Abstract
Objectives:
Small intestinal bacterial overgrowth (SIBO) is often present in patients with chronic pancreatitis (CP) with persistent steatorrhea, despite pancreatic enzyme replacement therapy (PERT). Overall prevalence of SIBO, diagnosed by glucose breath test (GBT), varies between 0–40% but 0–21% in those without upper gastrointestinal (GI) surgery. We investigated the prevalence and non-surgical independent predictors of SIBO in CP without upper GI surgery.
Methods:
273 patients ≥18 years-old had a presumptive diagnosis of CP and a GBT between 1989–2017. We defined CP by Mayo Score (0–16) ≥4 and a positive GBT for SIBO by Rome Consensus Criteria, and retrospectively collected data for 5 a priori variables (age, opiates, alcohol use, diabetes mellitus (DM), gastroparesis) and 41 investigational variables (demographics, GI symptoms, comorbidities, CP etiologies and co-factors, CP symptom duration, Mayo score and non-diabetes components, and biochemical variables).
Results:
98 of 273 patients had definite CP and 40.8% had SIBO. Five of 46 variables predicted SIBO: opiates, p=0.005; DM, p=0.04; total Mayo score, p<0.05, zinc, p=0.005, and albumin, p<0.05). Multivariable analysis of 3 non-correlated variables identified zinc level (OR=0.0001; p=0.03) as the sole independent predictor of SIBO (model C-statistic=0.89; p<0.001).
Conclusions:
SIBO, diagnosed by GBT, occurs in 40.8% of patients with CP without upper GI surgery. In CP patients, markers of more severe CP (low zinc level, DM and increased Mayo score) and opiate use should raise clinical suspicion for SIBO, particularly in patients with persistent steatorrhea or weight loss despite PERT.
Introduction
Chronic pancreatitis (CP) is a progressive clinical syndrome characterized by morphological evidence of chronic inflammation, fibrosis and parenchymal loss (of acinar and islet cells) resulting in loss of exocrine and endocrine function [1–3]. Abdominal pain is a hallmark feature of CP, but other manifestations, including steatorrhea and weight loss, also may be present [4], markedly impairing quality of life and contributing to significant morbidity and mortality [5,6].
Unfortunately, treatment options in CP are limited and palliative [7]. Pancreatic enzyme replacement therapy (PERT) is the most common therapy for CP associated with exocrine pancreatic insufficiency (EPI). In EPI, PERT improves digestive symptoms, nutrition, bone health, glycemic control and health related quality of life [1,4,8]. Steatorrhea, however, often responds only partially to PERT [9–12]. Initial management steps include assessing adherence to PERT, ingesting PERT throughout the meal, increasing the dose and adding co-treatment with an H2-blocker or PPI [1,3,8]. Persistent steatorrhea, despite addressing these factors, may indicate an alternate cause of diarrhea or malabsorption, which is most commonly due to small intestinal bacterial overgrowth (SIBO) [13], defined as a small intestine bacterial population of 105 colony-forming/mL on culture of small bowel aspirates [14–16]. Other less common causes are multiple and reviewed elsewhere [3,13].
SIBO decreases luminal fat digestion and absorption by deconjugating bile salts and causing some degree of secondary enteritis. Proposed mechanisms for causing SIBO in patients with CP include impaired small bowel motility due to opioids, gastrointestinal (GI) surgery, diabetic neuropathy and ileal braking secondary to nutrient malabsorption [3,17,18]. In addition, other factors may be important, including antibacterial effect of pancreatic secretions [19,20], and an altered host immune response [21], possibly involving diminished trypsin mediated conversion of gut antibiotic propeptides to an active form, as illustrated with human defensin-5 in other conditions [22,23].
The true prevalence of SIBO in CP is unclear largely due to variable methods to diagnose SIBO and whether patients with upper GI surgery were included in individual studies [13]. When small bowel aspiration and quantitative culture is the gold-standard diagnostic test, the prevalence of SIBO in CP is 50–77% [24–26]. This method, however, is invasive (requires endoscopy), costly and is hampered by sampling error, reproducibility and standardization and frequently is not used [27,28]. By hydrogen breath testing (HBT) the prevalence of SIBO in CP is 0–92% [29–39], highest (47–92%) with lactulose hydrogen breath tests (LBT) [33,36,37,39], which lack specificity [27,40,41], and lower (0–40%) with glucose breath tests (GBT) [29–32,34,35,38], which are more specific. As pointed out in a recent meta-analysis [42], pooled analyses of these data is limited by heterogeneity of the HBT protocol, variable inclusion of patients with prior upper GI surgery, small sample sizes and criteria for diagnosis of definite CP. Moreover, there are no defined definite predictive variables for SIBO in patients with CP, with the exception of prior upper GI surgery [13,31,42].
Our aims were to determine the prevalence of SIBO by GBT, in a well-defined cohort of patients with CP without prior upper GI surgery; and to create a predictive model for SIBO by examining five a priori and 41 investigational variables in a large population of patients with CP.
METHODS
Selection of case samples
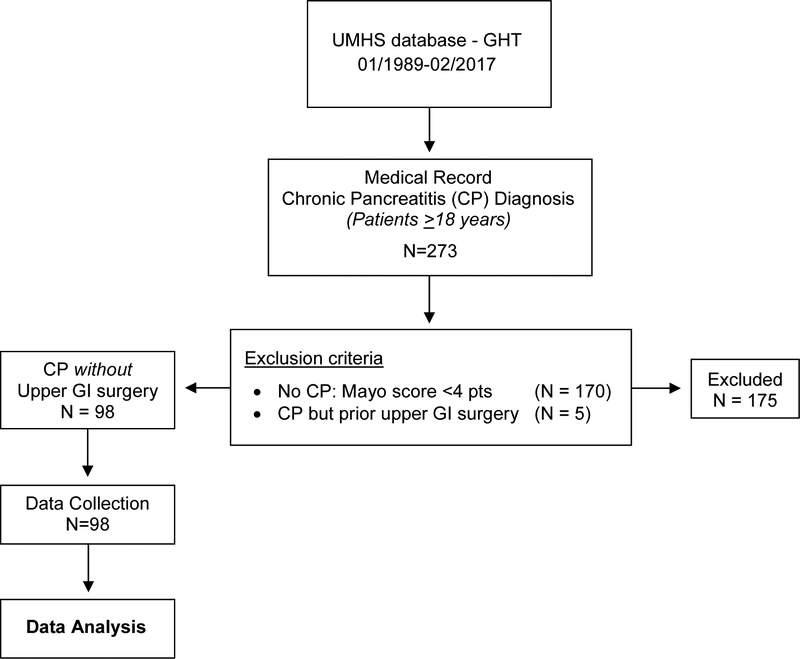
The study was approved by the University of Michigan Institutional Review Board (01/26/2016). We performed a retrospective review of adult patients (≥ 18 years of age) referred to the Gastrointestinal Physiology Laboratory at the University of Michigan for a GBT from January 1989 – February 2017 and identified 273 unique patients who had a clinical diagnosis of CP (Figure 1). Definite-CP (n=103) or Non-CP (n=170) was based on the Mayo clinical diagnostic criteria, the study gold standard (Figure 2). Five of 103 patients with CP were excluded for having prior upper GI surgery (small bowel surgery, gastric bypass, pancreaticoduodenectomy), an established risk factor for SIBO [13,25,31,42].
Figure 1.
Methodological Summary
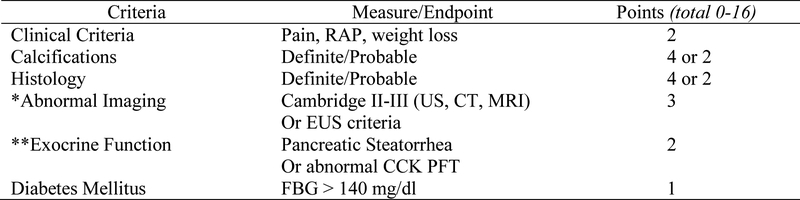
Figure 2. Mayo Clinical Diagnostic Criteria: A Scoring System for Definite Chronic Pancreatitis.
CCK, cholecystokinin; FBG, Fasting Blood Glucose; PFT, pancreatic function testing; RAP, Recurrent Acute Pancreatitis; EUS, Endoscopic Ultrasonography *EUS criteria for CP included having > 5 EUS criteria or EUS Rosemont criteria that were suggestive of or consistent with CP **Pancreatic steatorrhea was based on a positive qualitative fecal fat or quantitative fecal fat >7 grams/24 hours Adapted from (45).
Data collection
All GBT samples were analyzed using a Quintron MicroLyzer (Quintron Instrument Company Inc., Milwaukee, Wisconsin). The pre-test GBT protocol consisted of fasting, prohibition of fluids for 8 hours and avoidance of smoking for 6 hours, Pepto-Bismol products for 14 days, and antibiotics for 30 days prior to test. The two hour GBT was performed according to the Rome Consensus Conference Expert Group [27,40], which differs slightly from the new North American consensus document [43], published May 2017 and postdating the end of the study period. Samples were analyzed for hydrogen and methane concentrations, each measured in parts per million (ppm), the latter which predominates in 6–20% of the population [44] and increases the sensitivity of the GBT. The sample was considered measurable by evaluating the carbon dioxide level. A positive GBT was defined as an increase in breath hydrogen and/or methane by at least 12 ppm over basal concentration levels.
The existing GI Physiology Laboratory database had the following variables (Table 1): 4 demographic variables; 10 gastrointestinal (GI) symptoms self-reported at the time of GBT or documented by the ordering physician, 2 environmental exposures (current smoking and consuming alcoholic beverages); 2 prescribed medications (opiates and proton pump inhibitors (PPI)); and 6 comorbidities.
Table 1.
Univariable analysis of a positive GBT-SIBO in patients with definite chronic pancreatitis (CP)
| Variable | Total N (%) |
Negative GBT N (%) |
Positive GBT N (%) |
p |
|---|---|---|---|---|
| N | 58 (59.2) | 40 (40.8) | ||
| Demographics | ||||
| Age, mean (SD) | 51.6 (16.3) | 52.4 (12.8) | 0.78 | |
| Female | 32 (55.2) | 18 (45.0) | 0.32 | |
| Caucasian race | 51 (94.4) | 33 (97.1) | 0.566 | |
| BMI, mean (SD)(BMI) | 27.9 (7.1) | 26.1 (7.3) | 0.23 | |
| Reported gastrointestinal (GI) symptomsa | ||||
| Heart Burn | 30 (51.7) | 20 (50.0) | 0.87 | |
| Regurgitation | 25 (43.9) | 17 (42.5) | 0.90 | |
| Chest pain | 18 (31.0) | 12 (30.0) | 0.91 | |
| Nausea | 29 (50.9) | 22 (55.0) | 0.69 | |
| Vomit | 16 (27.6) | 16 (40.0) | 0.19 | |
| Bloating | 40 (69.0) | 28 (70.0) | 0.91 | |
| Gas | 42 (75.0) | 24 (61.5) | 0.16 | |
| Abdominal pain | 46 (79.3) | 28 (70.0) | 0.29 | |
| Diarrhea | 36 (62.1) | 26 (65.0) | 0.77 | |
| Constipation | 23 (39.7) | 12 (30.0) | 0.33 | |
| Comorbidities (see below for diabetes) | ||||
| Celiac disease (CEL) | 2 (3.4) | 0 (0.0) | 0.51 | |
| Irritable bowel syndrome | 14 (24.1) | 5 (12.8) | 0.17 | |
| Gastroparesis (GPR) | 1 (1.7) | 4 (10.0) | 0.09 | |
| Diverticulosis | 1 (1.8) | 0 (0.0) | 0.59 | |
| Cholecystectomy | 26 (45.6) | 17 (43.6) | 0.85 | |
| Prescribed medications | ||||
| PERT | 30 (56.6) | 27 (67.5) | 0.39 | |
| Opiates | 13 (22.4) | 20 (50.0) | 0.005 | |
| Proton pump inhibitor (PPI) | 28 (48.3) | 24 (61.5) | 0.20 | |
| Etiology of CP | ||||
| Genetic | 6 (11.1) | 1 (2.6) | 0.23 | |
| Otherb | 9 (16.7) | 4 (10.3) | 0.55 | |
| Idiopathic | 20 (37.0) | 17 (44.7) | 0.67 | |
| Alcohol | ||||
| Ever | 23 (42.6) | 18 (46.2) | 0.83 | |
| Current | 4 (17.4) | 4 (22.2) | 0.71 | |
| Former | 19 (82.6) | 13 (72.2) | 0.47 | |
| Unknown | 0 (0.0) | 1 (5.6) | 0.44 | |
| Additional Co-factors for CP | ||||
| History of pancreatic necrosis | 3 (5.2) | 1 (2.5) | 0.64 | |
| Tobacco | ||||
| Ever | 25 (50.0) | 20 (51.3) | >0.99 | |
| Current | 10 (40.0) | 9 (45.0) | 0.77 | |
| Former | 14 (56.0) | 11 (55.0) | >0.99 | |
| Unknown | 1 (4.0) | 0 (0.0) | >0.99 | |
| Mayo score (0–16) for CP, median (IQR) | 5.0 (1.0) | 6.0 (4.0) | <0.05 | |
| EPI | 10 (20.0) | 15 (37.5) | 0.10 | |
| Diabetes mellitus (DM) | 20 (37.0) | 24 (60.0) | 0.04 | |
| Subtypec | Type 1 | 1 (5.0) | 2 (8.3) | >0.99 |
| Type 3c | 16 (80.0) | 19 (79.2) | >0.99 | |
| Unknown | 3 (15.0) | 3 (12.5) | >0.99 | |
| Pancreatic calcifications | 14 (26.9) | 14 (35.9) | 0.37 | |
| Abnormal imaging | 41 (77.4) | 25 (65.8) | 0.24 | |
| Symptoms | 40 (100.0) | 54 (100.0) | >0.99 | |
| Histology | 0 (0) | 0 (0) | >0.99 | |
| Biochemical parametersd | ||||
| 25-hydroxy Vitamin D, mean (SE), ng/mL | 27.1 (2.0) | 22.8 (2.0) | 0.13 | |
| Vitamin A, mean (SE), mcg/dL | 50.5 (4.3) | 68.1 (18.8) | 0.36 | |
| Vitamin E, mean (SE), mg/L | 11.1 (1.0) | 10.0 (1.1) | 0.47 | |
| B12, mean (SE), pg/mL | 688.5 (79.7) | 825.9 (94.7) | 0.27 | |
| Zinc, mean (SE), mcg/mL | 0.9 (0.05) | 0.6 (0.04) | 0.005 | |
| Selenium, mean (SE), ng/mL | 137.5 (4.8) | 125.4 (8.5) | 0.22 | |
| Folate, mean (SE), ng/mL | 16.9 (1.6) | 16.9 (1.9) | 0.99 | |
| Ferritin, mean (SE), ng/mL | 143.9 (30.9) | 154.6 (46.3) | 0.84 | |
| Albumin, mean (SE), g/dL | 4.3 (0.07) | 4.0 (0.09) | <0.05 | |
| HgbA1c, mean (SE), % | 6.5 (0.4) | 8.1 (0.8) | 0.07 | |
| C-reactive protein, mean (SE), mg/dL | 0.7 (0.2) | 0.4 (0.2) | 0.39 |
Active patient reported gastrointestinal symptoms at the time of the GBT.
Includes single or multiple factors: hypertriglyceridemia, celiac disease, and autoimmune pancreatitis.
Type 3c includes 21 patients who were misclassified as Type 2 diabetes mellitus, including 11 patients who were SIBO-positive and 12 patients who were SIBO-negative.
Normal values for biochemical parameters: Vitamin D 25–100 ng/mL; vitamin A 32.5–78.0 mcg/dL; vitamin E 5.5–17.0 mg/L; vitamin B12 211–911 pg/mL; zinc 0.55–1.50 mcg/mL; selenium 70–150 ng/mL; folate 3.0–999.0 ng/mL; ferritin 6.0–155.0 ng/mL; albumin 3.5–4.9 g/dL; Hgb A1c 4.2–5.6%; C-reactive protein 0.0–0.6 mg/dL.
BMI, body mass index; CP, chronic pancreatitis; EPI, exocrine pancreatic insufficiency; PERT, pancreatic enzyme replacement therapy; SIBO, small intestinal bacterial overgrowth; Variables with P values in bold were included in the multivariable logistic regression model, except for HgbA1c due to confounding with DM.
Co-investigators AAL and JRB used methods similar to our previous studies [45] to extract additional data from chart notes (Gastroenterology clinic visits, inpatient consults and emergency department visits) and laboratory tests completed within 6 months prior to GBT. Eleven biochemical markers of SIBO were examined, including 25-hydroxy vitamin D, vitamin A, vitamin E, vitamin B12, folate, zinc, selenium, ferritin, albumin, hemoglobin A1c (Hgb A1c) and C-reactive protein levels. Diagnosis of definite CP, was defined as a Mayo Score (0 – 16) ≥4 points (Figure 2) [45,46], which required data collection for symptoms of abdominal pain and weight loss, steatorrhea (positive qualitative fecal fat or quantitative fecal fat >7 grams/24 hours) and diabetes mellitus (DM, fasting blood glucose > 126, hemoglobin A1C) and imaging. DM was subclassified as type 1, 2 and 3c based on current recommendations [47]. Data for pancreatic histology were available infrequently. Pancreatic imaging was reviewed and included endoscopic retrograde cholangiopancreatography (ERCP), transabdominal ultrasound (US), computed tomography (CT), magnetic resonance imaging (MRI) and endoscopic ultrasonography (EUS). Imaging criteria for CP were the Cambridge II-III criteria, based on ERCP, US, CT and/or MRI, and ≥ 5 EUS criteria for CP, or EUS Rosemont criteria that were “suggestive of” or “consistent with” CP. To calculate values for Mayo Score (Figure 2), we added no points for missing data.
Statistical Analyses
All calculations were computed using SPSS version 25 (IBM Inc., Armonk, NY). Univariable analyses for a positive GBT were performed for 5 a priori variables (age, opiate use, current alcohol use, DM, gastroparesis (GPR)) and 41 investigational variables consisting of 3 additional demographic variables, 10 individual GI symptoms, 4 other comorbidities (celiac disease, irritable bowel syndrome, diverticulosis, history of cholecystectomy), 2 other medications (pancreatic enzyme replacement therapy (PERT), PPI use), 3 additional non-alcohol etiologies of CP (genetic, idiopathic, and other (hypertriglyceridemia, celiac disease, autoimmune)), 2 other co-factors of CP (tobacco use, history of necrotizing pancreatitis), duration of CP symptoms, severity of CP features (Mayo Score 0–16), 5 non-DM components of the Mayo score (exocrine pancreatic insufficiency (EPI), presence of calcifications on imaging, abnormal imaging, symptoms, histology) and 11 biochemical variables(Table 1). We performed a multivariable logistic regression model to identify independent predictors for a positive GBT, by including variables with a p-value of < 0.10 in the univariable analyses, but limited the logistic regression model to a maximum of 4 variables to decrease the likelihood of overfitting of the model because only 40 patients had a positive GBT, and by not including significantly correlated factors in the logistic regression model. Correlations were examined within 2 groups of variables: markers for CP severity (Mayo Score, DM, Zinc levels and albumin) and GI dysmotility factors (DM, gastroparesis, opiate use). Area under the receiver operator characteristic (AUROC) curve with a C-statistic were calculated for the exploratory predictive models of SIBO in CP. Categorical and dichotomous variables were assessed by 2×2 contingency tables and χ2 -tests or two-tailed Fisher’s exact test when necessary. Continuous variables were assessed by two sample t-tests for normally distributed variables and the Wilcoxon rank-sum test for non-parametric data. A p-value of ≤ 0.05 was considered statistically significant.
RESULTS
Clinical Characteristics
Of the patients who underwent a GBT for SIBO between January 1989 and February 2017, 273 were labeled as having CP, 103 of 273 (37.7%) fulfilled the Mayo Clinical Criteria for definite CP, and 98 of 103 had no prior upper GI surgery (Figure 1). The population with definite CP was 51.0% female and 95.5% Caucasian with a mean (SD) age 51.9 (14.9) and body mass index 27.2 kg/m2 (7.2).
Prevalence of SIBO in CP
40.8% of 98 patients with definite CP and no prior upper GI surgery had a positive GBT, which is significantly greater than published data for similar older age (>61 years) healthy controls (15.8%; P<0.001) [21]. Of the 40 patients with a positive GBT, 38 were positive for hydrogen, 2 were positive for methane and none were positive for both.
Univariable Analyses of SIBO in CP
Five of 46 variables (Table 1) had a statistically significant association with a positive GBT in CP, including two a priori variables (prescribed opiates, p = 0.005; and DM, p = 0.04). In addition, three investigational variables, a higher Mayo score (p < 0.05), low zinc (p = 0.005), and low albumin (p < 0.05), suggest SIBO associates with more severe CP. The positive predictive value (PPV) of the two categorical variables for a positive GBT was 24/40 (60%) for DM, 20/40 (50%) for opiate use, and 29/40 (72.5%) for DM and/or opiate use.
Variable Correlations and Multivariable Analyses of SIBO in CP
For multivariable logistic regression analysis (Table 2) we identified a maximum of seven potential variables for inclusion on the basis of having a p value <0.1 in the univariable analyses. These included three a priori variables (prescribed opiates, p = 0.005; DM, p = 0.04; and GPR, p = 0.09), total Mayo score (p < 0.05), and three biochemical variables (zinc, p = 0.005; albumin, p < 0.05; Hgb A1c p = 0.07), of which we excluded Hgb A1c due to confounding effects with the variable DM. Of the remaining 6, multiple correlations were found between variables associated with CP severity, including one with Mayo score (DM [p < 0.001]); two with DM (Mayo score [p < 0.001], albumin [p = 0.003]); and one with zinc level (albumin [p = 0.001]) (Supplemental Table 1). Among motility factors, GPR correlated only with DM (p=0.003) and opiate was not correlated with DM or GPR.
Table 2.
Multivariable analysis of a positive GBT-SIBO in patients with definite chronic pancreatitis (CP)
| VARIABLE | OR | 95% CI | p | |
|---|---|---|---|---|
| Lower | Upper | |||
| Model #1 | ||||
| CP severity factors | ||||
| Diabetes mellitus (DM) | 8.23 | 0.76 | 89.30 | 0.08 |
| Prescribed Medications | ||||
| Opiates | 6.12 | 0.58 | 64.35 | 0.13 |
| Biochemical predictors of SIBO | ||||
| Zinc level (mcg/mL) | 0.0001 | 0.0001 | 0.42 | 0.03 |
| Model #2 | ||||
| CP severity factors | ||||
| Mayo score (0–16) for CP | 1.18 | 0.767 | 1.82 | 0.45 |
| Prescribed Medications | ||||
| Opiates | 7.03 | 0.666 | 74.25 | 0.11 |
| Biochemical predictors of SIBO | ||||
| Zinc mcg/mL | 0.0001 | 0.0001 | 0.62 | 0.04 |
Two exploratory logistic regression models were constructed from 7 variables with p<0.1 in the univariable analyses (Table 1), each excluding Hgb A1c due to confounding effects with the variable DM and accounting for correlations among the remaining 6 variables (Supplemental Table 1), resulting in 3 variables per model, each having zinc level and opiate use but differing by having either DM or Mayo score, which were significantly correlated (p < 0.001).
Area under the receiver operator characteristic (AUROC) curve was similar for Model #1 (C-statistic=0.89, 95% CI 0.758–1.000, p=0.001) and Model #2 (C-statistic=0.88, 95% CI 0.74–1.00; p=0.002)
CI, confidence interval; OR, odds ratio; SIBO, small intestinal bacterial overgrowth
Two exploratory logistic regression models were constructed, each accounting for correlations among 6 variables discussed above, resulting in 3 variables per model, each having zinc level and opiate use but differing by having either DM or Mayo score, which were significantly correlated (p < 0.001).
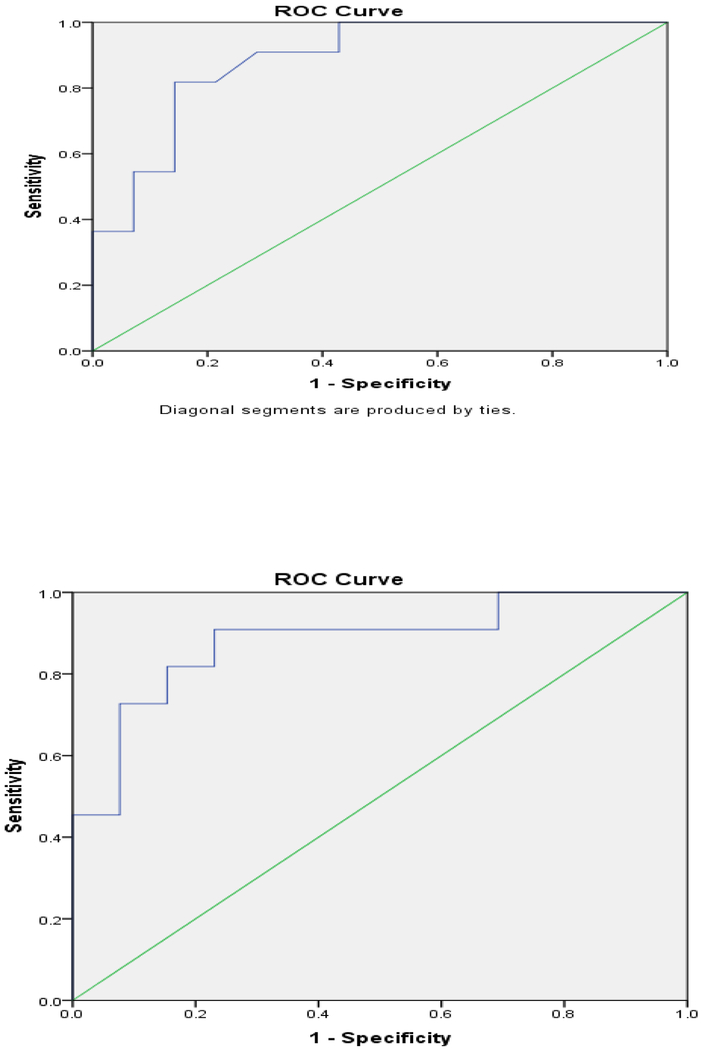
Model #1 included DM, zinc level, and opiate use and excluded Mayo score (correlated with DM), albumin (correlated with zinc), and GPR (correlated with DM and had few patients (n=5)). Zinc level had a significant negative association with SIBO in CP (OR = 0.0001; 95% CI 0.0001–0.423; p = 0.03) and DM had a positive but non-significant association (OR = 8.23; 95% CI 0.76–89.30; p= 0.08). The area under the receiver operator characteristic (AUROC) curve was constructed for a positive GBT in patients with CP, yielding a C-statistic of 0.89 (95% CI 0.76–1.00; p = 0.001) (Figure 3a).
Figure 3. ROC curve for two related models of SIBO in definite CP by multivariable logistic regression.
a) Area under the ROC curve for model #1 which included 3 variables: diabetes mellitus (DM), zinc level, and opiate use. This model of SIBO in patients with definite CP had a significant negative association with zinc (OR=0.0001, p=0.03), a positive but non-significant association with DM (OR=8.23, p=0.08) and a C-statistic of 0.89 (95% CI 0.76–1.00). b) ROC curve for model #2 which included 3 variables: total Mayo clinical score, zinc level, and opiate use. This model of SIBO in definite CP showed a significant negative association with zinc (OR=0.0001, p=0.04), no positive association with Mayo score, and a C-statistic of 0.88 (95% CI 0.74–1.00).
Model #2 included Mayo score, zinc level, and opiate use but excluded DM (correlated with Mayo score) and also albumin and GPR, the latter two the reasons discussed in Model #1. Zinc level had a significant negative association with SIBO in CP (OR = 0.0001; 95% CI 0.001–0.62; p = 0.04) (Table 2) and a C-statistic of 0.88 (95% CI 0.74–1.00; p=0.002) (Figure 3b), similar to model #1.
Outcomes to Antibiotics in SIBO Positive Patients
Thirty-three of 40 patients had identifiable data for assessing a response to antibiotics in definite CP with positive GBT. Symptomatic improvement, for which there was no standardized reporting, was defined broadly as yes or no within 3 months following treatment. Twenty-six of 33 (78.8%) had symptomatic improvement while 6 of 33 (18.2%) had no improvement in symptoms. There were no differences in baseline symptoms between antibiotic responders vs. non-responders (Table 3).
Table 3.
Clinical Characteristics of Patients with CP and SIBO Based on Response to Antibiotic therapy
| Clinical Characteristics | Positive Symptom Response to Antibiotics (n = 26) | Negative Symptom Response to Antibiotics (n = 7) | p-value |
|---|---|---|---|
| Mayo clinical score, median (IQR) | 6 (3.75) | 6 (3.5) | 0.82 |
| Abdominal pain, n (%) | 21 (80.8) | 6 (85.7) | >0.99 |
| Bloating, n (%) | 19 (73.1) | 4 (57.1) | 0.65 |
| Gas, n (%) | 9 (34.6) | 1 (14.3) | 0.40 |
| Diarrhea, n (%) | 17 (65.4) | 3 (42.9) | 0.39 |
| Constipation, n (%) | 4 (15.4) | 1 (14.3) | >0.99 |
| Nausea/Vomiting, n (%) | 8 (30.8) | 1 (14.3) | 0.64 |
| Weight loss/Malabsorption, n (%) | 7 (26.9) | 2 (28.6) | >0.99 |
DISCUSSION
40.8% of 98 patients with definite CP and no prior upper GI surgery had a positive GBT, an indirect but specific marker of SIBO. By univariable analyses we identified five variables significantly associated with a positive GBT in CP: prescribed opiates (p = 0.005), total Mayo score (p < 0.05), DM (p = 0.04), zinc (p = 0.005) and albumin (p < 0.05). By multivariable analysis, we identified low zinc level as an independent predictor of a positive GBT (OR = 0.0001; 95% CI 0.001–0.42; p = 0.03) and DM as having a non-significant trend towards a positive association (OR=8.23; 95% CI 0.76–89.3; p=0.80). The model for SIBO in CP has a moderate predictive value (C-statistic value of 0.89 (95% CI 0.76–0.92; p = 0.001).
In 1979 King and Toskes [14] referred to their unpublished data when they wrote “moderate degrees of small-intestinal colonization occur in some patients with chronic relapsing pancreatitis”. SIBO is known to associate with CP, but large, uniform studies are lacking [13]. The 40.8% prevalence of SIBO in our population of CP and no upper GI surgery is approximately two-fold higher than the 0–21% range in five studies with SIBO diagnosed by GBT [13,31,32,34,35,38]. We speculate this high prevalence of SIBO is mainly attributable to the application of a stringent definition of CP. The increased test sensitivity for SIBO by using both breath hydrogen and methane concentrations had a very minor effect because elevated breath methane was solely responsible for a positive GBT in only 2 of 98 patients (2.0%). Nevertheless, because methane predominates in 6–20% of the population [44], studies relying solely on breath hydrogen in the GBT could underestimate the prevalence of SIBO in CP. Only the small (n=11) study by Madsen et al [32] used both breath hydrogen and methane concentrations to diagnose SIBO, but for unclear reasons, they found no SIBO in CP. We found a lower prevalence of SIBO compared to three reports diagnosing SIBO by aspiration and culture [24–26], the gold standard, which may be attributable to lower sensitivity of GBT for SIBO [27].
There are no clearly established variables that associate with SIBO in CP, with the exception of upper GI surgery [13,31,42]. Evidence, however, from our study suggests that SIBO is more prevalent in more severe CP, with some but not all markers of advanced CP, as reviewed in a recent editorial [13]. We found by univariable analysis that a higher Mayo score, a marker of CP severity, had a significant association with SIBO. Of the six components of the Mayo score, DM, a marker of advanced CP, had a significant association with SIBO (p = 0.04) (discussed below), and EPI had a nonsignificant trend towards an association (p = 0.10). In addition, by an exploratory multivariable analysis, we report low zinc level, a marker of malabsorption and severe CP, was independently associated with a positive GBT in definite CP (OR = 0.0001, p=0.03) and DM had a positive trend towards significance (OR = 8.23, p=0.08). No prior study of SIBO in CP included a multivariable analysis, largely due to small sample size.
Low zinc level, a marker of nutritional status, correlated with low albumin level (p =0.001) and was an independent predictor of SIBO in definite CP. This observation supports the premise that more severe CP (with micronutrient deficiencies) increases the risk of SIBO. Zinc level has not been evaluated previously as a predictor of SIBO in definite CP but unrelated studies indicate that EPI may predispose to zinc deficiency [48–50]. For example, Boosalis et al. demonstrated that patients with EPI had impaired absorption of orally administered zinc sulfate compared to healthy controls, suggesting an important role for normal pancreatic function in zinc metabolism [48]. The relationship between low zinc level and SIBO may not be generalizable to non-pancreatic conditions based at least on observations in patients with celiac disease, in whom Lakhani et al reported zinc and other micronutrients had no significant association with SIBO [51].
A significant association between DM and SIBO (diagnosed by GBT) in CP populations has been previously reported [38], but in the largest of these studies (n=68) there was only a nonsignificant trend (p=0.07) [34]. In the non-CP literature, the association between DM and SIBO is robust and attributed to a combination of small bowel enteropathy, neuropathy, and resultant intestinal stasis [52,53]. In contrast to the largest study in CP [34], our results demonstrate an association between opiate use and SIBO (diagnosed by GBT). These discordant results may potentially be attributable to differences in sample size and in GBT methodology (e.g., omission of breath methane measurements).
Symptoms are not predictive of SIBO in CP. We found no significant association between 10 GI symptoms and SIBO in patients with CP without upper GI surgery, similar to findings from both studies of CP (without prior upper GI surgery and SIBO diagnosed by GBT) [29,32,34,35,38], and non-CP studies [54,55]. Although we found no association between SIBO and diarrhea in CP, a possible association may exist because antibiotic treatment of SIBO improves/resolves diarrhea in 40–100% of patients in 3 studies [30,31,34] and significantly reduces steatorrhea in another study [31]. However, 2 of 3 studies included patients with upper GI surgery [30,31].
Although the prevalence of SIBO in CP without upper GI surgery is 40.8%, it is uncertain whether EPI is a prerequisite for the presence of SIBO. Only two of the seven CP studies that diagnosed SIBO by GBT [29–32,34,35,38] investigated the relationship between SIBO and EPI, and neither reported a significant association [34,35]. Consistent with these prior observations, our data also shows only a non-significant trend towards an association between EPI and SIBO in CP (p=0.10).
The association between PERT and SIBO (diagnosed by GBT) is also unknown in CP patients without upper GI surgery. Data of two studies are conflicting [34,38], with no association reported in the larger of the two studies [34]. Consistent with this latter observation, we found no significant association between PERT and SIBO in CP. These data should be cautiously interpreted however because most patients with EPI receive PERT therapy, which may reduce the detection of SIBO based on evidence that PERT may have antibacterial properties [56].
High serum folate and low serum Vitamin D levels have been proposed as biochemical markers of SIBO in CP and non-CP conditions, but these associations appear weak in CP [35]. We observed no significant association between either variable and SIBO in CP. Nonetheless, measuring Vitamin D is important in CP populations due to an increased risk of non-traumatic fractures [57].
Regarding other variables of interest, there is no clearly established association of SIBO with age, sex, etiology (alcohol vs idiopathic) [34], pancreatic calcifications [31,35]; presence or degree of steatorrhea [31,35]; BMI; or prescriptions for PERT or PPIs [34,35]. Although we observed no significant association between PPI use and SIBO in CP, it should be noted that PPIs are commonly prescribed to CP patients who are not responding to PERT and PPI induced hypochlorhydria may promote conditions favorable for SIBO [58,59]. Based on this concern, it would be reasonable to test for SIBO in CP prior to adding PPI therapy.
There are several potential weaknesses of our study. One is that our cohort at a tertiary referral center may not represent a typical CP cohort in the community. Secondly, this is a retrospective study which may be limited by bias, confounding and in some instances incomplete data. Prospective, controlled studies are required to confirm these results and to refine our exploratory model. Thirdly, the methodology for performing GBT in our study was based on Rome Consensus guidelines [27,40], which were the accepted standard for performance of GBT at the time. In the interim, North American guidelines for GBT were published May 2017, postdating the end of our study period, and differs by recommending use of a higher dose of glucose as a substrate (75 g vs 50 g) and larger magnitude increase in breath H2 (>20 ppm vs > 12 ppm) as evidence of a positive test result [43]. The prevalence of SIBO in CP using new North American recommendations is unknown and should be examined in future studies.
In conclusion, we have examined the largest cohort of well-defined patients with CP without upper GI surgery using GBT to diagnose and identify predictors of SIBO to help guide management. The prevalence of SIBO is high (40.8%) in patients with CP without prior upper GI surgery. Univariable analyses identified five variables significantly associated with SIBO in CP (total Mayo score, DM, zinc level, albumin and opiate use). Multivariable analysis identified zinc level as an independent negative predictor for SIBO while DM had a nonsignificant trend towards a positive association, which conferred a moderate predictive value for SIBO (model AUROC = 0.89; 95% CI 0.76–1.00; p = 0.001). These data may help clinicians to gauge probability of SIBO and whether to test or empirically treat for SIBO in patients with CP without gastric surgery who have weight loss and/or steatorrhea persisting despite PERT. When patients with CP have one or more of 5 variables associated with SIBO, particularly low zinc level or a combination of diabetes and opiate use (PPV=72.5%), it would be reasonable to offer treatment for SIBO rather than to investigate alternative causes of persistent steatorrhea or weight loss with PERT therapy.
Supplementary Material
STUDY HIGHLIGHTS.
- WHAT IS CURRENT KNOWLEDGE
- Small intestinal bacterial overgrowth (SIBO) is relatively common in patients with chronic pancreatitis (CP) but the exact prevalence is unclear
- Upper GI surgery associates with an increased prevalence of SIBO in CP
- Gastrointestinal symptoms are not predictive of SIBO in CP
- WHAT IS NEW HERE
- SIBO, diagnosed by glucose breath test (GBT), is present in 40.8% of patients with CP without upper GI surgery
- Five variables significantly associate with SIBO in patients with CP without upper GI surgery (total Mayo score, DM, low zinc level, low albumin and opiate use).
- Zinc level is an independent negative predictor of SIBO while DM has a nonsignificant trend towards a positive association (AUROC = 0.89).
- SIBO should be considered in patients with CP having a low zinc level or the combination of diabetes and opiate use (PPV=72.5%), particularly with persistent steatorrhea or weight loss despite PERT
Financial support:
AAL and MJD receive research support from the National Institutes of Health (KL2TR002241 to AAL and DK106647 to MJD).
Abbreviations:
- BMI
body mass index
- CI
confidence interval
- CP
chronic pancreatitis
- CT
computed tomography
- DM
diabetes mellitus
- EPI
exocrine pancreatic insufficiency
- ERCP
endoscopic retrograde cholangiopancreatography
- EUS
endoscopic ultrasonography
- GBT
glucose hydrogen breath test
- GI
gastrointestinal
- GPR
gastroparesis
- HBT
hydrogen breath test
- MRCP
magnetic resonance cholangiopancreatography
- OR
odds ratio
- PERT
pancreatic enzyme replacement therapy
- PPI
proton pump inhibitor
- SIBO
small intestinal bacterial overgrowth
- US
ultrasonograpy
- AUROC
area under the receiver operator characteristic
Footnotes
Potential competing interests: No authors declare competing interests (AAL, EJW, JRB, MJD, RS).
Potential competing interests: All authors disclose no conflict of interest. M.J.D received honoraria from the British Medical Journal (BMJ) Publishing Group Limited for writing/updating a monograph on Chronic Pancreatitis published in BMJ Point of Care (http://online.epocrates.com) and Best Practice (http://bestpractice.bmj.com); Oakstone Publishing, LLC for podcasts entitled “The Best of DDW, Pancreatic Disorders”; and American Gastroenterological Association Institute (AGAI) Council for coauthoring a Technical Review on The Initial Medical Management of Acute Pancreatitis. MJD also served as consultant for the Cystic Fibrosis Foundation Therapeutics (CFFT), Inc. (Bethesda, MD, USA).
References
- 1.Forsmark CE: Management of chronic pancreatitis. Gastroenterology 2013, 144:1282–1291e1283. [DOI] [PubMed] [Google Scholar]
- 2.Conwell DL, Lee LS, Yadav D, Longnecker DS, Miller FH, Mortele KJ, Levy MJ, Kwon R, Lieb JG, Stevens T, et al. : American pancreatic association practice guidelines in chronic pancreatitis: evidence-based report on diagnostic guidelines. Pancreas 2014, 43:1143–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DiMagno MJ, Wamsteker EJ, Lee A: Chronic Pancreatitis. In BMJ Point-of-Care 2018. Edited by. www.pointofcare.bmj.com (Last accessed November 17, 2017); 2017. [Google Scholar]
- 4.Mullady DK, Yadav D, Amann ST, O’Connell MR, Barmada MM, Elta GH, Scheiman JM, Wamsteker EJ, Chey WD, Korneffel ML, et al. : Type of pain, pain-associated complications, quality of life, disability and resource utilisation in chronic pancreatitis: a prospective cohort study. Gut 2011, 60:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wehler M, Nichterlein R, Fischer B, Farnbacher M, Reulbach U, Hahn EG, Schneider T: Factors associated with health-related quality of life in chronic pancreatitis. Am J Gastroenterol 2004, 99:138–146. [DOI] [PubMed] [Google Scholar]
- 6.Yadav D, Timmons L, Benson JT, Dierkhising RA, Chari ST: Incidence, prevalence, and survival of chronic pancreatitis: a population-based study. Am J Gastroenterol 2011, 106:2192–2199. [DOI] [PubMed] [Google Scholar]
- 7.Uc A, Andersen DK, Bellin MD, Bruce JI, Drewes AM, Engelhardt JF, Forsmark CE, Lerch MM, Lowe ME, Neuschwander-Tetri BA, et al. : Chronic Pancreatitis in the 21st Century - Research Challenges and Opportunities: Summary of a National Institute of Diabetes and Digestive and Kidney Diseases Workshop. Pancreas 2016, 45:1365–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DiMagno EP, DiMagno MJ: Chronic pancreatitis: landmark papers, management decisions, and future. Pancreas 2016, 45:641–650. [DOI] [PubMed] [Google Scholar]
- 9.Waljee AK, DiMagno MJ, Wu BU, Schoenfeld PS, Conwell DL: Systematic review: pancreatic enzyme treatment of malabsorption associated with chronic pancreatitis. Aliment Pharmacol Ther 2009, 29:235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shafiq N, Rana S, Bhasin D, Pandhi P, Srivastava P, Sehmby SS, Kumar R, Malhotra S: Pancreatic enzymes for chronic pancreatitis. Cochrane Database Syst Rev 2009:CD006302. [DOI] [PubMed] [Google Scholar]
- 11.Taylor JR, Gardner TB, Waljee AK, Dimagno MJ, Schoenfeld PS: Systematic review: efficacy and safety of pancreatic enzyme supplements for exocrine pancreatic insufficiency. Aliment Pharmacol Ther 2010, 31:57–72. [DOI] [PubMed] [Google Scholar]
- 12.de la Iglesia-Garcia D, Huang W, Szatmary P, Baston-Rey I, Gonzalez-Lopez J, Prada-Ramallal G, Mukherjee R, Nunes QM, Dominguez-Munoz JE, Sutton R, et al. : Efficacy of pancreatic enzyme replacement therapy in chronic pancreatitis: systematic review and meta-analysis. Gut 2017, 66:1354–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DiMagno MJ, Forsmark CE: Chronic pancreatitis and small intestinal bacterial overgrowth. Pancreatology 2018:In press. [DOI] [PubMed] [Google Scholar]
- 14.King CE, Toskes PP, Spivey JC, Lorenz E, Welkos S: Detection of small intestine bacterial overgrowth by means of a 14C-D-xylose breath test. Gastroenterology 1979, 77:75–82. [PubMed] [Google Scholar]
- 15.Corazza GR, Menozzi MG, Strocchi A, Rasciti L, Vaira D, Lecchini R, Avanzini P, Chezzi C, Gasbarrini G: The diagnosis of small bowel bacterial overgrowth. Reliability of jejunal culture and inadequacy of breath hydrogen testing. Gastroenterology 1990, 98:302–309. [DOI] [PubMed] [Google Scholar]
- 16.Singh VV, Toskes PP: Small Bowel Bacterial Overgrowth: Presentation, Diagnosis, and Treatment. Curr Treat Options Gastroenterol 2004, 7:19–28. [DOI] [PubMed] [Google Scholar]
- 17.Shin HS, Ingram JR, McGill AT, Poppitt SD: Lipids, CHOs, proteins: can all macronutrients put a ‘brake’ on eating? Physiol Behav 2013, 120:114–123. [DOI] [PubMed] [Google Scholar]
- 18.Spiller RC, Trotman IF, Higgins BE, Ghatei MA, Grimble GK, Lee YC, Bloom SR, Misiewicz JJ, Silk DB: The ileal brake--inhibition of jejunal motility after ileal fat perfusion in man. Gut 1984, 25:365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubinstein E, Mark Z, Haspel J, Ben-Ari G, Dreznik Z, Mirelman D, Tadmor A: Antibacterial activity of the pancreatic fluid. Gastroenterology 1985, 88:927–932. [DOI] [PubMed] [Google Scholar]
- 20.Marotta F, Tajiri H, Li ZL, Barreto R, Bellini O, Barbi G: Pure pancreatic juice from patients with chronic pancreatitis has an impaired antibacterial activity. Int J Pancreatol 1997, 22:215–220. [DOI] [PubMed] [Google Scholar]
- 21.Dukowicz AC, Lacy BE, Levine GM: Small intestinal bacterial overgrowth: a comprehensive review. Gastroenterol Hepatol (N Y) 2007, 3:112–122. [PMC free article] [PubMed] [Google Scholar]
- 22.Ishikawa C, Tanabe H, Maemoto A, Ito T, Watari J, Kono T, Fujiya M, Ashida T, Ayabe T, Kohgo Y: Precursor processing of human defensin-5 is essential to the multiple functions in vitro and in vivo. J Innate Immun 2010, 2:66–76. [DOI] [PubMed] [Google Scholar]
- 23.Ghosh D, Porter E, Shen B, Lee SK, Wilk D, Drazba J, Yadav SP, Crabb JW, Ganz T, Bevins CL: Paneth cell trypsin is the processing enzyme for human defensin-5. Nat Immunol 2002, 3:583–590. [DOI] [PubMed] [Google Scholar]
- 24.Gubergrits NB, Linevskiy YV, Lukashevich GM, Fomenko PG, Moroz TV, Mishra T: Morphological and functional alterations of small intestine in chronic pancreatitis. JOP : Journal of the pancreas 2012, 13:519–528. [DOI] [PubMed] [Google Scholar]
- 25.Bang Jorgensen B, Thorsgaard Pedersen N, Worning H: Short report: lipid and vitamin B12 malassimilation in pancreatic insufficiency. Aliment Pharmacol Ther 1991, 5:207–210. [DOI] [PubMed] [Google Scholar]
- 26.Pongprasobchai S, DiMagno EP: Are small intestinal bacterial overgrowth and pancreatic diseases associated? Pancreatology 2002, 2:351:abstract [Google Scholar]
- 27.Saad RJ, Chey WD: Breath testing for small intestinal bacterial overgrowth: maximizing test accuracy. Clin Gastroenterol Hepatol 2014, 12:1964–1972; quiz e1119–1920. [DOI] [PubMed] [Google Scholar]
- 28.Hamilton I, Worsley BW, Cobden I, Cooke EM, Shoesmith JG, Axon AT: Simultaneous culture of saliva and jejunal aspirate in the investigation of small bowel bacterial overgrowth. Gut 1982, 23:847–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lembcke B, Kraus B, Lankisch PG: Small intestinal function in chronic relapsing pancreatitis. Hepatogastroenterology 1985, 32:149–151. [PubMed] [Google Scholar]
- 30.Casellas F, Guarner L, Vaquero E, Antolin M, de Gracia X, Malagelada JR: Hydrogen breath test with glucose in exocrine pancreatic insufficiency. Pancreas 1998, 16:481–486. [DOI] [PubMed] [Google Scholar]
- 31.Trespi E, Ferrieri A: Intestinal bacterial overgrowth during chronic pancreatitis. Curr Med Res Opin 1999, 15:47–52. [DOI] [PubMed] [Google Scholar]
- 32.Madsen JL, Graff J, Philipsen EK, Scharff O, Rumessen JJ: Bile acid malabsorption or disturbed intestinal permeability in patients treated with enzyme substitution for exocrine pancreatic insufficiency is not caused by bacterial overgrowth. Pancreas 2003, 26:130–133. [DOI] [PubMed] [Google Scholar]
- 33.Mancilla AC, Madrid SA, Hurtado HC, Orellana BC, Pena ZM, Tobar AE, Berger FZ: [Small intestine bacterial overgrowth in patients with chronic pancreatitis]. Rev Med Chil 2008, 136:976–980. [PubMed] [Google Scholar]
- 34.Kumar K, Ghoshal UC, Srivastava D, Misra A, Mohindra S: Small intestinal bacterial overgrowth is common both among patients with alcoholic and idiopathic chronic pancreatitis. Pancreatology 2014, 14:280–283. [DOI] [PubMed] [Google Scholar]
- 35.Signoretti M, Stigliano S, Valente R, Piciucchi M, Delle Fave G, Capurso G: Small intestinal bacterial overgrowth in patients with chronic pancreatitis. J Clin Gastroenterol 2014, 48 Suppl 1:S52–55. [DOI] [PubMed] [Google Scholar]
- 36.Kim DB, Paik CN, Sung HJ, Chung WC, Lee KM, Yang JM, Choi MG: Breath hydrogen and methane are associated with intestinal symptoms in patients with chronic pancreatitis. Pancreatology 2015, 15:514–518. [DOI] [PubMed] [Google Scholar]
- 37.Therrien A, Bouchard S, Sidani S, Bouin M: Prevalence of Small Intestinal Bacterial Overgrowth among Chronic Pancreatitis Patients: A Case-Control Study. Can J Gastroenterol Hepatol 2016, 2016:7424831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ni Chonchubhair HM, Bashir Y, Dobson M, Ryan BM, Duggan SN, Conlon KC: The prevalence of small intestinal bacterial overgrowth in non-surgical patients with chronic pancreatitis and pancreatic exocrine insufficiency (PEI). Pancreatology 2018. [DOI] [PubMed] [Google Scholar]
- 39.Grigor’eva Iu V, Iakovenko EP, Volosheinikova TV, Ovsiannikova IA, Lavrent’eva SA: [The clinical manifestations and duodenal mucosa in the patients with chronic pancreatitis and bacterial overgrowth in the small intestine]. Eksp Klin Gastroenterol 2010:29–34. [PubMed] [Google Scholar]
- 40.Gasbarrini A, Corazza GR, Gasbarrini G, Montalto M, Di Stefano M, Basilisco G, Parodi A, Usai-Satta P, Vernia P, Anania C, et al. : Methodology and indications of H2-breath testing in gastrointestinal diseases: the Rome Consensus Conference. Aliment Pharmacol Ther 2009, 29 Suppl 1:1–49. [DOI] [PubMed] [Google Scholar]
- 41.Vanner S: The lactulose breath test for diagnosing SIBO in IBS patients: another nail in the coffin. Am J Gastroenterol 2008, 103:964–965. [DOI] [PubMed] [Google Scholar]
- 42.Capurso G, Signoretti M, Archibugi L, Stigliano S, Delle Fave G: Systematic review and meta-analysis: Small intestinal bacterial overgrowth in chronic pancreatitis. United European Gastroenterol J 2016, 4:697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rezaie A, Buresi M, Lembo A, Lin H, McCallum R, Rao S, Schmulson M, Valdovinos M, Zakko S, Pimentel M: Hydrogen and Methane-Based Breath Testing in Gastrointestinal Disorders: The North American Consensus. Am J Gastroenterol 2017, 112:775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rana SV, Sinha SK, Sharma S, Kaur H, Bhasin DK, Singh K: Effect of predominant methanogenic flora on outcome of lactose hydrogen breath test in controls and irritable bowel syndrome patients of north India. Dig Dis Sci 2009, 54:1550–1554. [DOI] [PubMed] [Google Scholar]
- 45.Reddy NG, Nangia S, DiMagno MJ: The Chronic Pancreatitis International Classification of Diseases, Ninth Revision, Clinical Modification Code 577.1 Is Inaccurate Compared With Criterion-Standard Clinical Diagnostic Scoring Systems. Pancreas 2016, 45:1276–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Layer P, Yamamoto H, Kalthoff L, Clain JE, Bakken LJ, DiMagno EP: The different courses of early- and late-onset idiopathic and alcoholic chronic pancreatitis. Gastroenterology 1994, 107:1481–1487. [DOI] [PubMed] [Google Scholar]
- 47.Hart PA, Bellin MD, Andersen DK, Bradley D, Cruz-Monserrate Z, Forsmark CE, Goodarzi MO, Habtezion A, Korc M, Kudva YC, et al. : Type 3c (pancreatogenic) diabetes mellitus secondary to chronic pancreatitis and pancreatic cancer. Lancet Gastroenterol Hepatol 2016, 1:226–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boosalis MG, Evans GW, McClain CJ: Impaired handling of orally administered zinc in pancreatic insufficiency. Am J Clin Nutr 1983, 37:268–271. [DOI] [PubMed] [Google Scholar]
- 49.Dutta SK, Procaccino F, Aamodt R: Zinc metabolism in patients with exocrine pancreatic insufficiency. J Am Coll Nutr 1998, 17:556–563. [DOI] [PubMed] [Google Scholar]
- 50.Yu HH, Yang TM, Shan YS, Lin PW: Zinc deficiency in patients undergoing pancreatoduodenectomy for periampullary tumors is associated with pancreatic exocrine insufficiency. World journal of surgery 2011, 35:2110–2117. [DOI] [PubMed] [Google Scholar]
- 51.Lakhani SV, Shah HN, Alexander K, Finelli FC, Kirkpatrick JR, Koch TR: Small intestinal bacterial overgrowth and thiamine deficiency after Roux-en-Y gastric bypass surgery in obese patients. Nutr Res 2008, 28:293–298. [DOI] [PubMed] [Google Scholar]
- 52.Virally-Monod M, Tielmans D, Kevorkian JP, Bouhnik Y, Flourie B, Porokhov B, Ajzenberg C, Warnet A, Guillausseau PJ: Chronic diarrhoea and diabetes mellitus: prevalence of small intestinal bacterial overgrowth. Diabetes Metab 1998, 24:530–536. [PubMed] [Google Scholar]
- 53.Zietz B, Lock G, Straub RH, Braun B, Scholmerich J, Palitzsch KD: Small-bowel bacterial overgrowth in diabetic subjects is associated with cardiovascular autonomic neuropathy. Diabetes Care 2000, 23:1200–1201. [DOI] [PubMed] [Google Scholar]
- 54.Rezaie A, Pimentel M, Rao SS: How to Test and Treat Small Intestinal Bacterial Overgrowth: an Evidence-Based Approach. Curr Gastroenterol Rep 2016, 18:8. [DOI] [PubMed] [Google Scholar]
- 55.Baker J, Saad W: Common gastrointestinal symptoms do not predict the results of glucose breath testing in the evaluation of suspected small intestinal bacterial overgrowth. Am J Gastroenterol 2015, 110:S1004:abstract. [Google Scholar]
- 56.Simpson KW, Batt RM, Jones D, Morton DB: Effects of exocrine pancreatic insufficiency and replacement therapy on the bacterial flora of the duodenum in dogs. Am J Vet Res 1990, 51:203–206. [PubMed] [Google Scholar]
- 57.Tignor AS, Wu BU, Whitlock TL, Lopez R, Repas K, Banks PA, Conwell D: High prevalence of low-trauma fracture in chronic pancreatitis. Am J Gastroenterol 2010, 105:2680–2686. [DOI] [PubMed] [Google Scholar]
- 58.Saltzman JR, Kowdley KV, Pedrosa MC, Sepe T, Golner B, Perrone G, Russell RM: Bacterial overgrowth without clinical malabsorption in elderly hypochlorhydric subjects. Gastroenterology 1994, 106:615–623. [DOI] [PubMed] [Google Scholar]
- 59.Fried M, Siegrist H, Frei R, Froehlich F, Duroux P, Thorens J, Blum A, Bille J, Gonvers JJ, Gyr K: Duodenal bacterial overgrowth during treatment in outpatients with omeprazole. Gut 1994, 35:23–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.