Highlights
-
•
The RILA assay is the leading candidate biomarker for radiotherapy toxicity.
-
•
We describe work to standardise its use across multiple centres.
-
•
Patient factors including smoking and arthritis were found to affect RILA score.
-
•
RILA predicts acute breast pain but not other acute end-points.
-
•
This work establishes the basis for implementing the assay clinically.
Abstract
Predicting which patients will develop adverse reactions to radiotherapy is important for personalised treatment. Prediction will require an algorithm or nomogram combining clinical and biological data. The radiation-induced lymphocyte apoptosis (RILA) assay is the leading candidate as a biological predictor of radiotherapy toxicity. In this study we tested the potential of the assay for standardisation and use in multiple testing laboratories.
The assay was standardised and reproducibility determined using samples from healthy volunteers assayed concurrently in three laboratories in Leicester (UK), Mannheim (Germany) and Montpellier (France). RILA assays were performed on samples taken prior to radiotherapy from 1319 cancer patients enrolled in the REQUITE project at multiple centres. The patients were being treated for breast (n = 753), prostate (n = 506) or lung (n = 60) cancer.
Inter-laboratory comparisons identified several factors affecting results: storage time, incubation periods and type of foetal calf serum. Following standardisation, there was no significant difference in results between the centres. Significant differences were seen in RILA scores between cancer types (prostate > breast > lung), by smoking status (non-smokers > smokers) and co-morbidity with rheumatoid arthritis (arthritics > non-arthritics).
An analysis of acute radiotherapy toxicity showed as expected that RILA assay does not predict most end-points, but unexpectedly did predict acute breast pain. This result may elucidate the mechanism by which the RILA assay predicts late radiotherapy toxicity.
The work shows clinical trials involving multiple laboratory measurement of the RILA assay are feasible and the need to account for tumour type and other variables when applying to predictive models.
1. Introduction
Radiotherapy is an important modality for treating many cancers, but a minority of patients develop adverse reactions that may be long-lasting and lower quality-of-life. Predicting patients with increased risk for developing adverse reactions is of interest for personalised treatment because it would allow either modification of treatment or interventions to mitigate the risk of side effects.
Research aiming to develop predictive assays for radiotherapy adverse reactions involves approaches including proteomics [1], transcriptomics [2] and genomics [3]. Radiogenomic studies identified several replicated genetic associations with late reactions to radiotherapy, but those found so far only account for a small fraction of the genetic variance [4], [5], [6], [7], [8], [9].
Various assays for cellular radiosensitivity have been evaluated as predictive tests for late reactions, mainly using patient lymphocytes or skin fibroblasts [7], [10], [11], [12]. The approach with the best evidence for predictive value is the radiation-induced lymphocyte apoptosis (RILA) assay. In a prospective study of 399 patients with various cancers, individuals with low lymphocyte apoptosis were more likely to develop late toxicity, but there was no predictive value for acute toxicity [13]. Subsequent papers confirmed the finding of an inverse correlation between lymphocyte apoptosis and toxicity in cancers of the cervix, head and neck, prostate and breast [13], [14], [15], [16], [17], [18], [19], [20], [21]. A recent prospective multi-centre trial recruited 502 breast cancer patients from ten clinical centres and carried out the RILA assay in a single laboratory in Montpellier [22]. Patients with low RILA scores (bottom tertile) had higher toxicity, with the test having a high negative predictive value for low toxicity in patients with middle and upper tertile scores. A key-point was that RILA significantly predicted less toxicity with increasing values when taken as a continuous variable, leading to the integration of RILA in a multifactorial nomogram [23]. Larger datasets using uniform laboratory and clinical protocols are needed to incorporate RILA assay data into statistical models including the known prognostic patient, treatment and genetic factors. A barrier to collection of larger datasets is the lack of information on the transferability of the assay between laboratories. A previous study which compared the results from two different laboratories on 25 head and neck cancer patients found good agreement, but larger studies in more centres and cancer types are needed [24].
REQUITE is an EU-funded multi-centre observational study that has recruited >4400 patients with breast, lung or prostate cancer and is following them for at least two years to score radiotherapy side effects [25]. Three of the centres involved in REQUITE (ICM, Montpellier; UMM Mannheim and University of Leicester) carried out the RILA assay on recruited patients. The aims of the work described here were to: standardise the RILA protocol across three laboratories in different countries; identify experimental factors affecting RILA results; identify clinical/patient factors affecting data obtained using a standardised assay; and analyse relationships with acute toxicity.
2. Methods
2.1. Patient recruitment and ethics
Breast, prostate and non-small cell lung cancer patients were recruited according to inclusion and exclusion criteria defined by the REQUITE project [25]; further documentation available from www.requite.eu). All patients were to undergo radiotherapy with curative intent with no prior treatment except hormone therapy in some prostate cancer patients. Venepuncture was carried out prior to radiotherapy and peripheral blood collected in lithium heparin blood tubes (Becton Dickinson, 367526). Supplementary Table 1 summarises patient characteristics. Ethical approval was gained from regulatory authorities in each country and written informed consent received from each patient. Table 1 shows frequencies of acute radiotherapy side effects.
Table 1.
Frequency of acute radiotherapy toxicity. Change from baseline by two grades or higher at follow-up six weeks after end of radiotherapy, except where noted as G1 which is one grade higher than baseline. German patients were not asked questions on sexual health.
| Cancer site/end-point | End-point | Leicester | Mannheim | Montpellier | Combined |
|---|---|---|---|---|---|
| Breast | N | 180 | 142 | 382 | 635 |
| Pain | G1 33.5% | G1 24.8% | G1 21.5% | G1 24.8% | |
| Oedema | 0% | 0.7% | 1.6% | 0.9% | |
| Ulceration | 0% | 2.8% | 7.2% | 4.5% | |
| Erythema | 18.1% | 15.9% | 17.6% | 17.4% | |
| Hyperpigmentation | G1 1.1% | G1 48.3% | G1 10.3% | G1 15.5% | |
| Prostate | N | 196 | 52 | 216 | 430 |
| Rectal | Proctitis | 4.6% | 8.2% | 4.7% | 4.9% |
| Diarrhoea | 9.7% | 8.3% | 8.3% | 8.8% | |
| Rectal bleeding | 1.0% | 0% | 0.5% | 0.6% | |
| Flatus | 2.6% | 0% | 0% | 1.1% | |
| Urinary | Urinary obstruction | 0.5% | 0% | 5.6% | 2.8% |
| Urinary incontinence | 2.6% | 0% | 2.3% | 2.1% | |
| Urinary frequency | 12.2% | 6.3% | 3.2% | 7.5% | |
| Urinary urgency | 12.2% | 8.2% | 0.9% | 6.4% | |
| Urinary retention | 15.3% | 0% | 2.8% | 7.7% | |
| Sexual | Erectile dysfunction | 0% | N/A | 13.1% | 7.1% |
| Ejaculation disorder | 0% | N/A | 16.4% | 8.7% | |
| Orgasmic dysfunction | 0% | N/A | 19.2% | 10.1% | |
| Libido | 0% | N/A | 18.1% | 9.5% | |
| Skin | Radiation dermatitis | 2.0% | 2.9% | 0.5% | 1.3% |
2.2. Radiation-induced lymphocyte apoptosis (RILA) assay
The initial protocol was from the ICM Montpellier as described elsewhere [13], [22], [26]. The final standardised operating procedure used is available as form RQ7 on request.
In brief, the protocol involves incubating whole blood in tissue culture medium for one day, followed by X-irradiation or sham treatment and a further two days of culture. Red cells were then lysed and cytotoxic T lymphocytes labelled with CD8 antibody and propidium iodide. Flow cytometry was used to define cells as apoptotic based on reduced PI staining, and RILA score calculated as the difference in percentage of apoptotic cells between irradiated and non-irradiated control. More detail is available in the Supplementary Methods, together with details of the pre-experiment inter-laboratory standardisation.
2.3. Statistics
Statistical analysis was performed using IBM SPSS Statistics 22. RILA assay scores were expressed as mean ± standard deviation. Correlation analyses were either performed using the Pearson product-moment correlation (r) or Spearman’s rank correlation (rho) tests. Regression analyses were logistic, multinomial or linear as appropriate. Mean differences were analysed using Student’s t or Kruskal-Wallis tests as appropriate.
3. Results
3.1. Effect of protocol time parameters
Using the standardised assay, data were collected for 1319 patients enrolled in the REQUITE study. RILA data on 23 patients were excluded from analysis because they failed quality control, with the data on the remaining 1296 patients being analysed. Four time intervals were recorded for each sample: storage time (time from blood draw to setting up cultures), pre-irradiation incubation (time from setting up of cultures to irradiation), post-incubation (time from irradiation to cell lysis) and FACS lag (time from cell lysis to loading on FACS machine). Table 2 shows variation in timings at the three centres.
Table 2.
Length of time intervals for different stages of the RILA protocol achieved in practice. All times in hours.
| Cohort | Time interval | Mean (h) | Median (h) | Min (h) | Max (h) | Interquartile range (h) | Break-down (h) |
|---|---|---|---|---|---|---|---|
| Leicester (n = 415) | Storage | 15.4 | 20.1 | 0.3 | 47.1 | 2.9–25.3 | 0–12 h n = 182 12–36 h n = 227 36–48 h n = 6 |
| Pre-rad incubation | 18.9 | 17.8 | 0.5 | 25.3 | 16.5–21.3 | 0–12 h n = 1 12–36 h n = 414 |
|
| Post-rad incubation | 49.1 | 49.2 | 45.7 | 50.2 | 48.8–49.5 | ||
| FACS lag | 3.6 | 3.6 | 0.2 | 5.5 | 3.1–4.1 | ||
| Mannheim (n = 194) | Storage | 22.0 | 23.5 | 0.2 | 34.5 | 22.4–24.8 | 0–12 h n = 13 12–36 h n = 181 36–48 h n = 0 |
| Pre-rad incubation | 12.6 | 3.0 | 0.3 | 28.3 | 2.0–25.7 | 0–12 h n = 107 12–36 h n = 87 |
|
| Post-rad incubation | 43.7 | 44.0 | 19.6 | 69.0 | 43.5–44.3 | 12–36 h n = 4 36–48 h n = 187 >48 hr n = 3 |
|
| FACS lag | 1.5 | 1.3 | 0.3 | 4.5 | 1.0–1.9 | ||
| Montpellier (n = 678) | Storage | 10.4 | 5.5 | 0 | 32.8 | 2.5–24.5 | 0–12 h n = 498 12–36 h n = 180 36–48 h n = 0 |
| Pre-rad incubation | 20.1 | 20.0 | 16.8 | 28.7 | 18.3–21.2 | ||
| Post-rad incubation | 45.5 | 45.8 | 38.3 | 49.3 | 44.3–47.3 | ||
| FACS lag | 4.4 | 4.4 | 0.3 | 8.8 | 3.5–5.2 |
For 85 samples Mannheim performed the assay with both 0 h and 24 h pre-irradiation incubation (0/48 and 24/48 protocols). RILA scores were a mean 10.7% (range −14.7% to 32.7%) higher for the 24/48 protocol (or 0.45%/hour). The wide range of scores hampers application of a standard correction factor for comparison of results obtained using different protocols, but the correlation between 0/48 and 24/48 RILA scores was high (r = 0.71, p < 0.001).
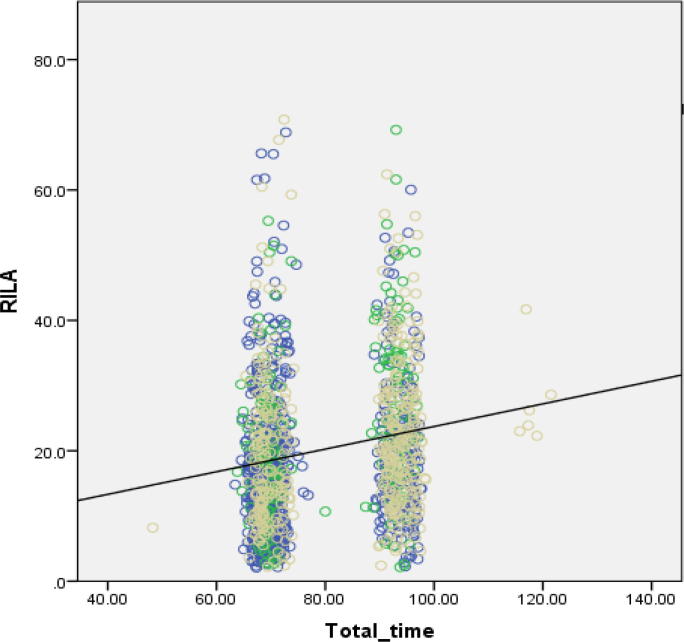
The effect of variation in protocol timings on RILA scores was investigated (Table 2 and Fig. 3). Different factors significantly affected RILA score in each cohort (Supplementary Table 2). Combining data across all three centres shows that RILA scores increased with total time from venepuncture to FACS (Supplementary Table 2 and Fig. 3).
Fig. 3.
RILA score against total time in hours from venepuncture to FACs lysis. Patients from all three clinical centres (n = 1190). Yellow circles are Leicester, green are Mannheim, blue are Montpellier. Slope = +0.17%/hour, p = <0.001. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2. Results by cancer type and centre
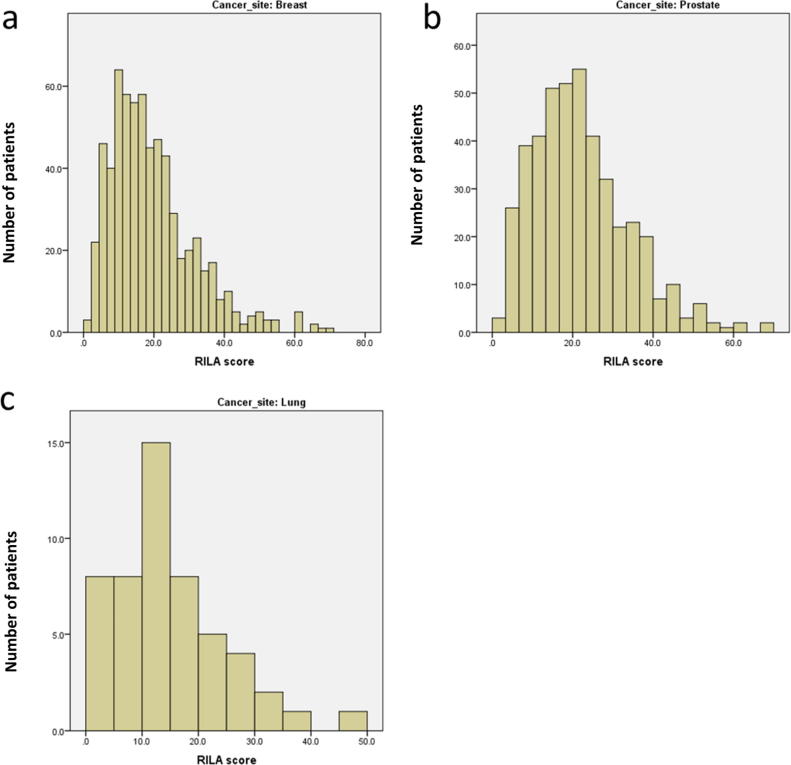
At the time of analysis full patient data were available on 1247 of the 1296 patients: 52 lung, 724 breast and 471 prostate cancer patients. The distributions of RILA scores for each cancer type were broadly similar (Fig. 1). There was a significant difference in RILA score between the three cancer types: breast cancer samples had mean RILA = 19.2 ± 11.8%, prostate samples 22.1 ± 12.0% and lung cancer samples 15.1 ± 9.5% (Kruskal-Wallis p < 0.001). Two-way comparisons were also significant: breast vs prostate p < 0.001, lung vs breast p = 0.008, prostate vs lung p < 0.001 (Mann-Whitney).
Fig. 1.
Distributions of RILA score (%) by cancer site. Panel A breast, B prostate, C lung.
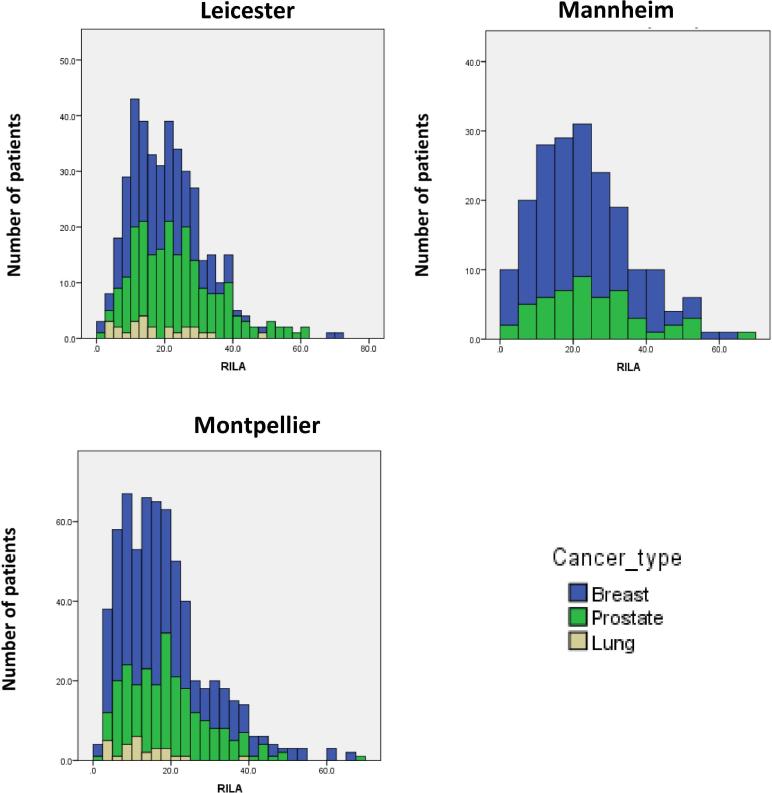
RILA scores on all REQUITE samples were significantly different between the three centres (Kruskal-Wallis p < 0.001) (Fig. 2 and Table 3). Comparing RILA scores between the centres for each of the three cancer types showed significant differences for breast and prostate (Kruskal-Wallis p < 0.001) but only a trend for lung (p = 0.07).
Fig. 2.
Distribution of RILA scores (%) by centre and cancer type (Blue: breast cancer, Green: prostate cancer, Yellow: lung cancer). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 3.
RILA scores by centre and cancer type.
| Centre | Cancer type | Number | Mean RILA (%) | Lower tertile cut-point (%) | Upper tertile cut-point (%) |
|---|---|---|---|---|---|
| UK | Combined | 414 | 21.4 | 14.8 | 24.7 |
| Breast | 190 | 19.4 | 13.6 | 22.6 | |
| Lung | 25 | 17.8 | 12.1 | 22.6 | |
| Prostate | 198 | 23.9 | 16.8 | 26.8 | |
| Germany | Combined | 194 | 23.1 | 16.4 | 26.7 |
| Breast | 142 | 22.3 | 15.4 | 26.1 | |
| Prostate | 52 | 25.3 | 18.2 | 28.9 | |
| France | Combined | 678 | 18.6 | 12.3 | 20.9 |
| Breast | 392 | 18.0 | 11.4 | 19.8 | |
| Lung | 27 | 12.5 | 8.7 | 14.7 | |
| Prostate | 221 | 19.7 | 14.2 | 22.8 |
A regression analysis was performed to compute a RILA score adjusted for the effect of incubation times and patient factors identified below (rRILA). This rRILA score was not significantly different between the three centres (Kruskal-Wallis p = 0.08), indicating that much of the difference between centres can be explained by these factors.
3.3. Effect of clinical patient factors
The characteristics of the patients for each cohort studied are shown in Supplementary Table 1.
In the combined data two patient factors were associated with RILA scores in a univariate analysis: smoking status and rheumatoid arthritis. Comparing never and ex-smokers (mean RILA 20.5 ± 12.2, n = 1025) against recent quitters and current smokers (mean RILA 18.1 ± 10.1, n = 222) gave a significant difference in RILA scores (Mann-Whitney p value = 0.02). Patients with rheumatoid arthritis had a significantly higher RILA (mean = 26.2 ± 16.4, n = 29) than those without (mean = 20.0 ± 11.7, n = 1218) (Mann-Whitney p = 0.04).
In a hierarchical linear regression analysis, smoking status (B = −2.1, p = 0.01) and rheumatoid arthritis (B = 8.2, p = 0.001) were both significant predictors of lymphocyte apoptosis. Including all the factors that significantly affect RILA score into a single hierarchical linear regression model showed that in the final model they explained 7.6% of the variation in RILA (by R squared) (Supplementary Table 3).
3.4. Analysis with acute toxicity
Acute radiotherapy toxicity endpoints were recorded six weeks after radiotherapy and the pre-radiotherapy baseline score deducted (Table 1). The endpoints were studied for association with rRILA score by regression analysis. rRILA did not predict any prostate cancer toxicity acute end-point, either singly or when combined into STAT scores for urinary, rectal and sexual dysfunction, or overall acute toxicity.
For breast cancer, rRILA did predict acute pain (Mann-Whitney p = 0.008), but none of the other end-points or a STAT score for overall acute toxicity. Patients with a grade 1 acute pain had a mean RILA score of 17.5%, while those with no increase in pain had mean 20.3%. The patients in the lowest tertile of rRILA score had a prevalence of acute breast pain of 27.4%, compared with 19.2% for the highest tertile group (OR 0.63 95% CI 0.40–0.99).
4. Discussion
The aim of the work presented here was to investigate the potential of the RILA assay for standardisation and use in multiple testing laboratories prior to attempting to validate its use as a predictive assay for late radiotherapy toxicity in a multi-centre study. We identified the steps required to generate comparable data in three laboratories across Europe. Despite the assay standardisation, mean RILA scores generated in a large multi-centre study differed across laboratories. However, the differences were lost by adjusting scores for cancer site, incubation times and patient factors. Our work shows that clinical trials involving multi-centre measurement of the RILA assay are feasible but its implementation in predictive models needs to account for tumour type and other variables.
4.1. Variability
The data show that the incubation times affects RILA scores, with time before cell lysis increasing the score by on average 0.17%/hour, but the time from lysis to flow cytometry (FACS lag) decreasing it by 1.0%/hour. Keeping all incubations strictly controlled presents practical difficulties since the blood samples are drawn at variable times during the day, but here we show that incubation time can be included as an adjustment factor.
4.2. Use for prediction
Previous studies defined the group at risk of radiotherapy side effects as the lower tertile of RILA scores. An important question to address is whether a common RILA score threshold can be used across cancer types and laboratories.
We showed that RILA scores varied across the three cancer types. Although previous studies have provided some evidence [18], [22], this is the first direct demonstration of a difference in lymphocyte apoptosis between cancer types, but the reason is currently unknown. Possible explanations are differences in age and sex of the three patient groups. However, our analysis showed no associations between RILA scores and age within any of the cancers and sex in lung cancer patients. Differences in treatment prior to radiotherapy might play a role: the breast tumours were resected prior to enrolment in this study, some of the prostate cancer patients had hormones prior to radiotherapy.
Taken together the results indicate that separate thresholds will be needed for each cancer type. An alternative approach is to include RILA as a continuous variable in a predictive model or nomogram, like that patented by ICM under EP #18305213 and #16306097-3 [22].
4.3. Biological factors
An inverse correlation between tobacco use and RILA score was observed. There was no observable effect of patient smoking habit on lymphocyte apoptosis at 0 Gy in our data, suggesting smoking has an interactive rather than a direct effect with radiation. This observation helps explain the apparent contradiction with published laboratory studies on the effect of smoking on lymphocyte apoptosis [27], [28], [29]. The observation of reduced radiation-induced apoptosis in smokers does however concord with the hypothesis that low apoptosis is associated with increased radiation toxicity, because smoking has been identified as a risk factor for toxicity by published clinical studies in a range of cancer types [30], [31], [32], [33], [34].
Rheumatoid arthritis is an inflammatory condition in which many cell types are involved, including the activation of cytotoxic T lymphocytes [35]. As activated effector cells have a higher tendency to undergo apoptosis t is consistent with the finding that rheumatoid arthritis patients have a higher RILA score. Despite somewhat mixed evidence [36], [37], individuals with rheumatoid arthritis are considered to have increased radiotherapy side effects, contrary to any expectation based on their higher RILA score.
It is possible that these factors are part of the, as yet unexplained, mechanism underlying the correlation between RILA and radiation toxicity. As such, whether to adjust RILA for these factors needs to consider the effect on predictive value of the test measured, for example as measured by multi-parameter nomograms or Receiver Operating Characteristic (ROC) curve analysis. The REQUITE late toxicity analysis should be well placed to consider this question.
4.4. Acute toxicity
Previous studies found no evidence that RILA predicts acute radiotherapy toxicity, and the present study confirmed that for all clinical endpoints with the exception of acute breast pain. The reason for the latter is unknown, although as the frequency of pain is higher than the other end-points there is a greater statistical power. It may also reflect a genuine distinction between acute toxicity endpoints: principal components analysis demonstrates that pain does not cluster with any other endpoint, suggesting it has a separate biological basis. Pain may be a marker of inflammation or deep tissue damage caused by radiotherapy, separate from the other acute breast end-points that reflect skin damage.
4.5. Limitations and strengths of the assay
A limitation of the current RILA protocol to routine implementation is the necessity for culturing the cells for one day prior to ex-vivo irradiation and for two days after irradiation. The current study presents preliminary evidence that using shorter incubation times causes reduced RILA levels but which are correlated with the three-day protocol. Further work is required to evaluate shorter protocols with no pre-irradiation incubation and a shortened post-irradiation incubation.
The strengths of RILA are multiple and facilitate its use in daily clinical practice: one simple blood sample (without any tissue biopsy); obtaining the results in four days, and generation of a significant continuous variable capable of being incorporated in a nomogram.
There is now sufficient evidence to underpin increased use of the RILA assay in daily practice as soon as possible before breast irradiation. For widespread clinical use, additional work is required to show implementation has patient benefit and is cost effective.
4.6. Mechanism
Identifying the mechanism by which RILA predicts late toxicity might be useful for the design of ameliorative interventions. Currently it is unknown whether RILA discriminates between consequential and generic late effects, but the lack of predictive power for acute reactions might suggest it is the latter [38]. The finding in this study that acute breast pain exceptionally is predicted by RILA, may provide a more nuanced dissection of pathological pathways. When toxicity data are available on the REQUITE patients it may be possible to discriminate between types of late reactions.
4.7. Recommendations
The SOP produced for the study presented here should be used by others carrying out the RILA assay. Any use of the protocol in multi-centre studies requires standardisation of the reagents and culture conditions. It is recommended that future inter-laboratory comparisons of radiosensitivity tests accumulate sufficient pilot data on comparable cancer patients. The pilot data can be used either to define centre and cancer-site specific tertile cut-offs or construct nomograms, prior to testing associations with clinical end-points.
5. Conclusion
This report presents data from the collection phase of a multi-national study to validate the RILA assay as a predictive test for adverse reactions to radiotherapy, part of the larger REQUITE study. Despite efforts to use a common protocol and reagents, there was variation in the results generated in different centres. Adjusting for some variables identified allowed results to be combined across centres. Evidence was found for different levels of radiation-induced lymphocyte apoptosis between cancer types, which may be of biological interest but would need to be accounted for in studies involving multiple malignancies. Lastly several experimental and lifestyle factors were found to affect RILA score and will need to be included in the future analysis of predictive value of the assay for acute and late toxicity.
Declaration of Competing Interest
Dr. Azria has a patent on RILA assay testing for breast and prostate cancer issued to NovaGray. The other authors have nothing to declare.
Acknowledgements
This work was supported by funding from the European Union Seventh Framework Programme for research, technological development and demonstration under grant agreement no. 601826 (REQUITE). We gratefully acknowledge the contribution of the physicians, study nurses and patients enrolled into the REQUITE study. These include in the UK: Teresa Beaver, Kiran Kancherla, Sarah Nicholson and Tim Rattay; Germany: Elena Sperk, Anke Keller, Annette Kipke, Katharina Fleckenstein, Martina Ottstadt, Michael Ehmann, Gritt Welzel, Daniel Bürgy, Yasser Aboumadian, Chrisian Neumaier, Katharina Heim, Mario Grimm, Anna Simeonova, Frank Giordano, Elisabeth Heesch, Georg Lars Hildenbrand, Benjamin Gauter-Fleckenstein, Tina Reis, Sabine Mai, Christiane Zimmermann, Stefanie Kolb and Markus Mihalko, in France: Jean-Pierre Bleuse, Laura Bourillon, Roxana Draghici, Anne Fenoglietto and Julie Grataloup. The FACS machine in Leicester was supported by Medical Research Council (UK) grant G0802524. Catharine West is supported by Cancer Research UK funding for the Manchester Cancer Research Centre and the NIHR Manchester Biomedical Research Centre (both UK).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2019.06.001.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Skiold S., Azimzadeh O., Merl-Pham J., Naslund I., Wersall P., Lidbrink E. Unique proteomic signature for radiation sensitive patients; a comparative study between normo-sensitive and radiation sensitive breast cancer patients. Mutant Res. 2015;776:128–135. doi: 10.1016/j.mrfmmm.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Mayer C., Popanda O., Greve B., Fritz E., Illig T., Eckardt-Schupp F. A radiation-induced gene expression signature as a tool to predict acute radiotherapy-induced adverse side effects. Cancer Lett. 2011;302:20–28. doi: 10.1016/j.canlet.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Kerns S.L., West C.M., Andreassen C.N., Barnett G.C., Bentzen S.M., Burnet N.G. Radiogenomics: the search for genetic predictors of radiotherapy response. Future Oncol. 2014;10:2391–2406. doi: 10.2217/fon.14.173. [DOI] [PubMed] [Google Scholar]
- 4.Andreassen C.N., Rosenstein B.S., Kerns S.L., Ostrer H., De Ruysscher D., Cesaretti J.A. Individual patient data meta-analysis shows a significant association between the ATM rs1801516 SNP and toxicity after radiotherapy in 5456 breast and prostate cancer patients. Radiother Oncol. 2016;121:431–439. doi: 10.1016/j.radonc.2016.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnett G.C., Thompson D., Fachal L., Kerns S., Talbot C., Elliott R.M. A genome wide association study (GWAS) providing evidence of an association between common genetic variants and late radiotherapy toxicity. Radiother Oncol. 2014;111:178–185. doi: 10.1016/j.radonc.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 6.Fachal L., Gomez-Caamano A., Barnett G.C., Peleteiro P., Carballo A.M., Calvo-Crespo P. A three-stage genome-wide association study identifies a susceptibility locus for late radiotherapy toxicity at 2q24.1. Nat Genet. 2014;46:891–894. doi: 10.1038/ng.3020. [DOI] [PubMed] [Google Scholar]
- 7.Herskind C., Talbot C.J., Kerns S.L., Veldwijk M.R., Rosenstein B.S., West C.M. Radiogenomics: a systems biology approach to understanding genetic risk factors for radiotherapy toxicity? Cancer Lett. 2016;382:95–109. doi: 10.1016/j.canlet.2016.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seibold P., Behrens S., Schmezer P., Helmbold I., Barnett G., Coles C. XRCC1 polymorphism associated with late toxicity after radiation therapy in breast cancer patients. Int J Radiat Oncol Biol Phys. 2015;92:1084–1092. doi: 10.1016/j.ijrobp.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 9.Talbot C.J., Tanteles G.A., Barnett G.C., Burnet N.G., Chang-Claude J., Coles C.E. A replicated association between polymorphisms near TNFalpha and risk for adverse reactions to radiotherapy. Br J Cancer. 2012;107:748–753. doi: 10.1038/bjc.2012.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.HPA Human Radiosensitivity . Health Protection Agency; 2013. Report of the independent advisory group on ionising radiation. [Google Scholar]
- 11.Nuta O., Somaiah N., Boyle S., Chua M.L., Gothard L., Yarnold J. Correlation between the radiation responses of fibroblasts cultured from individual patients and the risk of late reaction after breast radiotherapy. Cancer Lett. 2016;374:324–330. doi: 10.1016/j.canlet.2016.02.036. [DOI] [PubMed] [Google Scholar]
- 12.Somaiah N., Chua M.L., Bourne S., Daley F., A’Hern R., Nuta O. Correlation between DNA damage responses of skin to a test dose of radiation and late adverse effects of earlier breast radiotherapy. Radiother Oncol. 2016;119:244–249. doi: 10.1016/j.radonc.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 13.Ozsahin M., Crompton N.E., Gourgou S., Kramar A., Li L., Shi Y. CD4 and CD8 T-lymphocyte apoptosis can predict radiation-induced late toxicity: a prospective study in 399 patients. Clin Cancer Res. 2005;11:7426–7433. doi: 10.1158/1078-0432.CCR-04-2634. [DOI] [PubMed] [Google Scholar]
- 14.Azria D., Belkacemi Y., Romieu G., Gourgou S., Gutowski M., Zaman K. Concurrent or sequential adjuvant letrozole and radiotherapy after conservative surgery for early-stage breast cancer (CO-HO-RT): a phase 2 randomised trial. Lancet Oncol. 2010;11:258–265. doi: 10.1016/S1470-2045(10)70013-9. [DOI] [PubMed] [Google Scholar]
- 15.Bordon E., Henriquez Hernandez L.A., Lara P.C., Pinar B., Fontes F., Rodriguez Gallego C. Prediction of clinical toxicity in localized cervical carcinoma by radio-induced apoptosis study in peripheral blood lymphocytes (PBLs) Radiat Oncol. 2009;4:58. doi: 10.1186/1748-717X-4-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bordon E., Henriquez-Hernandez L.A., Lara P.C., Pinar B., Rodriguez-Gallego C., Lloret M. Role of CD4 and CD8 T-lymphocytes, B-lymphocytes and Natural Killer cells in the prediction of radiation-induced late toxicity in cervical cancer patients. Int J Radiat Biol. 2011;87:424–431. doi: 10.3109/09553002.2010.537433. [DOI] [PubMed] [Google Scholar]
- 17.Bordon E., Henriquez-Hernandez L.A., Lara P.C., Ruiz A., Pinar B., Rodriguez-Gallego C. Prediction of clinical toxicity in locally advanced head and neck cancer patients by radio-induced apoptosis in peripheral blood lymphocytes (PBLs) Radiat Oncol. 2010;5:4. doi: 10.1186/1748-717X-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foro P., Algara M., Lozano J., Rodriguez N., Sanz X., Torres E. Relationship between radiation-induced apoptosis of T lymphocytes and chronic toxicity in patients with prostate cancer treated by radiation therapy: a prospective study. Int J Radiat Oncol Biol Phys. 2014;88:1057–1063. doi: 10.1016/j.ijrobp.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Fuentes-Raspall M.J., Caragol I., Alonso C., Ramon y Cajal T., Fisas D., Seoane A. Apoptosis for prediction of radiotherapy late toxicity: lymphocyte subset sensitivity and potential effect of TP53 Arg72Pro polymorphism. Apoptosis. 2015;20:371–382. doi: 10.1007/s10495-014-1056-2. [DOI] [PubMed] [Google Scholar]
- 20.Henriquez-Hernandez L.A., Carmona-Vigo R., Pinar B., Bordon E., Lloret M., Nunez M.I. Combined low initial DNA damage and high radiation-induced apoptosis confers clinical resistance to long-term toxicity in breast cancer patients treated with high-dose radiotherapy. Radiat Oncol. 2011;6:60. doi: 10.1186/1748-717X-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schnarr K., Boreham D., Sathya J., Julian J., Dayes I.S. Radiation-induced lymphocyte apoptosis to predict radiation therapy late toxicity in prostate cancer patients. Int J Radiat Oncol Biol Phys. 2009;74:1424–1430. doi: 10.1016/j.ijrobp.2008.10.039. [DOI] [PubMed] [Google Scholar]
- 22.Azria D., Riou O., Castan F., Nguyen T.D., Peignaux K., Lemanski C. Radiation-induced CD8 T-lymphocyte apoptosis as a predictor of breast fibrosis after radiotherapy: results of the prospective multicenter french trial. EBioMedicine. 2015;2:1965–1973. doi: 10.1016/j.ebiom.2015.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Azria D., Lapierre A., Gourgou S., De Ruysscher D., Colinge J., Lambin P. Data-based radiation oncology: design of clinical trials in the toxicity biomarkers era. Front Oncol. 2017;7:83. doi: 10.3389/fonc.2017.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mirjolet C., Merlin J.L., Dalban C., Maingon P., Azria D. Correlation between radio-induced lymphocyte apoptosis measurements obtained from two French centres. Cancer Radiother. 2016;20:391–394. doi: 10.1016/j.canrad.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 25.West C., Azria D., Chang-Claude J., Davidson S., Lambin P., Rosenstein B. The REQUITE project: validating predictive models and biomarkers of radiotherapy toxicity to reduce side-effects and improve quality of life in cancer survivors. Clin Oncol (R Coll Radiol) 2014;26:739–742. doi: 10.1016/j.clon.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 26.Crompton N.E., Miralbell R., Rutz H.P., Ersoy F., Sanal O., Wellmann D. Altered apoptotic profiles in irradiated patients with increased toxicity. Int J Radiat Oncol Biol Phys. 1999;45:707–714. doi: 10.1016/s0360-3016(99)00256-4. [DOI] [PubMed] [Google Scholar]
- 27.El-Hodhod M.A., Hamdy A.M., Ahmed M.B., Youssef S.R., Aly S.M. Effect of passive smoking on blood lymphocyte apoptosis in children. Eur J Clin Invest. 2011;41:387–392. doi: 10.1111/j.1365-2362.2010.02420.x. [DOI] [PubMed] [Google Scholar]
- 28.Hernandez C.P., Morrow K., Velasco C., Wyczechowska D.D., Naura A.S., Rodriguez P.C. Effects of cigarette smoke extract on primary activated T cells. Cell Immunol. 2013;282:38–43. doi: 10.1016/j.cellimm.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mesic A., Nefic H. Assessment of the genotoxicity and cytotoxicity in environmentally exposed human populations to heavy metals using the cytokinesis-block micronucleus cytome assay. Environ Toxicol. 2015;30:1331–1342. doi: 10.1002/tox.22004. [DOI] [PubMed] [Google Scholar]
- 30.Barnett G.C., Wilkinson J.S., Moody A.M., Wilson C.B., Twyman N., Wishart G.C. The Cambridge breast intensity-modulated radiotherapy trial: patient- and treatment-related factors that influence late toxicity. Clin Oncol (R Coll Radiol) 2011;23:662–673. doi: 10.1016/j.clon.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 31.Cozzarini C., Rancati T., Carillo V., Civardi F., Garibaldi E., Franco P. Multi-variable models predicting specific patient-reported acute urinary symptoms after radiotherapy for prostate cancer: Results of a cohort study. Radiother Oncol. 2015;116:185–191. doi: 10.1016/j.radonc.2015.07.048. [DOI] [PubMed] [Google Scholar]
- 32.De Langhe S., Mulliez T., Veldeman L., Remouchamps V., van Greveling A., Gilsoul M. Factors modifying the risk for developing acute skin toxicity after whole-breast intensity modulated radiotherapy. BMC Cancer. 2014;14:711. doi: 10.1186/1471-2407-14-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kraus-Tiefenbacher U., Sfintizky A., Welzel G., Simeonova A., Sperk E., Siebenlist K. Factors of influence on acute skin toxicity of breast cancer patients treated with standard three-dimensional conformal radiotherapy (3D-CRT) after breast conserving surgery (BCS) Radiat Oncol. 2012;7:217. doi: 10.1186/1748-717X-7-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steinberger E., Kollmeier M., McBride S., Novak C., Pei X., Zelefsky M.J. Cigarette smoking during external beam radiation therapy for prostate cancer is associated with an increased risk of prostate cancer-specific mortality and treatment-related toxicity. BJU Int. 2015;116:596–603. doi: 10.1111/bju.12969. [DOI] [PubMed] [Google Scholar]
- 35.Wood K.L., Twigg H.L., 3rd, Doseff A.I. Dysregulation of CD8+ lymphocyte apoptosis, chronic disease, and immune regulation. Front Biosci (Landmark Ed) 2009;14:3771–3781. doi: 10.2741/3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dong Y., Li T., Churilla T.M., Shaikh T., Sigurdson E.R., Bleicher R.J. Impact of rheumatoid arthritis on radiation-related toxicity and cosmesis in breast cancer patients: a contemporary matched-pair analysis. Breast Cancer Res Treat. 2017;166:787–791. doi: 10.1007/s10549-017-4438-7. [DOI] [PubMed] [Google Scholar]
- 37.Lin A., Abu-Isa E., Griffith K.A., Ben-Josef E. Toxicity of radiotherapy in patients with collagen vascular disease. Cancer. 2008;113:648–653. doi: 10.1002/cncr.23591. [DOI] [PubMed] [Google Scholar]
- 38.Dorr W., Hendry J.H. Consequential late effects in normal tissues. Radiother Oncol. 2001;61:223–231. doi: 10.1016/s0167-8140(01)00429-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.