Abstract
Background:
Higher energy expenditure (EE) is associated with greater food intake, possibly because the human body senses EE and modifies eating behaviors to regulate food intake and ultimately achieve energy balance. As eating behaviors are also influenced by social and cultural factors, any association between EE and eating behavior may differ between ethnicities and sexes.
Objective:
To assess relationships between EE and eating behavior constructs of the Three-Factor Eating Questionnaire (TFEQ).
Subjects/Methods:
307 healthy adults (201M/106F, 160 Native Americans) completed the TFEQ and had measures of 24-h EE in a whole-room calorimeter during energy balance. Body composition was assessed by DXA.
Results:
On average, adjusted 24-h EE was lower (β=−229 kcal/day, CI: −309-−148, p<0.001) but cognitive restraint (Δ=+1.5; CI: 0.5–2.5, p=0.003) and disinhibition (Δ=+2.1, CI: 1.3–2.8, p<0.001) scores were higher in women compared to men. In Native Americans, adjusted 24-h EE (β=+94 kcal/day, CI: 48–139, p<0.001) and disinhibition scores (Δ=+1.0, CI: 0.1–2.0, p=0.003) were higher compared to other ethnicities. Higher 24-h EE associated with lower cognitive restraint in women (ρ=−0.20, p=0.04), but not men (p=0.71; interaction term p=0.01) with no ethnic differences. Greater 24-h EE associated with higher disinhibition (ρ=0.20, p=0.001) and hunger cues (ρ=0.16, p=0.004) with no gender differences. These associations were primarily present in non-Native Americans (ρ=0.23, p=0.006 and ρ=0.25, p=0.003) but not observed in Native Americans (both p>0.40).
Conclusions:
Higher EE is associated with psychological constructs of eating behaviors that favors overeating including lower cognitive restraint, higher dietary disinhibition, and greater susceptibility to hungers cues, supporting the existence of energy-sensing mechanisms influencing human eating behavior. These associations were observed in ethnicities other than Native Americans, possibly explaining the contradictory relationships reported between EE and weight change in different ethnic groups. We propose that increased EE may alter eating behaviors, potentially leading to uncontrolled overeating and weight gain.
Keywords: TFEQ, eating behavior, energy expenditure, energy sensing, dietary restraint
Introduction
The complex interplay between energy intake and expenditure (EE) determines daily energy balance and weight change. While energy intake varies considerably from day to day making it difficult to measure, EE is more stable as it largely depends on fat free mass (FFM) and can be accurately measured by indirect calorimetry; therefore, it has been extensively studied in relation to future weight change1–5. However, literature has provided conflicting results on the role of EE in future weight change across different ethnic groups6. Specifically, a relatively lower EE is associated with higher rates of weight gain over time in Native Americans of Southwest descent3, 7, 8 whereas the opposite is observed in Blacks2, indicating that ethnic-specific differences influencing the relationship between EE and energy intake may be an important factor in elucidating the role of EE in the etiology of obesity6.
Although energy balance is portrayed as a static regulatory system, daily alterations to one component (EE or energy intake) occur dynamically and may elicit physiological or behavioral compensation in the other component6, 9–11. Consequently, the autoregulatory responses to these perturbations of energy balance would act to regulate energy homeostasis and, in turn, body weight6. Consistent with the hypothesis of a complex interplay between energy metabolism and food consumption, EE is known to correlate strongly with energy intake1, 9, 11–15, supporting the existence of energy sensing mechanisms that may regulate energy intake in humans6, 16, and may ultimately explain inter-individual differences in weight change17. The EE-energy intake link may be driven by the body’s ability to sense EE and consequently alter eating behaviors to meet the body’s energy requirements by modulating energy intake9, 11, 12. Energy intake is often determined by eating behaviors, which in turn are also influenced by social and cultural factors18, 19. Therefore, it is plausible that EE, as an index of energy demands and recently shown to be the main determinant of energy intake1, 12, 14, 15, may be associated with social, cultural, and psychological constructs related to eating behavior, which may ultimately drive energy intake9, 11.
The Three-Factor Eating Questionnaire (TFEQ) is a widely used and validated tool for assessing three dimensions of eating behavior20, 21, including dietary restraint, disinhibition, and susceptibility to hunger22. Associations between cognitive restraint and frequent dieting, lower energy intake and drive for thinness have been reported23–27. Similarly, dietary disinhibition has previously been associated with overeating, loss of control over energy intake, eating in response to emotional distress, as well as greater intake of palatable energy dense foods, often sweet-tasting high-fat food items28. Notably, there are strong and consistent associations between disinhibition, obesity and weight gain over time29–31. Moreover, a greater susceptibility to hunger cues has been associated with the orexigenic hormone ghrelin32 and greater total energy intake14, 24, 33.
Higher degree of dietary restraint is associated with relatively lower ad libitum food intake25 and, in women, restrained eaters have relatively lower EE18. Cultural and societal factors related to gender and ethnicity likely influence the eating behavior constructs of the TFEQ and may further explain differing reports of the relationship between EE and weight change in different ethnicities. Given the putative association between EE and eating behaviors as well as the influential role of social and cultural factors on eating behaviors, we hypothesized that the relationship between EE and psychological constructs related to eating behaviors may differ between genders and ethnicities. Therefore, the aims of the current study were to assess the relationships between 24-h EE, as assessed in a whole-room calorimeter during weight maintenance thus representing an adequate measure of daily energy needs, and psychological constructs related to eating behavior, as assessed by the TFEQ, in a large and ethnically diverse cohort, and to investigate any potential differences that exist between genders or ethnicities.
Subjects and Methods
The present study represents a secondary analysis of 317 healthy individuals who were recruited from the greater Phoenix area through newspaper and Internet-based advertisement to participate in six different studies (Clinical Trial identifiers: NCT00523627, NCT00342732, NCT00856609, NCT01224704, NCT01237093, NCT00687115) designed to understand the behavioral and metabolic predictors of obesity, including assessment of eating behavior by the TFEQ and 24-h measurements of EE in whole-room indirect calorimeter. Inclusion criteria for the enrollment in these studies were an age range of 18–65 years, while exclusion criteria included diabetes, thyroid disease, hypertension, or cardiovascular disease; pregnancy or use of hormonal contraception. Ethnic groups included the following: 160 Native Americans of Southwestern heritage, 77 Whites, 27 Blacks, 22 Hispanics, and 21 subjects of mixed ethnicity. All participants were found to be healthy based on medical history, physical examinations, and laboratory tests, and had no evidence of active psychiatric illness including eating disorders. Participants were not taking any medications, were nonsmokers, and were excluded if they were not weight stable for the past six months (i.e., variation ≥2.3 kg) or had any significant health problems, including cancer, hypertension, or were current substance abusers. Prior to participation in any study, all volunteers were fully informed of the nature and purpose of the study and written informed consent was obtained. All studies were approved by the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
Upon admission to the clinical research unit, subjects were fed a weight-maintaining diet with a macronutrient distribution of 50% carbohydrate, 30% fat and 20% protein for at least 3 days prior to metabolic testing. Individual weight maintenance calories were initially calculated based on weight, gender and BMI, as previously described 34, and subsequently adjusted daily by the research dietitian to ensure a body weight within 1% of the admission weight throughout the baseline period. Body composition was determined by dual-energy X-ray absorptiometry (DXA, LUNAR Prodigy, GE). All participants did not have diabetes mellitus based on American Diabetes Association criteria35, as determined by a 75g oral glucose tolerance test conducted after at least 3 days on the weight-maintaining diet.
Three-Factor Eating Questionnaire
The TFEQ was administered within 48 hours of admission in the morning approximately 1-hour after breakfast. The TFEQ is a 51-item questionnaire that classifies eating behavior on the bases of three factors: cognitive dietary restraint, dietary disinhibition, and susceptibility to hunger. Cognitive restraint reflects the intent to restrict energy intake to control body weight. Disinhibition is the self-reported tendency to overeat in response to various stimuli. Hunger measures an individual’s inclination to eat in response to subjective feelings of hunger. Scores range from 0– 21 (restraint), 0–16 (disinhibition) and from 0–14 (hunger), where higher scores indicate greater disturbances in eating behavior. The TFEQ has demonstrated good internal consistently (Cronbach’s alpha ranging from .79 to .92)21. Based on established cutoffs 22, 25, individuals were classified as: restrained (score>10) or unrestrained eaters (0–10), disinhibited (>8) or non-disinhibited eaters (0–8), and susceptible to hunger cues (>7) or not susceptible to hunger cues (0–7).
Energy expenditure measurement
The 24-h EE was measured in a whole-room indirect calorimeter (respiratory chamber) during energy balance and after at least 4 days of weight stability, as previously described in detail 3, 36. The prescribed energy intake for the 24 hours in the respiratory chamber was calculated using unit-specific equations derived to achieve energy balance in this setting37. Four meals were provided at 8:00, 11:00, 16:00, and 19:00 through an airlock. Subjects were instructed to eat all food within 30 minutes and to return any uneaten food to metabolic kitchen for adjustment of intake calories. Carbon dioxide production, oxygen consumption, respiratory quotient (RQ), and the rate of EE calculated by the Lusk’s equation38 were measured continuously, calculated for each 15-minute interval, averaged, and then extrapolated to the 24-hour-interval. Spontaneous physical activity (SPA) was measured by a radar system based on the Doppler Effect and expressed as percentage of time in motion. Sleeping EE was defined as the average EE between 01:00 and 05:00 AM during which SPA was less than 1.5% 7. The EE in the inactive state (EE0 activity) was calculated as the intercept of the regression line between EE and SPA between 11:00 and 01:00 39. The awake and fed thermogenesis (AFT), as a measure of the thermic effect of food and the energy cost of being awake, was calculated as the difference between EE0 activity and sleeping EE 39.
Statistical Analysis
The primary outcome of this study was to assess the relationships between 24-h EE and TFEQ constructs, while the secondary outcome was to assess differences in these relationships according to gender and ethnicity. Alpha was set at 0.05 and 2-sided p-values were reported. All analyses were preplanned and performed using SAS Enterprise Guide (version 7.1) and SPSS (version 25). Normally distributed data are presented as mean±SD, while skewed data are presented as median with interquartile range (IQR). The Chi-squared test was used to assess gender differences across ethnicities. Gender and ethnic differences on average TFEQ scores (Δ) were evaluated by Mann-Whitney U test and Kruskal-Wallis H test, respectively. As there were no differences between TFEQ scores of Blacks, Whites, Hispanics, and mixed ethnicity, these individuals were merged in one group (n=147) and compared to Native Americans (n=160). Two-way ANOVA was used to test the interaction between gender and ethnicity on TFEQ scores. Levene’s test was used to test the homogeneity of variances between groups and, in case of heterogeneous variances, Welch’s ANOVA was conducted in place of ANOVA. Residuals of 24-h EE (adjusted 24-h EE) after adjustments for age, gender, ethnicity, FFM, and fat mass (FM) were calculated via linear regression analysis. Spearman’s rank order correlation was used to quantify associations between TFEQ scores, 24-h EE, and residuals of 24-h EE. Confirmatory analyses were also performed after adjusting 24-h EE for SPA, and results were unchanged so only findings using unaltered/residuals 24-h EE are reported. Linear regression models were used to evaluate the associations between 24-h EE and TFEQ scores, while testing for gender and ethnic differences by including the respective interactions terms. Logistic regression analyses were also performed to assess associations between 24-h EE and subgroups (restrained vs unrestrained, disinhibited vs non-disinhibited, and susceptible to hunger cues vs not susceptible) obtained by dichotomizing TFEQ dimensions using established cutoffs22.
Results
Demographic, anthropometric, metabolic, and eating behavior characteristics of the study population are shown in Table 1. Participants were in energy balance during the 24-h EE (mean deviation=0.5%), and the average RQ (=0.86) was similar to the FQ of the balanced diet provided (=0.87)40. On average, women had higher BMI, percent body fat, and FM, but were shorter, had less FFM and lower adjusted 24-h EE (β=−229 kcal/day, 95% CI: −309 to −148, p=5×10−8) compared to men. In terms of ethnic differences, Native Americans had higher body weight, BMI, percent body fat, FM, absolute (β=+219 kcal/day, CI: 136 to 302, p=4×10−7) and adjusted (β=+94 kcal/day, CI: 48 to 139, p=7×10−5) 24-h EE as compared to non-Native Americans. Frequency of genders was similar across ethnic groups (p=0.73).
Table 1.
Participant demographic, anthropometric, metabolic, and eating behavior characteristics in men (n = 201), women (n = 106) and in Native Americans (n = 160) and Other Ethnicities (n = 147)
| Variable | All | Men | Women | Native Americans | Other Ethnicities | Restrained eaters |
|---|---|---|---|---|---|---|
| n | 307 | 201 | 106 | 160 | 147# | 59 |
| Age (years) | 35.1 ± 9.7 | 35.6 ± 9.6 | 34.2 ± 9.9 | 34.2 ± 8.8 | 36.0 ± 10.6 | 35.8 ± 9.8 |
| Body weight (kg) | 92.1 ± 22.4 | 91.9 ± 22.0 | 92.3 ± 23.2 | 95.1 ± 22.5 | 88.8 ± 21.9* | 88.6 ± 20.9 |
| Height (cm) | 170 ± 9 | 175 ± 7 | 163 ± 5* | 169 ± 8 | 172 ± 9* | 169 ± 10 |
| BMI (kg/m2) | 31.8 ± 7.9 | 30.2 ± 7.1 | 34.9 ± 8.4* | 33.4 ± 7.9 | 30.0 ± 7.5* | 31.3 ± 7.4 |
| Body fat (%) | 30.7 ± 9.1 | 26.3 ± 7.1 | 39.2 ± 5.9* | 32.7 ± 8.0 | 28.6 ± 9.7* | 30.6 ± 10.0 |
| Fat mass (kg) | 29.4 ± 13.5 | 25.3 ± 11.8 | 37.2 ± 13.3* | 32.0 ± 13.1 | 26.6 ± 13.5* | 27.8 ± 12.6 |
| Fat free mass (kg) | 62.7 ± 12.5 | 66.7 ± 11.6 | 55.1 ± 10.6* | 63.1 ± 12.9 | 62.2 ± 12.2 | 60.8 ± 13.5 |
| Fasting glucose (mg/dL) | 93.4 ± 8.5 | 93.3 ± 8.5 | 93.5 ± 8.7 | 93.2 ± 9.5 | 93.7 ± 7.4 | 94.9 ± 9.2 |
| 2-h glucose (mg/dL) Respiratory chamber |
124 ± 30 | 122 ± 29 | 128 ± 31 | 127 ± 30 | 120 ± 29 | 126 ± 31 |
| 24-h INTAKE (kcal/day) | 2265 ± 344 | 2367 ± 292 | 2072 ± 354* | 2276 ± 347 | 2254 ± 342 | 2176 ± 333 |
| 24-h EE (kcal/day) | 2278 ± 384 | 2382 ± 370 | 2080 ± 330* | 2383 ± 377 | 2164 ± 359* | 2177 ± 391 |
| 24-h RQ (ratio) | 0.86 ± 0.04 | 0.85 ± 0.04 | 0.86 ± 0.04 | 0.85 ± 0.04 | 0.86 ± 0.04 | 0.86 ± 0.04 |
| 24-h energy balance (kcal/day) | −13 ± 286 | −15 ± 283 | −8 ± 293 | −107 ± 268 | 91 ± 271* | −1 ± 244 |
| 24-h energy balance (% of 24-h EE) | 0.5 ± 12.6 | 0.6 ± 12.0 | 0.4 ± 13.8 | −3.8 ± 11.2 | 5.2 ± 12.4* | 1.1 ± 11.5 |
| Sleeping EE (kcal/day) | 1695 ± 271 | 1764 ± 259 | 1565 ± 244* | 1736 ± 273 | 1652 ± 262* | 1652 ± 280 |
| Spontaneous physical activity (%) | 7.0 ± 4.5 | 6.1 ± 5.7 | 6.7 ± 4.9 | 7.2 ± 5.3 | 6.1 ± 4.5 | 6.0 ± 3.9 |
| EE0 activity (kcal/14∙hrs) | 1320 ± 219 | 1386 ± 207 | 1195 ± 187* | 1339 ± 228 | 1300 ± 209 | 1262 ± 222 |
| Awake and fed thermogenesis (kcal/14∙hrs) | 331 ± 119 | 357 ± 121 | 282 ± 98* | 327 ± 128 | 336 ± 108 | 303 ± 120 |
|
TFEQ |
||||||
| Cognitive Restraint (score) | 6.9 ±4.2 | 6.4 ± 4.0 | 7.9 ± 4.2* | 6.7 ± 3.8 | 7.2 ± 4.5 | |
| Restrained eaters (n) $ | 59 (19%) | 32 (16%) | 27 (25%)* | 27 (17%) | 32 (22%) | |
| Disinhibition (score) |
5.0 ± 3.3 | 4.3 ± 2.8 | 6.3 ± 3.7* | 5.2 ± 2.8 | 4.7 ± 3.8* | |
| Disinhibited eaters (n) $ | 52 (17%) | 21 (10%) | 31 (29%)* | 22 (14%) | 30 (20%) | |
| Hunger Cues (score) |
4.7 ± 3.3 | 4.5 ± 3.2 | 5.0 ± 3.4 | 4.7 ± 3.1 | 4.6 ± 3.5 | |
| Susceptible to hunger cues (n) $ | 64 (21%) | 40 (20%) | 24 (23%) | 30 (19%) | 34 (23%) |
Data are presented as mean±SD.
boldface indicates significant differences (p < 0.05) between groups as assessed by Students t-test, Mann-Whitney U test (for TFEQ scores), or Chi-squared test.
77 Whites, 27 Blacks, 22 Hispanics, and 21 subjects of mixed ethnicity.
Individuals were classified according to TFEQ cutoff values as: restrained (score>10), disinhibited (>8), and susceptible to hunger cues (>7).
When participants were categorized according to TFEQ cutoffs (Table 1), restrained and disinhibited subjects were more likely to be women (p=0.04 and p=3×10−5, respectively) with no differences by ethnic group (p=0.28 and p=0.12, respectively), BMI (p=0.62), or body fat (p=0.95). There were no gender (p=0.57) or ethnic (p=0.35) differences in scores of susceptibility to hunger cues. On average, restrained subjects had lower 24-h EE (Δ=−126 kcal/day, CI: −234 to −17, p=0.02) and AFT (Δ=−34 kcal/14∙hrs, CI: −69 to −1, p=0.05) but not sleeping EE (p=0.11) or SPA (p=0.65), while subjects who were disinhibited (Δ=+97 kcal/day, CI: −18 to 211, p=0.09) and susceptible to hunger cues (Δ=+95 kcal/day, CI: −10 to 201, p=0.08) tended to have higher 24-h EE.
TFEQ scores: gender and ethnic differences
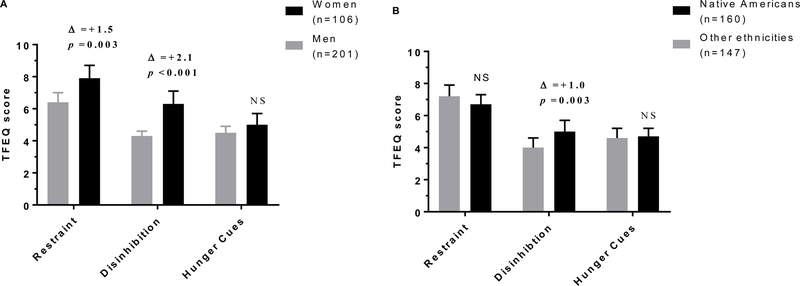
The average TFEQ scores of cognitive restraint, disinhibition and hunger between genders and ethnic groups are shown in Figure 1, respectively. Cognitive restraint scores were, on average, 23% higher in women compared to men (mean absolute difference, Δ=+1.5, 95% CI: 0.5 to 2.5, p=0.003), with no ethnic differences (p=0.35). No interaction between gender and ethnicity was observed for cognitive restraint (p=0.12). Disinhibition scores were higher in women (Δ=+2.1, 95% CI: 1.3 to 2.8, p=10−6) and Native Americans (Δ=+1.0, 95% CI: 0.1 to 2.0, p=0.003) compared to men and non-Native Americans, respectively, without any interaction between gender and ethnicity (p=0.07). Cognitive restraint and disinhibition scores were not related in the entire cohort (p=0.74), or separately in women (p=0.17) or men (p=0.62). No gender (p=0.18) or ethnic (p=0.52) differences were observed for hunger ratings.
Figure 1.
(A) Women had higher cognitive restraint and disinhibition scores as compared to men, and (B) Native Americans had higher disinhibition scores as compared to other ethnicities. Error bars represent 95% confidence interval of the mean. Δ: mean difference between groups.
Relationships between TFEQ scores and 24-h EE
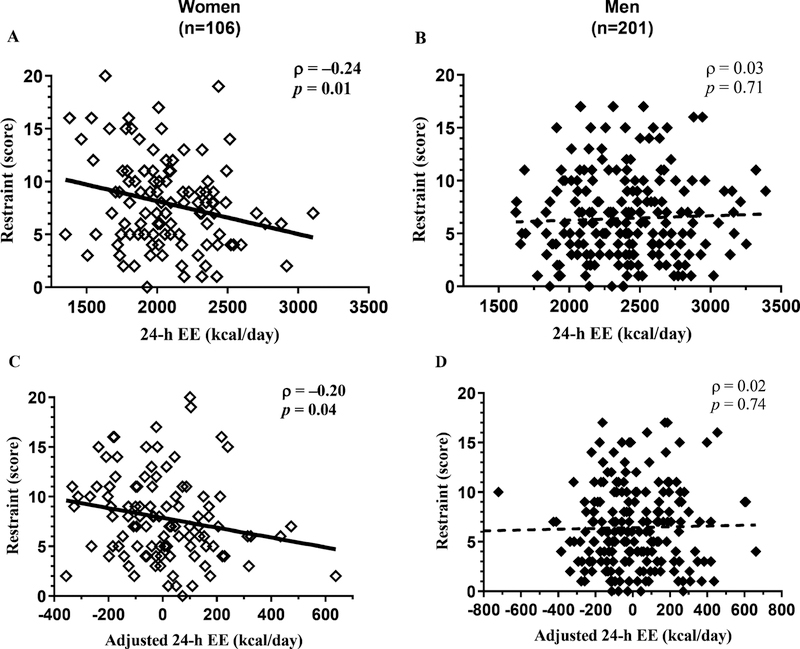
A higher 24-h EE tended to be associated with lower cognitive restraint (ρ= −0.10, p=0.07). However, this inverse relationship was only observed in women (ρ= −0.20, p=0.04, Figure 2A) but not in men (ρ =0.03, p=0.71, Figure 2B; interaction term p=0.01). Similar results were obtained for 24-h EE after adjustment for its known determinants (e.g., body composition, gender, etc.), such that a relatively higher 24-h EE was associated with lower cognitive restraint in women (ρ= −0.24, p=0.01, Figure 2C) but not in men (ρ=0.02, p=0.74, Figure 2D). Both lower restraint (p=0.03) and greater disinhibition (p=0.002) scores were found to be independent predictors of higher 24-h EE in women. There was no ethnic difference in the relationship between 24-h EE and cognitive restraint (interaction term p=0.58). There were no associations between restraint scores and BMI (p=0.26) or body fat (p=0.09).
Figure 2.
Higher 24-h EE was associated with lower dietary restraint in women (A) but not men (B). Higher residual 24-h EE (adjusted for age, gender, ethnicity, FFM and FM) was associated with lower dietary restraint in women (C) but not men (D). In each panel, the Spearman’s correlation coefficient (ρ) is reported along with its significance (p). The best-fit line is displayed in each panel.
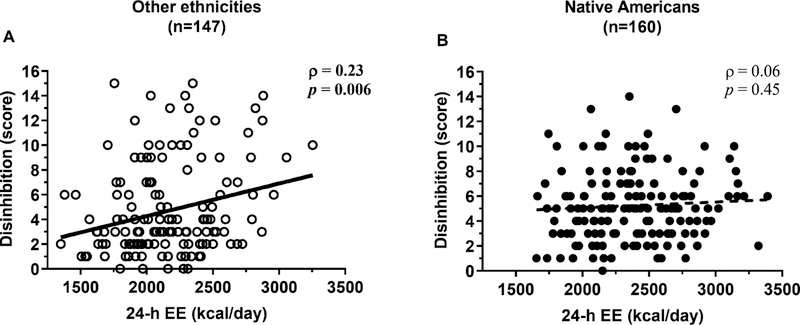
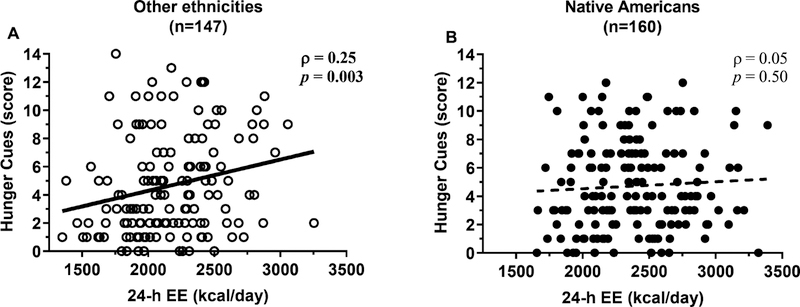
A greater 24-h EE was associated with higher disinhibition (ρ=0.20, p=0.001) in the whole cohort, and this relationship did not differ between genders (interaction term p=0.44). However, this direct association was driven by non-Native Americans (ρ=0.23, p=0.006, Figure 3A) as no relationship between 24-h EE and disinhibition scores was observed in Native Americans (ρ=0.06, p=0.45, Figure 3B; interaction term p=0.03). Higher hunger cue scores were associated with higher 24-h EE (ρ=0.16, p=0.004) with no differences by gender (interaction term p=0.58). This positive association between 24-h EE and hunger scores was primarily present in subjects not of Native American descent (ρ=0.25, p=0.003, Figure 4A) such as Blacks (ρ=0.46, p=0.02, n=27), but not observed in Native Americans (ρ=0.05, p=0.50, Figure 4B; interaction term p=0.09). Similar positive associations for disinhibition and hunger scores were also found for BMI and body fat, as well as for 24-h EE components including sleeping EE, diet-induced thermogenesis and physical activity-related EE, in ethnicities other than Native Americans but, again, not in Native Americans (data not shown). No associations were found between restraint or disinhibition scores and SPA (all p>0.20). In a multivariate model, higher disinhibition scores (β=+22 kcal/day, p=0.01) and lower restraint scores (β=−11 kcal/day, p=0.04), but not hunger scores (p=0.93), were independent predictors of 24-h EE in the whole cohort. Similar results were obtained in women (disinhibition: β=+33 kcal/day, p=0.006; restraint: β=−18 kcal/day, p=0.02), whereas only disinhibition (β=+55 kcal/day, p<0.001), but not restraint (p=0.91) or hunger (p=0.24), was a predictor of 24-h EE in men.
Figure 3.
Higher 24-h EE was associated with higher dietary disinhibition in non-Native Americans (A) but not in Native Americans (B). In both panels, the Spearman’s correlation coefficient (ρ) is reported along with its significance (p). The best-fit line is displayed in both panels.
Figure 4.
Higher 24-EE was associated with greater susceptibility to hunger cues in non-Native Americans (A) but not in Native Americans (B). In both panels, the Spearman’s correlation coefficient (ρ) is reported along with its significance (p). The best-fit line is displayed in both panels.
Discussion
We examined the relationships between daily energy needs as quantified by 24-h EE measured during weight maintenance and psychological constructs related to eating behavior as assessed by the TFEQ across genders and ethnicities in a large, ethnically diverse cohort. Individuals with higher daily EE reported higher levels of dietary disinhibition, greater susceptibility to hunger cues, and possessed less cognitive restraint of eating, suggesting that cognitive aspects of eating behavior may be partly determined by the underlying metabolism. However, these associations were highly dependent upon gender and ethnicity. Specifically, we found an inverse relationship between 24-h EE and cognitive restraint only in women, who had a relatively lower EE compared to men and were more likely to be restrained eaters, suggesting that cognitive restraint could be a female-specific learned behavior in response to lower daily energy needs. Overall, higher 24-h EE was associated with greater disinhibited eating behavior scores and increased susceptibility to hunger cues; however, these associations were not present in Native Americans and were primarily observed in other ethnicities. Thus, the putative behavioral effects exerted by EE on psychological constructs related to eating behavior may depend on gender, ethnic, or cultural differences. These gender and ethnic differences may partly explain the contradictory relationships reported between EE and future weight gain in different ethnic groups, as EE might alter eating behavior differentially determining daily food intake and ultimately long-term weight change.
The main aim of the present study was to better understand how EE, recently shown to be the foremost driver of energy intake in several independent studies 1, 12, 14, 27, 41, may regulate food intake via a putative effect on eating behavior as assessed by the psychological constructs of a well-validated research tool (TFEQ). We observed moderate associations between lower EE and higher cognitive dietary restraint, as well as between higher EE and both higher dietary disinhibition and susceptibility to hunger cues. Our current study provides further evidence on the potential metabolic mechanisms influencing these constructs of eating behavior. Energy expenditure, which has recently been proposed to be the main driver of energy intake in humans in multiple independent studies1, 9, 12–14, may influence psychological constructs of eating behaviors, thereby predisposing individuals to overeating 11. Specifically, an increased EE might signify an energy-sensing hunger signal that could alter eating behavior and may lead to greater-than-necessary intake 9, 42.
Our results show that the relationship between 24-h EE and cognitive restraint is contingent upon gender and, more importantly, this association was not driven by body size, as neither BMI nor body fat were associated with restraint scores. In line with previous literature, in our current study women have higher levels of cognitive restraint as compared to men19, 43. This may be due to the higher rates of dieting, as well as the widespread cultural emphasis on a thin ideal body image in women18, 44. Our finding that 24-h EE, even after accounting for differences in body size and composition, was inversely associated with restraint in women supports the results from a previous study in lean women, which showed that restrained women had lower energy intake and relatively lower EE compared to unrestrained women18. Similarly, restrained eaters with and without a history of dieting had lower resting EE 45, suggesting that restrained eating may be a behavioral adaptation to a lower EE which supports the hypothesis of energy sensing mechanisms that might drive hunger and appetite6, 16, 46. Alternatively, higher dietary restraint may be associated with habitual undereating and negative energy balance that could partly explain the lower EE observed in restrained eaters, although our measures of 24-h EE were obtained in energy balance after at least 4 days of weight maintenance. Nevertheless, given the cross-sectional nature of EE-restraint relationship, future studies including long-term assessments of eating behavior, energy intake and EE are warranted to elucidate the causality of this relationship.
Ethnic and cultural differences may account for difference in psychological constructs of eating behavior and might partly explain the previously reported conflicting associations between EE and weight change2, 3, 7, 39, where a relatively lower EE is associated with weight gain in Native Americans but not in other ethnicities. In our current study, a higher EE was associated both with greater dietary disinhibition and with increased susceptibility to hunger cues in those without self-reported Native American heritage. This might provide an explanation for the positive association between higher EE and long-term weight gain reported in populations such as Nigerian adults2, as in the current study higher 24-h EE was associated with greater hunger scores in Blacks. In contrast, the absence of associations between 24-h EE and both disinhibition and hunger cues in Native Americans could signify that a higher EE may not be associated with abnormal eating behavior in people of Native American heritage and possibly explain why, in this population, a lower EE is instead associated with weight gain3, 7 perhaps due to sustained positive daily energy balance caused by a deficit in 24-h EE. It is unclear whether the distinct relationships observed in different ethnicities are due to cultural, environmental, or genetic differences. As dietary disinhibition and hunger cues are consistently associated with overeating14, 24, 28, 33, it is possible that in certain populations the mechanism of overconsumption and sustained positive energy balance may occur via an effect of an internal sensing of higher EE on eating behaviors. Nevertheless, as no measures of free-living energy intake were available in the current study, future studies including actual measures of food intake and habitual eating behavior are warranted to demonstrate this energy-sensing mechanism.
Our study has several strengths including a large sample size, a highly diverse ethnic composition, and precise measurements of EE for 24 hours during energy balance and weight maintenance which likely reflects daily energy needs; nevertheless, it also has some important limitations including multiple testing which might have led to an inflated Type I error rate. Nonetheless, the main results of our primary research questions (i.e., the relationships between 24-h EE and the three TFEQ constructs) have p-values that meet the stringent Bonferroni’s threshold of 0.0167 calculated based on three independent tests (e.g., the unadjusted p-values for 24-h EE vs. disinhibition and hunger scores were 0.001 and 0.004, respectively). Nevertheless, as this study represents an exploratory analysis of the relationships between 24-h EE and TFEQ constructs, the currents results need to be confirmed in confirmatory studies done in ethnically cohorts, including the formal validation of TFEQ in Native Americans which is currently lacking. The average BMI was in the obese range (≥ 30 kg/m2) which may have led to a bias in quantifying the associations between EE and eating behavior constructs that can be confounded or blunted by adiposity. Yet, in this overweight cohort, we were still able to detect moderate associations for EE and replicate the previously reported association observed in restrained women18, without observing any association between restraint scores and measures of body size such as BMI or body fat. Further, our study lacks measures of habitual physical activity, which is an established determinant of eating behaviors and food intake47–49. Further, we did not have measure of energy intake in free-living conditions and were therefore not able to demonstrate that TFEQ constructs related to eating behavior may influence energy intake in real-life settings, although previous studies have shown consistent associations between TFEQ scores and energy intake14, 50, 51. Lastly, the recruiting method may have biased our findings based on cross-sectional associations, thus the casual relationships between EE and TFEQ constructs could be ascertained with current data that also do not include a control group. Therefore, intervention studies including manipulation of EE and subsequent measurement of eating behavior in intervention vs. control groups are warranted to elucidate the causality of these relationships. However, the present analysis that constitutes the largest study assessing the relationships between psychological constructs related to eating behaviors by the widely used TFEQ and EE using 24-h measures by a highly precise and reproducible method of indirect calorimetry 3, will serve as a basis to develop future studies that should take into account both gender and ethnicity as important factors in their design.
In summary, we observed that individuals with higher 24-h EE reported lower cognitive restraint, higher disinhibited eating, and greater susceptibility to hunger cues, all features likely associated with a propensity to overeating. However, these relationships were dependent upon gender and ethnicity. That is, women with relatively lower 24-h EE were more likely to be restrained eaters suggesting that dietary restraint may be a learned behavior to lower daily energy needs. Furthermore, higher 24-h EE was associated with self-reported increased disinhibited eating and susceptibility to hunger cues, but these associations varied by ethnicity. Our present results support recent findings implicating EE as the main determinant of energy intake, and suggest that EE may influence eating behavior in humans. Indeed, the observed associations between 24-h EE and psychological constructs related to eating behaviors may constitute one of the energy sensing mechanisms, which ultimately determines the propensity of one individual to weight gain.
Acknowledgements
Clinical Trial Registration Numbers (clinicaltrials.gov): NCT00523627, NCT00342732, NCT00856609, NCT01224704, NCT01237093, NCT00687115
Sources of Support: This study was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health.
The authors thank the volunteers who participated in our studies. They also thank the clinical staff of the Phoenix Epidemiology and Clinical Research Branch for conducting the examinations.
Funding
This study was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases.
Abbreviations:
- AFT
awake and fed thermogenesis
- EE
Energy Expenditure
- FFM
fat free mass
- FM
fat mass
- SPA
spontaneous physical activity
- TFEQ
three-factor eating questionnaire
Footnotes
Disclosure statement: The authors have nothing to disclose.
Prior presentation
This study was presented in abstract form at the 2015 Obesity Week, Los Angeles CA, November 2–7, 2015.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Piaggi P, Thearle MS, Krakoff J, Votruba SB. Higher Daily Energy Expenditure and Respiratory Quotient, Rather Than Fat-Free Mass, Independently Determine Greater ad Libitum Overeating. The Journal of clinical endocrinology and metabolism 2015; 100(8): 3011–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luke A, Durazo-Arvizu R, Cao G, Adeyemo A, Tayo B, Cooper R. Positive association between resting energy expenditure and weight gain in a lean adult population. The American journal of clinical nutrition 2006; 83(5): 1076–81. [DOI] [PubMed] [Google Scholar]
- 3.Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. The Journal of clinical investigation 1986; 78(6): 1568–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buscemi S, Verga S, Caimi G, Cerasola G. Low relative resting metabolic rate and body weight gain in adult Caucasian Italians. International journal of obesity (2005) 2005; 29(3): 287–91. [DOI] [PubMed] [Google Scholar]
- 5.Seidell JC, Muller DC, Sorkin JD, Andres R. Fasting respiratory exchange ratio and resting metabolic rate as predictors of weight gain: the Baltimore Longitudinal Study on Aging. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity 1992; 16(9): 667–74. [PubMed] [Google Scholar]
- 6.Piaggi P, Vinales KL, Basolo A, Santini F, Krakoff J. Energy expenditure in the etiology of human obesity: spendthrift and thrifty metabolic phenotypes and energy-sensing mechanisms. Journal of endocrinological investigation 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piaggi P, Thearle MS, Bogardus C, Krakoff J. Lower energy expenditure predicts long-term increases in weight and fat mass. The Journal of clinical endocrinology and metabolism 2013; 98(4): E703–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tataranni PA, Harper IT, Snitker S, Del Parigi A, Vozarova B, Bunt J et al. Body weight gain in free-living Pima Indians: effect of energy intake vs expenditure. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity 2003; 27(12): 1578–83. [DOI] [PubMed] [Google Scholar]
- 9.Blundell JE, Caudwell P, Gibbons C, Hopkins M, Naslund E, King N et al. Role of resting metabolic rate and energy expenditure in hunger and appetite control: a new formulation. Disease models & mechanisms 2012; 5(5): 608–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Economos CD, Hatfield DP, King AC, Ayala GX, Pentz MA. Food and physical activity environments: an energy balance approach for research and practice. American journal of preventive medicine 2015; 48(5): 620–9. [DOI] [PubMed] [Google Scholar]
- 11.Blundell JE, Dalton M, Gibbons C. Food intake and appetite in the aetiology of obesity. Advanced Nutrition and Dietetics in Obesity 2017: 97.
- 12.Caudwell P, Finlayson G, Gibbons C, Hopkins M, King N, Naslund E et al. Resting metabolic rate is associated with hunger, self-determined meal size, and daily energy intake and may represent a marker for appetite. The American journal of clinical nutrition 2013; 97(1): 7–14. [DOI] [PubMed] [Google Scholar]
- 13.Weise CM, Hohenadel MG, Krakoff J, Votruba SB. Body composition and energy expenditure predict ad-libitum food and macronutrient intake in humans. International journal of obesity (2005) 2014; 38(2): 243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McNeil J, Lamothe G, Cameron JD, Riou ME, Cadieux S, Lafreniere J et al. Investigating predictors of eating: is resting metabolic rate really the strongest proxy of energy intake? The American journal of clinical nutrition 2017; 106(5): 1206–1212. [DOI] [PubMed] [Google Scholar]
- 15.Lam YY, Ravussin E. Variations in energy intake: it is more complicated than we think. The American journal of clinical nutrition 2017; 106(5): 1169–1170. [DOI] [PubMed] [Google Scholar]
- 16.Dulloo AG, Jacquet J, Miles-Chan JL, Schutz Y. Passive and active roles of fat-free mass in the control of energy intake and body composition regulation. Eur J Clin Nutr 2017; 71(3): 353–357. [DOI] [PubMed] [Google Scholar]
- 17.Basolo A, Votruba SB, Heinitz S, Krakoff J, Piaggi P. Deviations in Energy Sensing Predict Long-term Weight Change in Overweight Native Americans. Metabolism: clinical and experimental 2018. [DOI] [PMC free article] [PubMed]
- 18.Tuschl RJ, Platte P, Laessle RG, Stichler W, Pirke KM. Energy expenditure and everyday eating behavior in healthy young women. The American journal of clinical nutrition 1990; 52(1): 81–6. [DOI] [PubMed] [Google Scholar]
- 19.Provencher V, Drapeau V, Tremblay A, Despres JP, Bouchard C, Lemieux S. Eating behaviours, dietary profile and body composition according to dieting history in men and women of the Quebec Family Study. The British journal of nutrition 2004; 91(6): 997–1004. [DOI] [PubMed] [Google Scholar]
- 20.Bohrer BK, Forbush KT, Hunt TK. Are common measures of dietary restraint and disinhibited eating reliable and valid in obese persons? Appetite 2015; 87: 344–51. [DOI] [PubMed] [Google Scholar]
- 21.Allison DB, Kalinsky LB, Gorman BS. A comparison of the psychometric properties of three measures of dietary restraint. Psychological Assessment 1992; 4(3): 391–398. [Google Scholar]
- 22.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. Journal of Psychosomatic Research 1985; 29(1): 71–83. [DOI] [PubMed] [Google Scholar]
- 23.Lawson OJ, Williamson DA, Champagne CM, DeLany JP, Brooks ER, Howat PM et al. The association of body weight, dietary intake, and energy expenditure with dietary restraint and disinhibition. Obesity research 1995; 3(2): 153–61. [DOI] [PubMed] [Google Scholar]
- 24.Goulet J, Provencher V, Piche ME, Lapointe A, John Weisnagel S, Nadeau A et al. Relationship between eating behaviours and food and drink consumption in healthy postmenopausal women in a real-life context. The British journal of nutrition 2008; 100(4): 910–7. [DOI] [PubMed] [Google Scholar]
- 25.Graham AL, Gluck ME, Votruba SB, Krakoff J, Thearle MS. Perseveration augments the effects of cognitive restraint on ad libitum food intake in adults seeking weight loss. Appetite 2014; 82: 78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laessle RG, Kikker S. Resting metabolic rate in young women classified as restrained or unrestrained eaters. Physiology & Behavior 2008; 95(3): 542–543. [DOI] [PubMed] [Google Scholar]
- 27.Hopkins M, Finlayson G, Duarte C, Gibbons C, Johnstone AM, Whybrow S et al. Biological and psychological mediators of the relationships between fat mass, fat-free mass and energy intake. International journal of obesity (2005) 2018. [DOI] [PubMed]
- 28.Finlayson G, Bordes I, Griffioen-Roose S, de Graaf C, Blundell JE. Susceptibility to overeating affects the impact of savory or sweet drinks on satiation, reward, and food intake in nonobese women. The Journal of nutrition 2012; 142(1): 125–30. [DOI] [PubMed] [Google Scholar]
- 29.Hays NP, Roberts SB. Aspects of Eating Behaviors “Disinhibition” and “Restraint” Are Related to Weight Gain and BMI in Women. Obesity 2008; 16(1): 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bryant EJ, King NA, Blundell JE. Disinhibition: its effects on appetite and weight regulation. Obesity reviews : an official journal of the International Association for the Study of Obesity 2008; 9(5): 409–19. [DOI] [PubMed] [Google Scholar]
- 31.Finlayson G, Cecil J, Higgs S, Hill A, Hetherington M. Susceptibility to weight gain. Eating behaviour traits and physical activity as predictors of weight gain during the first year of university. Appetite 2012; 58(3): 1091–8. [DOI] [PubMed] [Google Scholar]
- 32.Langlois F, Langlois MF, Carpentier AC, Brown C, Lemieux S, Hivert MF. Ghrelin levels are associated with hunger as measured by the Three-Factor Eating Questionnaire in healthy young adults. Physiol Behav 2011; 104(3): 373–7. [DOI] [PubMed] [Google Scholar]
- 33.French SA, Mitchell NR, Wolfson J, Finlayson G, Blundell JE, Jeffery RW. Questionnaire and laboratory measures of eating behavior. Associations with energy intake and BMI in a community sample of working adults. Appetite 2014; 72: 50–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferraro R, Boyce VL, Swinburn B, De Gregorio M, Ravussin E. Energy cost of physical activity on a metabolic ward in relationship to obesity. The American journal of clinical nutrition 1991; 53(6): 1368–71. [DOI] [PubMed] [Google Scholar]
- 35.Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2013; 36(Supplement 1): S67–S74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thearle MS, Pannacciulli N, Bonfiglio S, Pacak K, Krakoff J. Extent and determinants of thermogenic responses to 24 hours of fasting, energy balance, and five different overfeeding diets in humans. The Journal of clinical endocrinology and metabolism 2013; 98(7): 2791–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abbott WG, Howard BV, Christin L, Freymond D, Lillioja S, Boyce VL et al. Short-term energy balance: relationship with protein, carbohydrate, and fat balances. The American journal of physiology 1988; 255(3 Pt 1): E332–7. [DOI] [PubMed] [Google Scholar]
- 38.Lusk G ANIMAL CALORIMETRY Twenty-Fourth Paper. ANALYSIS OF THE OXIDATION OF MIXTURES OF CARBOHYDRATE AND FAT. Journal of Biological Chemistry 1924; 59(1): 41–42. [Google Scholar]
- 39.Piaggi P, Krakoff J, Bogardus C, Thearle MS. Lower “awake and fed thermogenesis” predicts future weight gain in subjects with abdominal adiposity. Diabetes 2013; 62(12): 4043–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kruger R, De Bray JG, Beck KL, Conlon CA, Stonehouse W. Exploring the Relationship between Body Composition and Eating Behavior Using the Three Factor Eating Questionnaire (TFEQ) in Young New Zealand Women. Nutrients 2016; 8(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hopkins M, Finlayson G, Duarte C, Whybrow S, Ritz P, Horgan GW et al. Modelling the associations between fat-free mass, resting metabolic rate and energy intake in the context of total energy balance. International journal of obesity (2005) 2016; 40(2): 312–8. [DOI] [PubMed] [Google Scholar]
- 42.Blundell JE, Gibbons C, Caudwell P, Finlayson G, Hopkins M. Appetite control and energy balance: impact of exercise. Obesity reviews : an official journal of the International Association for the Study of Obesity 2015; 16 Suppl 1: 67–76. [DOI] [PubMed] [Google Scholar]
- 43.Ernst B, Wilms B, Thurnheer M, Schultes B. Eating behaviour in treatment-seeking obese subjects - Influence of sex and BMI classes. Appetite 2015; 95: 96–100. [DOI] [PubMed] [Google Scholar]
- 44.Perez M, Joiner TE Jr. Body image dissatisfaction and disordered eating in black and white women. The International journal of eating disorders 2003; 33(3): 342–50. [DOI] [PubMed] [Google Scholar]
- 45.Platte P, Wurmser H, Wade SE, Mecheril A, Pirke KM. Resting metabolic rate and diet‐induced thermogenesis in restrained and unrestrained eaters. International Journal of Eating Disorders 1996; 20(1): 33–41. [DOI] [PubMed] [Google Scholar]
- 46.Dulloo AG. Collateral fattening: When a deficit in lean body mass drives overeating. Obesity (Silver Spring) 2017; 25(2): 277–279. [DOI] [PubMed] [Google Scholar]
- 47.Shook RP, Hand GA, Drenowatz C, Hebert JR, Paluch AE, Blundell JE et al. Low levels of physical activity are associated with dysregulation of energy intake and fat mass gain over 1 year. The American journal of clinical nutrition 2015; 102(6): 1332–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mayer J, Roy P, Mitra KP. Relation between caloric intake, body weight, and physical work: studies in an industrial male population in West Bengal. The American journal of clinical nutrition 1956; 4(2): 169–75. [DOI] [PubMed] [Google Scholar]
- 49.Beaulieu K, Hopkins M, Blundell J, Finlayson G. Does Habitual Physical Activity Increase the Sensitivity of the Appetite Control System? A Systematic Review. Sports Med 2016; 46(12): 1897–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leblanc V, Provencher V, Begin C, Gagnon-Girouard MP, Corneau L, Tremblay A et al. Associations between eating patterns, dietary intakes and eating behaviors in premenopausal overweight women. Eating behaviors 2012; 13(2): 162–5. [DOI] [PubMed] [Google Scholar]
- 51.de Lauzon B, Romon M, Deschamps V, Lafay L, Borys JM, Karlsson J et al. The Three-Factor Eating Questionnaire-R18 is able to distinguish among different eating patterns in a general population. The Journal of nutrition 2004; 134(9): 2372–80. [DOI] [PubMed] [Google Scholar]