Abstract
Prdm14 is a pluripotency regulator central to embryonic stem cell identity and primordial germ cell specification. Genomic regions containing PRDM14 are often amplified leading to mis-expression in human cancer. Prdm14 expression in mouse hematopoietic stem cells (HSCs) leads to progenitor cell expansion prior to the development of T-cell acute lymphoblastic leukemia (T-ALL), consistent with PRDM14’s role in cancer initiation. Here, we demonstrate mechanistic insight into PRDM14-driven leukemias in vivo. Mass spectrometry revealed novel PRDM14-protein interactions including histone H1, RNA binding proteins and the master hematopoietic regulator CBFA2T3. In mouse leukemic cells, CBFA2T3 and PRDM14 associate independently of the related ETO family member CBFA2T2, PRDM14’s primary protein partner in pluripotent cells. CBFA2T3 plays crucial roles in HSC self-renewal and lineage commitment, and participates in oncogenic translocations in acute myeloid leukemia. These results suggest a model whereby PRDM14 recruits CBFA2T3 to DNA, leading to gene mis-regulation causing progenitor cell expansion and lineage perturbations preceding T-ALL development. Strikingly, Prdm14-induced T-ALL does not occur in mice deficient for Cbfa2t3, demonstrating that Cbfa2t3 is required for leukemogenesis. Moreover, T-ALL develops in Cbfa2t3 heterozygotes with a significantly longer latency, suggesting that PRDM14-associated T-ALL is sensitive to Cbfa2t3 levels. Our study highlights how an oncogenic protein uses a native protein in progenitor cells to initiate leukemia, providing insight into PRDM14-driven oncogenesis in other cell types.
Implications
The pluripotency regulator PRDM14 requires the master hematopoietic regulator CBFA2T3 to initiate leukemia in progenitor cells, demonstrating an oncogenic role for CBFA2T3 and providing an avenue for targeting cancer initiating cells.
Keywords: T-cell acute lymphoblastic leukemia, PRDM14, CBFA2T3, mass spectrometry, mouse genetics
Introduction
Leukemia is the most common cancer in children1. Despite advances in chemotherapies that improve survival, most relapsed patients succumb to the disease1,2. Cancer initiation is difficult to study in human patients, as by the time of diagnosis multiple genetic lesions are often present, making the initiating event difficult to deduce2. Mouse models of cancer offer powerful tools that allow for the study of cancer initiation to improve the understanding of disease progression.
Our lab previously identified PR domain-containing 14 (Prdm14) as a potent mammalian oncogene3,4. Prdm14 is a pluripotency regulator central to embryonic stem cell (ESC) identity5–7 and primordial germ cell (PGC) specification8,9. Prdm14 is not expressed in adult tissues; however, its mis-expression, often as a result of genomic amplification, is found in multiple human cancers including leukemia4,10. A high level of expression of PRDM14 is associated with poor outcomes in both non-small cell lung carcinoma (NSCLC)11 and breast cancer12,13.
Prdm14 was mis-expressed in hematopoietic stem cells (HSCs) by mating mice carrying a Rosa26-floxed-stop-Prdm14-IRES-GFP transgene to mice carrying Tg(Mx1-cre) (R26PR;Mx1-cre)14. Upon inducing expression of Prdm14, R26PR;Mx1-cre mice rapidly succumb to a completely penetrant T-cell acute lymphoblastic leukemia (T-ALL) with a highly infiltrative CD8+ immature single positive T-cell immunophenotype14. Prior to the onset of leukemia, abnormal HSC-like and lymphoid progenitor cells accumulate in the bone marrow (BM) of the mice. Subsequent work showed that the T-ALL is driven by activating mutations on Notch115. In contrast, when Prdm14 is expressed in committed T-cells using a dLck-cre line (R26PR;dLck-cre), mice remain healthy and do not show signs of leukemia, suggesting that Prdm14 requires additional factor(s) present in progenitor cells to act as an oncogene14. However, the mechanism through which Prdm14 functions in progenitor cells to initiate cancer is poorly understood.
PRDM14 contains six zinc finger motifs, which bind the same consensus DNA sequence in both mouse and human, and a PR domain, which is related to the SET domain6,16. Certain PR family proteins have histone methyltransferase activity, however no methyltransferase activity has been reported for PRDM1415. Instead, it is likely that PRDM14 regulates gene expression through protein interaction partners. In support of this hypothesis, PRDM14 requires an interaction with the Core-binding factor, runt domain, alpha subunit 2 translocated to 2 (CBFA2T2) protein to promote ESC self-renewal and to establish pluripotent PGCs17,18. In this context, the CBFA2T2-PRDM14 protein interaction stabilizes the complex on chromatin to regulate gene expression. CBFA2T2 belongs to the eight-twenty-one (ETO) family of chromatin-associated proteins, which includes myeloid translocation gene 8 (MTG8, ETO) and CBFA2T3 (MTG16, ETO-2). These proteins each contain 4 Nervy Homology Region (NHR) domains, and form homo- or hetero-oligomeric ETO complexes via the NHR2 domain19–21. Each of the three ETO family members participate in oncogenic translocations with RUNX1 in acute myeloid leukemia (AML)22.
Despite evidence that protein binding partners are critical for PRDM14’s function, PRDM14’s functional interactors in a cancer model have not been described. We hypothesize that when Prdm14 is expressed in progenitor cells, outside its normal milieu in a pluripotent cell, it requires a protein partner to facilitate oncogenesis. Here, we report the first PRDM14-protein interaction in an in vivo cancer model and demonstrate how PRDM14 can hijack a hematopoietic regulatory protein, CBFA2T3, in progenitor cells to initiate leukemogenesis. The protein domains involved in the interaction suggest a model whereby PRDM14 recruits CBFA2T3 to chromatin, which is stabilized in a large chromatin complex to initiate leukemia. We show that CBFA2T3 has a critical role in PRDM14-induced leukemia initiation, as mice expressing Prdm14 in hematopoietic progenitor cells fail to develop T-ALL on a Cbfa2t3 null genetic background.
Materials & Methods
Mouse Strains/Animal Care
All mouse experiments were carried out under the approval of the Animal Use Committee (AUC), and housed at The Centre for Phenogenomics, which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. All mouse strains were maintained on a C57BL/6J congenic genetic background: B6.Gt(ROSA)26Sortm1(LSL-Prdm14-IRES-EGFP)Jus, abbreviated “R26PR”, B6.Gt(ROSA)26Sortm(LSL−3XFLAG-Prdm14-P2A-EGFP)Jus abbreviated “R26FLPR”,14,15 B6.Tg(Mx1-cre)1Cgn/J abbreviated ‘Mx1-cre’ (from Dr. Margaret A. Goodell, Baylor College of Medicine, Houston, Texas, USA), B6.Cbfa2t3tm1.1Swh abbreviated “Cbfa2t3−/−” (from Dr. Scott W. Hiebert, Vanderbilt University, Nashville, Tennessee, USA). Polyinosinic:polycytidylic acid (pIpC) administration to activate Mx1-cre was performed at 8 weeks of age with one intraperitoneal injection of 250μg. Mice were monitored for signs of cancer including enlarged lymph nodes and abdomen, lethargy and laboured breathing. Tissues from moribund mice were dissected and processed for protein, RNA, flow cytometry and/or blood analyses. Samples for pathology analysis were fixed in 10% neutral buffered formalin (NBF) overnight. Tissues were paraffin embedded and sectioned, and slides stained with hematoxylin and eosin (H&E). Images were acquired with an Eclipse E100 (Nikon) microscope and DS-Qi1Mc (Nikon) camera.
Protein Extraction
Protein lysates from HEK293T cells and R26FLPR or R26FLPR;Mx1-cre bone marrow and thymus single cell suspensions were extracted using NP-40 lysis buffer [50mM Tris-HCl pH 8.0, 150mM NaCl, 1% NP-40, 2mM EDTA, protease inhibitors (Roche)]. After 30 minute incubation on ice the lysates were centrifuged for 15 minutes at 16,000 x g. Protein concentrations were determined using the Pierce™ Coomassie (Bradford) Protein Assay Kit (Thermo Fisher Scientific).
Mass Spectrometry
Label-free pull downs were performed in triplicate per sample as previously described23. Briefly, 1mg of protein lysate per pull down was incubated with 10μL of FLAG M2 beads (Sigma-Aldrich) in a total volume of 600μL NP-40 lysis buffer containing 50μg/mL Ethidium Bromide. After incubation for 1.5 hours on a rotation wheel at 4°C, the pull downs were sequentially washed twice with 1mL of lysis buffer (300mM NaCl and 0.5% NP40), twice with 1mL PBS with 0.5% NP40 and three times with 1mL PBS. Bead-bound proteins were then subjected to trypsin digestion as previously described24. Finally, tryptic peptides were acidified and desalted with C18 Stagetips prior to mass spectrometry (MS) analyses25. Desalted tryptic peptides were separated on an Easy-nLC 1000 (Thermo Scientific) connected online to a Thermo scientific Orbitrap Fusion Tribid Mass spectrometer. MS and MS/MS spectra were recorded in a top speed modus with a run cycle of three seconds. Raw files were analyzed using MAXQuant software version 1.5.1.0. with default settings24. Raw MS data was searched against the Uniprot mouse proteome database, released 2015_12. Perseus version 1.3.0.426 was used to further analyze the data. Briefly, the protein list was filtered for contaminants and reverse hits. Label-free quantification (LFQ) values were transformed into log2 values and missing values were imputed by normal distribution. Volcano plots were generated with an in-house R script.
Immunoprecipitation
Tissue protein lysates (500mg) were incubated at 4°C overnight with Protein A Dynabeads (Invitrogen) and anti-FLAG (M2, Sigma), anti-ETO-2 (C20, Santa Cruz) or anti-MTGR1 (KT42, Abcam) antibodies. HEK293T lysates were incubated at 4°C overnight with anti-FLAG (M2, Sigma) or anti-HA (Thermo Scientific) magnetic beads. Beads were washed three times with PBST (PBS, 0.1% Tween-20). Laemmli Sample Buffer 2X (Bio-Rad) was added to the beads and boiled for 5 minutes at 95°C to obtain co-immunoprecipitated proteins.
Density Gradient
Protein lysate (100μL) was loaded onto a linear 15–40% glycerol gradient. The gradient was placed in a SW60 Ti swinging-bucket rotor (Beckman Coulter) and spun in an Optima XPN-80 Ultracentrifuge (Beckman Coulter) at 44,000 rpm for 16 hours at 4°C. The gradient was immediately fractionated into 21 fractions of 200μL.
Western Blot
Protein lysates were resolved on a 4–12% polyacrylamide gradient gel (Bio-Rad) and transferred to a polyvinylidene difluoride (PVDF) membrane (Bio-Rad) for blotting. Membranes were blocked in 5% skim milk for 1 hour and subsequently incubated with primary antibodies diluted in 5% skim milk for 2 hours. After washing with PBST, membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies diluted in 5% skim milk for 1 hour. Membranes were washed again with PBST, and chemiluminescence Clarity Western ECL Substrate (Bio-Rad) was added for signal detection with Amersham Hyperfilm ECL (GE Healthcare). The following primary antibodies were used: FLAG (M2, Sigma), ETO-2 (C20, Santa Cruz), MTGR1 (Abcam), HA (Sigma). The following secondary antibodies were used: Goat Anti-Rat IgG H&L HRP (Abcam), Rabbit Anti-Goat IgG H&L HRP (Abcam), Peroxidase AffiniPure Goat Anti-Mouse IgG Light Chain Specific (Jackson ImmunoResearch).
Protein Domain Interactions
Full-length coding sequences of mouse PRDM14 and CBFA2T3 were amplified using CloneAmp HiFi PCR Premix (ClonTech) with the following primer sequences (5’ to 3’): PRDM14 - GACGATGACGACAAGTTCCGCATGGCCTTACCGCCCTCTGGT and AACGGGCCCTCTAGACTCGAGCTAGCAGGTTTTATGAAGCCT; CBFA2T3 - CCAGACTACGCGGGAATAAGGATGTCCCAGGCATCCACCACC and GGGTTTAAACGGGCCCTCTAGATCAGCGGGGCACAGCAGCGTC. In-Fusion® HD Cloning Kit (Takara Bio) was used to clone fragments into the pcDNA3.1+ (Invitrogen) mammalian expression vector. The resulting plasmids were used to amplify and subclone the deletion constructs into pcDNA3.1+ vectors for expression with the following primer sequences (5’ to 3’): PRDM14-ΔZF – GACGATGACGACAAGTTCCGCATGGCCTTACCGCCCTCTGGT and AACGGGCCCTCTAGACTCGAGCTACTCATAGCCATTTCCGTACCA; PRDM14-ΔN-PR – GACGATGACGACAAGTTCCGCAAGTTCCTGGGCGTTCCCATG and AACGGGCCCTCTAGACTCGAGCTAGCAGGTTTTATGAAGCCT; PRDM14-PR – GACGATGACGACAAGTTCCGCGGCTTCAACTTCACAGAGGAG and AACGGGCCCTCTAGACTCGAGCTACTCATAGCCATTTCCGTACCA; CBFA2T3- ΔNterm – CCAGACTACGCGGGAATAAGGCAGCTGCTGCTGGACGCCAGC and GGGTTTAAACGGGCCCTCTAGATCAGCGGGGCACAGCAGCGTC; CBFA2T3-ΔCterm – CCAGACTACGCGGGAATAAGGATGTCCCAGGCATCCACCACC and GGGTTTAAACGGGCCCTCTAGACTATTCGTGCTGGGCCAGGTACTG; CBFA2T3-NHR1 – CCAGACTACGCGGGAATAAGGTGTCTCTTGATGAACGGCAGC and GGGTTTAAACGGGCCCTCTAGACTATTCGTGCTGGGCCAGGTACTG. Accurate plasmid sequence was confirmed by Sanger sequencing. To co-express constructs, HEK293T cells were cultured in Dulbecco’s Modified Eagle Media (DMEM, Wisent) with penicillin/streptomycin and 10% fetal bovine serum (FBS). Cells were transfected with Lipofectamine 2000 (Invitrogen) per manufacturer’s instructions and lysed 24 hours post-transfection.
RT-PCR
Total RNA extraction was performed with RNeasy Mini Kit (Qiagen). RNA was reverse transcribed using SuperScript™ VILO™ cDNA Synthesis Kit (Invitrogen). cDNA was amplified with AccuStart™ II PCR SuperMix (Quantabio). Primers used (5’ to 3’): Cbfa2t3 - TGGAAGCACCTCAACAGTCTTC and GTGGTTGAGTTCCTCACGGT; Tbp - CCTTGTACCCTTCACCAATGAC and ACAGCCAAGATTCACGGTAGA.
Flow Cytometry
Single-cell suspensions were prepared from thymus, spleen and bone marrow tissues. Red blood cells were lysed with Red Blood Cell Lysis Solution (Miltenyi Biotec) and washed with staining media [SM: Hanks’ Balanced Salt Solution (Thermo Fisher Scientific), 2% fetal bovine serum (FBS, Wisent), 10mM HEPES, pH 7.2]. One million cells of each sample were stained with fluorochrome-conjugated antibodies for 30 minutes on ice in the dark, and washed with SM. Samples were analyzed on a LSR II flow cytometer (BD Biosciences) in the Flow and Mass Cytometry Facility at the Hospital for Sick Children. Data collection and analysis was performed with FACSDiva (BD Biosciences) and FlowJo v10 (BD) software. The following antibodies were used: CD4-Alexa 700 (eBioscience), CD8a-eFluor 450 (eBioscience), CD24-PE (eBioscience), TCRBeta-APC (eBioscience), 7-AAD (BioLegend).
Statistical Analysis
A t-test was performed on LFQ values and the FDR-corrected p-value was calculated to determine significant proteins after AP/MS. Kaplan-Meier survival curves were compared using a logrank (Mantel-Cox) test for statistical significance in Prism (GraphPad). An unpaired t-test was performed to identify changes in quantified flow cytometry data in Prism (GraphPad).
Results
PRDM14 interacts with CBFA2T3 in leukemia cells
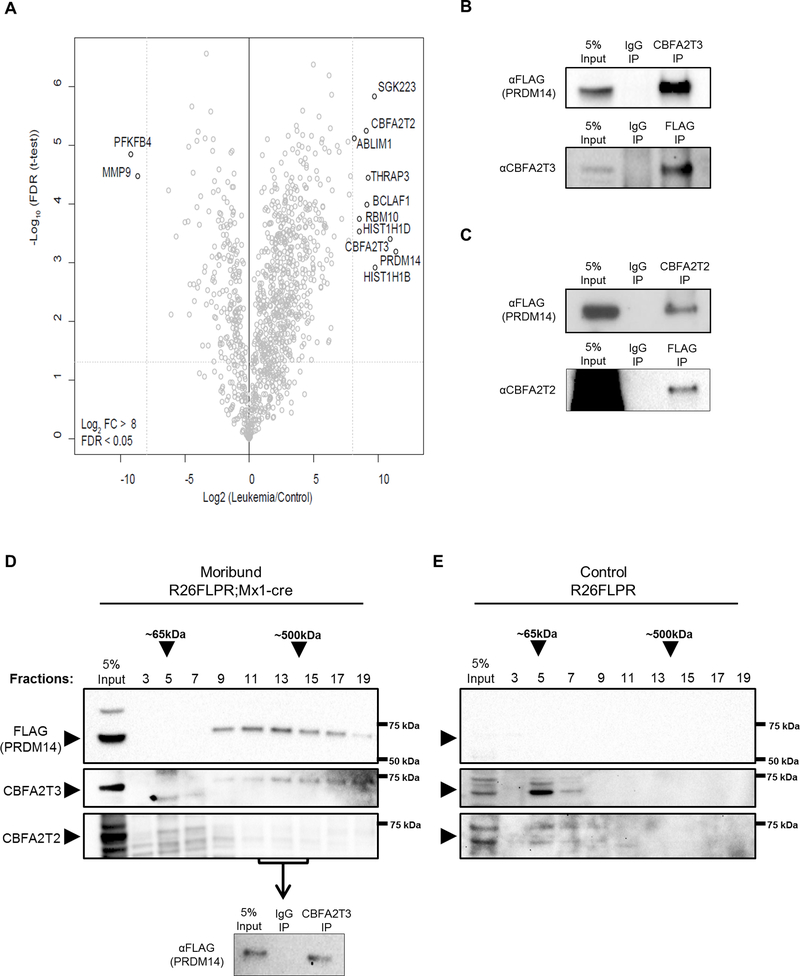
To determine the molecular mechanism for PRDM14-induced cancer initiation, we sought to identify PRDM14 protein interacting partners. Prdm14 expression was induced in 8-week old R26FLPR;Mx1-cre animals by injection of polyinosinic:polycytidylic acid (pIpC). Immunoprecipitation (IP) of FLAG-PRDM14 was conducted on bone marrow (BM) from moribund R26FLPR;Mx1-cre with anti-FLAG magnetic beads. Bead-bound proteins were digested with trypsin and subjected to quantitative liquid chromatography (LC)-MS/MS for identification. AP/MS analysis was performed using methods that are optimized for low protein inputs. Control BM from R26FLPR animals not exposed to cre and therefore not expressing Prdm14 was also subjected to LC-MS/MS to eliminate proteins pulled down through non-specific antibody binding. Label-free quantification (LFQ) analysis revealed several strong interactions with proteins involved in NOTCH1 activation (SGK223/PEAK1), splicing (THRAP3, RBM10) and chromatin binding (CBFA2T3, linker histone variants HIST1h1d/H1.3 and HIST1h1b/H1.5, CBFA2T2) (Figure 1A, Supplementary Table S1). The protein with the strongest fold change over control was ETO-family member CBFA2T3. PRDM14 interaction with both ETO family members was confirmed using reciprocal co-IP on thymus tissue with FLAG-PRDM14 and endogenous CBFA2T3 (Figure 1B) or CBFA2T2 (Figure 1C). The leukemias that develop in PRDM14-expressing mice are highly infiltrative, saturating all hematopoietic tissues, with monoclonal immature pre-T cells14,15. Therefore, thymus tissue was used to confirm the interaction in a second tissue type, producing high protein yields to reduce the number of animals required, and providing a more homogeneous wild-type control tissue than BM. Co-IP with IgG alone confirmed proteins were not being recovered through non-specific binding to the antibody (Figures 1B and 1C). The strength of the interaction with CBFA2T3 and its importance in hematopoietic cell regulation led us to further investigate its relationship with PRDM14.
Figure 1. PRDM14 interacts with CBFA2T3 in leukemia cells.
(A) Volcano plot of LFQ mass spectrometry data from R26FLPR;Mx1-cre leukemic BM performed technically in triplicate. Data points represent individual proteins with significant hits in the top right corner having –Log False Discovery Rate (FDR) >1.301 and Log2 fold change (FC) >8 over control R26FLPR animals. (B) Co-IP in leukemic thymus confirms protein interaction between PRDM14 and CBFA2T3, which is not due to unspecific IgG binding. Representative image from four experiments. (C) Co-IP in leukemic thymus confirms protein interaction between PRDM14 and CBFA2T2, which is not due to unspecific IgG binding. Representative image from four experiments. (D, E) Western blot probing for FLAG (PRDM14), CBFA2T3 and CBFA2T2 after separation of protein complexes via density gradient. Top arrows indicate approximate size of complexes running at fraction 5 or between fractions 13–15 based on purified protein standards, and side arrows indicate expected size of the indicated protein. (D) FLAG (PRDM14) and CBFA2T3 proteins, but not CBFA2T2, peak in fraction 13. Fractions 12–14 were pooled and FLAG-PRDM14 was pulled down with αCBFA2T3 antibody confirming an association in those fractions. Representative images from four experiments. (E) In control R26FLPR tissue not expressing PRDM14, CBFA2T3 peaks in fraction 5. Representative images from three experiments.
To further characterize the interaction with PRDM14, we ran R26FLPR;Mx1-cre thymus protein lysates through a density gradient to separate potential protein complexes. Western Blot analysis showed a partial co-fractionation of PRDM14 (64 kDa) and CBFA2T3 (68 kDa) interacting in a fraction corresponding to approximately 400–500 kDa in size, suggesting that additional complex members or CBFA2T3 oligomerization may contribute to the formation of multimeric protein associations (Figure 1D). CBFA2T2, PRDM14’s primary interaction partner in pluripotent cells17,18, peaked in the first fractions (5 and 7), but did not co-fractionate with PRDM14 or CBFA2T3 in the later fractions, indicating that it does not participate abundantly in the same CBFA2T3-containing complex in murine leukemia cells. Co-IP performed with an αCBFA2T3 antibody from lysates from fractions 12–14 confirmed an association between FLAG-PRDM14 and CBFA2T3 in those peak fractions. In contrast, a density gradient carried out in R26FLPR control thymus lacking cre revealed that CBFA2T3 shows a peak in fraction 5 with no detectable signal in later fractions when PRDM14 is absent, demonstrating that CBFA2T3 is predominantly found as a monomer in wild-type thymus (Figure 1E).
PRDM14 and CBFA2T3 interact through the PR and NHR1 domains
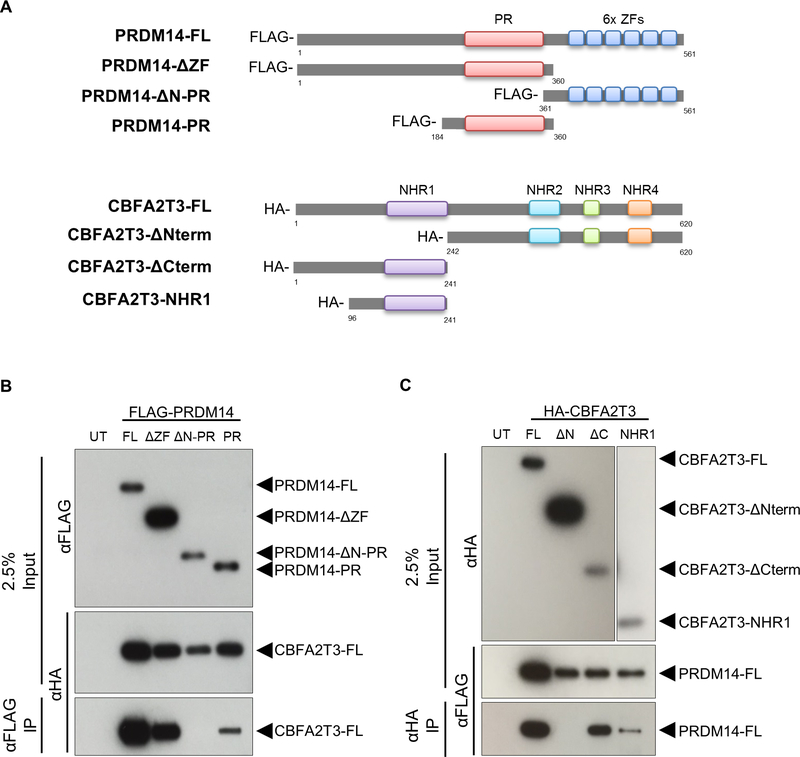
CBFA2T3 contains four NHR domains that bind diverse types of proteins, enabling it to act as a bridge between transcription factors that bind NHR1 and chromatin modifying enzymes that bind NHR3–427. PRDM14 interacts with other proteins primarily via its PR domain17. To identify the protein domain at which the PRDM14-CBFA2T3 interaction occurs, a series of deletion constructs lacking individual functional domains was created for each protein (Figure 2A). To test the interacting domains, tagged expression plasmids were co-transfected into HEK293T cells followed by protein extraction and co-IP with either anti-FLAG (PRDM14) or anti-HA (CBFA2T3) magnetic beads. PRDM14 bound to full length CBFA2T3 without its C-terminal zinc fingers, but the N-PR region was required for interaction. PRDM14’s PR domain alone was sufficient to pull down CBFA2T3 (Figure 2B). CBFA2T3 bound full length PRDM14 without the C-terminal half of the protein, but the N-terminal half was required for interaction. Further, the NHR1 domain of CBFA2T3 alone was sufficient to pull down PRDM14 (Figure 2C, Supplementary Figure S1), even though this small region of the protein appeared to be less stable than the larger components. Together, these data demonstrate that PRDM14 and CBFA2T3 interact through the PR and NHR1 domains, respectively.
Figure 2. PRDM14 and CBFA2T3 interact through the PR and NHR1 domains, respectively.
(A) Deletion constructs lacking key functional domains were constructed for PRDM14 (FLAG-tagged) and CBFA2T3 (HA-tagged). Numbers indicate beginning and ending amino acid positions for each corresponding full length protein. (B, C) Western blot results after co-IP with anti-FLAG (B) or anti-HA (C) antibodies. The constructs important for facilitating interaction are detected in the bottom panels. The NHR1 panel of the HA blot in (C) shows a longer exposure due to decreased expression. Representative images from three experiments. PRDM14-FL (67kDa), PRDM14-ΔZF (42kDa), PRDM14-ΔN-PR (25kDa), PRDM14-PR (21kDa), CBFA2T3-FL (70kDa), CBFA2T3-ΔNterm (45kDa), CBFA2T3-ΔCterm (25kDa), CBFA2T3-NHR1 (13kDa). UT, untransfected; FL, full length.
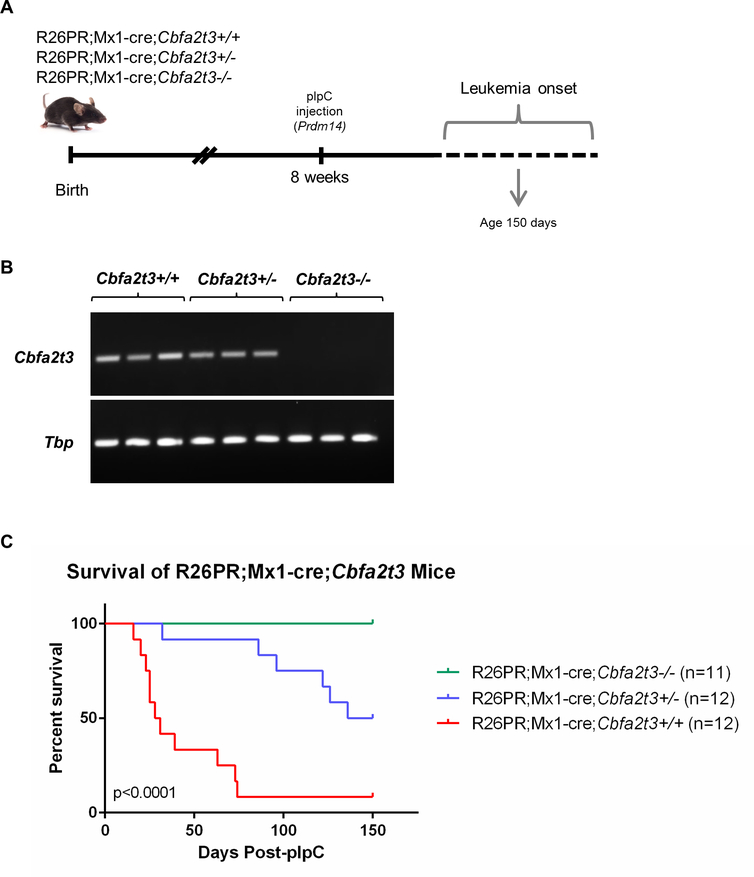
Altering levels of Cbfa2t3 can prevent or slow T-ALL development
Cbfa2t3 is important for HSC self-renewal and is most highly expressed in hematopoietic stem and progenitor cells27–30. Therefore, we hypothesized that a misappropriation of its role in BM when PRDM14 is ectopically present is critical for T-ALL pathogenesis. To test this idea, we placed the R26PR and Mx1-cre constructs together on a Cbfa2t3−/− genetic background (R26PR;Mx1-cre;Cbfa2t3) prior to pIpC induction (Figures 3A and 3B) and assessed leukemia phenotypes over time. Strikingly, although R26PR;Mx1-cre;Cbfa2t3+/+ animals (11/12) develop a very penetrant T-ALL as early as 16 days post pIpC-injection, R26PR;Mx1-cre;Cbfa2t3−/− animals (11/11) did not develop any signs of leukemia over 150 days post-pIpC injection, suggesting that Cbfa2t3 is essential for Prdm14-induced T-ALL (Figure 3C). Unexpectedly, R26PR;Mx1-cre;Cbfa2t3+/− mice showed a significant delay in leukemia onset with 6/12 mice succumbing to disease in 32–136 days post pIpC-injection, and 6/12 mice remaining healthy by 150 days post pIpC-injection, in contrast to R26PR;Mx1-cre;Cbfa2t3+/+ mice. These data suggest that the leukemia that develops due to expression of Prdm14 is sensitive to levels of Cbfa2t3.
Figure 3. Deletion of Cbfa2t3 prevents T-ALL development in R26PR;Mx1-cre mice.
(A) Mouse cross of R26PR;Mx1-cre mice with Cbfa2t3−/− mice and experimental timeline. (B) RT-PCR detecting Cbfa2t3 and Tbp expression (internal control) in Cbfa2t3+/+ and Cbfa2t3+/− mice, but not Cbfa2t3-/−. (C) Kaplan-Meier survival curve of R26PR;Mx1-cre;Cbfa2t3 mice presented as percent survival by days post-pIpC injection (Prdm14 expression) for each genotype. Significance was determined by comparing the three curves using a logrank (Mantel-Cox) test for statistical significance. None of the R26PR;Mx1-cre;Cbfa2t3−/− mice died by 150 days (n=11); R26PR;Mx1-cre;Cbfa2t3+/− mice died between 32–136 days post-pIpC (n=12, mean = 100 days); R26PR;Mx1-cre;Cbfa2t3+/+ mice died between 16–74 days post-pIpC (n=12, mean = 38 days). Tbp, TATA-box binding protein.
T-ALL has a similar disease phenotype in both Cbfa2t3+/− and Cbfa2t3+/+ backgrounds
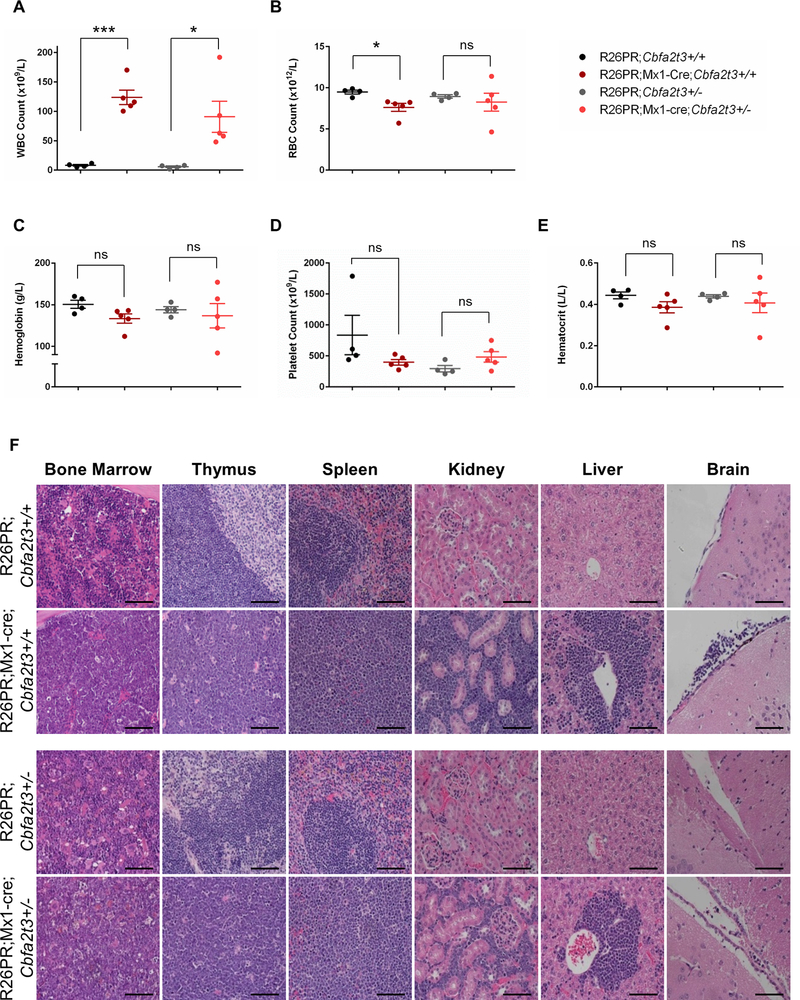
The significant difference in survival between Prdm14-expressing mice on a Cbfa2t3+/+ or Cbfa2t3+/− background led us to consider that the cell type may also differ in leukemias that arose on the two genetic backgrounds. However, the difference was restricted to penetrance and the timing of onset, because few differences in leukemia subtype or pathology were noted between moribund R26PR;Mx1-cre;Cbfa2t3+/+ and R26PR;Mx1-cre;Cbfa2t3+/− mice. All moribund animals presented with lethargy, distended abdomens and laboured breathing and upon dissection had grossly enlarged thymi, spleens and livers. However, only 67% of R26PR;Mx1-cre;Cbfa2t3+/− mice (4/6) developed enlarged lymph nodes compared to 100% of R26PR;Mx1-cre;Cbfa2t3+/+ mice (11/11). Complete blood counts in R26PR;Mx1-cre;Cbfa2t3+/− mice showed a significant increase in white blood cell counts (48–192 × 109/L, mean 90.74 ± 26.37) over control animals (2.1–8.2 × 109/L, mean 5.63 ± 1.38) (Figure 4A). Although R26PR;Mx1-cre;Cbfa2t3+/+ mice showed a significant decrease in red blood cell numbers from control animals (5.71–8.3 × 1012/L, mean 7.62 ± 0.49 in moribund animals versus 8.8–9.9 × 1012/L, mean 9.49 ± 0.24 in control animals), those in R26PR;Mx1-cre;Cbfa2t3+/− mice were more variable, and did not reach significance (4.6–11.4 × 1012/L, mean 8.25 ± 1.10 in moribund animals versus 8.4–9.3 × 1012/L, mean 8.95 ± 0.20 in control animals) (Figure 4B). Hemoglobin, hematocrit and platelet values were statistically unchanged between moribund and control mice (Figures 4C, 4D and 4E). Histopathological analyses of both R26PR;Mx1-cre;Cbfa2t3+/+ and R26PR;Mx1-cre;Cbfa2t3+/− mice revealed a loss of normal architecture in the BM, spleen, thymus, liver and kidney associated with an extensive infiltration of lymphoblast cells. Lymphoblasts could also be detected in the meninges and blood vessel linings within the brain (Figure 4F).
Figure 4. R26PR;Mx1-cre;Cbfa2t3+/− moribund mice develop T-ALL with a similar disease phenotype compared to R26PR;Mx1-cre;Cbfa2t3+/+ moribund mice.
(A) White blood cell (WBC), (B) red blood cell (RBC), (C) hemoglobin, (D) platelet, and (E) hematocrit counts in peripheral blood of control (n=4) and moribund (n=5) mice. Significance was determined using an unpaired t-test. (F) Representative images of hematoxylin and eosin-stained sections of fixed bone marrow, thymus, spleen, kidney, liver and brain tissues. All images were taken with a 40X objective. Scale bar, 100μM. ***p<0.0006; *p<0.05; ns, not significant.
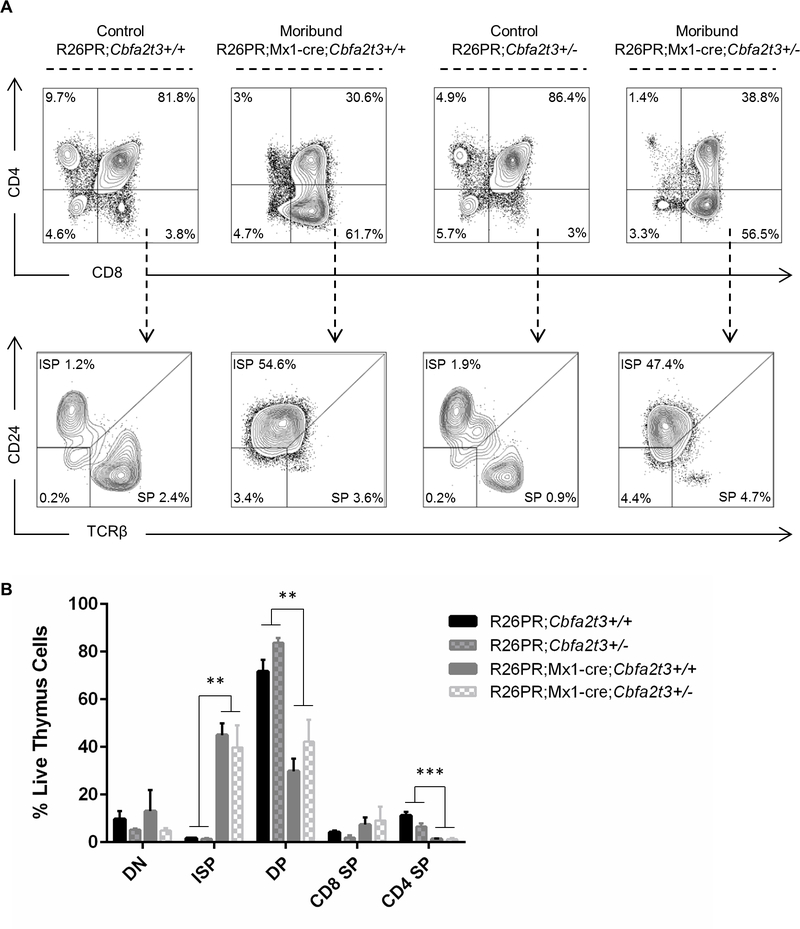
We have previously shown that cells mis-expressing PRDM14 have a block in T-cell development leading to an accumulation of CD8+ immature single positive (ISP) T-cells (CD4-CD8+CD24+TCRβ lo/-) at the expense of double positive (DP) T-cells (CD4+CD8+)14. We confirmed this result using flow cytometry immunophenotyping of R26PR;Mx1-cre;Cbfa2t3+/+ mice and showed that R26PR;Mx1-cre;Cbfa2t3+/− mice develop a corresponding accumulation of CD8+ ISP T-cells in the thymus (Figure 5A and 5B). Taken together, these results indicate that the T-ALL that develops in R26PR;Mx1-cre;Cbfa2t3+/− mice is similar in cell type to that in R26PR;Mx1-cre;Cbfa2t3+/+ mice but with a significantly delayed onset. Strikingly, the absence of Cbfa2t3 prevents T-ALL when Prdm14 is mis-expressed in HSCs.
Figure 5. Lymphoblasts have a block in T-cell development.
(A) Representative plots from flow cytometry analysis of thymus cells using markers of T-cell development. (B) Quantification of T-cell populations in thymus from 3–6 biological replicates per genotype. **p<0.03; ***p<0.003. DN, double negative; DP, double positive; ISP, immature single positive; SP, single positive.
Discussion
Our work suggests that the presence of CBFA2T3 in hematopoietic progenitors is required for PRDM14 to initiate T-ALL. The protein-protein interaction between PRDM14 and CBFA2T3 that occurs in conserved family motifs suggests a model where this complex, which likely contains multiple CBFA2T3 oligomers, provides a platform that recruits chromatin modifying enzymes to initiate leukemia (Figure 6). The master hematopoietic regulator CBFA2T3 is crucial for HSC quiescence and hematopoietic lineage decisions. Cbfa2t3 homozygous mice are viable and fertile31, developing a mild anemia due to reduced erythroid progenitor cells32. Homozygous mutant mice exhibit defects in stem cell self-renewal with fewer quiescent Lin−Sca-1+c-Kit+ (LSK) cells, and failure of BM to repopulate in transplant assays30,31. CBFA2T3 controls the expression of many hematopoietic transcription factors, including a key complex that includes the E2A transcription factor, T-cell acute lymphocytic leukemia 1 (TAL1), LIM domain only 2 (LMO2), and LIM Domain Binding 1 (LDB1)33, which regulates long term (LT)-HSC quiescence. Relevant to the initiation of leukemia by PRDM14, both TAL1 and LMO2 are required for leukemia initiation34,35. Homozygous Cbfa2t3 mutant mice also have lower numbers of T-cells, with primary perturbations in the DN1 populations29. After BM transplantation, Cbfa2t3 null mice fail to repopulate the T-lineage because lymphoid-primed multipotent progenitors (LMPP) and early T-cell precursor (ETP) cells are reduced29. In contrast, Prdm14 mis-expression in BM expands progenitor cells, including cells that resemble LT-HSCs and common lymphoid progenitors, in which Cbfa2t3 is overexpressed4,14. Therefore the expansion of LT-HSC-like cells and lymphoid progenitors in PRDM14-expressing mice is consistent with Cbfa2t3’s role in HSC self-renewal and T-cell development.
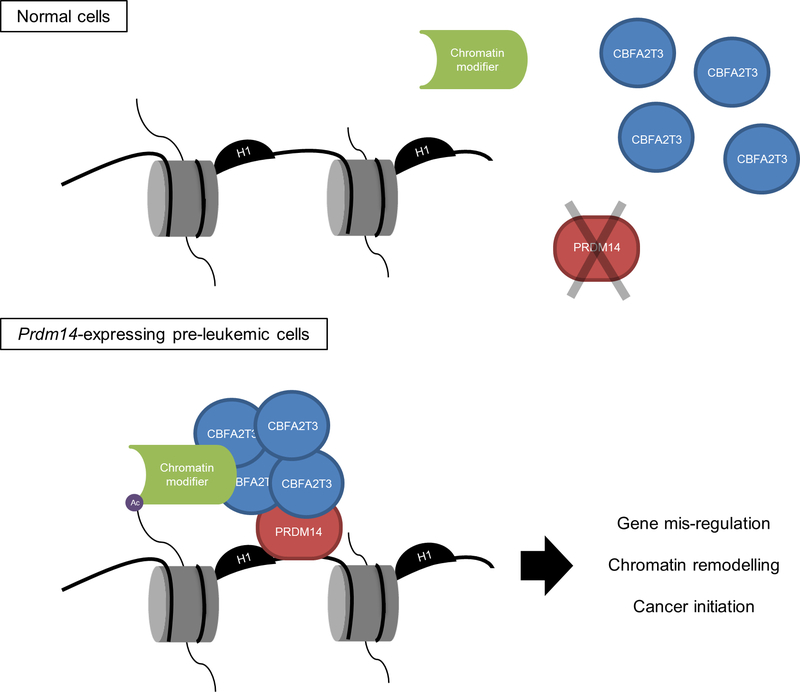
Figure 6. Model of PRDM14/CBFA2T3-mediated cancer initiation.
In normal hematopoietic cells lacking PRDM14, CBFA2T3 exists predominantly as a monomer or in transient protein complexes. Upon aberrant Prdm14 expression in pre-leukemic cells, PRDM14 can hijack CBFA2T3 and potentially additional proteins via CBFA2T3 to PRDM14-target genes, leading to chromatin remodeling, gene mis-regulation and cancer initiation.
CBFA2T3’s family member CBFA2T2, which is required for PRDM14 to reset potency in PGCs, also interacted with PRDM14 in leukemia cells. The protein structure and function of the ETO family members overlap, leading to the idea that the different members interact with a common set of transcription factors and co-repressors to provide functional redundancy, and explaining rather mild phenotypes in knockout mice. ETO proteins can form multimers, including heterodimeric and tetrameric structures. However, specificity arises because they are normally expressed in different cell types. Consistently, CBFA2T3 expression is high in hematopoietic stem and progenitor cells, and decreases during differentiation, remaining high in megakaryocyte-erythroid and B220+ progenitor cells27,28. In mouse HSCs, Cbfa2t3 is expressed at twice the level of Cbfa2t236, which could give CBFA2T3 a competitive advantage over CBFA2T2 for interaction with PRDM14. Importantly, density gradient experiments show that CBFA2T3 and PRDM14 associate and potentially assemble into a protein complex in leukemic tissue without CBFA2T2, supporting the idea that PRDM14 preferentially associates with the predominant ETO family member present in HSCs. Moreover, Cbfa2t3 is highly expressed in Prdm14-driven pre-leukemia cells compared with immunophenotype-matched progenitor cells4. A partnership with overexpressed CBFA2T3, then, is consistent with an overabundance of LT- HSC-like and LMPP-like cells in PRDM14 expressing pre-leukemia cells14.
Altogether, these data support the idea that Cbfa2t3 is required for Prdm14-induced leukemogenesis. Strikingly, T-ALL did not occur on a Cbfa2t3−/− genetic background within 150 days of inducing Prdm14 expression. Further, leukemia onset was significantly delayed on the heterozygous Cbfa2t3+/− genetic background. CBFA2T3 is poised to interact with PRDM14 in immature cell types in the event that Prdm14 becomes mis-expressed and is likely the essential factor mediating PRDM14-induced cancer initiation in progenitor hematopoietic cell types. The presence of Cbfa2t3 in progenitors could explain why Prdm14 transforms hematopoietic stem and progenitor cells but lacks this capacity in committed cells14.
CBFA2T3’s NHR1 domain is required for the interaction with PRDM14 in cultured cells. PRDM14 binds DNA at a specific sequence that is identical between mouse and human (GGTC[TC]CTAA) through its six zinc fingers7,8. ETO family members do not bind DNA directly, so it is likely that PRDM14 serves as a bridge between DNA and CBFA2T3, while the NHR3 and NHR4 domains complex with other chromatin modifying proteins27, similar to other related family members that contain Nervy homology domains (Figure 6). CBFA2T3 may form a co-repressor complex with histone deacetylases (HDACs) 1–3 as well as 6 and 8 to regulate gene expression in this context as well18,27. Thus, PRDM14 may recruit repressive CBFA2T3 and indirectly HDAC proteins that are capable of depositing epigenetic marks onto chromatin at specific DNA targets. This mechanism suggests that PRDM14 hijacks CBFA2T3 to mis-regulate gene expression, expanding stem and progenitor cells as it perturbs normal lineage development to initiate T-ALL.
NOTCH signaling is critical to T-cell development and for the transition from HSCs to common lymphoid progenitors. Activating mutations at the NOTCH1 locus are found in greater than 50% of T-ALL cases37. We previously showed that in mouse pre-leukemia cells, PRDM14 binds DNA adjacent to cryptic recombination signal sequence sites at Notch1 to recruit RAG recombinase enzymes15 that allow for illegitimate recombination, creating a constitutively active NOTCH1 that drives T-ALL38. PRDM14-induced T-ALLs are extremely aggressive and early onset, more-so than in models that overexpress Notch115, suggesting that other factors are at play to promote NOTCH-driven tumorigenesis. The Notch intracellular domain (ICD) and CBFA2T3 NHR1 are critical for integrating NOTCH functions during T-cell development. Similar to other hematopoietic transcription factors, the level of CBFA2T3 affects appropriate transient NOTCH signaling, and perturbations can lead to activation of NOTCH39. Thus, both constitutive activation of NOTCH1 through illegitimate recombination driven by PRDM14 and high levels of CBFA2T3 may lead to this aggressive T-ALL.
AP/MS identified several new partners for PRDM14 that may reveal additional functions for this intriguing protein. Bcl2-associated transcription factor 1 (BCLAF1) is an apoptosis regulator associated with repressor complexes, whose expression is linked to poor outcomes in AML40. RBM10 and THRAP3 are RNA-binding factors that potentially associate with CBFA2T3, which can bind RNA through the NHR4- and NHR2-proximal regions41. Noncoding RNAs have a well-established role in recruiting chromatin-binding complexes to remodel the genome42,43. Finally, two Histone 1 (H1) variants, H1.3 and H1.5, were also recovered. H1 is not involved in the core nucleosome, but rather binds as a linker histone that is important for higher order chromatin structure. H1.3 binds at the imprinting control regions of the imprinted long non-coding RNAs H19 and maternally expressed gene 3 (Meg3), altering DNA methylation at these sites in ovarian cancer cells44. Many imprinted genes, among them Meg3 and its overlapping imprinted transcript Deiodinase-2 (Dio2), are mis-expressed in Prdm14 pre-leukemia cells4,45. In vitro assays have not detected histone H3 methyltransferase activity for the PR domain of PRDM1415,17; however, given its association with H1, it will be prudent to determine if PRDM14 can directly modify H1.
Previous studies of PRDM14 protein interactions using a co-IP technique have identified chromatin remodelers that were not identified here. Two studies in ESCs reported that PRDM14 interacts with the Polycomb Repressor Complex 2 (PRC2), which confers the H3K27me3 mark to repress gene transcription46,47. A separate study in ESCs showed an interaction with Coactivator Associated Arginine Methyltransferase 1 (CARM1), a histone arginine methyltransferase important for cell fate specification in the early embryo48. However, it is noteworthy that these interactions were not found in the AP/MS analysis from mouse leukemic tissues reported here, nor in AP/MS experiments carried out in ESC and human germ cell tumour-derived (NCCIT) cell lines17,18. Although a complete overlap in binding partners for PRDM14 and ETO family members was not found in this experiment, an alternative protein interaction approach may be needed to more readily detect less stable interactions or complex members indirectly bound via interaction with CBFA2T3.
Together, our results demonstrate how an oncogenic protein can hijack proteins already present in a given cell type to initiate leukemia. Although CBFA2T3 has been associated with tumour suppressor functions in cancers of epithelial origin, it is reported to have oncogenic properties in hematopoietic cancers49,50. Ours is the first study to definitively show an oncogenic function for CBFA2T3 as a partner with PRDM14. PRDM14 overexpression and amplification is also implicated in cancer initiation in breast and other solid tumours, and the association with CBFA2T3 may be a common mechanism in PRDM14-driven oncogenesis that extends to many other cancer subtypes. Because Prdm14 is not expressed in mature cells, targeting its interaction with CBFA2T3 could be specific to cancer cells, thereby avoiding detrimental off-target effects and allowing for therapeutic strategies for a subset of tumours.
Supplementary Material
Acknowledgements
The authors thank Dr. Scott W. Hiebert of Vanderbilt University and Dr. Margaret Goodell for supplying mice, Christine Taylor and Julie Ruston for help with mouse colony management, Alexandra Khozin and Stephanie Tran for technical assistance, and all members of the Justice lab for helpful discussions. This work was supported by the National Institutes of Health (R01CA163849 to M.J. Justice); NSERC (Discovery Grant to E.I. Campos); CIHR (Project Grant PJT-159683 to E.I. Campos.); Cancer Research Society (Operating Grant to E.I. Campos.); Garron Family Cancer Centre (Pitblado Discovery Grant to E.I. Campos). The Vermeulen lab is part of the Oncode Institute, which is partly funded by the Dutch Cancer Society (KWF).
Footnotes
Conflict of interest statement: The authors declare no conflicts of interest.
References
- 1.Roberts KG & Mullighan CG Genomics in acute lymphoblastic leukaemia : insights and treatment implications. Nat. Rev. Clin. Oncol 12, 344–357 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Mullighan CG et al. Genomic analysis of the clonal origins of relapsed acute lymphoblastic leukemia. Science. 322, 1377–1380 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dettman EJ & Justice MJ The zinc finger SET domain gene Prdm14 Is overexpressed in lymphoblastic lymphomas with retroviral insertions at Evi32. PLoS One 3, 1–9 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dettman EJ et al. Prdm14 initiates lymphoblastic leukemia after expanding a population of cells resembling common lymphoid progenitors. Oncogene 30, 2859–2873 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsuneyoshi N et al. PRDM14 suppresses expression of differentiation marker genes in human embryonic stem cells. Biochem. Biophys. Res. Commun 367, 899–905 (2008). [DOI] [PubMed] [Google Scholar]
- 6.Chia N-Y et al. A genome-wide RNAi screen reveals determinants of human embryonic stem cell identity. Nature 468, 316–320 (2010). [DOI] [PubMed] [Google Scholar]
- 7.Ma Z, Swigut T, Valouev A, Rada-Iglesias A & Wysocka J Sequence-specific regulator Prdm14 safeguards mouse ESCs from entering extraembryonic endoderm fates. Nat. Struct. Mol. Biol 18, 120–127 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Yamaji M et al. Critical function of Prdm14 for the establishment of the germ cell lineage in mice. Nat. Genet 40, 1016–1022 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Magnúsdóttir E et al. A tripartite transcription factor network regulates primordial germ cell specification in mice. Nat. Cell Biol 15, 905–915 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gell JJ, Zhao J, Chen D, Hunt TJ & Clark AT PRDM14 is expressed in germ cell tumors with constitutive overexpression altering human germline differentiation and proliferation. Stem Cell Res. 27, 46–56 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang T et al. High expression of PRDM14 correlates with cell differentiation and is a novel prognostic marker in resected non-small cell lung cancer. Med. Oncol 30, 1–7 (2013). [DOI] [PubMed] [Google Scholar]
- 12.Nishikawa N et al. Gene amplification and overexpression of PRDM14 in breast cancers. Cancer Res. 67, 9649–9657 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Taniguchi H et al. Silencing PRDM14 expression by an innovative RNAi therapy inhibits stemness, tumorigenicity, and metastasis of breast cancer. Oncotarget 8, 46856–46874 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carofino BL, Ayanga B & Justice MJ A mouse model for inducible overexpression of Prdm14 results in rapid-onset and highly penetrant T-cell acute lymphoblastic leukemia (T-ALL). Dis. Model. Mech 6, 1494–506 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carofino BL, Ayanga B, Tracey LJ, Brooke-bisschop T & Justice MJ PRDM14 promotes RAG-dependent Notch1 driver mutations in mouse T-ALL. Biol. Open 1–9 (2016). doi: 10.1242/bio.017699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma Z, Swigut T, Valouev A, Rada-Iglesias A & Wysocka J Sequence-specific regulator Prdm14 safeguards mouse ESCs from entering extraembryonic endoderm fates. Nat. Struct. Mol. Biol 18, 120–127 (2011). [DOI] [PubMed] [Google Scholar]
- 17.Nady N et al. ETO family protein Mtgr1 mediates Prdm14 functions in stem cell maintenance and primordial germ cell formation. Elife 4, e10150 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tu S et al. Co-repressor CBFA2T2 regulates pluripotency and germline development. Nature 534, 387–390 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amann JM et al. ETO, a target of t(8;21) in acute leukemia, makes distinct contacts with multiple histone deacetylases and binds mSin3A through its oligomerization domain. Mol. Cell. Biol 21, 6470–83 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindberg S, Olsson A, Persson A-M & Olsson I Interactions between the leukaemia- associated ETO homologues of nuclear repressor proteins. Eur. J. Haematol 71, 439–447 (2003). [DOI] [PubMed] [Google Scholar]
- 21.Hug BA & Lazar MA ETO interacting proteins. Oncogene 23, 4270–4274 (2004). [DOI] [PubMed] [Google Scholar]
- 22.Guastadisegni M et al. CBFA2T2 and C20orf112 : two novel fusion partners of RUNX1 in acute myeloid leukemia. Leukemia 24, 1516–1519 (2010). [DOI] [PubMed] [Google Scholar]
- 23.Smits AH, Jansen PWTC, Poser I, Hyman AA & Vermeulen M Stoichiometry of chromatin-associated protein complexes revealed by label-free quantitative mass spectrometry-based proteomics. Nucleic Acids Res. 41, 1–8 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cox J & Mann M MaxQuant enables high peptide individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol 26, 1367–1372 (2008). [DOI] [PubMed] [Google Scholar]
- 25.Rappsilber J, Mann M & Ishihama Y Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat. Protoc 2, 1896–1906 (2007). [DOI] [PubMed] [Google Scholar]
- 26.Tyanova S et al. The Perseus computational platform for comprehensive analysis of ( prote ) omics data. Nat. Methods 13, 731–740 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Steinauer N, Guo C & Zhang J Emerging Roles of MTG16 in Cell-Fate Control of Hematopoietic Stem Cells and Cancer. Stem Cells Int. 2017, 1–12 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindberg SR, Olsson A, Persson AM & Olsson I The Leukemia-associated ETO homologues are differently expressed during hematopoietic differentiation. Exp. Hematol 33, 189–198 (2005). [DOI] [PubMed] [Google Scholar]
- 29.Hunt A, Fischer M, Engel ME & Hiebert SW Mtg16/Eto2 contributes to murine T-cell development. Mol. Cell. Biol 31, 2544–2551 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fischer MA, Moreno-Miralles I, Hunt A, Chyla BJ & Hiebert SW Myeloid translocation gene 16 is required for maintenance of haematopoietic stem cell quiescence. EMBO J. 31, 1494–505 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chyla BJ et al. Deletion of Mtg16, a target of t(16;21), alters hematopoietic progenitor cell proliferation and lineage allocation. Mol. Cell. Biol 28, 6234–6247 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamlett I et al. Characterization of megakaryocyte GATA1-interacting proteins: The corepressor ETO2 and GATA1 interact to regulate terminal megakaryocyte maturation. Blood 112, 2738–2749 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schuh AH et al. ETO-2 Associates with SCL in Erythroid Cells and Megakaryocytes and Provides Repressor Functions in Erythropoiesis. Mol. Cell. Biol 25, 10235–10250 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCormack MP et al. The Lmo2 Oncogene Initiates Leukemia in Mice by Inducing Thymocyte Self-Renewal. Science. 327, 879–883 (2010). [DOI] [PubMed] [Google Scholar]
- 35.Gerby B, Tremblay CS, Tremblay M, Rojas-sutterlin S & Sauvageau G SCL, LMO1 and Notch1 Reprogram Thymocytes into Self-Renewing Cells. PLoS Genet. 10, 1–19 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heng TSP, Painter MW & The Immunological Genome Project. The Immunological Genome Project : networks of gene expression in immune cells. Nat. Immunol 9, 1091–1094 (2008). [DOI] [PubMed] [Google Scholar]
- 37.Weng AP et al. Activating Mutations of NOTCH1 in Human T Cell Acute Lymphoblastic Leukemia. Science. 306, 269–271 (2004). [DOI] [PubMed] [Google Scholar]
- 38.Ashworth TD et al. Deletion-based mechanisms of Notch1 activation in T-ALL : key roles for RAG recombinase and a conserved internal translational start site in Notch1. Blood 116, 5455–5465 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Butko E, Pouget C & Traver D Complex regulation of HSC emergence by the Notch signaling pathway. Dev. Biol 409, 129–138 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dell’Aversana C et al. miR-194–5p / BCLAF1 deregulation in AML tumorigenesis. Leukemia 1–11 (2017). doi: 10.1038/leu.2017.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rossetti S, Unen L Van, Sacchi N & Hoogeveen AT Novel RNA-binding properties of the MTG chromatin regulatory proteins. BMC Mol. Biol 9, 9–11 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsai M et al. Long Noncoding RNA as Molecular Scaffold of Histone Modification Complexes. Science. 329, 689–694 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hendrickson DG, Kelley DR, Tenen D, Bernstein B & Rinn JL Widespread RNA binding by chromatin- associated proteins. Genome Biol. 17, 1–18 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Medrzycki M et al. Histone H1. 3 Suppresses H19 Noncoding RNA Expression and Cell Growth of Ovarian Cancer Cells. Cancer Res. 74, 6463–6474 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simko SJ, Voicu H, Carofino BL & Justice MJ Mouse lymphoblastic leukemias induced by aberrant Prdm14 expression demonstrate widespread copy number alterations also found in human ALL. Cancers (Basel). 4, 1050–1066 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamaji M et al. PRDM14 ensures naive pluripotency through dual regulation of signaling and epigenetic pathways in mouse embryonic stem cells. Cell Stem Cell 12, 368–382 (2013). [DOI] [PubMed] [Google Scholar]
- 47.Chan Y-S et al. A PRC2-dependent repressive role of PRDM14 in human embryonic stem cells and induced pluripotent stem cell reprogramming. Stem Cells 31, 682–92 (2013). [DOI] [PubMed] [Google Scholar]
- 48.Burton A et al. Single-Cell Profiling of Epigenetic Modifiers Identifies PRDM14 as an Inducer of Cell Fate in the Mammalian Embryo. Cell Rep. 5, 687–701 (2013). [DOI] [PubMed] [Google Scholar]
- 49.Thirant C et al. ETO2-GLIS2 Hijacks Transcriptional Complexes to Drive Cellular Identity and Self-Renewal in Pediatric Acute Megakaryoblastic Leukemia. Cancer Cell 31, 452–465 (2017). [DOI] [PubMed] [Google Scholar]
- 50.Salaverria I et al. The CBFA2T3 / ACSF3 Locus Is Recurrently Involved in IGH Chromosomal Translocation t(14;16)(q32;q24) in Pediatric B-Cell Lymphoma with Germinal Center Phenotype. Genes, Chromosom. Cancer 51, 338–343 (2012). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.