Abstract
With the large number of women diagnosed and treated for breast cancer each year, the importance of studying recurrence has become evident due to most deaths from breast cancer resulting from tumor recurrence following therapy. To mitigate this, cellular and molecular pathways used by residual disease prior to recurrence must be studied. An altered metabolism has long been considered a hallmark of cancer, and several recent studies have gone further to report metabolic dysfunction and alterations as key to understanding the underlying behavior of dormant and recurrent cancer cells.
Our group has used two probes: 2-[N-(7-nitrobenz-2-oxa-1, 3-diaxol-4-yl) amino]-2-deoxyglucose (2-NBDG) and tetramethyl rhodamine, ethyl ester (TMRE), to image glucose uptake and mitochondrial membrane potential, respectively, to report changes in metabolism between primary tumors, regression, residual disease, and after re-growth in genetically engineered mouse (GEM) derived mammospheres. Imaging revealed unique metabolic phenotypes across the stages of tumor development. While primary mammospheres, overexpressing Her2, maintained increased glucose uptake (“Warburg effect”), after Her2 downregulation, during regression and residual disease, mammospheres appeared to switch to oxidative phosphorylation. Interestingly, in mammospheres where Her2 overexpression was turned back on to model recurrence, glucose uptake was lowest, indicating a potential change in substrate preference following the re-activation of Her2, re-eliciting growth. Our findings highlight the importance of imaging metabolic adaptions to gain insight into the fundamental behaviors of residual and recurrent disease.
Implications
This study demonstrates these functional fluorescent probes’ ability to report metabolic adaptations during primary tumor growth, regression, residual disease, and re-growth in Her2 breast tumors.
Keywords: Confocal imaging, fluorescence microscopy, metabolism, tumor dormancy, tumor recurrence
Introduction
Over 3 million women in the U.S. who have had breast cancer are at risk for developing recurrence [1]. This risk is higher for women showing overexpression of human epidermal growth factor receptor 2 (Her2) [2]. Women with Her2-positive (Her2+) disease typically receive chemotherapy together with the anti-Her2 targeted therapy, trastuzumab, often in the neoadjuvant setting [3]. Neoadjuvant therapy is used to make large or advanced tumors more operable and can also provide information on a tumor’s response to therapy. At surgical resection the extent of residual disease can be assessed using various metrics [4, 5]. The absence of residual invasive disease in both the breast and axillary lymph nodes constitutes pathologic complete response (pCR) and is positively associated with long-term relapse-free survival following adjuvant therapy [3]. Almost half of Her2+ patients who undergo the standard regime of neoadjuvant chemo- and anti-Her2 (trastuzumab) therapy, however, have residual disease at the time of surgery [6, 7]. This partial response following neoadjuvant therapy leads to increased risk of relapse [4, 8]. Further, approximately 30% of breast cancer patients lacking any clinical or pathological signs of metastasis at the time of diagnosis have disseminated tumor cells present in their bone marrow which go undetected [9]. Thus, residual disease owing to partial response at the primary tumor site may be viewed a surrogate for micro-metastasis elsewhere in the body that may have also evaded neoadjuvant therapy and has the potential to eventually emerge as recurrent disease [10]. The presence of residual disease at the primary tumor location or lack thereof not only informs local response to treatment but also distant disseminated tumor cells’ therapy response. Residual tumor cells can persist in a dormant state for years prior to re-emerging as a recurrent tumor [6, 8, 11]. Given the large number of women at risk for recurrence, it is imperative to understand the fundamental behavior of these dormant residual cells; i.e. to develop and adapt methodologies to visualize the pathways that are required for survival and recurrence of these cells in vivo, in hopes of one day designing and testing therapies to target these pathways in order to mitigate recurrence.
Metabolism may hold clues to dormant cell survival and recurrence since metabolic reprogramming has been observed in tumor cells that survive stressors such as changes in the environment or oncogene expression [12-14]. Our group has previously shown acute Her2 oncogene downregulation results in a rapid decrease in fluorodeoxyglucose ([18 F]FDG) uptake in Positron Emission Tomography (PET) in vivo in mammary tumors as well as in tumors driven by the Akt1, c-MYC, Wnt1, or H-Ras oncogenes in genetically engineered mouse (GEM) models, irrespective of the baseline FDG uptake [15]. In another study, researchers used FDG-PET imaging of Hodgkin’s lymphoma patients with suspected residual disease to determine recurrence risk. Here, elevated FDG intensity due to hypermetabolism was used to improve the diagnostic accuracy of determining whether a residual mass had relapsed; however, for one patient, relapsed residual disease was missed due to its small size [16]. Moving beyond measuring glycolysis alone, other groups have shown transcriptome analysis of isolated residual cancer cell populations following MYC/Erbb2 oncogene inactivation in 3-dimensional culture. Here they demonstrate increased fatty acid synthase (FASN), oxidative phosphorylation, and elevated reactive oxygen species (ROS) compared to both normal and MYC/Erbb2 overexpressing tumor cells, highlighting the importance of mitochondrial metabolism following oncogene downregulation [17]. These shifts were also found in transcriptomic and histological changes between patient-matched diagnostic core-needle biopsies and neoadjuvant-treated surgical samples. Direct treatment of the increased ROS and dysregulated lipid metabolism with C75, an inhibitor of FASN, and etomoxir, a mitochondrial fatty acid oxidation inhibitor, in the experimental model attenuated tumor recurrence [17]. Additionally, others have reported the importance of the mitochondria in the regulation of the cell during periods of energy deprivation resulting in cell dormancy [18]. Finally, recent work has shown potential metabolic vulnerabilities of the mitochondria in Her2 breast cancer tumors during treatment with Her2 targeted therapies [19]. Taken together, these studies point to the significance of both mitochondrial metabolism and glycolysis in the metabolism of dormant cells.
In the studies described above, PET imaging was used to report on glucose uptake in vivo, and transcriptomics was used to examine oxidative phosphorylation of isolated residual cells [15, 17]. Although transcriptomics, proteomics, and metabolomics provide a comprehensive approach to characterizing metabolic phenotypes of residual disease, they are limited to in vitro or ex vivo studies and cannot be used for repeated imaging sessions with multiple time points. While PET imaging complements the “omic” technologies through live metabolic imaging, it is most commonly used to image a single endpoint, glucose uptake, providing a limited understanding of in vivo metabolism. Further, PET imaging, with a spatial resolution of 1-2 mm, does not have the resolution to specifically image the selective, highly localized alterations of a small population of dormant cells [20].
To achieve higher resolution, optical imaging has emerged as a strategy to quantify multiple metabolic pathways [21]. Relying on endogenous fluorescence alone allows for imaging of flavin adenine dinucleotide (FAD) and reduced nicotinamide adenine dinucleotide (NADH) to provide insight in the reduction-oxidation state in the electron transport chain with increased NADH fluorescence suggesting increased glycolysis and increased FAD fluorescence reflecting greater reliance on oxidative phosphorylation; however they do not directly report on glucose uptake nor mitochondrial activity [22]. Previously, our group has developed a novel technique to image glucose uptake and mitochondrial membrane potential (mitochondrial metabolism) using the fluorescent probes 2-NBDG (2-(N-(7-Nitrobenz-2-oxa-1,3-diazol-4-yl)Amino)-2-Deoxyglucose) and TMRE (Tetramethylrhodamine Ethyl Ester), respectively [23]. Our group [24-27] and others [28-30] have extensively validated 2-NBDG imaging in cells [25], window chambers [24, 26], and flank tumor models [27]. TMRE has been extensively used to study oxidative phosphorylation in vitro [31]. Recently, we performed foundational studies to translate this indicator to quantitatively image oxidative phosphorylation in vivo [23, 32]. This platform could be valuable in informing the evolution of disease and can inform which stages of the cancer cascade could be most susceptible to metabolically-targeted therapy.
To study tumor dormancy and recurrence, we have developed a genetically engineered mouse (GEM) model, MMTV-rtTA;TetO-NeuNT (MTB;TAN) [33]. Doxycycline (Dox) administration induces expression of Her2/neu, leading to mammary tumor formation. Withdrawal of Dox leads to Her2 downregulation and tumor regression, mimicking sustained use of targeted therapies as would be the case in clinical practice [34]. Residual cells surviving Her2 downregulation eventually re-initiate proliferation to form a recurrent tumor after 4 to 6 months, a similar timeframe to human breast cancer when correcting for mouse lifespan. Using this model, we and others have previously identified several genes that promote the survival and recurrence of residual cells, including par-4 [35], Snail [36], Ssb-1 [34], and Notch1 [37], which are all associated with recurrence in human breast cancer.
In this study, we demonstrate a strategy to dynamically image metabolic adaptions occurring during a tumor’s transition from a primary tumor to residual disease using our Her2 model of tumor dormancy and previously validated metabolic probes. As a first step, we sought to examine these metabolic reporters with the GEM model in vitro. We generated primary mammosphere cultures from independent tumors harvested from MTB;TAN mice. Mammospheres were imaged first in the presence of Dox (Her2 on) to mimic a proliferating, primary tumor, and then 2 and 4 days following Dox withdrawal (Her2 off), to emulate tumor regression. Surviving residual tumor cells (14 and 28 days after Dox withdrawal) were also imaged. Finally, we resupplied Dox to residual cells and imaged the changes in metabolism during the transition from residual disease to recurrence. These time points were confirmed to model different stages of tumor development by immunofluorescent staining of Ki67 and Cleaved Caspase 3 (CC3). With mammospheres on Dox overexpressing Her2 (primary and re-activated), Ki67 expression was high, confirming proliferation. On the other hand, short-term Dox withdrawal resulted in increased CC3 expression, confirming apoptosis due to Her2 downregulation. Consistent with these findings, we show that mammospheres cultured continuously with Dox demonstrated the highest glycolytic rates compared to all other treatment groups while short term Dox removal (regression) yielded a dramatic increase in mitochondrial membrane potential. Increased mitochondrial membrane potential and decreased glucose uptake, were maintained during dormancy. Interestingly, after the re-addition of Dox to residual cells, mammospheres showed decreased glucose uptake but increased mitochondrial activity compared to the baseline proliferative state, indicating two metabolically distinct phenotypes. Interestingly, these two groups had similar ATP levels and growth rates. This study reports the functional flexibility of these probes to report on different and distinct stages of tumor evolution and identifies key metabolic changes that occur over the course of tumor progression, dormancy, and recurrence with the ultimate goal of informing the potential impact of metabolically-targeted therapies on this cancer cascade. Using these probes, we aim to create a non-destructive assay that can ultimately be translated to in vivo imaging using a mammary window chamber to image either GEM derived or patient-derived xenograft (PDX) models.
Methods
GEM model derived mammospheres
Genetically engineered breast tumors were harvested from MMTV-rtTA;TetO-NeuNT (MTB;TAN) mice, minced, and digested at 37°C in Earle’s Balanced Salt Solution (EBSS) without phenol red media (Gibco 14155-063) supplemented with 300U/mL collagenase and 100U/mL hyaluronidase (StemCell 7912), 2% FBS (MediaTech 35-010-CV), 100μg/mL gentamycin (Sigma G1264), 1% PenStrep (Gibco 15140-122) and 2 μg/mL doxycycline (RPI D43020). Dissociated tumor cells were cultured on Poly(2-hydroxyethyl methacrylate) (Sigma P3932) coated dishes using RPMI media (Sigma R8758) supplemented with B27 (Invitrogen 17504-044), murine EGF (murine E4127), bFGF (Invitrogen 13256-029), PenStrep (Gibco 15140-122) and L-Glutamine (Gibco 25030-081) [15, 34, 35]. 2 μg/mL doxycycline (Dox) (RPI D43020) was added to the media to maintain expression of Her2, a well-known oncogenic driver often amplified in aggressive breast cancers [38]. To passage mammospheres, cells were collected, spun down, and trypsinized for 5 minutes until most cells were present as single cells. Trypsin was quenched using whole media, and cells were split 1:3. Mammospheres were passaged every 3 days. For experiments, mammospheres were trypsinized as above and allowed to grow for 2 days prior to experimental manipulations. To downregulate Her2 expression, mammospheres were transferred to media without Dox. In order to focus on the metabolic effects of modulating Her2 protein expression specifically, cells were cultured without EGF and bFGF for at least a week prior to experiments unless otherwise stated.
Metabolic imaging
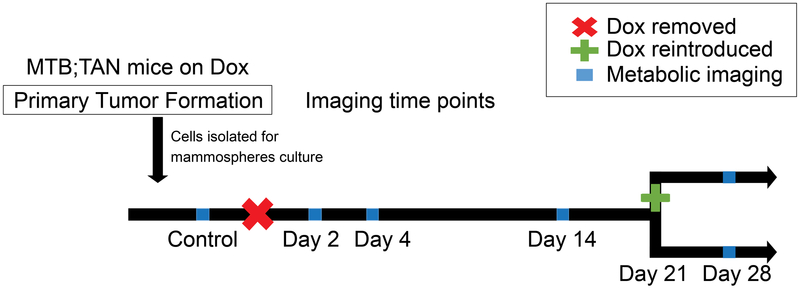
Figure 1 details the different time points for metabolic imaging of glucose uptake and mitochondrial membrane potential. Cells were cultured with Dox to maintain Her2 tumor expression. Cells were grown without Dox in the media for each of the different time points (2, 4, 14, and 28 days). A separate culture was grown without Dox for 21 days and then returned to Dox 7 days before imaging. A total of 10 representative mammospheres from each time point were imaged, and all time points were measured on at least two different days, each as an independent experiment.
Figure 1: Time course for metabolic imaging in 3D culture.
Breast cancer cells were isolated and cultured with Dox to maintain Her2 overexpression. To mimic therapeutic intervention, Dox was withdrawn for 2, 4, 14, or 28 days (indicated by red x) to model tumor regression and dormancy. Imaging was performed before Dox withdrawal and at each time point using both 2-NBDG and TMRE as indicated by blue squares along the time course. After 21 days of Dox withdrawal, a separate culture had Dox added back to their growth media for 7 days (green plus sign) to model recurrence.
The fluorescent indicators, 2-NBDG and TMRE were chosen for our imaging due to their translatability to in vivo experiments our group have previously published [23, 26, 39, 40]. 2-NBDG (2-(N-(7- Nitrobenz-2-oxa-1,3-diazol-4-yl)Amino)-2-Deoxyglucose, Duke University Small Molecule Facility) is a fluorescent glucose analog that enters the cell via glucose transporters (GLUT) as a function of glucose uptake and causes an increase in fluorescence intensity [25, 41]. TMRE (Tetramethylrhodamine Ethyl Ester, Life Technologies/ThermoFisher) is a cationic dye that accumulates in the mitochondrial inner membrane as a function of mitochondrial membrane potential and causes an increase in fluorescence intensity [39, 42]. During incubation, 2-NBDG and TMRE were diluted to final concentrations of 200 μM and of 50 nM, respectively, in media. Concentrations were chosen based on standard manufacturer protocols as to not affect the metabolism of cells and minimize each probe’s toxicity [25].
Before imaging, the mammospheres were removed from media and washed with PBS. Cells were incubated at 37°C, 5% CO2 for 30 minutes with either 200 μM 2-NBDG or 50 nM TMRE dissolved in glucose-free cell media. To prevent metabolite competition and minimize optical and biological crosstalk between metabolic probes, mammospheres were only stained with one of the two probes and different mammospheres were imaged at each time point. Mammospheres were then washed with PBS, returned to their normal media, and imaged immediately.
All images were collected on a Zeiss 780 Upright confocal microscope at the Duke University Light Microscopy Core Facility using a 20x/1.0 Water Zeiss W Plan-Apochromat 421452-9800 WD 1.8 mm objective and 2.0 digital zoom providing sub-cellular resolution (~0.4 μm). For 2-NBDG imaging, mammospheres were excited using a 488 nm argon laser source (Lasos) with emission collected from 494-560 nm. For TMRE imaging, mammospheres were excited using a 561 nm laser diode (Lasos) with emission collected from 565-691 nm. Fluorescence was detected using a GaAsP array and cooled photomultiplier tube. Image z stacks were taken starting from 1.5 μm above the mammosphere, through the entire sphere, and ending 1.5 μm below the mammosphere using a 1.5 μm step size for each image to adequately sample the entire sphere (Supplemental Figure 1). The thickness of each image slice was 3.0 μm. The scan area at each plane was 212.5 μm × 212.5 μm (512 × 512 pixels) and took approximately 9 seconds to image per slice. A 512 × 512 × n data set, where n represents the number of slices needed to capture the entire mammosphere, was used for image processing. A fluorescence calibration standard slide (DeltaVision, Ex/Em: 488 nm/519 nm) was imaged at each session to account for day-to-day system variation using both imaging settings. Background fluorescence of media-only plates and unstained cell plates were obtained using each imaging setting to ensure background noise from the media, plate, or autofluorescence from endogenous fluorescence was negligible (< 4%) over the linear range of the detector (data not shown).
Image Processing
Overall uptake of 2-NBDG or TMRE was analyzed using MATLAB (MathWorks, USA). A 3 × 3 × 3 median filter cube was applied to the entire cube to suppress the effects of shot noise. Due to the anisotropic resolution of confocal microscopy, the voxel size is anisotropic. In order to improve segmentation and image projections, a spline interpolation was performed in the z-direction to allow for a cubic voxel size of 0.42 um3 [17]. From here, using MATLAB’s Image Processing Toolbox, mammospheres were automatically segmented using the Fast Marching Method and intensities were corrected by a fluorescence calibration standard slide [43]. A threshold of 1% of the maximum pixel value of the image was selected for the overall z-stack. All pixel values less than this threshold value were set to zero. Probability density functions (pdfs) were created from all non-zero pixels from each image sack for each group. All representative mammospheres shown throughout the manuscript are mean projections.
Immunofluorescence Staining
Immunofluorescent staining of Ki67 and cleaved caspase 3 (CC3) was performed to phenotype our mammospheres based on proliferation (Ki67 staining) and apoptosis (CC3 staining) levels. Mammospheres were centrifuged (5 min, 1500G) onto poly-D-lysine coated coverslips (Neuvitro H-12-1.5-PDL). Cells were fixed with 4% paraformaldehyde (ThermoFischer 28908) in PBS for 10 minutes, permeabilized with 0.05% Triton-X-100 (Sigma X-100) for 20 minutes, and then non-specific binding was blocked with 10% normal goat serum (Invitrogen PCN5000) for one hour. To detect Ki67, cells were incubated with an anti-Kit67 antibody conjugated to Alexa Fluor 488 (1:100 dilution; BD Pharmingen #561165). To detect cleaved caspase-3, cells were incubated with rabbit anti-Cleaved caspase 3 antibody (1:300; Cell Signaling Technologies 9661), followed by a goat anti-rabbit secondary antibody conjugated to Alexa Fluor 568 (1:200; ThermoFischer #A-11011). Following incubation with antibodies, cells were mounted with ProLong® Gold Antifade Reagent with DAPI (Cell Signaling Technology 8961).
CellTiter-Glo Assay
To compare differences in 2-NBDG and TMRE uptake to changes in energy production, CellTiter-Glo Luminescent Cell Viability Assay (Promega) was used to quantify ATP levels following manufacturer instruction. Specifically, 200,000 cells per well were plated and grown for 2 days. Cells were harvested; half of all cells were used for ATP quantification, and half were used for protein content quantification. Luminescence was read on a luminometer and normalized to protein content for three biological replicates (n=3). Data is shown as mean ± SEM.
RT-qPCR
RT-qPCR was completed to measure Her2 expression levels. Total RNA was initially extracted from cell pellets and reverse transcribed to cDNA. Threshold cycles (CTs) of Erbb2 (Thermo, 4331182, Rn00566561_m1) were collected and normalized to β-actin (Thermo, 4331182, Mm02619580_g1) to calculate the ΔCT values. ΔΔCT were calculated to determine the changes in Erbb2 levels between time points. 2−ΔΔCT was used to calculate fold changes in the target gene expression from three biological replicates, with each sample plated with 3 technical replicates (n=3). To determine if change in expression was biologically relevant, cells were plated at uniform density and counted after 1 and 4 days to identify proliferation rates. Data is shown as mean ± SEM from three independent experiments. (n=3).
Statistical analysis
Probability density functions (pdfs) were chosen to visualize the changes in fluorescence intensity distribution across different metabolic phenotypes. Empirical p-values were calculated for a Kolmogorov-Smirnov statistic using blocked permutation (n = 1000 random permutations per tests) to compare distributions, prior to binning data for graphing [44]. To determine statistical significant changes in average probe uptake, Erbb2 expression, ATP production, and growth rates, mean values between time points were compared with Student’s t-tests. Exact two-sided P-values <0.05 were considered significant and are indicated as *. MATLAB (MathWorks, USA) Statistics Toolbox was used for all tests.
Results
Media formulation and passage protocol validation produce reproducible metabolic endpoints.
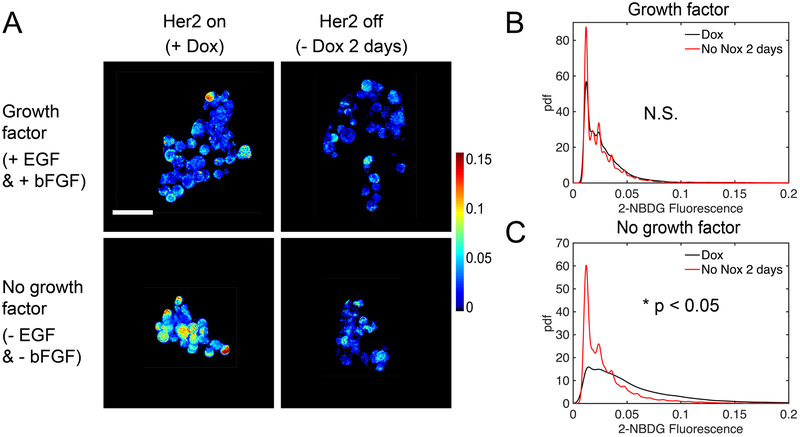
Before studying metabolism during regression, dormancy, and recurrence, we validated media formulation and cell culture methodology to ensure reproducible and robust results. Mammospheres are normally maintained in culture media supplemented with basic fibroblast growth factor (b-FGF) and epidermal growth factor (EGF) [45, 46]. Vander Heiden et al. have shown that growth factors can affect glycolysis [47], so we first evaluated the effect of these growth factors on 2-NBDG uptake between mammospheres cultured in Dox (primary mammospheres - control) and those removed from Dox for 2 days (regressing phenotype - test). Dox withdrawal leads to rapid and total loss of Her2 protein (Supplemental Figure 2). Given that growth factors promote tumor growth [48], we predicted that their presence would mask differences in tumor metabolism between the test and control group, specifically, glucose uptake between proliferative and regressing phenotypes. Imaging was performed on mammospheres continuously cultured with EFG and bFGF and on mammospheres cultured without EGF and bFGF for at least a week prior to imaging. Figure 2A shows representative images of mammospheres incubated with 200 μM 2-NBDG as previously described. Qualitatively, only the mammospheres cultured without the added growth factors show a decrease in 2-NBDG intensity following Dox removal. To analytically assess 2-NBDG uptake, probability density functions (pdfs) of 2-NBDG pixel intensities were created from images of mammospheres cultured with (Figure 2B) and without growth factor in the media (Figure 2C). There was no significant difference in 2-NBDG uptake between Dox and no Dox mammospheres grown in media with added growth factors. There was, however, a significant difference in 2-NBDG intensity between mammospheres on Dox and mammospheres following Dox removal when both were cultured without added growth factors (p<0.05). The no growth factor condition was concordant with our previous in vivo study showing that Her2 downregulation caused decreased glucose uptake [15]. Since the presence of growth factors masked metabolic changes caused by Dox withdrawal, all subsequent imaging studies were performed under conditions free of supplemental growth factors.
Figure 2: Growth factors mask changes in glucose uptake following acute Dox withdrawal.
2-NBDG uptake was measured in cultures with Dox (control group) and cultures with Dox removed 2 days prior to imaging (test group). Fluorescence intensities were calibrated using a standard fluorescent slide and corrected for gain adjustments. (A) Representative mean projection images of 2-NBDG intensity. (B) Probability density functions of 2-NBDG pixel intensities from the 3D mammospheres show no significant difference between Dox and no Dox for 2-day cultures with growth factors in the media. (C) Probability density functions of 2-NBDG pixel intensities from the 3D mammospheres show significant differences between Dox and no Dox for 2 day cultures without the presence of growth factors in the media. For all groups n=5 mammospheres. (* is p<0.05, N.S. is not significant).
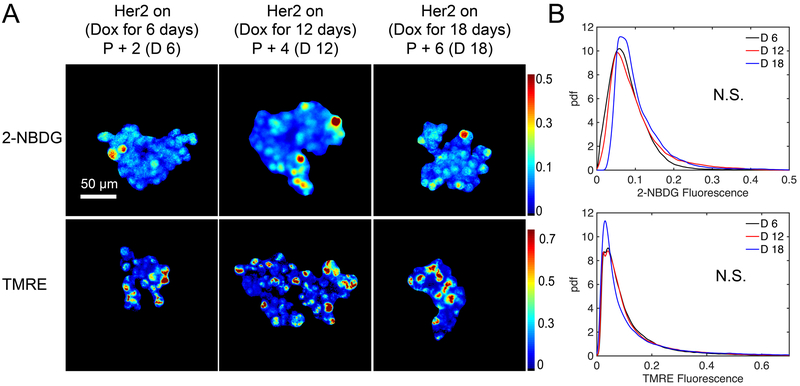
We next investigated whether continued passaging affects mammosphere metabolism. To explore how mammosphere phenotype is affected by the duration of time in culture, we varied the length of time mammospheres were serially passaged (every 3 days) on Dox prior to Dox withdrawal. Mammospheres were then incubated with either 200 μM 2-NBDG or 50 nM TMRE as previously described. Representative 2-NBDG and TMRE images in Figure 3A show mammospheres serially passaged with Dox for variable lengths of time (passaged two times, four times, and six times) before Dox removal 2 days prior to imaging and show no qualitative differences in uptake. Pdfs of 2-NBDG and TMRE in Figure 3B show no significant differences between passage times, indicating length of passaging does not affect mammosphere metabolism. All subsequent imaging studies were performed with mammospheres cultured with Dox for 18 days or less.
Figure 3: Length of time in culture does not affect 2 day Dox withdrawal phenotype.
Cells were cultured with Dox for 6, 12, or 18 days and passaged (P) every third day (P+2 / D6, P+4 / D12, or P+6 / D18). Dox was then removed from the media and metabolic imaging was performed two days later on days 8, 14, or 20, respectively. Fluorescence intensities were calibrated using a standard fluorescent slide and corrected for gain adjustments. (A) Representative mean projection images of 2-NBDG and TMRE intensities 2 days following Dox withdrawal. (B) Probability density functions of 2-NBDG and TMRE pixel intensities from the 3D mammospheres show no significant differences across varying lengths of time cells remained on Dox prior to removal. For all groups n=5 mammospheres. (* is p<0.05, N.S. is not significant).
2-NBDG and TMRE report on metabolic adaptations during transitions between primary tumors, regressing tumors, residual disease, and re-initiated tumor cells.
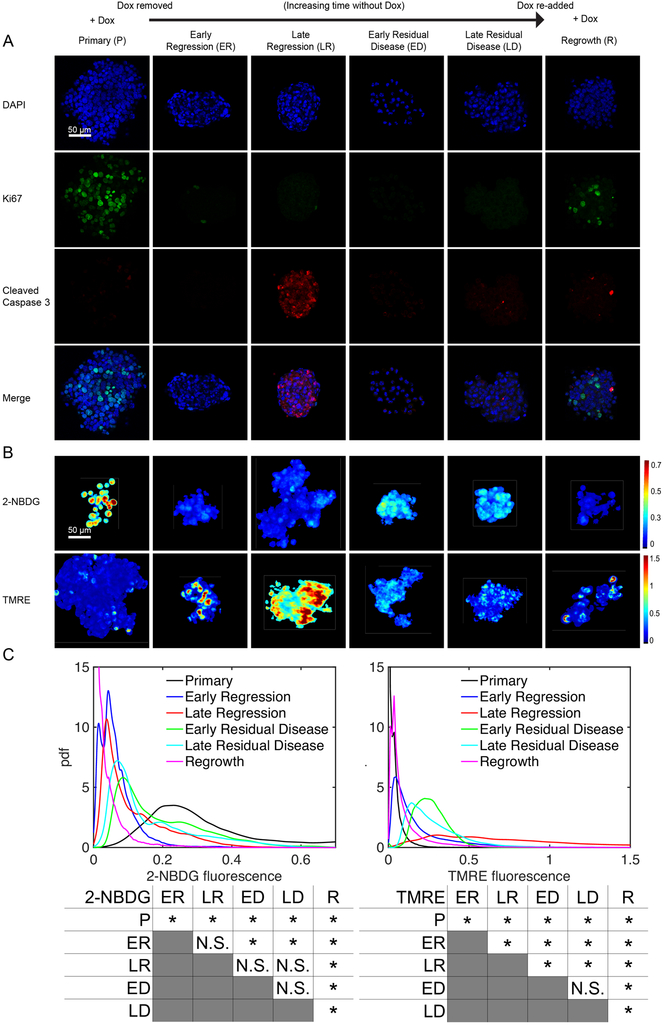
Having an optimized methodology to ensure biologically relevant, reproducible results, we next sought to assess how the metabolic properties of mammospheres change following Dox withdrawal (Her2 downregulation) and subsequent Dox re-addition (Her2 re-activation). We performed immunofluorescence staining for Ki67 and cleaved caspase-3 (CC3) to measure proliferation and apoptosis, respectively, as shown in Figure 4A, to ensure the selected time points for our probes reported biologically relevant phenomena (primary tumor, regression, residual disease, and re-growth). Primary mammospheres (“P”) grown in the presence of Dox (Her2 on) exhibited a proliferative phenotype with high Ki67 (p<0.05) and low CC3 staining. (Supplemental Figure 3 reports the quantification of Ki67 and CC3 results). Her2 downregulation led to decreased proliferation and increased apoptosis at 2 and 4 days following Dox withdrawal (Early Regression, “ER” and Late Regression, “LR”). Following these acute changes of regression, a population of residual mammosphere cells can survive Her2 downregulation [37]. These residual mammospheres (“ED” and “LD”), assayed at 14 or 28 days following Dox withdrawal, exhibited low proliferation and low apoptosis, suggestive of a dormant state. Re-addition of Dox led to the re-initiation of proliferation (p<0.05) and regrowth of the mammospheres (“R”).
Figure 4: Tumor metabolism reprograms during the different stages of a tumor’s life cycle.
Mammospheres stained for either Ki67 and cleaved caspase 3 (CC3), 2-NBDG, or TMRE were imaged from cultures with Dox in the media (Primary, P), without Dox in the media for 2 days prior to imaging (Early Regression, ER), without Dox in the media for 4 days prior to imaging (Late Regression, LR), without Dox in the media for 14 days prior to imaging (Early Residual Disease, ED), and without Dox in the media for 28 days prior to imaging (Late Residual Disease, LD). Cells were also cultured without Dox for 21 days followed by Dox added back in for 7 days (Regrowth, R). (A) Representative image planes of DAPI, Ki67, cleaved caspase 3 (CC3) staining. (B) Representative mean projection images of 2-NBDG and TMRE intensities. (C) Probability density functions of 2-NBDG and TMRE intensities from the 3D mammospheres show significantly decreased 2-NBDG and significantly increased TMRE uptake after Dox withdrawal. After re-addition of Dox to dormant tumors, 2-NBDG uptake remains significantly decreased. Significant comparisons between time points for both 2-NBDG and TMRE are included. For all groups n=10 mammospheres. (* is p<0.05, N.S. is not significant).
We next performed metabolic imaging at each time point as described previously. Figure 4B shows representative 2D projection images of 2-NBDG and TMRE intensities for mammospheres at baseline as well as 2, 4, 14 and 28 days following Dox removal and mammospheres cultured without Dox for 21 days and subsequently with Dox for 7 days (for comparison with the 28 day No Dox mammospheres). Qualitatively, primary mammospheres (“P”, on Dox) show high 2-NBDG uptake and low TMRE uptake, characteristic of a primarily glycolytic phenotype; the proliferative nature of primary mammospheres is supported by Ki67 expression. On the other hand, mammospheres show a spike in mitochondrial membrane potential during late regression (“LR”, 4 days off Dox) characterized by high TMRE uptake. During the LR stage, there also is increased CC3 expression. Taken together, this indicates a state of possible oxidative stress and metabolic alternations [49]. Residual disease mammospheres (“ED” and “LD”, 14 and 28 days off Dox) report moderate, stable signal levels of both probes with little Ki67 or CC3 expression. Following the re-addition of Dox, Ki67 expression increases, indicating a stage of re-growth (“R”, 21 days off Dox + 7 days on Dox), but decreased 2-NBDG and slightly elevated TMRE levels relative to the baseline proliferative state.
Pdfs of 2-NBDG and TMRE pixel intensities followed by a summary of all statistical comparisons for each probe across disease phases further indicate distinct changes throughout the tumor life cycle as shown in Figure 4C. Pdfs were chosen to display and quantify the spatial heterogeneity that exists in each mammosphere; however, averages of each mammospheres’ fluorescence are reported in Supplemental Figure 4. In primary mammospheres (“P”, on Dox), 2-NBDG intensity maximizes (p<0.05) relative to all time points while TMRE intensity is significantly decreased relative to mammospheres in all regressing (“ER” and “LR”, 2 and 4 days off Dox) and residual disease (“ED” and “LD”, 14 and 28 days off Dox) time points as determined by KS testing. During regression (“ER” and “LR”, 2 and 4 days off Dox), 2-NBDG intensity is significantly lower than primary mammospheres (on Dox) (p<0.05) while TMRE intensity increases and reaches a maximum (p<0.05) in the late regression phase (“LR”, 4 days off Dox). These cells with increased TMRE uptake do not correspond to cells undergoing apoptosis (Supplemental Figure 5). During the residual disease stages (“ED” and “LD”, 14 and 28 days off Dox), 2-NBDG is increased relative to the regression phase, though not significantly, but remains decreased relative to that of the primary mammospheres (p<0.05). On the other hand, TMRE is increased relative to baseline (p<0.05) and the early regression phase (p<0.05) and decreased relative to the late regression phase (p<0.05). At 7 days after the re-addition of Dox (“R”), mammospheres show both a significant decrease in 2-NBDG relative to all phases (p<0.05) and TMRE uptake relative to regression and residual disease phases (p<0.05), though higher TMRE uptake relative to primary mammospheres (p<0.05).
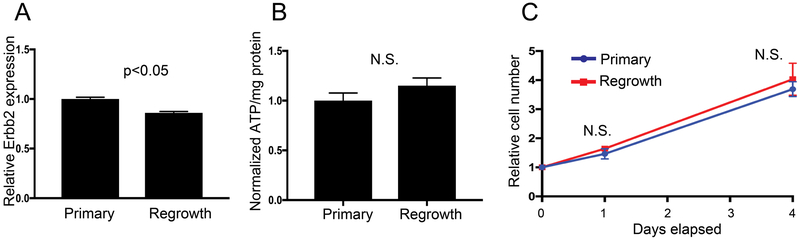
Additionally, primary and re-growing mammospheres had similar Erbb2 (i.e. Her2) gene expression, similar ATP content, and similar growth rates (Figure 5A-C). Taken together, these findings suggest that tumor cell regrowth is associated with altered tumor cell metabolism but not with differences in the ability to proliferate in response to Her2 expression following long-term Dox withdrawal.
Figure 5: ATP production, Her2 expression, and proliferation rate comparing mammospheres continuously cultured with Dox and No Dox 21 days + Dox 7 days (primary vs regrowth).
Mammospheres were either continuously cultured with Dox (Primary) or removed from Dox for 21 days followed by the re-addition of Dox for 7 days prior to assays (Regrowth). (A) RT-pPCR was used to measure Erbb2 (Her2) expression. Both Primary and Regrowth cultures show elevated Errb2 expression. For both groups: n=3 biological replicates with 3 technical replicates. (B) CellTiter-Glo was used to measure ATP production normalized to protein content and show no significant difference between Primary and Regrowth time points. For both groups n=3 biological replicates. (C) Growth rate curves show no difference in proliferation between the two time points. For all groups n=3 biological replicates. Bars and markers are shown as the mean +/− SEM (* is p<0.05, N.S. is not significant).
Discussion
Despite the increased interest in the regulation and function of metabolism in cancer, there still exists a need for a translational technique with the ability to link in vitro and in vivo results in biomedical research. Currently, gold standard techniques fail to directly connect in vitro cell culture results to preclinical in vivo models without changing endpoints. For example, the Seahorse Bioscience extracellular flux analyzer, the gold standard for metabolic studies in vitro, uses special perturbations to measure oxygen consumption rate (OCR) to indicate oxidative phosphorylation and extracellular acidification rate (ECAR) to indicate glycolysis [50]; however, this technique lacks the spatial information provided by imaging techniques such as the methodology described here. To move in vivo, typically FDG-PET or hyperpolarized MRI is used. FDG-PET is widely used to image glucose uptake leaving out methods of measuring mitochondrial metabolism completely, and hyperpolarized MRI is used to image hyperpolarized carbon compounds to report on glycolysis or mitochondrial metabolism endpoints. This piecemeal approach does not allow for direct comparison between models across both glycolytic and mitochondrial pathways. Metabolic imaging using 2-NBDG and TMRE is advantageous to fill this void due to its ability to directly measure key pathways in metabolism and translate endpoints in vitro, as in this study, to in vivo models. Additionally, the use of 3D confocal microscopy with a thin (3 μm) sectioning depth followed by a 3D segmentation algorithm in this study allowed for the uptake of either probe throughout the full mammosphere to be captured and compared across time points.
A GEM mouse model was chosen due to its relevance to the current metabolic research community and its consistent, well-defined timeline for studying different stages of tumor progression, treatment, dormancy, and recurrence [15, 34, 36, 37]. In order to asses our method’s ability to metabolically differentiate these life stages, we performed immunofluorescent staining of Ki67 and CC3 in parallel. These metrics highlighted the changes in phenotype across the time course with and without Dox: highly proliferative periods due to Her2 overexpression in primary and re-growing mammospheres, high levels of apoptosis following acute Her2 withdrawal mimicking a treatment response, and viable mammospheres lacking proliferative or apoptotic behavior following long-term Her2 downregulation modeling stable residual disease. Importantly, glucose uptake and mitochondrial membrane potential collectively allowed for differentiation between these time points highlighted by the differing immunofluorescent staining phenotypes. During initial Dox withdrawal, 2-NBDG uptake decreased (p<0.05), which is consistent with a previous study in this model that reports a decrease in FDG-PET following acute Dox withdrawal [15]. Also, TMRE uptake is significantly increased during early regression (p<0.05) and again further during late regression (p<0.05). Additionally, during late regression, CC3 expression is increased (p<0.05). In support of these observations, following acute Dox withdrawal in a similar model, populations of cells reported increased CC3 expression and an upregulation of the oxidative phosphorylation pathway [17]. Another possibility, however, could be that this initial hyperpolarization seen in the early regression time point could be the beginnings of the apoptotic pathway. Several studies have shown that hyperpolarization of the mitochondrial inner membrane can precede a depolarization step that catalyzes caspase 3 activity [51-53].
In the late regression phase (4 days off Dox), mammospheres showed their highest levels of both TMRE uptake (p<0.05) and CC3 expression. At this point, many cells are likely under stress from acute Her2 downregulation resulting in high CC3 expression [49, 54, 55]. Further analysis of the late regression population highlights the existence of two populations of cells: a high TMRE non-apoptotic cell population and a low TMRE population of apoptotic cells (Supplemental Figure 5). Further, it has previously been established that apoptosis and caspase 3 activity are both associated with a loss of mitochondrial membrane potential, or depolarization prior to cell death [56-58]. Because of this, the increase in mitochondrial membrane potential measured by TMRE uptake in these mammospheres may be linked to a population of cells transitioning to a larger reliance on mitochondrial metabolism over glycolysis in order to survive Her2 downregulation. In fact, previous work has indicated that hyperpolarization of the mitochondrial membrane is a potential method to avoid apoptosis and that cancer cells with a high mitochondrial membrane potential report a heightened tolerance to treatment [32, 59]. Particular cells within the mammospheres could be rewiring their metabolism following Dox withdrawal as a tactic to survive the harsh conditions of oncogene downregulation. This switch is consistent with a pancreatic cancer study implicating a reliance on mitochondrial metabolism to survive periods of regression [60]. 2-NBDG and TMRE together not only differentiated baseline from regression but also illustrated a dynamic metabolic process of cells undergoing metabolic adaptations and potentially showing a deterrent mechanism to escape cell death. The population of cells that remained viable 14 days following Dox withdrawal resisted apoptosis and were deemed residual diseased cells.
These surviving mammospheres cultured without Dox for 14 and 28 days showed no Ki67 or CC3 expression further indicating their label as residual disease. Having already survived Her2 downregulation, these cells did not show as extreme TMRE uptake levels as the late regression mammospheres reported (p<0.05), but their levels were higher than primary tumors indicating a switch to oxidative phosphorylation instead of only glycolysis (p<0.05). They also maintained a moderate level of 2-NBDG uptake, indicating the use of glucose as a fuel source for the citric acid cycle. This trend held for both time points and highlights the changes in preferred metabolic pathways required for long-term survival in Her2 downregulated environments. This is consistent with previous work, which indicated a switch to oxidative phosphorylation from glycolysis during dormancy [60].
We hypothesized that upon re-addition of Dox to dormant mammospheres to induce re-growth, the metabolic phenotype of these mammospheres would be indistinguishable from baseline, primary mammospheres. Therefore, the finding that re-growing mammospheres exhibit reduced 2-NBDG uptake compared to all time points while also reporting a lower TMRE uptake compared to all time points off Dox was surprising. To rule out trivial differences between primary and re-growing mammospheres, ATP content, HER2 expression, and growth rate were measured. Primary and re-growing mammospheres reported similar ATP output and HER2 expression. Further, both time points showed no difference in Ki67 expression levels and the same growth rate, indicating a return to a highly proliferative phenotype. The cells that survived long-term Her2 downregulation were still responsive to Dox and behaved similarly to baseline mammospheres consistently cultured with Dox, except with respect to their glucose uptake and mitochondrial membrane potential.
Therefore, it is likely that these re-growing mammospheres have a distinct metabolic profile from primary mammospheres. Re-growing mammospheres exhibited the lowest glucose uptake compared to all other time points (p<0.05). Additionally, these mammospheres showed a decrease in mitochondrial membrane potential compared to residual disease (p<0.05) and increase compared to primary mammospheres (p<0.05). While the mechanistic basis for this remains unknown, it suggests that the process of regression, dormancy, and re-growth may have profound effects on tumor metabolism. These effects could be due to adaptive changes, or result from the selection for cells with altered metabolic pathways. [20]. Previous studies have reported that oncogenic transformations can cause permanent changes to expression or activity levels of enzymes or transporters, which could have occurred with the re-addition of Dox [14]. One possibility for the decrease in glucose uptake is that after the re-addition of Dox, cells switch the substrate used for energy. These substrates would provide carbon for oxidative phosphorylation, similar to how glucose did in the residual disease population. The TCA cycle can rely on a host of other sources of carbon besides glucose alone [14]. A previous study showed an increase in fatty acid degradation or glutamine uptake following therapy [17, 61]. Further study is required to determine the specific change in substrate between the baseline and recurrent mammospheres. It is important to note that our strategy can only report on the downstream effect of such substrates, increased or decreased mitochondrial membrane potential. In order to robustly report on this change in substrate, metabolomics, hyperpolarized magnetic resonance imaging, or more fluorescent substrate analogues, like Bodipy FL C16 to measure long-chain fatty acid uptake, should be employed to pinpoint this metabolic shift [20].
This, however, leaves the question of the decreased mitochondrial membrane potential levels in the re-growing cells compared to other time points reliant on oxidative phosphorylation instead of glycolysis alone. It is possible that the mammospheres retained metabolic memory of the pathways required to survive long term Her2 downregulation and rewired their metabolism to more efficiently use resources thus requiring less mitochondrial membrane potential compared to other time points also energetically reliant on oxidative phosphorylation (residual disease population). This along with the use of an alternative substrate could serve as rationale for the equal ATP output between primary and re-growth mammospheres.
Upon further examination of the TMRE fluorescence distribution, there exist two peaks, signifying two populations of cells within the time point with differing mitochondrial membrane potential. Previous work has shown increased heterogeneity in recurrent or relapsed tumors due to treatment-induced mutations or clonal evolution [62, 63]. One possibility is that the cells with depressed mitochondrial membrane potential could be in the initial stages of apoptosis. As these mammospheres begin to recur, there is likely another selection process for the strongest cells to adapt in order to proliferate and survive, similar to the transition seen from regression to residual disease. It is likely that some residual cells cannot make the metabolic changes required for survival after the re-expression of Her2 [56]. Following the death of these cells, the overall mitochondrial membrane potential of the population would increase. This unstable population may be a key metabolic window to target for future treatment studies due to their susceptibility to death [59]. Further work should also be completed to examine the differences in the primary mammospheres compared to these two metabolic phenotypes within the regrowth cultures to identify key pathways involved with acquired drug resistance to Her2 therapies. Not only could these high membrane potential cells found in the regrowth population serve as potential therapy targets for metabolic inhibitors as key substrates are identified, but also this work indicates that mitochondrial membrane potential could serve as a metric to identify recurrent populations for further study.
A final possibility that could explain the metabolic differences between primary mammospheres and re-growing cells is that the latter cells were exposed to Her2 signaling for a shorter period of time. That is, the metabolism of these re-growing cells could still be changing, and they may eventually resemble more closely the primary tumor cells. This, in turn, would suggest the intriguing possibility that the metabolic profile of tumors at early stages of recurrence is unique and may provide opportunities for therapeutic intervention.
Metabolism is highly dependent on the surrounding environment. While in vitro culture is critically important in modern cancer research, it cannot fully replicate the tumor microenvironment and the role of vasculature in these tumor stages. Finally, caution should be considered regarding the use of TMRE: because it is directly correlated to mitochondrial membrane potential but does not explicitly report on a voltage due to the proton gradient, our method relies on a relative measurement. Next steps to further understand the transition of metabolism occurring within a tumor’s life should be to image each stage with 2-NBDG and TMRE in vivo using a GEM-derived mammary transplant and a mammary window chamber. These methods still allow for the high resolution seen with the mammospheres but include other valuable sources of information such as hemoglobin saturation and angiogenesis to aid in a deeper understanding of aerobic respiration’s role in dormant and recurrent populations and the tumor’s interplay with tumor-associated fibroblasts. Additionally, we have shown here that 2-NBDG and TMRE can be used to stain superficial cell aggregates, but the limitations of dye permeability in larger cell aggregates in vitro remains uncharacterized. Finally, the use of 3D confocal microscopy was selected for this study in order to reduce out-of-focus fluorescence and multi-scattering events in each image; however, scattering may still affect fluorescence as a function of depth. The work described here presents a new technique for the direct study of two key metabolic endpoints related to tumor metabolism and has the potential to be expanded to image other endpoints in the future.
Supplementary Material
Acknowledgments
This work is supported by generous funding from Duke School of Medicine Core Facility Voucher Program, NIH Grant 1R01CA195500-01, V Foundation Grant, and the Duke Medical Imaging Training Program NIH Grant T32-EB001040. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Thank you to Dr. Yasheng Gao at Duke University’s Light Microscopy Core Facility for imaging trouble shooting. Many thanks to Dr. Brandon Nichols for discussion of image processing methodologies and Marianne Lee for assistance in preliminary experiments.
Financial Support
This work is supported by generous funding from Duke School of Medicine Core Facility Voucher Program, NIH Grant 1R01CA195500-01, V Foundation Grant, and the Duke Medical Imaging Training Program NIH Grant T32-EB001040. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Disclosure of Potential Conflicts of Interest
Dr. Ramanujam has founded a company called Zenalux Biomedical and she and other team members have developed technologies related to this work where the investigators or Duke may benefit financially if this system is sold commercially.
References
- [1].Howlader N et al. , “SEER Cancer Statistics Review, 1975-2014,” N. C. Institute, Ed., ed. Bethesda, MD, 2017. [Google Scholar]
- [2].Gonzalez-Angulo AM et al. , “High risk of recurrence for patients with breast cancer who have human epidermal growth factor receptor 2-positive, node-negative tumors 1 cm or smaller,” J Clin Oncol, vol. 27, no. 34, pp. 5700–6, December 1 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Buzdar AU et al. , “Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer,” J Clin Oncol, vol. 23, no. 16, pp. 3676–85, June 01 2005. [DOI] [PubMed] [Google Scholar]
- [4].Debska-Szmich S, Krakowska M, Czernek U, Habib-Lisik M, Zieba A, and Potemski P, “The role of preoperative systemic treatment in patients with breast cancer,” Contemp Oncol (Pozn), vol. 20, no. 2, pp. 93–101, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Symmans WF et al. , “Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy,” J Clin Oncol, vol. 25, no. 28, pp. 4414–22, October 1 2007. [DOI] [PubMed] [Google Scholar]
- [6].Mougalian SS et al. , “Ten-Year Outcomes of Patients With Breast Cancer With Cytologically Confirmed Axillary Lymph Node Metastases and Pathologic Complete Response After Primary Systemic Chemotherapy,” JAMA Oncol, vol. 2, no. 4, pp. 508–16, April 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wuerstlein R and Harbeck N, “Neoadjuvant Therapy for HER2-positive Breast Cancer,” Rev Recent Clin Trials, vol. 12, no. 2, pp. 81–92, 2017. [DOI] [PubMed] [Google Scholar]
- [8].Hayashi N et al. , “The Prognostic Effect of Changes in Tumor Stage and Nodal Status After Neoadjuvant Chemotherapy in Each Primary Breast Cancer Subtype,” Clin Breast Cancer, October 03 2017. [DOI] [PubMed] [Google Scholar]
- [9].Diel I et al. , “Micrometastatic breast cancer cells in bone marrow at primary surgery: prognostic value in comparison with nodal status.,” J Natl Cancer Inst, vol. 88, no. 22, pp. 1652–8, 1996. [DOI] [PubMed] [Google Scholar]
- [10].Saphner T, Tormey D, and Gray R, “Annual hazard rates of recurrence for breast cancer after primary therapy,,” J Clin Oncol, vol. 14, pp. 2738–46, 1996. [DOI] [PubMed] [Google Scholar]
- [11].Symmans WF et al. , “Long-Term Prognostic Risk After Neoadjuvant Chemotherapy Associated With Residual Cancer Burden and Breast Cancer Subtype,” J Clin Oncol, vol. 35, no. 10, pp. 1049–1060, April 01 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Weinberg SE and Chandel NS, “Targeting mitochondria metabolism for cancer therapy,” Nat Chem Biol, vol. 11, no. 1, pp. 9–15, January 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Nagarajan A, Malvi P, and Wajapeyee N, “Oncogene-directed alterations in cancer cell metabolism,” Trends Cancer, vol. 2, no. 7, pp. 365–377, July 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Keenan MM and Chi JT, “Alternative fuels for cancer cells,” Cancer J, vol. 21, no. 2, pp. 49–55, Mar-Apr 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Alvarez JV et al. , “Oncogene pathway activation in mammary tumors dictates FDG-PET uptake,” Cancer Res, vol. 74, no. 24, pp. 7583–98, December 15 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bjurberg M, Gustavsson A, Ohlsson T, and Brun E, “FDG-PET in the detection of residual disease and relapse in patients with Hodgkin’s lymphoma. Experience from a Swedish centre,” Acta Oncol, vol. 45, no. 6, pp. 743–9, 2006. [DOI] [PubMed] [Google Scholar]
- [17].Havas KM et al. , “Metabolic shifts in residual breast cancer drive tumor recurrence,” (in English), Journal of Clinical Investigation, vol. 127, no. 6, pp. 2091–2105, June 1 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Scatena R, Bottoni P, and Giardina B, “Mitochondria, metabolism and cancer: a growing role in cancer cell differentiation and cancer cell dormancy,” Cancer & Metabolism, vol. 2, no. Suppl 1, 2014. [Google Scholar]
- [19].Kurmi K et al. , “Tyrosine Phosphorylation of Mitochondrial Creatine Kinase 1 Enhances a Druggable Tumor Energy Shuttle Pathway,” Cell Metabolism, 2018/August/30/ 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Youn H and Hong KJ, “In vivo non invasive molecular imaging for immune cell tracking in small animals,” Immune Netw, vol. 12, no. 6, pp. 223–9, December 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Solomon M, Liu Y, Berezin MY, and Achilefu S, “Optical Imaging in Cancer Research: Basic Principles, Tumor Detection, and Therapeutic Monitoring,” (in English), Medical Principles and Practice, vol. 20, no. 5, pp. 397–415, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hou J et al. , “Correlating two-photon excited fluorescence imaging of breast cancer cellular redox state with seahorse flux analysis of normalized cellular oxygen consumption,” (in English), Journal of Biomedical Optics, vol. 21, no. 6, June 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhu C et al. , “Near-simultaneous intravital microscopy of glucose uptake and mitochondrial membrane potential, key endpoints that reflect major metabolic axes in cancer,” Sci Rep, vol. 7, no. 1, p. 13772, October 23 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rajaram N et al. , “Delivery Rate Affects Uptake of a Fluorescent Glucose Analog in Murine Metastatic Breast Cancer,” (in English), Plos One, vol. 8, no. 10, October 18 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Millon SR, Ostrander JH, Brown JQ, Raheja A, Seewaldt VL, and Ramanujam N, “Uptake of 2-NBDG as a method to monitor therapy response in breast cancer cell lines,” Breast Cancer Res Treat, vol. 126, no. 1, pp. 55–62, February 2011. [DOI] [PubMed] [Google Scholar]
- [26].Frees AE, Rajaram N, McCachren SS, Fontanella AN, Dewhirst MW, and Ramanujam N, “Delivery-Corrected Imaging of Fluorescently-Labeled Glucose Reveals Distinct Metabolic Phenotypes in Murine Breast Cancer,” (in English), Plos One, vol. 9, no. 12, December 19 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rajaram N, Reesor AF, Mulvey CS, Frees AE, and Ramanujam N, “Non-Invasive, Simultaneous Quantification of Vascular Oxygenation and Glucose Uptake in Tissue,” (in English), Plos One, vol. 10, no. 1, January 30 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yamada K, Saito M, Matsuoka H, and Inagaki N, “A real-time method of imaging glucose uptake in single, living mammalian cells,” (in English), Nature Protocols, vol. 2, no. 3, pp. 753–762, 2007. [DOI] [PubMed] [Google Scholar]
- [29].Tsytsarev V, Maslov KI, Yao JJ, Parameswar AR, Demchenko AV, and Wang LHV, “In vivo imaging of epileptic activity using 2-NBDG, a fluorescent deoxyglucose analog,” (in English), Journal of Neuroscience Methods, vol. 203, no. 1, pp. 136–140, January 15 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cai HW and Peng FY, “2-NBDG Fluorescence Imaging of Hypermetabolic Circulating Tumor Cells in Mouse Xenograft model of Breast Cancer,” (in English), Journal of Fluorescence, vol. 23, no. 1, pp. 213–220, January 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Perry SW, Norman JP, Barbieri J, Brown EB, and Gelbard HA, “Mitochondrial membrane potential probes and the proton gradient: a practical usage guide,” Biotechniques, vol. 50, no. 2, pp. 98–115, February 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Martinez AF et al. , “Metaboloptics: Visualization of the tumor functional landscape via metabolic and vascular imaging,” Sci Rep, vol. 8, no. 1, p. 4171, March 8 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Moody SE et al. , “Conditional activation of Neu in the mammary epithelium of transgenic mice results in reversible pulmonary metastasis,” Cancer Cell, vol. 2, no. 6, pp. 451–61, 2002. [DOI] [PubMed] [Google Scholar]
- [34].Feng Y et al. , “SPSB1 promotes breast cancer recurrence by potentiating c-MET signaling,” Cancer Discov, vol. 4, no. 7, pp. 790–803, July 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Alvarez JV et al. , “Par-4 downregulation promotes breast cancer recurrence by preventing multinucleation following targeted therapy,” Cancer Cell, vol. 24, no. 1, pp. 30–44, July 8 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Moody SE et al. , “The transcriptional repressor Snail promotes mammary tumor recurrence,” Cancer Cell, vol. 8, no. 3, pp. 197–209, September 2005. [DOI] [PubMed] [Google Scholar]
- [37].Abravanel DL et al. , “Notch promotes recurrence of dormant tumor cells following HER2/neu-targeted therapy,” J Clin Invest, vol. 125, no. 6, pp. 2484–96, June 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Slamon D, Clark G., Wong S, Levin W, Ullrich A, and McGuire W, “Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene,” Science, vol. 235, no. 4785, pp. 177–82, 1987. [DOI] [PubMed] [Google Scholar]
- [39].Scaduto R and Grotyohann L, “Measurement of mitochondrial membrane potential using fluorescent rhodamine derivatives,” Biophysical Journal, vol. 76, pp. 469–477, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Martinez A et al. , “Metaboloptics: Visualization of the tumor functional landscape via metabolic and vascular imaging,” Scientific Reports, vol. Under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].O’Neil RG, Wu L, and Mullani N, “Uptake of a fluorescent deoxyglucose analog (2-NBDG) in tumor cells,” Mol Imaging Biol, vol. 7, no. 6, pp. 388–92, Nov-Dec 2005. [DOI] [PubMed] [Google Scholar]
- [42].Crowley LC, Christensen ME, and Waterhouse NJ, “Measuring Mitochondrial Transmembrane Potential by TMRE Staining,” Cold Spring Harb Protoc, vol. 2016, no. 12, p. pdb prot087361, December 1 2016. [DOI] [PubMed] [Google Scholar]
- [43].Sethian JA, Level set methods and fast marching methods: evolving interfaces in computational geometry, fluid mechanics, computer vision, and materials science. Cambridge University Press, 1999. [Google Scholar]
- [44].Aguirre GK, Zarahn E, and D’Esposito M, “A Critique of the Use of the Kolmogorov-Smirnov (KS) Statistic for the Analysis of BOLD fMRI Data,” Magnetic Resonance in Medicine, vol. 39, no. 3, pp. 500–5, 1998. [DOI] [PubMed] [Google Scholar]
- [45].Lombardo Y, de Giorgio A, Coombes CR, Stebbing J, and Castellano L, “Mammosphere formation assay from human breast cancer tissues and cell lines,” J Vis Exp, no. 97, March 22 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wang R et al. , “Comparison of mammosphere formation from breast cancer cell lines and primary breast tumors,” J Thorac Dis, vol. 6, no. 6, pp. 829–37, June 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Vander Heiden MG, Plas DR, Rathmell JC, Fox CJ, Harris MH, and Thompson CB, “Growth Factors Can Influence Cell Growth and Survival through Effects on Glucose Metabolism,” Molecular and Cellular Biology, vol. 21, no. 17, pp. 5899–5912, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Bos R, van Diest PJ, de Jong JS, van der Groep P, van der Valk P, and van der Wall E, “Hypoxia-inducible factor-1alpha is associated with angiogenesis, and expression of bFGF, PDGF-BB, and EGFR in invasive breast cancer,” Histopathology, vol. 46, no. 1, pp. 31–6, January 2005. [DOI] [PubMed] [Google Scholar]
- [49].Kannan K and Jain S, “Oxidative stress and apoptosis,” Pathophysiology, vol. 7, no. 27, pp. 153–63, 2000. [DOI] [PubMed] [Google Scholar]
- [50].TeSlaa T and Teitell MA, “Techniques to monitor glycolysis,” Methods Enzymol, vol. 542, pp. 91–114, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Banki K, Hutter E, Gonchoroff NJ, and Perl A, “Elevation of mitochondrial transmembrane potential and reactive oxygen intermediate levels are early events and occur independently from activation of caspases in Fas signaling,” J Immunol, vol. 162, no. 3, pp. 1466–79, February 1 1999. [PMC free article] [PubMed] [Google Scholar]
- [52].Jo WS et al. , “Loss of mitochondrial membrane potential and caspase activation enhance apoptosis in irradiated K562 cells treated with herbimycin A,” Int J Radiat Biol, vol. 81, no. 7, pp. 531–43, July 2005. [DOI] [PubMed] [Google Scholar]
- [53].Perl A, Gergely P Jr., Nagy G, Koncz A, and Banki K, “Mitochondrial hyperpolarization: a checkpoint of T-cell life, death and autoimmunity,” Trends Immunol, vol. 25, no. 7, pp. 360–7, July 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Takahashi A, Masuda A, Sun M, Centonze VE, and Herman B, “Oxidative stress-induced apoptosis is associated with alterations in mitochondrial caspase activity and Bcl-2-dependent alterations in mitochondrial pH (pHm),” Brain Res Bull, vol. 62, no. 6, pp. 497–504, February 15 2004. [DOI] [PubMed] [Google Scholar]
- [55].Satoh T, Enokido Y, Aoshima H, Uchiyama Y, and Hatanaka H, “Changes in mitochondrial membrane potential during oxidative stress-induced apoptosis in PC12 cells,” J Neurosci Res, vol. 50, no. 3, pp. 413–20, November 1 1997. [DOI] [PubMed] [Google Scholar]
- [56].Heiskanen KM, Bhat MB, Wang HW, Ma J, and Nieminen AL, “Mitochondrial depolarization accompanies cytochrome c release during apoptosis in PC6 cells,” J Biol Chem, vol. 274, no. 9, pp. 5654–8, February 26 1999. [DOI] [PubMed] [Google Scholar]
- [57].Zamzami N et al. , “Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death,” J Exp Med, vol. 182, no. 2, pp. 367–77, August 1 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Gutscher M et al. , “Real-time imaging of the intracellular glutathione redox potential,” Nat Methods, vol. 5, no. 6, pp. 553–9, June 2008. [DOI] [PubMed] [Google Scholar]
- [59].Zhang BB, Wang DG, Guo FF, and Xuan C, “Mitochondrial membrane potential and reactive oxygen species in cancer stem cells,” Fam Cancer, vol. 14, no. 1, pp. 19–23, March 2015. [DOI] [PubMed] [Google Scholar]
- [60].Viale A et al. , “Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function,” (in English), Nature, vol. 514, no. 7524, pp. 628–32, October 30 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Fan J et al. , “Glutamine-driven oxidative phosphorylation is a major ATP source in transformed mammalian cells in both normoxia and hypoxia,” Mol Syst Biol, vol. 9, p. 712, December 3 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Johnson BE et al. , “Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma,” Science, vol. 343, no. 6167, pp. 189–193, January 10 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Ding L et al. , “Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing,” Nature, vol. 481, no. 7382, pp. 506–10, January 11 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.