Abstract
Local antimicrobial delivery is a promising strategy for improving treatment of deep surgical site infections (SSIs) by eradicating bacteria that remain in the wound or around its margins after surgical debridement. Eradication of biofilm bacteria can require sustained exposure to high antimicrobial concentrations (we estimate 100–1,000 μg/mL sustained for 24 hr) which are far in excess of what can be provided by systemic administration. We have previously reported the development of temperature-responsive hydrogels based on poly(N-isopropylacrylamide-co-dimethylbutyrolactone acrylate-co-Jeffamine M-1000 acrylamide) (PNDJ) that provide sustained antimicrobial release in vitro and are effective in treating a rabbit model of osteomyelitis when instilled after surgical debridement. In this work, we sought to measure in vivo antimicrobial release from PNDJ hydrogels and the antimicrobial concentrations provided in adjacent tissues. PNDJ hydrogels containing tobramycin and vancomycin were administered in 4 dosing sites in rabbits (intramedullary in the femoral canal, soft tissue defect in the quadriceps, intramuscular injection in the hamstrings, and intraarticular injection in the knee). Gel and tissue were collected up to 72 hr after dosing and drug levels were analyzed. In vivo antimicrobial release (43–95% after 72 hr) was markedly faster than in vitro release. Drug levels varied significantly depending on the dosing site but not between polymer formulations tested. Notably, total antimicrobial concentrations in adjacent tissue in all dosing sites were sustained at estimated biofilm-eradicating levels for at least 24 hr (461–3,161 μg/mL at 24 hr). These results suggest that antimicrobial-loaded PNDJ hydrogels are promising for improving the treatment of biofilm-based SSIs.
Keywords: N-isopropylacrylamide, surgical site infection, local delivery, sustained release, tobramycin, vancomycin
Introduction
Surgical site infections (SSIs) are among the most common and costly complications following surgery, particularly when involving deep tissues or organ spaces. Deep SSIs are devastating for patients, often requiring multiple surgeries with prolonged recovery and uncertain outcomes, and the majority are caused by bacteria that form biofilm on foreign bodies and compromised dysvascular tissue [1,2]. Biofilm harbors non-dividing bacterial persister cells which are both multidrug-tolerant and protected from host immune cells within the biofilm’s extracellular polymeric substance (EPS) matrix [3,4]. Thus, biofilm infections are treatable only by thorough surgical removal (debridement) of all surfaces that harbor biofilm, supplemented with antimicrobial chemotherapy at high concentrations to eradicate all bacteria in retained biofilm fragments [3,4]. The minimum inhibitory concentration (MIC) is the concentration of an antimicrobial required to inhibit the overnight growth of individual bacteria in fluid (planktonic growth). Although MIC can routinely be achieved by systemic administration, it is not effective against biofilm. The minimum biofilm eradication concentration (MBEC), which is the level required to kill all bacteria in a biofilm, is frequently at least 10–100x greater than MIC and cannot be provided safely by systemic administration [5–7]. Microbiological [8–10], animal [11,12], and clinical [13–16] reports support that exposure at MBEC antimicrobial levels (generally, 100–1,000 μg/mL) sustained for at least 24 hr is critical for eradication of bacteria in biofilm. To achieve sustained high levels, antimicrobials are administered locally to the infection site to maximize drug efficacy while minimizing toxicity from systemic exposure.
Sustained local delivery of antimicrobials represents a significant clinical drug delivery challenge. Because the location of any residual biofilm fragments or liberated planktonic bacteria is unknown at the time of surgery, MBEC levels should be provided throughout the entire surgical site. Aminoglycosides, which are among the most common locally delivered agents, are rapidly cleared from local tissue [17,18], necessitating a high rate of sustained release to maintain MBEC levels. Moreover, the in vivo drug release profile and tissue clearance of antimicrobials may not be accurately replicated using simplified in vitro release experiments. Therefore, it is critical that evaluation of local antimicrobial delivery includes determination of drug concentrations in local tissue.
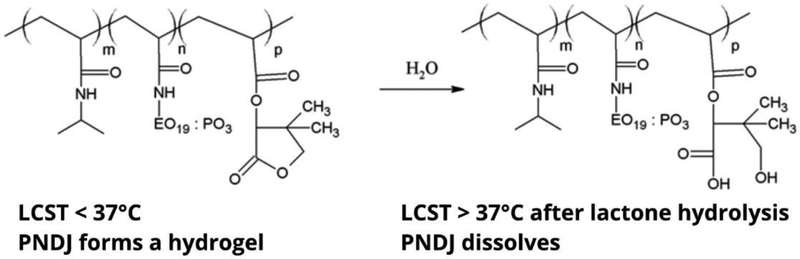
We have previously reported the use of temperature-responsive hydrogels based on the polymer poly(N-isopropylacrylamide-co-dimethylbutyrolactone acrylate-co-Jeffamine M-1000 acrylamide) (PNDJ; structure provided in Fig 1) that form upon heating to physiological temperature [19]. An aqueous solution of PNDJ forms a degradable hydrogel based on the lower critical solution temperature (LCST) property of PNDJ. The LCST is initially below body temperature, leading to precipitation and gel formation when the polymer solution is injected and warms to body temperature. Subsequently, hydrolysis of lactone moieties in the dimethylbutyrolactone acrylate (DBLA) repeat unit side-group cause an increase in the polymer LCST. When the LCST increases to above body temperature, the polymer spontaneously dissolves (Fig 1) [19,20]. PNDJ gels are soft and viscous, conforming to the site in which they are placed. The gels provide antimicrobial release over multiple days in vitro and are configured to dissolve within 4–6 weeks. Gentamicin-loaded PNDJ gels were effective in treating radial osteomyelitis caused by S. aureus in rabbits when applied to the infection site after debridement [21]. In a separate study using the same model, PNDJ gels containing tobramycin were effective in a total of 12/12 cases against S. aureus and MRSA, whereas daily systemic tobramycin or local antimicrobials (low-dose ALBC) achieved curative results in only 3/7 or 5/7 cases, respectively [22]. Additionally, preliminary assessments of gel toxicity showed only transient increases in systemic markers for renal toxicity and no signs of local toxicity at the injection sites [21,23]. However, the local antimicrobial concentrations provided by PNDJ hydrogels in vivo have not been measured. Therefore, we sought to identify whether PNDJ hydrogels provide local antimicrobial concentrations in tissue that meet or exceed MBEC levels. The primary goal of this work was to determine the effects of polymer formulation (specifically, the density of Jeffamine M-1000 which controls gel water content and drug release in vitro [24]), initial drug concentration, and dosing site on antimicrobial concentrations in the hydrogel and in local tissue over 72 hr after dosing. A secondary goal was to identify PNDJ polymer compositions which provide sustained local antimicrobial concentrations in tissue on the order of MBEC for at least 24 hr. Four dosing sites (soft tissue resection cavity, in bone, intramuscular injection, and in a joint space) for the antimicrobial-loaded PNDJ gels were chosen as clinically relevant for musculoskeletal infection. Tobramycin and vancomycin co-delivery from the gels was chosen for this work, because the combination is commonly used in local delivery [25] and has broad coverage against the vast majority of bacteria that cause SSIs. Direct measurement of antimicrobial concentrations within recovered hydrogels and in adjacent tissues up to 72 hr after application was performed to assess reaching clinical antimicrobial concentration targets.
Fig 1.
PNDJ structure and mechanism of dissolution. The polymer is composed of N-isopropylacrylamide (NIPAAm), Jeffamine M-1000 acrylamide (JAAm), and dimethylbutyrolactone acrylate (DBLA) monomers (pictured left to right in the structure). The JAAm side-chain is made up of ethylene oxide (EO) and propylene oxide (PO) repeat units in an approximate 19:3 ratio. PNDJ forms a hydrogel in vivo when the LCST is below body temperature. Hydrolytic ring-opening of the lactone in the DBLA side-chain increases the polymer LCST and leads to dissolution when the LCST exceeds body temperature
Materials and Methods
Materials
Materials were reagent grade and obtained from Sigma-Aldrich unless otherwise noted. N-isopropylacrylamide (NIPAAm) was recrystallized from hexane. Azobisisobutyronitrile (AIBN) was recrystallized from methanol. HPLC grade tetrahydrofuran (THF) was used for polymerizations and as the mobile phase for molecular weight determination. Jeffamine® M-1000 was obtained from Huntsman Corporation (The Woodlands, TX, USA). Jeffamine® M-1000 is a random copolymer of ethylene oxide and propylene oxide units in a 19:3 ratio and approximately 1,000 g/mol molecular weight, with one methoxy end group and one primary amine end group. Tobramycin sulfate USP was obtained from Spectrum Chemical (New Brunswick, NJ). Vancomycin hydrochloride USP was obtained from ChemImpex International (Wood Dale, IL).
Preparation of antimicrobial loaded hydrogels
Polymer synthesis
Dimethylbutyrolactone acrylate (DBLA) and Jeffamine M-1000 acrylamide (JAAm) monomers were synthesized by reaction of acryloyl chloride with DL-pantolactone and Jeffamine M-1000, respectively, according to published methods [26,27]. PNDJ polymers were prepared by free radical polymerization of NIPAAm, DBLA, and JAAm in THF/dioxane solution (50/50) with AIBN as the initiator, purified by dialysis, and obtained as a lyophilized powder as previously reported [21]. NIPAAm provides temperature responsive gelation; DBLA content primarily controls the degradation profile; JAAm content affects the water content, drug release properties, and degradation time in vitro [19,24]. The monomer feed ratios were adjusted to produce 9 different polymer formulations between 1.02–1.50 mol% JAAm (polymers are identified by JAAm content e.g. PNDJ1.02, PNDJ1.50; see Table 1). The polymer DBLA content was held approximately constant (6.4–7.4 mol% DBLA) for all batches based on prior work. These monomer ratios control the initial gelation temperature to be slightly above ambient temperature and allows for dissolution at body temperature over time via hydrolytic degradation [20].
Table 1.
PNDJ polymers used for in vitro and in vivo studies. Polymer composition was determined by 1H NMR and JAAm content is denoted by numerical subscript in the polymer name. The weight average (Mw) and number average (Mn) molecular weights were determined by size exclusion chromatography
| Composition (mol%) | Molecular weight (g/mol) | ||||
|---|---|---|---|---|---|
| Study | Polymer | JAAm | DBLA | Mw | Mn |
| In Vitro Release | PNDJ1.02 | 1.02% | 6.78% | 35,870 | 19,330 |
| PNDJ1.19 | 1.19% | 7.02% | 39,450 | 23,850 | |
| PNDJ1.30 | 1.30% | 7.40% | 34,040 | 19,810 | |
| PNDJ1.45 | 1.45% | 5.48% | 36,320 | 17,400 | |
| In Vivo Delivery Study 1 | PNDJ1.09 | 1.09% | 6.62% | 35,110 | 16,290 |
| PNDJ1.36 | 1.36% | 6.69% | 34,040 | 19,810 | |
| In Vivo Delivery Study 2 | PNDJ1.02 | 1.02% | 6.78% | 35,870 | 19,330 |
| PNDJ1.13 | 1.13% | 6.42% | 39,450 | 23,850 | |
| PNDJ1.31 | 1.31% | 7.40% | 36,870 | 23,150 | |
| PNDJ1.50 | 1.50% | 6.63% | 36,320 | 17,400 | |
Polymer characterization
All polymers were characterized for composition by 1H NMR spectroscopy (Bruker, 400 MHz) in methanol-d4. Molecular weight was determined by size exclusion chromatography in conjunction with multi-angle light scattering (Mini-DAWN; Wyatt Technology Corp, Santa Barbara, CA, USA) with THF as the mobile phase. The lower critical solution temperature (LCST), the temperature above which the polymer precipitates in aqueous solution, was determined for each polymer batch at 3 mg/mL in PBS according to a previously published cloud point method [19].
Gel preparation
Polymers were dissolved at 38 wt% in 0.2 M sodium acetate-acetic acid buffer (pH 4.0) and stored at 4°C. After dissolution, one gram of polymer solution was loaded into a 3 mL syringe and stored upright to allow air bubbles to resolve. Tobramycin sulfate and vancomycin hydrochloride were co-dissolved in aqueous HCl buffer at a total concentration of 22 wt% of total active drug, and the solution was measured into a second 3 mL syringe. A Luer-lock female-to-female coupler was used to connect the syringe containing polymer solution and the second syringe containing antimicrobial solution. The solutions were rapidly mixed by pushing the contents completely from one side to the other for 10–12 strokes, resulting in even distribution of the antimicrobials. For animal studies, lyophilized PNDJ polymers were sterilized by ethylene oxide gas and drug solutions were sterilized by syringe filtration prior to syringe loading.
In vitro antimicrobial release
Drug release kinetics from PNDJ hydrogels were measured to determine the effects of JAAm content and vancomycin/tobramycin drug loading ratios. Hydrogels of four PNDJ polymers (JAAm content of 1.02%, 1.19%, 1.30%, 1.45%; Table 1) were prepared with 2.35–3.14 wt% tobramycin and 0.78–2.35 wt% vancomycin in 9 different ratios (Online Resource Fig 1a) to obtain a gel with polymer concentration between 28.5–32.6 wt%. Approximately 350 mg of mixed gel was dispensed through a 20G needle to coat the bottom of a tared 8 mL glass vial and the mass was recorded. Three fractions were obtained from each set of coupled syringes, and each fraction was treated as a replicate in release testing. After dispensing, gels were formed in a 37°C water bath, then 8 mL of pre-warmed PBS was added to each vial, and vials were incubated at 37°C for release studies. At selected time points, vials were inverted and pipette-mixed to evenly mix the PBS release medium, aliquots of release medium were collected and then completely exchanged. Aliquots were taken at 1, 6, 24, 48, and 70 hr to maintain infinite-sink conditions and stored at −20°C until measurement. Vancomycin concentration was determined by UV absorbance (280 nm) and tobramycin by ninhydrin assay [28].
In vivo antimicrobial delivery and concentrations in tissue
Study design
Antimicrobial delivery from 6 PNDJ formulations loaded with 3% tobramycin and 2% vancomycin (3T2V) or 3% tobramycin and 1% vancomycin (3T1V) was evaluated in 2 studies. The goal of the first study was to determine whether polymer formulation, initial drug concentration, and dosing site affected antimicrobial concentrations in the hydrogel and in local tissue over 72 hr after dosing. The first study evaluated all combinations of two polymer formulations (PNDJ1.09 and PNDJ1.36, see Table 1), two drug formulations (3T2V and 3T1V), and four dosing sites (a soft tissue defect in the quadriceps, femoral canal, intramuscular injection in the biceps femoris, and intra-articular injection in the knee). Drug concentrations in tissues and recovered hydrogels were determined at 8, 24, and 72 hr postoperatively, with two replicates per polymer-drug formulation-dosing site-time point combination. In the second study, we tested a wider range of polymer JAAm content than in the first study to determine whether antimicrobial concentrations in tissue were affected specifically in the first 24 hr after dosing. The second study utilized dosing with four PNDJ formulations (PNDJ1.02, PNDJ1.13, PNDJ1.31, PNDJ1.50; Table 1), each loaded with 3T1V dosed in the same four dosing sites as the previous study, and drug concentrations in tissue and recovered hydrogels were evaluated at 24 hr only.
Animal studies
New Zealand White rabbits (female, 3.1–3.5 kg, Western Oregon Rabbit Co., Philomath, OR, USA) were used for animal studies. All animals were operated on under general anesthesia using aseptic surgical techniques. Rabbits received PNDJ gel in both hindlimbs, in three sites: a soft tissue surgical defect in the quadriceps, an intramuscular injection in the hamstring, and an intraarticular injection in the knee. Additionally, gel was injected in the intramedullary canal of the right femur only; both femurs were not dosed to reduce the risk of fat embolus. The soft tissue defect was created in the mid-thigh region of the quadriceps through an anterolateral incision and resection of cubic sections of approximately 1 gram of muscle. The surgical incision was then closed and 1 g of antimicrobial-loaded hydrogel was injected into the site via a 14-gauge catheter inserted percutaneously into the site prior to closure. The right femoral canal was entered distally with a drill hole in the patellar groove through a separate incision. The marrow was removed by irrigation with saline. Approximately 1.5 mL of antimicrobial loaded gel was injected to fill the right femoral canal, and the drill hole was sealed with bone wax. The incision at the knee was then closed. Lastly, 1 mL of gel was injected in each biceps femoris muscle and intraarticularly in each knee using an 18-gauge needle. In the first study which included two drug concentrations, each rabbit received the same polymer formulation in each hind limb; gels dosed in the right leg contained 3T2V and gels dosed in the left leg contained 3T1V. In the second study, each rabbit received gel in the right hind limb only. All rabbits were given subcutaneous sustained-release buprenorphine for analgesia. Rabbits surviving for 24 or 72 hr returned to unconstrained cage ambulation postoperatively.
Rabbits were euthanized by sodium pentobarbital injection at either 8, 24, or 72 hr in the first study or at 24 hr in the second study. Immediately following euthanasia, dissection, and recovery of gel and tissue specimens was performed under a heat-lamp to minimize cooling and potential dissolution of the gel. Samples of gel and adjacent tissue (at least 2–3 pieces, 1–2 mm thick) from each site were collected in tared vials. Samples were stored at −20°C and measured within 4 weeks of collection. Most or all of the gel was recovered from the quadriceps surgical wound and femoral canal, but only a fraction of the gel was collected from the intramuscular biceps femoris injection and intra-articular knee injection sites. All specimens were weighed, diluted in sterile water, and homogenized to liberate antimicrobials contained in the specimens.
Determination of antimicrobials in hydrogels and tissue
Hydrogel and tissue specimen homogenates were evaluated for tobramycin content by agar diffusion microbiological assay against E. coli (ATCC #25922) and for vancomycin content against S. epidermidis (ATCC #35984). E. coli is gram-negative and not susceptible to vancomycin. The strain of S. epidermidis used is susceptible to vancomycin but highly resistant to tobramycin; the bioassay was not sensitive to tobramycin as vancomycin standard curves were indistinguishable with or without a 3-fold excess of tobramycin (results not shown). For each bioassay, turbid overnight liquid cultures were diluted (1:100) in tryptic soy agar solution at 50–55°C during cooling after autoclave sterilization. The bacterial suspension was poured into bioassay dishes (Biodish XL, Becton Dickinson, Franklin Lakes, NJ, USA) and allowed to solidify. The reverse end of a sterile Pasteur pipette was used to core evenly spaced wells in the agar. Samples (40 μL each) were dispensed into each well and the plate was incubated for 24 hr at 37°C. The zone of inhibition of bacterial growth was imaged and the radius was quantified in ImageJ. The average distance in pixels from the edge of the well to the edge of the zone of inhibition was determined from at least 3 measurements in each image. The zone of inhibition size for each gel or tissue specimen was compared to standard solutions of the antimicrobial being measured ranging from 16–8,000 μg/mL. The antimicrobial content of each gel or tissue sample was determined by multiplying the concentration measured in each assay by the dilution factor used to prepare the homogenate. When multiple gel samples were recovered from a single dosing site, the average antimicrobial concentration remaining in the gel was calculated using an average weighted according to the mass of each gel sample. For tissue samples, the average was not weighted according to sample mass. Concentrations were calculated assuming gel and tissue densities of 1 g/mL.
Data analysis
Figures were created in Prism 5 (GraphPad). Differences in antimicrobial concentrations in tissue and gel were evaluated by multifactor ANOVA testing (Minitab 18) with the polymer, time point, dosing site, and initial drug concentration in the gel used as input factors, and the measured concentrations of tobramycin and vancomycin in recovered gel or tissue used as model responses. Factors that were not statistically significant were excluded from the analysis and data within that factor were grouped for further analysis. For example, if the effect of polymer composition was not significant, then the corresponding data for different polymer compositions with all other factors being equal (time point, dosing site, and initial drug concentration) were analyzed as replicates. Additionally, vancomycin and tobramycin concentrations measured in gels and tissue were initially summed to provide a simplified analysis of the total antimicrobial concentrations in the different tissue sites over time. This analysis was then stratified to the individual drug levels measured in each dosing site at 24 and 72 hr for both gels and tissues. Here, differences across dosing sites at specific times were determined using multiple pairwise comparisons tests according to Bonferroni’s method. Significant differences are reported for p < 0.05; in multiple comparison tests, significance is reported for adjusted p < 0.05.
Results
In vitro antimicrobial release.
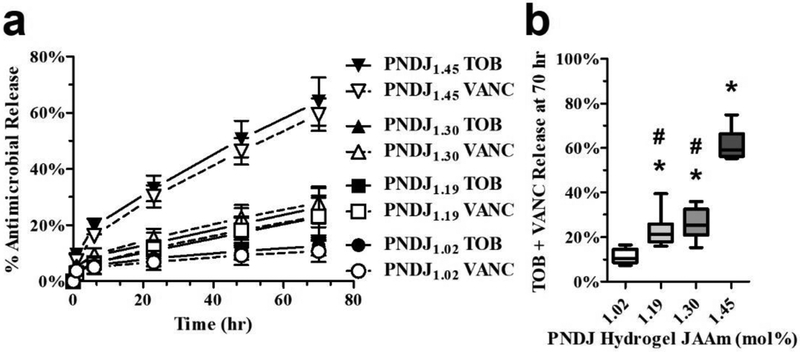
Tobramycin and vancomycin release profiles were determined for 4 PNDJ formulations containing each of 9 different drug loadings (Fig 2; Online Resource Fig 1a for formulation details). PNDJ gels with higher JAAm content provided faster release over 70 hr (Fig 2a, Online Resource Fig 1b). The release profile consisted of a small burst release during the first 6 hr followed by approximately linear release profile thereafter. Gels with the highest JAAm content, PNDJ1.45, released 59.9% tobramycin and 50.7% vancomycin, compared to gels with the lowest JAAm content, PNDJ1.02, which released 11.3% tobramycin and 7.5% vancomycin within 70 hr. The fraction of each drug released was not affected by the initial drug concentration within the range tested (Fig 2b, Online Resource Fig 1b). In addition, the release of the two drugs was approximately proportional to their relative initial concentration in the gel for all of the tested PNDJ hydrogels (Online Resource Fig 1c).
Fig 2.
In vitro release of tobramycin (TOB) and vancomycin (VANC) from PNDJ hydrogels is affected by JAAm content but not by drug loading ratio. (a) Antimicrobial release from PNDJ hydrogels with varying JAAm content loaded with 9 drug loading combinations (2.35–3.14% tobramycin, 0.78–2.35% vancomycin; see Online Resource Fig 1a). (b) Percentage of antimicrobials released from PNDJ hydrogels with varying JAAm content (1.02–1.45 mol%) and drug loading (2.35–3.14% tobramycin, 0.78–2.35% vancomycin) at 70 hr. Each box-and-whisker plot represents the distribution of data from 9 drug loading combinations within the gel (described further in Online Resource Fig 1a). Significant differences in antimicrobial release (adjusted p < 0.01) were identified for gels in comparison to PNDJ1.02 (*) and PNDJ1.45 (#) using a oneway ANOVA with Bonferroni post-test for multiple comparisons
In vivo antimicrobial delivery and concentrations in tissue
Gel distribution
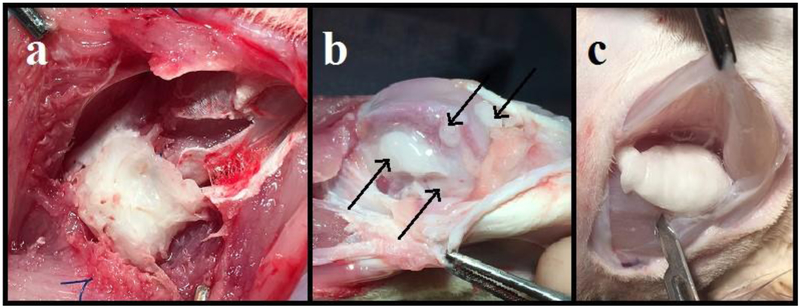
PNDJ hydrogels were identified and recovered from all dosing sites (Fig 3). In the femoral canal and quadriceps defect, gels assumed the shape of the site in which they were instilled, filling the intramedullary canal or the cube-shaped soft tissue resection cavity (Fig 3a), respectively. In the IM injection in the biceps femoris, gels adopted elongated shapes along the longitudinal axis of the muscle; about half of the gels formed a single cohesive mass between tissue planes and the other half were evenly distributed amongst the muscle fascicles (Fig 3c). In the knee site, gels were distributed throughout the entire joint space including the intercondylar notch, medial and lateral gutters, suprapatellar pouch, and posterior recesses (Fig 3b). After 72 hr, the majority of the gel recovered from the knee was found in the posterior recesses.
Fig 3.
Images of PNDJ hydrogels (white mass) recovered after 24 hr from 3 sites: (a) injection in a quadriceps muscle resection cavity; (b) intraarticular injection in the knee; (c) intramuscular injection in hamstrings. PNDJ hydrogels conformed to the potential space, and gel shape was determined by pressure from surrounding tissues. The resection site (a) was cubic because a cubic mass of muscle was removed by transection of part of a muscle belly. Intraarticular gel (b; black arrows) distributed to pouches gutters and intercondylar areas, and intramuscular injections (c) distributed between muscle bellies as in this case or amongst muscle fibers (not shown)
Polymer composition, drug loading and dosing site effects on antimicrobial delivery
In the first in vivo study dosing with PNDJ1.09 and PNDJ1.36 hydrogels, antimicrobial concentrations declined both in tissue and recovered gels over time (Online Resource Figs 2, 3). Dosing site was a significant factor in determining local drug concentrations (p < 0.001 for both gels and tissues). However, ANOVA testing indicated that polymer composition (JAAm content) did not have a significant effect on the concentration of either drug remaining in the gels (tobramycin: p = 0.652; vancomycin: p = 0.913) nor in tissue (tobramycin: p = 0.406; vancomycin: p = 0.267; Online Resource Figs 2, 3). Since the polymer composition did not have a significant effect on the results and given that the number of animals was limited to n = 2 per group, measurements of antimicrobial levels provided by different polymers for the same time point, dosing site, and drug formulation were grouped as replicates (akin to hidden replication in a designed experiment) for this analysis of antimicrobial delivery. Drug loading ratio also did not have a significant effect on antimicrobial concentrations in tissue (tobramycin p = 0.307; vancomycin p = 0.699), and PNDJ hydrogels loaded with 3T1V produced similar drug concentration profiles compared to those loaded with 3T2V (Online Resource Figs 3–5). We therefore focused further analysis of antimicrobial delivery on sites receiving hydrogels loaded with 3T2V, because those hydrogels were dosed in all four dosing sites.
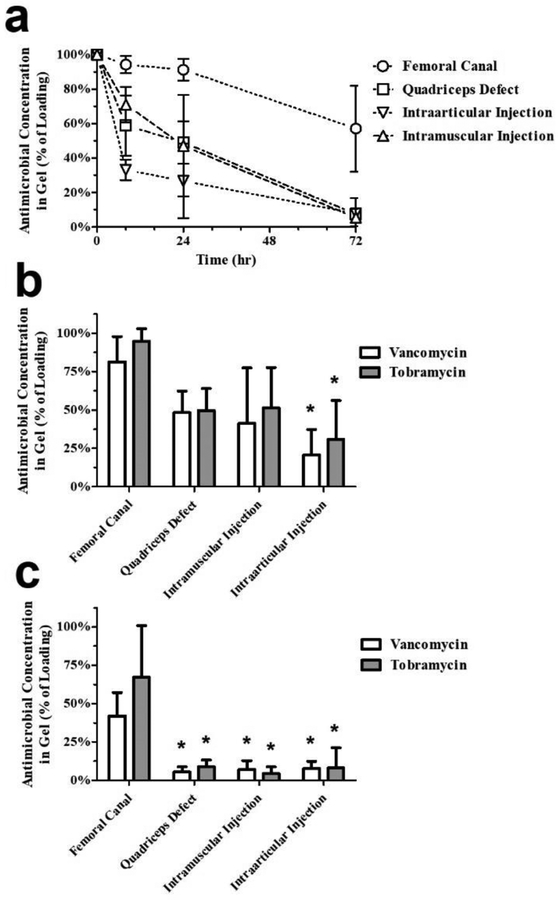
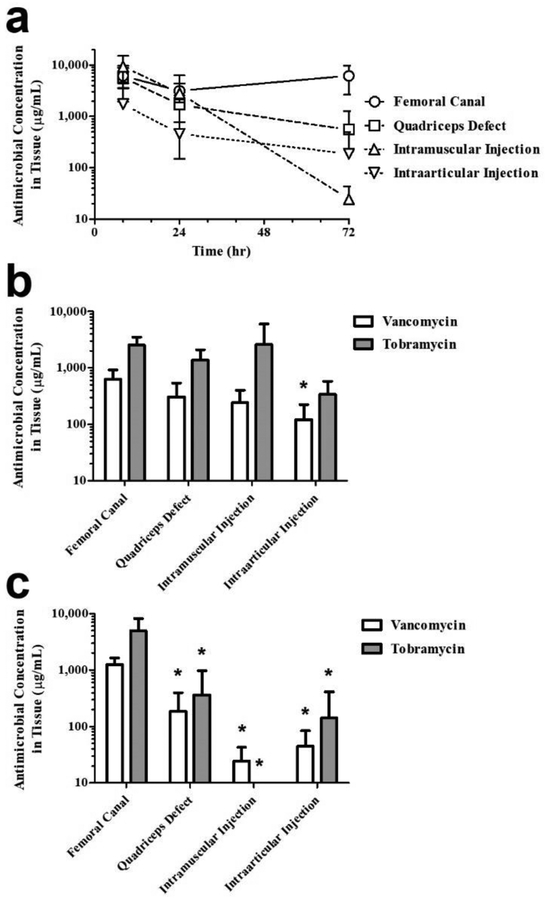
The effect of dosing site on antimicrobial levels remaining in PNDJ gels was significant (p < 0.001 for both drugs). The total drug concentration (tobramycin + vancomycin) was greatest in gels in the femoral canal at all time points compared to the other dosing sites (Fig 4a). Among the remaining sites, gels in the quadriceps defect and intramuscular injection retained 49% and 47% of the loaded drugs, respectively, compared to gels in the intraarticular injection which retained 27% within the first 24 hr. By 72 hr, the total drug concentration for gels in these three sites was about 5% of the initial concentration compared to 57% for gels in the femoral canal. Comparison of individual drug concentrations in the gel across dosing sites at 24 hr shows that concentrations of both drugs are significantly lower in the knee compared to the femur sites (p < 0.05; Fig 4b). At 72 hr, concentrations of both drugs were significantly decreased in in all other dosing sites compared to the femoral canal (p < 0.05; Fig 4c).
Fig 4.
Antimicrobial concentrations in PNDJ hydrogels recovered from 4 dosing sites after dosing with hydrogels containing 3% tobramycin and 2% vancomycin. (a) Total antimicrobials (tobramycin + vancomycin) measured at 6, 24, and 72 hr after dosing. Comparison of individual antimicrobial concentrations across dosing sites at (b) 24 hr and (c) 72 hr showed significant differences in tobramycin or vancomycin levels (adjusted p < 0.05) in sites compared to the femoral canal (*). Data are averaged across 2 hydrogel compositions (PNDJ1.09, PNDJ1.36) applied in 4 sites in 4 rabbits; error bars indicate standard deviation
Dosing site also had a significant effect on drug levels in tissue at all time points (p < 0.001 for both drugs; Fig 5). The highest total drug levels in tissue were observed in the femoral canal, similar to the gel drug levels (Fig 5a). At 24 hr, average total drug levels were higher in the femoral canal (3,160 μg/mL), quadriceps defect (1,683 μg/mL), and intramuscular injection (2,846 μg/mL) compared to levels measured in the intraarticular injection site (461 μg/mL). By 72 hr, average tissue levels in the IM injection declined sharply to 24 μg/mL, a decline of 99.2% from concentrations measured at 24 hr. Conversely, total drug concentration in the femur was 6,231 μg/mL, 97% greater than at 24 hr, while levels in the quadriceps defect and knee injection decreased but remained at 548 μg/mL and 187 μg/mL, respectively, a decline of 67.4% and 59.4% from the levels at 24 hr. Comparison of individual drug concentrations in tissue showed that tobramycin levels were greater than vancomycin (p = 0.001). Across all dosing sites where tobramycin was detected, the average tissue level of tobramycin was 2.8–10.8 fold greater than that of vancomycin at 24 hr (Fig 5b), and 1.9–4.0 fold greater at 72 hr (Fig 5c).
Fig 5.
Antimicrobial concentrations in tissue recovered from 4 dosing sites after dosing with hydrogels containing 3% tobramycin and 2% vancomycin. (a) Total antimicrobials (tobramycin + vancomycin) measured at 6, 24, and 72 hr after dosing. Comparison of individual antimicrobial concentrations across dosing sites at (b) 24 hr and (c) 72 hr showed significant differences in tobramycin or vancomycin levels (adjusted p < 0.05) in sites compared to the femoral canal (*). Data are averaged across 2 hydrogel compositions (PNDJ1.09, PNDJ1.36) applied in 4 sites in 4 rabbits; error bars indicate standard deviation
In a second in vivo study, antimicrobial elution from hydrogels of PNDJ formulations with JAAm content range of from 1.02 – 1.50 mol% at 24 hr showed that JAAm content of the hydrogel polymer again did not have a significant effect on antimicrobial levels either in the tissue (p = 0.368) or in the gel (p = 0.184) (Online Resource Fig 6). Tobramycin tissue levels were greater than vancomycin levels (p = 0.001), by an average factor of 8.62 (e.g. IM tobramycin range: 157–1,321 μg/mL vs. IM vancomycin range: 48–88 μg/mL). Additionally, similar to the results of the prior study, antimicrobial levels varied depending on dosing site in tissue (p = 0.002) and in the hydrogels (p < 0.001). For example, average tobramycin concentrations in femoral canal gels and tissue were 24,397 μg/mL and 3,399 μg/mL, respectively, compared to average tobramycin concentrations in intraarticular injected gels and tissue which were 2,425 μg/mL and 60 μg/mL, respectively.
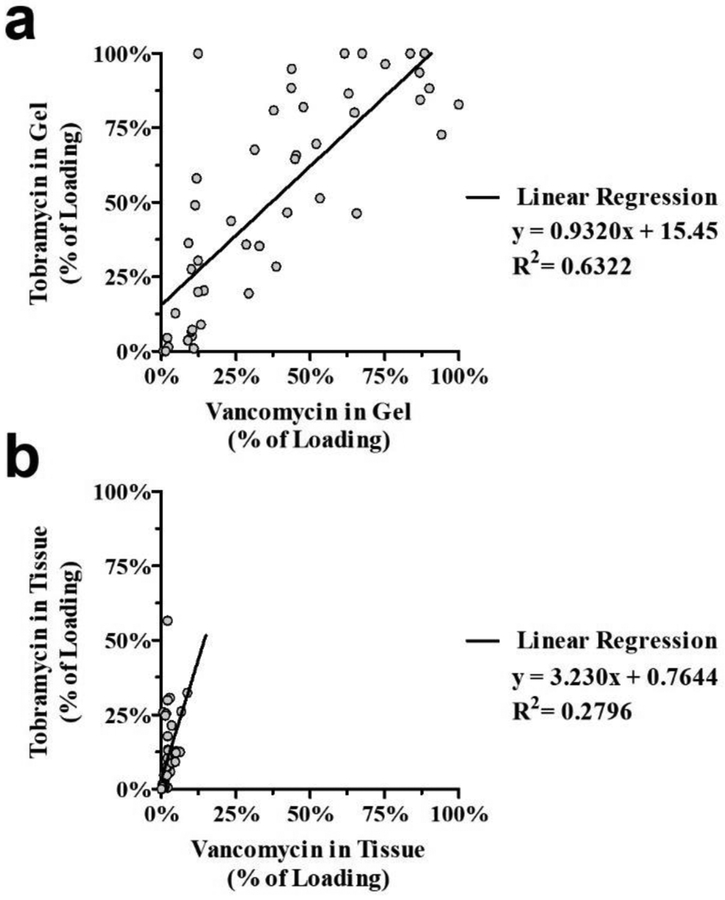
Antimicrobial ratios in gel and tissue
The ratio of tobramycin to vancomycin in both recovered gel and tissue samples was analyzed for comparison to in vitro release studies which identified proportional release of the two antimicrobials. In this analysis, vancomycin and tobramycin concentrations were expressed as a percentage of their initial concentration within the gel, and data from 3T2V loaded hydrogels applied in all dosing sites and exposure times were pooled for levels measured in either gels (Fig 6a) or tissues (Fig 6b). Tissues contained a disproportionately high amount of tobramycin relative to vancomycin, whereas the levels of each drug in the gel were more proportional to their initial content. In 3T2V-loaded hydrogels, the fractions of the initial load of tobramycin and vancomycin remaining in the gel were similar, indicating approximately proportional release of tobramycin and vancomycin (regression slope = 0.923) and reasonable correlation between tobramycin and vancomycin levels in the gel (R2 = 0.6322, Fig 6a). Conversely, tissue levels of tobramycin were significantly higher than vancomycin (p < 0.001; regression slope = 3.230), and the correlation between tobramycin and vancomycin levels in tissue samples was poor (R2 = 0.276; Fig 6b). Put another way, the tobramycin levels were on average 4.85 times the vancomycin levels (3.23 × 1.5), despite the fact there was only 1.5 times as much tobramycin compared to vancomycin in the gels. Similar antimicrobial ratios in gels and tissues were observed in 3T1V-loaded hydrogels (Online Resource Fig 7). In these samples, tissue tobramycin levels were again higher than vancomycin (p = 0.001; regression slope = 2.236; R2 = 0.439; Online Resource Fig 7a), despite a relatively lower proportion of tobramycin remaining in the gels (regression slope = 0.548; R2 = 0.474; Online Resource Fig 7b).
Fig 6.
Ratios of vancomycin and tobramycin measured in (a) PNDJ hydrogels or (b) adjacent tissues expressed as a percentage of the initial drug loading of 3% tobramycin and 2% vancomycin. Data points represent concentration measurements taken at 6, 24, and 72 hr after dosing with 2 hydrogel compositions (PNDJ1.09, PNDJ1.36) in 4 sites in 4 rabbits
Discussion
The goal of local antimicrobial delivery in treating SSIs is to provide high and sustained drug levels in tissue at the debridement margins in a surgical wound and in surgical dead space. The complexity and patient-to-patient variability of infected surgical sites greatly impacts the transport and clearance of antimicrobials in tissue. While drug release from different delivery vehicles may be evaluated using standardized methodologies in vitro, those data do not accurately represent the levels that occur in vivo. Here, we evaluated PNDJ hydrogels loaded with tobramycin and vancomycin as a platform for local antimicrobial delivery with a focus on direct measurement of drug concentrations in local delivery sites over a timeframe relevant for clinical management of deep SSI. This study has three primary findings: 1) Antimicrobial release from PNDJ hydrogels was faster in vivo compared to in vitro; 2) Drug delivery varied significantly depending on the dosing site in vivo; and 3) Local antimicrobial levels in tissue on the order of reported MBEC values were sustained for 24–72 hr, suggesting that antimicrobial-loaded PNDJ hydrogels are promising for improving the treatment of biofilm-based SSIs.
This study has some key limitations. First, our data were generated from small surgical sites in animal models. Although human and animal physiology is similar in the selected dosing sites, the gel volumes that were delivered in rabbits are much smaller than the volumes required to fill a wound in a human. A larger gel volume having a lower surface area to volume ratio would be expected to release antimicrobials over a longer time, but the effect of this difference on local antimicrobial exposure would need to be investigated. Local concentrations in human patients could also be affected by the timing of postoperative activity, including motion of nearby joints and strengthening of adjacent muscles. The relatively low surface area of hydrogels used in these animal studies combined with external pressures applied by surrounding tissue most likely accelerated drug release in the intraarticular and intramuscular injection sites. In these sites, gel shape was significantly altered compared to the initial conformation from hydraulic loads caused by muscle contraction and joint position in constrained spaces with limited compliance. Conversely, small dosing site dimensions are unlikely to have affected tissue drug levels measured in the quadriceps defect or femoral canal dosing sites as gels did not exhibit altered shapes compared to their initial conformation.
A second limitation of this study is that in some cases we observed high variability in drug concentrations among tissue samples treated with the same hydrogel, and even among multiple samples collected from the same site. This variability may be explained by differences in the sampling of tissues including their precise location or thickness. For example, drug concentration data for tissues collected near the gel interface will be greater than those at greater depth. This is particularly important since drug levels were determined via bacterial susceptibility which is measured on a logarithmic basis. In this study, the number of replicates for each group was limited to 2 for each gel type/dosing site/antimicrobial loading group per time point, and a much larger study would be necessary to reduce the effect of potential sampling errors. Extreme care was taken by a skilled orthopaedic surgeon to collect specimens at uniform depth from the initial injection site. This was likely very successful in cortical bone which was very uniform and in the quadriceps defect where the tissue-gel interface was easily identifiable. However, the margins of the intramuscular sites were at times difficult to identify, and collecting tissue that did not contain traces of gel dispersed amongst muscle fascicles was likely imperfect and may have produced to artificially elevated tissue levels in some cases. Similarly, in the intraarticular injection site, the synovial tissues and periarticular knee tissues adjacent to bone, fat pads, or in the gutters were often thin and difficult to harvest. These tissues produced notably low and variable antimicrobial concentrations, which may be attributed to sampling variability as well as drug clearance following high release resulting from repeated gel compression. The low levels reported in knee tissues likely underestimate the antimicrobial concentrations provided at the surface of these tissues.
A third limitation of this study is that a significant number of animals were required to perform direct sampling of gels and tissues. We attempted to mitigate this by using multiple dosing sites per animal. In the first in vivo study, we also grouped drug level data generated from different hydrogels as replicates because the number of animals in each group was limited. An alternative approach could be to monitor local drug concentration noninvasively by MRI using a contrast agent as a drug surrogate or a custom-labeled antimicrobial. This would offer the advantage of measuring concentration throughout a larger volume of tissue at multiple time points in a single animal [29–31]. However, this approach relies on assumptions that the contrast agent is released and transports throughout tissues similarly to the active drug(s) which cannot be directly monitored, and concentrations in sites near anatomic structures like joints or bones cannot be accurately calculated due to imaging interference from tissue.
A fourth limitation of this study is that the in vivo dissolution time and toxicity of PNDJ hydrogels with tobramycin and vancomycin were not evaluated. The studies presented here were too short (≤ 3 days) to include a meaningful evaluation of gel dissolution time in vivo, although we did observe intact gels in all dosing sites in this study. In a previous study, a PNDJ hydrogel with an in vitro dissolution time of 2–3 weeks was fully dissolved by 4 weeks after dosing in vivo, in agreement with in vitro results [21]. Systemic toxicity could be caused by the hydrogels in this work as a result of either polymer clearance or systemic exposure to released antimicrobials. After PNDJ polymer is dissolved, it is expected to be cleared by renal excretion, similarly to other linear, water-soluble polymers of similar molecular weight [32,33]. Further characterization of PNDJ biocompatibility and toxicity are important topics of future work.
In vitro evaluation of antimicrobial release from PNDJ hydrogels showed that increasing JAAm content produced a corresponding increase in drug release (Fig 2). Here, the proportion of total drug content released ranged from 9–55% at 70 hr depending on JAAm content. JAAm contains hydrophilic poly(ethylene) moieties that increase water content within the hydrogel leading to faster release, softer gels, and faster gel degradation [19]. In pilot studies, polymers with high JAAm content (> 1.6 mol%) produced very weak gels in injection sites after just 24 hr, and as such JAAm content was limited in this work (≤ 1.5 mol%) to improve the mechanical properties of the gel. Importantly, the ratio of tobramycin and vancomycin loaded into PNDJ gels did not measurably affect drug release, and the two antimicrobials eluted at approximately the same rate (Fig 2b, Online Resource Fig 1).
However, in vitro release data did not accurately model the performance of PNDJ hydrogels in vivo in some key respects. First, the JAAm content of PNDJ hydrogels within the range that we used did not affect release in any in vivo study. Second, drug release was much faster in vivo. In the first in vivo study, hydrogel drug levels were depleted of both antimicrobials in all dosing sites at 70 hr (< 11% of initial dose) except for the gel in the femoral canal, which retained over 50% of its initial drug concentration. (Fig 4a,c). Rapid drug release was most pronounced in the intraarticular injection where 73% of the total drug content was released by 24 hr, while release in the quadriceps defect and intramuscular injection was 51% and 53% respectively (Fig 4a). In contrast, in vitro release experiments indicate 28% release at 70 hr from similar hydrogels (PNDJ1.30, Fig 2). Fast in vivo drug release is likely caused by fluid exchange within the hydrogel, gel compression from movement of surrounding tissue, and tissue clearance which are not accurately replicated in vitro. The comparatively lower release in the femur was likely due to the dosing site being confined and sequestered from high rates of fluid exchange in the soft tissue, and from the lack of compressive forces acting on the gel. Still, the effect of the in vivo microenvironment outweighed the effect polymer JAAm content on drug release.
The in vivo data did also show some similarities to in vitro data. First, the initial drug loading did not significantly affect drug release, and as such, we focused on the results generated for hydrogels loaded with 3% tobramycin and 2% vancomycin (data for gels loaded with 3% tobramycin and 1% vancomycin were similar and are shown in Online Resource Figs 3–5). Second, comparison of tobramycin and vancomycin concentrations within PNDJ hydrogels showed that the drugs released at comparable rates, similar to what was observed in vitro (Figs 4, 6, and Online Resource Figs 1, 7). Overall, high dose antimicrobial delivery from PNDJ hydrogels was sustained in all sites for at least 24–72 hr, but these data illustrate that drug delivery from sustained-release depots, especially those based on soft materials, must be evaluated in vivo.
In this study, analysis of local tissue drug levels showed that the dosing site was the most important factor affecting antimicrobial release. The dosing sites were selected to represent a range of potential tissue types, administration methods, and mechanical environments that might be encountered in SSI treatment. The data further illustrate that while in vitro studies are certainly valuable, they should not be relied on to predict achievable drug concentrations in tissue in vivo. Release from PNDJ gels maintained high antimicrobial tissue levels for 24 hr in all sites, but these levels decreased notably (by 59.4–99.2%) between 24 and 72 hr in all sites other than the femur tissue, in which concentrations approximately doubled (Fig 5a). These results suggest that the rate of drug clearance away from the soft tissue and knee joint surgical sites exceeds the rate of delivery, while the opposite may be true in the femur. Still, the increase in femoral drug levels may have been caused by sampling error as previously described. Maintaining tissue drug levels present at 24 hr for 72 hr or longer would require much higher drug loading than the 4–5 wt% loading used in this study which would also increase the risk of systemic toxicity.
Tobramycin concentrations in tissue were 340–2,607 μg/mL at 24 hr, about 2–3 orders of magnitude above typical MIC levels (0.5–4 μg/mL) (Fig 5b). In the femur, the average concentration was no less than 2,000 μg/mL in any rabbit at any time point. In the quadriceps defect and intramuscular injection site, tobramycin levels exceeded 1,000 μg/mL at 24 hr, while in the knee tissue levels were measured at 340 μg/mL. By 72 hr, tobramycin in the quadriceps defect and knee decreased but remained above 140 μg/mL, but comparatively was not detected in tissue from the intramuscular injection (Fig 5c). Again, these data suggest rapid clearance of tobramycin in soft tissue sites. Vancomycin tissue levels were significantly lower and never reached the peak levels achieved by tobramycin (p < 0.001), but remained more steady over the duration of the study (Fig 5b,c). The measured vancomycin levels were greater than 100 μg/mL for at least 24 hr in all sites tested and 72 hr in the femur and quadriceps defect. The highest average vancomycin concentration in tissue in any group across all sites and time points was 1,360 μg/mL (PNDJ1.36, 72 hr, femur), whereas tobramycin concentrations in excess of 4,000 μg/mL were measured in several tissue samples. Furthermore, at 24 hr, tobramycin comprised on average 80% of the drug content in the tissues. Thus, the proportion of vancomycin relative to tobramycin measured in tissue samples was lower than would be expected based on the amount released from the gel. It is unclear whether this discrepancy is due to differences in vancomycin transport, clearance, or some other cause. A possible explanation is that vancomycin, which has a higher molecular weight compared to tobramycin, may have been present at comparable levels in fluid within the surgical site but had more limited transport into adjacent tissue. Further studies are needed to investigate the distribution and possible clearance of vancomycin in these tissues.
Recently, we performed microbiological studies evaluating the sterilization of biofilm-infected muscle, we found that combined concentrations of tobramycin and vancomycin at 100–750 μg/mL sustained for 24 hr were sufficient to eradicate tissue-bound biofilms of 10 different bacteria (including multidrug-resistant S. aureus and S. epidermidis) representing pathogens commonly found in SSIs [34]. Based on these data, we identified a target for local delivery of up to 750 μg/mL total antimicrobial concentration sustained for at least 24 hr. Among the dosing sites evaluated in this work, the quadriceps defect site is a reasonable model for the most common surgical wounds following debridement in treatment of an established deep tissue SSI in humans. Combined tissue levels in this site were 1,683 μg/mL at 24 hr and 548 μg/mL at 72 hr which are promising compared to the target estimated by microbiological studies. The only tissue in which the upper limit of the MBEC target was not met was in the intraarticular injection site, where levels were 451 μg/mL at 24 hr. This may present a concern for treating especially virulent infections in this site.
Based on current clinical understanding, successful treatment of deep SSIs relies on thorough removal of foreign material along with infected and dysvascular tissue. If debridement procedures were capable of removing all biofilm and planktonic bacteria, then adjuvant chemotherapy would not be required. Realistically, however, even when performed by the most skilled surgeons, debridement is likely to be imperfect due to the inevitable dislodgment of microscopic biofilm fragments or liberating planktonic bacteria. Thus, local delivery is implemented to achieve drug concentrations capable of eradicating residual planktonic bacteria and biofilm in the surgical wound or at the debridement margins which may otherwise lead to recurrence. Because of variation in the extent of infection, extent of surgical debridement, antimicrobial susceptibility of the pathogen(s), and patient-specific host defense factors, the required concentration of antimicrobials will vary. While clinical outcomes data are needed to confirm eradication of biofilm-based SSIs, it is reasonable to expect that providing sustained tissue levels above MBEC across the entire diversity of complex tissue and implant surfaces within an orthopaedic surgical site is the strategy that has the highest likelihood of success.
Some of the most serious SSIs occur following orthopaedic surgery. Orthopaedic SSIs present a challenge for local drug delivery technologies because of the irregular shape and movement of articulating surfaces, compatibility with postoperative healing of a variety of tissues, and the requirement for local delivery to sustain biofilm-eradicating levels. High-dose formulations of ALBC (poly(methylmethacrylate)) are the clinical standard treatment for prosthetic joint infections, but ALBC is implanted as a temporary spacer that must be removed and replaced in an additional procedure several weeks later. Clinical studies report peak local levels from ALBC formulations that exceed MBEC levels (>100 μg/mL) within the first 24 hr and decline thereafter, providing long-term exposure at sub-MIC levels [35,36]. Resorbable non-gel polymers (e.g., PLGA, polyanhydrides) [37–39] or bone graft substitutes (e.g., calcium sulfate, calcium phosphate, hydroxyapatite [40–42], may also be used in orthopaedic applications. but are incompatible with bone-implant fixation interfaces and articulating surfaces. Alternatively, materials with high water content (e.g. collagen sponge [43–46]), are more compatible with the diverse tissues within an orthopaedic site, but are also prone to fast release of commonly used low molecular weight hydrophilic antimicrobials which precludes their use for infection treatment. For example, in vivo evaluation of gentamicin-loaded collagen sponge in canine stifles [17] and rabbit hindlimbs [47] found local levels transiently exceeded 1,000 μg/mL in the first few hours after dosing, but declined to near MIC (<10 μg/mL) by 24 hr after implantation. To our knowledge, the only new formulation in clinical studies for orthopaedic infection treatment is a bismuth-thiol in a polypropylene glycol-based gel. However, preclinical animal models showed no clear dose response and only 30–90% success in preventing planktonic bacterial infections when applied in combination with systemic cefazolin [48].
PNDJ hydrogels have a number of properties that suggest promise in improving treatment of orthopaedic SSIs. PNDJ gels are capable of conforming to the complex architecture of surgical sites, and they are compatible with application in hard, soft, and articulating tissues as well as implant surfaces. Additionally, PNDJ hydrogels have tunable degradation properties to completely dissolve within 6 weeks of administration [19], may be loaded with numerous antimicrobials (gentamicin, vancomycin, and tobramycin have been directly demonstrated), has shown high effectiveness in preclinical infection models [21,22], and as described in this work, provide sustained antimicrobial release that produced tissue drug levels that are expected to be effective against both planktonic and biofilm-based bacteria.
In addition to potentially improving the treatment of established SSIs, antimicrobial-loaded PNDJ hydrogels also hold promise for SSI prevention, an area of high unmet need. Gentamicin-loaded collagen sponges have been shown to provide antimicrobial levels that exceed MIC levels over 22 hr in animal models [17], but clinical trial data have failed to show efficacy in prevention of SSI following colorectal surgery [44,45] and mixed results have been reported in prevention of SSI following cardiac surgery [46,49,50]. Gentamicin and vancomycin loaded into a sesame oil-lecithin carrier was also recently found to not provide significant reduction of SSI following abdominal surgery relative to the current standard of care [51]. These materials likely do not provide antimicrobial exposure in local tissue at high multiples of MIC for more than a few hours. We suspect that the drug payloads, although high, are rapidly released and may be cleared from tissue prior to eradicating all bacteria in the wound [43,52]. Intrawound application of unencapsulated vancomycin powder has been recently adopted for SSI prophylaxis, but wound drain levels in total hip and total knee arthroplasty have been reported to be highly variable [53], and vancomycin only has activity against Gram-positive microorganisms. Based on its advantageous properties and strength of the preclinical data, PNDJ hydrogels have the potential to become a standard component of SSI prophylaxis because they provide high, sustained, and reliable antimicrobial concentrations that cover a broad spectrum of pathogens.
Conclusion
In this work, we evaluated in vivo antimicrobial delivery from PNDJ hydrogels by direct measurements of drug concentrations in tissue. We demonstrated that in vitro drug release experiments, while informative, did not directly predict in vivo drug release, which was faster. Tissue drug levels varied significantly depending on the site of PNDJ application, but in all tested tissues, we observed drug exposure that is expected to be effective against biofilm. Future studies will focus on formulation optimization and studies to evaluate the safety of the gels. Overall, this work supports the feasibility of temperature-responsive degradable PNDJ hydrogels as a novel sustained release carrier for antimicrobials to eliminate residual post-debridement bacteria from biofilm-based surgical site infections including orthopaedic infections.
Supplementary Material
Acknowledgements:
We thank Pamela Bortz and the animal care group at St. Joseph’s Hospital and Medical Center (Phoenix, AZ) for their assistance with this project. Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R44AR070685. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Compliance with Ethical Standards: The authors declare that the experiments described in this manuscript were conducted in compliance with the current laws of the United States.
Conflict of Interest Disclosure: DO is an employee of, owns stock in, and is an inventor on pending patents owned by, Sonoran Biosciences. BV owns stock in and has received research support from Sonoran Biosciences. Alex McLaren has served as a consultant for and owns stock in Sonoran Biosciences. VB, RM, EC, and JH are current or former employees of Sonoran Biosciences.
Animal Studies: All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- 1.Gbejuade HO, Lovering AM, Webb JC. The role of microbial biofilms in prosthetic joint infections: A review. Acta Orthopaedica. 2015;86:147–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McConoughey SJ, Howlin R, Granger JF, Manring MM, Calhoun JH, Shirtliff M, et al. Biofilms in periprosthetic orthopedic infections. Future Microbiology. 2014;9:987–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewis K Multidrug tolerance of biofilms and persister cells. Curr Top Microbiol Immunol. 2008;322:107–31. [DOI] [PubMed] [Google Scholar]
- 4.Lewis K Persister Cells. Annual Review of Microbiology. 2010;64:357–72. [DOI] [PubMed] [Google Scholar]
- 5.Fux C, Wilson S, Stoodley P. Detachment characteristics and oxacillin resistance of Staphyloccocus aureus biofilm emboli in an in vitro catheter infection model. J Bacteriol. 2004;186:4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olson ME, Ceri H, Morck DW, Buret AG, Read RR. Biofilm bacteria: formation and comparative susceptibility to antibiotics. Can J Vet Res. 2002;66:86–92. [PMC free article] [PubMed] [Google Scholar]
- 7.Patel R. Biofilms and antimicrobial resistance. Clin Orthop Relat Res. 2005;41–7. [DOI] [PubMed] [Google Scholar]
- 8.Castaneda P, McLaren A, Tavaziva G, Overstreet D. Biofilm Antimicrobial Susceptibility Increases With Antimicrobial Exposure Time. Clin Orthop Relat Res [Internet]. 2016. [cited 2016 Jan 25]; Available from: http://link.springer.com/10.1007/s11999-016-4700-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith K, Perez A, Ramage G, Gemmell CG, Lang S. Comparison of biofilm-associated cell survival following in vitro exposure of meticillin-resistant Staphylococcus aureus biofilms to the antibiotics clindamycin, daptomycin, linezolid, tigecycline and vancomycin. Int J Antimicrob Agents. 2009;33:374–8. [DOI] [PubMed] [Google Scholar]
- 10.Raad I, Hanna H, Jiang Y, Dvorak T, Reitzel R, Chaiban G, et al. Comparative Activities of Daptomycin, Linezolid, and Tigecycline against Catheter-Related Methicillin-Resistant Staphylococcus Bacteremic Isolates Embedded in Biofilm. Antimicrob Agents Chemother. 2007;51:1656–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson CL, Hickmon SG, Skinner RA. Treatment of experimental osteomyelitis by surgical debridement and the implantation of bioerodable, polyanhydride-gentamicin beads. J Orthop Res. 1997;15:249–255. [DOI] [PubMed] [Google Scholar]
- 12.Ambrose CG, Clyburn TA, Louden K, Joseph J, Wright J, Gulati P, et al. Effective Treatment of Osteomyelitis with Biodegradable Microspheres in a Rabbit Model. Clin Orthop Relat Res. 2004;421:293–9. [DOI] [PubMed] [Google Scholar]
- 13.Langlais F Can we improve the results of revision arthroplasty for infected total hip replacement? J Bone Joint Surg Br. 2003;85:637–640. [PubMed] [Google Scholar]
- 14.Ostermann PAW, Seligson D, Henry SL. Local antibiotic therapy for severe open fractures. J Bone Joint Surg Br. 1995;77-B:93–7. [PubMed] [Google Scholar]
- 15.Zalavras CG, Patzakis MJ, Holtom P. Local antibiotic therapy in the treatment of open fractures and osteomyelitis. Clin Orthop Relat Res. 2004;86–93. [DOI] [PubMed] [Google Scholar]
- 16.Cabrita HB, Croci AT, Camargo OP de, Lima ALLM de. Prospective study of the treatment of infected hip arthroplasties with or without the use of an antibiotic-loaded cement spacer. Clinics (Sao Paulo). 2007;62:99–108. [DOI] [PubMed] [Google Scholar]
- 17.Hayes GM, Gibson TWG, Moens NMM, Monteiro B, Johnson RJ. Intra-articular pharmacokinetics of a gentamicin impregnated collagen sponge in the canine stifle: an experimental study. Vet Surg. 2014;43:166–73. [DOI] [PubMed] [Google Scholar]
- 18.Wilson RC, Duran SH, Horton CR Jr, Wright LC. Bioavailability of gentamicin in dogs after intramuscular or subcutaneous injections. Am J Vet Res. 1989;50:1748–50. [PubMed] [Google Scholar]
- 19.Overstreet DJ, Huynh R, Jarbo K, McLemore RY, Vernon BL. In situ forming, resorbable graft copolymer hydrogels providing controlled drug release. J Biomed Mater Res A. 2013;101:1437–46. [DOI] [PubMed] [Google Scholar]
- 20.Cui Z, Lee BH, Vernon BL. New hydrolysis-dependent thermosensitive polymer for an injectable degradable system. Biomacromolecules. 2007;8:1280–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Overstreet D, McLaren A, Calara F, Vernon B, McLemore R. Local gentamicin delivery from resorbable viscous hydrogels is therapeutically effective. Clin Orthop Relat Res. 2015;473:337–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McLaren AC, Castaneda P, Overstreet DJ. Post-Debridement Local Antimicrobial Delivery from PNDJ Gel is Effective for Orthopaedic Infections: A Rabbit Study. ORS Annual Meeting [Internet]. 2017. p. 1319 Available from: https://www.ors.org/Transactions/63/1319.pdf [Google Scholar]
- 23.Moore R, Badha V, McLaren A, Overstreet D, Moore MR. Toxicity Assessment of a Novel Temperature-Responsive Antimicrobial Loaded Hydrogel for Biofilm Treatment. Society of Toxicology Annual Meeting 2018 San Antonio, TX; 2018. [Google Scholar]
- 24.Overstreet DJ, McLemore RY, Doan BD, Farag A, Vernon BL. Temperature-Responsive Graft Copolymer Hydrogels for Controlled Swelling and Drug Delivery. Soft Materials. 2013;11:294–304. [Google Scholar]
- 25.Parvizi J, Gehrke T, Chen AF. Proceedings of the International Consensus Meeting on Periprosthetic Joint Infection. The Bone & Joint Journal. 2013;95-B:1450–2. [DOI] [PubMed] [Google Scholar]
- 26.Raghavan S, Babu VS. Enantioselective synthesis of oseltamivir phosphate. Tetrahedron. 2011;67:2044–50. [Google Scholar]
- 27.Kizhakkedathu JN, Janzen J, Le Y, Kainthan RK, Brooks DE. Poly(oligo(ethylene glycol)acrylamide) Brushes by Surface Initiated Polymerization: Effect of Macromonomer Chain Length on Brush Growth and Protein Adsorption from Blood Plasma. Langmuir. 2009;25:3794–801. [DOI] [PubMed] [Google Scholar]
- 28.Frutos P, Torrado S, Perez-Lorenzo ME, Frutos G. A validated quantitative colorimetric assay for gentamicin. J Pharm Biomed Anal. 2000;21:1149–1159. [DOI] [PubMed] [Google Scholar]
- 29.Giers MB, Estes CS, McLaren AC, Caplan MR, McLemore R. Jeannette Wilkins Award: Can Locally Delivered Gadolinium Be Visualized on MRI? A Pilot Study. Clin Orthop Relat Res. 2012;470:2654–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giers MB, McLaren AC, Plasencia JD, Frakes D, McLemore R, Caplan MR. Spatiotemporal quantification of local drug delivery using MRI. Comput Math Methods Med. 2013;2013:149608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McLaren A, Giers MB, Fraser J, Hosack L, Caplan MR, McLemore R. Antimicrobial Distribution From Local Delivery Depends on Dose: A Pilot Study With MRI. Clin Orthop Relat Res. 2014;472:3324–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bertrand N, Fleischer JG, Wasan KM, Leroux J-C. Pharmacokinetics and biodistribution of N-isopropylacrylamide copolymers for the design of pH-sensitive liposomes. Biomaterials. 2009;30:2598–605. [DOI] [PubMed] [Google Scholar]
- 33.DeNardo SJ, Yao Z, Lam KS, Song A, Burke PA, Mirick GR, et al. Effect of Molecular Size of Pegylated Peptide on the Pharmacokinetics and Tumor Targeting in Lymphoma-Bearing Mice. Clin Cancer Res. 2003;9:3854S–3864S. [PubMed] [Google Scholar]
- 34.Badha V, Moore R, McLaren A, Castaneda P, Overstreet D. Antimicrobial Susceptibility of Tissue-Based Bacterial Biofilms. Boston, MA; 2017. p. 53. [Google Scholar]
- 35.Anagnostakos K, Wilmes P, Schmitt E, Kelm J. Elution of gentamicin and vancomycin from polymethylmethacrylate beads and hip spacers in vivo. Acta Orthopaedica. 2009;80:193–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Regis D, Sandri A, Samaila E, Benini A, Bondi M, Magnan B. Release of Gentamicin and Vancomycin from Preformed Spacers in Infected Total Hip Arthroplasties: Measurement of Concentrations and Inhibitory Activity in Patients’ Drainage Fluids and Serum. The Scientific World Journal. 2013;2013:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gollwitzer H Antibacterial poly(D,L-lactic acid) coating of medical implants using a biodegradable drug delivery technology. J Antimicrob Chemother. 2003;51:585–91. [DOI] [PubMed] [Google Scholar]
- 38.Lucke M, Schmidmaier G, Sadoni S, Wildemann B, Schiller R, Haas N, et al. Gentamicin coating of metallic implants reduces implant-related osteomyelitis in rats. Bone. 2003;32:521–531. [DOI] [PubMed] [Google Scholar]
- 39.Price JS, Tencer AF, Arm DM, Bohach GA. Controlled release of antibiotics from coated orthopedic implants. J Biomed Mater Res. 1996;30:281–6. [DOI] [PubMed] [Google Scholar]
- 40.Barralet J, Gaunt T, Wright A, Gibson I, Knowles J. Effect of porosity reduction by compaction on compressive strength and microstructure of calcium phosphate cement. J Biomed Mater Res A. 2002;63:1–9. [DOI] [PubMed] [Google Scholar]
- 41.Hak DJ. The Use of Osteoconductive Bone Graft Substitutes in Orthopaedic Trauma. J Am Acad Orthop Surg. 2007;15:525–36. [DOI] [PubMed] [Google Scholar]
- 42.Brooks BD, Sinclair KD, Grainger DW, Brooks AE. A resorbable antibiotic-eluting polymer composite bone void filler for perioperative infection prevention in a rabbit radial defect model. PloS one. 2015;10:e0118696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sorensen TS, Sorensen AI, Merser S. Rapid release of gentamicin from collagen sponge. Acta Orthop Scand. 1990;61:353–356. [DOI] [PubMed] [Google Scholar]
- 44.Bennett-Guerrero E, Pappas TN, Koltun WA, Fleshman JW, Lin M, Garg J, et al. Gentamicin–Collagen Sponge for Infection Prophylaxis in Colorectal Surgery. N Engl J Med. 2010;363:1038–49. [DOI] [PubMed] [Google Scholar]
- 45.Pochhammer J, Zacheja S, Schäffer M. Subcutaneous application of gentamicin collagen implants as prophylaxis of surgical site infections in laparoscopic colorectal surgery: a randomized, double-blinded, three-arm trial. Langenbeck’s Archives of Surgery. 2015;400:1–8. [DOI] [PubMed] [Google Scholar]
- 46.Kowalewski M, Pawliszak W, Zaborowska K, Navarese EP, Szwed KA, Kowalkowska ME, et al. Gentamicin-collagen sponge reduces the risk of sternal wound infections after heart surgery: Meta-analysis. The Journal of Thoracic and Cardiovascular Surgery. 2015;149:1631–1640.e6. [DOI] [PubMed] [Google Scholar]
- 47.Mehta S, Humphrey JS, Schenkman DI, Seaber AV, Vail TP. Gentamicin distribution from a collagen carrier. Journal of Orthopaedic Research. 1996;14:749–54. [DOI] [PubMed] [Google Scholar]
- 48.Penn-Barwell JG, Baker B, Wenke JC. Local bismuth thiols potentiate antibiotics and reduce infection in a contaminated open fracture model. J Orthop Trauma. 2015;29:e73–78. [DOI] [PubMed] [Google Scholar]
- 49.Schimmer C, Özkur M, Sinha B, Hain J, Gorski A, Hager B, et al. Gentamicin-collagen sponge reduces sternal wound complications after heart surgery: A controlled, prospectively randomized, double-blind study. The Journal of Thoracic and Cardiovascular Surgery. 2012;143:194–200. [DOI] [PubMed] [Google Scholar]
- 50.Bennett-Guerrero E, Ferguson TB, Lin M, Garg J, Mark DB, Scavo VA, et al. Effect of an Implantable Gentamicin-Collagen Sponge on Sternal Wound Infections Following Cardiac Surgery: A Randomized Trial. JAMA. 2010;304:755. [DOI] [PubMed] [Google Scholar]
- 51.Bennett-Guerrero E, Berry SM, Bergese SD, Fleshner PR, Minkowitz HS, Segura-Vasi AM, et al. A randomized, blinded, multicenter trial of a gentamicin vancomycin gel (DFA-02) in patients undergoing abdominal surgery. The American Journal of Surgery. 2017;213:1003–9. [DOI] [PubMed] [Google Scholar]
- 52.Penn-Barwell JG, Murray CK, Wenke JC. Local antibiotic delivery by a bioabsorbable gel is superior to PMMA bead depot in reducing infection in an open fracture model. J Orthop Trauma. 2014;28:370–5. [DOI] [PubMed] [Google Scholar]
- 53.Johnson JD, Nessler JM, Horazdovsky RD, Vang S, Thomas AJ, Marston SB. Serum and Wound Vancomycin Levels After Intrawound Administration in Primary Total Joint Arthroplasty. The Journal of Arthroplasty. 2017;32:924–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.