Abstract
Background:
Nosocomial infections (NI) can be a major cause of morbidity and mortality in cirrhosis.
Aim:
Define the determinants of NI development and 30-day outcomes among hospitalized patients with cirrhosis.
Methods:
NACSELD (North American Consortium for the Study of End-Stage Liver Disease) enrolled cirrhotics admitted non-electively. Admission variables and 30-day outcomes were compared between patients with/without NI. These were also compared based on whether there was an isolated admission infection, NI or both. Models were created for NI development using admission variables and for 30-day mortality.
Results:
2864 patients were included, of which 15% (n=436) developed NI. NI vs no-NI: 1866 patients were infection-free, 562 had admission infections only, 228 had only NI while 208 had both infections. At admission, NI patients were more likely to be infected and have advanced cirrhosis. NIs were associated with higher rates of ACLF, death and transplant regardless of admission infections. Infection details: NI patients had higher respiratory, UTI, C.difficile, fungal infections and vancomycin-resistant Enterococci compared to patients without NI. NI development: Risk factors for NI were admission infections, MELD>20, SIRS criteria, PPI, rifaximin and lactulose use but the regression model (sensitivity 0.67, specificity 0.63) was not robust. Mortality: Age, alcohol etiology, admission MELD, lactulose use, ACLF, AKI, ICU and NI increased the risk while rifaximin decreased the risk of death.
Conclusions:
Nosocomial infections are prevalent in hospitalized cirrhotic patients and are associated with poor outcomes. Although higher MELD and SIRS are associated with NI, all hospitalized cirrhotic patients require vigilance and preventive strategies.
Introduction:
Infections are a major cause of morbidity and mortality in cirrhosis(1, 2). In addition to precipitating complications such as hepatic encephalopathy (HE), infections are often responsible for the development of acute kidney injury (AKI) and acute-on-chronic liver failure (ACLF), can increase the risk of delisting for liver transplant and hasten death(3, 4). An important aspect in hospitalized patients with cirrhosis is the development of nosocomial infections, which may potentially be a preventable cause of organ failure, ACLF, and death(5, 6). Prior smaller and single-center studies have shown that nosocomial infections are common in cirrhosis and can negatively impact short-term outcomes(7–9). However, a larger multi-center approach focused on North America with models to predict the development of, and outcomes from nosocomial infections is required.
Therefore, our aim was to define the determinants of developing a nosocomial infection and to evaluate their influence on 30-day outcomes in a large prospective, multi-center, inpatient cohort with cirrhosis.
Methods:
The data of unique patients with cirrhosis enrolled in the North American Consortium for the Study of End-Stage Liver Disease (NACSELD) were analyzed for this study. NACSELD consists of 14 tertiary-care hepatology centers across North America, which prospectively recruited patients with cirrhosis hospitalized for non-elective reasons from April 2013 to February 2017. We excluded subjects who were post-transplant, had HIV infection, had non-hepatocellular carcinoma malignancies, and were unable to provide consent or did not have a representative who could give consent. All consented patients had data recorded securely in a REDCAP database(10). The data collected was demographic variables, details of cirrhosis and severity, admission medications and reasons for the hospitalization and hospital course. Admissions were divided into those due or not due to an infection. Infections first diagnosed within the first 48 hours after admission were considered non-nosocomial, and those diagnosed 48 hours or greater after admission were considered nosocomial. In patients admitted with an admission infection, a subsequent nosocomial infection was only diagnosed if the PI and team were satisfied that this was not an unresolved prior infection.
Data collected on admission pertained to demographics, prior hospitalizations, diabetes, concomitant medications (beta-blockers, rifaximin, lactulose, SBP prophylaxis, PPI), prior cirrhosis complications, laboratory values for MELD score and Child class, SIRS criteria, WBC count, platelet count, serum albumin and serum sodium.
Specific data regarding the site of infection, causative organism and resistance patterns of the admission infection and nosocomial infections were analyzed. Infections were defined as follows(8, 11) a) spontaneous bacteremia: positive blood cultures without a source of infection, (b) SBP: ascitic fluid polymorphonuclear cells >250/μL with/without a positive fluid culture, (c) lower respiratory tract infections: new pulmonary infiltrate in the presence of: (i) at least one respiratory symptom (cough, sputum production, dyspnea, pleuritic pain) with (ii) at least one finding on auscultation (rales or crepitation) or one sign of infection (core body temperature >38°C or < 36 °C, shivering or leucocyte count >10,000/mm3 or <4,000/mm3) in the absence of antibiotics, (d) Clostridium difficile: diarrhea with a positive C. difficile assay (e) bacterial entero-colitis: diarrhea or dysentery with a positive stool culture for Salmonella, Shigella, Yersinia, Campylobacter, or pathogenic E. coli, (f) skin Infection: fever with cellulitis, (g) urinary tract infection (UTI): urine WBC >15/high power field with either positive urine gram stain or culture in a symptomatic patient, (h) intra-abdominal infections: diverticulitis, appendicitis, cholangitis, etc., (i) secondary bacterial peritonitis: >250 polymorphonuclear cells/μL of ascitic fluid in the presence of an intra-abdominal source of peritonitis and multiple organisms cultured from ascitic fluid. In addition, we recorded all instrumentations and procedures that occurred throughout the hospitalization. The timing of procedures and instrumentation vis-à-vis NI were also recorded. Hospital course data collected in aggregate pertained to individual extra-hepatic organ failures, ACLF according to NACSELD criteria, transfer to the intensive care unit (ICU), length of stay (LOS), development of acute kidney injury (AKI according to International Ascites Club consensus criteria(12)), liver transplant and 30-day mortality. NACSELD-ACLF is defined as 2 or more of the following, brain failure: grade 3 / 4 hepatic encephalopathy, respiratory failure: mechanical ventilation or BiPAP, renal failure: renal replacement therapy and circulatory failure: shock(13) any time during the admission. These outcomes were compared between those with and without NI using appropriate parametric (unpaired t-tests) or non-parametric tests (Chi-square test).
In addition, the group was also divided into 1) patients who never had an infection on admission and did not develop an NI, 2) those who only had an admission infection without a NI, 3) those without an admission infection but who developed a NI and 4) those with both types of infections. Outcomes were compared between these four groups as well, using appropriate parametric (ANOVA) and non-parametric (Kruskal-Wallis) tests.
Determinants of nosocomial infection development were studied using binary logistic regression with variables on admission. Specific variables considered were age, gender, race, alcoholic etiology, diabetes, admitted with infection, refractory ascites on admission, hospitalized in the last 6 months, admission medications (PPI, non-selective beta-blockers, lactulose, rifaximin), admission laboratory values (albumin, WBC, serum sodium, MELD), SIRS criteria on admission, admission mean arterial pressure and being on dialysis at admission for the admission models. Lactulose and rifaximin were used as surrogates for hepatic encephalopathy. Variables significant at p<0.10 on univariate analysis were included in the final models created using backwards elimination procedures. The final models were tested using ROC curves for AUC, sensitivity and specificity for the development of a nosocomial infection. We also studied differences in rate of NI between centers using random effects for center in the models.
Separate models for variables independently associated with nosocomial infection using admission values were also created for patients who were admitted with infection and for those who were not admitted with infection. We also performed analyses of odd ratios of variables that were significantly different between patients who ultimately developed NI vs others. Determinants of 30-day mortality were also analyzed using multi-variable logistic regression. In addition to NI, variables included were again admission variables related to liver disease, medications, demographics, and inpatient. Models were also created using nosocomial infection as a yes/no variable and using the ordinal variable of: no infections, admission yes but no nosocomial infection, no admission infection but nosocomial infection and both infection types). Variables significant at p<0.10 on univariate analysis were included in the final models created using backwards elimination procedures. The final models were tested using ROC curves for AUC, sensitivity and specificity for 30-day mortality. We also then performed bootstrap analysis to determine the robustness of the models.
Results:
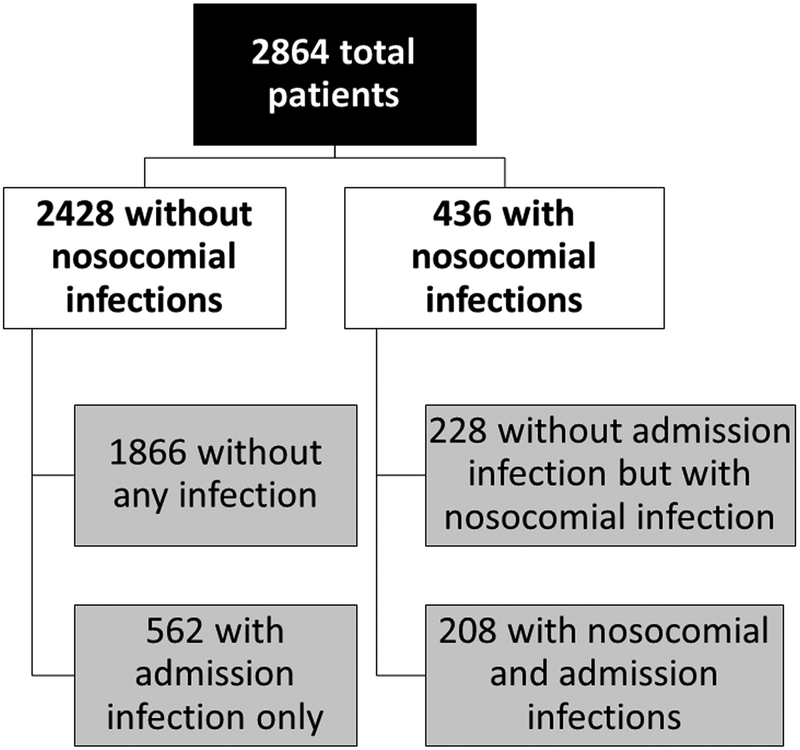
We included 2864 patients, of whom 436 (15%) developed a nosocomial infection during hospitalization. As shown in figure 1, 208 of the 436 (48%) were not infected on admission, while the remaining 208 (52%) had also been diagnosed with an infection at admission. 1866 patients had no infection during the entire hospitalization, while 562 patients had infections only on admission without having developed a nosocomial infection. Since all our 2,864 patients with cirrhosis had contact with the health system within 6 months, all non-nosocomial infections were healthcare-associated.
Figure 1:
Flowchart of patients with and without admission and nosocomial infections
Comparison of patients with and without nosocomial infections:
As shown in Table 1, patients with and without a nosocomial infection has similar demographics, prevalence of diabetes and cirrhosis etiology, with the exception of more women in the NI group. A higher number of patients with NI were admitted with an infection, had more advanced liver diseases defined as a higher MELD score, were more likely to be on PPI, SBP prophylaxis and lactulose and were more likley to have been hospitalized in the prior 6-months. The major non-infectious reasons for admission were similar between groups with liver-related being the major cause [anasarca (21%), hepatic encephalopathy (17%), renal insufficiency (12%), GI bleeding (6%) and other (3%)] followed by liver unrelated [cardiovascular (6%), pulmonary (5%) and others (3%)].
Table 1:
Comparison of patients without and with a nosocomial infection
| No Nosocomial Infection (n = 2428) |
With Nosocomial Infection (n = 436) |
p-value |
|
|---|---|---|---|
| Admission Data | |||
| Age (years) | 57.22 (10.85) | 57.23 (10.39) | 0.70 |
| Gender (Male) | 63% (1527) | 57% (249) | 0.03 |
| Race (Caucasian) | 79% (1906) | 81% (351) | 0.30 |
| BMI | 29.93 (7.79) | 30.10 (11.45) | 0.18 |
| Etiology Alcoholic cirrhosis HCV HCV + alcoholic cirrhosis NASH Other |
30% (734) 21% (503) 14% (349) 21% (498) 14% (333) |
32% (139) 18% (80) 12% (52) 24% (106) 13% (57) |
0.23 |
| Diabetes | 34% (805) | 38% (164) | 0.09 |
| Admitted with infection | 23% (555) | 48% (208) | <0.0001 |
| Ascites | 69% (1674) | 75% (325) | 0.02 |
| Refractory ascites | 32% (770) | 40% (175) | 0.0006 |
| Hospitalized in last 6 months | 67% (1482) | 74% (281) | 0.005 |
| Medication used: PPI NSBB SBP prophylaxis Rifaximin Lactulose |
54% (1174) 41% (964) 17% (404) 35% (842) 54% (1293) |
59% (234) 38% (160) 23% (96) 41% (172) 62% (267) |
0.05 0.32 0.005 0.03 0.003 |
| On dialysis at admission | 3% (69) | 5% (21) | 0.05 |
| Admission SIRS (n, %) | 25% (592) | 37% (157) | <0.0001 |
| Temperature Component | 8% (204/2414) | 16% (70/432) | <0.0001 |
| Heart Rate Component | 39% (947/2418) | 47% (204/436) | 0.0028 |
| Respiratory Rate Component | 15% (367/2411) | 26% (114/433) | <0.0001 |
| WBC Component | 32% (781/2408) | 45% (193/429) | <0.0001 |
| Sepsis | 9% (213/2428) | 30% (131/236) | <0.0001 |
| Admission Lab values | |||
| Bilirubin | 5.74 (8.48) | 7.52 (9.14) | <0.0001 |
| Albumin | 2.86 (0.670 | 2.75 (0.69) | 0.003 |
| WBC | 7.49 (4.970 | 9.00 (7.24) | <0.0001 |
| INR | 1.67 (0.640 | 1.78 (0.60) | 0.0001 |
| Serum Sodium | 134.33 (7.05) | 133.35 (6.55) | <0.0001 |
| Serum creatinine | 1.49 (1.30) | 1.86 (1.56) | <0.0001 |
| Child-Pugh score | 9.46 (2.14) | 10.32 (2.11) | <0.0001 |
| MELD score | 19.05 (7.52) | 22.17 (8.06) | <0.0001 |
| Hospital Course | |||
| Episodes of AKI | 37% (777) | 69% (277) | <0.0001 |
| Length of hospital stay | 9.64 (10.51) | 28.04 (61.29) | <0.0001 |
| ICU admission | 18% (439) | 52% (227) | <0.0001 |
| Renal Failure | 5% (129) | 21% (91) | <0.0001 |
| Brain Failure | 12% (297) | 28% (118) | <0.0001 |
| Circulatory Failure | 6% (140) | 25% (106) | <0.0001 |
| Respiratory Failure | 9% (218) | 34% (147) | <0.0001 |
| Number of Organ Failures 0 1 2 3 4 |
76% (1850) 17% (416) 5% (113) 2% (38) 0.4% (7) |
46% (200) 22% (96) 16% (69) 13% (56) 3% (15) |
<0.0001 |
| NACSELD-ACLF | 7% (158) | 32% (140) | <0.0001 |
| Liver transplant | 3% (74) | 9% (38) | <0.0001 |
| 30-day survival | 92% (2244) | 72% (313) | <0.0001 |
ACLF defined as NACSELD-ACLF (2 or more of the following, brain failure: grade 3 / 4 hepatic encephalopathy, respiratory failure: mechanical ventilation or BiPAP, renal failure: renal replacement therapy and circulatory failure: shock) AKI: acute kidney injury, NSBB: non-selective beta-blockers
Instrumentations and interventions occurred at higher rates in NI patients compared to those without NI and these were comparable before or after the NI development (Supplementary table 1). The hospital course for patients with NI was associated with a greater probability of developing AKI, organ failures and ACLF. Patients with nosocomial infections had a longer hospital length of stay (LOS) and had a lower 30-day survival. Patients with NI received a liver transplant at a higher rate than patients without a NI.
Infection details:
Between groups with and without NI, the relative rates of SBP, spontaneous bacteremia and skin/soft-tissue infections were similar. In contrast, more NIs tended to be respiratory, urinary, procedure-related, fungal and C.difficile. There was also a greater culture positivity rate across all types, especially for gram-positive organisms and vancomycin-resistant Enterococci in patients with NI (Table 3).
Table 3:
Details of Infections
|
Type of infection |
No Nosocomial Infection (n = 562) |
With Nosocomial Infection (n = 436) |
p-value |
Type of infection |
Admission Infection present without Nosocomial Infection (n = 562) |
No Admission Infection but with Nosocomial Infection (n = 228) |
Both admission Infection and Nosocomial Infection (n = 208) |
p-value |
|---|---|---|---|---|---|---|---|---|
| SBP | 23% (125) | 21% (93) | 0.70 | SBP* | 23% (125) | 16% (36) | 27% (57) | 0.02 |
| Spontaneous Bacteremia | 10% (56) | 12% (54) | 0.24 | Spontaneous Bacteremia | 10% (56) | 11% (24) | 14% (30) | 0.23 |
| Respiratory | 9% (50) | 19% (82) | <0.0001 | Respiratory | 9% (50) | 17% (39) | 21% (43) | <0.0001 |
| Skin/Soft-tissue | 10% (55) | 8% (34) | 0.26 | Skin/Soft-tissue* | 10% (55) | 4% (8) | 13% (26) | 0.003 |
| UTI | 19% (107) | 34% (149) | <0.0001 | UTI | 19% (107) | 33% (74) | 36% (75) | <0.0001 |
| C.difficile | 5% (29) | 8% (36) | 0.05 | C.difficile | 5% (29) | 6% (14) | 11% (22) | 0.03 |
| Procedural Related Infection | 1% (4) | 6% (26) | <0.0001 | Procedural Related Infection | 1% (4) | 4% (10) | 8% (16) | <0.0001 |
| Other bacterial infection | 9% (5) | 5%(22) | 0.02 | Other bacterial infection | 9% (50) | 5%(10) | 6% (12) | 0.06 |
| Organism details | ||||||||
| Gram Positive | 29% (160) | 42% (181) | <0.0001 | Gram Positive | 29% (160) | 39% (87) | 45% (94) | <0.0001 |
| Gram Negative | 22% (124) | 28% (122) | 0.04 | Gram Negative* | 22% (124) | 23% (52) | 34% (70) | 0.004 |
| Fungus | 2% (9) | 14% (58) | <0.0001 | Fungus | 2% (9) | 13% (29) | 14% (29) | <0.0001 |
| No Organism Isolated | 38% (209) | 34% (145) | 0.18 | No Organism Isolated* | 38% (209) | 25% (57) | 43% (88) | <0.0001 |
| VRE | 4% (19) | 11% (37) | 0.0005 | VRE | 4% (19) | 14% (21) | 9% (16) | 0.0004 |
| MRSA | 5% (23) | 7% (22) | 0.44 | MRSA | 5% (23) | 7% (10) | 7% (12) | 0.74 |
| Fluoroquinolone Resistance | 5% (21) | 8% (25) | 0.11 | Fluoroquinolone Resistance* | 5% (21) | 6% (8) | 10% (17) | 0.10 |
The numbers of infectious organisms and sites of infections may add up to >100% since there were multiple organisms isolated in patients with >1 infections. VRE Vancomycin-resistant Enterococcus, MRSA: Methicillin-resistant Staphylococcus aureus. Procedure-related infections are bloodstream infections related to central lines and peripheral lines,
=p<0.05 comparing patients with admission and NI vs those with NI alone
Comparison of patients according to admission infections and nosocomial infections (Table 2):
Table 2:
Comparison of patients based on admission and nosocomial infections
| No admission Infection or Nosocomial Infection (n = 1866) |
Admission Infection present without Nosocomial Infection (n = 562) |
No Admission Infection but with Nosocomial Infection (n = 228) |
Both admission Infection and Nosocomial Infection (n = 208) |
p-value for the comparison between all four groups | |
|---|---|---|---|---|---|
| Admission Data | |||||
| Age (years) | 57.38 (10.61) | 56.65 (11.62) | 57.73 (10.21) | 56.94 (10.45) | 0.74 |
| Gender (Male) | 64% (1181) | 61% (338) | 58% (130) | 57% (119) | 0.12 |
| Race (Caucasian) | 79% (1464) | 78% (431) | 83% (185) | 79% (164) | 0.50 |
| BMI | 29.34 (7.69) | 29.36 (8.15) | 30.59 (14.05) | 29.62 (7.28) | 0.55 |
| Etiology Alcoholic cirrhosis HCV HCV + alcoholic cirrhosis NASH Other |
31% (577) 21% (394) 14% (265) 21% (389) 12% (224) |
27% (152) 19% (108) 15% (81) 19% (108) 19% (105) |
34% (77) 14% (32) 13% (29) 26% (58) 13% (28) |
29% (61) 23% (48) 11% (23) 23% (47) 14% (28) |
0.004 |
| Diabetes | 34% (624) | 33% (177) | 37% (83) | 38% (80) | 0.35 |
| Ascites | 68% (1260) | 73% (403) | 75% (168) | 75% (156) | 0.02 |
| Refractory ascites | 31% (566) | 36% (197) | 41% (93) | 39% (82) | 0.0005 |
| Hospitalized in last 6 months* | 66% (1119) | 70% (354) | 67% (126) | 80% (152) | 0.0005 |
| Medication used: PPI NSBB SBP prophylaxis Rifaximin Lactulose |
54% (888) 40% (736) 16% (298) 34% (627) 53% (977) |
55% (280) 42% (224) 20% (103) 39% (212) 57% (309) |
57% (116) 37% (80) 21% (46) 38% (85) 60% (133) |
61% (116) 39% (79) 24% (48) 43% (85) 65% (132) |
0.22 0.67 0.02 0.03 0.005 |
| On dialysis at admission | 3% (54) | 3% (15) | 6% (12) | 5% (9) | 0.23 |
| Admission SIRS (n, %)* | 22% (399) | 35% (189) | 32% (70) | 41% (84) | <0.0001 |
| Admission Lab values | |||||
| Bilirubin | 5.69 (8.73) | 5.91 (7.68) | 7.65 (9.85) | 7.47 (8.39) | <0.0001 |
| Albumin* | 2.90 (0.66) | 2.73 (0.71) | 2.83 (0.68) | 2.67 (0.69) | <0.0001 |
| WBC* | 7.11 (4.59) | 8.76 (5.89) | 8.13 (4.80) | 9.92 (9.11) | <0.0001 |
| INR | 1.65 (0.60) | 1.75 (0.77) | 1.74 (0.58) | 1.82 (0.62) | <0.0001 |
| Serum Na | 134.56 (6.77) | 133.55 (7.92) | 133.16 (6.87) | 133.57 (6.21) | <0.0001 |
| Serum creatinine | 1.48 (1.29) | 1.54 (1.33) | 1.87 (1.61) | 1.84 (1.50) | <0.0001 |
| Child-Pugh score* | 9.34 (2.13) | 9.88 (2.15) | 10.06 (2.06) | 10.66 (2.12) | <0.0001 |
| MELD score | 18.76 (7.46) | 20.04 (7.68) | 21.92 (8.13) | 22.54 (7.99) | <0.0001 |
| Outcomes | |||||
| Episodes of AKI | 34% (552) | 46% (222) | 71% (146) | 68% (130) | <0.0001 |
| Length of hospital stay | 9.36 (10.69) | 10.64 (9.91) | 27.47 (23.73) | 28.82 (85.53) | <0.0001 |
| ICU admission | 17% (324) | 21% (115) | 52% (118) | 52% (107) | <0.0001 |
| Renal Failure | 5% (93) | 7% (36) | 23% (51) | 19% (40) | <0.0001 |
| Brain Failure | 12% (218) | 14% (77) | 27% (60) | 28% (58) | <0.0001 |
| Circulatory Failure | 5% (93) | 9% (47) | 27% (58) | 24% (48) | <0.0001 |
| Respiratory Failure | 9% (163) | 10% (55) | 34% (76) | 34% (70) | <0.0001 |
| Number of Organ Failures 0 1 2 3 4 |
77% (1427) 17% (323) 4% (78) 1% (24) 0% (4) |
74% (411) 16% (91) 6% (35) 3% (14) 1% (3) |
44% (99) 23% (52) 16% (36) 14% (31) 3% (7) |
48% (99) 21% (43) 16% (33) 12% (25) 4% (8) |
<0.0001 |
| NACSELD-ACLF | 6% (106) | 9% (52) | 33% (74) | 32% (66) | <0.0001 |
| Liver transplant | 3% (54) | 4% (20) | 9% (21) | 8% (17) | <0.0001 |
| 30-day survival* | 93% (1725) | 91% (505) | 68% (152) | 75% (157) | <0.0001 |
ACLF defined as NACSELD-ACLF (2 or more of the following, brain failure: grade 3 / 4 hepatic encephalopathy, respiratory failure: mechanical ventilation or BiPAP, renal failure: renal replacement therapy and circulatory failure: shock), AKI: acute kidney injury, NSBB: non-selective beta-blockers,
=p<0.05 comparing patients with admission and NI vs those with NI alone
The groups were not different with respect to demographics, including gender. A higher proportion of patients with infection regardless of NI or admission, had ascites and refractory ascites compared to uninfected patients. Patients with both admission and nosocomial infections had a higher probability of prior hospitalization compared to the rest.
The outcomes of patients with NI, regardless of whether they had an admission infection or not, were worse than uninfected patients and those with only admission infections. There was a longer LOS, higher rate of ICU transfer, more individual organ failures and more frequent ACLF in patients with NI compared to those without infections or with admission infections only. This resulted in a lower 30-day survival and higher liver transplant rate in patients with any NI compared to the non-NI groups.
Comparison of NI alone compared to those with both admission and NI (table 2):
Demographics, etiology, diabetes, ascites/refractory ascites and medication use were similar. Patients with both admission and NI had higher rates of hospitalization in prior 6 moths and presented with SIRS, higher WBC count, lower albumin and higher Child score. Remaining laboratory values on admission, including creatinine, sodium and MELD score were similar. Comparing outcomes, patients with both infections had a significantly lower survival than those with a single NI but other outcomes, including liver transplant, LOS, individual organ failures, NACSELD-ACLF, ICU transfer and AKI episodes were statistically similar regardless of whether the NI was associated with an admission infection or not.
Specific infection details:
Between the three infected groups, there was a lower SBP and skin/soft-tissue infection rate in those with only NI compared to the rest, while spontaneous bacteremia rates were similar. Respiratory, urinary, C.difficile and fungal infections were significantly higher in both NI groups compared to the rest. The NI patients had more gram-positive and fungal organisms and vancomycin-resistant Enterococci compared to those without NI. When patients with only NI vs those with admission and NI were compared, there were higher rates of SBP, skin/soft-tissue infections with higher isolation of gram-negative organisms or no growth on cultures. When specific organisms were studied, patients with both infection types had higher fluoroquinolone resistance than those with only NI (Table 3).
Models for nosocomial infection development:
Using the admission variables found that diabetes, admission lactulose use, WBC count, MELD score, and admission with infection were associated with development of NI (Table 4). However, the model was relatively poor with an AUC of 0.70 and sensitivity and specificity in the 60% range even at the Youden index. Similar patterns were seen even when groups with and without admission infection were studied independently (Supplementary tables 2–3).
Table 4:
Model for Predicting Nosocomial Infections using Admission Variables
| Variable | p-value | OR (95% CI) | AUC (95%CI) | Sensitivity/ Specificity |
|---|---|---|---|---|
| Diabetes | 0.02 | 1.33 (1.05, 1.68) |
0.70 (0.67–0.73) |
0.64/ 0.67 |
| Admitted with Infection | <0.0001 | 2.93 (2.33, 3.68) |
||
| Lactulose at Admission | 0.003 | 1.43 (1.13, 1.81) |
||
| WBC | 0.018 | 1.02 (1.00, 1.04) |
||
| MELD | <0.0001 | 1.04 (1.03, 1.06) |
For this analysis, n = 2,597
There were significant differences in the rate of NI between the centers (X2 = 72.91, d.f. = 16, p<0.0001). To accommodate this difference, we fit the model for NI including a random effect for center, which despite having a significant difference in the rate of NI between centers, the overall model is not substantially different when we take the random center effect into account (Table S4).
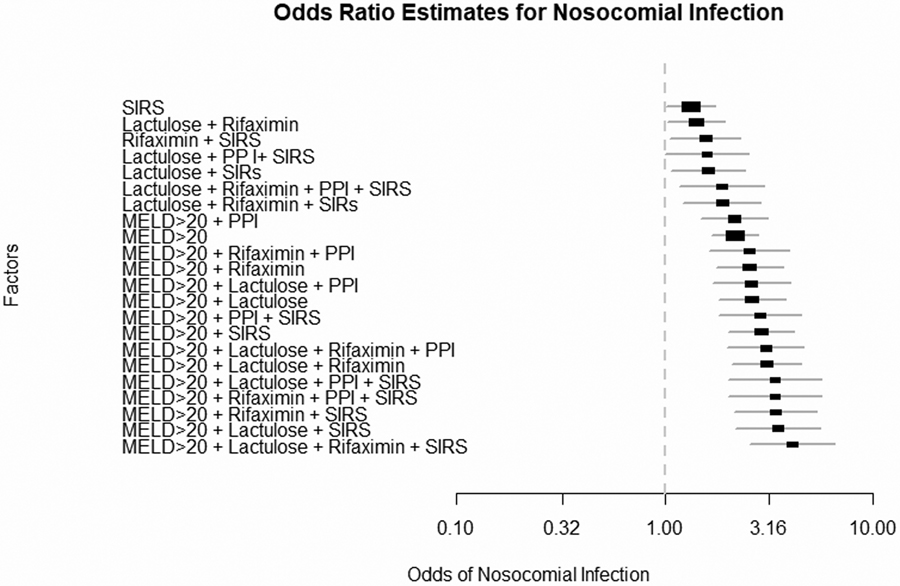
Odds ratios: We found that specific factors related to MELD >20 and SIRS on admission were independently associated with NI development. The addition of PPI, rifaximin and lactulose use increased the odds ratios (Figure 2) for NI development. The greatest OR was for patients with MELD>20 +Lactulose +Rifaximin+PPI+SIRS of 4.08 (95%CI 2.44–6.79, Supplementary table 5). Of note, medications individually without MELD>20 or SIRS did not significantly affect NI development.
Figure 2:
Odds ratio estimates for development of nosocomial infections based on values on hospital admission. PPI: proton pump inhibitor, SIRS: systemic inflammatory response syndrome
Models for 30-day mortality:
As shown in table 5, the first model demonstrated that any nosocomial infection was associated with higher 30-day mortality independent of age, admission MELD, rifaximin and lactulose use, AKI episodes and ACLF development. The AUC was 0.84 with 83% sensitivity and 72% specificity. The second model showed that any nosocomial infection (regardless of whether there was an admission infection or not), was associated with increased 30-day mortality independent of age, alcoholic etiology, admission MELD, PPI, lactulose and rifaximin use, and episodes of AKI, ACLF and ICU admission during the hospitalization. The AUC was again 0.84 with 73% sensitivity and 83% specificity at the Youden Index. Using 2,500 bootstrap samples, for Model 1(NI yes/no) the mean AUC was 0.83 with a 95% Bootstrap CI of (0.80, 0.85) and for Model 2(Ordinal Infection Variable) the mean AUC was 0.83 with a 95% Bootstrap CI of (0.79, 0.85)
Table 5:
Models for Predicting 30-day mortality
| Model incorporating Nosocomial infections as Yes/No | Model incorporating Infections as ordinal variables | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | p-value | OR (95% CI) | AUC (95%CI) | Sensitivity/ Specificity |
Variable | p-value | OR (95% CI) | AUC (95%CI) | Sensitivity/ Specificity |
| Age | <0.0001 | 1.04 (1.03, 1.06) |
0.84 (0.82, 0.89) |
0.83 /0.72 |
Age | <0.0001 | 1.05 (1.03, 1.06) |
0.84 (0.81, 0.87) |
0.73/ 0.83 |
| Rifaximin at Admission | 0.0282 | 0.68 (0.48, 0.96) |
Alcoholic Etiology | 0.0240 | 1.45 (1.05, 2.00) |
||||
| Lactulose at Admission | 0.0445 | 1.42 (1.01, 2.0) |
PPI at Admission | 0.0133 | 0.67 (0.49, 0.92) |
||||
| Episodes of AKI | <0.0001 | 2.17 (1.51, 3.14) |
Rifaximin at Admission | 0.0064 | 0.61 (0.42, 0.87) |
||||
| MELD | <0.0001 | 1.07 (1.05, 1.09) |
Lactulose at Admission | 0.0203 | 1.52 (1.07, 2.17) | ||||
| ICU Admission | 0.0003 | 1.99 (1.38, 2.87) |
Episodes of AKI | <0.0001 | 2.27 (1.56, 3.30) | ||||
| ACLF | <0.0001 | 3.34 (2.23, 5.01) |
MELD | <0.0001 | 1.31 (1.04, 1.08) |
||||
| Nosocomial Infection | 0.0002 | 1.96 (1.37, 2.80) |
ICU Admission | 0.0012 | 1.89 (1.29, 2.77) |
||||
| ACLF | <0.0001 | 3.57 (2.34, 5.44) |
|||||||
| Infection Variable 0 1 2 3 |
0.0047 |
--- 1 vs 0 1.14 (0.75, 1.73) 2 vs 0 2.22 (1.42,3.50) 3 vs 0 1.56 (1.01, 2.56) |
|||||||
Ordinal variable; 0=no infections, 1=admission infection only, 2=no admission infection but nosocomial infection, 3=both admission and nosocomial infections
For Yes/No model n = 2,198. For ordinal infection model, n = 2,149
Discussion:
The results of this large multi-center study underline the high prevalence and prognostic importance of nosocomial infections in hospitalized patients with cirrhosis. These infections were more likely to be procedure related, associated with organ failures, death and were more likely to be gram-positive and fungal in origin than infections present on admission. Importantly, the occurrence of NIs was independently associated with admission MELD score, SIRS and medication use but the multi-variable models for NI development are not robust enough to accurately define who will develop NI. Therefore, all inpatients with cirrhosis are potentially at risk. Therefore, nosocomial infections are common, are hard to predict and negatively affect outcomes. Screening for these infections any time there is a significant clinical change could potentially improve outcome in hospitalized patients with cirrhosis.
Our large cohort found NIs were common and found in 15% of included patients. Moreover, the patients who already have one infection on admission are at high risk for NI development since almost half developed an NI. These infections were associated with poor outcomes in the form of greater AKI, extra-hepatic organ failures, ACLF and poorer survival. This was even more striking when patients with any NI (either alone or with an infection on admission) were compared to patients who only had an admission infection. The findings also demonstrate that ACLF in the North American cohort is not only influenced by fungal and bacterial infections but is found more so with NI than admission infections(14). Nosocomial infections, despite being caused to a greater extent by fungi and C.difficile that are typically associated with inferior transplant free survival because of the increased risk for delisting and higher rate of liver transplant(4). However, the overall transplant rate remained low at <10% of the included cohort(15). This high prevalence of NI and their negative impact on outcomes follows prior smaller observations and is now validated in a large North American multi-center study (5, 7–9).
With these negative outcomes, it would be ideal to define on admission the group(s) of patients likely to develop NI. While patients with admission infections were clearly at risk, the models generated for NI development were not robust. However, MELD >20 with SIRS criteria and use of PPI, lactulose and rifaximin emerged as risk factors. These medications, apart from PPI use, are corollaries of worsening liver disease that is not measurable by the MELD score and importantly were only contributory when linked with the MELD score and SIRS(16). This points towards a combined impact of inflammation, liver disease and potential alteration in gut microbiota leading to NI and ACLF(17–20).
Unlike infections on admission, NIs were more likely to be urinary, respiratory, C.difficile, skin/soft-tissue infections, SBP and procedure-related with greater fungi and infection with VRE. The pattern of differentiation of the site and causative organism also differed between patients with NI versus admission infections. The evolving resistance pattern of bacterial and recently, fungal infections in cirrhosis, has sparked keen debate about the appropriate use of antibiotics and antifungal measures in this population(21, 22). The higher proportion of patients with fungal and culture-negative infections in patients with NI suggests difficulties in culturing the NI organisms in the background of antibiotic therapy for the admission infection. This is challenging because the lack of appropriate therapy can worsen the impact of infections on organ failures and worsen survival. Studies with a priori use of advanced spectrum antibiotics have demonstrated better outcomes compared to traditional antibiotic measures(23). The current observations reinforce the challenges that inpatients with cirrhosis face with changing bacteriology and mycology that require a dedicated inpatient monitoring strategy.
Prevention of these NI could be performed by more aggressive monitoring of inpatients admitted with infections and following guidelines for NI prevention. These could be related to less frequent use of PPIs, urinary catheterization, use of stricter measures to prevent aspiration during HE and upper gastrointestinal bleeding episodes, local site antiseptic measures to prevent central line associated blood stream infections, and strict adherence to guidelines on SBP prophylaxis(6). Many of these are procedure-related and are listed by the Centers for Medicare and Medicaid services as preventable using evidence-based guidelines(24). NI were associated with mortality independent of other relevant clinical variables and it is possible that prevention of NI could also reduce ACLF development and subsequent mortality(6).
Experiencing more than one infection, i.e. admission and NI, culminated in a lower survival in this group despite a similar LOS, AKI rate, ACLF rate and ICU transfer to patients with only a NI. The mechanisms behind this higher rate of death without the antecedent changes in organ failures in patients with two infections compared to those with only NI is unclear but could be related to higher inflammation and potential delays in recognizing and addressing these infections in the background of the already treated admission infection. Therefore, any change in clinical status of patients with cirrhosis should prompt investigation for NI, which could reduce the delay in management of these infections.
The current study is limited by including a convenience sample of patients who provided informed consent. We also performed a retrospective analysis of a prospectively collected dataset but the analysis of NI was an a priori aim of this consortium. Also, patients included were in tertiary-care centers and may not be generalizable. We only performed analyses of NI based on admission variables but not at other time points. There was a significantly longer LOS in patients who developed NI compared to the rest, and NI therefore could be a marker of prolongation of hospitalization. Given the data, it is also difficult to separate whether sicker patients developed more NI or NI made patients sicker over the course of the hospitalization. Mechanical ventilation could make it difficult to diagnose HE in the same patient, which remains a limitation of our data set. The procedures and instrumentation vis-à-vis NI were similar pre and post-NI development. However, despite this, NI emerged as an independent predictor of mortality.
We conclude that nosocomial infections are prevalent, potentially preventable, and independently associated with ACLF and mortality in a large prospective multi-center cohort of inpatients with cirrhosis. Models based on admission variables are not robust to be associated with the development of nosocomial infections. However, patients with infections are the reason for admission, with a MELD score>20, positive SIRS criteria and PPI use on admission may be a higher risk. Therefore, all hospitalized patients with cirrhosis, especially those already admitted with an infection, should be considered at risk for NI development and changes to their underlying status should prompt timely investigation for NIs. Novel and cirrhosis-specific monitoring strategies are needed to prevent these infections that may have a bearing on costs, additional morbidity, and mortality.
Supplementary Material
WHAT IS KNOWN
Infections are one of the leading causes of morbidity and mortality in cirrhosis
Nosocomial infections have been found to affect prognosis in smaller studies outside of North America
A large multi-center prospective approach towards the predictors of development of nosocomial infections and their impact on prognosis is needed
WHAT IS NEW HERE
Using the North American Consortium for the study of end-stage Liver Disease (NACSELD), we found that 15% of the 2864 patients had evidence of nosocomial infections
Nosocomial infections were associated with higher mortality independent of ACLF, ICU, admission infections and were more likely to be respiratory, urinary, C.difficile and fungal infections with higher Vancomycin resistant Enterococci and gram-positive infections.
The occurrence of nosocomial infections in this large dataset was higher in those with admission infections, MELD>20 and SIRS criteria on admission but the multi-variable analysis was not robust, indicating that every hospitalized patient with cirrhosis should be treated as at risk.
Financial support:
Partly supported by investigator-initiated grant from Grifols Pharmaceuticals
Footnotes
Presentations: Portions of this manuscript were an oral presentation at the Liver Meeting 2018 in San Francisco
Guarantor of the article: JSB is the guarantor of this article
Potential competing interests: None in the last year
References:
- 1.Piano S, Brocca A, Mareso S, et al. Infections complicating cirrhosis. Liver Int 2018;38 Suppl 1:126–133. [DOI] [PubMed] [Google Scholar]
- 2.Arvaniti V, D’Amico G, Fede G, et al. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology 2010;139:1246–56, 1256 e1–5. [DOI] [PubMed] [Google Scholar]
- 3.Bajaj JS, O’Leary JG, Wong F, et al. Bacterial infections in end-stage liver disease: current challenges and future directions. Gut 2012;61:1219–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reddy KR, O’Leary JG, Kamath PS, et al. High risk of delisting or death in liver transplant candidates following infections: Results from the North American Consortium for the Study of End-Stage Liver Disease. Liver Transpl 2015;21:881–8. [DOI] [PubMed] [Google Scholar]
- 5.Merli M, Lucidi C, Giannelli V, et al. Cirrhotic patients are at risk for health care-associated bacterial infections. Clin Gastroenterol Hepatol 2010;8:979–85. [DOI] [PubMed] [Google Scholar]
- 6.Bajaj JS, Moreau R, Kamath PS, et al. Acute-on-Chronic Liver Failure: Getting ready for prime-time. Hepatology 2018. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez J, Acevedo J, Wiest R, et al. Bacterial and fungal infections in acute-on-chronic liver failure: prevalence, characteristics and impact on prognosis. Gut 2017. [DOI] [PubMed] [Google Scholar]
- 8.Bajaj JS, O’Leary JG, Reddy KR, et al. Second infections independently increase mortality in hospitalized patients with cirrhosis: the North American consortium for the study of end-stage liver disease (NACSELD) experience. Hepatology 2012;56:2328–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sargenti K, Prytz H, Strand A, et al. Healthcare-associated and nosocomial bacterial infections in cirrhosis: predictors and impact on outcome. Liver Int 2014. [DOI] [PubMed] [Google Scholar]
- 10.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cazzaniga M, Dionigi E, Gobbo G, et al. The systemic inflammatory response syndrome in cirrhotic patients: relationship with their in-hospital outcome. J Hepatol 2009;51:475–82. [DOI] [PubMed] [Google Scholar]
- 12.Angeli P, Rodriguez E, Piano S, et al. Acute kidney injury and acute-on-chronic liver failure classifications in prognosis assessment of patients with acute decompensation of cirrhosis. Gut 2015;64:1616–22. [DOI] [PubMed] [Google Scholar]
- 13.O’Leary JG, Reddy KR, Garcia-Tsao G, et al. NACSELD Acute-on-Chronic Liver Failure (NACSELD-ACLF) Score Predicts 30-Day Survival in Hospitalized Patients with Cirrhosis. Hepatology 2018. [DOI] [PubMed] [Google Scholar]
- 14.Bajaj JS, Reddy KR, Tandon P, et al. Prediction of Fungal Infection Development and Their Impact on Survival using the NACSELD Cohort. Am J Gastroenterol 2017. [DOI] [PubMed] [Google Scholar]
- 15.Artru F, Louvet A, Ruiz I, et al. Liver transplantation in the most severely ill cirrhotic patients: A multicenter study in acute-on-chronic liver failure grade 3. J Hepatol 2017;67:708–715. [DOI] [PubMed] [Google Scholar]
- 16.O’Leary JG, Reddy KR, Wong F, et al. Long-term use of antibiotics and proton pump inhibitors predict development of infections in patients with cirrhosis. Clin Gastroenterol Hepatol 2015;13:753–9 e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bajaj JS, Vargas HE, Reddy KR, et al. Association Between Intestinal Microbiota Collected at Hospital Admission and Outcomes of Patients with Cirrhosis. Clin Gastroenterol Hepatol 2018. [DOI] [PubMed] [Google Scholar]
- 18.Claria J, Stauber RE, Coenraad MJ, et al. Systemic inflammation in decompensated cirrhosis: Characterization and role in acute-on-chronic liver failure. Hepatology 2016;64:1249–64. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y, Guo J, Qian G, et al. Gut dysbiosis in acute-on-chronic liver failure and its predictive value for mortality. J Gastroenterol Hepatol 2015. [DOI] [PubMed] [Google Scholar]
- 20.Bajaj JS, Heuman DM, Hylemon PB, et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol 2014;60:940–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brumble L, Keaveny AP. Editorial: The Risky Business of Fungal Infections in Patients with Cirrhosis. Am J Gastroenterol 2018;113:564–566. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez J, Tandon P, Mensa J, et al. Antibiotic prophylaxis in cirrhosis: Good and bad. Hepatology 2016;63:2019–31. [DOI] [PubMed] [Google Scholar]
- 23.Merli M, Lucidi C, Di Gregorio V, et al. An empirical broad spectrum antibiotic therapy in health-care-associated infections improves survival in patients with cirrhosis: A randomized trial. Hepatology 2016;63:1632–9. [DOI] [PubMed] [Google Scholar]
- 24. http://www.cdc.gov/HAI/pdfs/hai/infections_deaths.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.