Abstract
Background
Strong and broad antiviral T-cell responses targeting vulnerable sites of HIV-1 will likely be a critical component for any effective cure strategy.
Methods
BCN01 trial was a phase I, open-label, non-randomized, multicenter study in HIV-1-positive individuals diagnosed and treated during early HIV-1 infection to evaluate two vaccination regimen arms, which differed in the time (8 versus 24 week) between the ChAdV63.HIVconsv prime and MVA.HIVconsv boost vaccinations. The primary outcome was safety. Secondary endpoints included frequencies of vaccine-induced IFN-γ+ CD8+ T cells, in vitro virus-inhibitory capacity, plasma HIV-1 RNA and total CD4+ T-cells associated HIV-1 DNA. (NCT01712425).
Findings
No differences in safety, peak magnitude or durability of vaccine-induced responses were observed between long and short interval vaccination arms. Grade 1/2 local and systemic post-vaccination events occurred in 22/24 individuals and resolved within 3 days. Weak responses to conserved HIV-1 regions were detected in 50% of the individuals before cART initiation, representing median of less than 10% of their total HIV-1-specific T cells. All participants significantly elevated these subdominant T-cell responses, which after MVA.HIVconsv peaked at median (range) of 938 (73-6,805) IFN-γ SFU/106 PBMC, representing on average 58% of their total anti-HIV-1 T cells. The decay in the size of the HIV-1 reservoir was consistent with the first year of early cART initiation in both arms.
Interpretation
Heterologous prime-boost vaccination with ChAdV63-MVA/HIVconsv was well-tolerated and refocused pre-cART T-cell responses towards more protective epitopes, in which immune escape is frequently associated with reduced HIV-1 replicative fitness and which are common to most global HIV-1 variants.
Funding
HIVACAT Catalan research program for an HIV vaccine and Fundació Gloria Soler. Vaccine manufacture was jointly funded by the Medical Research Council (MRC) UK and the UK Department for International Development (DFID) under the MRC/DFID Concordat agreements (G0701669.
Research in Context
Evidence Before this Study: T cells play an important role in the control of HIV infection and may be particularly useful for HIV-1 cure by killing cells with reactivated HIV-1. Evidence is emerging that not all T-cell responses are protective and mainly only those targeting conserved regions of HIV-1 proteins are effective, but typically immunologically subdominant, while those recognizing hypervariable, easy-to-escape immunodominant ‘decoys’ do not control viremia and do not protect from a loss of CD4 T cells. We pioneered a vaccine strategy focusing T-cell responses on the most conserved regions of the HIV-1 proteome using an immunogen designated HIVconsv. T cells elicited by the HIVconsv vaccines in HIV-uninfected UK and Kenyan adults inhibited in vitro replication of HIV-1 isolates from 4 major global clades A, B, C and D.
Added Value of this Study: The present study demonstrated the concept that epitopes subdominant in natural infection, when taken out of the context of the whole HIV-1 proteome and presented to the immune system by a potent simian adenovirus prime-poxvirus MVA boost regimen, can induce strong responses in patients on antiretroviral treatment and efficiently refocus HIV-1-specific T-cells to the protective epitopes delivered by the vaccine.
Implications of all the Available Evidence: Nearly all HIV-1 vaccine strategies currently emphasize induction of broadly neutralizing Abs. The HIVconsv vaccine is one of a very few approaches focussing exclusively on elicitation of T cells and, therefore, can complement antibody induction for better prevention and cure. Given the cross-clade reach on the HIVconsv immunogen design, if efficient, the HIVconsv vaccines could be deployed globally. Effective vaccines will likely be a necessary component in combination with other available preventive measures for halting the HIV-1/AIDS epidemic
1. Introduction
Cytotoxic T lymphocytes (CTL) play a key role in the control of chronic HIV-1 and simian immunodeficiency virus infections [1], [2], [3]. HIV-1-specific CD8+ T cells in acute infection appear to be uniquely able to efficiently suppress viral replication and force escape mutations [4], whereas CD8+ T-cell responses generated in the chronic infection often lack this capacity [5]. Multiple strategies to achieve optimal immune control of HIV-1 infection in the absence of cART have been evaluated, including a wide range of therapeutic T-cell vaccines [6], [7], [8], [9], [10], [11]. The largest, although still suboptimal, clinical effect demonstrated to date by active vaccination was obtained by transfer of antigen-pulsed autologous dendritic cells, which resulted in a 10-fold, but only transient reduction in the viral set-point after treatment discontinuation [12].
The failures of past therapeutic vaccines to control HIV-1 replication in the absence of cART might have been caused by suboptimal specificity and limited breadth of vaccine-induced T-cell responses, due to either inadequate immunogen design, its delivery strategy or both [13]. In particular, inclusion of full-length HIV-1 proteins in vaccine immunogens may drive CTL responses towards immunodominant, but often variable and therefore non-protective ‘decoy’ epitopes similar to those elicited by natural HIV-1 infection [14]. Thus, some groups have refined immunogen design by selecting protein segments able to focus T-cell immunodominance towards more beneficial and conserved determinants [15], [16], [17] in order to tackle HIV-1 viral diversity and escape [18], [19], [20], [21], [22] and circumvent the effect of host-genetics in natural response to HIV-1 [23] (reviewed in [1]).
The HIVconsv T-cell immunogen was constructed by assembling 14 regions that are highly conserved among the four major HIV-1 clades A, B, C and D into one chimeric protein based on alternating clade consensus sequences [19]. The HIVconsv immunogen delivered by combined plasmid DNA, non-replicating simian adenovirus and non-replicating poxvirus MVA regimens was tested in European and African trials in HIV-1-negative adult volunteers, and demonstrated a good safety profile and induction of high frequencies of CD8+ T cells capable of strong in vitro HIV-1 inhibition [22], [24], [25], especially when compared to those reported in the STEP [26] and RV144 [27] studies. For the HIVconsv delivery, modified simian (chimpanzee) adenovirus serotype 63 (ChAdV-63) was chosen for its low pre-existing seroprevalence in human subjects [28], thus avoiding limitations associated with some of the human adenovirus vectored vaccines [29], [30]. MVA was chosen as the subsequent boost vaccination, since poxviruses were shown to strongly boost existing CD8+ T-cell responses [31].
Several studies demonstrated that early cART in acute/recent HIV-1 infection improved immune recovery [32], [33], reduced the incidence of AIDS and non-AIDS-related diseases [34], restricted immune escape [35] and limited the size of latent reservoir [36], [37], [38], [39]. However, recent clinical examples suggested that in the absence of a targeted immune therapy, HIV-1 could rebound even from an undetectable and/or very low-level viral reservoir: the “Mississippi baby” [40], [41], and individuals treated in Fiebig I from the SEARCH 010/RV254 Acute HIV Infection cohort [42]. Thus, future HIV-1 cure approaches are likely to require a potent T-cell vaccine component [43] that is able to stimulate CTL responses capable of eliminating early cells, in which the virus is reactivated [44], thereby containing viral rebound after treatment interruption.
The BCN 01 trial evaluated for the first time the safety and immunogenicity of a heterologous prime/boost regimen of the ChAdV63.HIVconsv and MVA.HIVconsv vaccines in a cohort of 24 individuals, who were diagnosed with well-documented acute/recent HIV-1 infection and were treated immediately with cART including Tenofovir/Emtricitabine/Raltegravir. In this population, we demonstrate a marked shift in immunodominance profiles of HIV-1-specific T-cell responses towards conserved T-cell epitopes, along with high in vitro viral inhibition capacity without signs of immune exhaustion. These findings may prove critical for future successful combination ‘kick and kill’ eradication strategies [44], [45], [46].
2. Methods
2.1. Trial Design and Study Participants
Trial BCN 01 was a phase I, open-label, non-randomized, multicenter prime/boost therapeutic vaccination study in acute and recently HIV-1-infected individuals to evaluate the safety and immunogenicity of a vaccination regimen of the ChAdV63.HIVconsv and MVA.HIVconsv vaccines using two intervals between the prime and boost. Study participants were recruited at two HIV-1 units (Fundació Lluita contra la Sida at Hospital Universitari Germans Trias i Pujol, Badalona and Hospital Clínic, Barcelona). Individuals with confirmed acute/recent HIV-1 infection (< 6 months from estimated HIV-1 acquisition), who fulfilled all eligibility criteria, were enrolled and started on cART with an INSTI-based regimen (Tenofovir/Emtricitabine plus Raltegravir). Complete list of inclusion/exclusion criteria is available at NCT01712425. Patients were sequentially allocated into the Long arm first (the first participants 1–12) followed by the Short (participants 13–24) arm later. These two arms had a 24- or 8-week interval between the ChAdV63.HIVconsv prime and MVA.HIVconsv boost administrations in the Long and Short arm, respectively, and were chosen to assess whether or not the MVA.HIVconsv boost given 24 weeks after the ChAdV63.HIVconsv vaccine instead of the previously reported 8-week interval [25] was able to induce higher and/or longer-lasting HIVconsv-specific responses. The rational for this is that ChAd vaccines may persist for longer period of time compared to other (viral) vectored vaccines and could thus induce a more robust memory T cell responses. Sequential recruitment was performed to reduce the total duration of the trial and ensure that participants were suppressed for a similar period of time when entering into future roll-over study BCN 02-Romi (NCT02616874) involving analytical cART interruption. An amendment was approved to enroll 24 additional individuals who commenced the same cART regimen and were followed for 60 weeks, but did not receive any vaccine (Controls). This group was only introduced to address potential differences in viral reservoir decay between any of the two vaccination arms and the natural decay of the 1st year of INSTI-based cART when started at acute/recent stages of HIV-1 infection. No safety or immunological analyses were conducted in the control arm as HIV-1-specific T-cell responses were expected to contract once cART mediated viral suppression was achieved. Flow chart of the study design and patient disposition is depicted in Fig. 1a, and the chronogram of the study is shown in Fig. 1b.
Fig. 1.
Disposition of participants and flow chart of the study. Consolidated Standards of Reporting Trials (CONSORT) flow diagram for the trial. Trial BCN 01 was a non-randomized, open label, sequence allocation study. CONSORT diagram delineates the study enrollment of 54 subjects who underwent sequential allocation to the long, short vaccination arms and the control group. Three subjects withdrew from the study before vaccination and/or week 24 and were replaced during enrollment period. All the participants in the Long and Short arms (b) received the ChAdV63.HIVconsv and MVA.HIVconsv vaccines, while 24 individuals in the control arm did not receive any vaccine. All 48 subjects completed the study as per protocol. The 24 vaccinated individuals were included in the safety and immunological analyses. Latent viral reservoir was measured in all 48 participants.
Criteria for documented acute and recent infection included plasma HIV-1 RNA with Ab (EIA) negativity, Gag p24 antigen assay positivity, an indeterminate Western immunoblot signal or the absence of p31 band in a positive Western blot. Days since estimated HIV-1 acquisition and Fiebig staging at treatment initiation time point were defined according to the time since known exposure, acute antiretroviral syndrome and/or results of clinical laboratory tests at the time of HIV-1 diagnosis [47].
The study protocol as well as patient information sheet and consent forms were discussed, reviewed and approved by the Community Advisory Committee of the HIVACAT program. All patients provided written informed consent before enrolment. The study was approved by the institutional ethical review board of both Hospital Universitari Germans Trias i Pujol and Hospital Clinic (Reference Nr AC-11-027) and by the Spanish Regulatory Authorities (EudraCT 2011-000846-39, IND approvals with Nr 12-016 & 12-017). Risk of Genetically Modified Organism release to the environment was evaluated by the Ministry of Environment (B/ES/12/10 & B/ES/12/09). ClinicalTrials.gov identifier: NCT01712425
2.2. Procedures
Vaccinations started after 6 months on stable cART and with plasma virus load (pVL) < 50 copies/mL (week 24 of trial = C0). The ChAdV63.HIVconsv and MVA.HIVconsv vaccines were GMP manufactured at the Clinical Biomanufacturing Facility, University of Oxford, UK and IDT Biologika GmbH, Germany, respectively [19], [24], [48]. All vaccines were stored below − 65 °C until use. Vaccines were thawed no more than 30 min prior to injection. Vaccination with 5 × 10 [10] virus particles (vp) of ChAdV63.HIVconsv at week 0 (C0) was followed by 2 × 10 [8] plaque-forming units (PFU) of MVA.HIVconsv at weeks 24 or 8 (M0). Vaccines were administered by intramuscular needle injection into the deltoid region of both arms (each dose was divided between two arms). The study entailed a total of 24 weeks of clinical and laboratory follow-up after the last vaccination. Visits at 1, 4, 12 and 24 weeks after MVA.HIVconsv boost are designated M1, M4, M12 and M24, respectively. Antiretroviral treatment was maintained during the trial in all 48 individuals.
2.3. Outcomes
The primary objective of the study was to evaluate the safety of ChAdV63.HIVconsv and MVA.HIVconsv vaccines administered sequentially in a heterologous prime-boost regimen with two intervals to HIV-1-positive adults. Local and systemic events were solicited prospectively for a minimum of 7 days following each immunization. Both local and systemic events were graded according to NIH Division of AIDS. Adverse Events (AEs) were specified as unrelated, unlikely, probably or definitely related to the vaccination.
Secondary endpoints included several immunological and virological readouts. Total HIV-1 and HIVconsv-specific T cells were assessed using cryopreserved peripheral blood mononuclear cells (PBMC) obtained at week 0 as baseline (BL: at day of cART initiation); week 24 (C0: pVL < 50 copies/mL, before vaccination); weeks 1 and 4 after ChAdV63.HIVconsv (C1 and C4); and at and 1, 4 and 24 weeks after MVA.HIVconsv (M0, M1, M4 and M24) using an IFN-γ-detecting enzyme-linked immunoabsorbent spot (ELISpot) assay. The IFN-γ Mabtech kit was used according to manufacturer's instructions. All peptides used in the study were 15-mer peptides overlapping by 11 amino acid residues covering the full HIV-1 proteome consensus for subtype B and were obtained through the NIH AIDS Reagent Program, except for the HIVconsv immunogen sequence, for which a matching 199-peptide set (Ana Spec, San José, CA; 95,131) was generously donated by the International AIDS Vaccine Initiative. Peptides were combined into 6 pools P1-P6 of 32–33 peptides per pool corresponding to the HIVconsv vaccine insert (IN pools for ‘inside’ the immunogen) and 12 pools of 39–67 peptides per pool spanning the rest of the HIV-1 viral protein sequences (OUT pools for outside the immunogen). All peptides pools were tested in duplicates. The final concentration of individual peptides in the ELISpot assay was 1.57 μg/mL. Medium only was used as no-peptide negative control in quadruplicate wells, and PHA (1 μg/mL) and a CEF peptide pool (2 μg/mL) consisting of 23 previously defined human CD8+ T-cell epitopes from cytomegalovirus, Epstein–Barr virus and influenza virus (C.T.L. OH, USA) were added as positive controls. All peptide stocks were stored at − 80 °C until use. Spots were counted using an automated Cellular Technology Limited (C.T.L., OH, USA) ELISpot Reader Unit. The threshold for positive responses was defined as at least 50 spot-forming units (SFU)/106 PBMC (5 spots per well) and responses exceeding the mean number of SFU in negative control wells plus 3 SD of the negative control wells, or 3x the mean of negative control wells, whichever was higher.
For flow-cytometry and CD8+ T-cell suppression assays, cryopreserved PBMCs were used from the vaccination time point (C0), at peak of vaccine-induced T cells (M1 or M4) and at the end of trial visit (M24). PBMC were CD8+ T-cell depleted by magnetic bead separation (MACS Milteny Biotec) and stimulated with PHA (5 μg/mL) in RPMI 10% fetal bovine serum (R10). After 3 days of stimulation, the CD4-enriched fraction was infected by spinoculation with HIV-1 BaL and IIIB isolates at a multiplicity of infection (MOI) of 0.01. HIV-1-infected cells were cultured in duplicates or triplicates in R10 medium with 20 IU/mL of interleukin 2 in 96-well round-bottomed plates, alone or together with unstimulated CD8+ T cells obtained by positive magnetic bead separation from an additional vial of PBMC thawed on the same day. Cultures at different CD8+ effector:CD4+ target ratios (E:T of 1:1, 1:2 and 1:10) were harvested after 6 days. Cells were stained first with Aqua Live/Dead and fixed with 1% paraformaldehyde/20 μg/mL lysolecithin at room temperature, permeabilized with cold 50% methanol followed by 0.1% Nonidet P-40, and finally stained with anti-Gag p24 (KC-57-FITC; Beckman Coulter), anti-CD3 (APC-Cy7, BD Biosciences), anti-CD4 (PerCP, BD Biosciences), and anti-CD8 (APC, BD Biosciences) mAbs. CD8+ T-cell antiviral activity was expressed as percentage of inhibition calculated as: [(fraction of p24+ cells in CD4+ T cells cultured alone) – (fraction of p24+ in CD4+ T cells cultured with CD8+ T cells)]/(fraction of p24+ cells in CD4+ T cells cultured alone) × 100. Data previously generated from cART-suppressed individuals treated during chronic HIV-1 infection [49] and from a longitudinal observational cohort of elite and viremic controller individuals (EO-09-042) were used for comparison of CD8+ T-cell antiviral activity from early cART-treated BCN 01 participants. Elite and viremic controller individuals were defined as individuals with sustained levels of pVL < 50 or < 2000 copies/mL, respectively, for more than 10 years in the absence of cART. Total CD8-depleted cells from day 0 and CD4-enriched fraction from day 3 were stained for the exhaustion marker PD-1 using mAb conjugated to PE (BioLegend).
To quantify the size of the peripheral blood proviral reservoir, lysed extracts from 2.5 × 106 CD4+ T cells were used to measure total cell-associated HIV-1 DNA by ddPCR with primers and probes binding within 5′-LTR and Gag [50]. The RPP30 cellular gene was quantified in parallel to normalize sample input [39], [50], [51].
To evaluate HIV-1 RNA below 40 copies/mL, 4–8 mL of plasma samples were ultracentrifugated at 170,000g at 4 °C for 30 min before quantification using the Abbott Real-Time HIV-1 assay (Abbott Molecular Inc.). Serial dilutions of a positive control down to 5 HIV-1 RNA copies/mL were used as a standard curve to calculate quantitative values from raw qPCR 51CT data [39].
2.4. Statistical Analysis
Trial BCN 01 was an exploratory pilot study and, because of the small sample size of the study, was not powered to detect significant differences between arms and only allowed to detect trends in safety, immunological and virological effects, which collectively inform the design of future studies. The safety endpoints were described in the Long and Short arms and summarized by the number and percentage of AEs and their grading. Immunogenicity endpoints were described in the Long and Short arms. The minimum breadth of the T-cell response was estimated as the number of peptide pools eliciting a positive response. The total frequency of HIV-1-specific IFN-γ+ T cells was calculated as the sum of the SFU/106 PBMC stimulated with individual peptide pools.
Differences in the breadth and frequency of the HIV-1-specific responses and virologic determinations between two longitudinal determinations in the same individual were assessed using a Wilcoxon signed-rank test. Differences between groups were compared using a Mann–Whitney U-test or Kruskal-Wallis, ANOVA tests as indicated. Only the reservoir measurement (proviral DNA) was performed in the Control arm. Correlations between the HIV-1-specific responses, proviral DNA and clinical parameters were performed using non-parametric Spearman's rank correlation. Missing data due to technical problems were censored from the analysis, and no imputation techniques were used. Censored matched pairs of ultrasensitive viral load were analyzed using the paired Prentice-Wilcoxon test. Statistical significance was set at 5% for all the univariate tests. The analyses were performed with R (v3.0.2) and GraphPad Prism (v5.01) for Mac OS X (San Diego, CA). Flow data were analyzed using FlowJo software.
3. Results
3.1. Participants Enrolled in the Study
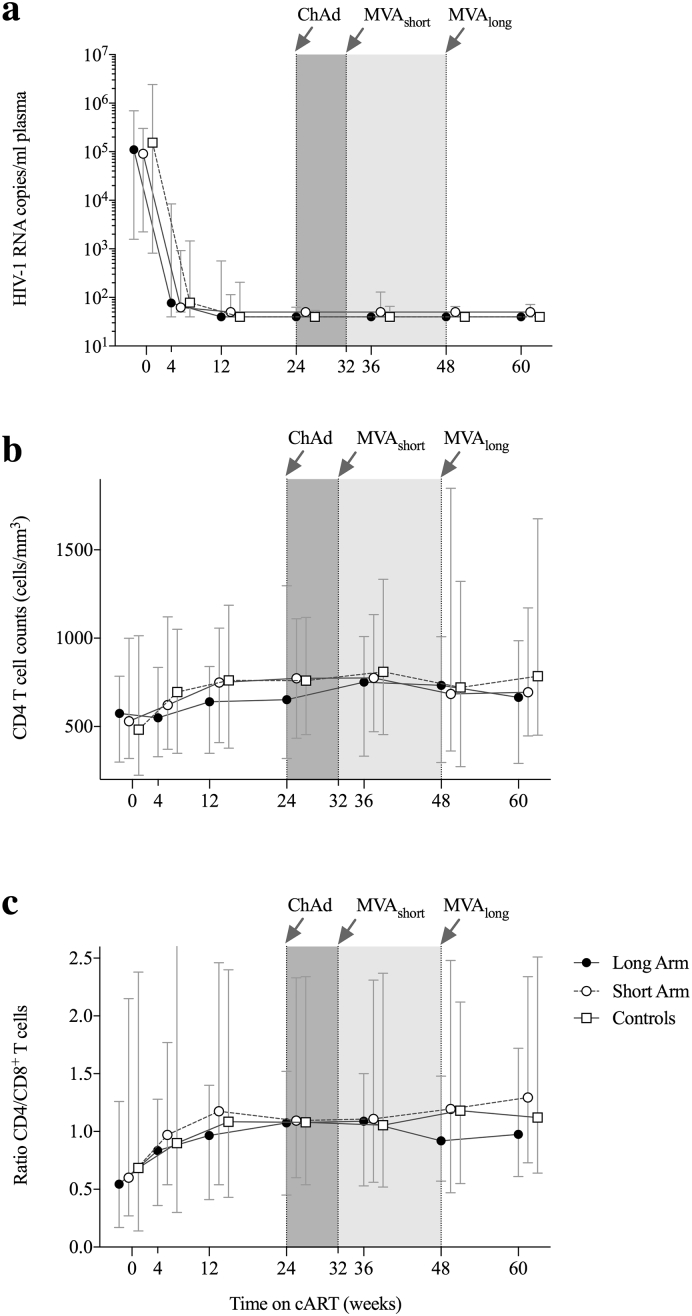
Between October 5, 2012 and April 15, 2013, 27 acute/early HIV-1-infected individuals were sequentially enrolled into the Long and Short arms of BCN 01. Three participants withdrew consent before vaccinations due to suboptimal compliance expectations for follow-up visits or reasons unrelated to the trial. Between May 27, 2013 and April 10, 2014 individuals were enrolled into the no-vaccine arm (Fig. 1b). Recruitment into the BCN 01 trial was enhanced by an intensified MSM testing campaign, which resulted in a gradually earlier-diagnosed and younger population over the recruitment periods. All the 48 participants showed over 99% visit adherence and all completed the study. Study participants' demographics are given in Table 1. Plasma viral loads < 50 copies/mL were reached within median of 12 weeks in all groups as expected for an integrase inhibitor-based regimen (INSTI) (Fig. 2a). Baseline CD4+ T-cell counts (Fig. 2b) and CD4+/CD8+ T-cell ratio (Fig. 2c) significantly increased during the trial during the first year of cART. There was a tendency in older individuals towards lower CD4+ T-cell counts at baseline (Spearman r = − 0.27; p = 0.06) and poorer CD4+ T-cell recovery over the first 60 weeks after treatment initiation (Spearman r = − 0.24; p = 0.09) (Appendix Fig. 1).
Table 1.
Clinical characteristics of the patients included in the BCN 01 study.
| Long arm (A) 0–24 weeks (n = 12) |
Short arm (B) 0–8 weeks (n = 12) |
Controls No vaccine (n = 24) |
|
|---|---|---|---|
| Median age at HIV-1 diagnose | 41 (30–54) | 38 (27–48) | 34 (19–62) |
| Sex (M/F) | 11/1 | 12/0 | 24/0 |
| MSM/HTSa | 9/3 | 12/0 | 24/0 |
| Days since HIV-1 to cART, median (range) | 91 (28–203) | 82 (32–116) | 73 (17–130) |
| Fiebig stage at cART initiation, (numer and Percent of individuals) |
V (6, 50%) VI (6, 50%) |
V (8, 66%) VI (4, 33%) |
I (1, 4%) IV (1, 4%) V (18, 75%) VI (4, 17%) |
| Log10 of pVL before cART, median (range) | 5.04 (3.20–5.84) | 4.80 (3.35–5.48) | 5.18 (2.91–6.39) |
| CD4 (cells/mm3) before cART, median (range) | 574 (299–785) | 519 (309–990) | 482 (224–1014) |
| CD4 (cells/mm3) at week 60, median (range) | 664 (291–986) | 684 (437–1161) | 785 (451–1876) |
| CD4/CD8 ratio before cART, median (range) | 0.54 (0.17–1.26) | 0.60 (0.27–2.15) | 0.68 (0.14–2.38) |
| CD4/CD8 ratio at week 60, median (range) | 1.10 (0.68–1.66) | 1.29 (0.73–2.34) | 1.12 (0.53–2.34) |
| Number of individuals with B*27/B*57/B*58 | 3 (3/0/0) | 0 (0,0,0) | 3 (1/3/0) |
MSM (men who have sex with men)/HTS (heterosexual).
Fig. 2.
Immune recovery after early treatment initiation.
Evolution of pVL (a) and CD4 T-cell counts (b) and CD4/CD8 ratio (c) over the first 60 weeks after early-cART start with TDF/FTC/RAL in study participants.
3.2. Both ChAdV63.HIVconsv and MVA.HIVconsv vaccines were safe and well tolerated
All 24 subjects from the Long and Short arms were included in the safety analysis. Overall, the vaccines were well tolerated. One participant experienced a severe adverse event (SAE) prior to the administration of the ChAdV63.HIVconsv vaccine (acute pancreatitis requiring hospitalization). No other serious adverse reactions or suspected unexpected adverse reactions occurred during the study. A total of 334 AE were recorded during the study (182 in the Long Arm and 152 in the Short Arm, Chi-square, p = 0.25), the majority of which (300, 90%) were mild or moderate (Grade 1–2). The number of AEs per patient was not significantly different between the Long and Short vaccination Arm (Chi-square, p = 0.590).
The summary of local and systemic AEs related to vaccination is shown in Table 2. Local and systemic events after vaccination occurred in 22/24 individuals. Peak of reactogenicity was observed at 24–48 h after vaccination and resolved spontaneously within 3 days. The most frequently reported local reactogenicity AE was a Grade-1 pain reported in both the Long and Short arms, and more frequently reported after the MVA.HIVconsv vaccination than after the ChAdV63.HIVconsv. The most frequently reported systemic reactogenicity event was malaise. As for laboratory abnormalities, no Grade 3 or 4 abnormalities were observed in the performed hematological and biochemical tests (data not shown). Overall, the vaccines were safe and consistent with safety and tolerability data previously observed in HIV-1-negative volunteers [22], [25].
Table 2.
Number and proportion of volunteers suffering local or systemic side effects related to vaccination.
| ChAdV63.HIVconsv |
MVA.HIVconsv |
|||||||
|---|---|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Any | Grade 1 | Grade 2 | Grade 3 | Any | |
| Local reactogenicity | ||||||||
| Redness/Erythema (≥ 2.5 cm diameter)a | ||||||||
| Left arm | 0 | 0 | 0 | 0 (0%) | 1 | 0 | 0 | 1 (4%) |
| Right arm | 0 | 0 | 0 | 0 (0%) | 1 | 1 | 0 | 2 (8%) |
| Induration (≥ 2.5 cm diameter)b | ||||||||
| Left arm | 0 | 0 | 0 | 0 (0%) | 0 | 0 | 0 | 0 (0%) |
| Right arm | 0 | 0 | 0 | 0 (0%) | 0 | 0 | 0 | 0 (0%) |
| Local pain | ||||||||
| Left arm | 13 | 1 | 1 | 15 (62%) | 13 | 9 | 1 | 23 (96%) |
| Right arm | 12 | 1 | 0 | 13 (54%) | 11 | 9 | 0 | 20 (83%) |
| Systemic adverse events | ||||||||
| Fever | 0 | 0 | 0 | 0 (0%) | 2 | 0 | 1 | 3 (12%) |
| Headache | 8 | 2 | 1 | 11 (46%) | 7 | 5 | 1 | 13 (54%) |
| Malaise | 5 | 5 | 1 | 11 (46%) | 8 | 8 | 2 | 18 (75%) |
| Nausea | 0 | 0 | 0 | 0 (0%) | 0 | 0 | 0 | 0 (0%) |
| Diarrhea | 1 | 1 | 0 | 2 (8%) | 3 | 0 | 1 | 4 (17%) |
| Sweating | 2 | 2 | 0 | 4 (17%) | 7 | 0 | 0 | 7 (29%) |
| Myalgia | 2 | 5 | 0 | 7 (29%) | 8 | 5 | 2 | 15 (62%) |
| Anorexia | 2 | 0 | 0 | 2 (8%) | 1 | 2 | 0 | 3 (12%) |
| Abdom pain | 0 | 1 | 0 | 1 (4%) | 3 | 3 | 0 | 6 (25%) |
Note to local AE:
Injection-site erythema not reaching Grade 1 DAIDS criteria (< 2.5 cm in diameter) was observed in 4 (17%) and 4 (17%) participants in the left/right arm, respectively after the ChAdV63.HIVconsv vaccination, and in 12 (50%) and 9 (37%) participants on the left/right arms, respectively after the MVA.HIVconsv vaccination.
Injection-site induration not reaching Grade 1 DAIDS criteria (< 2.5 cm in diameter) was observed in 2 (8%) and 3 (12%) participants in the left/right arm, respectively after the ChAdV63.HIVconsv vaccination, and in 17 (71%) and 14 (58%) participants on the left/right arm, respectively after the MVA.HIVconsv vaccination.
3.3. ChAdV63.HIVconsv-MVA.HIVconsv regimen induced high frequencies of T cells against conserved regions of HIV-1 in all vaccine recipients
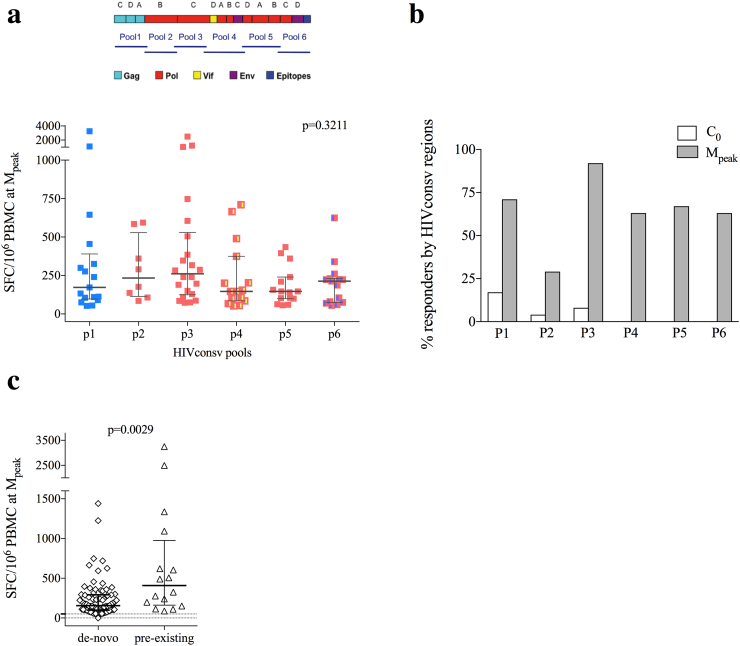
Peptide pools P1-P6 covering the HIVconsv immunogen (778 amino acids) contained 15-mer peptides overlapping by 11 amino acids (Fig. 3a). Out of the 8 time points analyzed for each vaccine recipient (total of 192 time points), 4 samples (2% of evaluated samples) were censored due to low positive controls and/or high background. T-cells to the HIVconsv immunogen of median (range) frequency of 286 (55-2,640) SFU/106 PBMC were detected in 50% of study participants in the Long (Fig. 3b) and Short (Fig. 3c) arms equally at HIV-1 diagnosis and before cART (BL). Individuals with and without HIVconsv pre-existing responses did not differ in duration with untreated infection or pre-cART viremia. All HIV-1-specific T cells were significantly reduced after initiation of cART and viral suppression to frequencies of median of 75 (0–476) SFU/106 PBMC (BL vs C0; Wilcoxon signed-rank, p = 0.0173). All vaccine recipients completed ChAdV63.HIVconsv-MVA.HIVconsv immunizations and all 24 (100%) showed an absolute increase in HIVconsv-specific IFN-γ-producing T cells during the study; in 15 individuals (63%) showed increase already after ChAdV63.HIVconsv and the remaining 9 individuals (37%) after MVA.HIVconsv. There was no difference between the Long and Short arms, whereby 8 and 7 subjects responded to ChAdV63.HIVconsv, and 4 and 5 responded only after MVA.HIVconsv, respectively. For all individuals, the peak of total T-cell frequencies (sum of frequencies to HIVconsv P1-P6 pools) was detected after MVA.HIVconsv. Individuals in the Long arm showed a tendency towards reaching peak responses more slowly than subjects in the Short Arm with 2 vs 6 individuals peaking at 1 week (M1) and 9 vs 6 individuals peaking at 4 weeks after MVA.HIVconsv (M4), respectively (Chi-square, p = 0.08). Altogether, median (range) total peak total frequencies of HIVconsv-specific T cells reached 938 (73-6,805) SFU/106 PBMC, which represented an absolute increase in the frequencies of 750 (124-1,948) and 1,015 (73-6,535) SFU/106 PBMC from the time point before vaccination (C0) in the Long and Short arm, respectively (Wilcoxon signed-rank, p < 0.0001) (Fig. 3d). The longevity of vaccine-elicited responses was not statistically different between the Long and Short Arms as frequencies of total HIVconsv-specific T cells were 390 (60-1,701) and 140 (0-1,567) SFU/106 PBMC at 24 weeks after the MVA.HIVconsv administration in these arms (M24), respectively (Fig. 3e).
Fig. 3.
Vaccination immunogenicity.
Cryopreserved, unexpanded PBMC were stimulated with pools P1–P6 of overlapping 15-mer peptides across the HIVconsv immunogen in an IFN-γ ELISPOT assay. (a) Schematic representation of the employed conserved regions in the HIV proteome from different HIV-1 clades included in the HIVconsv immunogen and distribution of the set of 6 peptide pools used for immunogenicity studies. Magnitude of total HIVconsv-specific responses (sum of SFU/106 PBMC to pools P1-P6) over trial duration in the Long (b) and Short (c) vaccination arms are shown. (d) Total magnitude of HIVconsv-specific responses before and at peak immunogenicity in all vaccinated individuals. Median total frequency for the entire cohort is shown in red. Wilcoxon signed-rank p value is shown (e) Comparison of total magnitude of HIVconsv-specific responses between Long and Short vaccination arms at different time points of the clinical study. Mann–Whitney U-test is used for comparisons between Long and Short arms, and Wilcoxon signed-rank for comparisons within timepoints in the same individual.
3.4. ChAdV63.HIVconsv-MVA.HIVconsv vaccination elicited responses to multiple epitopes within the HIVconsv immunogen
Effector CD8+ T-cell recognition of multiple conserved epitopes will likely be one of the key features of a successful HIV-1 vaccine. Vaccine recipients responded to median (range) of 4 [1], [2], [3], [4], [5], [6] peptide pools out of 6 vaccine peptide pools P1-P6 (Appendix Fig. 2). There was no significant difference in the specific T-cell frequencies for the different pools (ANOVA test, p = 0.3211, Fig. 4a), indicating balanced and polyspecific vaccine-induced responses across the HIVconsv immunogen. Most (83%) of the HIVconsv peptide pool-specific T cells detected at peak immunogenicity were considered de novo responses as they were not detectable before cART initiation (BL), when high levels of plasma viremia were present. This was the case for peptide pools P4-P6 (Fig. 4b), which covered the conserved regions of polymerase, integrase, Vif, and Env gp41 and gp120 regions included in the HIVconsv immunogen. The highest proportion of pre-vaccination responses was directed against pool P1 covering partial Gag sequences (Fig. 4b). Finally, in individuals who showed response to HIVconsv peptide pools at the time of HIV-1 diagnosis (12 individuals, 16 responses in total), vaccination effectively boosted their pre-existing responses. Indeed, these presumably expanded T cells reached higher frequencies of median (range) of 497 (87–3250) SFU/106 PBMC than de novo vaccine-induced T cells of median (range) of 156 (0–1440) SFU/106 PBMC (Mann–Whitney U-test, p = 0.0029) (Fig. 4c).
Fig. 4.
Breadth of vaccine-elicited HIVconsv-specific T-cells.
(a) Comparison (ANOVA p-value) of the frequency of each participant's response to individual peptide pools at the peak immunogenicity time point after MVA.HIVconsv booster vaccination and (b) percentages of participants showing a detectable response (‘responders’) to HIVconsv peptide pools either before any vaccination (white bars) or at the peak immunogenicity time point (gray bars). (c) Comparison of the frequency of responses detected at the peak immunogenicity time point reflecting either de-novo induced or vaccine-boosted (‘pre-existing’) responses. Mann–Whitney U-test p value is shown.
3.5. ChAdV63.HIVconsv-MVA.HIVconsv vaccination shifts immunodominance T-cell patterns towards conserved regions of the HIV-1 proteome without induction of responses against junctional regions
Next, we assessed whether or not the HIVconsv vaccinations led to a non-specific expansion of T cells targeting HIV-1 regions not present in the HIV-1 immunogen or T cells specific for unrelated co-pathogens. The non-HIVconsv HIV-1-specific responses referred to as the OUT peptide pools and those directed to the CEF peptide pool were determined in an IFN-γ ELISPOT assay. The dominance of HIVconsv-specific responses was calculated at each time point as the percentage of HIVconsv-specific T-cell frequencies divided by the total HIV-1 proteome-specific (HIVconsv + OUT) T-cell frequencies. A significant reduction in total HIV-1-specific T cells after viral suppression by cART and elimination of HIV-1 antigenemia was observed (Wilcoxon signed-rank, p < 0.001 at C0 (Fig. 5a). No expansion of T cells targeting HIV-1 regions outside of the immunogen was noted over the vaccination time points, indicating that the vaccines selectively expanded HIVconsv-specific T cells. An effective shift of response patterns towards conserved T-cell epitopes reached its peak after the MVA.HIVconsv vaccination with median (range) of 58% (7%–100%) of the total anti-HIV-1 immune responses. Despite some decay of vaccine-elicited T-cell frequencies, this focus was maintained over time and was still 33% (0%–100%) of all detectable HIV-1 responses 24 weeks after the last vaccination (M24) (Fig. 5b). Similarly, no increases in the frequency of responses to the CEF peptides were observed over the vaccination time points (Fig. 5c).
Fig. 5.
Changes in T-cell dominance patterns. (a) Schematic representation of the average distribution of total HIV-1 T-cells among different HIV-1 proteins at baseline (BL), its decrease during viral suppression and its expansion at peak responses. HIVconsv-specific responses are shown in purple. Sizes of pie charts are to scale with total frequencies of responses. Acc - Accessory proteins. (b) Median frequency of total HIVconsv-specific T cells (green bars) and changes in median HIVconsv immunodominance (red line) are shown over time. (c) Mean ± SD frequencies of T cells specific for OUT and CEF peptide pools are shown over time. (d) Comparison of the frequency of individual HIVconsv peptide pool responses detected in 15 study subjects using two sets of 15-mer peptides covering (P1-P6, + junctions) or avoiding (P1-P6, − junctions) the junctional regions is shown. Wilcoxon signed-rank t-test is used.
Additionally, we determined the immunogenicity of potential junctional neo-epitopes located across the adjacent segment junctions of the HIVconsv immunogen [19] by comparing response rates to two sets of peptide pools with and without junctional peptides. In contrast to what was observed in an HIV-1-negative cohort where ~ 20% of induced responses were specific for junctions [24], responses to both sets of peptide pools were of comparable frequency all individuals (Fig. 5d), indicating that responses against junctional regions were minimal if induced at all.
3.6. Early cART preserves antiviral function of CD8+ T cells and might contribute to an efficacious vaccine response
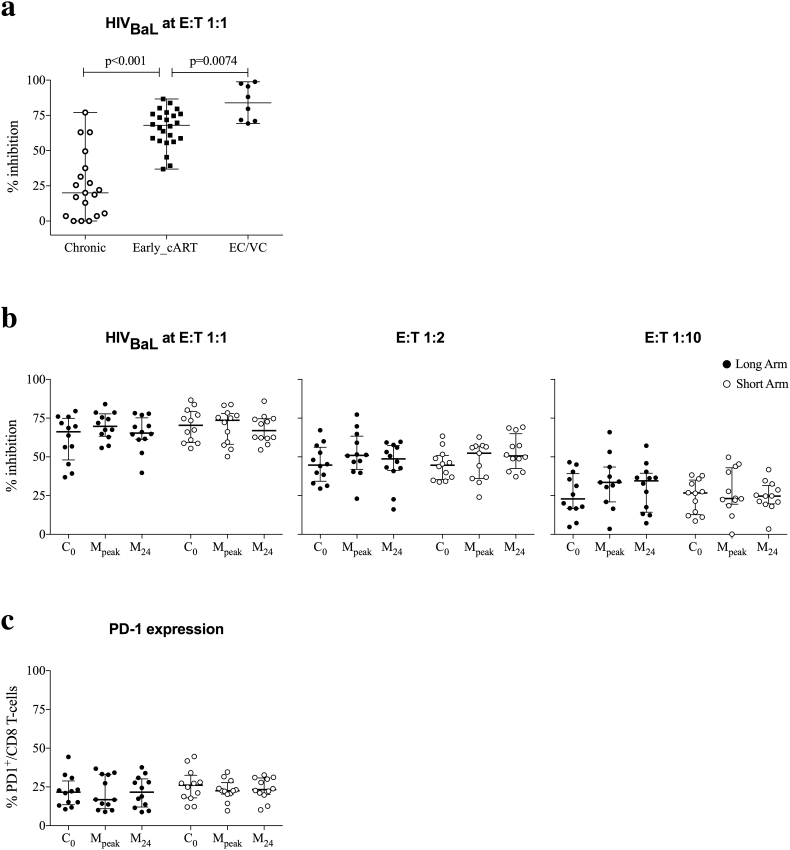
To determine CD8+ T-cell mediated viral inhibitory capacity against HIV-1 BaL and IIIB isolates at different CD8:CD4 ratios (E:T = 1:1, 1:2 and 1:10), longitudinal cryopreserved-and-thawed samples obtained at 6 months on stable cART and just before vaccination (C0), at the peak of vaccine-induced immunogenicity (M1 or M4, collectively Mpeak) and at the end of trial in all individuals from the Long and Short arms (M24) were used in in vitro viral replication inhibition assays. Data previously generated from individuals virologically suppressed, who were treated during chronic HIV-1 infection [49] and from eight long-term controller individuals were used for comparison with the CD8+ T-cell antiviral activity from early-cART-treated BCN 01 participants. CD8+ T-cell viral inhibition of the BaL virus was higher in vaccinated individuals at C0 at median (range) of 68% (37–87%) viral inhibition when compared to the same day levels described in individuals treated during chronic HIV-1 infection (median 20%, Mann–Whitney U-test, p < 0.0001) and closer to levels observed in cohorts of elite and viremic controllers (median 84%, Mann–Whitney U-test, p = 0.0074, Fig. 6a). This high suppressive capacity was maintained during the entire vaccination period, was also observed at lower E:T ratios and was confirmed with both 2 clade B viruses tested (Fig. 6b). In line with an effective antiviral T-cell response, the levels of the PD-1 exhaustion markers on total CD8+ T cells were maintained at low levels throughout the study period with median (range) of 23% (10–45%) of CD8+ T cells suggesting that early cART initiation might preserve CD8+ T-cell function and consequently allow for an effective vaccine response (Fig. 6c).
Fig. 6.
High CD8+ T-cell viral inhibitory capacity and low levels of PD-1-expressing CD8+ T cells in early-treated individuals.
(a) Comparison (Mann–Whitney U-test) of levels of CD8+ T-cell viral inhibition is shown for HIV-1Bal (E:T 1:1) in individuals, who started cART during chronic infection (Chronic), participants in BCN 01 (Early_cART) 24 weeks after cART initiation and before any vaccination (C0), and elite and viremic controllers (EC/VC). (b) Levels of CD8+ T-cell viral inhibition are shown for HIV-1Bal (E:T ratio 1:1, 1:2 and 1:10) for individuals in the Long and Short Arms before vaccination (C0), at peak of vaccine-induced immunogenicity (Mpeak) and at the end of trial (M24). (c) Expression of PD-1 by CD8+ T cells for the same time points.
3.7. Early cART, but not HIVconsv vaccination limits the size of latent viral reservoir
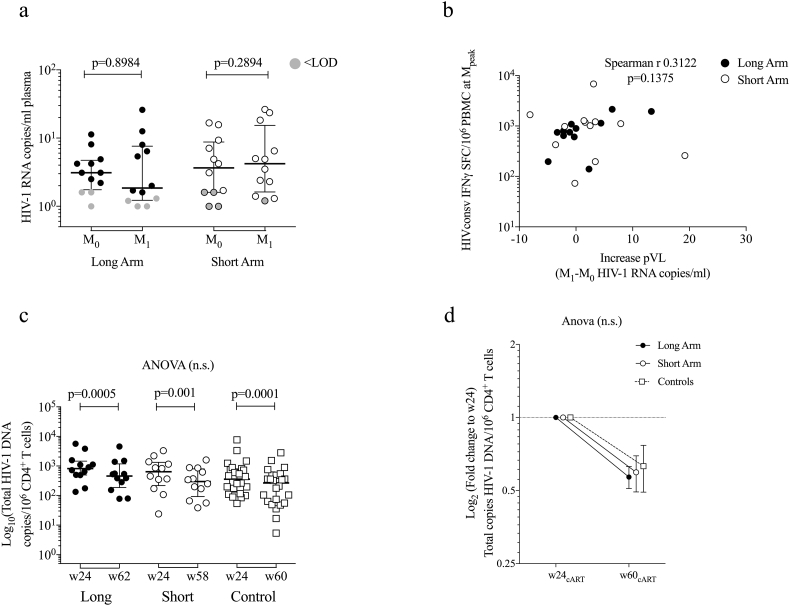
To determine whether vaccine-induced immune activation resulted in an increased HIV-1 replication despite cART, pVL was measured just prior to and at 1 week after the MVA.HIVconsv administration (M0 and M1) in all vaccinated subjects using both standard and ultrasensitive assays. Overall, 6 participants had plasma HIV-1 RNA “blips” of median (range) of 200 (37-119) RNA copies/mL, whereby 40 copies/mL is the detection limit, while on reported optimal cART adherence along the entire trial follow-up period (data not shown). Using the ultrasensitive pVL assay, plasma viremia was detectable in 92% of individuals at M0 or M1 (Fig. 7a). We observed 8 patients with a 2-fold increase in ultrasensitive pVL 1 week after vaccination (M1), but changes in the ultrasensitive pVL in the overall cohort were not statistically significant between the two tested time points (Fig. 7a). In addition, there was no correlation between the frequencies of HIVconsv-specific IFN-γ-producing cells at peak and the detected increases in plasma viremia (Spearman r 0.3122, p = 0.1375; Fig. 7b).
Fig. 7.
Changes in ultrasensitive pVL after MVA.HIVconsv booster vaccination and proviral DNA decay dynamics.
(a) Copies of HIV-1 RNA per mL of plasma are shown for each vaccine group just before MVA.HIVconsv (M0) and 1 week later (M1) with censored values below the limit of detection shown in gray. Prentice-Wilcoxon p values are shown. (b) Correlation (Spearman r) between total frequency of HIVconsv-specific T cells at the peak immunogenicity timepoint and the absolute increase in pVL. (c) Total HIV-1 DNA copies/106 CD4+ T cells for each vaccination group are shown at week 24 (before vaccination) and at week 56/60 after treatment initiation (Wilcoxon signed-rank p values are shown for comparisons within timepoints in same individual) (d) Comparison (ANOVA) of fold change of proviral DNA over 1 year after viral suppression from week 24 of cART in all groups (mean ± SE).
To determine the proportion of total circulating CD4+ T cells harboring proviral DNA and to identify potential changes in the viral reservoir size induced by vaccination, total HIV-1 DNA copies in purified PBMC-derived CD4+ T cells were quantified longitudinally. Total HIV-1 DNA was measured just prior to vaccination when all subjects had achieved pVL < 50 copies/mL after 24 weeks under stable cART and at 12 and 24 weeks post MVA.HIVconsv for both the Long and Short arms (~ 1 year after treatment initiation). Proviral DNA from 24 early treated, but not vaccinated individuals (Control) was measured for comparison with the natural decay due to cART initiation. At pre-vaccination, total HIV-1 DNA was not statistically different between the Long, Short and Control arms with median (range) of 815 (134-5,660), 639 (24-3,289) and 358 (54-7,665) copies of total HIV-1 DNA/106 CD4+ cells, respectively (Fig. 7c). Peripheral blood CD4+ T-cell proviral reservoir showed similar decay kinetics in all groups. Overall, in the compiled BCN 01 cohort (n = 48), we observed median (range) of 342 (5.4-4,565) copies of HIV-1 DNA/106 CD4+ cells at 1 year after treatment initiation. These levels of proviral DNA were 3-fold lower than values in virally suppressed individuals who started cART during chronic HIV-1 infection (median of 1065 copies/106 CD4+ T cells) [49]. The proviral DNA decay was consistent with a decay over the first year of early initiated cART [50], but was not further accelerated by the ChAdV63.HIVconsv-MVA.HIVconsv vaccinations (Fig. 7d).
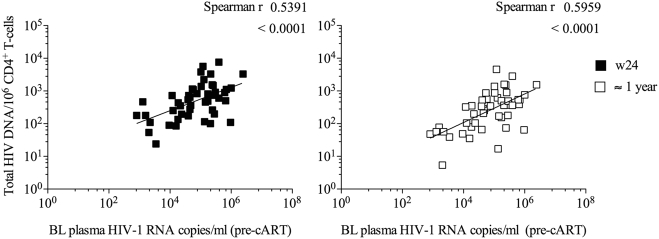
Levels of proviral DNA in PBMC-derived CD4+ T cells measured at pre-vaccination (BL) and at the end of study (M24) did not correlate with estimated days since HIV-1 acquisition to cART initiation and were not associated with either the HIVconsv-specific nor the total HIV-1-specific T-cell frequencies (data not shown). Overall, the frequency of CD4+ T cells harboring HIV-1 DNA correlated well with pre-cART viremia in acute/recent infection (Spearman r = 0.54, p < 0.0001 at C0, and Spearman r = 0.59, p < 0.0001, at M24; see Appendix Fig. 3).
4. Discussion
The present BCN 01 trial data show that a heterologous regimen using the ChAdV63.HIVconsv and MVA.HIVconsv vaccines in a cohort of early-treated individuals with acute or recent HIV-1 infection was a safe and effective strategy to shift the immunodominance of the total HIV-1-specific T-cell response towards conserved regions of HIV-1. Vaccination led to a marked and highly selective expansion of pre-existing as well as induction of new T-cell responses to HIVconsv, but failed to impact the size of the in vivo latent viral reservoir.
A successful therapeutic vaccination may ultimately be achieved by an optimal T-cell immunogen and the use of highly immunogenic vaccine vector or vector combination, which can refocus HIV-1-specific CTL response to vulnerable sites of the virus [15], [19], [20], [21], [52]. Here, we tested the immunogenicity of two heterologous prime-boost regimens with simian adenovirus and MVA-vectored vaccines expressing the first-generation conserved immunogen HIVconsv in a population of early-diagnosed and early-treated HIV-1-positive individuals in Barcelona, Catalonia, Spain. Overall, the vaccines were well tolerated with side effects limited to Grade 1 and 2, whereby most AEs judged to be related to the vaccination were transient and resolved spontaneously within 3 days. These data support the previously reported safety profile of the combined ChAdV63.HIVconsv and MVA.HIVconsv vaccination in HIV-1-negative individuals immunized with the same regimen [25].
Our analyses show that T-cell responses to conserved regions of HIV-1 were sub-dominant in natural HIV-1 infection [53], [54]. Administration of HIVconsv vaccines was able to induce broad and potentially novel T-cell responses by strongly refocusing the HIV-1-specific T cells to conserved epitopes contained in the immunogen insert. These responses were of high frequency, although lower than vaccine responses seen in the HIV-1-negative individuals included in the earlier HIV-CORE 02 trial [24]. In the HIV-CORE 02 trial, median (range) frequency of HIVconsv-induced peak responses induced in the ChAdV63.HIVconsv-MVA.HIVconsv arm was 5150 (1475–16,510) SFC/106 PBMC24. However, it is important to note that HIV-CORE 02 used freshly isolated PBMC IFN-γ ELISPOT assays, which has been shown by reference labs and some of the co-authors using HIV-CORE 02 samples to lead to approximately 5-times higher frequency when compared to frozen-and-thawed cells (Spearman r = 0.9156; P < 0.0001). Therefore, peak vaccine-induced T-cell levels observed in BCN 01 were comparable to those observed in HIV-1-negative individuals in HIV-CORE 02 and considerably stronger than responses reported in past therapeutic vaccine trials such as the ones using canarypox ALVAC-HIV vaccine [6] or homologous vaccinations with MVA-B [55] in chronically suppressed individuals. Thus, our data strongly suggest that potent heterologous prime-boost vaccination regimens including an adenovirus-vectored prime as well as early cART initiation contribute critically to the effective refocus and boost of T-cell responses to relevant epitopes in BCN 01. Although limited by the non-randomized nature of the study and the small size of the clinical trial, we did not observe statistically separable differences in the frequency or breadth of induced responses, functionality or longevity between the longer and the shorter vaccination intervals. This does not exclude the possibility that larger, randomized trials may reveal some differences between different dosing intervals though, and that these differences could have a potential effect on viral control.
The frequency and kinetics of CD8+ T cell activation during acute HIV-1 infection has recently been related to viral setpoint [4], suggesting that early treatment initiation could help maintain necessary HIV-1-specific CD8+ T-cell responses associated with subsequent control of viremia. In line with this hypothesis, we observed also in our cohort a more marked immune restoration (normal CD4/CD8 T-cell ratios) [56], a reduced expression of immune exhaustion markers and stronger CD8+ T-cell viral inhibitory capacity than those reported for individuals who started treatment in the chronic phase of HIV-1 infection [49]. Some or all these factors might have contributed to an increased immune responsiveness to the therapeutic vaccination strategy tested in this population.
Aside from the non-randomized nature of the trial, one potential caveat of the present study was that the relative contribution of HIVconsv vaccine-induced T cells to the total in vitro CD8+ T-cell inhibition activity could not be accurately estimated. Thus, although we document a strong refocus of the specificity of the T-cell response after vaccination, it remains unclear how much these responses added to the observed inhibition of viral replication in vitro. The increase in viral inhibition capacity could be limited due to the continuous presence of CD8+ T cells targeting non-protective epitopes, the existence of other vulnerable viral epitopes not covered by HIVconsv immunogen and/or insufficient T-cell help stimulation, which improved vaccine immunogen designs may overcome [15], [19], [21], [57]. On the other hand, it is likely that the vaccination contributed to the prolonged maintenance of responses able to suppress HIV-1 replication in vitro, which would have been gradually lost if no vaccine were administered. While in trial BCN 01, the non-vaccinated control arm could not be tested for antiviral activity, this scenario concurs with recently reported data from the London-based RIVER trial (NCT02336074 [58]). In that trial, using the same vaccine combination along with vorinostat, the vaccinees maintained in vitro inhibitory activity over time whereas placebo controls showed indeed a progressive decay, suggesting that also in BCN 01, the vaccine was responsible for the continuously high levels of antiviral activity observed.
Despite a strong expansion of T-cell responses to HIV-1, a prolonged maintenance of antiviral activity of these responses with vaccination and relatively low reservoir levels at the end of the trial [38], [59], we did not observe a more pronounced reduction in the size of the HIV-1 reservoir associated with vaccination when compared to non-vaccinated individuals. Moreover, no differences in the viral reservoir decay dynamics between the longer and the shorter vaccination regimen were noted. This does not exclude however the possibility that more sensitive assays or larger, randomized trials and specially with longer follow-up could reveal differences between vaccinated and non-vaccinated individuals. However, the same was reported in other therapeutic vaccine trials, where less immunogenic regimens were tested in chronic cART-suppressed subjects [49], [55]. In that sense, the results may not be surprising as no latency reversing agent was administered in BCN-01 and vaccination did not induce robust signals of virus reactivation either. In addition, the effect of vaccine-induced T-cells on residual replicating cells in the tissue is unknown. Whether or not if effective, such elimination of HIV-1-producing cells in tissues would be detectable by reservoir analyses in the peripheral blood remains to be assessed.
Importantly, a change in T-cell immunodominance towards targets of interest would not occur if the vaccination regimen led to a broad, non-specific expansion of all HIV-1-specific T cells that existed before vaccination. We assessed this by measuring longitudinally T-cell responses to regions of HIV-1 that both were and were not covered by the HIVconsv immunogen, by documenting T cells to epitopes from unrelated viruses such as CMV, EBV and influenza virus as well as by determining potential responses to junctional regions of the HIVconsv chimeric protein. These analyses showed no signs of expansion of pre-existing T-cell responses to HIV-1 regions not covered by the immunogen or of responses to other viral infections. Interestingly, a marked absence of responses to junctional regions contained in the HIVconsv sequence was also noted, which is in contrast to findings with the same vaccination regimen in HIV-1-uninfected subjects [24] and which may suggest that de novo responses may be primed differently in HIV-1-negative versus already HIV-1-positive individuals. Alternatively, another possible explanation could be that low-levels of HIVconsv immunogen-specific T-cell responses already existed at pre-vaccination time points in the already HIV-1-infected subjects and that vaccination expanded these responses rather than induced them de novo. Detailed T-cell receptor analyses on total T cells and epitope-specific populations will be needed to address this question. Nevertheless, regardless of whether vaccination induced de novo responses or expanded T cells from very subdominant populations, the results in BCN 01 document for the first time an impressive plasticity in the HIV-1-specific T-cell response patterns upon potent vaccination in early-treated individuals. Thus, and to the best of our knowledge, this is the first therapeutic HIV-1 vaccine trial able to demonstrate a shift in the immunodominance of the virus-specific T cells towards conserved regions of HIV-1 in a cohort of early-treated individuals. The data provide strong rationale for the further development of this vaccine strategy and incorporation of this regimen into future kick-and-kill approaches to address whether or not these responses can control rebounding virus after cART interruption.
Declaration of Competing Interest
BM, PC, ASB, MR, MCP, SML, BC, JMP and CB report grants from the HIVACAT Catalan research program for an HIV vaccine and Fundació Gloria Soler. BM holds a post-doctoral fellowship grant from ISCIII (JR 13/00024) from 2014 to 2016 during the conduct of the study and is a consultant for AELIX THERAPEUTICS, S.L., outside the submitted work. SML holds a PhD grant from DGR (2013FI_B 00275) from 2013 to 2016 during the conduct of the study. JMM received a personal 80:20 research grant from the Institut d'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Barcelona, Spain, during 2017–19 and reports grants and personal fees from Abbvie, Angelini, Contrafect, Genentech, Gilead, Jansen, Medtronic, MSD, Pfizer, ViiV Healthcare, outside the submitted work. LD reports being employer of Immunocore. TH reports grants from Medical Research Council UK, during the conduct of the study, and has a patent US 7981430B2 issued. CB is founder, CSO and shareholder of AELIX THERAPEUTIC, S.L. BC is founder, consultant and shareholder of AELIX THERAPEUTICS, S.L. TH reports grants from Medical Research Council UK, during the conduct of the study, and has a patent US 7981430B2 issued. SML hold a PhD grant from DGR (2013FI_B 00275) from 2013 to 2016 during the conduct of the study. CM, ASB, PCo, RE, NP, IR, CR, MM, AC, NB, EW, HY have nothing to disclose.
Acknowledgments
Acknowledgments
This study was presented in part at the IAS 2015 Conference, Vancouver, 2015 (Abstract Nr. MOPEA036); at the 7th International Workshop on HIV-1 Persistence, HIV-1 Reservoirs and Strategies Towards HIV-1 Cure, Miami, 8-11th Dec 2015 (Abstract Nr. OP 9.3) and at CROI 2016 meeting (Abstract Nr. 320). We especially thank Rafaela Ayen and Carmen Ligero for their technical assistance. We thank the operational support of Okairos on the management with ChAdV63 vaccines given under the clinical trial agreement. After acquisition of Okairos, GSK now co-owns the ChAdV63.HIVconsv vaccine and allowed the BCN 01 trial to be completed. Special thanks to all the volunteers participating in this study for their perseverance and dedication, without whom this trial would not have been possible.
Role of the Funding Source
BCN01 study was funded by the HIVACAT Catalan research program for an HIV vaccine and Fundació Gloria Soler. The GMP manufacture of the ChAdV63.HIVconsv and MVA.HIVconsv vaccines was jointly funded by the Medical Research Council (MRC) UK and the UK Department for International Development (DFID) under the MRC/DFID Concordat agreements (G0701669). The funders had no role in the study design, data collection, data analysis, data interpretation, writing of the manuscript, or in the decision to submit the results for publication.
Members of the BCN 01 Study group
IrsiCaixa AIDS Research Institute-HIVACAT, Hospital Universitari Germans Trias i Pujol, Badalona, Spain: Beatriz Mothe, Pep Coll, Alvaro Sanchez-Bernabeu, Miriam Rosás, Maria C. Puertas, Sara Morón-López, Bonaventura Clotet, Javier Martinez-Picado and Christian Brander.
Fundació Lluita contra la Sida, Hospital Universitari Germans Trias i Pujol, Badalona, Spain: Patricia Cobarsi, Jordi Puig, Isabel Bravo, Roser Escrig, Jessica Toro, Aintzane Ayestaran-Loinaz, Silvia Gel, Nuria Perez-Alvarez, Arelly Ornelas, Cristina Perez-Reche and Ana Maria Barriocanal.
Hospital Clinic-HIVACAT, IDIBAPS, University of Barcelona, Spain: Christian Manzardo, Juan Ambrosioni, David Nicolas, Irene Ruiz, Cristina Rovira, Carmen Ligero, Josep M. Gatell and Jose M. Miro.
Projecte dels noms, BCN Checkpoint, Barcelona, Spain: Jorge Saz, Michael Meulbroek, Ferran Pujol.
The Jenner Institute, The Nuffield Department of Medicine, University of Oxford, UK: Nicola Borthwick, Alison Crook, Edmund G. Wee, Lucy Dorrell and Tomáš Hanke.
Author's Contributions
BM, LD, TH, BC and CB conceived and designed the study. BM, CM, PCo, PC, RE, IR, MM, AC, JA, DN, JMM, LD, BC and CB contributed to the study design and data management. BM, ASB, CR, MR, MCP, SML performed the experiments. NP, AO, BM, MCP and SML undertook the statistical analysis. NB, AC, EW, MR, LD, TH contributed with reagents/materials/analysis tools. BM, SML, CB and TH drafted the manuscript. CM, PCo, MCP, JMM, JMG, LD, JMP and BC participated in study analyses and revised the manuscript critically for important intellectual content. All authors reviewed and approved the final version of the manuscript.
Appendix A.
Appendix Fig. 1.
Correlation between baseline CD4 T-cell counts (left), CD4 T-cell counts increase at week 60 (right) and age of individuals at study entry is shown.
Appendix Fig. 2.
Net frequencies of cells specific for each pool (color-coded) are shown for each participant. Participant's numbers are shown above the graphs, A and B corresponding to the Long and Short vaccination arms, respectively.
Appendix Fig. 3.
Correlation between the baseline plasma viremia (log10 HIV-1 RNA copies/mL of plasma) and proviral HIV-1 DNA levels (log10 copies/106 CD4 + T cells) 24 (left) and 60 weeks (right) after treatment initiation in all vaccinated and non-vaccinated individuals (Spearman r).
References
- 1.Mothe B., Ibarrondo J., Llano A., Brander C. Virological, immune and host genetics markers in the control of HIV infection. Dis Markers. 2009;27(3):105–120. doi: 10.3233/DMA-2009-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mothe B, Ibarrondo J, Llano A, Brander C. Virological, immune and host genetics markers in the control of HIV infection. Dis Markers. 2009;27(3):105-120. doi:10.3233/DMA-2009-0655 [DOI] [PMC free article] [PubMed]
- 2.Schmitz J.E., Kuroda M.J., Santra S. Control of viremia in simian immunodeficiency virus infection by CD8 + lymphocytes. Science. 1999;283(5403):857–860. doi: 10.1126/science.283.5403.857. http://www.ncbi.nlm.nih.gov/pubmed/9933172 [DOI] [PubMed] [Google Scholar]; Schmitz JE, Kuroda MJ, Santra S, et al. Control of viremia in simian immunodeficiency virus infection by CD8 + lymphocytes. Science. 1999;283(5403):857-860. http://www.ncbi.nlm.nih.gov/pubmed/9933172. Accessed November 25, 2015. [DOI] [PubMed]
- 3.Cartwright E.K., Spicer L., Smith S.A. CD8(+) lymphocytes are required for maintaining viral suppression in SIV-infected macaques treated with short-term antiretroviral therapy. Immunity. 2016;45(3):656–668. doi: 10.1016/j.immuni.2016.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cartwright EK, Spicer L, Smith SA, et al. CD8(+) Lymphocytes Are Required for Maintaining Viral Suppression in SIV-Infected Macaques Treated with Short-Term Antiretroviral Therapy. Immunity. 2016;45(3):656-668. doi:10.1016/j.immuni.2016.08.018 [DOI] [PMC free article] [PubMed]
- 4.Ndhlovu Z.M., Kamya P., Mewalal N. Magnitude and kinetics of CD8 + T cell activation during hyperacute HIV infection impact viral set point. Immunity. 2015;43(3):591–604. doi: 10.1016/j.immuni.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ndhlovu ZM, Kamya P, Mewalal N, et al. Magnitude and Kinetics of CD8 + T Cell Activation during Hyperacute HIV Infection Impact Viral Set Point. Immunity. 2015;43(3):591-604. doi:10.1016/j.immuni.2015.08.012 [DOI] [PMC free article] [PubMed]
- 5.Streeck H., Jolin J.S., Qi Y. Human immunodeficiency virus type 1-specific CD8 + T-cell responses during primary infection are major determinants of the viral set point and loss of CD4 + T cells. J Virol. 2009;83(15):7641–7648. doi: 10.1128/JVI.00182-09. [DOI] [PMC free article] [PubMed] [Google Scholar]; Streeck H, Jolin JS, Qi Y, et al. Human immunodeficiency virus type 1-specific CD8 + T-cell responses during primary infection are major determinants of the viral set point and loss of CD4 + T cells. J Virol. 2009;83(15):7641-7648. doi:10.1128/JVI.00182-09 [DOI] [PMC free article] [PubMed]
- 6.Autran B., Murphy R.L., Costagliola D. Greater viral rebound and reduced time to resume antiretroviral therapy after therapeutic immunization with the ALVAC-HIV vaccine (vCP1452) AIDS. 2008;22(11):1313–1322. doi: 10.1097/QAD.0b013e3282fdce94. [DOI] [PubMed] [Google Scholar]; Autran B, Murphy RL, Costagliola D, et al. Greater viral rebound and reduced time to resume antiretroviral therapy after therapeutic immunization with the ALVAC-HIV vaccine (vCP1452). AIDS. 2008;22(11):1313-1322. doi:10.1097/QAD.0b013e3282fdce94 [DOI] [PubMed]
- 7.Schooley R.T., Spritzler J., Wang H. AIDS clinical trials group 5197: a placebo-controlled trial of immunization of HIV-1-infected persons with a replication-deficient adenovirus type 5 vaccine expressing the HIV-1 core protein. J Infect Dis. 2010;202(5):705–716. doi: 10.1086/655468. [DOI] [PMC free article] [PubMed] [Google Scholar]; Schooley RT, Spritzler J, Wang H, et al. AIDS clinical trials group 5197: a placebo-controlled trial of immunization of HIV-1-infected persons with a replication-deficient adenovirus type 5 vaccine expressing the HIV-1 core protein. J Infect Dis. 2010;202(5):705-716. doi:10.1086/655468 [DOI] [PMC free article] [PubMed]
- 8.Harrer T., Plettenberg A., Arastéh K. Safety and immunogenicity of an adjuvanted protein therapeutic HIV-1 vaccine in subjects with HIV-1 infection: a randomised placebo-controlled study. Vaccine. 2014;32(22):2657–2665. doi: 10.1016/j.vaccine.2013.10.030. [DOI] [PubMed] [Google Scholar]; Harrer T, Plettenberg A, Arastéh K, et al. Safety and immunogenicity of an adjuvanted protein therapeutic HIV-1 vaccine in subjects with HIV-1 infection: a randomised placebo-controlled study. Vaccine. 2014;32(22):2657-2665. doi:10.1016/j.vaccine.2013.10.030 [DOI] [PubMed]
- 9.Caskey M., Schoofs T., Gruell H. Antibody 10-1074 suppresses viremia in HIV-1-infected individuals. Nat Med. 2017 doi: 10.1038/nm.4268. January. [DOI] [PMC free article] [PubMed] [Google Scholar]; Caskey M, Schoofs T, Gruell H, et al. Antibody 10-1074 suppresses viremia in HIV-1-infected individuals. Nat Med. January 2017. doi:10.1038/nm.4268 [DOI] [PMC free article] [PubMed]
- 10.Scheid J.F., Horwitz J.A., Bar-On Y. HIV-1 antibody 3BNC117 suppresses viral rebound in humans during treatment interruption. Nature. 2016 doi: 10.1038/nature18929. June. [DOI] [PMC free article] [PubMed] [Google Scholar]; Scheid JF, Horwitz JA, Bar-On Y, et al. HIV-1 antibody 3BNC117 suppresses viral rebound in humans during treatment interruption. Nature. June 2016. doi:10.1038/nature18929 [DOI] [PMC free article] [PubMed]
- 11.Sneller M.C., Justement J.S., Gittens K.R. A randomized controlled safety/efficacy trial of therapeutic vaccination in HIV-infected individuals who initiated antiretroviral therapy early in infection. Sci Transl Med. 2017;9(419):eaan8848. doi: 10.1126/scitranslmed.aan8848. [DOI] [PMC free article] [PubMed] [Google Scholar]; Sneller MC, Justement JS, Gittens KR, et al. A randomized controlled safety/efficacy trial of therapeutic vaccination in HIV-infected individuals who initiated antiretroviral therapy early in infection. Sci Transl Med. 2017;9(419):eaan8848. doi:10.1126/scitranslmed.aan8848 [DOI] [PMC free article] [PubMed]
- 12.García F., Climent N., Guardo A.C. A dendritic cell-based vaccine elicits T cell responses associated with control of HIV-1 replication. Sci Transl Med. 2013;5(166):166ra2. doi: 10.1126/scitranslmed.3004682. [DOI] [PubMed] [Google Scholar]; García F, Climent N, Guardo AC, et al. A dendritic cell-based vaccine elicits T cell responses associated with control of HIV-1 replication. Sci Transl Med. 2013;5(166):166ra2. doi:10.1126/scitranslmed.3004682 [DOI] [PubMed]
- 13.Hanke T. Conserved immunogens in prime-boost strategies for the next-generation HIV-1 vaccines. Expert Opin Biol Ther. 2014;14(5):601–616. doi: 10.1517/14712598.2014.885946. [DOI] [PubMed] [Google Scholar]; Hanke T. Conserved immunogens in prime-boost strategies for the next-generation HIV-1 vaccines. Expert Opin Biol Ther. 2014;14(5):601-616. doi:10.1517/14712598.2014.885946 [DOI] [PubMed]
- 14.Kulkarni V., Valentin A., Rosati M. Altered response hierarchy and increased T-cell breadth upon HIV-1 conserved element DNA vaccination in macaques. PLoS One. 2014;9(1) doi: 10.1371/journal.pone.0086254. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kulkarni V, Valentin A, Rosati M, et al. Altered Response Hierarchy and Increased T-Cell Breadth upon HIV-1 Conserved Element DNA Vaccination in Macaques. PLoS One. 2014;9(1):e86254. doi:10.1371/journal.pone.0086254 [DOI] [PMC free article] [PubMed]
- 15.Mothe B., Llano A., Ibarrondo J. Definition of the viral targets of protective HIV-1-specific T cell responses. J Transl Med. 2011;9:208. doi: 10.1186/1479-5876-9-208. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mothe B, Llano A, Ibarrondo J, et al. Definition of the viral targets of protective HIV-1-specific T cell responses. J Transl Med. 2011;9:208. doi:10.1186/1479-5876-9-208 [DOI] [PMC free article] [PubMed]
- 16.Pereyra F., Heckerman D., Carlson J.M. HIV control is mediated in part by CD8 + T-cell targeting of specific epitopes. J Virol. 2014;88(22):12937–12948. doi: 10.1128/JVI.01004-14. [DOI] [PMC free article] [PubMed] [Google Scholar]; Pereyra F, Heckerman D, Carlson JM, et al. HIV Control Is Mediated in Part by CD8 + T-Cell Targeting of Specific Epitopes. J Virol. 2014;88(22):12937-12948. doi:10.1128/JVI.01004-14 [DOI] [PMC free article] [PubMed]
- 17.Murakoshi H., Akahoshi T., Koyanagi M. Clinical control of HIV-1 by cytotoxic T cells specific for multiple conserved epitopes. J Virol. 2015 doi: 10.1128/JVI.00020-15. March. JVI.00020-15- [DOI] [PMC free article] [PubMed] [Google Scholar]; Murakoshi H, Akahoshi T, Koyanagi M, et al. Clinical Control of HIV-1 by Cytotoxic T Cells Specific for Multiple Conserved Epitopes. J Virol. March 2015:JVI.00020-15-. doi:10.1128/JVI.00020-15 [DOI] [PMC free article] [PubMed]
- 18.Hancock G., Yang H., Yorke E. Identification of effective subdominant anti-HIV-1 CD8 + T cells within entire post-infection and post-vaccination immune responses. PLoS Pathog. 2015;11(2) doi: 10.1371/journal.ppat.1004658. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hancock G, Yang H, Yorke E, et al. Identification of effective subdominant anti-HIV-1 CD8 + T cells within entire post-infection and post-vaccination immune responses. PLoS Pathog. 2015;11(2):e1004658. doi:10.1371/journal.ppat.1004658 [DOI] [PMC free article] [PubMed]
- 19.Létourneau S., Im E.-J., Mashishi T. Design and pre-clinical evaluation of a universal HIV-1 vaccine. PLoS One. 2007;2(10):e984. doi: 10.1371/journal.pone.0000984. [DOI] [PMC free article] [PubMed] [Google Scholar]; Létourneau S, Im E-J, Mashishi T, et al. Design and pre-clinical evaluation of a universal HIV-1 vaccine. PLoS One. 2007;2(10):e984. doi:10.1371/journal.pone.0000984 [DOI] [PMC free article] [PubMed]
- 20.Rolland M., Nickle D.C., Mullins J.I. HIV-1 group M conserved elements vaccine. PLoS Pathog. 2007;3(11):e157. doi: 10.1371/journal.ppat.0030157. [DOI] [PMC free article] [PubMed] [Google Scholar]; Rolland M, Nickle DC, Mullins JI. HIV-1 group M conserved elements vaccine. PLoS Pathog. 2007;3(11):e157. doi:10.1371/journal.ppat.0030157 [DOI] [PMC free article] [PubMed]
- 21.Ondondo B., Murakoshi H., Clutton G. Novel conserved-region T-cell mosaic vaccine with high global HIV-1 coverage is recognized by protective responses in untreated infection. Mol Ther. 2016;24(4):832–842. doi: 10.1038/mt.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ondondo B, Murakoshi H, Clutton G, et al. Novel Conserved-region T-cell Mosaic Vaccine With High Global HIV-1 Coverage Is Recognized by Protective Responses in Untreated Infection. Mol Ther. 2016;24(4):832-842. doi:10.1038/mt.2016.3 [DOI] [PMC free article] [PubMed]
- 22.Mutua G., Farah B., Langat R. Broad HIV-1 inhibition in vitro by vaccine-elicited CD8(+) T cells in African adults. Mol Ther Methods Clin Dev. 2016;3 doi: 10.1038/mtm.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mutua G, Farah B, Langat R, et al. Broad HIV-1 inhibition in vitro by vaccine-elicited CD8(+) T cells in African adults. Mol Ther Methods Clin Dev. 2016;3:16061. doi:10.1038/mtm.2016.61 [DOI] [PMC free article] [PubMed]
- 23.Pereyra F., Jia X., McLaren P.J. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science. 2010;330(6010):1551–1557. doi: 10.1126/science.1195271. [DOI] [PMC free article] [PubMed] [Google Scholar]; Pereyra F, Jia X, McLaren PJ, et al. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science. 2010;330(6010):1551-1557. doi:10.1126/science.1195271 [DOI] [PMC free article] [PubMed]
- 24.Borthwick N., Ahmed T., Ondondo B. Vaccine-elicited human T cells recognizing conserved protein regions inhibit HIV-1. Mol Ther. 2014;22(2):464–475. doi: 10.1038/mt.2013.248. [DOI] [PMC free article] [PubMed] [Google Scholar]; Borthwick N, Ahmed T, Ondondo B, et al. Vaccine-elicited human T cells recognizing conserved protein regions inhibit HIV-1. Mol Ther. 2014;22(2):464-475. doi:10.1038/mt.2013.248 [DOI] [PMC free article] [PubMed]
- 25.Hayton E.-J., Rose A., Ibrahimsa U. Safety and tolerability of conserved region vaccines vectored by plasmid DNA, simian adenovirus and modified vaccinia virus ankara administered to human immunodeficiency virus type 1-uninfected adults in a randomized, single-blind phase I trial. PLoS One. 2014;9(7) doi: 10.1371/journal.pone.0101591. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hayton E-J, Rose A, Ibrahimsa U, et al. Safety and tolerability of conserved region vaccines vectored by plasmid DNA, simian adenovirus and modified vaccinia virus ankara administered to human immunodeficiency virus type 1-uninfected adults in a randomized, single-blind phase I trial. PLoS One. 2014;9(7):e101591. doi:10.1371/journal.pone.0101591 [DOI] [PMC free article] [PubMed]
- 26.McElrath M.J., De Rosa S.C., Moodie Z. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet. 2008;372(9653):1894–1905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]; McElrath MJ, De Rosa SC, Moodie Z, et al. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet. 2008;372(9653):1894-1905. doi:10.1016/S0140-6736(08)61592-5 [DOI] [PMC free article] [PubMed]
- 27.Rerks-Ngarm S., Pitisuttithum P., Nitayaphan S. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361(23):2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]; Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361(23):2209-2220. doi:10.1056/NEJMoa0908492 [DOI] [PubMed]
- 28.Colloca S., Barnes E., Folgori A. Vaccine vectors derived from a large collection of simian adenoviruses induce potent cellular immunity across multiple species. Sci Transl Med. 2012;4(115):115ra2. doi: 10.1126/scitranslmed.3002925. [DOI] [PMC free article] [PubMed] [Google Scholar]; Colloca S, Barnes E, Folgori A, et al. Vaccine Vectors Derived from a Large Collection of Simian Adenoviruses Induce Potent Cellular Immunity Across Multiple Species. Sci Transl Med. 2012;4(115):115ra2-115ra2. doi:10.1126/scitranslmed.3002925 [DOI] [PMC free article] [PubMed]
- 29.Buchbinder S.P., Mehrotra D.V., Duerr A. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372(9653):1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]; Buchbinder SP, Mehrotra D V, Duerr A, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372(9653):1881-1893. doi:10.1016/S0140-6736(08)61591-3 [DOI] [PMC free article] [PubMed]
- 30.Gray G., Buchbinder S., Duerr A. Overview of STEP and Phambili trial results: two phase IIb test of concept studies investigating the efficacy of MRK ad5 gag/pol/nef sub-type B HIV vaccine. Curr Opin HIV AIDS. 2010;5(5):357–361. doi: 10.1097/COH.0b013e32833d2d2b. [DOI] [PMC free article] [PubMed] [Google Scholar]; Gray G, Buchbinder S, Duerr A. Overview of STEP and Phambili trial Results: two phase IIb test of concept studies investigating the efficacy of MRK ad5 gag/pol/nef sub-type B HIV vaccine. Curr Opin HIV AIDS. 2010;5(5):357-361. doi:10.1097/COH.0b013e32833d2d2b.Overview [DOI] [PMC free article] [PubMed]
- 31.Hanke T., Goonetilleke N., McMichael A.J., Dorrell L. Clinical experience with plasmid DNA- and modified vaccinia virus Ankara-vectored human immunodeficiency virus type 1 clade a vaccine focusing on T-cell induction. J Gen Virol. 2007;88(Pt 1):1–12. doi: 10.1099/vir.0.82493-0. [DOI] [PubMed] [Google Scholar]; Hanke T, Goonetilleke N, McMichael AJ, Dorrell L. Clinical experience with plasmid DNA- and modified vaccinia virus Ankara-vectored human immunodeficiency virus type 1 clade A vaccine focusing on T-cell induction. J Gen Virol. 2007;88(Pt 1):1-12. doi:10.1099/vir.0.82493-0 [DOI] [PubMed]
- 32.Fidler S., Porter K., Ewings F. Short-course antiretroviral therapy in primary HIV infection. N Engl J Med. 2013;368(3):207–217. doi: 10.1056/NEJMoa1110039. [DOI] [PMC free article] [PubMed] [Google Scholar]; Fidler S, Porter K, Ewings F, et al. Short-course antiretroviral therapy in primary HIV infection. N Engl J Med. 2013;368(3):207-217. doi:10.1056/NEJMoa1110039 [DOI] [PMC free article] [PubMed]
- 33.Le T., Wright E.J., Smith D.M. Enhanced CD4 + T-cell recovery with earlier HIV-1 antiretroviral therapy. N Engl J Med. 2013;368(3):218–230. doi: 10.1056/NEJMoa1110187. [DOI] [PMC free article] [PubMed] [Google Scholar]; Le T, Wright EJ, Smith DM, et al. Enhanced CD4 + T-cell recovery with earlier HIV-1 antiretroviral therapy. N Engl J Med. 2013;368(3):218-230. doi:10.1056/NEJMoa1110187 [DOI] [PMC free article] [PubMed]
- 34.Lundgren J.D., Babiker A.G., Gordin F. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373(9):795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lundgren JD, Babiker AG, Gordin F, et al. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med. 2015;373(9):795-807. doi:10.1056/NEJMoa1506816 [DOI] [PMC free article] [PubMed]
- 35.Deng K., Pertea M., Rongvaux A. Broad CTL response is required to clear latent HIV-1 due to dominance of escape mutations. Nature. 2015;517(7534):381–385. doi: 10.1038/nature14053. [DOI] [PMC free article] [PubMed] [Google Scholar]; Deng K, Pertea M, Rongvaux A, et al. Broad CTL response is required to clear latent HIV-1 due to dominance of escape mutations. Nature. 2015;517(7534):381-385. doi:10.1038/nature14053 [DOI] [PMC free article] [PubMed]
- 36.Ananworanich J., Puthanakit T., Suntarattiwong P. Reduced markers of HIV persistence and restricted HIV-specific immune responses after early antiretroviral therapy in children. AIDS. 2014;28(7):1015–1020. doi: 10.1097/QAD.0000000000000178. [DOI] [PubMed] [Google Scholar]; Ananworanich J, Puthanakit T, Suntarattiwong P, et al. Reduced markers of HIV persistence and restricted HIV-specific immune responses after early antiretroviral therapy in children. AIDS. 2014;28(7):1015-1020. doi:10.1097/QAD.0000000000000178 [DOI] [PubMed]
- 37.Hocqueloux L., Avettand-Fènoël V., Jacquot S. Long-term antiretroviral therapy initiated during primary HIV-1 infection is key to achieving both low HIV reservoirs and normal T cell counts. J Antimicrob Chemother. 2013;68(5):1169–1178. doi: 10.1093/jac/dks533. [DOI] [PubMed] [Google Scholar]; Hocqueloux L, Avettand-Fènoël V, Jacquot S, et al. Long-term antiretroviral therapy initiated during primary HIV-1 infection is key to achieving both low HIV reservoirs and normal T cell counts. J Antimicrob Chemother. 2013;68(5):1169-1178. doi:10.1093/jac/dks533 [DOI] [PubMed]
- 38.Hey-Cunningham W.J., Murray J.M., Natarajan V. Early antiretroviral therapy with raltegravir generates sustained reductions in HIV reservoirs but not lower T-cell activation levels. AIDS. 2015 doi: 10.1097/QAD.0000000000000625. February. [DOI] [PubMed] [Google Scholar]; Hey-Cunningham WJ, Murray JM, Natarajan V, et al. Early antiretroviral therapy with raltegravir generates sustained reductions in HIV reservoirs but not lower T-cell activation levels. AIDS. February 2015. doi:10.1097/QAD.0000000000000625 [DOI] [PubMed]
- 39.Martínez-Bonet M., Puertas M.C., Fortuny C. Establishment and replenishment of the viral reservoir in perinatally HIV-1-infected children initiating very early antiretroviral therapy. Clin Infect Dis. 2015;61(7):1169–1178. doi: 10.1093/cid/civ456. [DOI] [PMC free article] [PubMed] [Google Scholar]; Martínez-Bonet M, Puertas MC, Fortuny C, et al. Establishment and Replenishment of the Viral Reservoir in Perinatally HIV-1-infected Children Initiating Very Early Antiretroviral Therapy. Clin Infect Dis. 2015;61(7):1169-1178. doi:10.1093/cid/civ456 [DOI] [PMC free article] [PubMed]
- 40.Luzuriaga K., Gay H., Ziemniak C. Viremic relapse after HIV-1 remission in a perinatally infected child. N Engl J Med. 2015;372(8):786–788. doi: 10.1056/NEJMc1413931. [DOI] [PMC free article] [PubMed] [Google Scholar]; Luzuriaga K, Gay H, Ziemniak C, et al. Viremic relapse after HIV-1 remission in a perinatally infected child. N Engl J Med. 2015;372(8):786-788. doi:10.1056/NEJMc1413931 [DOI] [PMC free article] [PubMed]
- 41.Persaud D., Gay H., Ziemniak C. Absence of detectable HIV-1 viremia after treatment cessation in an infant. N Engl J Med. 2013;369(19):1828–1835. doi: 10.1056/NEJMoa1302976. [DOI] [PMC free article] [PubMed] [Google Scholar]; Persaud D, Gay H, Ziemniak C, et al. Absence of detectable HIV-1 viremia after treatment cessation in an infant. N Engl J Med. 2013;369(19):1828-1835. doi:10.1056/NEJMoa1302976 [DOI] [PMC free article] [PubMed]
- 42.Colby D.J., Trautmann L., Pinyakorn S. Rapid HIV RNA rebound after antiretroviral treatment interruption in persons durably suppressed in Fiebig I acute HIV infection. Nat Med. 2018;24(7):923–926. doi: 10.1038/s41591-018-0026-6. [DOI] [PMC free article] [PubMed] [Google Scholar]; Colby DJ, Trautmann L, Pinyakorn S, et al. Rapid HIV RNA rebound after antiretroviral treatment interruption in persons durably suppressed in Fiebig I acute HIV infection. Nat Med. 2018;24(7):923-926. doi:10.1038/s41591-018-0026-6 [DOI] [PMC free article] [PubMed]
- 43.Anderson J.L., Fromentin R., Corbelli G.M., Østergaard L., Ross A.L. Progress towards an HIV cure: update from the 2014 International AIDS Society Symposium. AIDS Res Hum Retroviruses. 2015;31(1):36–44. doi: 10.1089/aid.2014.0236. [DOI] [PMC free article] [PubMed] [Google Scholar]; Anderson JL, Fromentin R, Corbelli GM, Østergaard L, Ross AL. Progress Towards an HIV Cure: Update from the 2014 International AIDS Society Symposium. AIDS Res Hum Retroviruses. 2015;31(1):36-44. doi:10.1089/AID.2014.0236 [DOI] [PMC free article] [PubMed]
- 44.Shan L., Deng K., Shroff N.S. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity. 2012;36(3):491–501. doi: 10.1016/j.immuni.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]; Shan L, Deng K, Shroff NS, et al. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity. 2012;36(3):491-501. doi:10.1016/j.immuni.2012.01.014 [DOI] [PMC free article] [PubMed]
- 45.Søgaard O.S., Graversen M.E., Leth S. The depsipeptide romidepsin reverses HIV-1 latency in vivo. PLoS Pathog. 2015;11(9) doi: 10.1371/journal.ppat.1005142. [DOI] [PMC free article] [PubMed] [Google Scholar]; Søgaard OS, Graversen ME, Leth S, et al. The Depsipeptide Romidepsin Reverses HIV-1 Latency In Vivo. PLoS Pathog. 2015;11(9):e1005142. doi:10.1371/journal.ppat.1005142 [DOI] [PMC free article] [PubMed]
- 46.Leth S., Schleimann M.H., Nissen S.K. Combined effect of Vacc-4x, recombinant human granulocyte macrophage colony-stimulating factor vaccination, and romidepsin on the HIV-1 reservoir (REDUC): a single-arm, phase 1B/2A trial. Lancet HIV. 2016;3(10):e463–e472. doi: 10.1016/S2352-3018(16)30055-8. [DOI] [PubMed] [Google Scholar]; Leth S, Schleimann MH, Nissen SK, et al. Combined effect of Vacc-4x, recombinant human granulocyte macrophage colony-stimulating factor vaccination, and romidepsin on the HIV-1 reservoir (REDUC): a single-arm, phase 1B/2A trial. Lancet HIV. 2016;3(10):e463-e472. doi:10.1016/S2352-3018(16)30055-8 [DOI] [PubMed]
- 47.Cohen M.S., Chen Y.Q., McCauley M. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493-505. doi:10.1056/NEJMoa1105243 [DOI] [PMC free article] [PubMed]
- 48.Rosario M., Bridgeman A., Quakkelaar E.D. Long peptides induce polyfunctional T cells against conserved regions of HIV-1 with superior breadth to single-gene vaccines in macaques. Eur J Immunol. 2010;40:1973–1984. doi: 10.1002/eji.201040344. [DOI] [PubMed] [Google Scholar]; Rosario M, Bridgeman A, Quakkelaar ED, et al. Long peptides induce polyfunctional T cells against conserved regions of HIV-1 with superior breadth to single-gene vaccines in macaques. Eur J Immunol. 2010;40:1973-1984. doi:10.1002/eji.201040344 [DOI] [PubMed]
- 49.Hancock G., Morón-López S., Kopycinski J. Evaluation of the immunogenicity and impact on the latent HIV-1 reservoir of a conserved region vaccine, MVA.HIVconsv, in antiretroviral therapy-treated subjects. J Int AIDS Soc. 2017;20(1):1–11. doi: 10.7448/IAS.20.1.21171. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hancock G, Morón-López S, Kopycinski J, et al. Evaluation of the immunogenicity and impact on the latent HIV-1 reservoir of a conserved region vaccine, MVA.HIVconsv, in antiretroviral therapy-treated subjects. J Int AIDS Soc. 2017;20(1):1-11. doi:10.7448/IAS.20.1.21171 [DOI] [PMC free article] [PubMed]
- 50.Puertas M.C., Massanella M., Llibre J.M. Intensification of a raltegravir-based regimen with maraviroc in early HIV-1 infection. AIDS. 2014;28(3):325–334. doi: 10.1097/QAD.0000000000000066. [DOI] [PubMed] [Google Scholar]; Puertas MC, Massanella M, Llibre JM, et al. Intensification of a raltegravir-based regimen with maraviroc in early HIV-1 infection. AIDS. 2014;28(3):325-334. doi:10.1097/QAD.0000000000000066 [DOI] [PubMed]
- 51.Hindson B.J., Ness K.D., Masquelier D.A. High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Anal Chem. 2011;83(22):8604–8610. doi: 10.1021/ac202028g. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hindson BJ, Ness KD, Masquelier DA, et al. High-Throughput Droplet Digital PCR System for Absolute Quantitation of DNA Copy Number. Anal Chem. 2011;83(22):8604-8610. doi:10.1021/ac202028g [DOI] [PMC free article] [PubMed]
- 52.Mothe B., Hu X., Llano A. A human immune data-informed vaccine concept elicits strong and broad T-cell specificities associated with HIV-1 control in mice and macaques. J Transl Med. 2015;13(1):60. doi: 10.1186/s12967-015-0392-5. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mothe B, Hu X, Llano A, et al. A human immune data-informed vaccine concept elicits strong and broad T-cell specificities associated with HIV-1 control in mice and macaques. J Transl Med. 2015;13(1):60. doi:10.1186/s12967-015-0392-5 [DOI] [PMC free article] [PubMed]
- 53.Bansal A., Gough E., Sabbaj S. CD8 T-cell responses in early HIV-1 infection are skewed towards high entropy peptides. AIDS. 2005;19(3):241–250. http://www.ncbi.nlm.nih.gov/pubmed/15718834 [PubMed] [Google Scholar]; Bansal A, Gough E, Sabbaj S, et al. CD8 T-cell responses in early HIV-1 infection are skewed towards high entropy peptides. AIDS. 2005;19(3):241-250. http://www.ncbi.nlm.nih.gov/pubmed/15718834. Accessed August 12, 2018. [PubMed]
- 54.Yang O.O., Daar E.S., Ng H.L., Shih R., Jamieson B.D. Increasing CTL targeting of conserved sequences during early HIV-1 infection is correlated to decreasing viremia. AIDS Res Hum Retroviruses. 2011;27(4):391–398. doi: 10.1089/aid.2010.0183. [DOI] [PMC free article] [PubMed] [Google Scholar]; Yang OO, Daar ES, Ng HL, Shih R, Jamieson BD. Increasing CTL targeting of conserved sequences during early HIV-1 infection is correlated to decreasing viremia. AIDS Res Hum Retroviruses. 2011;27(4):391-398. doi:10.1089/aid.2010.0183 [DOI] [PMC free article] [PubMed]
- 55.Mothe B., Climent N., Plana M. Safety and immunogenicity of a modified vaccinia Ankara-based HIV-1 vaccine (MVA-B) in HIV-1-infected patients alone or in combination with a drug to reactivate latent HIV-1. J Antimicrob Chemother. February 2015 doi: 10.1093/jac/dkv046. [DOI] [PubMed] [Google Scholar]; Mothe B, Climent N, Plana M, et al. Safety and immunogenicity of a modified vaccinia Ankara-based HIV-1 vaccine (MVA-B) in HIV-1-infected patients alone or in combination with a drug to reactivate latent HIV-1. J Antimicrob Chemother. February 2015. doi:10.1093/jac/dkv046 [DOI] [PubMed]
- 56.Thornhill J., Inshaw J., Kaleebu P. Brief report. JAIDS J Acquir Immune Defic Syndr. 2016;73(1):69–73. doi: 10.1097/QAI.0000000000001013. [DOI] [PMC free article] [PubMed] [Google Scholar]; Thornhill J, Inshaw J, Kaleebu P, et al. Brief Report. JAIDS J Acquir Immune Defic Syndr. 2016;73(1):69-73. doi:10.1097/QAI.0000000000001013 [DOI] [PMC free article] [PubMed]
- 57.Ahmed T., Borthwick N.J., Gilmour J., Hayes P., Dorrell L., Hanke T. Control of HIV-1 replication in vitro by vaccine-induced human CD8(+) T cells through conserved subdominant Pol epitopes. Vaccine. 2016;34(9):1215–1224. doi: 10.1016/j.vaccine.2015.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ahmed T, Borthwick NJ, Gilmour J, Hayes P, Dorrell L, Hanke T. Control of HIV-1 replication in vitro by vaccine-induced human CD8(+) T cells through conserved subdominant Pol epitopes. Vaccine. 2016;34(9):1215-1224. doi:10.1016/j.vaccine.2015.12.021 [DOI] [PMC free article] [PubMed]
- 58.Fidler S., Stohr W., Pace M. 22nd International AIDS Conference; Amsterdam, July 23–27. 2018. A randomised controlled trial comparing the impact of antiretroviral therapy (ART) with a “kick-and-kill” approach to ART alone on HIV reservoirs in individuals with primary HIV infection (PHI); RIVER trial. [Google Scholar]; Fidler S, Stohr W, Pace M, et al. A randomised controlled trial comparing the impact of antiretroviral therapy (ART) with a “Kick-and-Kill” approach to ART alone on HIV reservoirs in individuals with primary HIV infection (PHI); RIVER trial. In: 22nd International AIDS Conference; Amsterdam, July 23-27 2018.
- 59.Ananworanich J., Chomont N., Fletcher J.L. Markers of HIV reservoir size and immune activation after treatment in acute HIV infection with and without raltegravir and maraviroc intensification. J Virus Erad. 2017;1(2):116–122. http://www.ncbi.nlm.nih.gov/pubmed/26835516.nnnnJanuary15 [PMC free article] [PubMed] [Google Scholar]; Ananworanich J, Chomont N, Fletcher JL, et al. Markers of HIV reservoir size and immune activation after treatment in acute HIV infection with and without raltegravir and maraviroc intensification. J virus Erad. 1(2):116-122. http://www.ncbi.nlm.nih.gov/pubmed/26835516.nnnnJanuary15,2017. [PMC free article] [PubMed]