Abstract
This study examined effects of the α2/α3-subtype-selective GABAA receptor positive allosteric modulator KRM-II-81 in an assay of pain-related behavioral depression. Adult, male Sprague-Dawley rats responded for electrical brain stimulation in a frequency-rate intracranial self-stimulation (ICSS) procedure. Intraperitoneal injection of 1.8% lactic acid served as an acute noxious stimulus to depress ICSS. Effects of KRM-II-81 were evaluated in the absence and presence of the acid noxious stimulus. The nonsteroidal anti-inflammatory drug ketorolac and the benzodiazepine diazepam were tested as comparators. Neither ketorolac nor KRM-II-81 altered ICSS in the absence of the acid noxious stimulus; however, diazepam produced facilitation consistent with its abuse liability. Ketorolac blocked acid-induced depression of ICSS, and effects of 1.0 mg/kg ketorolac lasted for at least 5 hours. KRM-II-81 (1.0 mg/kg) produced significant antinociception after 30 min that dissipated by 60 min. Diazepam also attenuated acid-depressed ICSS, but only at doses that facilitated ICSS when administered alone. The lack of ketorolac or KRM-II-81 effects on ICSS in the absence of the acid noxious stimulus suggests low abuse liability for both compounds. The effectiveness of ketorolac to block acid-induced ICSS depression agrees with clinical analgesic efficacy of ketorolac. KRM-II-81 produced significant but less consistent and shorter-acting antinociception than ketorolac.
Keywords: drug abuse, analgesic, intracranial self-stimulation, GABA receptors, positive allosteric modulator, rat
Introduction
The treatment of pain continues to be a clinical challenge, and despite concerted efforts to develop novel analgesics, opioids and non-steroidal anti-inflammatory drugs (NSAIDs) have remained the standard of care for decades. There are limitations to the utility of these drugs: for example, opioids have abuse liability (Ling et al., 2011) and can produce lethal respiratory depression (Duthie and Nimmo, 1987), whereas NSAIDs increase the risk of cardiovascular disease (Funk and FitzGerald, 2007) and can cause ulcers and other gastrointestinal problems (Sostres et al., 2010). GABAA receptor positive allosteric modulators (GABAAR PAMs), such as the benzodiazepine diazepam, are in widespread clinical use for a number of indications, including anxiety (Bellantuono et al., 1980) and sleep dysfunction (Wilt et al., 2016), but they are not used as stand-alone analgesics. There is evidence, however, suggesting that analgesic effects of GABAAR PAMs may be masked by concurrent sedation (Ralvenius et al., 2015).
GABAA receptors are pentameric ligand-gated ion channels composed of five subunits around a central pore (Olsen and Sieghart, 2009), and GABAA receptor subtypes can be defined by the type of α subunit they contain. Like opioids, clinically available GABAAR PAMs have abuse liability, an effect that, in addition to sedation, appears to be mediated primarily by α1-containing GABAA receptors (Rowlett et al., 2005; Crestani and Rudolph, 2015; Schwienteck et al., 2017). Thus, one strategy for identifying GABAAR PAMs with reduced side effects has been to develop compounds that act selectively to modulate α2/α3-containing receptor activity without having effects at α1 receptors (Atack, 2011; Poe et al., 2016). To date, α1-sparing compounds have greatly reduced or eliminated sedation and motor impairment characteristic of less selective GABAAR PAMs (Di Lio et al., 2011; Poe et al., 2016; Knutson et al., 2018). Furthermore, there is evidence that compounds selectively targeting α2/α3-containing GABAA receptors may have applications for the treatment of pain (Munro et al., 2008; Lewter et al., 2017).
Many preclinical studies of candidate analgesics measure drug effects on reflexive withdrawal responses from noxious stimuli; however, behavioral depression is a clinical hallmark of pain and an important target in pain treatment (Dworkin et al., 2005; Negus et al., 2006; Negus et al., 2010). Consequently, preclinical assays have been developed in rodents to assess the expression and treatment of pain-related depression of unconditioned behaviors, such as feeding (Kwilasz and Negus, 2012), wheel running (Stevenson et al., 2011; Kandasamy et al., 2016) and nesting (Negus et al., 2015). Pain states can also decrease the expression of positively reinforced operant behaviors, and intracranial self-stimulation (ICSS) is one type of operant responding that has been used in assays of pain-depressed behavior (Negus, 2013). In ICSS, subjects equipped with an electrode targeting a brain-reward area such as the lateral hypothalamus are trained to press a lever for pulses of electrical brain stimulation (Carlezon and Chartoff, 2007; Negus and Miller, 2014). Noxious stimuli can produce a pain-related depression of ICSS, and this ICSS depression can be blocked by analgesic drugs such as opioids and NSAIDS (Negus et al., 2010; Negus, 2013; Ewan and Martin, 2014). Furthermore, in the absence of a noxious stimulus, ICSS can also be used to examine the abuse potential of drugs; most drugs of abuse increase (or “facilitate”) ICSS responding, and as a result, ICSS facilitation is considered an abuse-related drug effect (Negus and Miller, 2014). Thus, ICSS can be used to evaluate both antinociceptive drug effects (by evaluating drug effectiveness to block pain-related depression of ICSS in the presence of a noxious stimulus) and abuse-related drug effects (by evaluating drug effectiveness to facilitate ICSS in the absence of a noxious stimulus).
The goal of the current study was to evaluate antinociceptive and abuse-related effects of the α2/α3-subtype-selective GABAAR PAM KRM-II-81 (Poe et al., 2016) on ICSS in the presence and absence of a noxious stimulus. Intraperitoneal injection of dilute acid served as an acute visceral noxious stimulus to produce pain-related depression of ICSS responding, and we have shown previously that acid-induced ICSS depression can be alleviated by a range of clinically effective opioid and non-opioid analgesics but not by many drug classes that fail to function as clinically effective analgesics (e.g. kappa agonists) (Negus, 2013). The NSAID ketorolac was included as a positive control because it is a clinically effective analgesic that has not been tested previously in this assay of pain-related ICSS depression (Litvak and McEvoy, 1990). The non-selective GABAAR PAM diazepam was included as a negative control because it is not a clinically effective analgesic but produces an abuse-related facilitation of ICSS in the absence of pain manipulations (Schwienteck et al., 2017). We hypothesized that ketorolac and KRM-II-81 would block acid-induced depression of ICSS without altering ICSS in the absence of the acid noxious stimulus, whereas diazepam would block acid-induced ICSS depression only at doses similar to or greater than doses that increase ICSS in the absence of the acid stimulus.
Methods
Subjects
Adult male Sprague-Dawley rats were obtained from Envigo (Indianapolis, IN), weighed 310–350 g at the time of surgery, and were individually housed and maintained on a 12-h light/dark cycle with lights on from 06:00 to 18:00 h. All rats had free access to food and water except during testing. Animal maintenance and research were in compliance with National Institutes of Health guidelines, and all animal-use protocols were approved by the Institutional Animal Care and Use Committee.
Surgery
Subjects were anesthetized with isoflurane gas (3–4% in oxygen; Webster Veterinary, Phoenix, AZ) until unresponsive to toe-pinch and placed in a stereotaxic apparatus (Kopf Instruments, Tujunga, CA). The cathode of a stainless-steel electrode (0.25 mm diameter and insulated except at the flattened tip; MS303/1-AIU/SPC, Plastics One, Roanoke, VA) was implanted in the medial forebrain bundle at the level of the lateral hypothalamus (2.8 mm posterior to bregma, 1.7 mm lateral to the midsagittal suture, 8.8 mm ventral to the skull). Electrodes were secured to the skull using screws (Plastics One, Inc., Roanoke, VA) and orthodontic resin (Butler Schein, Dublin, OH), and the anode of the electrode (0.125 mm diameter, uninsulated) was wrapped around one of the screws to act as a ground. Animals were allowed at least seven recovery days prior to initiation of ICSS training.
Apparatus
Experiments were conducted in sound-attenuating chambers that contained modular acrylic test chambers (29.2 × 30.5 × 24.1) equipped with a response lever (4.5 cm wide, extended 2.0 cm through the center of one wall, 3 cm off the floor), stimulus lights (three lights colored red, yellow and green positioned 7.6 cm directly above the lever), a 2-W white house light, and an ICSS stimulator (Med Associates, St. Albans, VT). Electrodes were connected to the stimulator via bipolar cables and a commutator (Model SL2C, Plastics One, Roanoke, VA). A computer and software program (Med Associates, St. Albans, VT) controlled the stimulator, programming parameters and data collection.
Training
Rats were trained under a fixed-ratio 1 (FR 1) schedule of brain stimulation using procedures similar to those described previously (Negus et al., 2012; Rosenberg et al., 2013; Schwienteck et al., 2015). Each lever press resulted in the delivery of a 0.5-s train of square wave cathodal pulses (0.1-ms pulse duration), and stimulation was accompanied by illumination of the stimulus lights above the lever. Responses during the 0.5-s stimulation period did not result in additional stimulation. During the initial phase of training, sessions lasted 30 to 60 min, the frequency of stimulation was held constant at 158 Hz, and the stimulation intensity was adjusted to the lowest value that would sustain responding for at least 30 stimulations per minute. Frequency manipulations were then introduced during sessions that consisted of sequential 10-min components. During each component, a descending series of 10 current frequencies (1.75–2.2 log Hz in 0.05 log increments) was presented, with a 60-s trial at each frequency. A frequency trial began with a 5-s time out followed by a 5-s “priming” phase, during which five non-contingent stimulations were delivered at a rate of one per second. This non-contingent stimulation was followed by a 50-s “response” phase, during which responding produced electrical stimulation under a FR 1 schedule. Training continued with 3 to 12 sequential components per day, and the current intensity was adjusted until rats reliably responded during the first four to five frequency trials of all components for at least three consecutive days. This intensity (range: 80–250 µA) was held constant for the remainder of the study.
Testing
Once training was completed, ICSS testing began. Three sets of experiments were conducted in different groups of rats. An initial group of eight rats was used to determine the effects of KRM-II-81 (n=8) and ketorolac (n=6) in the presence and absence of acid. Dose-effect test sessions consisted of three sequential baseline components followed by an i.p. injection of vehicle, ketorolac (0.1–10 mg/kg) or KRM-II-81 (0.32–10 mg/kg) and then a 15-min pretreatment interval,l followed by a second i.p. injection of vehicle or 1.8% lactic acid immediately before a pair of test components. Dose order was randomized across rats using a Latin Square design. A second set of rats was used for time course experiments with ketorolac or KRM-II-81. Time-course test sessions consisted of three baseline components followed by i.p. injection of 1.0 mg/kg ketorolac (n=8) or 1.0 mg/kg KRM-II-81 (n=9), followed by a pretreatment interval and then by a pair of test components. The pretreatment interval durations were 1, 5, and 20 h (ketorolac) or 15, 30 and 60 min (KRM-II-81), and 1.8% lactic acid was administered i.p. at the end of the pretreatment interval and immediately before two test components. In both groups, rats were also treated with drug vehicle 15 min before 1.8% lactic acid. Only a subset of the original group of rats were included in the ketorolac (n=4) and KRM-II-81 (n=1) time-course experiment data due to incomplete data sets in individuals that lost their head caps. To complete these data sets, animals were added to the study to yield final group sizes of n=8 (ketorolac) and n=9 (KRM-II-81).
A third set of rats (n=6) was used to evaluate effects of diazepam in the presence and absence of acid using dose-effect procedures identical to those used for ketorolac and KRM-II-81. Effects of vehicle and 0.32–10 mg/kg diazepam i.p. were examined alone and in combination with 1.8% lactic acid, and treatments were randomized across rats using a Latin Square design. For all rats, test days were preceded by at least two training days during which rats responded for brain stimulation in a three-component training session.
Drugs
Lactic acid and ketorolac tris salt were purchased from Sigma Chemical Co (St. Louis, MO) and prepared in sterile water and sterile saline, respectively. Diazepam was purchased from Tocris Bioscience (Minneapolis, MN), and KRM-II-81 (5-(8-ethynyl-6-(pyridine-2-yl)-4H-benzo[f]imidazo[1,5-a][1,4]diazepin-3-yl) oxazole) was synthesized at the University of Wisconsin-Milwaukee and generously provided by Dr. James Cook. Diazepam and KRM-II-81 were dissolved in 2:1:7 DMSO:emulphor:saline. All drugs were injected i.p. in a volume of 1 ml/kg.
Data analysis
The first baseline component of each session was considered an acclimation component, and data were discarded. The primary dependent variable for all remaining components was the reinforcement rate in stimulations per trial during each frequency trial. To normalize these raw data, reinforcement rates from each trial in each rat were converted to percent Maximum Control Rate (% MCR), which was defined as the mean of the maximal rates observed during the second and third “baseline” components for that rat on that day. Thus, % MCR = [(rate during a frequency trial) / (MCR)] x 100. Normalized ICSS rates at each frequency were averaged across test components within each rat and then across rats to yield a “frequency-rate” curve for each experimental manipulation. Two-way ANOVA was used to compare frequency-rate curves, with ICSS frequency as one variable and dose or time as the second variable. A significant ANOVA was followed by the Holm-Sidak post hoc test, and the criterion for significance was set at p<0.05.
An additional summary measure of ICSS performance was calculated to collapse data across brain-stimulation frequencies and facilitate comparison of drug effects in the absence or presence of the lactic acid noxious stimulus. For this summary measure, the total number of stimulations per component was calculated as the average of the total stimulations delivered across all 10-frequency trials of each component. Data were expressed as a percentage of the total stimulations per component earned during the daily baseline. Thus, % Baseline Total Stimulations was calculated as (Mean Total Stimulations During Test Components / Mean Total Stimulations During Baseline Components) × 100. T-test, one-way ANOVA, or two-way ANOVA was used as appropriate to compare % Baseline Total Stimulations across test conditions. A significant ANOVA was followed by the Holm-Sidak post hoc test, and the criterion for significance for all statistical analyses was set at p<0.05.
Results
ICSS baseline
Under baseline conditions, electrical brain stimulation maintained a frequency-dependent increase in ICSS rates of responding. For the 22 rats used in these studies, the mean ± S.E.M. baseline maximum control rate was 61.32 ± 0.58 stimulations per trial, and the mean ± S.E.M. number of total baseline stimulations was 286.03 ± 5.42 stimulations per component.
Effects of the acid noxious stimulus in ICSS
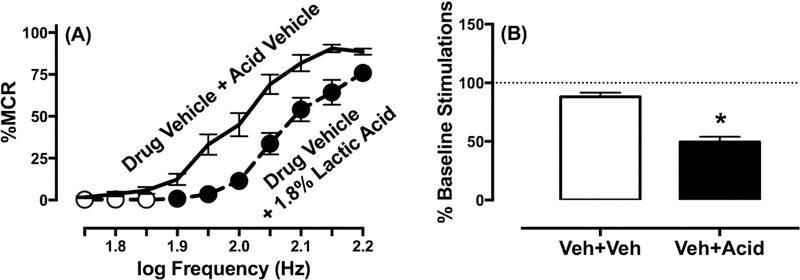
Figure 1 shows that administration of 1.8% lactic acid as a noxious stimulus significantly depressed ICSS behavior and produced both a rightward shift in the ICSS frequency-rate curve (Figure 1A) (frequency: F(9,171)=141.7, p<0.001; treatment: F(1,19)=103, p<0.001; frequency x treatment: F(9,171)=9.317, p<0.001) and a decrease in the total number of stimulations per component collapsed across all frequencies (Figure 1B) (t(19)=9.36, p<0.001). Test drugs were examined in the absence or presence of 1.8% lactic acid to evaluate their effectiveness to block this acid-induced depression of ICSS.
Figure 1.
Effects of acute 1.8% lactic acid, i.p. on ICSS in rats. (A) Left panel shows frequency-rate curves. Horizontal axis: electrical brain stimulation frequency in Hertz (Hz, log scale). Vertical axis: ICSS reinforcement rate expressed as a percentage of the maximum control rate (% MCR). Filled symbols indicate significantly (p<0.05) different from drug vehicle + acid vehicle treatment as indicated by the Holm-Sidak post hoc test after a significant two-way ANOVA interaction between brain-stimulation frequency and absence/presence of acid treatment. (B) Right panel shows summary results for total stimulations per component earned across all frequencies. Horizontal axis: Treatment condition. Vertical axis: % Baseline number of total stimulations per component. Asterisk indicates significantly different from drug vehicle + acid vehicle (Veh+Veh) treatment as indicated by a t test. All data show mean ± S.E.M. from all 22 rats in the study.
Effects of ketorolac in the absence and presence of the acid noxious stimulus
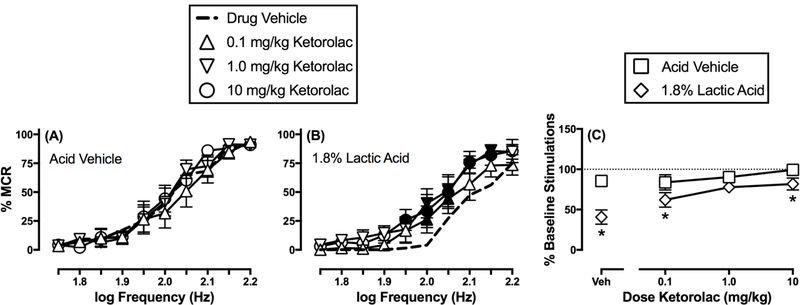
Ketorolac (0.1–10 mg/kg) did not significantly alter ICSS frequency-rate curves in the absence of the acid noxious stimulus (Figure 2A) (frequency: F(9,45)=45.58, p<0.001; dose: F(3,15)=1.34, NA; frequency x dose: F(27,135)=1.31, NS), but it did attenuate acid-induced depression of ICSS (Figure 2B) (frequency: F(9,45)=33.11, p<0.001; dose: F(3,15)=13.09, p=0.001; frequency x dose: F(27,135)=1.15, NS). Specifically, Figure 2B shows that acid-depressed ICSS was increased at two frequencies (2.0–2.05 log Hz) at the lowest dose (0.1 mg/kg) and at a larger range of frequencies following both 1.0 mg/kg (2.0–2.15 log Hz) and 10 mg/kg (1.95–2.15 log Hz) ketorolac. Figure 2C compares effects of each ketorolac dose on the summary measure of ICSS in the absence and presence of the acid noxious stimulus. Two-way ANOVA indicated a significant main effect of acid treatment and ketorolac dose and a significant interaction (treatment: F(1,5)=37.58, p=0.002; dose: F(3,15)=13.67, p=0.001; treatment x dose: F(3,15)=4.37, p=0.025). All ketorolac doses tended to reduce acid-induced ICSS depression, but post hoc analysis indicated that only 1.0 mg/kg ketorolac eliminated the difference between acid vehicle and acid treatment conditions.
Figure 2.
Effects of ketorolac on ICSS in the absence or presence of the acid noxious stimulus. Left and center panels show frequency-rate curves in the absence (A) or presence (B) of 1.8% lactic acid. Horizontal axes: electrical brain stimulation frequency in Hertz (Hz, log scale). Vertical axes: ICSS reinforcement rate expressed as a percentage of the maximum control rate (% MCR). Filled points indicate a significant (p<0.05) difference from “Drug Vehicle” as indicated by the Holm-Sidak post hoc test after a significant two-way ANOVA interaction between brain-stimulation frequency and drug dose. Right panel (C) shows summary results for total stimulations per component earned across all brain-stimulation frequencies. Horizontal axis: Dose ketorolac in mg/kg. Vertical axis: % Baseline number of total stimulations per component. Asterisks indicate a significant (p<0.05) difference from the acid vehicle condition for each ketorolac dose as indicated by the Holm-Sidak post hoc test after a significant two-way ANOVA interaction between drug dose and absence/presence of acid. All points represent the mean ± S.E.M. for n=6 rats.
Effects of KRM-II-81 in the absence and presence of the acid noxious stimulus
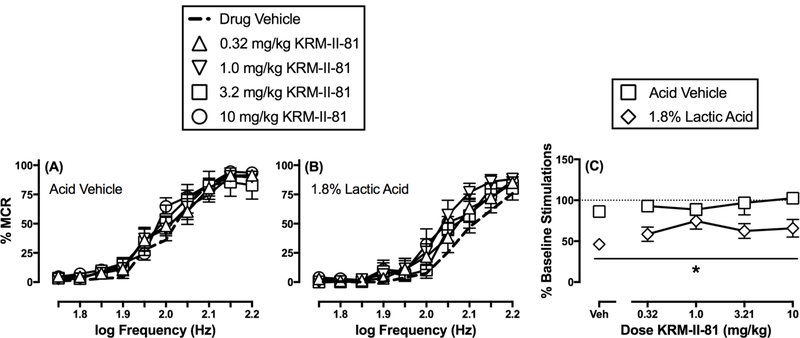
Figure 3 shows that KRM-II-81 (0.32–10 mg/kg) did not significantly alter ICSS in the absence of the acid noxious stimulus (frequency: F(9,63)=104.3, p<0.0001; dose: F(4,28)=0.5803, p=0.6794; frequency x dose: F(36,252)=1.01, NS). Unlike ketorolac, KRM-II-81 (0.32–10 mg/kg) was not effective in blocking acid-depressed ICSS in the initial dose-effect study (frequency: F(9,63)=66.35, p<0.001; dose: F(4,28)=1.65, NS; frequency x dose: F(36,252)=1.01, NS). However, the intermediate dose of 1.0 mg/kg KRM-II-81 did appear to alleviate acid-induced ICSS depression, and two-way ANOVA comparing effects only of vehicle and 1.0 mg/kg KRM-II-81 did reveal a significant main effect of KRM-II-81 (frequency: F(9,63)=67.35, p<0.001; dose: F(1,7)=12.47, p=0.01; frequency x dose: F(9,63)=2.00, p=0.054). Figure 3C compares effects of each KRM-II-81 dose on the summary measure of ICSS in the absence and presence of the acid noxious stimulus. There was a significant main effect of acid treatment, but not a significant main effect of KRM-II-81 dose or a significant interaction (treatment: F(1,7)=81.53, p<0.001; dose: F(4,28)=2.04, NS; treatment x dose: F(4,28)=1.11, NS). However, as in the frequency-rate curve analysis, there was a trend for antinociception at the intermediate dose of 1.0 mg/kg, and a t-test comparing effects of 1.0 mg/kg KRM-II-81 in the absence and presence of acid found that this KRM-II-81 dose blocked acid-induced ICSS depression (t(8)=3.68, p<=0.01)
Figure 3.
Effects of KRM-II-81 on ICSS in the absence or presence of the acid noxious stimulus. Left and center panels show frequency-rate curves in the absence (A) or presence (B) of 1.8% lactic acid. Horizontal axes: electrical brain stimulation frequency in Hertz (Hz, log scale). Vertical axes: ICSS reinforcement rate expressed as a percentage of the maximum control rate (% MCR). Filled points indicate a significant (p<0.05) difference from “Drug Vehicle” as indicated by the Holm-Sidak post hoc test after a significant two-way ANOVA interaction between brain-stimulation frequency and drug dose. Right panel (C) shows summary results for total stimulations per component earned across all brain-stimulation frequencies. Horizontal axis: Dose KRM-II-81 in mg/kg. Vertical axis: % Baseline number of total stimulations per component. Asterisk indicates a significant (p<0.05) difference from the acid vehicle condition as indicated by a main effect of acid treatment in the two-way ANOVA of effects produced by drug dose and absence/presence of acid. All points represent mean ± S.E.M. for n=8 rats.
Effects of diazepam in the absence and presence of the acid noxious stimulus.
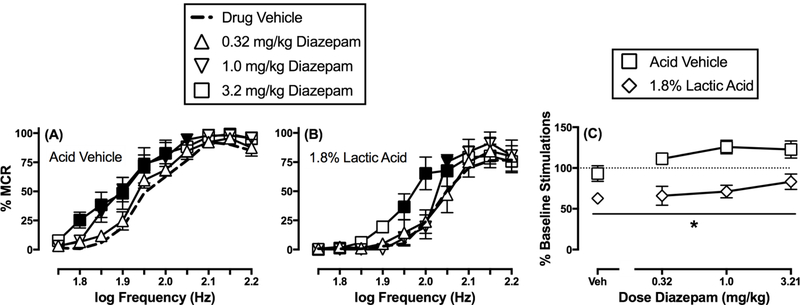
Figure 4 shows that diazepam produced dose-dependent increases in ICSS responding in the absence of the acid noxious stimulus (frequency: F(9,45)=106.3, p<0.0001; dose: F(3,15)=3.33, p<0.05; frequency x dose: F(27,135)=1.48, p=0.076). Specifically, the lowest dose of 0.32 mg/kg did not significantly alter ICSS, whereas responding was increased across a range of frequencies at both 1.0 mg/kg (1.85–2.05 log Hz) and 3.2 mg/kg (1.8–2.0 log Hz) diazepam. Diazepam also dose-dependently blocked acid-depressed ICSS (frequency: F(9,45)=61.16, p<0.001; dose: F(3,15)=2.76, p=0.079; frequency x dose: F(27,135)=2.85, p<0.001). Specifically, responding was not altered at any frequency after the lowest dose of diazepam (0.32 mg/kg); however, responding was increased at single frequency (2.05 log Hz) at a dose of 1.0 mg/kg, and at a range of frequencies (1.95–2.05 log Hz) after 3.2 mg/kg diazepam. In addition, rats were given a dose of 10 mg/kg diazepam in combination with 1.8% lactic acid, but the effect was not different from that observed after 3.2 mg/kg diazepam (data not shown). Figure 4C compares effects of each diazepam dose on the summary measure of ICSS in the absence and presence of the acid noxious stimulus. There was a significant main effect of acid treatment, but not a significant main effect of diazepam dose or a significant interaction (treatment: F(1,5)=43.02, p<0.002; dose: F(3,15)=3.11, p=0.058; treatment x dose: F(3,15)=2.78, p=0.077). Note that the main effect of dose approached statistical significance, and in general, there was a trend for diazepam to non-selectively increase ICSS regardless of the absence or presence of the acid noxious stimulus.
Figure 4.
Effects of diazepam on ICSS in the absence or presence of the acid noxious stimulus. Left and center panels show frequency-rate curves in the absence (A) or presence (B) of 1.8% lactic acid. Horizontal axes: electrical brain stimulation frequency in Hertz (Hz, log scale). Vertical axes: ICSS reinforcement rate expressed as a percentage of the maximum control rate (% MCR). Filled points indicate a significant (p<0.05) difference from “Drug Vehicle” as indicated by the Holm-Sidak post hoc test after a significant two-way ANOVA interaction between brain-stimulation frequency and drug dose. Right panel (C) shows summary results for total stimulations per component earned across all brain-stimulation frequencies. Horizontal axis: Dose diazepam in mg/kg. Vertical axis: % Baseline number of total stimulations per component. Asterisk indicates a significant (p<0.05) difference from the acid vehicle condition as indicated by a main effect of acid treatment in the two-way ANOVA of effects produced by drug dose and absence/presence of acid. All points represent mean ± S.E.M. for n=6 rats.
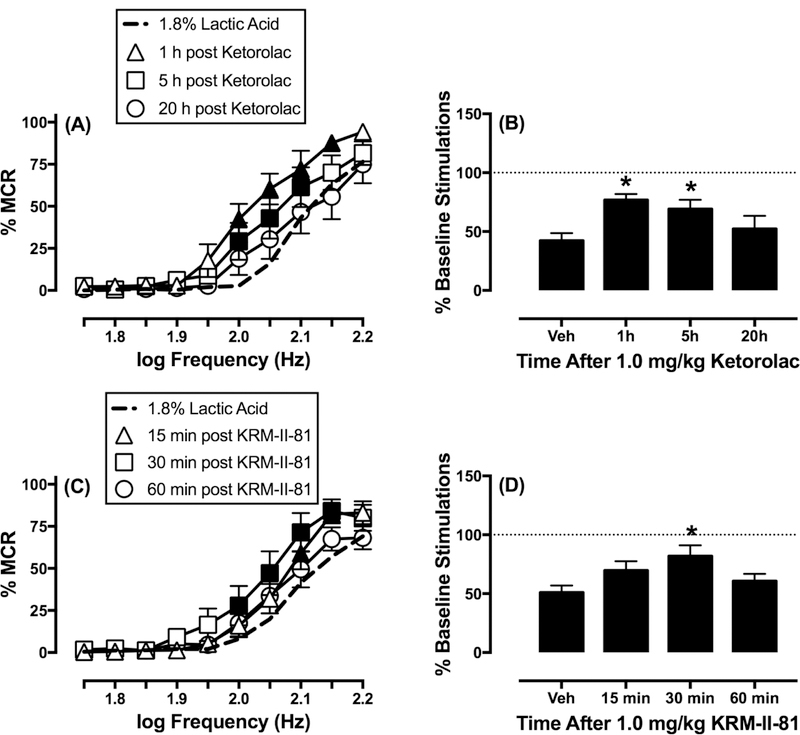
Time course of ketorolac and KRM-II-81 to block acid-depressed ICSS.
Figure 5 shows the time course of ketorolac and KRM-II-81 effects on acid-depressed ICSS. In the time-course study, 1.0 mg/kg ketorolac produced maximal blockade of acid-depressed ICSS at the earliest time points (1 and 5 h), and this effect was no longer apparent after 20 h (frequency: F(9,63)=52.28, p<0.001; time: F(3,21)=7.60, p<0.002; frequency x time: F(27,189)=1.94, p<0.01). As there a trend for antinociception was observed at the intermediate dose of 1.0 mg/kg KRM-II-81, this dose was evaluated for time-course studies. KRM-II-81 (1.0 mg/kg) was sufficient to produce time-dependent blockade of acid-depressed ICSS that was present 30 min following acid administration, but was no longer evident 1 h later (frequency: F(9,72)=74.29, p<0.001; time: F(3,24)=6.14, p<0.005; frequency x time: F(27,216)=1.59, p<0.05).
Figure 5.
Time course of ketorolac (top) and KRM-II-81 (bottom) effects on ICSS in the presence of the acid noxious stimulus in rats. Left panels show frequency-rate curves for ketorolac (A) or KRM-II-81 (C). Horizontal axes: electrical brain stimulation frequency in Hertz (Hz, log scale). Vertical axes: ICSS reinforcement rate expressed as a percentage of the maximum control rate (% MCR). Filled points indicate a significant (p<0.05) difference from “15 min post Vehicle” as indicated by the Holm-Sidak post hoc test after a significant two-way ANOVA interaction between brain-stimulation frequency and treatment. Right panels show summary results for total stimulations per component earned across all brain-stimulation frequencies. Horizontal axes: Time in min or h after drug vehicle (Veh), 1.0 mg/kg ketorolac (Ket), or 1.0 mg/kg KRM-II-81 (KRM). Vertical axes: % Baseline number of total stimulations per component. Asterisks indicate a significant difference from “Veh” as indicated by one-way ANOVA followed by the Dunnett’s post hoc test. All points and bars represent the mean ± S.E.M. for n=8 (A,B) or n=9 (C,D) rats.
Discussion
The present study compared the effects of ketorolac, diazepam and the novel α2/α3-subtype-selective GABAAR PAM KRM-II-81 in ICSS models of abuse-related behavior and analgesic-like activity in rats. There were two main findings. First, diazepam, but not ketorolac or KRM-II-81, produced facilitation of ICSS responding, consistent with the known abuse liability of diazepam. Second, all three drugs attenuated acid-induced depression of ICSS, but only KRM-II-81 and ketorolac produced this antinociceptive effect at doses that had no effect on ICSS in the absence of acid. These findings are consistent with the hypothesis that α2/α3-subtype-selective GABAAR PAMs have potential as candidate analgesics with low abuse liability.
Despite decades of research on analgesic drug development, opioids like morphine and NSAIDs like ketorolac overwhelmingly dominate clinical use (Kissin, 2010), and in the present study, the NSAID ketorolac served as a positive control. Ketorolac is one of a limited number of injectable non-opioid alternatives clinically available for treatment of moderate-to-severe pain (Baley et al., 2014). Consistent with other preclinical evidence suggesting that it can alleviate pain-depressed behavior without producing signs of abuse liability (Martin et al., 2004; Xie et al., 2014), ketorolac in the current study attenuated acid-induced depression of ICSS at doses that did not alter ICSS in the absence of the acid noxious stimulus. These results are also consistent with the clinical utility of ketorolac as an analgesic with low abuse liability (Estape et al., 1990; White et al., 1997). We have shown previously that acid-induced depression of ICSS can also be alleviated by relatively low doses of opioid analgesics such as morphine that do not produce abuse-related ICSS facilitation (Pereira Do Carmo et al., 2009; Altarifi et al., 2015). Unfortunately, both NSAIDs and opioids have serious side effects. Identifying new targets for pain treatment is one of the core initiatives in the National Institutes of Health’s plan to address the “opioid crisis” in the United States (Volkow and Collins, 2017; Collins et al., 2018).
Evidence from studies of chronic pain states in animal models suggests that GABAAR PAMs could provide analgesic benefits (Melzack and Wall, 1965; Moore et al., 2002; Coull et al., 2005; Zeilhofer and Zeilhofer, 2008), and yet, despite widespread use for other indications, clinically available GABAAR PAMs are not effective for treating pain in humans (Enna and McCarson, 2006; Friedman et al., 2017). Diazepam is a prototypical GABAAR PAM primarily prescribed as an anxiolytic; it is not used clinically as a stand-alone medication for the treatment of pain but does have abuse liability. In the current study, diazepam was the only drug to produce facilitation in ICSS in the absence of the acid noxious stimulus, which is consistent with previous work from our lab (Schwienteck et al., 2017). ICSS facilitation can be characterized as an abuse-related effect (Negus and Miller, 2014); as such, the effects of diazepam in the current study are consistent with other preclinical evidence for the abuse liability of diazepam (Rowlett and Woolverton, 1998; Rowlett et al., 2005) as well as abundant clinical evidence identifying diazepam as a drug of abuse (Griffiths et al., 1979; Evans et al., 1990; Woods and Winger, 1995). As with ketorolac, diazepam was also effective in alleviating acid-induced depression of ICSS; however, this effect was observed only at doses of diazepam that also facilitated ICSS in the absence of acid. This suggests that reversal of acid-depressed ICSS by diazepam reflects non-selective facilitation instead of antinociception, a conclusion consistent with poor clinical effectiveness of diazepam as an analgesic (Friedman et al., 2017).
There are at least two variables that contribute to the lack of analgesic effectiveness of currently approved GABAAR PAMs. The first is the route of administration: particularly for clinical applications, GABAAR PAMs are routinely administered systemically and this does not appear to produce significant analgesia. However, it has been shown that in both humans (Tucker et al., 2004) and rodents (Luger et al., 1995; Knabl et al., 2008) GABAAR PAMs are effective in producing analgesic effects when administered by the intrathecal route. This suggests that analgesic effects potentially mediated by spinal GABAA receptors may be masked by other effects mediated by GABAA receptors located in other regions of the peripheral or central nervous system (CNS). The second is lack of selectivity: different effects of GABAAR PAMs are mediated by different GABAA receptor subtypes, but classical GABAAR PAMs like diazepam are relatively non-selective. Importantly, the sedative and abuse-related effects of GABAAR PAMs appear to be mediated by the α1 GABAA receptor subtype (Harvey et al., 2002; Rowlett et al., 2005; Crestani and Rudolph, 2015), which predominates in both abundance and distribution of expression in brain as opposed to spinal cord (Persohn et al., 1992). Furthermore, novel GABAAR PAMs that lack activity at the α1 GABAA receptor subtype both fail to produce motoric impairment (Di Lio et al., 2011; Poe et al., 2016; Knutson et al., 2018) and reverse hyperalgesia in mice (Di Lio et al., 2011; Fischer et al., 2017). Although there is heterogeneity of GABAA receptor subtype expression in the spinal cord, multiple studies have found evidence for lamina-specific distribution of α subunits, with α2 predominating in superficial layers of dorsal horn, presumably on primary afferent terminals (Bohlhalter et al., 1996; Paul et al., 2012). Additionally, studies using GABAA PAMs in genetically modified mice have implicated α2/α3 GABAA receptors to be of primary importance in mediating the antinociceptive effects of GABAAR PAMs (Knabl et al., 2008; Munro et al., 2008, 2009; Knabl et al., 2009; Di Lio et al., 2011; Paul et al., 2014; Ralvenius et al., 2015, 2016; Fischer et al., 2017; Witkin et al., 2018). Taken together, these results suggest that α2/α3-subtype-selective GABAAR PAMs may produce relatively selective antinociception with minimal side effects by positively modulating spinal α2/α3 GABAA receptors that mediate antinociception but not α1 GABAA receptors in the CNS that mediate undesirable effects.
KRM-II-81 represents an improvement over previous generations of α2/α3-subtype-selective GABAAR PAMs due to improved pharmacokinetics (Poe et al., 2016) and evidence of analgesic-like effects in mice in assays of both pain-stimulated and pain-depressed behaviors (Lewter et al., 2017). In light of the current directive by the NIH to develop novel treatments for pain that lack the abuse liability characteristic of opioids, a procedure like ICSS may be particularly useful to examine both analgesic-like and abuse-related effects of candidate analgesics (Negus et al., 2006; Negus, 2013). KRM-II-81, like ketorolac, failed to facilitate ICSS in the absence of acid, consistent with other evidence to suggest low abuse liability of α2/α3-subtype-selective GABAAR PAMs (Ator et al., 2010; Shinday et al., 2013; Schwienteck et al., 2017). Moreover, the effectiveness of KRM-II-81 to alleviate acid-induced depression of ICSS in rats is consistent with data showing effectiveness of KRM-II-81 to alleviate lactic acid-induced depression of nesting in mice (Lewter et al., 2017). However, in the current study, KRM-II-81 was effective across a narrower range of doses and had a shorter time course than ketorolac. Similarly, KRM-II-81 was not effective to alleviate acid-induced depression of locomotor activity in mice, whereas acetaminophen was effective (Lewter et al., 2017). Taken together, these results support the general thesis that α2/α3-subtype-selective GABAAR PAMs warrant further study as candidate analgesics. Although antinociceptive effects of KRM-II-81 are less reliable and shorter acting that those of the NSAID ketorolac or acetaminophen, it may be possible to develop higher efficacy and/or longer acting α2/α3-subtype-selective GABAAR PAMs than KRM-II-81. Additionally, KRM-II-81 or other α2/α3-subtype-selective GABAAR PAMs may be useful as alternatives or adjuncts to non-opioid or opioid analgesics in patients who cannot tolerate side-effects of these compounds.
Acknowledgements
We want to acknowledge the NIH for financial support (R01NS070715, R01MH096463, R01NS076517, T32DA007027). We also thank the Milwaukee Institute for Drug Discovery and University of Wisconsin-Milwaukee’s Shimadzu Laboratory for Advanced and Applied Analytical Chemistry for the help with spectroscopy.
Source of Funding: R01NS070715, R01NS076517, R01MH096463 and T32DA007027
Footnotes
Conflict of Interest: No conflicts of interest to declare
References
- Altarifi AA, Rice KC, Negus SS (2015). Effects of mu-opioid receptor agonists in assays of acute pain-stimulated and pain-depressed behavior in male rats: role of mu-agonist efficacy and noxious stimulus intensity. J Pharmacol Exp Ther 352:208–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atack JR (2011). GABAA receptor subtype-selective modulators. I. alpha2/alpha3-selective agonists as non-sedating anxiolytics. Curr Top Med Chem 11:1176–202. [DOI] [PubMed] [Google Scholar]
- Ator NA, Atack JR, Hargreaves RJ, Burns HD, Dawson GR (2010). Reducing abuse liability of GABAA/benzodiazepine ligands via selective partial agonist efficacy at alpha1 and alpha2/3 subtypes. J Pharmacol Exp Ther 332:4–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baley K, Michalov K, Kossick MA, McDowell M (2014). Intravenous acetaminophen and intravenous ketorolac for management of pediatric surgical pain: a literature review. AANA J 82:53–64. [PubMed] [Google Scholar]
- Bellantuono C, Reggi V, Tognoni G, Garattini S (1980). Benzodiazepines: clinical pharmacology and therapeutic use. Drugs 19:195–219. [DOI] [PubMed] [Google Scholar]
- Bohlhalter S, Weinmann O, Mohler H, Fritschy JM (1996). Laminar compartmentalization of GABAA-receptor subtypes in the spinal cord: an immunohistochemical study. J Neurosci 16:283–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA Jr., Chartoff EH (2007). Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc 2:2987–95. [DOI] [PubMed] [Google Scholar]
- Collins FS, Koroshetz WJ, Volkow ND (2018). Helping to end addiction over the long-term: The research plan for the NIH HEAL initiative. JAMA [DOI] [PMC free article] [PubMed]
- Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, et al. (2005). BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 438:1017–21. [DOI] [PubMed] [Google Scholar]
- Crestani F, Rudolph U (2015). Behavioral functions of GABAA receptor subtypes--the Zurich experience. Adv Pharmacol 72:37–51. [DOI] [PubMed] [Google Scholar]
- Di Lio A, Benke D, Besson M, Desmeules J, Daali Y, Wang ZJ, et al. (2011). HZ166, a novel GABAA receptor subtype-selective benzodiazepine site ligand, is antihyperalgesic in mouse models of inflammatory and neuropathic pain. Neuropharmacology 60:626–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duthie DJ, Nimmo WS (1987). Adverse effects of opioid analgesic drugs. Br J Anaesth 59:61–77. [DOI] [PubMed] [Google Scholar]
- Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, et al. (2005). Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 113:9–19. [DOI] [PubMed] [Google Scholar]
- Enna SJ, McCarson KE (2006). The role of GABA in the mediation and perception of pain. Adv Pharmacol 54:1–27. [DOI] [PubMed] [Google Scholar]
- Estape J, Vinolas N, Gonzalez B, Ingles F, Bofill T, Guzman MC, Tarrago E (1990). Ketorolac, a new non-opioid analgesic: a double-blind trial versus pentazocine in cancer pain. J Int Med Res 18:298–304. [DOI] [PubMed] [Google Scholar]
- Evans SM, Funderburk FR, Griffiths RR (1990). Zolpidem and triazolam in humans: behavioral and subjective effects and abuse liability. J Pharmacol Exp Ther 255:1246–55. [PubMed] [Google Scholar]
- Ewan EE, Martin TJ (2014). Differential suppression of intracranial self-stimulation, food-maintained operant responding, and open field activity by paw incision and spinal nerve ligation in rats. Anesth Analg 118:854–62. [DOI] [PubMed] [Google Scholar]
- Fischer BD, Schlitt RJ, Hamade BZ, Rehman S, Ernst M, Poe MM, et al. (2017). Pharmacological and antihyperalgesic properties of the novel alpha2/3 preferring GABAA receptor ligand MP-III-024. Brain Res Bull 131:62–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman BW, Irizarry E, Solorzano C, Khankel N, Zapata J, Zias E, Gallagher EJ (2017). Diazepam Is No Better Than Placebo When Added to Naproxen for Acute Low Back Pain. Ann Emerg Med 70:169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CD, FitzGerald GA (2007). COX-2 inhibitors and cardiovascular risk. J Cardiovasc Pharmacol 50:470–9. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Bigelow G, Liebson I (1979). Human drug self-administration: double-blind comparison of pentobarbital, diazepam, chlorpromazine and placebo. J Pharmacol Exp Ther 210:301–10. [PubMed] [Google Scholar]
- Harvey SC, Foster KL, McKay PF, Carroll MR, Seyoum R, Woods JE 2nd, et al. (2002). The GABA(A) receptor alpha1 subtype in the ventral pallidum regulates alcohol-seeking behaviors. J Neurosci 22:3765–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy R, Calsbeek JJ, Morgan MM (2016). Home cage wheel running is an objective and clinically relevant method to assess inflammatory pain in male and female rats. J Neurosci Methods 263:115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissin I (2010). The development of new analgesics over the past 50 years: a lack of real breakthrough drugs. Anesth Analg 110:780–9. [DOI] [PubMed] [Google Scholar]
- Knabl J, Witschi R, Hosl K, Reinold H, Zeilhofer UB, Ahmadi S, et al. (2008). Reversal of pathological pain through specific spinal GABAA receptor subtypes. Nature 451:330–4. [DOI] [PubMed] [Google Scholar]
- Knabl J, Zeilhofer UB, Crestani F, Rudolph U, Zeilhofer HU (2009). Genuine antihyperalgesia by systemic diazepam revealed by experiments in GABAA receptor point-mutated mice. Pain 141:233–8. [DOI] [PubMed] [Google Scholar]
- Knutson DE, Kodali R, Divovic B, Treven M, Stephen MR, Zahn NM, et al. (2018). Design and Synthesis of Novel Deuterated Ligands Functionally Selective for the gamma-Aminobutyric Acid Type A Receptor (GABAAR) alpha6 Subtype with Improved Metabolic Stability and Enhanced Bioavailability. J Med Chem 61:2422–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwilasz AJ, Negus SS (2012). Dissociable effects of the cannabinoid receptor agonists Delta9-tetrahydrocannabinol and CP55940 on pain-stimulated versus pain-depressed behavior in rats. J Pharmacol Exp Ther 343:389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewter LA, Fisher JL, Siemian JN, Methuku KR, Poe MM, Cook JM, Li JX (2017). Antinociceptive Effects of a Novel alpha2/alpha3-Subtype Selective GABAA Receptor Positive Allosteric Modulator. ACS Chem Neurosci 8:1305–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling W, Mooney L, Hillhouse M (2011). Prescription opioid abuse, pain and addiction: clinical issues and implications. Drug Alcohol Rev 30:300–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvak KM, McEvoy GK (1990). Ketorolac, an injectable nonnarcotic analgesic. Clin Pharm 9:921–35. [PubMed] [Google Scholar]
- Luger TJ, Hayashi T, Weiss CG, Hill HF (1995). The spinal potentiating effect and the supraspinal inhibitory effect of midazolam on opioid-induced analgesia in rats. Eur J Pharmacol 275:153–62. [DOI] [PubMed] [Google Scholar]
- Martin TJ, Buechler NL, Kahn W, Crews JC, Eisenach JC (2004). Effects of laparotomy on spontaneous exploratory activity and conditioned operant responding in the rat: a model for postoperative pain. Anesthesiology 101:191–203. [DOI] [PubMed] [Google Scholar]
- Melzack R, Wall PD (1965). Pain mechanisms: a new theory. Science 150:971–9. [DOI] [PubMed] [Google Scholar]
- Moore KA, Kohno T, Karchewski LA, Scholz J, Baba H, Woolf CJ (2002). Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J Neurosci 22:6724–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro G, Lopez-Garcia JA, Rivera-Arconada I, Erichsen HK, Nielsen EO, Larsen JS, et al. (2008). Comparison of the novel subtype-selective GABAA receptor-positive allosteric modulator NS11394 [3’-[5-(1-hydroxy-1-methyl-ethyl)-benzoimidazol-1-yl]-biphenyl-2-carbonitrile] with diazepam, zolpidem, bretazenil, and gaboxadol in rat models of inflammatory and neuropathic pain. J Pharmacol Exp Ther 327:969–81. [DOI] [PubMed] [Google Scholar]
- Munro G, Ahring PK, Mirza NR (2009). Developing analgesics by enhancing spinal inhibition after injury: GABAA receptor subtypes as novel targets. Trends Pharmacol Sci 30:453–9. [DOI] [PubMed] [Google Scholar]
- Negus SS (2013). Expression and treatment of pain-related behavioral depression. Lab Anim (NY) 42:292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Miller LL (2014). Intracranial self-stimulation to evaluate abuse potential of drugs. Pharmacol Rev 66:869–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Vanderah TW, Brandt MR, Bilsky EJ, Becerra L, Borsook D (2006). Preclinical assessment of candidate analgesic drugs: recent advances and future challenges. J Pharmacol Exp Ther 319:507–14. [DOI] [PubMed] [Google Scholar]
- Negus SS, Bilsky EJ, Do Carmo GP, Stevenson GW (2010). Rationale and methods for assessment of pain-depressed behavior in preclinical assays of pain and analgesia. Methods Mol Biol 617:79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, O’Connell R, Morrissey E, Cheng K, Rice KC (2012). Effects of peripherally restricted kappa opioid receptor agonists on pain-related stimulation and depression of behavior in rats. J Pharmacol Exp Ther 340:501–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Neddenriep B, Altarifi AA, Carroll FI, Leitl MD, Miller LL (2015). Effects of ketoprofen, morphine, and kappa opioids on pain-related depression of nesting in mice. Pain 156:1153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W (2009). GABA A receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology 56:141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul J, Zeilhofer HU, Fritschy JM (2012). Selective distribution of GABA(A) receptor subtypes in mouse spinal dorsal horn neurons and primary afferents. J Comp Neurol 520:3895–911. [DOI] [PubMed] [Google Scholar]
- Paul J, Yevenes GE, Benke D, Di Lio A, Ralvenius WT, Witschi R, et al. (2014). Antihyperalgesia by alpha2-GABAA receptors occurs via a genuine spinal action and does not involve supraspinal sites. Neuropsychopharmacology 39:477–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira Do Carmo G, Stevenson GW, Carlezon WA, Negus SS (2009). Effects of pain-and analgesia-related manipulations on intracranial self-stimulation in rats: further studies on pain-depressed behavior. Pain 144:170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persohn E, Malherbe P, Richards JG (1992). Comparative molecular neuroanatomy of cloned GABAA receptor subunits in the rat CNS. J Comp Neurol 326:193–216. [DOI] [PubMed] [Google Scholar]
- Poe MM, Methuku KR, Li G, Verma AR, Teske KA, Stafford DC, et al. (2016). Synthesis and Characterization of a Novel gamma-Aminobutyric Acid Type A (GABAA) Receptor Ligand That Combines Outstanding Metabolic Stability, Pharmacokinetics, and Anxiolytic Efficacy. J Med Chem 59:10800–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralvenius WT, Benke D, Acuna MA, Rudolph U, Zeilhofer HU (2015). Analgesia and unwanted benzodiazepine effects in point-mutated mice expressing only one benzodiazepine-sensitive GABAA receptor subtype. Nat Commun 6:6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralvenius WT, Acuna MA, Benke D, Matthey A, Daali Y, Rudolph U, et al. (2016). The clobazam metabolite N-desmethyl clobazam is an alpha2 preferring benzodiazepine with an improved therapeutic window for antihyperalgesia. Neuropharmacology 109:366–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg MB, Carroll FI, Negus SS (2013). Effects of monoamine reuptake inhibitors in assays of acute pain-stimulated and pain-depressed behavior in rats. J Pain 14:246–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlett JK, Woolverton WL (1998). Discriminative stimulus effects of benzodiazepine agonists and partial agonists in pentobarbital-trained rhesus monkeys. Behav Pharmacol 9:81–92. [PubMed] [Google Scholar]
- Rowlett JK, Platt DM, Lelas S, Atack JR, Dawson GR (2005). Different GABAA receptor subtypes mediate the anxiolytic, abuse-related, and motor effects of benzodiazepine-like drugs in primates. Proc Natl Acad Sci U S A 102:915–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwienteck KL, Negus SS, Poklis JL, Banks ML (2015). Effects of continuous nicotine treatment and subsequent termination on cocaine versus food choice in male rhesus monkeys. Exp Clin Psychopharmacol 23:395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwienteck KL, Li G, Poe MM, Cook JM, Banks ML, Stevens Negus S (2017). Abuse-related effects of subtype-selective GABAA receptor positive allosteric modulators in an assay of intracranial self-stimulation in rats. Psychopharmacology (Berl) 234:2091–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinday NM, Sawyer EK, Fischer BD, Platt DM, Licata SC, Atack JR, et al. (2013). Reinforcing effects of compounds lacking intrinsic efficacy at alpha1 subunit-containing GABAA receptor subtypes in midazolam-but not cocaine-experienced rhesus monkeys. Neuropsychopharmacology 38:1006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sostres C, Gargallo CJ, Arroyo MT, Lanas A (2010). Adverse effects of non-steroidal anti-inflammatory drugs (NSAIDs, aspirin and coxibs) on upper gastrointestinal tract. Best Pract Res Clin Gastroenterol 24:121–32. [DOI] [PubMed] [Google Scholar]
- Stevenson GW, Mercer H, Cormier J, Dunbar C, Benoit L, Adams C, et al. (2011). Monosodium iodoacetate-induced osteoarthritis produces pain-depressed wheel running in rats: implications for preclinical behavioral assessment of chronic pain. Pharmacol Biochem Behav 98:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker AP, Mezzatesta J, Nadeson R, Goodchild CS (2004). Intrathecal midazolam II: combination with intrathecal fentanyl for labor pain. Anesth Analg 98:1521–7. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Collins FS (2017). The Role of Science in Addressing the Opioid Crisis. N Engl J Med 377:391–4. [DOI] [PubMed] [Google Scholar]
- White PF, Joshi GP, Carpenter RL, Fragen RJ (1997). A comparison of oral ketorolac and hydrocodone-acetaminophen for analgesia after ambulatory surgery: arthroscopy versus laparoscopic tubal ligation. Anesth Analg 85:37–43. [DOI] [PubMed] [Google Scholar]
- Wilt TJ, MacDonald R, Brasure M, Olson CM, Carlyle M, Fuchs E, et al. (2016). Pharmacologic Treatment of Insomnia Disorder: An Evidence Report for a Clinical Practice Guideline by the American College of Physicians. Ann Intern Med 165:103–12. [DOI] [PubMed] [Google Scholar]
- Witkin JM, Smith JL, Ping X, Gleason SD, Poe MM, Li G, et al. (2018). Bioisosteres of ethyl 8-ethynyl-6-(pyridin-2-yl)-4H-benzo[f]imidazo [1,5-a][1,4]diazepine-3-carboxylate (HZ-166) as novel alpha 2,3 selective potentiators of GABAA receptors: Improved bioavailability enhances anticonvulsant efficacy. Neuropharmacology 137:332–43. [DOI] [PubMed] [Google Scholar]
- Woods JH, Winger G (1995). Current benzodiazepine issues. Psychopharmacology (Berl) 118:107–15. [DOI] [PubMed] [Google Scholar]
- Xie JY, Qu C, Patwardhan A, Ossipov MH, Navratilova E, Becerra L, et al. (2014). Activation of mesocorticolimbic reward circuits for assessment of relief of ongoing pain: a potential biomarker of efficacy. Pain 155:1659–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeilhofer HU, Zeilhofer UB (2008). Spinal dis-inhibition in inflammatory pain. Neurosci Lett 437:170–4. [DOI] [PubMed] [Google Scholar]