Abstract
The γ-retroviral vector is a gene delivery vehicle that is commonly used in gene therapy. Despite its efficacy, its strong enhancers contributed to malignant transformations in some hematopoietic stem cell (HSC) gene therapy trials. A safer version without viral enhancers (SIN) is available, but its production is cumbersome, as high titers can only be obtained in transient transfection. Our aim was to develop a system that could easily generate high-titer SIN vectors from stable producer cells. The use of the cytomegalovirus enhancer-promoter sequence to generate the full-length genomic RNA combined to sequences that decrease transcriptional readthrough (WPRE and strong polyadenylation sequences) led to 6 × 106 infectious units (IU)/mL of a SIN GFP vector in transient transfection. The incorporation of a blasticidin selection cassette to the retroviral plasmid allowed the generation of stable clones in the 293Vec packaging cells that release 2 × 107 IU/mL and 1.4 × 107 IU/mL of a SIN GFP and a SIN PIGA vector, respectively. A titer of 1.8 × 106 IU/mL was obtained with a SIN vector containing the long 8.9-kb COL7A1 cDNA. Thus, an efficient process was established for the generation of stable 293Vec-derived retrovirus producer cells that release high-titer SIN vectors.
Keywords: SIN γ-retroviral vector, packaging cell, gene transfer, type VII collagen, epidermolysis bullosa
Introduction
The first viral vector developed for gene delivery was derived from the Moloney murine leukemia (MLV) γ-retrovirus.1 This vector integrates in the genome of dividing cells that can then be passed to daughter cells,2 a property that is required for approaches involving hematopoietic stem cell (HSC) and T cell genetic engineering.
The γ-retroviral vector can be produced by transient transfections or with stable packaging cell lines. The latest option is preferable, as the manufacturing is easier, more reproducible, less expensive, and more suitable for large-scale productions needed for late-phase trials and commercialization.3, 4
The γ-retroviral vector is widely used in ex vivo gene therapy applications for the treatment of genetic diseases and cancer. Successful examples of therapies using this vector are Strimvelis, Yescarta, and Zalmoxis, which have been recently approved for commercialization.5, 6, 7 Strimvelis is a HSC gene therapy treatment for adenosine deaminase (ADA) deficiency,5 and Yescarta is a treatment for non-Hodgkin lymphoma in which the patient’s own T cells are genetically modified to express a chimeric antigen receptor directed toward CD19.8 Zalmoxis is a gene therapy treatment that controls graft versus host disease (GVHD) in patients undergoing haploidentical bone marrow transplantation. In this approach, allogeneic T cells are engineered in vitro to express the herpes simplex virus thymidine kinase (HSV-TK) gene. After grafting, these cells would be eliminated in the presence of the nucleoside analog ganciclovir if the patient develops GVHD.9
In 2000, Cavazzano-Calvo and colleagues used a γ-retroviral vector to successfully treat X-linked severe combined immunodeficiency (SCID-X1) patients.10 Mutations in the interleukin-2 receptor γC (IL2RG) gene in SCID-X1 lead to an absence of T cell and natural killer cell development and B cell dysfunction.11 In the gene therapy trial, harvested HSCs were transduced in vitro with a γ-retroviral vector containing the IL2RG cDNA. Cells were subsequently reinfused in patients, and a normal hematopoiesis with a full immune reconstitution was observed shortly after in the majority of patients.10, 12 Unfortunately, 4 patients in the original French trial, as well as another one in a similar trial in London, developed T cell acute lymphoblastic leukemia between 2 and 6 years after gene therapy.13, 14, 15 Other cases of leukemias and myelodisplastic syndromes were reported in HSC γ-retroviral gene therapy for Wiskott-Aldrich syndrome and chronic granulomatous disease.16, 17 In all these patients, the integration site of the vector was near proto-oncogene sequences like LMO2, CCND2, PRDM16, SETBP1, and MDS1-EVI1 that became transcriptionally activated, owing to the strong viral enhancers present in the vector. In addition, several chromosomic alterations were observed in the leukemic cells of these patients.14, 15, 16, 17, 18 Surprisingly, no leukemia cases have been reported in HSC gene therapy for ADA,5, 19 suggesting that other factors, like the nature of the transgene or of the disease treated could have also contributed to the malignant transformation mediated by the γ-retroviral vector. Thereafter, several groups have clearly shown in vitro and in animal models that a self-inactivated (SIN) γ-retroviral or lentiviral vector that has its enhancers deleted was less oncogenic than its wild-type counterpart, as long as it contains a relatively weak internal promoter.20, 21, 22, 23, 24 Based on these results, a new SCID-X1 gene therapy clinical trial was launched with a SIN γ-retroviral vector. More than 6 years after treatment, there were no leukemia cases reported in the 7 patients who have been followed, and the efficacy was comparable to that of previous trials in which a regular γ-retroviral vector was used.25
Like the γ-retroviral vector, the lentiviral vector is integrative, and it is used in HSC gene therapy. The SIN lentiviral vector has a good safety profile with, so far, no oncogenic events reported in HSC clinical trials.26, 27, 28 The toxicity of some lentiviral proteins and the vesicular stomatitis virus-G (VSV-G) envelope that is commonly used to pseudotype the vector limits the manufacturing to transient systems.29 The SIN γ-retroviral vector manufactured for the SCID-X1 trial was pseudotyped with the non-toxic envelope from the gibbon ape leukemia virus (Galv); but it was still produced by transient transfections in order to get relatively good titers, around 106 infectious units per milliliter (IU/mL).30
A few years ago, we constructed three retrovirus packaging cell lines that release high-titer viral particles pseudotyped with the amphotropic (293Vec-Ampho), the RD114 (293Vec-RD114), and the Galv (293Vec-Galv) envelopes. These cell lines can produce regular γ-retroviral vectors with titers in the range of 107 IU/mL, and they have the properties to grow in suspension and serum-free media.31, 32, 33, 34 In this study, our goal was to design an optimized SIN vector that could lead to high-titer vectors with the 293Vec packaging cell lines. The SIN γ-retroviral vector that was constructed led to high-titer vector production in transient transfection. High-titer retrovirus producer clones were also easily established from the 293Vec-RD114 and 293Vec-Ampho cells with three different SIN retroviral vectors containing the GFP gene and two therapeutic transgenes.
Results
Optimization of a SIN γ-Retroviral Vector
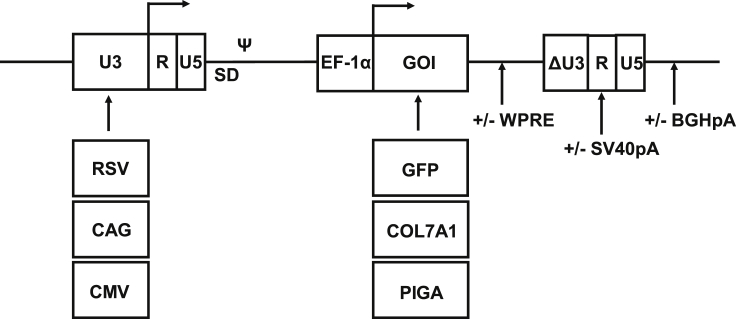
The introduction of strong enhancer-promoter sequences in place of U3 in the 5′ long terminal repeat (5′ LTR) sequence has been proposed to increase titers of SIN γ-retroviral and lentiviral vectors.35, 36, 37 In this study, the enhancer-promoter sequences of the cytomegalovirus (CMV) and of the Rous sarcoma virus (RSV) were compared in the context of a SIN MFG-derived γ-retroviral vector.38 The synthetic sequence composed of the CMV enhancer sequence fused to the chicken β-actin promoter (CAG) was also tested, as its strength is superior to that of the CMV and the RSV enhancer-promoter sequences in several eukaryotic cell lines.39 Vector optimization was conducted with the GFP gene under the control of the human elongation factor-1 α promoter (EF-1α), and their designs are displayed in Figure 1.
Figure 1.
Design of SIN γ-Retroviral Vectors Used in This Study
SIN vectors contain a gene of interest (GOI; GFP, COL7A1, or PIGA) under the control of the EF-1α promoter. Modifications include three enhancer-promoter sequences (RSV, CAG, and CMV), the WPRE, and two poly(A) sequences (SV40pa and BGHpa). SD and Ψ splice donor site and packaging signal sequence, respectively.
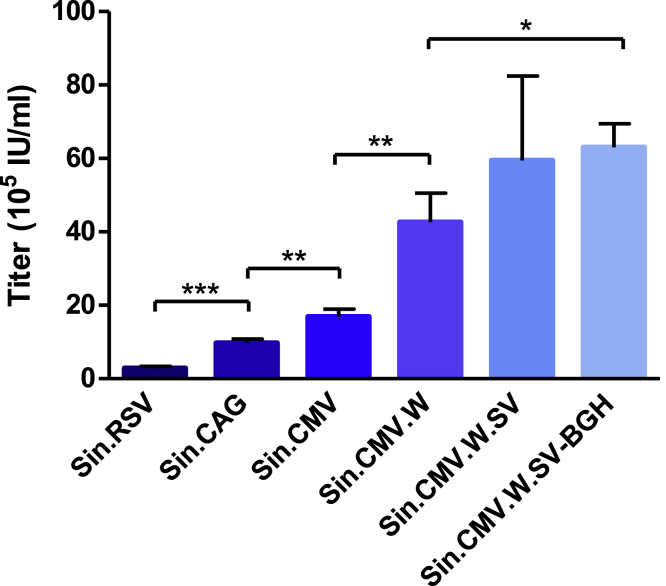
Transient productions of SIN.RSV generated a titer of 3 × 105 IU/mL, and a 5.6-fold higher titer was obtained with the SIN.CMV vector. The titer of the SIN.CAG vector that was also tested was intermediary at 9.6 × 105 IU/mL. The woodchuck hepatitis posttranscriptional regulatory element (WPRE) sequence and strong polyadenylation (poly(A)) sequences were next incorporated to the SIN.CMV vector to counteract the readthrough of the MLV poly(A).40 WPRE increased the titer from 1.7 × 106 to 4.2 × 106 IU/mL, and a titer of 6 × 106 IU/mL was obtained with the incorporation of the simian virus 40 (SV40) poly(A) in the R region. The addition of the bovine growth hormone (BGH) poly(A) downstream to the 3′ LTR of the SIN.CMV.W.SV gave a titer of 6.2 × 106 IU/mL (Figure 2). These results showed that the CMV sequence is superior to the RSV and CAG sequences for the production of the MFG-derived SIN γ-retroviral vector, and that the subsequent additions of WPRE and poly(A) sequences have a positive effect on viral production.
Figure 2.
Titers of Different SIN GFP Vectors Produced in Transient Transfections
Titers were assessed by measuring GFP fluorescence by FACS analysis of HT-1080-transduced cells. Values presented are the mean ± SD of three independent experiments. *p < 0.05; **p < 0.01; ***p < 0.005.
Production of SIN GFP γ-Retroviral Vectors in Stable Packaging Cell Lines
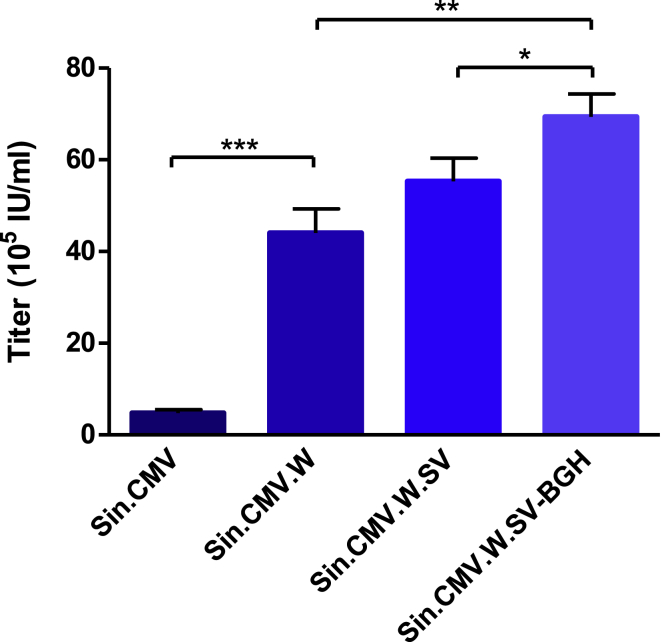
Vectors containing the CMV enhancer-promoter sequences were next tested during stable viral productions. The 293Vec-RD114 packaging cells were co-transfected with each construct and a hygromycin resistance (Hygr) plasmid, and viral titers from supernatants of sorted GFP-positive cell populations were measured. Results were comparable to those obtained by transient transfections, as each modification added to the vector increased titers. The addition of the WPRE sequence to SIN.CMV increased the titer from 4.8 × 105 to 4.4 × 106 IU/mL. The successive incorporation of the SV40 and the BGH poly(A) sequences further increased the titers to 5.6 × 106 and 7 × 106 IU/mL, respectively (Figure 3). Thus, the increased titers observed in transient transfections with the optimized SIN vectors were also found with stable productions.
Figure 3.
Titers of SIN GFP Vectors Produced from Stable 293Vec-RD114 Producer Cell Lines
Titers were assessed by measuring GFP fluorescence by FACS analysis of HT-1080-transduced cells. Values presented are the mean ± SD of three independent experiments. *p < 0.05; **p < 0.01; ***p < 0.005.
SIN γ-Retroviral Vectors Containing Therapeutic Transgenes
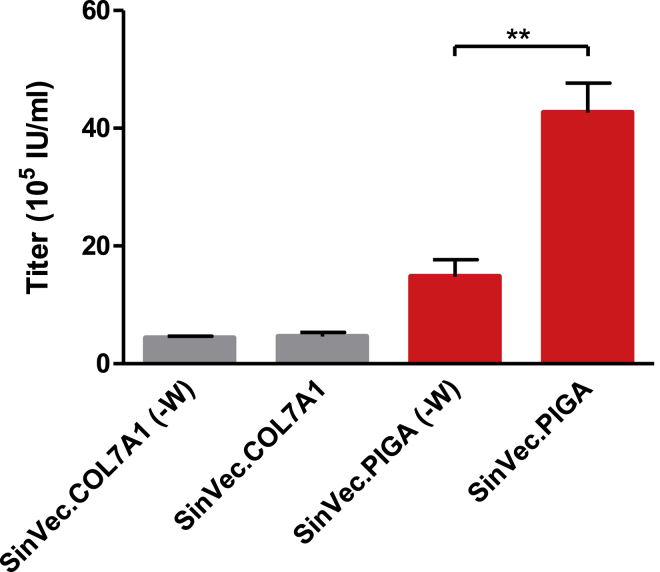
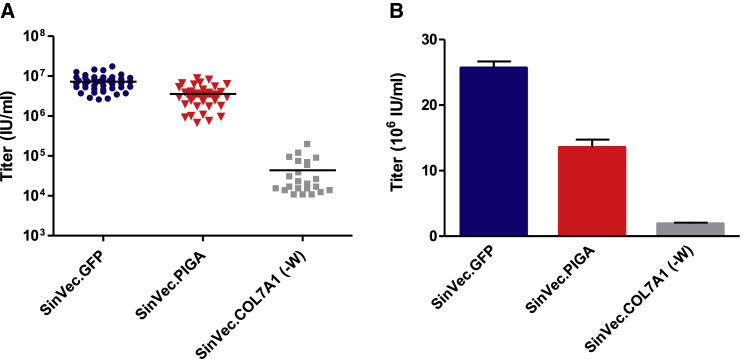
The performance of the SIN γ-retroviral vector was then assessed with 2 different therapeutic transgenes. The first one is the 8.9-kb COL7A1 cDNA that could be used to correct keratinocytes and fibroblasts of recessive dystrophic epidermolysis bullosa (RDEB) patients. A vector with such a long transgene would be less efficiently packaged and would lead to low titers.41, 42, 43 We hypothesized that the benefit of WPRE could be cancelled by its size (700 bp), which would further decrease the packaging ability of a COL7A1 vector. Two SIN vectors with or without WPRE were then constructed, and their titers were compared in transient productions. Similar vectors were also constructed with a smaller therapeutic transgene: the phosphatidylinositol glycan-class A (PIGA) cDNA (1.4 kb). HSC gene transfer of the PIGA cDNA has been proposed as a treatment for paroxysmal nocturnal hemoglobinuria patients.44 As expected, titers with COL7A1 vectors were lower than those obtained with PIGA and GFP vectors. Titers of 4 × 105 IU/mL were measured for both COL7A1 vectors (with and without WPRE). Higher titers were obtained with the PIGA vectors that were measured on the K562(PIG-) cell line, a PIGA knockout cell line with a lack of CD59 anchored at the cell surface (Figure S1): 1.4 × 106 IU/mL for the version without WPRE, and 4 × 106 IU/mL when WPRE was added to the vector (Figure 4). These results indicated that the titer increase mediated by WPRE with the GFP SIN vectors could be reproduced with the PIGA vector. On the contrary, WPRE had no significant effect on the titer of the COL7A1 vector. For simplicity, SIN vector final versions used in the study will be referred to hereinafter as SINVec.GFP, SINVec.PIGA, and SINVec.COL7A1 (-W).
Figure 4.
Effect of WPRE in SIN COL7A1 and PIGA Vectors Produced in Transient Transfections
Titers of COL7A1 vectors were assessed by measuring collagen VII expression by FACS analysis of HT-1080-transduced cells. Titers of PIGA vectors were assessed by measuring CD59 expression by FACS analysis of K562(PIG-)-transduced cells. Values presented are the mean ± SD of three independent experiments. **p < 0.01.
An Efficient Selection Method for the Stable Production of SIN Vectors
A blasticidin resistance (BSDr) cassette was introduced in the SIN GFP retroviral plasmid to facilitate the generation of stable retrovirus producer cells (Figure S2). The BSDr gene was placed under the control of a minimal promoter fragment from the HSV-TK gene, and this cassette was ligated to the retroviral plasmid. Our hypothesis was that several copies of vectors would be needed to confer blasticidin resistance; this would lead to more integrated plasmid copies in positive cells as well as cells that release vector at higher titers. To validate our hypothesis, packaging cells were transfected with the GFP SIN plasmid containing the blasticidin cassette or co-transfected with the GFP SIN plasmid and a Hygror plasmid for comparison. Cells selected with blasticidin were 91% GFP positive, while only 48% of the cells were fluorescent after hygromycin selection (Figure S3). Furthermore, sorted GFP-positive cells were 2.1-fold more fluorescent if cells had been selected in blasticidin versus hygromycin. There were also 9.9 plasmid copies integrated per cell in the BSDr cell population, while only 2.9 copies were present in the Hygror cells. These differences between the two cell populations led to the production of vectors at 7 × 106 IU/mL from the Hygror cells and 1.7 × 107 IU/mL from the BSDr cells (Table 1). Thus, the addition of a stringent blasticidin selection cassette to our SIN retroviral plasmid was an efficient strategy to select high-titer retrovirus producer cells.
Table 1.
Comparison between Hygromycin and Blasticidin Selection Systems in 293Vec-RD114-Transfected Packaging Cells
| Experiment | Hygromycin Selection |
Blasticidin Selection |
||||
|---|---|---|---|---|---|---|
| PCN | GFP Mean Fluorescence | Titer (106 IU/mL) | PCN | GFP Mean Fluorescence | Titer (106 IU/mL) | |
| 1 | 2.5 | 16.8 | 3.4 | 13.7 | 36.8 | 8.1 |
| 2 | 2.4 | 14.2 | 3.7 | 7 | 32.3 | 7.7 |
| 3 | 3.9 | 16.7 | 3.5 | 9.1 | 31.8 | 9.2 |
PCN, plasmid copy number.
Generation of Stable Retrovirus Producer Clones with SIN Vectors
SIN vectors with GFP, COL7A1, and PIGA were next tested for stable vector productions in packaging cell clones. WPRE was kept for the SIN GFP and PIGA vectors but not for the COL7A1 vector, as this sequence had no positive effect on titers (Figure 4). The blasticidin cassette was introduced in each of these retroviral plasmids that were transfected in the 293Vec-RD114 packaging cells for the GFP and the PIGA vectors and in the 293Vec-Ampho packaging cells for the COL7A1 vector, as amphotropic pseudotyped vectors are more efficient for transducing human fibroblasts.43 All the 43 clones screened with the GFP vector had titers above 2 × 106 IU/mL, and for the PIGA vector, 90% had titers above 6 × 105 IU/mL. For the COL7A1 vector, 80% of the clones were above 2 × 103 IU/mL, with an average titer of 6 × 104 IU/mL (Figure 5A). Vectors produced from the best clones generated titers of 2.6 × 107 IU/mL for the GFP vector, 1.4 × 107 IU/mL for the PIGA vector, and 1.8 × 106 IU/mL for the COL7A1 vector (Figure 5B). These packaging cell clones had an average of 6.5 plasmid copies per cell and demonstrated a stable viral vector production for a 3-month period (Figure S4). High-titer retrovirus producer clones could then be easily established with three different SIN vectors in the 293Vec packaging cells.
Figure 5.
Production of SIN Vectors from Stable Packaging Cell Clones Selected in Blasticidin
(A) Screening titers of vectors produced from 293Vec-RD114 clones transfected with SINVec.GFP and SINVec.PIGA and from 293Vec-Ampho clones transfected with SINVec.COL7A (-W). (B) Titrations of SINVec.GFP and SINVec.PIGA vectors produced from the best 293Vec-RD114 clones and titration of the SINVec.COL7A1 (-W) produced from the best 293Vec-Ampho clone.
Transduction of Primary Cells with SIN Vectors
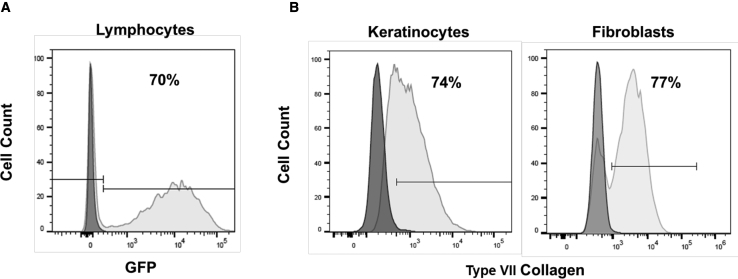
We next tested the transduction efficacy of the SIN GFP and COL7A1 vectors on primary cells. Human primary T lymphocytes were activated and transduced with the SIN GFP vector produced from the best 293Vec-RD114 clone in the presence of retronectin. A high transduction efficiency of 70% was achieved at a MOI of 5 (Figure 6A). Next, fibroblasts and keratinocytes from one RDEB patient negative for collagen VII were transduced with virus produced from our best 293Vec-Ampho clone. A strong transduction was achieved, as keratinocytes and fibroblasts were 74% and 77% positive for collagen VII expression, respectively (Figure 6B). We could conclude that SIN vectors produced from stable 293Vec clones have the potential to be used in clinical gene therapy trials.
Figure 6.
Transduction of Human Lymphocytes, RDEB Keratinocytes and Fibroblasts with SIN Vectors
(A and B) FACS analysis of primary cells was measured 3 days after transduction (A) of lymphocytes with the SINVec.GFP vector at a MOI of 5 and (B) of RDEB keratinocytes and fibroblasts with the SINVec.COL7A1 (-W) vector at a MOI of 1.
Discussion
Retroviral and lentiviral vectors are commonly used in ex vivo approaches that involve the genetic modification of HSCs and T lymphocytes. Although these vectors have been extremely safe when used with T cells,45, 46 leukemias and myelodysplastic syndroms have been reported in some HSC gene therapy clinical trials with the γ-retroviral vector.13, 14, 15, 17, 18 The absence of genotoxicity with the lentiviral vector is mainly due to its SIN design that also leads to a good safety profile when it is applied to the γ-retroviral vector.20, 25 The main objective of this study was to design a SIN γ-retroviral plasmid that could easily generate high-titer stable retrovirus producer cells, a feature that is currently not achievable with the lentiviral vector.
In this study, several modifications introduced in the MFG-derived γ-retroviral plasmid to increase viral titers were tested in transient transfections. The CMV enhancer-promoter sequence in place of U3 in the 5′ LTR and the addition of the WPRE sequence and two strong poly(A) sequences increased the titer of a SIN GFP vector up to 6 × 106 IU/mL. The addition of a BSDr cassette in the SIN plasmid allowed the easy selection of high-titer stable virus producer cells with the 293Vec-RD114 and 293Vec-Ampho packaging cells. Titers of 2.6 × 107 and 1.4 × 107 IU/mL were obtained with retrovirus producer cell clones with the SINVec.GFP and the SINVec.PIG-A vectors, respectively. SINVec.COL7A1 (-W) was produced at a titer of 1.8 × 106 IU/mL.
It has been well established that internal promoters in SIN γ-retroviral vectors interfere with the activity of the 5′ LTR. This promoter competition limits the production of the genomic RNA that leads to low vector titers.35 In addition, the deletion of U3 in the 3′ LTR of the SIN γ-retroviral vector leads to a defect in genomic RNA exportation.47, 48 The use of strong enhancer-promoter sequences in place of the U3 region in the 5′ LTR is a strategy that has been proposed to overcome this limitation with γ-retroviral as well as with lentiviral vectors.35, 36, 37 It has been previously shown with both vectors that the RSV LTR sequence led to higher titers than the CMV enhancer-promoter sequence.35, 36 In this study, the results were opposite as a 6-fold higher titer was found with the SIN.CMV vector versus the SIN.RSV vector in transient transfections (Figure 2). This difference could be explained by different vector backbones used among the studies: lentiviral from HIV type 1,36 γ-retroviral derived from the friend spleen focus-forming virus,35 and γ-retroviral derived from MLV (our study). Small nucleotide differences in the enhancer-promoter sequences used among the studies could also explain this discrepancy.
The WPRE sequence is often incorporated in lentiviral and retroviral vectors to increase viral titers as well as transgene expression in transduced cells.49, 50, 51 Its effect has been mainly attributed to a decrease from transcriptional readthrough that occurs with the relatively weak retrovirus poly(A) sites.40, 52 In our study, WPRE increased by 3-fold the titer of a SIN GFP vector produced by transient transfections, and this effect was 10-fold more pronounced when vectors were produced from stable packaging cell lines (Figures 2 and 3). This difference could be due to the limited amount of genomic RNAs available in the stable packaging cell lines that could be strongly increased by the addition of WPRE. On the contrary, the amount of genomic RNAs should not be that limiting in transient viral productions, as large quantities of plasmids would be present in the transfected cells. A similar trend has been observed with the incorporations of the two poly(A) signals: significant increases in titers were only obtained with stable retrovirus producer cells (Figure 3).
It has been shown that retroviral vectors that exceed the wild-type proviral RNA in size are produced at lower titers.41, 53 The 10-fold lower titers obtained in transient transfections with the SINVec.COL7A1 or the SINVec.COL7A1 (-W) vector versus the SINVec.GFP or the SINVec.PIGA vector were then expected (Figures 2 and 4). Indeed, the size of the SINVec.COL7A1 (-W) vector leads to a genomic RNA of 10.2 kb that exceeds by 1.9 kb the size of the wild-type genomic RNA. The addition of WPRE did not increase the titer of the SIN COL7A1 vector, but its effect was observed with the GFP and the PIGA vectors with a 2.5-fold and a 2.9-fold titer increase, respectively (Figures 2 and 4). One logical explanation for this result could be that the positive effect of WPRE is cancelled by its size, which would further decrease the packaging ability of the SIN COL7A1 vector.
Sorted GFP packaging cells co-transfected with the SIN.CMV.W.SV-BGH plasmid and a Hygror plasmid produced vectors with titers of 7 × 106 IU/mL (Figure 3). We hypothesized that the genomic RNAs were still limiting, because the 293Vec packaging cell line can produce non-SIN vectors with titers above 107 IU/mL.31, 32, 33 The addition of the BSDr cassette driven by a weak promoter sequence allowed the selection of cells with 3 times more copies that led to an increase in titers by 3-fold (Table 1). This strategy had another advantage, as fewer clones have to be screened since more than 90% of the cells were harboring the vector, as compared to 50% with the co-transfection strategy (Figure S3).
The generation of stable retrovirus producer cells with SIN γ-retroviral vectors had already been reported by two groups. In the first study, SIN retroviral plasmids were stably introduced in the Phoenix packaging cells with the piggybac transposon system. Viral titers obtained with this method were around 106 IU/mL and were comparable to the titer of a non-SIN vector produced in PG13 cells. It was not clear whether the piggybac transposon system was superior to a standard transfection method, as there was no such comparison in the study.54 Titers around 107 IU/mL were achieved with our SIN GFP and PIGA vectors in the 293Vec cells, which are comparable to titers obtained with non-SIN vectors in the same packaging cells.31, 32, 33, 34 Then, we assume that 10-fold higher titers were achieved with SIN vectors produced from 293Vec cells, as they release at least 10 times more virus than PG13 cells.33 In the second study, the SIN vector was introduced in the packaging cells by recombinase-mediated cassette exchange in a predefined locus of elevated transcription activity. With this strategy, SIN vector titers of 2 × 107 IU/mL and 4 × 106 IU/mL were obtained with a T cell receptor and a COL7A1 cDNA transgene, respectively. These titers are very similar to those found in our study. The advantage of this strategy is that only one copy of the vector is present in the packaging cell, which would facilitate its characterization. However, this strategy is cumbersome, as two cloning steps were necessary to obtain the final vector producer cells. The first cloning was for the exchange of the vector, and the second one was to remove the residual sequences that were required for targeting and selection.55 The 293Vec cells were also used in this study, suggesting that the potency of this packaging cell line to release high-titer vectors is key for the production of SIN vectors.
In conclusion, this work showed that high-titer stable producer cells could be easily established with an optimized SIN γ-retroviral vector in 293Vec cells. We conclude that the stable production of SIN γ-retroviral vectors with the 293Vec platform will be a more efficient, a more reliable, and a cheaper option for late-stage trials and commercialization than the production of SIN γ-retroviral or lentiviral vectors in transient transfection.
Materials and Methods
Ethics
The study was approved by the ethics committee of the CHU de Québec - Université Laval Research Centre, Quebec City, QC, Canada, for the protection of human subjects and was conducted in accordance with the Helsinki Declaration of 1975.
Plasmids
SIN retroviral vectors were constructed based on the MFG plasmid.38 Briefly, the viral enhancers (379 bp) located in the 3′ LTR region were deleted by NheI/SacI digestion. The digested vector was then blunted with T4 DNA polymerase and ligated. The 5′ LTR was modified by replacing the HindIII/SacI U3 region by different enhancer-promoter regions: the CMV (509 bp), the RSV (232 bp), and the CAG (644 bp).39 The human EF-1α, short version (245 bp), was then cloned in the three vectors as an internal promoter in XhoI/BamHI. In each vector, the GFP gene (720 bp) was inserted downstream of the EF-1α promoter in BamHI to generate SIN.CMV, SIN.RSV and SIN.CAG. Three other versions of the SIN.CMV were also constructed. First, the WPRE sequence (611 bp) was cloned downstream of GFP to generate SIN.CMV.W. To further improve the transcriptional termination, the SIN.CMV.W.SV vector was constructed by inserting a SV40 poly(A) blunt fragment (222 bp) in the R sequence of the 3′ LTR in SmaI. A BGH poly(A) fragment (256 bp) was then cloned downstream of the 3′ LTR in AflII to also improve the termination efficacy of the genomic RNA.
A cassette containing a BSDr gene under the control of the HSV-TK promoter was introduced in the SIN.CMV.W.SV-BGH plasmid to facilitate the generation of stable retrovirus packaging cell clones.
SIN therapeutic retroviral vectors were also constructed with PIGA and COL7A1 optimized cDNA sequences (GenScript, Township, NJ, USA). The cDNA fragments were introduced in BamHI in place of GFP in the plasmids containing WPRE and the two poly(A) sequences. Versions without WPRE were also constructed similarly.
Fragments used in the constructions of all SIN vectors have been isolated from existing plasmids by restriction digests or PCR amplifications or have been synthesized by GenScript.
The pMD2iHygror plasmid was constructed to select stable retrovirus producer cells by co-transfection with SIN GFP vectors. A SalI/XbaI iHygror cassette was cloned instead of the iZeor cassette in the PMD2iZeor plasmid digested by XhoI/XbaI.31 The iHygror fragment is a Hygror gene linked to an encephalomyocarditis virus internal ribosomal entry site.
For the inactivation of the PIGA gene, the PX330-Pig plasmid was constructed by inserting a guide pair of the following annealed oligonucleotides: 5′-caccgctcagtgcctgattgaaaga-3′ and 5′-aaactctttcaatcaggcactgagc-3′ synthesized by Integrated DNA Technologies (Coralville, IA, USA) in the BbsI site of pX330.56 This plasmid co-expresses the S. pyogenes Cas9 nuclease along with the PIGA guide RNA.
Cell Lines and Primary Cells
Human RDEB keratinocytes and fibroblasts used in this study were isolated from a punch biopsy by the two-step thermolysine and trypsin method from one RDEB patient as described elsewhere.43
All cell lines and primary fibroblasts used in this study were cultured with media supplemented with 10% fetal calf serum (FCS) (Life Technologies, Grand Island, NY, USA) and antibiotics. Human primary fibroblasts, HT-1080 cells (ATCC, CCL-121), 293T cells (ATCC, CRL-11268), 293Vec-RD114 cells, 293Vec-Ampho cells,31, 32 and their derivatives containing the GFP, the COL7A1, and the PIGA SIN vectors were cultured with DMEM (Wisent, Saint-Jean-Baptiste, QC, Canada). Human peripheral blood lymphocytes, K562 cells, and K562(PIG-) cells were cultured with RPMI (Wisent). Human primary keratinocytes were cultured with DMEM/Ham’s F12 (3:1) (Life Technologies) supplemented with 5% FetalClone II Serum (Hyclone, Logan, UT, USA), insulin (5 μg/mL; Sigma, St. Louis, MO, USA), hydrocortisone (0.4 μg/mL; Calbiochem, San Diego, CA, USA), isoproterenol hydrochloride (0.212 μg/mL; Sandoz Canada, Boucherville, QC, Canada), epidermal growth factor (10 ng/mL; Austral Biologicals, San Ramon, CA, USA), penicillin G (100 IU/mL), and gentamicin (25 μg/mL; Sigma). For all experiments, keratinocytes were co-cultured with irradiated human fibroblast feeder layers as previously described.43
Transfections and Virus Productions
The K562(PIG-) cell line was established by electroporating K562 cells with the PX-330-Pig plasmid using a Gene Pulser electroporation system (Bio-Rad Laboratories, Hercules, CA, USA).57 A few days after recovery, cells were stained with a CD59-antibody-labeled fluorescein isothiocyanate (FITC; Cederlane, Burlington, ON, Canada), and CD59-negative cells were selected by fluorescence-activated cell sorting (FACS) with the BD FACSAria II (BD Biosciences, San Jose, CA, USA). One week later, a second enrichment by FACS was performed, followed by cloning in 96-well plates. After a 3-week culture, the clone K562(PIG-) was selected and used for subsequent experiments.
Recombinant retroviruses were generated by transient transfection of 293T cells in 6-well plates using the polyethyleneimine transfection procedure. A total amount of 2 μg plasmid DNA containing 0.5 μg pMD2GPiZeor (gag/pol expression plasmid), 0.5 μg pMD2.G (VSV-G envelope expression plasmid), and 1 μg transfer vector was transfected. Sixteen hours after transfection, the medium was replaced by 1 mL DMEM. On the next day, the culture supernatant was harvested, aliquoted, and stored at −80°C for later use.
Stable retrovirus producer cells were generated by transfection using the calcium phosphate procedure. Subconfluent 293Vec-RD114 or 293Vec-Ampho cells plated in a 10-cm dish were transfected with 20 μg BSDr SIN vector plasmids. Two days later, cells were selected in media supplemented with blasticidin (8 μg/mL; Invitrogen, Carlsbad, CA, USA) for 2 weeks and then cloned by limiting dilution in 96-well plates. After 2 to 3 weeks, cells were transferred in 24-well plates and screened at confluency for an overnight virus production in 1 mL medium.
For the co-transfection experiments, 1 μg pMD2iHygror and 19 μg SIN GFP plasmids were used to transfect 293Vec-RD114 cells. Two days later, cells were selected in media supplemented with 200 μg/mL hygromycin (Calbiochem) for 2 weeks.
For the production of virus from stable producer cells, 5 × 106 cells were plated in a 10-cm dish, and 2 days later, 5 mL supernatant was harvested and kept frozen until use. Vectors were produced from 293Vec-RD114 transfected with SIN GFP plasmids selected in blasticidin or hygromycin and sorted for GFP fluorescence by FACS. Viruses from individual clones were also produced similarly.
Titrations
For all vectors, titers were determined by scoring fluorescent-positive target cells by FACS analysis. For the GFP and COL7A1 vectors, HT-1080 cells were inoculated at a density of 105 cells per well in 24-well plates. On the next day, the medium from each well was replaced with 1 mL serial dilutions of virus supernatants containing polybrene (PB; 8 μg/mL). Forty-eight to 72 h later, cells transduced with GFP vectors were trypsinized and analyzed for fluorescence by FACS. Cells transduced with the COL7A1 vectors were trypsinized and permeabilized with Perm/Wash Buffer (BD Biosciences). Cells were incubated with a mouse monoclonal antibody raised against human collagen VII (LH7.2; 1:1,000, Sigma), followed by a phycoerytrin antibody (1:1,000; BioLegend), and the fluorescence was analyzed by FACS. For the titration of the PIGA vectors, 100,000 K562(PIG-) cells per well in 48-well plates were transduced with serial dilutions of vectors in the presence of PB. Two days later, cells were stained with a FITC-labeled CD59 antibody (1:1,000; Cedarlane).
Vector titers were calculated using the following formula (N × P) × 2/(V × D), in which N is the cell number at the day of infection, p is the percentage of fluorescent-positive cells determined by flow cytometry, V is the viral volume applied, and D is the virus dilution factor. Titers were calculated when the percentage of fluorescent-positive cells constituted between 2% to 20%.
Transduction of Human Primary T Lymphocytes and RDEB Human Keratinocytes and Fibroblasts
Purification and transduction of human primary T lymphocytes were performed as previously described.32 Briefly, T cells isolated from a healthy donor were purified by Ficoll-Hypaque sedimentation of peripheral blood lymphocytes. Cells were then incubated for 2 h in medium with 10% FCS in a 10-cm dish, and non-adherent cells were then harvested and activated by beads coated with anti-CD3 and anti-CD28 antibodies for 48 h (Invitrogen) at a cell/bead ratio of 1/3 in medium supplemented with interleukin-2 (30 U/mL; Peprotech, Montreal, QC, Canada). Activated lymphocytes were then added onto retronectin-coated 96-well plates at 2.5 × 104 cells per well with virus at a MOI of 5.
RDEB human fibroblasts and keratinocytes null for collagen type VII were transduced with the SIN COL7A1 vector. Cells were plated at a density of 13,000 cells per square centimeter in 12-well plates, as previously described.43 Briefly, fibroblasts were incubated for 2 h, and keratinocytes were incubated for 4 h at 37°C at 8% CO2. Then, fibroblasts and keratinocytes were transduced at a MOI of 1 in the presence of 10 μg/mL of the EF-C peptide (GenScript), a better transduction enhancer than PB.43
Primary cell transduction efficiency was evaluated by FACS analysis, as described earlier in the section titled Titrations.
Real-Time qPCR
Genomic DNA (gDNA) was extracted using the DNeasy Tissue Kit (QIAGEN, Valencia, CA, USA). Real-time qPCR was performed using a Platinum SYBR Green qPCR SuperMix UDG kit (Thermo Fisher Scientific, Waltham, MA, USA) with a LightCycler 480 (Roche, Laval, QC, Canada). The plasmid integrated copy number was determined from a standard curve generated with the linearized plasmid SinVec.GFP, using primers specific for the WPRE sequence, or with gDNA from 293Vec-RD114 cells containing a single SinVec.GFP copy per cell, using primers specific for the LTR. Serial dilutions of each gDNA were tested and run in triplicates. Amplification of the endogenous reference gene COL7A1 was done in parallel to standardize for the starting amount of gDNA inputs. For the GFP cell populations (Hygror and BSDr), primer sequences specific for WPRE were as follows: forward, 5′-CCGTTGTCAGGCAACGTG-3′; reverse, 5′-AGCTGACAGGTGGTGGCAAT-3′. For the three retrovirus producer clones, primers sequences specific for LTR were as follows: forward, 5′-GTTGCATCCGACTTGTGGTCT-3′; reverse, 5′-CGCTGACGGGTAGTCAATCA-3′. Primer sequences for COL7A1 were as follows: forward, 5′-CCTTTACGCCGCTGACATTG-3′; and reverse, 5′- CACTGGCTGCTCCAGAGAAA-3′. Primers were obtained from Integrated DNA Technologies.
Statistical Analyses
All statistical tests were performed using GraphPad Prism (GraphPad Software, La Jolla, CA, USA). Data from experiments are expressed as mean ± SD. Student’s t test was used for comparison of differences between indicated groups. Significant differences are indicated as follows: *p < 0.05, **p < 0.01, and ***p < 0.005.
Author Contributions
K.G. and M.C. designed the experiments. K.G., M.B.-W., S.R., A.D.-P., M.B., and M.C. generated reagents, performed the experiments, and analyzed data. M.C. wrote the manuscript. All authors revised the manuscript.
Conflicts of Interest
M.C. and K.G. are inventors of a patent on the 293Vec technology and are shareholders of Biovec Pharma. The remaining authors declare no competing interests.
Acknowledgments
This work was supported by the Réseau de thérapie cellulaire, tissulaire et génique du Québec (ThéCell), supported by the Fonds de la Recherche du Québec en Santé (FRQS), Fondation du Grand défi Pierre Lavoie, and the Canadian Institutes of Health Research (CIHR) (MOP-133639 to M.C. and FDN-143213 to L.G.). L.G. is holder of the Canada Research Chair I in stem cells and tissue engineering from CIHR.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omtm.2019.05.013.
Supplemental Information
References
- 1.Mann R., Mulligan R.C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983;33:153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- 2.Miller D.G., Adam M.A., Miller A.D. Gene transfer by retrovirus vectors occurs only in cells that are actively replicating at the time of infection. Mol. Cell. Biol. 1990;10:4239–4242. doi: 10.1128/mcb.10.8.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coroadinha A.S., Gama-Norton L., Amaral A.I., Hauser H., Alves P.M., Cruz P.E. Production of retroviral vectors: review. Curr. Gene Ther. 2010;10:456–473. doi: 10.2174/156652310793797739. [DOI] [PubMed] [Google Scholar]
- 4.van der Loo J.C., Wright J.F. Progress and challenges in viral vector manufacturing. Hum. Mol. Genet. 2016;25(R1):R42–R52. doi: 10.1093/hmg/ddv451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aiuti A., Roncarolo M.G., Naldini L. Gene therapy for ADA-SCID, the first marketing approval of an ex vivo gene therapy in Europe: paving the road for the next generation of advanced therapy medicinal products. EMBO Mol. Med. 2017;9:737–740. doi: 10.15252/emmm.201707573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gancberg D. Twenty years of European Union support to gene therapy and gene transfer. Hum. Gene Ther. 2017;28:951–953. doi: 10.1089/hum.2017.110. [DOI] [PubMed] [Google Scholar]
- 7.Salmikangas P., Kinsella N., Chamberlain P. Chimeric antigen receptor T-cells (CAR T-cells) for cancer immunotherapy - moving target for industry? Pharm. Res. 2018;35:152. doi: 10.1007/s11095-018-2436-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brudno J.N., Kochenderfer J.N. Chimeric antigen receptor T-cell therapies for lymphoma. Nat. Rev. Clin. Oncol. 2018;15:31–46. doi: 10.1038/nrclinonc.2017.128. [DOI] [PubMed] [Google Scholar]
- 9.Greco R., Oliveira G., Stanghellini M.T., Vago L., Bondanza A., Peccatori J., Cieri N., Marktel S., Mastaglio S., Bordignon C. Improving the safety of cell therapy with the TK-suicide gene. Front. Pharmacol. 2015;6:95. doi: 10.3389/fphar.2015.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cavazzana-Calvo M., Hacein-Bey S., de Saint Basile G., Gross F., Yvon E., Nusbaum P., Selz F., Hue C., Certain S., Casanova J.L. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288:669–672. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- 11.Cavazzana M., Six E., Lagresle-Peyrou C., André-Schmutz I., Hacein-Bey-Abina S. Gene therapy for X-linked severe combined immunodeficiency: where do we stand? Hum. Gene Ther. 2016;27:108–116. doi: 10.1089/hum.2015.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hacein-Bey-Abina S., Le Deist F., Carlier F., Bouneaud C., Hue C., De Villartay J.P., Thrasher A.J., Wulffraat N., Sorensen R., Dupuis-Girod S. Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. N. Engl. J. Med. 2002;346:1185–1193. doi: 10.1056/NEJMoa012616. [DOI] [PubMed] [Google Scholar]
- 13.Hacein-Bey-Abina S., Garrigue A., Wang G.P., Soulier J., Lim A., Morillon E., Clappier E., Caccavelli L., Delabesse E., Beldjord K. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J. Clin. Invest. 2008;118:3132–3142. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hacein-Bey-Abina S., Von Kalle C., Schmidt M., McCormack M.P., Wulffraat N., Leboulch P., Lim A., Osborne C.S., Pawliuk R., Morillon E. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 15.Howe S.J., Mansour M.R., Schwarzwaelder K., Bartholomae C., Hubank M., Kempski H., Brugman M.H., Pike-Overzet K., Chatters S.J., de Ridder D. Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J. Clin. Invest. 2008;118:3143–3150. doi: 10.1172/JCI35798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braun C.J., Boztug K., Paruzynski A., Witzel M., Schwarzer A., Rothe M., Modlich U., Beier R., Göhring G., Steinemann D. Gene therapy for Wiskott-Aldrich syndrome--long-term efficacy and genotoxicity. Sci. Transl. Med. 2014;6:227ra33. doi: 10.1126/scitranslmed.3007280. [DOI] [PubMed] [Google Scholar]
- 17.Ott M.G., Schmidt M., Schwarzwaelder K., Stein S., Siler U., Koehl U., Glimm H., Kühlcke K., Schilz A., Kunkel H. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat. Med. 2006;12:401–409. doi: 10.1038/nm1393. [DOI] [PubMed] [Google Scholar]
- 18.Stein S., Ott M.G., Schultze-Strasser S., Jauch A., Burwinkel B., Kinner A., Schmidt M., Krämer A., Schwäble J., Glimm H. Genomic instability and myelodysplasia with monosomy 7 consequent to EVI1 activation after gene therapy for chronic granulomatous disease. Nat. Med. 2010;16:198–204. doi: 10.1038/nm.2088. [DOI] [PubMed] [Google Scholar]
- 19.Cassani B., Montini E., Maruggi G., Ambrosi A., Mirolo M., Selleri S., Biral E., Frugnoli I., Hernandez-Trujillo V., Di Serio C. Integration of retroviral vectors induces minor changes in the transcriptional activity of T cells from ADA-SCID patients treated with gene therapy. Blood. 2009;114:3546–3556. doi: 10.1182/blood-2009-02-202085. [DOI] [PubMed] [Google Scholar]
- 20.Modlich U., Bohne J., Schmidt M., von Kalle C., Knöss S., Schambach A., Baum C. Cell-culture assays reveal the importance of retroviral vector design for insertional genotoxicity. Blood. 2006;108:2545–2553. doi: 10.1182/blood-2005-08-024976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montini E., Cesana D., Schmidt M., Sanvito F., Ponzoni M., Bartholomae C., Sergi Sergi L., Benedicenti F., Ambrosi A., Di Serio C. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat. Biotechnol. 2006;24:687–696. doi: 10.1038/nbt1216. [DOI] [PubMed] [Google Scholar]
- 22.Modlich U., Navarro S., Zychlinski D., Maetzig T., Knoess S., Brugman M.H., Schambach A., Charrier S., Galy A., Thrasher A.J. Insertional transformation of hematopoietic cells by self-inactivating lentiviral and gammaretroviral vectors. Mol. Ther. 2009;17:1919–1928. doi: 10.1038/mt.2009.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zychlinski D., Schambach A., Modlich U., Maetzig T., Meyer J., Grassman E., Mishra A., Baum C. Physiological promoters reduce the genotoxic risk of integrating gene vectors. Mol. Ther. 2008;16:718–725. doi: 10.1038/mt.2008.5. [DOI] [PubMed] [Google Scholar]
- 24.Montini E., Cesana D., Schmidt M., Sanvito F., Bartholomae C.C., Ranzani M., Benedicenti F., Sergi L.S., Ambrosi A., Ponzoni M. The genotoxic potential of retroviral vectors is strongly modulated by vector design and integration site selection in a mouse model of HSC gene therapy. J. Clin. Invest. 2009;119:964–975. doi: 10.1172/JCI37630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hacein-Bey-Abina S., Pai S.Y., Gaspar H.B., Armant M., Berry C.C., Blanche S., Bleesing J., Blondeau J., de Boer H., Buckland K.F. A modified γ-retrovirus vector for X-linked severe combined immunodeficiency. N. Engl. J. Med. 2014;371:1407–1417. doi: 10.1056/NEJMoa1404588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biffi A., Montini E., Lorioli L., Cesani M., Fumagalli F., Plati T., Baldoli C., Martino S., Calabria A., Canale S. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science. 2013;341:1233158. doi: 10.1126/science.1233158. [DOI] [PubMed] [Google Scholar]
- 27.Aiuti A., Biasco L., Scaramuzza S., Ferrua F., Cicalese M.P., Baricordi C., Dionisio F., Calabria A., Giannelli S., Castiello M.C. Lentiviral hematopoietic stem cell gene therapy in patients with Wiskott-Aldrich syndrome. Science. 2013;341:1233151. doi: 10.1126/science.1233151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson A.A., Walters M.C., Kwiatkowski J., Rasko J.E.J., Ribeil J.A., Hongeng S., Magrin E., Schiller G.J., Payen E., Semeraro M. Gene therapy in patients with transfusion-dependent β-thalassemia. N. Engl. J. Med. 2018;378:1479–1493. doi: 10.1056/NEJMoa1705342. [DOI] [PubMed] [Google Scholar]
- 29.Merten O.W., Hebben M., Bovolenta C. Production of lentiviral vectors. Mol. Ther. Methods Clin. Dev. 2016;3:16017. doi: 10.1038/mtm.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Loo J.C., Swaney W.P., Grassman E., Terwilliger A., Higashimoto T., Schambach A., Baum C., Thrasher A.J., Williams D.A., Nordling D.L. Scale-up and manufacturing of clinical-grade self-inactivating γ-retroviral vectors by transient transfection. Gene Ther. 2012;19:246–254. doi: 10.1038/gt.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghani K., Cottin S., Kamen A., Caruso M. Generation of a high-titer packaging cell line for the production of retroviral vectors in suspension and serum-free media. Gene Ther. 2007;14:1705–1711. doi: 10.1038/sj.gt.3303039. [DOI] [PubMed] [Google Scholar]
- 32.Ghani K., Wang X., de Campos-Lima P.O., Olszewska M., Kamen A., Rivière I., Caruso M. Efficient human hematopoietic cell transduction using RD114- and GALV-pseudotyped retroviral vectors produced in suspension and serum-free media. Hum. Gene Ther. 2009;20:966–974. doi: 10.1089/hum.2009.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X., Olszewska M., Qu J., Wasielewska T., Bartido S., Hermetet G., Sadelain M., Rivière I. Large-scale clinical-grade retroviral vector production in a fixed-bed bioreactor. J. Immunother. 2015;38:127–135. doi: 10.1097/CJI.0000000000000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Straetemans T., Kierkels G.J.J., Doorn R., Jansen K., Heijhuurs S., Dos Santos J.M., van Muyden A.D.D., Vie H., Clemenceau B., Raymakers R. GMP-grade manufacturing of T cells engineered to express a defined γδTCR. Front. Immunol. 2018;9:1062. doi: 10.3389/fimmu.2018.01062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schambach A., Mueller D., Galla M., Verstegen M.M., Wagemaker G., Loew R., Baum C., Bohne J. Overcoming promoter competition in packaging cells improves production of self-inactivating retroviral vectors. Gene Ther. 2006;13:1524–1533. doi: 10.1038/sj.gt.3302807. [DOI] [PubMed] [Google Scholar]
- 36.Dull T., Zufferey R., Kelly M., Mandel R.J., Nguyen M., Trono D., Naldini L. A third-generation lentivirus vector with a conditional packaging system. J. Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu C., Lu Y. High-titre retroviral vector system for efficient gene delivery into human and mouse cells of haematopoietic and lymphocytic lineages. J. Gen. Virol. 2010;91:1909–1918. doi: 10.1099/vir.0.020255-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rivière I., Brose K., Mulligan R.C. Effects of retroviral vector design on expression of human adenosine deaminase in murine bone marrow transplant recipients engrafted with genetically modified cells. Proc. Natl. Acad. Sci. USA. 1995;92:6733–6737. doi: 10.1073/pnas.92.15.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niwa H., Yamamura K., Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 40.Zaiss A.K., Son S., Chang L.J. RNA 3′ readthrough of oncoretrovirus and lentivirus: implications for vector safety and efficacy. J. Virol. 2002;76:7209–7219. doi: 10.1128/JVI.76.14.7209-7219.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gélinas C., Temin H.M. Nondefective spleen necrosis virus-derived vectors define the upper size limit for packaging reticuloendotheliosis viruses. Proc. Natl. Acad. Sci. USA. 1986;83:9211–9215. doi: 10.1073/pnas.83.23.9211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Titeux M., Pendaries V., Zanta-Boussif M.A., Décha A., Pironon N., Tonasso L., Mejia J.E., Brice A., Danos O., Hovnanian A. SIN retroviral vectors expressing COL7A1 under human promoters for ex vivo gene therapy of recessive dystrophic epidermolysis bullosa. Mol. Ther. 2010;18:1509–1518. doi: 10.1038/mt.2010.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dakiw Piaceski A., Larouche D., Ghani K., Bisson F., Cortez Ghio S., Larochelle S., Moulin V.J., Caruso M., Germain L. Translating the combination of gene therapy and tissue engineering for treating recessive dystrophic epidermolysis bullosa. Eur. Cell. Mater. 2018;35:73–86. doi: 10.22203/eCM.v035a06. [DOI] [PubMed] [Google Scholar]
- 44.Robert D., Mahon F.X., Richard E., Etienne G., de Verneuil H., Moreau-Gaudry F. A SIN lentiviral vector containing PIGA cDNA allows long-term phenotypic correction of CD34+-derived cells from patients with paroxysmal nocturnal hemoglobinuria. Mol. Ther. 2003;7:304–316. doi: 10.1016/s1525-0016(03)00011-x. [DOI] [PubMed] [Google Scholar]
- 45.Recchia A., Bonini C., Magnani Z., Urbinati F., Sartori D., Muraro S., Tagliafico E., Bondanza A., Stanghellini M.T., Bernardi M. Retroviral vector integration deregulates gene expression but has no consequence on the biology and function of transplanted T cells. Proc. Natl. Acad. Sci. USA. 2006;103:1457–1462. doi: 10.1073/pnas.0507496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scholler J., Brady T.L., Binder-Scholl G., Hwang W.T., Plesa G., Hege K.M., Vogel A.N., Kalos M., Riley J.L., Deeks S.G. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci. Transl. Med. 2012;4:132ra53. doi: 10.1126/scitranslmed.3003761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Volkova N.A., Fomina E.G., Smolnikova V.V., Zinovieva N.A., Fomin I.K. The U3 region of Moloney murine leukemia virus contains position-independent cis-acting sequences involved in the nuclear export of full-length viral transcripts. J. Biol. Chem. 2014;289:20158–20169. doi: 10.1074/jbc.M113.545855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dougherty J.P., Temin H.M. A promoterless retroviral vector indicates that there are sequences in U3 required for 3′ RNA processing. Proc. Natl. Acad. Sci. USA. 1987;84:1197–1201. doi: 10.1073/pnas.84.5.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zufferey R., Donello J.E., Trono D., Hope T.J. Woodchuck hepatitis virus posttranscriptional regulatory element enhances expression of transgenes delivered by retroviral vectors. J. Virol. 1999;73:2886–2892. doi: 10.1128/jvi.73.4.2886-2892.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klein R., Ruttkowski B., Knapp E., Salmons B., Günzburg W.H., Hohenadl C. WPRE-mediated enhancement of gene expression is promoter and cell line specific. Gene. 2006;372:153–161. doi: 10.1016/j.gene.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 51.Hlavaty J., Schittmayer M., Stracke A., Jandl G., Knapp E., Felber B.K., Salmons B., Günzburg W.H., Renner M. Effect of posttranscriptional regulatory elements on transgene expression and virus production in the context of retrovirus vectors. Virology. 2005;341:1–11. doi: 10.1016/j.virol.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 52.Higashimoto T., Urbinati F., Perumbeti A., Jiang G., Zarzuela A., Chang L.J., Kohn D.B., Malik P. The woodchuck hepatitis virus post-transcriptional regulatory element reduces readthrough transcription from retroviral vectors. Gene Ther. 2007;14:1298–1304. doi: 10.1038/sj.gt.3302979. [DOI] [PubMed] [Google Scholar]
- 53.Kumar M., Keller B., Makalou N., Sutton R.E. Systematic determination of the packaging limit of lentiviral vectors. Hum. Gene Ther. 2001;12:1893–1905. doi: 10.1089/104303401753153947. [DOI] [PubMed] [Google Scholar]
- 54.Feldman S.A., Xu H., Black M.A., Park T.S., Robbins P.F., Kochenderfer J.N., Morgan R.A., Rosenberg S.A. Use of the piggyBac transposon to create stable packaging cell lines for the production of clinical-grade self-inactivating γ-retroviral vectors. Hum. Gene Ther. Methods. 2014;25:253–260. doi: 10.1089/hgtb.2014.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hennig K., Raasch L., Kolbe C., Weidner S., Leisegang M., Uckert W., Titeux M., Hovnanian A., Kuehlcke K., Loew R. HEK293-based production platform for γ-retroviral (self-inactivating) vectors: application for safe and efficient transfer of COL7A1 cDNA. Hum. Gene Ther. Clin. Dev. 2014;25:218–228. doi: 10.1089/humc.2014.083. [DOI] [PubMed] [Google Scholar]
- 56.Cong L., Ran F.A., Cox D., Lin S., Barretto R., Habib N., Hsu P.D., Wu X., Jiang W., Marraffini L.A., Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Delgado-Cañedo A., Santos D.G., Chies J.A., Kvitko K., Nardi N.B. Optimization of an electroporation protocol using the K562 cell line as a model: role of cell cycle phase and cytoplasmic DNAses. Cytotechnology. 2006;51:141–148. doi: 10.1007/s10616-006-9028-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.