Abstract
Background
Surgical audit, sometimes including public reporting, is an important foundation of high quality health care. We aimed to assess the validity of a novel outcome metric, days at home up to 30 days after surgery, as a surgical outcome measure in clinical trials and quality assurance.
Methods
This was a multicentre, registry-based cohort study. We used prospectively collected hospital and national healthcare registry data obtained from patients aged 18 years or older undergoing a broad range of surgeries in Sweden over a 10-year period. The association between days at home up to 30 days after surgery and patient (older age, poorer physical status, comorbidity) and surgical (elective or non-elective, complexity, duration) risk factors, process of care outcomes (re-admissions, discharge destination), clinical outcomes (major complications, 30-day mortality) and death up to 1 year after surgery were measured.
Findings
From January, 2005, to December, 2014, we obtained demographic and perioperative data on 636,885 patients from 21 Swedish hospitals. Mortality at 30 days and one year was 1.8% and 7.3%, respectively. The median (IQR) days at home up to 30 days after surgery was 27 (23–29), being significantly lower among high-risk patients, those recovering from more complex surgical procedures, and suffering serious postoperative complications (all p < 0.0001). Patients with 8 days or less at home up to 30 days after surgery had a nearly 7-fold higher risk of death up to 1 year postoperatively when compared with those with 29 or 30 days at home (adjusted HR 6.78 [95% CI: 6.44–7.13]).
Interpretation
Days at home up to 30 days after surgery is a valid, easy to measure patient-centred outcome metric. It is highly sensitive to changes in surgical risk and impact of complications, and has prognostic importance; it is therefore a valuable endpoint for perioperative clinical trials and quality assurance.
Funding
Swedish National Research Council Medicine and Stockholm County Council ALF-project grant (LE), and the Australian National Health and Medical Research Council (PM).
Keywords: Anaesthesia, Audit, Postoperative complications, Patient-reported outcome measures, Surgery, Survival
Research in context
Evidence before this study
In 2017, Myles and colleagues published a retrospective study of clinical trial data (7 trials, 2109 patients) demonstrating that the number of days at home within 30 days of surgery (DAH30) was a valid outcome metric that integrates length of hospital stay, re-admission, discharge to a nursing facility, and death up to 30 days after surgery, and was associated with higher risk status and serious complications after surgery. However, this was a single-centre study with incomplete discharge destination data, and there was no longer-term follow-up.
Added value of this study
In this analysis of hospital and national healthcare registry data that included 636,885 adults undergoing elective and non-elective surgery, the number of postoperative days at home up to 30 days after surgery was lowest in patients at higher surgical risk and in those with complications. Patients with 8 days or less at home up to 30 days after surgery had a higher risk of death up to 1 year postoperatively when compared with those with 29 or 30 days at home (adjusted HR 6.78, 95% CI: 6.44–7.13). There was an incremental increase in 30-day complication rates, and decrease in 1-year survival, as days at home decreased.
Implications of all the available evidence
DAH30 is a valid and readily-obtainable generic patient-centred outcome measure. It is highly sensitive to comorbidity burden, differences in surgical risk, process of care outcomes, and impact of perioperative complications, and is associated with mortality up to 1 year after surgery. DAH30 is an ideal, patient-centred outcome measure for perioperative clinical trials and quality assurance. In addition, DAH30, as numerical data, provides greater statistical power and so can reduce the sample size required to evaluate new treatments in perioperative practice. Future studies should elucidate the value of DAH30 in surgical audit.
Alt-text: Unlabelled Box
1. Introduction
High quality surgery and perioperative care minimises preventable complications and improves the patient experience after surgery, lowering health care costs [1], [2], [3], [4], [5], [6]. Medical research, and in particular clinical trials, make an important contribution to these goals [7]. Outcome measures should be patient-centred [1], [4], while simultaneously valid, reliable and clinically meaningful in order to inform best practice [8], [9], [10], [11]. While postoperative complications occur too frequently, not all are serious and most can be managed to avoid early death or long-term patient harm [12]. It is unlikely that measuring complications alone fully captures the patient experience or eventual recovery after surgery. Collection and reporting of data on the quality of health care is expensive in terms of both time and resources [13], [14].
Hospital length of stay, by itself, is an inadequate measure of the success of surgery [15]. Patients discharged too early or in poor condition are more likely to require re-admission [16]. Premature hospital discharge may also be associated with increased 30-day mortality [17]. These outcomes may be masked by a reported reduction in hospital length of stay. Conversely, perioperative complications prolong hospital stay. Some major complications result in early death or patient discharge to a nursing facility. Surgical or serious illness outcomes leading to loss of the ability to live independently is a major concern for the elderly [18], [19].
An ideal healthcare quality indicator should be valid, reliably collected, and also reflect the patient perspective [1], [2], [3], [9]. Avoiding extra days in hospital after surgery or acute illness is highly valued by most patients [4], [19], [20], [21], [22], [23]. Accordingly, home days, home-to-home days [24], and days alive and out of hospital [25], [26], [27], [28] are related metrics that have been suggested to characterise the overall success of healthcare. We previously devised a modification of these metrics for the surgical setting, validating “days at home up to 30 days after surgery” (DAH30) [29]. Our initial study was done in a single-centre in Australia, where we did not have complete and reliable data on post-acute care hospitalisation or longer term survival. In the current study, we aimed to demonstrate criterion and broader predictive validity of DAH30, this time using Swedish national health system data.
2. Methods
2.1. Study Design and Participants
This was a multicentre cohort study using prospectively collected data from 21 Swedish hospitals. The study was approved by the Regional Ethics Committee of Stockholm, Sweden, which waived the need for informed consent from participants. We included patients aged 18 years or older who underwent elective or non-elective inpatient surgery. We excluded patients who were resident in a nursing home or other nursing facility immediately prior to surgery.
2.2. Data Sources
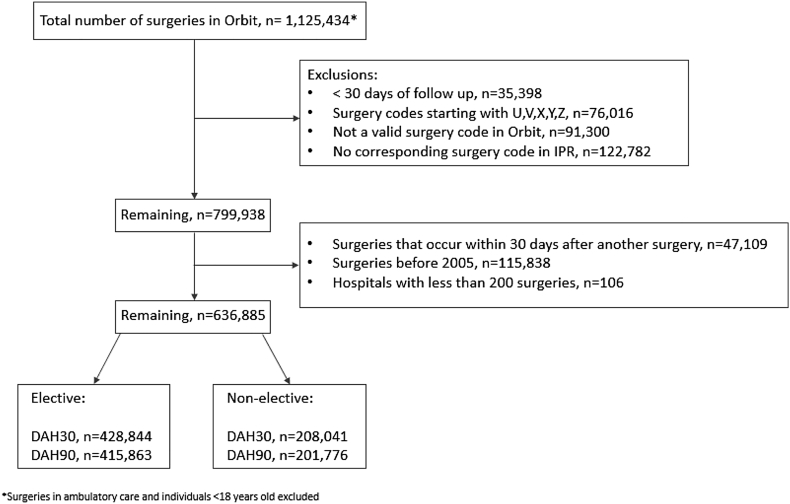
Data were obtained from Swedish hospitals that used the Orbit surgical planning system (EVRY, Stockholm, Sweden) from January 2005 to December 2014. Mandatory Orbit data include Swedish personal identity number, patient demographics, elective or non-elective status, type, extent and duration of surgery. The annual number of patients reported in Orbit at each participating hospital is detailed in e-table 1 in the online appendix. We further excluded patients lacking 30-day follow-up data, those with invalid surgery codes, and those in whom the Orbit coding did not match the code in the national inpatient registry (IPR) (Fig. 1).
Fig. 1.
Patient flow.
Orbit data were matched with the Swedish death registry and the National Inpatient Registry (IPR), using the unique 10-digit Swedish identity number assigned after birth or immigration [30]. The Swedish death registry includes the deaths of all Swedish citizens and residents with a national identity number; it is highly reliable with over 99% of all deaths recorded [31]. The IPR provided data on baseline health up to five years prior to the index surgery, allowing us to calculate the Charlson comorbidity index using ICD codes [32]. Additionally, the IPR contained index hospital admission and discharge dates, re-admission (and subsequent discharge) dates, and major complications following surgery. The IPR has high sensitivity for most surgical procedures, and current data suggest that the overall positive predictive value of diagnoses in the registry is approximately 85–95% [33]. The IPR provided data on hospital admission source and discharge destination, including whether patients were admitted from and discharged to their own home, another hospital or a nursing facility. No data were available on admission to a rehabilitation facility after surgery or any associated length of stay.
The American Society of Anesthesiologists physical status (ASA-PS) describes patients' baseline health prior to surgery, where I = a normal healthy patient, II = a patient with mild systemic disease, III = a patient with severe systemic disease, IV = a patient with severe systemic disease that is a constant threat to life, and V = a moribund patient who is not expected to survive without the operation [34]. The Charlson comorbidity index predicts 10-year mortality according to a patient's comorbid conditions; each condition is assigned a score of 1, 2, 3 or 6 depending on the associated risk of death [32]. The detection and reporting of complications was part of routine clinical care at each hospital and reported to the IPR in accordance with standard practice in Sweden.
2.3. Calculation of DAH30 and DAH90
DAH30 was calculated as previously described [29]. In short, DAH30 is calculated from the date of index surgery (Day 0) using hospitalisation and mortality data. The date of surgery and hospital discharge date are used to calculate hospital length of stay (ignoring any days in hospital prior to the index surgery). If a patient died in hospital or after discharge on any day within the first 30 days after surgery, the patient is assigned 0 DAH30; if a patient was discharged from hospital on Day 5 after surgery but was subsequently readmitted for 5 days before their second hospital discharge, then the patient would be assigned 20 DAH30. Postoperative days in a post-discharge nursing facility were not counted as days at home.
Given that major postoperative complications may impact patients beyond day 30, we also evaluated days at home up to 90 days after surgery (DAH90). Data on discharge destination were obtained from the IPR (e-tables 2 and 3 in the online appendix). We did not have data on the number of days spent in a rehabilitation facility before eventual discharge home, and as such only the hospital inpatient stay contributed to the calculation of DAH30 and DAH90 in this study.
2.4. Validity Testing
In the present context validity refers to whether DAH measures what it purports to measure: known associations with quality of care. DAH30 clearly has face validity because of its composition and patient-centeredness. We thus focussed on assessing criterion validity, and tested DAH30 in multiple ways: (i) association with known patient risk factors (age, ASA-PS, and Charlson comorbidity index); (ii) association with surgical risk factors (elective/non-elective status, duration and extent of surgery); (iii) association with process of care outcomes (length of stay, re-admissions, discharge destination); and (iv) association with clinical outcomes (major postoperative complications and 30-day mortality).
2.5. Predictive Validity
We evaluated predictive validity of DAH as a quality metric for long-term mortality after surgery. We correlated DAH30 with one-year mortality, after excluding patients who died within 30 days of surgery and after adjustment for the patient and surgical risk factors detailed in Table 1.
Table 1.
Patient and perioperative characteristics with respect to days at home up to 30 days after surgery (DAH30).
| DAH30 |
Still hospitalised on Day 30 |
Hospitalised up to Day 30 and readmitted |
Died within 30 days of surgery |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | No. of Individuals | Mean (SD) | Median (IQR) | P value | N | % | N | % | N | % |
| Total (All) | 636885 | 24 (7.6) | 27 (23–29) | 34688 | 5.4 | 14889 | 2.3 | 11451 | 1.8 | |
| Non-elective surgery | 208041 | 22 (9.1) | 26 (19–28) | 16062 | 7.7 | 5686 | 2.7 | 7868 | 3.8 | |
| Elective surgery | 428844 | 25 (6.6) | 27 (24–29) | 18626 | 4.3 | 9203 | 2.1 | 3583 | 0.8 | |
| Operation by organ system | < 0.0001 | |||||||||
| 01 Nervous system | 41669 | 20.4 (10) | 25 (15–28) | 5842 | 14 | 1582 | 3.8 | 1146 | 2.8 | |
| 02 Endocrine, Breast | 35925 | 27.9 (2.7) | 29 (28–29) | 392 | 1.1 | 333 | 0.9 | 36 | 0.1 | |
| 03 Eyes | 9989 | 28.0 (3.1) | 29 (28–29) | 190 | 1.9 | 171 | 1.7 | 18 | 0.2 | |
| 04 Ear, Nose, Throat, Jaw | 36964 | 27.9 (3.8) | 29 (28–29) | 718 | 1.9 | 468 | 1.3 | 63 | 0.2 | |
| 05 Heart, Major vessels | 20893 | 20.7 (9.1) | 23 (16–29) | 1630 | 7.8 | 613 | 2.9 | 590 | 2.8 | |
| 06 Lung, Trachea | 7257 | 17.9 (10) | 22 (11–26) | 1115 | 15.4 | 270 | 3.7 | 474 | 6.5 | |
| 07 Gastrointestinal | 109503 | 23.1 (8.4) | 27 (21–29) | 7093 | 6.5 | 3025 | 2.8 | 2805 | 2.6 | |
| 08 Urology, Sex organs | 101555 | 26.4 (5.0) | 28 (26–29) | 2817 | 2.8 | 2080 | 2 | 529 | 0.5 | |
| 09 Obstetrics | 55410 | 26.8 (2.1) | 27 (26–28) | 156 | 0.3 | 121 | 0.2 | 10 | 0 | |
| 10 Musculoskeletal | 169430 | 22.3 (7.9) | 25 (20–27) | 9558 | 5.6 | 3741 | 2.2 | 4064 | 2.4 | |
| 11 Peripheral vessels, lymphatics | 24007 | 23.3 (8.3) | 27 (22–29) | 1861 | 7.8 | 971 | 4 | 648 | 2.7 | |
| 12 Other surgeries | 24283 | 21.4 (10) | 27 (17–29) | 3316 | 13.7 | 1514 | 6.2 | 1068 | 4.4 | |
| Age, years | < 0.0001 | |||||||||
| 01 18–29 | 64230 | 26.9 (4.7) | 28 (27–29) | 1390 | 2.2 | 608 | 0.9 | 90 | 0.1 | |
| 02 30–39 | 74623 | 26.6 94.5) | 28 (26–29) | 1477 | 2 | 689 | 0.9 | 109 | 0.1 | |
| 03 40–49 | 66447 | 25.9 (6.1) | 28 (26–29) | 2551 | 3.8 | 1100 | 1.7 | 258 | 0.4 | |
| 04 50–59 | 83864 | 24.9 (7.0) | 28 (24–29) | 4325 | 5.2 | 1832 | 2.2 | 678 | 0.8 | |
| 05 60–69 | 132007 | 23.9 (7.6) | 27 (23–29) | 8131 | 6.2 | 3471 | 2.6 | 1818 | 1.4 | |
| 06 70–79 | 121375 | 22.6 (8.3) | 26 (21–28) | 8709 | 7.2 | 3692 | 3 | 2778 | 2.3 | |
| 07 80–89 | 78229 | 20.2 (9.3) | 23 (16–28) | 6696 | 8.6 | 2878 | 3.7 | 3916 | 5 | |
| 08 90 + | 16110 | 17.4 (9.9) | 20 (11–26) | 1409 | 8.7 | 619 | 3.8 | 1804 | 11.2 | |
| Sex | < 0.0001 | |||||||||
| 01 Male | 268921 | 23.5 (8.3) | 27 (22–29) | 17996 | 6.7 | 7496 | 2.8 | 6051 | 2.3 | |
| 02 Female | 367964 | 24.4 (7.1) | 27 (24–29) | < 0.0001 | 16692 | 4.5 | 7393 | 2 | 5400 | 1.5 |
| Duration of surgery | < 0.0001 | |||||||||
| 01 Up to 59 minutes | 254846 | 25.1 (7.3) | 28 (25–29) | 12519 | 4.9 | 6201 | 2.4 | 5233 | 2.1 | |
| 02 60 minutes or more | 382039 | 23.2 (7.8) | 26 (22–28) | 22169 | 5.8 | 8688 | 2.3 | 6218 | 1.6 | |
| ASA physical status | < 0.0001 | |||||||||
| Missing | 167851 | 01 1 | 137691 | 27.1 (3.7) | 28 (26–29) | 01 1 | 137691 | |||
| 01 1 | 137691 | 27.1 (3.7) | 28 (26–29) | 02 2 | 200985 | 24.9 (6.1) | 27 (24–29) | 02 2 | 200985 | |
| 02 2 | 200985 | 24.9 (6.1) | 27 (24–29) | 03 3 | 119278 | 20.1 (9.5) | 24 (16–28) | 03 3 | 119278 | |
| 03 3 | 119278 | 20.1 (9.5) | 24 (16–28) | 03 4 | 11080 | 11.5 (11) | 11 (0–22) | 03 4 | 11080 | |
| 03 4 | 11080 | 11.5 (11) | 11 (0–22) | 01 1 | 137691 | 27.1 (3.7) | 28 (26–29) | 01 1 | 137691 | |
| Charlson comorbidity index 1 year including cancer | < 0.0001 | |||||||||
| 01 0p | 399016 | 25.1 (6.6) | 27 (25–29) | 14534 | 3.6 | 5506 | 1.4 | 4130 | 1 | |
| 02 1p | 44287 | 22.2 (8.6) | 26 (20–28) | 3249 | 7.3 | 1332 | 3 | 1238 | 2.8 | |
| 03 2–3p | 128415 | 23.3 (8.1) | 27 (22–29) | 8800 | 6.9 | 4298 | 3.3 | 2569 | 2 | |
| 04 4p– | 65167 | 20.1 (10) | 24 (15–28) | 8105 | 12.4 | 3753 | 5.8 | 3514 | 5.4 | |
| Charlson comorbidity index 1 year excluding cancer | < 0.0001 | |||||||||
| 01 0p | 382464 | 25.3 (6.3) | 27 (25–29) | 12294 | 3.2 | 4492 | 1.2 | 3387 | 0.9 | |
| 02 1p | 42343 | 22.3 (8.5) | 26 (20–28) | 2950 | 7 | 1196 | 2.8 | 1096 | 2.6 | |
| 03 2–3p | 57464 | 22.6 (8.4) | 26 (20–29) | 4147 | 7.2 | 1886 | 3.3 | 1383 | 2.4 | |
| 04 4p– | 32435 | 20.2 (9.8) | 24 (15–28) | 3862 | 11.9 | 1677 | 5.2 | 1468 | 4.5 | |
| Charlson comorbidity index 5 year including cancer | < 0.0001 | |||||||||
| 01 0p | 337329 | 25.5 (6.2) | 28 (25–29) | 10877 | 3.2 | 4065 | 1.2 | 2430 | 0.7 | |
| 02 1p | 58772 | 22.8 (8.2) | 26 (21–28) | 3679 | 6.3 | 1499 | 2.6 | 1422 | 2.4 | |
| 03 2–3p | 144028 | 23.3 (8.0) | 27 (22–29) | 9376 | 6.5 | 4434 | 3.1 | 2924 | 2 | |
| 04 4p– | 96756 | 20.6 (9.7) | 25 (16–28) | 10756 | 11.1 | 4891 | 5.1 | 4675 | 4.8 | |
| Charlson comorbidity index 5 year excluding cancer | < 0.0001 | |||||||||
| 01 0p | 325081 | 25.7 (5.9) | 28 (25–29) | 9147 | 2.8 | 3285 | 1 | 1936 | 0.6 | |
| 02 1p | 56426 | 22.9 (8.1) | 26 (21–28) | 3344 | 5.9 | 1342 | 2.4 | 1255 | 2.2 | |
| 03 2-3p | 77813 | 22.9 (8.2) | 26 (21–29) | 5029 | 6.5 | 2175 | 2.8 | 1825 | 2.3 | |
| 04 4p- | 55386 | 20.8 (9.5) | 25 (16–28) | 5733 | 10.4 | 2449 | 4.4 | 2318 | 4.2 | |
2.6. Supplementary Analyses
To further test the criterion validity of DAH30 as an outcome metric, we undertook two supplementary analyses using proxies for high-quality versus poorer-quality perioperative care. Firstly, we compared DAH30 in patients who had undergone elective open aortic aneurysm surgery to those who underwent endovascular repair. Endovascular repair has been shown to reduce short-term mortality [35], complications [36], and hospital length of stay [37]. We thus hypothesised that DAH30 would be higher in patients undergoing endovascular repair compared to open repair.
Secondly, we compared DAH30 in patients who had undergone elective hip or knee arthroplasty in two high volume specialised orthopaedic hospitals (Trelleborg County Hospital and Hässleholm County Hospital) to those who underwent the same procedures in a university hospital setting (the two sites of Karolinska University Hospital, Solna and Huddinge). Trelleborg and Hässleholm County Hospitals have similar perioperative care strategies, restricting admission to low-risk patients (a majority being ASA-PS 1 or 2) without serious comorbidity, thereby facilitating a very high degree of standardisation of care. Patients receive a standardised perioperative care pathway typically including admission on the day of surgery, regional anaesthesia and multimodal opioid-sparing postoperative analgesia. Mobilisation occurs within 2 h of surgery and patients are often discharged on the first or second postoperative day. In contrast, patients in the university hospital setting are a heterogeneous population with multiple comorbidities, often corresponding to ASA-PS 3 or 4. Further, the university hospital population contains patients referred from other hospitals due to pre-existing coagulation abnormalities or the need for admission to intensive care after surgery. Consequently, patients at university hospitals receive more individualised perioperative care, including complex fluid management and more frequent blood transfusion. Duration of surgery is approximately 40% longer than at the specialised orthopaedic hospitals. We thus hypothesised that DAH30 would be higher in the specialised orthopaedic hospital setting.
Additionally, we compared the statistical efficiency of DAH30, hospital length of stay, 30-day mortality, and a composite of 30-day complications and mortality. We used the observed difference between elective aortic stent graft and open aortic surgery for each metric as a proxy for a clinically important improvement in surgical performance - a typical goal in surgical audit and quality improvement, or when evaluating new interventions in a perioperative clinical trial. A key aspect of clinical trial design is sample size calculation, and we used observed differences between both aortic surgery interventions to model this. For surgical audit, given that individual surgeons typically undertake only 2 to 5 specific major operative procedures each week, we modelled how long it would take before underperformance (poor outcomes) was detected if using an alert level of 1% (i.e. crossing a p < 0.01 boundary in a quality outcome analysis [38]).
2.7. Statistical Analysis
Descriptive data are presented as medians with IQR or frequencies with proportions. The p values for difference in DAH30/DAH90 by patient age, sex, ASA-PS, comorbidity, operation by organ type, and duration of surgery were calculated using Spearman correlations for ordered data and Kruskal–Wallis or Mann–Whitney for categorical data. Adjusted average DAH30 and DAH90 (i.e. hospital and calendar year) and adjusted differences with respect to covariates (i.e. presence of complications) were estimated by means of ANOVA. One-year mortality was analysed, restricted to patients alive at 30 days to avoid co-correlation, using Cox proportional hazard models; results are presented as hazards ratio (HR) with 95% confidence interval (CI). The HRs for 1-year mortality were adjusted for patient age and sex, comorbidity, ASA-PS, organ system, elective/acute and duration of surgery. Adjusted survival curves were estimated by the average covariate method by conditioning on the categorised DAH30 variable. Non-proportional hazards with respect to DAH30 were evaluated by estimating time-specific HRs for the periods 2–4 months, 5–7 months and 8–12 months. The lowest value for each variable was used as the reference. All analyses were performed using PROC PHREG, PROC GLM, PROC CORR and PROC NPAR1WAY using SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
2.8. Role of the Funding Sources
The study was conceived and designed by PM, LE and MB. TS and FG did the statistical analysis and all authors contributed to the interpretation of data and drafting of the manuscript. The funders had no role in study design, data collection, management, data analysis, data interpretation, preparation of the manuscript, or the decision to submit the manuscript for publication. MB had full access to all the data in the study and the corresponding author had final responsibility for the decision to submit for publication.
3. Results
3.1. Study Population
We identified 1,125,434 eligible patients who underwent surgery at any one of the 21 hospitals in Sweden from January 2005 to December 2014; after exclusions, data on 636,885 operations among 488,160 patients were available for complete analysis (Fig. 1). The median (IQR) age was 62 (43–74) years and 367,964 (57.7%) patients were female. A broad range of surgical procedures were represented and 208,041 (32.7%) procedures were non-elective (Fig. 1, Table 1). Overall, 13,551 (1.8%) patients died within 30 days of surgery, 26,349 (3.5%) died within 90 days and 55,709 (7.4%) died within one year after surgery. The discharge destination according to patient and surgical factors is reported in e-Table 1 in the Appendix.
The median (IQR) DAH30 was 27 [23], [24], [25], [26], [27], [28], [29] (Table 1) and the median (IQR) DAH90 was 87 (82 to 89); both varied according to the type and extent of surgery (Table 1). DAH30 varied across the Swedish hospitals (e-table 2 in the appendix), and generally increased over time (e-table 3 in the Appendix). Ongoing hospitalisation at day 30 or 90, hospital readmission, and mortality at 30 and 90 days all varied according to type of surgery (Table 1, e-tables 4 and 5 in the Appendix). The median DAH30 and DAH90 were significantly lower in elderly patients, those with significant medical conditions as measured by increasing Charlson Comorbidity Index, and at higher surgical risk (ASA-PS). The overall patterns for DAH30 and DAH90 in elective and non-elective subcohorts for the various surgical procedures did not differ from those in the whole cohort (e-tables 4 to 8 in the Appendix).
Table 4.
Association between days at home up to 30 days after surgery (DAH30) and one year mortality (excluding deaths that occur within the first 30 days): both elective and non-elective surgery.
| DAH30 | No. of individuals | No. of deaths | Person years | Rate per 1000 person years | Unadjusted HR (95% CI) |
Adjusteda HR (95% CI) |
Adjustedb HR (95% CI) |
|---|---|---|---|---|---|---|---|
| 29–30 | 199,169 | 4653 | 197,073 | 23.61 | Reference | Reference | Reference |
| 26–28 | 207,241 | 4939 | 204,863 | 24.11 | 1.02 (0.98–1.06) | 1.43 (1.36–1.51) | 1.35 (1.28–1.42) |
| 23–25 | 83,638 | 4664 | 81,276 | 57.38 | 2.43 (2.33–2.53) | 2.33 (2.2–2.46) | 2.25 (2.14–2.37) |
| 20–22 | 38,928 | 3564 | 37,044 | 96.21 | 4.07 (3.89–4.25) | 2.89 (2.73–3.07) | 2.76 (2.61–2.92) |
| 17–19 | 25,002 | 2873 | 23,434 | 122.6 | 5.18 (4.94–5.42) | 3.41 (3.21–3.62) | 3.23 (3.04–3.42) |
| 9–16 | 35,581 | 5527 | 32,465 | 170.24 | 7.18 (6.91–7.47) | 4.35 (4.12–4.59) | 4.22 (4.01–4.44) |
| 0–8 | 35,875 | 8662 | 30,403 | 284.91 | 12.01 (11.59–12.44) | 6.78 (6.44–7.13) | 6.73 (6.42–7.07) |
Abbreviations: HR, hazard ratio; CI, confidence interval.
Adjusted for all variables in Table 1 (for Charlson Comorbidity Index, using the 1 year including cancer data).
Adjusted for all variables in Table 1 except operation by organ system (for Charlson Comorbidity Index, using the 1 year including cancer data).
The associations between separate strata of DAH30 and the incidence of major complications are reported in Table 2. Lower DAH30 was coupled with increased major complication rates in all strata; for example, the incidence of pneumonia ranged from 0 to 12.1% from highest to lowest DAH30 strata. Patients with major complications had a substantially lower DAH30 when compared to those without complications (Table 3). The same patterns of associations were observed when analysing elective and non-elective patients separately (e-tables 9 to 12 in the Appendix).
Table 2.
Complications during the first 30 days after surgery and resultant days at home up to 30 days after surgery (DAH30), including both elective and non-elective surgeries.
| AKI |
ARDS |
Arrhythmia |
Cardiac Arrest |
DVT |
Delirium |
Infection, source uncertain |
Stroke |
MI |
Pneumonia |
Paralytic ileus |
Pulmonary embolism |
Cardiogenic pulmonary oedema |
ICD10 = T81a |
Any major complication |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DAH30 | Total N | # | % | # | % | # | % | # | % | # | % | # | % | # | % | # | % | # | % | # | % | # | % | # | % | # | % | # | % | # | % |
| 29–30 | 199,439 | 59 | 0 | 3 | 0 | 1944 | 1 | 98 | 0 | 85 | 0 | 79 | 0 | 53 | 0 | 199 | 0.1 | 277 | 0.1 | 131 | 0.1 | 143 | 0.1 | 141 | 0.1 | 17 | 0 | 4836 | 2.4 | 3113 | 1.6 |
| 26–28 | 207,762 | 112 | 0.1 | 2 | 0 | 965 | 0.5 | 107 | 0.1 | 127 | 0.1 | 191 | 0.1 | 81 | 0 | 580 | 0.3 | 1045 | 0.5 | 412 | 0.2 | 996 | 0.5 | 245 | 0.1 | 41 | 0 | 7924 | 3.8 | 4690 | 2.3 |
| 23–25 | 84,154 | 156 | 0.2 | 7 | 0 | 453 | 0.5 | 82 | 0.1 | 135 | 0.2 | 313 | 0.4 | 110 | 0.1 | 352 | 0.4 | 617 | 0.7 | 801 | 1 | 1572 | 1.9 | 342 | 0.4 | 68 | 0.1 | 5629 | 6.7 | 4715 | 5.6 |
| 20–22 | 39,321 | 161 | 0.4 | 10 | 0 | 289 | 0.7 | 55 | 0.1 | 101 | 0.3 | 307 | 0.8 | 67 | 0.2 | 272 | 0.7 | 685 | 1.7 | 893 | 2.3 | 1180 | 3 | 322 | 0.8 | 90 | 0.2 | 3408 | 8.7 | 4129 | 10.5 |
| 17–19 | 25,270 | 155 | 0.6 | 4 | 0 | 215 | 0.9 | 56 | 0.2 | 52 | 0.2 | 345 | 1.4 | 76 | 0.3 | 216 | 0.9 | 697 | 2.8 | 866 | 3.4 | 896 | 3.5 | 242 | 1 | 83 | 0.3 | 2679 | 10.6 | 3544 | 14 |
| 9–16 | 35,943 | 476 | 1.3 | 25 | 0.1 | 363 | 1 | 104 | 0.3 | 146 | 0.4 | 580 | 1.6 | 175 | 0.5 | 574 | 1.6 | 1000 | 2.8 | 1808 | 5 | 1499 | 4.2 | 554 | 1.5 | 182 | 0.5 | 4963 | 13.8 | 6574 | 18.3 |
| 0–8 | 44,996 | 1966 | 4.4 | 337 | 0.7 | 535 | 1.2 | 898 | 2 | 229 | 0.5 | 1015 | 2.3 | 365 | 0.8 | 2124 | 4.7 | 1903 | 4.2 | 5439 | 12.1 | 2385 | 5.3 | 1304 | 2.9 | 1344 | 3 | 7725 | 17.2 | 15,355 | 34.1 |
Abbreviations: AKI, acute kidney injury; ARDS, acute respiratory distress syndrome, DVT, deep vein thrombosis; MI, myocardial infarction; PE, pulmonary embolism.
Includes additional postoperative complications (not included in definition of any major complication).
Table 3.
Complications during the first 30 days after surgery and resultant mean (95% CI) days at home up to 30 days after surgery: both elective and non-elective surgery.
| Type of complication | No complication |
Complication |
Difference (no-yes) in days |
Adjustedb difference (no-yes) in days |
||
|---|---|---|---|---|---|---|
| N | Mean (95% CI) | N | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | |
| AKI | 633,800 | 24.16 (24.14–24.18) | 3085 | 7.22 (6.96–7.48) | 16.94 (16.68–17.20) | 11.00 (10.79–11.22) |
| ARDS | 636,497 | 24.09 (24.07–24.11) | 388 | 2.60 (1.86–3.35) | 21.49 (20.74–22.23) | 12.94 (12.34–13.54) |
| Arrhythmia | 632,121 | 24.09 (24.07–24.10) | 4764 | 23.07 (22.85–23.28) | 1.02 (0.81–1.23) | 1.00 (0.81–1.19) |
| Cardiac arrest | 635,485 | 24.11 (24.09–24.13) | 1400 | 8.30 (7.91–8.69) | 15.81 (15.42–16.20) | 10.32 (10.01–10.64) |
| DVT | 636,010 | 24.09 (24.07–24.11) | 875 | 16.46 (15.96–16.95) | 7.63 (7.14–8.13) | 4.30 (3.90–4.69) |
| Delirium | 634,055 | 24.13 (24.11–24.15) | 2830 | 13.05 (12.77–13.32) | 11.08 (10.80–11.35) | 5.84 (5.61–6.06) |
| Infection, source uncertain | 635,958 | 24.09 (24.08–24.11) | 927 | 12.92 (12.44–13.40) | 11.17 (10.69–11.66) | 6.89 (6.51–7.28) |
| Stroke | 632,568 | 24.17 (24.15–24.18) | 4317 | 11.34 (11.12–11.56) | 12.83 (12.60–13.05) | 8.40 (8.22–8.58) |
| MI | 630,661 | 24.17 (24.15–24.19) | 6224 | 15.01 (14.82–15.19) | 9.16 (8.98–9.35) | 4.83 (4.66–5.00) |
| Pneumonia | 626,535 | 24.32 (24.30–24.34) | 10,350 | 9.40 (9.26–9.54) | 14.92 (14.78–15.07) | 8.95 (8.83–9.06) |
| Paralytic ileus | 628,214 | 24.20 (24.18–24.22) | 8671 | 15.34 (15.18–15.49) | 8.86 (8.70–9.02) | 4.46 (4.32–4.59) |
| Pulmonary embolism | 633,735 | 24.14 (24.12–24.15) | 3150 | 12.36 (12.10–12.63) | 11.77 (11.51–12.03) | 7.57 (7.36–7.78) |
| Pulmonary oedema | 635,060 | 24.13 (24.11–24.15) | 1825 | 5.38 (5.04–5.72) | 18.75 (18.41–19.09) | 12.41 (12.14–12.69) |
| ICD10 = T81 | 599,721 | 24.42 (24.40–24.44) | 37,164 | 18.52 (18.45–18.60) | 5.90 (5.82–5.98) | 4.71 (4.65–4.78) |
| Any major complicationa | 594,765 | 24.80 (24.78–24.82) | 42,120 | 13.90 (13.83–13.97) | 10.90 (10.83–10.97) | 7.03 (6.97–7.10) |
Abbreviations: AKI, acute kidney injury; ARDS, acute respiratory distress syndrome, DVT, deep vein thrombosis; MI, myocardial infarction.
Major complication includes all types except ICD10 = T81.
Comparison adjusted for year of surgery, age, sex, duration of surgery, American Society of Anesthesiologists physical status, Charlson comorbidity index (1 year), hospital, and type of surgery (according to the two first positions in ORBIT-opcode 1).
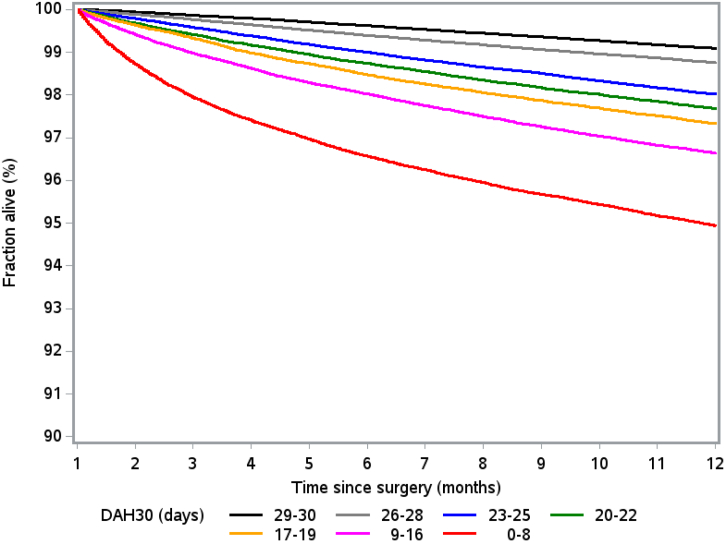
Decreasing DAH30 was significantly associated with a gradual increase in one-year mortality, after adjustment for all associated preoperative and intraoperative variables (Table 4 and Fig. 2). Patients with DAH30 ≤ 8 had a nearly 7-fold increased risk of death by one year, with a hazard ratio of 6.78 (95% CI: 6.44–7.13), accompanied by an incidence rate of 284.9 per 1000 person years. Similar patterns of results were obtained when analysing elective and non-elective patients separately (e-tables 13 and 14 in the Appendix). The risk of dying in those with low DAH30 was most apparent in the first 4 months after surgery (e-tables 15a and 15b). A statistical frailty model, taking clustering into account, could not be performed on this large dataset due to lack of computational resources. Instead an analysis restricted to the first operation occurring in the dataset was performed (e-table 15b). This analysis reveals that the effect of decreasing DAH30 is slightly stronger in the first operation (occurring in the database). Compared with hospital length of stay, DAH30 had stronger prognostic utility for 1-year survival (e-table 16 in the Appendix). Increased hospital length of stay was associated with a higher incidence of hospital re-admission and one-year mortality (e-table 17 in the Appendix).
Fig. 2.
One-year Kaplan–Meier survival plots according to the number of days at home up to 30 days after surgery (DAH30), excluding deaths that occur within the first 30 days (both elective and non-elective surgery).
Patients who underwent elective endovascular compared with open aortic surgery had DAH30 of 26 vs 20 respectively, and DAH90 of 86 vs 80 respectively (e-table 18 in the Appendix), both p < 0.0001. Similarly, patients undergoing elective hip or knee arthroplasty in the specialised orthopaedic hospitals had higher DAH30 compared with those admitted to university hospitals (e-tables 19a-f in the Appendix), all p < 0.0001.
A power calculation for elective aortic stent-graft vs. open aortic surgery based on the observed differences from the years 2010–2014 shows that using DAH30 as a quality metric requires far fewer patients to detect a clinically important difference between groups compared to 30-day mortality or a composite of 30-day mortality and complications (30 participants vs. 856 and 180 participants, respectively; e-Table 20 in the appendix). Using these data, a clinical trial enrolling 856 patients in each arm can detect a delta DAH30 of 1·2 days with 90% power at a 1% significance level. To detect an outlier surgeon or surgical team (p < 0.01) in a publicly-reporting surgical audit program, based on treating 5 patients per week, such a difference would be detected after 6 weeks using DAH30, 42 months using 30-day mortality, and 9 months when using a composite of 30-day mortality and complications.
4. Discussion
We found that DAH30 is a valid and easily-obtainable patient-centred outcome metric. While content (face) validity of this metric can be justified on the basis of previous work by others [4], [19], [20], [21], [22], [23], our results demonstrate criterion validity from multiple perspectives, and importantly, also demonstrate predictive validity for one-year survival. DAH30 is maximised when patients recover from surgery free of major complications, with early return of independence and ability to return home [2]. DAH30 can be calculated from readily available data. It captures the impact of patient and surgical risk factors, process of care outcomes, and clinical outcomes; it therefore has ideal attributes as a clinical trial outcome measure and quality indicator [3], [39].
Our 1-year survival analysis identified that the risk of dying in those with low DAH30 was most apparent up to 4 months of surgery. Patients having less days at home after surgery have prolonged hospital stays and/or need for specialised nursing facilities for a reason, most often because of severe complications. However, if they survive their complications/primary surgical illness it seems they have a reasonable prognosis in the subsequent 5–12 months after surgery, suggesting this later phase reflects ongoing deconditioning and underlying chronic co-morbidities.
In line with DAH30, we found that DAH90 was a similarly valid outcome-quality metric. Although some postoperative complications and poor survival manifest months after surgery [40], [41], [42], we could not identify any apparent advantage with DAH90 over and above DAH30. Given the additional work and potential for missing data, we do not believe DAH90 provides additional benefit over DAH30 as a quality metric in the perioperative setting but may provide valuable information for non-surgical patients with chronic medical disorders.
We found that DAH30 is a superior measure of quality of surgery and perioperative care over standard complication and mortality rates. It includes, and in a sense bypasses, otherwise undetected and/or unreported process of care issues and clinical outcomes. DAH30 thus has the potential to uncover a hidden or systemic failure to detect or report clinical pathway deviations. This is particularly important in the frail or elderly patient, where even minor complications may have negative consequences for discharge readiness. However, discharge planning and other practice patterns vary across different hospital and country settings, and so DAH30 should be interpreted with these in mind; statistical adjustment (for quality assurance [11]) or random patient selection (in clinical trials) can account for such variation.
High quality healthcare depends on trained, experienced and well-resourced staff, working within a team-based safety culture [43]. Deficiencies in any or many of these features will manifest as avoidable complications and failure to rescue [12]. Public reporting of accurate outcomes data is needed to help providers of healthcare remain accountable, and for informed decision-making by patients, families and primary care physicians [9], [44], [45]. However, reporting requires extremely large patient numbers to detect deficiencies in care if mortality is used as the outcome metric [44]; it is costly, and could perhaps be better focussed [13], [14], [46]. Quality of life and disability-free survival are arguably better patient-centred measures of longer-term outcome after surgery than mortality [41], [47]. DAH30 is ideally suited as an additional metric for this purpose.
There are many examples of substandard clinical practice by individuals [48], [49], hospitals [50], and perhaps countries [51]. Mortality rates are often used to detect divergence from acceptable clinical practice [44], but as surgical mortality is very uncommon, deviations may not be detected until very late [12], [50], [52]. DAH30 is a robust, readily-obtainable, and statistically efficient metric ideally suited for ongoing surveillance and hospital-level and public reporting. As individual hospital characteristics and case-mix independently affect ratings irrespective of quality of care [53], DAH30 should be risk-adjusted for bench-marking purposes [11]; this is readily achieved.
Postoperative complications may be underdiagnosed and/or under-reported. More importantly, the impact of such complications on patient recovery and survival is not always obvious when simply coded as dichotomous outcomes [54]. Some major complications, especially mortality, are rare, and so the ability to detect poor care or worse outcomes in a clinical trial is dependent on a large sample size. Similar issues have arisen in critical care research, for which ‘ventilator-free days’ and ‘ICU-free days’ are often reported [55]. Our supplementary modelling clearly demonstrates that DAH30 can be used as an outcome measure to improve the efficiency of clinical research and earlier detection of poor care.
Given that a hospital bed day costs approximately $1800 in the US, DAH30 is also an indicator of value-based care [6]. Hospitals in many countries are increasingly receiving a fixed reimbursement per episode of care according to the patient's diagnosis and perhaps comorbidities, regardless of hospital length of stay [56]. Hospitals may therefore minimise costs by implementing early discharge policies, with or without enhanced recovery pathways. Bundled payment models typically provide an incentive for early hospital discharge, but if patients and their families, or even nursing facilities, take on the burden of postoperative care too soon or in sub-optimal circumstances, there is an increased risk of unplanned readmission [56], [57], [58].
This study has important strengths, primarily based on very detailed and complete surgical data across a large number of Swedish hospitals, including smaller regional institutions and large university hospitals. The overall coverage of the health care registries in Sweden uniquely allowed us to characterise preoperative comorbidities, patient risk factors and surgical risk factors, and to capture process of care outcomes, clinical outcomes and long term mortality outcomes. As such, generalisability should be high, at least in developed countries. This study has several limitations which can be individually addressed in future applications of our method. Hospital discharge may be delayed for a variety of reasons unrelated to complications or quality of care, and these and other factors such as varying availability of home-based care by qualified health care personnel may also affect re-admissions. Within individual centres or health systems, these factors are unlikely to change in the short-term and DAH30 can be used for ongoing quality assurance. Between centres or health systems, as long as local practices are clearly described, DAH30 can be used to compare outcomes across similar settings. Case-mix will affect DAH30 and so we recommend it be risk-adjusted for bench-marking purposes [59]. The duration of time spent in a rehabilitation facility after discharge from hospital may not be recorded in most hospital record systems. Better integration of electronic records and data sharing could resolve this. “Home” in most situations refers to a person's usual place of abode; this could include residing with a family member or in a retirement village or nursing facility. Such variations need to be considered when designing a study or interpreting quality of care.
5. Conclusion
DAH30 is a valid and readily-obtainable, generic, patient-centred outcome measure. DAH30 accounts for major complications, prolonged hospital stay, discharge to any post-acute care nursing facility, post-discharge complications needing hospital readmission, and early death after surgery. Patients will spend more days at home in the first 30 days after surgery when effective and efficient care is provided. DAH30 is therefore a valuable perioperative outcome measure in clinical trials and quality assurance.
Contributors
PM, LE and MB initiated the study. PM, MB and LE wrote the first and final drafts of the report. TS and FG analysed the data. MB, LE, TS, LH, FG, JR, and PM contributed to each draft of the report.
All authors read and agreed to the final version of the manuscript.
Declaration of Interests
We declare no competing interests.
Funding Sources
This study was funded by the Stockholm County Council ALF in Sweden, an Australian National Health and Medical Research Council Practitioner Fellowship (PM), and an Australian Government Research Training Program Scholarship (JR).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.eclinm.2019.04.011.
Appendix A. Supplementary data
Supplementary tables
References
- 1.Lynn J., McKethan A., Jha A.K. Value-based payments require valuing what matters to patients. JAMA. 2015;314(14):1445–1446. doi: 10.1001/jama.2015.8909. [DOI] [PubMed] [Google Scholar]
- 2.Lavallee D.C., Chenok K.E., Love R.M. Incorporating patient-reported outcomes into health care to engage patients and enhance care. Health Aff (Millwood) 2016;35(4):575–582. doi: 10.1377/hlthaff.2015.1362. [DOI] [PubMed] [Google Scholar]
- 3.Porter M.E. What is value in health care? N Engl J Med. 2010;363(26):2477–2481. doi: 10.1056/NEJMp1011024. [DOI] [PubMed] [Google Scholar]
- 4.Hinami K., Bilimoria K.Y., Kallas P.G., Simons Y.M., Christensen N.P., Williams M.V. Patient experiences after hospitalizations for elective surgery. Am J Surg. 2014;207(6):855–862. doi: 10.1016/j.amjsurg.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 5.Birkmeyer J.D., Gust C., Dimick J.B., Birkmeyer N.J., Skinner J.S. Hospital quality and the cost of inpatient surgery in the United States. Ann Surg. 2012;255(1):1–5. doi: 10.1097/SLA.0b013e3182402c17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berwick D.M., Nolan T.W., Whittington J. The triple aim: care, health, and cost. Health Aff (Millwood) 2008;27(3):759–769. doi: 10.1377/hlthaff.27.3.759. [DOI] [PubMed] [Google Scholar]
- 7.Horton R. Surgical research or comic opera: questions, but few answers. Lancet. 1996;347(9007):984–985. doi: 10.1016/s0140-6736(96)90137-3. [DOI] [PubMed] [Google Scholar]
- 8.Bruce J., Russell E.M., Mollison J., Krukowski Z.H. The measurement and monitoring of surgical adverse events. Health Technol Assess. 2001;5(22):1–194. doi: 10.3310/hta5220. [DOI] [PubMed] [Google Scholar]
- 9.Austin J.M., McGlynn E.A., Pronovost P.J. Fostering transparency in outcomes, quality, safety, and costs. JAMA. 2016;316(16):1661–1662. doi: 10.1001/jama.2016.14039. [DOI] [PubMed] [Google Scholar]
- 10.Macleod M.R., Michie S., Roberts I. Biomedical research: increasing value, reducing waste. Lancet. 2014;383(9912):101–104. doi: 10.1016/S0140-6736(13)62329-6. [DOI] [PubMed] [Google Scholar]
- 11.Nashef S.A.M., Powell S., Jenkins D.P., Fynn S., Hall R. Crying wolf: the misuse of hospital data. Lancet. 2017;390(10091):227–228. doi: 10.1016/S0140-6736(17)31609-4. [DOI] [PubMed] [Google Scholar]
- 12.Ghaferi A.A., Birkmeyer J.D., Dimick J.B. Variation in hospital mortality associated with inpatient surgery. N Engl J Med. 2009;361(14):1368–1375. doi: 10.1056/NEJMsa0903048. [DOI] [PubMed] [Google Scholar]
- 13.Schuster M.A., Onorato S.E., Meltzer D.O. Measuring the cost of quality measurement: a missing link in quality strategy. Jama. 2017;318(13):1219–1220. doi: 10.1001/jama.2017.11525. [DOI] [PubMed] [Google Scholar]
- 14.Chernew M.E., Landrum M.B. Targeted supplemental data collection — addressing the quality-measurement conundrum. N Engl J Med. 2018;378(11):979–981. doi: 10.1056/NEJMp1713834. [DOI] [PubMed] [Google Scholar]
- 15.Hyder J.A., Hirschberg R.E., Nguyen L.L. Home discharge as a performance metric for surgery. JAMA Surg. 2015;150(2):96–97. doi: 10.1001/jamasurg.2014.1725. [DOI] [PubMed] [Google Scholar]
- 16.Glance L.G., Kellermann A.L., Osler T.M. Hospital readmission after noncardiac surgery: the role of major complications. JAMA Surg. 2014;149(5):439–445. doi: 10.1001/jamasurg.2014.4. [DOI] [PubMed] [Google Scholar]
- 17.Gupta A., Allen L.A., Bhatt D.L. Association of the hospital readmissions reduction program implementation with readmission and mortality outcomes in heart failure. JAMA Cardiol. 2018;3(1):44–53. doi: 10.1001/jamacardio.2017.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berian J.R., Mohanty S., Ko C.Y., Rosenthal R.A., Robinson T.N. Association of loss of independence with readmission and death after discharge in older patients after surgical procedures. JAMA Surg. 2016;151(9) doi: 10.1001/jamasurg.2016.1689. [DOI] [PubMed] [Google Scholar]
- 19.Fried T.R., Bradley E.H., Towle V.R., Allore H. Understanding the treatment preferences of seriously ill patients. N Engl J Med. 2002;346(14):1061–1066. doi: 10.1056/NEJMsa012528. [DOI] [PubMed] [Google Scholar]
- 20.Boney O., Bell M., Bell N. Identifying research priorities in anaesthesia and perioperative care: final report of the joint National Institute of Academic Anaesthesia/James Lind Alliance Research Priority Setting Partnership. BMJ Open. 2015;5(12) doi: 10.1136/bmjopen-2015-010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burns K.E., Jacob S.K., Aguirre V., Gomes J., Mehta S., Rizvi L. Stakeholder engagement in trial design: survey of visitors to critically ill patients regarding preferences for outcomes and treatment options during weaning from mechanical ventilation. Ann Am Thorac Soc. 2016;13(11):1962–1968. doi: 10.1513/AnnalsATS.201606-445OC. [DOI] [PubMed] [Google Scholar]
- 22.Hannah D., Lindholm B., Maisch L. Certain uncertainty: life after stroke from the patient's perspective. Circ Cardiovasc Qual Outcomes. 2014;7(6):968–969. doi: 10.1161/CIRCOUTCOMES.114.001315. [DOI] [PubMed] [Google Scholar]
- 23.Xian Y., O'Brien E.C., Fonarow G.C. Patient-centered research into outcomes stroke patients prefer and effectiveness research: implementing the patient-driven research paradigm to aid decision making in stroke care. Am Heart J. 2015;170(1):36–45. doi: 10.1016/j.ahj.2015.04.008. [.e1-11] [DOI] [PubMed] [Google Scholar]
- 24.Barnett M.L., Grabowski D.C., Mehrotra A. Home-to-home time — measuring what matters to patients and payers. N Engl J Med. 2017;377(1):4–6. doi: 10.1056/NEJMp1703423. [DOI] [PubMed] [Google Scholar]
- 25.Ariti C.A., Cleland J.G., Pocock S.J. Days alive and out of hospital and the patient journey in patients with heart failure: insights from the candesartan in heart failure: assessment of reduction in mortality and morbidity (CHARM) program. Am Heart J. 2011;162(5):900–906. doi: 10.1016/j.ahj.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Wasywich C.A., Gamble G.D., Whalley G.A., Doughty R.N. Understanding changing patterns of survival and hospitalization for heart failure over two decades in New Zealand: utility of 'days alive and out of hospital' from epidemiological data. Eur J Heart Fail. 2010;12(5):462–468. doi: 10.1093/eurjhf/hfq027. [DOI] [PubMed] [Google Scholar]
- 27.Xian Y., Wu J., O'Brien E.C. Real world effectiveness of warfarin among ischemic stroke patients with atrial fibrillation: observational analysis from Patient-Centered Research into Outcomes Stroke Patients Prefer and Effectiveness Research (PROSPER) study. BMJ. 2015;351 doi: 10.1136/bmj.h3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ellis G., Whitehead M.A., Robinson D., O'Neill D., Langhorne P. Comprehensive geriatric assessment for older adults admitted to hospital: meta-analysis of randomised controlled trials. Bmj. 2011;343:d6553. doi: 10.1136/bmj.d6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Myles P.S., Shulman M.A., Heritier S. Validation of days at home as an outcome measure after surgery: a prospective cohort study in Australia. BMJ Open. 2017;7(8) doi: 10.1136/bmjopen-2017-015828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lunde A.S., Lundeborg S., Lettenstrom G.S., Thygesen L., Huebner J. vol. 84. 1980. The person-number systems of Sweden, Norway, Denmark, and Israel; pp. 1–59. (Vital and health statistics series 2, data evaluation and methods research). [PubMed] [Google Scholar]
- 31.Johansson L.A., Bjorkenstam C., Westerling R. Unexplained differences between hospital and mortality data indicated mistakes in death certification: an investigation of 1,094 deaths in Sweden during 1995. J Clin Epidemiol. 2009;62(11):1202–1209. doi: 10.1016/j.jclinepi.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 32.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 33.Ludvigsson J.F., Andersson E., Ekbom A. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. doi: 10.1186/1471-2458-11-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.ASA House of Delegates ASA physical status classification system. 2014. http://www.asahq.org/resources/clinical-information/asa-physical-status-classification-system
- 35.Powell J.T., Sweeting M.J., Ulug P. Meta-analysis of individual-patient data from EVAR-1, DREAM, OVER and ACE trials comparing outcomes of endovascular or open repair for abdominal aortic aneurysm OVER 5 years. Br J Surg. 2017;104(3):166–178. doi: 10.1002/bjs.10430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lovegrove R.E., Javid M., Magee T.R., Galland R.B. A meta-analysis of 21,178 patients undergoing open or endovascular repair of abdominal aortic aneurysm. Br J Surg. 2008;95(6):677–684. doi: 10.1002/bjs.6240. [DOI] [PubMed] [Google Scholar]
- 37.Greenhalgh R.M., Brown L.C., Kwong G.P., Powell J.T., Thompson S.G. Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results: randomised controlled trial. Lancet. 2004;364(9437):843–848. doi: 10.1016/S0140-6736(04)16979-1. [DOI] [PubMed] [Google Scholar]
- 38.Collins G.S., Jibawi A., McCulloch P. Control chart methods for monitoring surgical performance: a case study from gastro-oesophageal surgery. Eur J Surg Oncol. 2011;37(6):473–480. doi: 10.1016/j.ejso.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 39.Groff A.C., Colla C.H., Lee T.H. Days spent at home - a patient-centered goal and outcome. N Engl J Med. 2017;375(17):1610–1612. doi: 10.1056/NEJMp1607206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khuri S.F., Henderson W.G., DePalma R.G., Mosca C., Healey N.A., Kumbhani D.J. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg. 2005;242(3):326–341. doi: 10.1097/01.sla.0000179621.33268.83. [discussion 41-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shulman M.A., Myles P.S., Chan M.T., McIlroy D.R., Wallace S., Ponsford J. Measurement of disability-free survival after surgery. Anesthesiology. 2015;122(3):524–536. doi: 10.1097/ALN.0000000000000586. [DOI] [PubMed] [Google Scholar]
- 42.Myles P.S., Peyton P., Silbert B., Hunt J., Rigg J.R., Sessler D.I. Perioperative epidural analgesia for major abdominal surgery for cancer and recurrence-free survival: randomised trial. BMJ. 2011;342:d1491. doi: 10.1136/bmj.d1491. [DOI] [PubMed] [Google Scholar]
- 43.Haynes A.B., Weiser T.G., Berry W.R. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med. 2009;360(5):491–499. doi: 10.1056/NEJMsa0810119. [DOI] [PubMed] [Google Scholar]
- 44.Lovegrove J., Valencia O., Treasure T., Sherlaw-Johnson C., Gallivan S. Monitoring the results of cardiac surgery by variable life-adjusted display. Lancet. 1997;350(9085):1128–1130. doi: 10.1016/S0140-6736(97)06507-0. [DOI] [PubMed] [Google Scholar]
- 45.Public reporting of surgical outcomesLancet. 2011;377(9772):1126. doi: 10.1016/S0140-6736(11)60446-7. [DOI] [PubMed] [Google Scholar]
- 46.Meyer G.S., Nelson E.C., Pryor D.B. More quality measures versus measuring what matters: a call for balance and parsimony. BMJ Qual Saf. 2012;21(11):964–968. doi: 10.1136/bmjqs-2012-001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gan F.F., Tang X., Zhu Y., Lim P.W. Monitoring the quality of cardiac surgery based on three or more surgical outcomes using a new variable life-adjusted display. International J Qual Health Care. 2017;29(3):427–432. doi: 10.1093/intqhc/mzx033. [DOI] [PubMed] [Google Scholar]
- 48.Aylin P., Alves B., Best N. Comparison of UK paediatric cardiac surgical performance by analysis of routinely collected data 1984–96: was Bristol an outlier? Lancet. 2001;358(9277):181–187. doi: 10.1016/S0140-6736(01)05404-6. [DOI] [PubMed] [Google Scholar]
- 49.Carter D.J. Correcting the record: Australian prosecutions for manslaughter in the medical context. J Law Med. 2015;22(3):588–609. [PubMed] [Google Scholar]
- 50.Dekker S.W., Hugh T.B. A just culture after mid Staffordshire. BMJ Qual Saf. 2014;23(5):356–358. doi: 10.1136/bmjqs-2013-002483. [DOI] [PubMed] [Google Scholar]
- 51.Pearse R.M., Moreno R.P., Bauer P. Mortality after surgery in Europe: a 7-day cohort study. Lancet. 2012;380(9847):1059–1065. doi: 10.1016/S0140-6736(12)61148-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stark J., Gallivan S., Lovegrove J. Mortality rates after surgery for congenital heart defects in children and surgeons' performance. Lancet. 2000;355(9208):1004–1007. doi: 10.1016/s0140-6736(00)90001-1. [DOI] [PubMed] [Google Scholar]
- 53.DeLancey J.O., Softcheck J., Chung J.W., Barnard C., Dahlke A.R., Bilimoria K.Y. Associations between hospital characteristics, measure reporting, and the Centers for Medicare & Medicaid Services Overall Hospital Quality Star Ratings. JAMA. 2017;317(19):2015–2017. doi: 10.1001/jama.2017.3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nishikawa G., Prasad V. Diagnostic expansion in clinical trials: myocardial infarction, stroke, cancer recurrence, and metastases may not be the hard endpoints you thought they were. Bmj. 2018;362 doi: 10.1136/bmj.k3783. [DOI] [PubMed] [Google Scholar]
- 55.Terragni P.P., Antonelli M., Fumagalli R. Early vs late tracheotomy for prevention of pneumonia in mechanically ventilated adult ICU patients: a randomized controlled trial. Jama. 2010;303(15):1483–1489. doi: 10.1001/jama.2010.447. [DOI] [PubMed] [Google Scholar]
- 56.Barnett M.L., Hsu J., McWilliams J.M. Patient characteristics and differences in hospital readmission rates. JAMA Intern Med. 2015;175(11):1803–1812. doi: 10.1001/jamainternmed.2015.4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ahmed J., Khan S., Lim M., Chandrasekaran T.V., MacFie J. Enhanced recovery after surgery protocols — compliance and variations in practice during routine colorectal surgery. Colorectal Dis. 2012;14(9):1045–1051. doi: 10.1111/j.1463-1318.2011.02856.x. [DOI] [PubMed] [Google Scholar]
- 58.Group EC The impact of enhanced recovery protocol compliance on elective colorectal cancer resection: results from an international registry. Ann Surg. 2015;261(6):1153–1159. doi: 10.1097/SLA.0000000000001029. [DOI] [PubMed] [Google Scholar]
- 59.Sheingold S.H., Zuckerman R., Shartzer A. Understanding Medicare hospital readmission rates and differing penalties between safety-net and other hospitals. Health Aff (Millwood) 2016;35(1):124–131. doi: 10.1377/hlthaff.2015.0534. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables