Abstract
Background
Sickle cell anemia may be associated with cognitive dysfunction, and some complications of sickle cell anemia might affect those with sickle cell trait (SCT), so we hypothesized that SCT is a risk factor for cognitive impairment.
Methods
The Reasons for Geographic and Racial Differences in Stroke (REGARDS) study enrolled a national cohort of 30,239 white and black Americans from 2003 to 7, who are followed every 6 months. Baseline and annual global cognitive function testing used the Six-Item Screener (SIS), a validated instrument (scores range 0–6; ≤ 4 indicates cognitive impairment). Participants with baseline cognitive impairment and whites were excluded. Logistic regression was used to calculate the association of SCT with incident cognitive impairment, adjusted for risk factors. Linear mixed models assessed multivariable-adjusted change in test scores on a biennially administered 3-test battery measuring learning, memory, and semantic and phonemic fluency.
Findings
Among 7743 participants followed for a median of 7·1 years, 85 of 583 participants with SCT (14·6%) developed incident cognitive impairment compared to 902 of 7160 (12·6%) without SCT. In univariate analysis, the odds ratio (OR) of incident cognitive impairment was 1·18 (95% CI: 0·93, 1·51) for those with SCT vs. those without. Adjustment did not impact the OR. There was no difference in change on 3-test battery scores by SCT status (all p > 0·11).
Interpretation
In this prospective cohort study of black Americans, SCT was not associated with incident cognitive impairment or decline in test scores of learning, memory and executive function.
Funding
National Institutes of Health, American Society of Hematology.
Keywords: Sickle cell trait, Cognitive dysfunction, Cognition, Prospective studies, Risk factors, Epidemiology
Research in context
Evidence before this study
Patients with sickle cell disease can develop impaired cognitive function. We are not aware of prior evidence on the association of sickle cell trait, the carrier state of sickle cell disease, with risk of cognitive impairment in older adults, the group at greatest risk of cognitive impairment.
Added value of this study
Findings showed no relationship of sickle cell trait with adverse changes in several measures of cognitive function over 7·1 years follow up among 7743 African-Americans age 45 and over. This new data is reassuring that unlike some disorders attributed to sickle cell trait, including kidney disease and venous thrombosis, cognitive decline is not.
Implications of the available evidence
It is not likely that patients aged 45 and older with sickle cell trait develop pathological changes in the brain that lead to cognitive impairment.
Alt-text: Unlabelled Box
1. Introduction
Vascular risk factors are also risk factors for cognitive decline and impairment, mediated partly by small, subclinical strokes [1], [2], [3], [4]. Patients with sickle cell anemia (SCA) may develop impaired cognitive function. Specifically, children [5] and adults [6] with SCA score lower on cognitive test scores than controls and children with SCA are at risk for lower academic attainment [7]. Silent cerebral infarction is common in children and adults with SCA [8], and white matter hyperintensities, a measure of silent cerebral infarction, correlate with poorer neurocognitive outcomes in these children [9]. SCA has also been linked with cognitive processing speed independent of silent infarcts, but related to MRI-defined white matter integrity [10]. In a study of adults with SCA cortical and subcortical brain volumes were lower than controls, and these lower volumes correlated with lower cognitive performance [11].
Taken together, these findings suggest a hypothesis that SCA is a risk factor for cognitive impairment via multiple potential mechanisms such as sickling in small vessels causing occlusion, subclinical or clinical stroke, or other metabolic abnormalities like increased inflammation [12], coagulation activation [13] or hypoxemia [14], [15]. Carriers of SCA, i.e. those with sickle cell trait (SCT), have increased risk of kidney disease [16], [17], venous thromboembolism [18], [19] and perhaps stroke [20], [21], [22], [23]. Proposed mechanisms for these associations include sickling in small vessels under conditions of hypoxia, and inflammation, hypercoagulability or other biochemical effects of subclinical sickling [13], [24], [25].
We hypothesized that, given the known vascular contribution to cognitive dysfunction (including small vessel disease of the brain), associations of SCA with cognitive impairment and structural brain abnormalities, and the emerging evidence of associations of SCT with other types of organ dysfunction, that adults aged 45 years and older with SCT and normal cognitive function will be more likely to develop cognitive impairment over several years follow-up than similar SCT-free individuals. Four longitudinally-administered cognitive performance tests were evaluated.
2. Methods
2.1. Study participants and data collection
The REGARDS study is a longitudinal observational study investigating racial and geographic variation in incidence of stroke and acquired cognitive impairment in the contiguous United States. Details of the study design were published elsewhere [26]. Cohort participants were randomly selected by mail and enrolled by telephone followed by an in-home visit between 2003 and 7. The aim was to enroll 50% non-Hispanic black and 50% non-Hispanic white participants aged 45 and older, with 50% residing in the southeast. Exclusion criteria included individuals who indicated race other than black or white, cognitive impairment precluding ability to complete a telephone interview, active cancer within 1 year or undergoing treatment for cancer, a medical condition preventing long term follow-up, residing in or waiting for nursing home residence, or inability to communicate in English [26]. Enrollment results yielded a cohort with 51% female and 42% black participants [26].
Baseline participant characteristics were obtained via a computer-assisted telephone interview followed by in-home examination using a standard protocol that included phlebotomy and shipment of blood and urine samples to a central laboratory for storage and measurement of glucose, lipid profile and kidney function [27]. SCT was determined via genotyping using a TaqMan SNP Genotyping Assay (Applied Biosystems/ThermoFisher Scientific) [16]. Among a subset of participants with available genomic data, ten principal components of ancestry were determined using EIGENSTRAT to control for population stratification [17], [28].
The study methods were approved by the institutional review boards at all participating institutions and all participants provided informed consent. Boards included the University of Alabama at Birmingham Institutional Review Board for Human Use, the University of Vermont Research Protections Office, the University of Cincinnati Human Research Protection Program, the Wake Forest University Institutional Review Board, and the Columbia University Human Research Protection Office,
2.2. Covariate measurements
Race was determined by participant self-report as black or white. Age, sex, education, household income level, and region of residence were determined by self-report. Education was categorized as less than high school, high school graduate, some college, or college graduate and above. Income was categorized as $20,000/yr, $20,000 to $34,999/yr, $35,000 to $74,999/yr, or >$75,000/yr. Current cigarette smoking, alcohol use, exercise frequency, and use of medications were determined by interview. Hypertension was defined as baseline systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg or self-reported use of antihypertensive medications. Hyperlipidaemia was defined as low-density lipoprotein > 130 mg/dl, or self-reported use of a cholesterol-lowering medication. Diabetes was defined by a fasting glucose > 126 mg/dl, nonfasting glucose > 200 mg/dl, or self-reported use of antidiabetes medications. Coronary heart disease was determined by self-reported myocardial infarction (MI), coronary artery bypass graft, angioplasty or stenting, or evidence of MI from baseline electrocardiogram. Atrial fibrillation was defined as self-reported or via electrocardiogram evidence. Left ventricular hypertrophy (LVH) was defined by electrocardiogram. Estimated glomerular filtration rate (eGFR) was calculated from serum creatinine using the Chronic Kidney Disease Epidemiology Collaboration equation [29].
2.3. Cognitive function assessments
Cognitive outcomes were studied in all participants in two ways, considered here as co-primary outcomes: incident cognitive impairment on a test of global cognitive function and longitudinal change of cognitive domain test scores reflecting learning, memory and executive function.
The study conducted global cognitive function testing using the Six-item Screener (SIS), a validated telephone-administered instrument for global cognitive function that assesses 3-item recall and orientation to year, month, and day of the week, yielding a score from 0 to 6 correctly answered questions [30], [31]. A score ≤ 4 is considered positive for cognitive impairment. The SIS was administered at baseline and then annually to all participants. The outcome of incident cognitive impairment was defined at the most recent assessment as of April 1, 2015, when our analysis data set was closed.
Participants also completed a 3-test battery every two years that evaluated measures of learning, memory and executive function [27]. Validated instruments were telephone-administered, including the Consortium to Establish a Registry for Alzheimer's Disease (CERAD) battery to assess word list learning (WLL), delayed recall by word list recall (WLR), semantic fluency by the Animal Fluency Test (AFT) score, and phonemic fluency by the Letter F test [32], [33], [34]. WLL is the sum of words learned over 3 trials, where participants are tested on immediate recall of 10 words, with scores ranging from 0 to 30. WLR is the sum of the same words recalled after a delay filled with intervening questions, with a range of 0–10 words. The AFT score is determined by the number of animals a participant can name within 1-min. The Letter F score is determined by the number of words beginning with the letter F that a participant can say within 1-min.
2.4. Inclusion/Exclusion criteria for analysis
We included black participants who had available data on baseline and at least one follow up SIS, and with SCT genotyping. Participants with hemoglobin SS or SC genotype, baseline cognitive impairment (SIS ≤ 4), or who developed incident stroke were excluded from all analyses.
2.5. Statistical analysis
Differences in baseline characteristics by SCT and incident cognitive impairment on the SIS were analyzed by t-tests for continuous measures and chi-square tests for categorical measures. For incident cognitive impairment based on the SIS, we used logistic regression to calculate odds ratios (OR) with 95% confidence intervals (CI) by SCT status. Statistical significance was defined as p ≤ 0·05. Linear mixed models were used to study association of SCT with repeated measures of WLL, WLR and semantic and verbal fluency over time. Similar to prior REGARDS reports, no random effects accounting for time between tests were included [35]. For both types of analysis, multivariable models were fitted to adjust for age, sex, education, income, region of residence, eGFR, systolic blood pressure, alcohol use, smoking status, exercise frequency, coronary heart disease, LVH, atrial fibrillation, hyperlipidaemia, statin use, diabetes, and hypertension. Models were repeated following removal of variables that were not significantly associated with the cognitive outcome. Censoring occurred at death or withdrawal from the study.
The first sensitivity analysis addressed whether observed associations might be explained by other genetic factors associated with African ancestry that are in linkage with SCT. Specifically, this was done on the subset with available genetic ancestry information by adding adjustment for African ancestry using the first ten principal components of ancestry. A second sensitivity analysis for the association of cognitive impairment by the SIS only, evaluated the cross-sectional association of SCT with a SIS ≤ 4 compared to 5 or 6 using analogous regression models to those for incident cognitive impairment in 9549 participants. This analysis involved baseline SIS values, so included participants whose baseline impairment or lack of follow up assessments excluded them from the analysis of of incident impairment.
Interactions between SCT and age (continuous variable), sex, and diabetes status were tested for in all analyses. Analysis was performed with SAS 9.4.
2.6. Role of the funding source
The funding source had no role in the study design, collection, analysis and interpretation of data, authorship or decision to submit for publication.
2.7. Data statement
There are restrictions to the open publication of a REGARDS dataset but data may be requested per study policies at www.regardsstudy.org.
3. Results
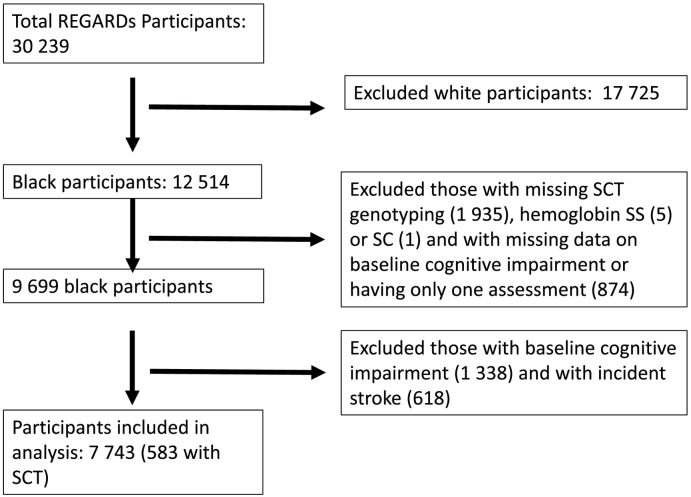
Fig. 1 shows a flow diagram of participant inclusion in the analysis of incident cognitive impairment by the SIS. Baseline characteristics by SCT status are shown in Table 1. Among 7743 participants, 583 had SCT and 4898 were women. Participants with SCT had a lower eGFR compared to those without SCT (86 vs. 90 ml/min/1·73 m2, p < 0·0001). The only other covariates to differ significantly by SCT were alcohol use and smoking status.
Fig. 1.
Flow diagram of participant inclusion.
Table 1.
Baseline characteristics by sickle cell trait status.
| Covariate (N missing) | All participants (N = 7743) | SCT (N = 583) |
No SCT (N = 7160) | p-Value |
|---|---|---|---|---|
| Age, years (0) | 63·1 (8·9) | 62·9 (9·0) | 63·1 (8·9) | 0·48 |
| eGFR, ml/min/1·73 m2 (48) | 90 (22) | 86 (24) | 90 (22) | < 0·0001 |
| Systolic Blood Pressure, mm Hg (21) | 130 (17) | 131 (17) | 130 (17) | 0·33 |
| Log(ACR), mg/g (282) | 2·4 (1·) | 2·7 (1·4) | 2·4 (1·3) | < 0·0001 |
| Sex (0) | ||||
| Female | 4898 (63·0%) | 388 (66·6%) | 4510 (63·0%) | 0·09 |
| Male | 2845 (37·0%) | 195 (33·4%) | 2650 (37·0%) | |
| Education (5) | ||||
| < High School | 1225 (15·8%) | 90 (15·4%) | 1135 (15·9%) | 0·98 |
| High School graduate | 2110 (27·3%) | 158 (27·3%) | 1952 (27·3%) | |
| Some College | 2165 (28·0%) | 167 (28·6%) | 1998 (27·9%) | |
| College graduate | 2238 (28·9%) | 168 (28·8%) | 2070 (28·9%) | |
| Income (0) | ||||
| <$20 k | 1814 (23·4%) | 153 (26·2%) | 1661 (23·2%) | 0·26 |
| $20 k-$34 k | 2018 (26·1%) | 145 (24·9%) | 1873 (26·2%) | |
| $35 k-$74 k | 2213 (28·6%) | 150 (25·7%) | 2063 (28·8%) | |
| >$75 k | 833 (10·8%) | 62 (10·6%) | 771 (10·8%) | |
| Refused | 865 (11·2%) | 73 (12·5%) | 792 (11·1%) | |
| Region (0) | ||||
| Belt | 2565 (33·1%) | 198 (34·0%) | 2367 (33·1%) | 0·58 |
| Buckle | 1359 (17·6%) | 109 (18·7%) | 1250 (17·5%) | |
| NonBelt | 3819 (49·3%) | 276 (47·3%) | 3543 (49·5%) | |
| Alcohol use (199) | ||||
| Heavy | 189 (2·5%) | 23 (4·0%) | 166 (2·4%) | 0·01 |
| Moderate | 2047 (27·1%) | 133 (23·3%) | 1914 (27·4%) | |
| None | 5308 (70·4%) | 414 (72·6%) | 4894 (70·2) | |
| Smoking status (36) | ||||
| Current | 1283 (16·6%) | 91 (15·7%) | 1192 (16·7%) | 0·03 |
| Past | 2833 (36·8%) | 189 (32·5%) | 2644 (37·1%) | |
| Never | 3591 (46·6%) | 301 (51·8%) | 3290 (46·2%) | |
| Exercise (93) | ||||
| 1–3 Times/Week | 2934 (38·4%) | 221 (38·2%) | 2713 (38·4%) | 0·81 |
| 4 + Times/Week | 2018 (26·4%) | 159 (27·5%) | 1859 (26·3%) | |
| None | 2698 (35·3%) | 199 (34·4%) | 2499 (35·3%) | |
| Coronary heart disease (135) | ||||
| No | 6616 (87·0%) | 495 (86·7%) | 6121 (87·0%) | 0·84 |
| Yes | 992 (13·0%) | 76 (13·3%) | 916 (13·0%) | |
| Left ventricular hypertrophy (131) | ||||
| No | 6582 (86·3%) | 484 (84·6%) | 6098 (86·5%) | 0·22 |
| Yes | 1, 044 (13·7%) | 88 (15·4%) | 956 (13·5%) | |
| Diabetes (78) | ||||
| No | 5563 (72·6%) | 409 (71·1%) | 5154 (72·7%) | 0·42 |
| Yes | 2102 (27·4%) | 166 (28·9%) | 1936 (27·3%) | |
| Atrial fibrillation (192) | ||||
| No | 7001 (92·7%) | 518 (91·4%) | 6483 (92·8%) | 0·20 |
| Yes | 550 (7·3%) | 49 (8·6%) | 501 (7·2%) | |
| Hyperlipidaemia (73) | ||||
| No | 5366 (70·0%) | 398 (68·6%) | 4968 (70·1) | 0·46 |
| Yes | 2304 (30·0%) | 182 (31·4%) | 2122 (29·9%) | |
| Statin Use (25) | ||||
| No | 5576 (72·2%) | 411 (70·5%) | 5165 (72·4%) | 0·33 |
| Yes | 2142 (27·8%) | 172 (29·5%) | 1970 (27·6%) | |
| Hypertension (289) | ||||
| No | 2700 (36%) | 214 (38·7%) | 2486 (36·0%) | 0·21 |
| Yes | 4754 (64%) | 339 (61·3%) | 4415 (64·0%) |
With median follow up of 7·1 years (range 0.4, 10.3 years), among the 583 participants with SCT, 85 (14·6%) experienced incident cognitive impairment, compared to 902 (11·7%) of those without SCT. Table 2 shows baseline characteristics by incident impairment on the SIS. Many baseline covariates had adverse levels in those with incident impairment, including age, eGFR, systolic blood pressure, hypertension, coronary heart disease, LVH, hyperlipidaemia and statin use. Those with incident impairment also had lower education and income at baseline, and were more likely to be men, than those without incident impairment.
Table 2.
Baseline characteristics by incident cognitive impairment on the six-item screener.
| Covariate (N missing) | All participants (N = 7743) | Incident impairment (N = 987) |
No incident impairment (N = 6756) |
|---|---|---|---|
| Age, years (0) | 63·1 (8·9) | 68·6 (9·2) | 62·3 (8·6) |
| eGFR, ml/min/1·73 m2 (48) | 90 (22) | 82 (23) | 91 (22) |
| Systolic Blood Pressure, mm Hg (21) | 130 (17) | 133 (18) | 130 (17) |
| Log(ACR), mg/g (282) | 2·40 (1·3) | 2·64 (1·5) | 2·34 (1·3) |
| Sex (0) | |||
| Female | 4898 (63·0%) | 541 (54·8%) | 4357 (64·5%) |
| Male | 2845 (37·0%) | 446 (45·2%) | 2399 (35·5%) |
| Education (5) | |||
| < High School | 1225 (15·8%) | 271 (27·5%) | 954 (14·1%) |
| High School graduate | 2110 (27·3%) | 288 (29·2%) | 1822 (27·0) |
| Some College | 2165 (28·0%) | 236 (24·0%) | 1929 (28·6%) |
| College graduate | 2238 (28·9%) | 190 (19·3%) | 2048 (30·3%) |
| Income (0) | |||
| <$20 k | 1814 (23·4%) | 335 (33·9%) | 1479 (21·9) |
| $20 k-$34 k | 2018 (26·1%) | 283 (28·7%) | 1735 (25·7%) |
| $35 k-$74 k | 2213 (28·6%) | 179 (18·1%) | 2034 (30·1%) |
| >$75 k | 833 (10·8%) | 49 (5·0%) | 784 (11·6%) |
| Refused | 865 (11·2%) | 141 (14·3%) | 724 (10·7%) |
| Region (0) | |||
| Belt | 2565 (33·1%) | 323 (32·7%) | 2242 (33·2%) |
| Buckle | 1359 (17·6%) | 163 (16·5%) | 1196 (17·7%) |
| NonBelt | 3819 (49·3%) | 501 (50·8%) | 3318 (49·1%) |
| Alcohol use group (199) | |||
| Heavy | 189 (2·5%) | 26 (2·7%) | 163 (2·5%) |
| Moderate | 2047 (27·1%) | 213 (22·2%) | 1834 (27·9%) |
| None | 5308 (70·4%) | 721 (75·1%) | 4587 (69·7%) |
| Smoking status (36) | |||
| Current | 1283 (16·6%) | 157 (16·0%) | 1126 (16·7%) |
| Past | 2833 (36·8%) | 370 (37·7%) | 2463 (36·6%) |
| Never | 3591 (46·6%) | 455 (46·3%) | 3136 (46·6%) |
| Exercise (93) | |||
| 1–3 Times/Week | 2934 (38·4%) | 346 (35·6%) | 2588 (38·7%) |
| 4 + Times/Week | 2018 (26·4%) | 259 (26·7%) | 1759 (26·3%) |
| None | 2698 (35·3%) | 367 (37·8%) | 2331 (34·9%) |
| Coronary heart disease (135) | |||
| No | 6616 (87·0%) | 785 (80·8%) | 5831 (87·9%) |
| Yes | 992 (13·0%) | 187 (19·2%) | 805 (12·1%) |
| Left ventricular hypertrophy (131) | |||
| No | 6582 (86·3%) | 810 (83·3%) | 5772 (86·8%) |
| Yes | 1, 044 (13·7%) | 163 (16·8%) | 881 (13·2%) |
| Diabetes (78) | |||
| No | 5563 (72·6%) | 661 (67·4%) | 4902 (73·3%) |
| Yes | 2102 (27·4%) | 320 (32·6%) | 1782 (26·7%) |
| Atrial fibrillation (192) | |||
| No | 7001 (92·7%) | 885 (92·4%) | 6116 (92·8%) |
| Yes | 550 (7·3%) | 73 (7·6%) | 477 (7·2%) |
| Hyperlipidaemia (73) | |||
| No | 5366 (70·0%) | 632 (64·6%) | 4734 (70·7%) |
| Yes | 2304 (30·0%) | 346 (35·4%) | 1958 (29·3) |
| Statin (25) | |||
| No | 5576 (72·2%) | 677 (68·7%) | 4899 (72·8%) |
| Yes | 2142 (27·8%) | 308 (31·3%) | 1834 (27·2%) |
| Hypertension (289) | |||
| No | 2700 (36%) | 306 (32·7%) | 2394 (36·7%) |
| Yes | 4754 (64%) | 629 (67·3%) | 4125 (63·3) |
In the univariate analysis, the OR of incident cognitive impairment by the SIS for those with versus without SCT was 1·18 (CI: 0·93, 1·51). Controlling for covariates, results were similar (OR 1·21; 95% CI: 0·92, 1·60). Removal of covariates with p > 0·05 did not change interpretation of the findings (OR 1·24 (95% CI 0·94, 1·64)). With additional adjustment for principal components of ancestry (available in 5638 participants) the adjusted OR of incident impairment was 1·23 (95% CI: 0·88, 1·71). In the cross-sectional sensitivity analysis (done due to the null findings) the OR of baseline cognitive impairment with SCT was 0·99 (95% CI: 0·79, 1·25) in an unadjusted model and 1·13 (95% CI: 0·87, 1·46) after adjustment. Interaction p-values for age, sex and diabetes status in all models were > 0·10.
The baseline characteristics of participants assessed for trajectories of WLL and WLR, AFT, and the letter F test are shown in e-Tables 1–3. There were 7078 participants who had at least 1 assessment of WLL and WLR, of whom 517 had SCT. Those with SCT were more likely than those without to have lower eGFR (86 vs 90 ml/min/1·73 m2; p < 0·0001), hypertension (64% vs 60%; p = 0·04), and no alcohol use (75% vs 70%, p = 0·03) and were similar in other characteristics. There were 7296 (549 with SCT) participants included in the AFT analysis and 5946 for Letter F (446 with SCT). The smaller numbers for some outcomes were due to lags in results availability due to timing of results scoring. Fig. 2, Fig. 3 show that, both without and with multivariable adjustment, there were no differences in the longitudinal change in scores on any of these tests by SCT status. Fig. 2 indicates that WLL and WLR remained relatively stable over time and did not show a difference in decline by SCT status. Fig. 3 shows that AFT and Letter F performance worsened over time similarly in those with and without SCT. For these cognitive domain score outcomes, there were no interactions of SCT status and age, sex or diabetes status (all p > 0·26).
Fig. 2.
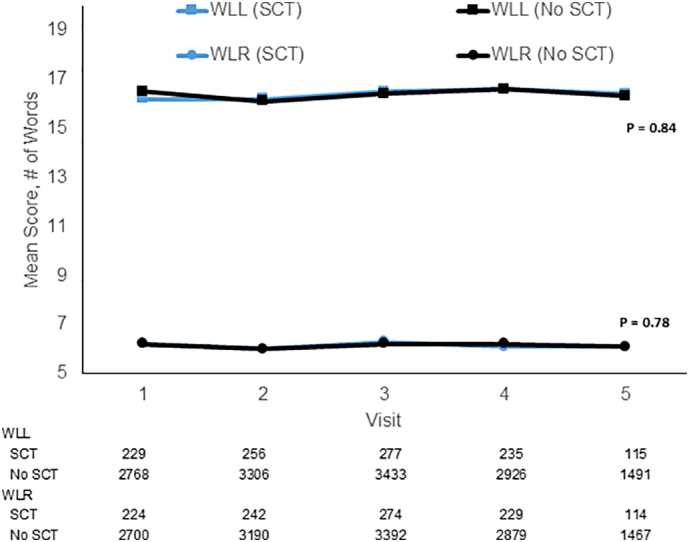
Sickle cell trait and longitudinal change in Word List Learning (WLL) and Delayed Recall (WLR).
Blue lines represent those with sickle cell trait and black lines those without sickle cell trait. Models were adjusted for age, sex, education, income, region, eGFR, systolic blood pressure, diabetes, alcohol use, smoking status, exercise frequency, coronary heart disease, left ventricular hypertrophy, atrial fibrillation, hyperlipidaemia, statin use, and hypertension. Note, y axis starts with a score of 5 words for ease of displaying the data. Numbers below the figure show the sample sizes.
Baseline SD of Scores: WLL (SCT) = 5.0; WLL (No SCT) = 5.0; WLR (SCT) = 2.3; WLR (No SCT) = 2.2. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 3.
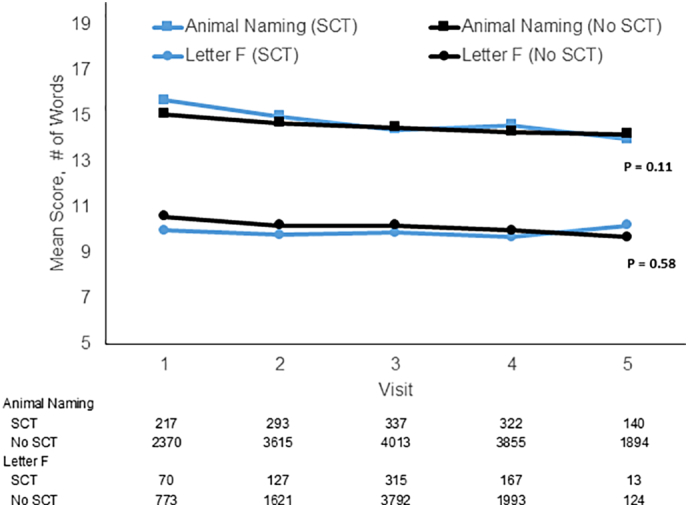
Sickle cell trait and longitudinal change in semantic fluency (Animal Fluency Test) and phonemic fluency (Letter F Test).
Blue lines represent those with sickle cell trait and black lines those without sickle cell trait. Models were adjusted for age, sex, education, income, region, eGFR, systolic blood pressure, diabetes, alcohol use, smoking status, exercise, coronary heart disease, left ventricular hypertrophy, atrial fibrillation, hyperlipidaemia, statin use, and hypertension. Note, y axis starts with a score of 5 words for ease of displaying the data. Numbers below the figure show the sample sizes.
Baseline SD of Scores: Animal Fluency Test (SCT) = 5.3; Animal Fluency Test (No SCT) = 5.2; Letter F (SCT) = 4.6; Letter F (No SCT) = 4.8. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
In the sensitivity analysis adjusting for the principal components of ancestry, the sample sizes were as follows: WLL and WLR: n = 5192; AFT: n = 5327; Letter F: n = 4353). The null associations of SCT with change in cognitive test scores were not impacted by adjustment for principal components (data not shown).
4. Discussion
In this large contemporary cohort of black Americans, there were no associations of SCT with incidence of cognitive impairment or with changes over time in test scores included in a 3-test battery assessing learning, memory, and semantic and phonemic fluency. These findings suggest that, unlike findings to date in SCA, biological consequences of SCT do not appear to cause cognitive dysfunction.
While there is literature suggestive that SCA patients experience cognitive dysfunction, to our knowledge, no prior studies evaluated SCT and cognitive impairment [5], [6], [7], [8], [9]. It is established that SCT is associated with hypercoagulability [25] and risk of both venous thromboembolism [19] and kidney disease [16], [17], [18], [36], [37]. In an autopsy series of 128 SCT patients, there appeared to be higher rates of visceral infarcts, including in the brain, than those without SCT (18% vs. < 1%) [22], [38]. Additionally, SCT has been linked to mild cerebral vasculopathy in children on imaging [39]. Although specific mechanisms are unclear, and the findings are still debated, SCT may also be a risk factor for ischemic stroke, but not in the largest study of older adults to date, which included REGARDS participants [20], [21], [22], [23]. Despite these findings, our results suggest that SCT does not lead to clinically significant cognitive dysfunction in adults aged 45 and older.
Regardless of the null findings here, and lack of differences in associations by age in this population age 45 and older, we would advocate for a detailed study examining cognitive impairment in a younger cohort of adults or in children. An estimated 11% of individuals with SCA experience a stroke before the age of 20, with the high risk period occurring between ages 2–5; approximately 24% experience stroke by age 45 [40], [41]. Additionally, there are imaging findings suggestive of silent cerebral infarction, including decreased brain volume [42] and increased white matter hyperintensities [8], [9], [11] in pediatric SCA patients. While there isn't similar data on cognitive function, a meta-analysis on the impact of stroke and silent cerebral infarction on the intelligence quotient of patients with SCA indicated that those with prior stroke had an intelligence quotient 10 points lower than those with silent cerebral infarction, while those with silent cerebral infarction had an intelligence quotient 6 points lower than those without radiographic indications of silent cerebral infarction [43]. Given the evidence of early cognitive impairment and silent cerebral infarction in patients with SCA, it is possible that the childhood brain could be more susceptible to effects of SCT.
Limitations of this study should be considered. Cognitive function assessment ascertainment relied on participant contact by phone and incident cases may have been missed [26]. This issue was partly mitigated since we had a very large cohort, continuous decline in test scores could be examined with high precision and cohort retention was high with 87.3% cumulative retention from enrollment in 2003–7 to January 2011 [44]. Regardless, this type of bias would be predicted to bias results toward the null. Although SCT is less common among non-blacks, exclusion of other racial groups means that our findings may not be generalized to these groups. In order to minimize the impact of stroke itself on the associations of SCT with cognitive outcomes, we censored participants during follow up at the time of stroke. This could have contributed to the null findings we observed under an assumption that stroke is a mechanism whereby SCT might lead to cognitive impairment. However, this should not have been a factor in our findings since we recently reported no association of SCT with risk of stroke in REGARDS, a finding corroborated in three other cohorts including 19,000 participants [23]. It is possible that patients with SCT have undetected mild cognitive decline or subtle differences in parameters such as cognitive processing speed, that might be important to their functioning [10] and insensitive to the cognitive measures used. It is also possible that there is clinical relevance to the 20% increased odds of cognitive impairment based on the six-item screener, although this association was not statistically significant; the finding could be subject to type II error, but is consistent with the rest of the null findings. Other risk factors for cognitive impairment in REGARDS using this endpoint have had much higher relative risk estimates (e.g., 1.6 for male sex, 2.1 for black race and 2.2 for low education). Ongoing REGARDS research will classify participants on dementia status and re-analysis of sickle cell trait with this endpoint will be important. Finally, we did not include participants below age 45 or oversample very old people, groups where any impact of SCT on cognitive function might be different.
The strengths of this study include the prospective design and large geographically dispersed cohort of over 8000 black Americans with representation of men and women. Incident cognitive impairment and longitudinal cognitive performance were carefully determined through a variety of measures with robust null results across all of these. We included people aged 45 and older, the age group with the highest likelihood of cognitive impairment, allowing us a better possibility to detect an association if one exists. Indeed, over a median of 7.1 years of follow up we had nearly 1000 cases of incident cognitive impairment based on the SIS.
We present a detailed examination of the association of SCT with incident cognitive impairment and change in cognitive scores over time in a large population sample of blacks, showing no important association. The findings we present are clinically relevant, as they might be reassuring to patients with SCT who are worried about cognitive function. The results suggest there is not cause for concern about cognitive dysfunction for SCT patients in this age group and their primary care providers. Further research is warranted to confirm our findings, particularly in younger individuals.
Declaration of Interest
The authors have no relevant relationships to disclose.
Author contributions
C.C., J.M.L., L.A.M., M.R.I., N.A.Z., R.N., F.U., V.G.W., S.E.J., J.M., H.H., C.W. and M.C. drafted/revised the manuscript for content.
C.C., L.A.M., N.A.Z., R.N., F.U., V.G.W., S.E.J., C.W. H.H. and M.C contributed to study concept and design.
J.M.L., and L.A.M. performed statistical analysis.
C.C., J.M.L., L.A.M., M.R.I., N.A.Z., R.N., F.U., V.G.W., S.E.J., J.M., H.H., C.W. and M.C interpreted data.
C.W. contributed vital reagent for genotyping.
F.U., V.G.W., S.E.J., C.W. and M.C acquired data.
M.C. M.R.I., and R.N. supervised and coordinated the study.
L.A.M., R.N., S.E.J., C.W. and M.C. obtained funding.
Acknowledgements
The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org. Study funding was by U01NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health (NIH); American Society of Hematology HONORS Award; and HHSN26120080001E from the Intramural Research Program of the National Cancer Institute Center for Cancer Research, NIH. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.eclinm.2019.05.003.
Appendix A. Supplementary data
Baseline characteristics tables
References
- 1.Daviglus M.L., Bell C.C., Berrettini W. National Institutes of Health State-of-the-Science Conference statement: preventing Alzheimer's disease and cognitive decline. NIH Consens State Sci Statements. 2010;27(4):1–30. [PubMed] [Google Scholar]; Daviglus ML, Bell CC, Berrettini W, et al. National Institutes of Health State-of-the-Science Conference statement: preventing Alzheimer's disease and cognitive decline. NIH Consens State Sci Statements 2010; 27(4): 1-30. [PubMed]
- 2.Lorius N., Locascio J.J., Rentz D.M. Vascular disease and risk factors are associated with cognitive decline in the Alzheimer disease spectrum. Alz Dis Assoc Disord. 2015;29(1):18–25. doi: 10.1097/WAD.0000000000000043. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lorius N, Locascio JJ, Rentz DM, et al. Vascular disease and risk factors are associated with cognitive decline in the Alzheimer disease spectrum. Alz Dis Assoc Disord 2015; 29(1): 18-25. [DOI] [PMC free article] [PubMed]
- 3.Duron E., Hanon O. Vascular risk factors, cognitive decline, and dementia. Vasc Health Risk Manag. 2008;4(2):363–381. doi: 10.2147/vhrm.s1839. [DOI] [PMC free article] [PubMed] [Google Scholar]; Duron E, Hanon O. Vascular risk factors, cognitive decline, and dementia. Vasc Health Risk Manag 2008; 4(2): 363-81. [DOI] [PMC free article] [PubMed]
- 4.Blom K., Emmelot-Vonk M.H., Koek H.L. The influence of vascular risk factors on cognitive decline in patients with dementia: a systematic review. Maturitas. 2013;76(2):113–117. doi: 10.1016/j.maturitas.2013.06.011. [DOI] [PubMed] [Google Scholar]; Blom K, Emmelot-Vonk MH, Koek HL. The influence of vascular risk factors on cognitive decline in patients with dementia: a systematic review. Maturitas 2013; 76(2): 113-7. [DOI] [PubMed]
- 5.Schatz J., Finke R.L., Kellett J.M., Kramer J.H. Cognitive functioning in children with sickle cell disease: a meta-analysis. J Pediatr Psychol. 2002;27(8):739–748. doi: 10.1093/jpepsy/27.8.739. [DOI] [PubMed] [Google Scholar]; Schatz J, Finke RL, Kellett JM, Kramer JH. Cognitive functioning in children with sickle cell disease: a meta-analysis. J Pediatr Psychol 2002; 27(8): 739-48. [DOI] [PubMed]
- 6.Vichinsky E.P., Neumayr L.D., Gold J.I. Neuropsychological dysfunction and neuroimaging abnormalities in neurologically intact adults with sickle cell anemia. JAMA. 2010;303(18):1823–1831. doi: 10.1001/jama.2010.562. [DOI] [PMC free article] [PubMed] [Google Scholar]; Vichinsky EP, Neumayr LD, Gold JI, et al. Neuropsychological dysfunction and neuroimaging abnormalities in neurologically intact adults with sickle cell anemia. JAMA 2010; 303(18): 1823-31. [DOI] [PMC free article] [PubMed]
- 7.Schatz J. Brief report: academic attainment in children with sickle cell disease. J Pediatr Psychol. 2004;29(8):627–633. doi: 10.1093/jpepsy/jsh065. [DOI] [PubMed] [Google Scholar]; Schatz J. Brief report: Academic attainment in children with sickle cell disease. J Pediatr Psychol 2004; 29(8): 627-33. [DOI] [PubMed]
- 8.Pegelow C.H., Macklin E.A., Moser F.G. Longitudinal changes in brain magnetic resonance imaging findings in children with sickle cell disease. Blood. 2002;99(8):3014–3018. doi: 10.1182/blood.v99.8.3014. [DOI] [PubMed] [Google Scholar]; Pegelow CH, Macklin EA, Moser FG, et al. Longitudinal changes in brain magnetic resonance imaging findings in children with sickle cell disease. Blood 2002; 99(8): 3014-8. [DOI] [PubMed]
- 9.van der Land V., Hijmans C.T., de Ruiter M. Volume of white matter hyperintensities is an independent predictor of intelligence quotient and processing speed in children with sickle cell disease. Br J Haematol. 2015;168(4):553–556. doi: 10.1111/bjh.13179. [DOI] [PubMed] [Google Scholar]; van der Land V, Hijmans CT, de Ruiter M, et al. Volume of white matter hyperintensities is an independent predictor of intelligence quotient and processing speed in children with sickle cell disease. Br J Haematol 2015; 168(4): 553-6. [DOI] [PubMed]
- 10.. Stotesbury H, Kirkham FJ, Kolbel M, et al. White matter integrity and processing speed in sickle cell anemia. Neurology 2018; 90(23): e2042-e50. [DOI] [PMC free article] [PubMed]
- 11.Mackin R.S., Insel P., Truran D. Neuroimaging abnormalities in adults with sickle cell anemia: associations with cognition. Neurology. 2014;82(10):835–841. doi: 10.1212/WNL.0000000000000188. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mackin RS, Insel P, Truran D, et al. Neuroimaging abnormalities in adults with sickle cell anemia: associations with cognition. Neurology 2014; 82(10): 835-41. [DOI] [PMC free article] [PubMed]
- 12.Torres L.S., Okumura J.V., Silva D.G. Correction: inflammation in sickle cell disease: differential and down-expressed plasma levels of annexin A1 protein. PLoS One. 2017;12(2) doi: 10.1371/journal.pone.0172659. [DOI] [PMC free article] [PubMed] [Google Scholar]; Torres LS, Okumura JV, Silva DG, et al. Correction: Inflammation in sickle cell disease: differential and down-expressed plasma levels of annexin A1 protein. PLoS One 2017; 12(2): e0172659. [DOI] [PMC free article] [PubMed]
- 13.Naik R.P., Wilson J.G., Ekunwe L. Elevated D-dimer levels in African Americans with sickle cell trait. Blood. 2016;127(18):2261–2263. doi: 10.1182/blood-2016-01-694422. [DOI] [PMC free article] [PubMed] [Google Scholar]; Naik RP, Wilson JG, Ekunwe L, et al. Elevated D-dimer levels in African Americans with sickle cell trait. Blood 2016; 127(18): 2261-3. [DOI] [PMC free article] [PubMed]
- 14.Caboot J.B., Allen J.L. Hypoxemia in sickle cell disease: significance and management. Paediatr Respir Rev. 2014;15(1):17–23. doi: 10.1016/j.prrv.2013.12.004. [DOI] [PubMed] [Google Scholar]; Caboot JB, Allen JL. Hypoxemia in sickle cell disease: significance and management. Paediatr Respir Rev 2014; 15(1): 17-23. [DOI] [PubMed]
- 15.Iampietro M., Giovannetti T., Tarazi R. Hypoxia and inflammation in children with sickle cell disease: implications for hippocampal functioning and episodic memory. Neuropsychol Rev. 2014;24(2):252–265. doi: 10.1007/s11065-014-9259-4. [DOI] [PubMed] [Google Scholar]; Iampietro M, Giovannetti T, Tarazi R. Hypoxia and inflammation in children with sickle cell disease: implications for hippocampal functioning and episodic memory. Neuropsychol Rev 2014; 24(2): 252-65. [DOI] [PubMed]
- 16.Naik R.P., Derebail V.K., Grams M.E. Association of sickle cell trait with chronic kidney disease and albuminuria in African Americans. JAMA. 2014;312(20):2115–2125. doi: 10.1001/jama.2014.15063. [DOI] [PMC free article] [PubMed] [Google Scholar]; Naik RP, Derebail VK, Grams ME, et al. Association of sickle cell trait with chronic kidney disease and albuminuria in African Americans. JAMA 2014; 312(20): 2115-25. [DOI] [PMC free article] [PubMed]
- 17.Naik R.P., Irvin M.R., Judd S. Sickle cell trait and the risk of ESRD in blacks. J Am Soc Nephrol. 2017;28(7):2180–2187. doi: 10.1681/ASN.2016101086. [DOI] [PMC free article] [PubMed] [Google Scholar]; Naik RP, Irvin MR, Judd S, et al. Sickle cell trait and the risk of ESRD in blacks. J Am Soc Nephrol 2017; 28(7): 2180-7. [DOI] [PMC free article] [PubMed]
- 18.Austin H., Key N.S., Benson J.M. Sickle cell trait and the risk of venous thromboembolism among blacks. Blood. 2007;110(3):908–912. doi: 10.1182/blood-2006-11-057604. [DOI] [PubMed] [Google Scholar]; Austin H, Key NS, Benson JM, et al. Sickle cell trait and the risk of venous thromboembolism among blacks. Blood 2007; 110(3): 908-12. [DOI] [PubMed]
- 19.Folsom A.R., Tang W., Roetker N.S. Prospective study of sickle cell trait and venous thromboembolism incidence. J Thromb Haemost. 2015;13(1):2–9. doi: 10.1111/jth.12787. [DOI] [PMC free article] [PubMed] [Google Scholar]; Folsom AR, Tang W, Roetker NS, et al. Prospective study of sickle cell trait and venous thromboembolism incidence. J Thromb Haemost 2015; 13(1): 2-9. [DOI] [PMC free article] [PubMed]
- 20.Golomb M.R. Sickle cell trait is a risk factor for early stroke. Arch Neurol. 2005;62(11):1778–1779. doi: 10.1001/archneur.62.11.1778. [DOI] [PubMed] [Google Scholar]; Golomb MR. Sickle cell trait is a risk factor for early stroke. Arch Neurol 2005; 62(11): 1778-9. [DOI] [PubMed]
- 21.Dowling M.M. Sickle cell trait is not a risk factor for stroke. Arch Neurol. 2005;62(11):1780–1781. doi: 10.1001/archneur.62.11.1780. [DOI] [PubMed] [Google Scholar]; Dowling MM. Sickle cell trait is not a risk factor for stroke. Arch Neurol 2005; 62(11): 1780-1. [DOI] [PubMed]
- 22.Caughey M.C., Loehr L.R., Key N.S. Sickle cell trait and incident ischemic stroke in the atherosclerosis risk in communities study. Stroke. 2014;45(10):2863–2867. doi: 10.1161/STROKEAHA.114.006110. [DOI] [PMC free article] [PubMed] [Google Scholar]; Caughey MC, Loehr LR, Key NS, et al. Sickle cell trait and incident ischemic stroke in the Atherosclerosis Risk in Communities study. Stroke 2014; 45(10): 2863-7. [DOI] [PMC free article] [PubMed]
- 23.Hyacinth H.I.C.C., Seals R.S., Irvin M.R., Naik R.P., Burke G.L., Zakai N.A. Sickle cell trait and ischemic stroke in African Americans. JAMA Neurol. 2018;75(7):802–807. doi: 10.1001/jamaneurol.2018.0571. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hyacinth HI CC, Seals RS, Irvin MR, Naik RP, Burke GL, Zakai NA, Wilson JG, Franceschini N, Winkler CA, David VA, Kopp J, Judd SE, Adams RJ, Longstreth Jr. WT, Egede L, Lackland DT, Taylor H, Manson JE, Howard V, Allison M, Gee BE, Correa A, Safford MM, Arnett DK, Howard G, Reiner AP, Cushman M. Sickle cell trait and ischemic stroke in African Americans. JAMA Neurol 2018; 75(7): 802-7. [DOI] [PMC free article] [PubMed]
- 24.Martin T.W., Weisman I.M., Zeballos R.J., Stephenson S.R. Exercise and hypoxia increase sickling in venous blood from an exercising limb in individuals with sickle cell trait. Am. J Med. 1989;87(1):48–56. doi: 10.1016/s0002-9343(89)80482-6. [DOI] [PubMed] [Google Scholar]; Martin TW, Weisman IM, Zeballos RJ, Stephenson SR. Exercise and hypoxia increase sickling in venous blood from an exercising limb in individuals with sickle cell trait. Am J Med 1989; 87(1): 48-56. [DOI] [PubMed]
- 25.Westerman M.P., Green D., Gilman-Sachs A. Coagulation changes in individuals with sickle cell trait. Am J Hematol. 2002;69(2):89–94. doi: 10.1002/ajh.10021. [DOI] [PubMed] [Google Scholar]; Westerman MP, Green D, Gilman-Sachs A, et al. Coagulation changes in individuals with sickle cell trait. Am J Hematol 2002; 69(2): 89-94. [DOI] [PubMed]
- 26.Howard V.J., Cushman M., Pulley L. The Reasons for Geographic and Racial Differences in Stroke study: objectives and design. Neuroepidemiol. 2005;25(3):135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]; Howard VJ, Cushman M, Pulley L, et al. The Reasons for Geographic and Racial Differences in Stroke study: objectives and design. Neuroepidemiol 2005; 25(3): 135-43. [DOI] [PubMed]
- 27.Gillett S.R., Boyle R.H., Zakai N.A., McClure L.A., Jenny N.S., Cushman M. Validating laboratory results in a national observational cohort study without field centers: the reasons for geographic and racial differences in stroke cohort. Clin Biochem. 2014;47(16–17):243–246. doi: 10.1016/j.clinbiochem.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]; Gillett SR, Boyle RH, Zakai NA, McClure LA, Jenny NS, Cushman M. Validating laboratory results in a national observational cohort study without field centers: the Reasons for Geographic and Racial Differences in Stroke cohort. Clin Biochem 2014; 47(16-17): 243-6. [DOI] [PMC free article] [PubMed]
- 28.Price A.L., Patterson N.J., Plenge R.M., Weinblatt M.E., Shadick N.A., Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38(8):904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]; Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet 2006; 38(8): 904-9. [DOI] [PubMed]
- 29.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]; Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150(9): 604-12. [DOI] [PMC free article] [PubMed]
- 30.Unverzagt F.W., McClure L.A., Wadley V.G. Vascular risk factors and cognitive impairment in a stroke-free cohort. Neurology. 2011;77(19):1729–1736. doi: 10.1212/WNL.0b013e318236ef23. [DOI] [PMC free article] [PubMed] [Google Scholar]; Unverzagt FW, McClure LA, Wadley VG, et al. Vascular risk factors and cognitive impairment in a stroke-free cohort. Neurology 2011; 77(19): 1729-36. [DOI] [PMC free article] [PubMed]
- 31.Callahan C.M., Unverzagt F.W., Hui S.L., Perkins A.J., Hendrie H.C. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care. 2002;40(9):771–781. doi: 10.1097/00005650-200209000-00007. [DOI] [PubMed] [Google Scholar]; Callahan CM, Unverzagt FW, Hui SL, Perkins AJ, Hendrie HC. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care 2002; 40(9): 771-81. [DOI] [PubMed]
- 32.Morris J.C., Heyman A., Mohs R.C. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. clinical and neurop/sychological assessment of Alzheimer's disease. Neurology. 1989;39(9):1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]; Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology 1989; 39(9): 1159-65. [DOI] [PubMed]
- 33.Mirra S.S., Heyman A., McKeel D. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41(4):479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]; Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology 1991; 41(4): 479-86. [DOI] [PubMed]
- 34.Seo E.H., Lee D.Y., Lee J.H. Total scores of the CERAD neuropsychological assessment battery: validation for mild cognitive impairment and dementia patients with diverse etiologies. Am J Geriatr Psychiatry. 2010;18(9):801–809. doi: 10.1097/JGP.0b013e3181cab764. [DOI] [PubMed] [Google Scholar]; Seo EH, Lee DY, Lee JH, et al. Total scores of the CERAD neuropsychological assessment battery: validation for mild cognitive impairment and dementia patients with diverse etiologies. Am J Geriatr Psychiatry 2010; 18(9): 801-9. [DOI] [PubMed]
- 35.Levine D.A., Galecki A.T., Langa K.M. Trajectory of cognitive decline after incident stroke. JAMA. 2015;314(1):41–51. doi: 10.1001/jama.2015.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]; Levine DA, Galecki AT, Langa KM, et al. Trajectory of cognitive decline after incident stroke. JAMA 2015; 314(1): 41-51. [DOI] [PMC free article] [PubMed]
- 36.Folsom A.R., Tang W., George K.M. Prospective study of gamma' fibrinogen and incident venous thromboembolism: the Longitudinal Investigation of Thromboembolism Etiology (LITE) Thromb Res. 2016;139:44–49. doi: 10.1016/j.thromres.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]; Folsom AR, Tang W, George KM, et al. Prospective study of gamma' fibrinogen and incident venous thromboembolism: The Longitudinal Investigation of Thromboembolism Etiology (LITE). Thromb Res 2016; 139: 44-9. [DOI] [PMC free article] [PubMed]
- 37.Heller P., Best W.R., Nelson R.B., Becktel J. Clinical implications of sickle-cell trait and glucose-6-phosphate dehydrogenase deficiency in hospitalized black male patients. New Engl J Med. 1979;300(18):1001–1005. doi: 10.1056/NEJM197905033001801. [DOI] [PubMed] [Google Scholar]; Heller P, Best WR, Nelson RB, Becktel J. Clinical implications of sickle-cell trait and glucose-6-phosphate dehydrogenase deficiency in hospitalized black male patients. New Engl J Med 1979; 300(18): 1001-5. [DOI] [PubMed]
- 38.McCormick W.F., Kashgarian M. Abnormal hemoglobins. V. Age at death of patients with sickle cell trait. Am J Hum Genet. 1965;17:10 1–3. [PMC free article] [PubMed] [Google Scholar]; McCormick WF, Kashgarian M. Abnormal hemoglobins. V. Age at death of patients with sickle cell trait. Am J Hum Genet 1965; 17: 10 1-3. [PMC free article] [PubMed]
- 39.Steen R.G., Hankins G.M., Xiong X. Prospective brain imaging evaluation of children with sickle cell trait: initial observations. Radiology. 2003;228(1):208–215. doi: 10.1148/radiol.2281020600. [DOI] [PubMed] [Google Scholar]; Steen RG, Hankins GM, Xiong X, et al. Prospective brain imaging evaluation of children with sickle cell trait: initial observations. Radiology 2003; 228(1): 208-15. [DOI] [PubMed]
- 40.Ohene-Frempong K., Weiner S.J., Sleeper L.A. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998;91(1):288–294. [PubMed] [Google Scholar]; Ohene-Frempong K, Weiner SJ, Sleeper LA, et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood 1998; 91(1): 288-94. [PubMed]
- 41.Verduzco L.A., Nathan D.G. Sickle cell disease and stroke. Blood. 2009;114(25):5117–5125. doi: 10.1182/blood-2009-05-220921. [DOI] [PubMed] [Google Scholar]; Verduzco LA, Nathan DG. Sickle cell disease and stroke. Blood 2009; 114(25): 5117-25. [DOI] [PubMed]
- 42.Steen R.G., Emudianughe T., Hunte M. Brain volume in pediatric patients with sickle cell disease: evidence of volumetric growth delay? Am J Neuroradiol. 2005;26(3):455–462. [PMC free article] [PubMed] [Google Scholar]; Steen RG, Emudianughe T, Hunte M, et al. Brain volume in pediatric patients with sickle cell disease: evidence of volumetric growth delay? Am J Neuroradiol 2005; 26(3): 455-62. [PMC free article] [PubMed]
- 43.Kawadler J.M., Clayden J.D., Clark C.A., Kirkham F.J. Intelligence quotient in paediatric sickle cell disease: a systematic review and meta-analysis. Dev Med Child Neurol. 2016;58(7):672–679. doi: 10.1111/dmcn.13113. [DOI] [PubMed] [Google Scholar]; Kawadler JM, Clayden JD, Clark CA, Kirkham FJ. Intelligence quotient in paediatric sickle cell disease: a systematic review and meta-analysis. Dev Med Child Neurol 2016; 58(7): 672-9. [DOI] [PubMed]
- 44.Cushman M., Callas P.W., McClure L.A. N-terminal pro-B-type natriuretic peptide and risk of future cognitive impairment in the REGARDS cohort. J Alzheimers Dis. 2016;54(2):497–503. doi: 10.3233/JAD-160328. [DOI] [PubMed] [Google Scholar]; Cushman M, Callas PW, McClure LA, et al. N-terminal pro-B-type natriuretic peptide and risk of future cognitive impairment in the REGARDS cohort. J Alzheimers Dis 2016; 54(2): 497-503. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Baseline characteristics tables