Introduction
A KRT5 disease-causing variant was first associated with epidermolysis bullosa simplex by Dowling-Meara in 1992.1 Additional variants throughout the KRT5 and KRT14 genes have subsequently been associated with phenotypic variants of EBS as well as other dermatologic diseases. KRT5 variants alone have been associated with Dowling-Degos disease, EBS-mottled pigmentation, EBS-migratory circinate erythema, EBS-localized (Weber-Cockayne), EBS-generalized intermediate (Koebner), and EBS-generalized severe (Dowling-Meara).2, 3, 4 Most EBS cases from KRT5 and KRT14 variants are autosomal dominant diseases, although autosomal recessive cases have been reported. To our knowledge, loss-of-function KRT5 variants associated with autosomal recessive EBS have not been described previously.2, 3
Case report
The proband was a 2-year-old male with a history of epidermolysis bullosa presenting at birth with blistering and sloughing of his “near transparent” skin. At the time of evaluation, 90% of his body surface area was affected by a combination of blisters, erosions, crusting, and hyperpigmentation. Although his fingernails were intact, teeth were slow to erupt, oral blistering and lesions were common, and his hands and feet demonstrated pseudosyndactyly development. He reportedly experienced recurrent upper respiratory infections. The proband also presented with symptoms outside of the EB spectrum, including developmental delays, speech and motor deficits, pectus carinatum, hearing loss, and growth retardation. The patient died of septic shock at age 26 months.
The family history revealed a first cousin once-removed with unspecified EB-like symptoms at birth, alive and well at age 30 years. The proband's mother, father, and older brother were in reportedly good overall health, without a history of skin disease or other more subtle findings of EB. The parents were cousins and of Middle Eastern descent. Next generation sequencing and copy number variation analysis of the EB-associated genes COL17A1, COL7A1, DSP, ITGA6, ITGB4, KRT14, KRT5, LAMA3, LAMB3, LAMC2, PKP1, and PLEC, a single-nucleotide polymorphism microarray analysis, and trio whole exome sequencing were performed.
Testing determined the proband was homozygous for the novel c.817delG (p.Va1273*) variant in the KRT5 gene. Familial studies revealed that both parents and the proband's brother were heterozygous for the KRT5 variant. Testing also revealed heterozygosity for a maternally inherited c.3418+2delT variant in the COL17A1 gene and 2 variants in the LAMA3 gene (c.9717A>G [p.Gly3239Gly] and c.5663T>C [p.Ile1888Thr]). Familial studies demonstrated that the proband's father was homozygous for the LAMA3 c.9717A>G variant. Given his lack of EB-associated symptoms, this variant was considered unlikely to be associated with EB. The LAMA3 c.5663T>C variant was maternally inherited.
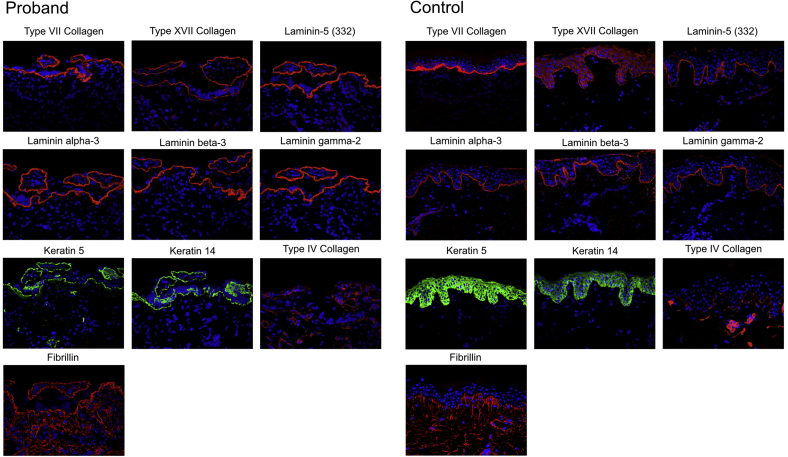
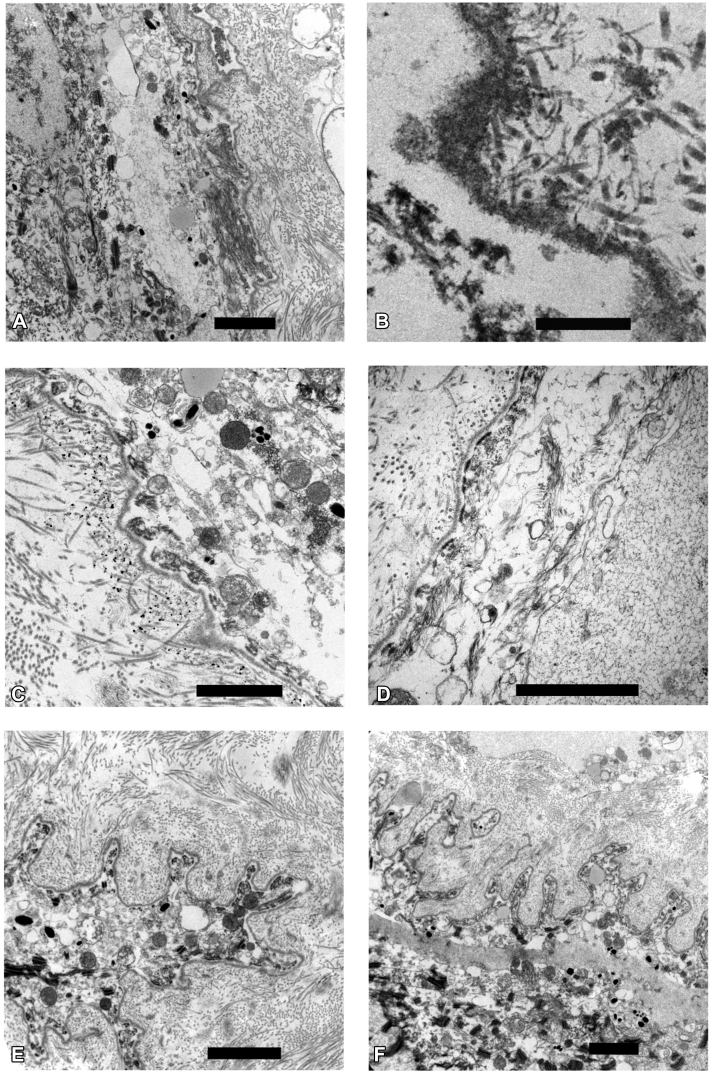
Subsequent evaluation of a full-thickness skin biopsy specimen from the proband revealed normal immunofluorescence staining for type VII and XVII collagen, and laminin-A3, -B3, and -C2. However the epidermis was thin, with discontinuous, sparse staining for keratin 5 and 14 (Fig 1). Closer examination of ultrastructure by immunoelectron microscopy (Fig 2) showed disorganization of cytoplasmic contents of the basal keratinocytes. Epidermal tonofilaments, or intermediate filaments, were largely absent except in tufts associated with hemidesmosomes near tight junctions. This lack of tonofilament structure resulted in wild undulations, or folds, of the lamina densa. Anchoring fibrils were well banded and arching, although some appeared to be free floating from the lamina densa.
Fig 1.
Immunofluorescence reveals thin epidermis with abnormal keratin 5 and 14 staining comparing the proband with normal control skin.
Fig 2.
Immunoelectron microscopy of full-thickness proband skin biopsy sample reveals (A) disorganized cytoplasmic contents of basal keratinocytes, (B) free-floating anchoring fibrils, (C, D) abnormal tonofilaments, and (E, F) undulating lamina densa. Black bar = 2 μm.
A chromosomal microarray showed copy number neutral-absence of heterozygosity in 9% of the autosomal genome. Subsequent whole exome sequencing revealed the proband was homozygous, and his parents and brother were heterozygous, for the GNS gene variant of uncertain significance called c.1262G>A (p.Arg421His).
Discussion
The c.817delG variant in the KRT5 gene resides in the 1B domain of the keratin 5 protein. Case reports of variants in this 1B region are limited to substitution variants resulting in autosomal dominant disease (interfil.org). Truncating variants upstream and downstream of the c.817delG variant have been reported in individuals with Dowling-Degos disease5 and various autosomal dominant EBS phenotypes,2, 3, 4 all outside of the 1B domain.
Cases of KRT5 homozygotes are limited. Stephens et al6 reported a patient homozygous for the K173N variant in the 1A region of the KRT5 gene. Because heterozygous family members exhibited similar symptom severity and keratin 5 immunofluorescent findings, the authors concluded that the K173N variant is fully dominant.
Yasukawa et al7 reported a family with the E170K variant in KRT5 resulting in autosomal dominant Weber-Cockayne type EBS. The proband presented with a more severe EBS-Koebner phenotype than his paternal heterozygous relatives and was found to have a second variant, E418K, that was inherited from his asymptomatic mother, indicating a likely autosomal recessive inheritance.
Another study8 reported 2 siblings with the generalized intermediate phenotype from the E170K and V143A variants. Parental testing revealed that the asymptomatic father carried the E170K variant and that the asymptomatic mother carried the V143A variant that had been reported previously in individuals with the localized form of EBS.8 The E170K variant has been reported in the homozygous state in multiple probands with the generalized intermediate form of EBS, while heterozygous parents presented with EBS-localized8, 9 or with mildly dystrophic toenails, micronychia, thickening of the index toenail plate, and horizontal ridging of the great toenail.10
The only reported autosomal recessive KRT5-associated case that did not include the E170K variant involved a female patient with EBS-Koebner. Genetic studies revealed that the proband inherited the G476D variant from her father who had EBS-Webber Cockayne and the G183E variant from her asymptomatic mother.11
To our knowledge, no cases of loss-of-function KRT5 variants resulting in autosomal recessive disease have been reported previously. In contrast, multiple individuals have been reported with loss-of-function variants in 1B region of the KRT14 gene resulting in autosomal recessive EBS.3, 12, 13, 14 Previous studies in knockout mouse models for KRT5 and KRT14 led to the prediction that KRT5 deficiency in humans may be lethal.15 The genetic and skin biopsy findings in this case support the prediction that a loss of functional keratin 5 resulted in the EB symptoms in this proband, providing evidence that KRT5 deficiency in humans is not universally incompatible with life.
Pathogenic variants in the GNS gene are associated with mucopolysaccharidosis type IIID. Whether the homozygous variant in the GNS gene was associated with the proband's symptoms outside the typical EB phenotype is unclear. Because the proband died before whole exome sequencing was finalized, additional assays to verify a possible MPS type IIID diagnosis were not completed.
Conclusion
To our knowledge, this is the first case of a homozygous KRT5 frameshift variant resulting in a severe, autosomal recessive EBS phenotype.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Lane E.B., Rugg E.L., Navsaria H. A mutation in the conserved helix termination peptide of keratin 5 in hereditary skin blistering. Nature. 1992;356(6366):244–246. doi: 10.1038/356244a0. [DOI] [PubMed] [Google Scholar]
- 2.Kim E.N., Harris A.G., Bingham L.J., Yan W., Su J.C., Murrell D.F. A review of 52 pedigrees with epidermolysis bullosa simplex identifying ten novel mutations in KRT5 and KRT14 in Australia. Acta Derm Venereol. 2017;97(9):1114–1119. doi: 10.2340/00015555-2715. [DOI] [PubMed] [Google Scholar]
- 3.Bolling M.C., Lemmink H.H., Jansen G.H.L., Jonkman M.F. Mutations in KRT5 and KRT14 cause epidermolysis bullosa simplex in 75% of the patients. Br J Dermatol. 2011;164(3):637–644. doi: 10.1111/j.1365-2133.2010.10146.x. [DOI] [PubMed] [Google Scholar]
- 4.Arin M.J., Grimberg G., Schumann H. Identification of novel and known KRT5 and KRT14 mutations in 53 patients with epidermolysis bullosa simplex: correlation between genotype and phenotype. Br J Dermatol. 2010;162(6):1365–1369. doi: 10.1111/j.1365-2133.2010.09657.x. [DOI] [PubMed] [Google Scholar]
- 5.Betz R.C., Planko L., Eigelshoven S. Loss-of-function mutations in the keratin 5 gene lead to Dowling-Degos disease. Am J Hum Genet. 2006;78(3):510–519. doi: 10.1086/500850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stephens K., Zlotogorski A., Smith L. Epidermolysis bullosa simplex : a keratin 5 mutation is dominant allele in epidermal cytoskeleton function fully. Hum Mol Genet. 1995;14:577–585. [PMC free article] [PubMed] [Google Scholar]
- 7.Yasukawa K., Sawamura D., McMillan J.R., Nakamura H., Shimizu H. Dominant and recessive compound heterozygous mutations in epidermolysis bullosa simplex demonstrate the role of the stutter region in keratin intermediate filament assembly. J Biol Chem. 2002;277(26):23670–23674. doi: 10.1074/jbc.M200974200. [DOI] [PubMed] [Google Scholar]
- 8.Wertheim-Tysarowska K., Ołdak M., Giza A. Novel sporadic and recurrent mutations in KRT5 and KRT14 genes in Polish epidermolysis bullosa simplex patients: further insights into epidemiology and genotype–phenotype correlation. J Appl Genet. 2016;57(2):175–181. doi: 10.1007/s13353-015-0310-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ołdak M., Szczecińska W., Przybylska D. Gene dosage effect of p.Glu170Lys mutation in the KRT5 gene in a Polish family with epidermolysis bullosa simplex. J Dermatol Sci. 2011;61(1):64–67. doi: 10.1016/j.jdermsci.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 10.González-Cantero Á., Sánchez-Moya A.I., Pérez-Hortet C., Martínez-Lorenzo E., Gómez-Dorado B., Schoendorff-Ortega C. “Nails only” phenotype and partial dominance of p.Glu170Lys mutation in a family with epidermolysis bullosa simplex. Pediatr Dermatol. 2017;34(4):e205–e206. doi: 10.1111/pde.13146. [DOI] [PubMed] [Google Scholar]
- 11.Kowalewski C., Hamada T., Wozniak K. A novel autosomal partially dominant mutation designated G476D in the keratin 5 gene causing epidermolysis bullosa simplex Weber-Cockayne type: a family study with a genetic twist. Int J Mol Med. 2007;20(1):75–78. [PubMed] [Google Scholar]
- 12.García M., Santiago J.L., Terrõn A. Two novel recessive mutations in KRT14 identified in a cohort of 21 Spanish families with epidermolysis bullosa simplex. Br J Dermatol. 2011;165(3):683–692. doi: 10.1111/j.1365-2133.2011.10428.x. [DOI] [PubMed] [Google Scholar]
- 13.Chan Y.M., Anton-Lamprecht I., Yu Q.C. A human keratin 14 “knockout”: the absence of K14 leads to severe epidermolysis bullosa simplex and a function for an intermediate filament protein. Genes Dev. 1994;8(21):2574–2587. doi: 10.1101/gad.8.21.2574. [DOI] [PubMed] [Google Scholar]
- 14.Yiasemides E., Trisnowati N., Su J. Clinical heterogeneity in recessive epidermolysis bullosa due to mutations in the keratin 14 gene, KRT14. Clin Exp Dermatol. 2008;33(6):689–697. doi: 10.1111/j.1365-2230.2008.02858.x. [DOI] [PubMed] [Google Scholar]
- 15.Peters B., Kirfel J., Büssow H., Vidal M., Magin T.M. Complete cytolysis and neonatal lethality in keratin 5 knockout mice reveal its fundamental role in skin integrity and in epidermolysis bullosa simplex. Mol Biol Cell. 2001;12(6):1775–1789. doi: 10.1091/mbc.12.6.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]