOI] [PubMed] [Google Scholar]
- 8.Ichihara A., Jinnin M., Ihn H. Treatment of psoriasis with ustekinumab improved skin tightening in systemic sclerosis. Clin Exp Rheumatol. 2017;35 Suppl 106(4):208–210. [PubMed] [Google Scholar]
- 9.Speeckaert R., Lambert J., Grine L., Van Gele M., De Schepper S., van Geel N. The many faces of interleukin-17 in inflammatory skin diseases. Br J Dermatol. 2016;175(5):892–901. doi: 10.1111/bjd.14703. [DOI] [PubMed] [Google Scholar]
- 10.Peroni A., Zini A., Braga V., Colato C., Adami S., Girolomoni G. Drug-induced morphea: report of a case induced by balicatib and review of the literature. J Am Acad Dermatol. 2008;59(1):125–129. doi: 10.1016/j.jaad.2008.03.009. [DOI] [PubMed] [Google Scholar]


