Abstract
Background and Purpose
The role played by post-stroke inflammation after an ischemic event in limiting functional recovery remains unclear. One component of post-stroke inflammation is disruption of the blood-brain barrier (BBB). This study examines the relationship between post-stroke BBB disruption and functional outcome.
Methods
Acute stroke patients treated with thrombolysis underwent MRI scanning 24 hours and 5 days after their initial event. BBB permeability maps were generated from perfusion weighted imaging. Average permeability was calculated in the affected hemisphere. Good functional outcome, defined as a modified Rankin score of 0 or 1, was compared with average permeability using logistic regression.
Results
Of the 131 patients enrolled, 76 patients had the necessary data to perform the analysis at 24 hours and 58 patients had data for the 5-day assessment. Higher BBB permeability measured at 24 hours (OR 0.57; 95% CI 0.33– 0.99, p=0.045) and at 5 days (OR 0.24; 95% CI 0.09:0.66, p=0.005) was associated with worse functional outcome one to three months after the acute ischemic stroke. For every percentage increase in BBB disruption at 5 days, there was a 76% decrease in the chance of achieving a good functional outcome after stroke. Multivariate analysis found this to be independent of age, stroke volume or clinical stroke severity.
Conclusions
Post-stroke BBB disruption appears to be predictive of functional outcome irrespective of stroke size.
Keywords: Blood-brain barrier, Functional outcome, Ischemic Stroke, MRI, Post-stroke inflammation
Introduction
Acute ischemic stroke (AIS) causes immediate brain injury due to a lack of sufficient blood flow to the affected brain tissue. Treatments for AIS aim to restore blood flow either mechanically with thrombectomy or pharmacologically with thrombolysis using intravenous tissue plasminogen activator (tPA). Successful early reperfusion is associated with better functional outcomes. In the days after an AIS, a post-stroke inflammatory response occurs;1 however, the role of this post-stroke inflammation in functional outcome has yet to be established.
In a healthy brain, the blood-brain barrier (BBB) regulates the entry of cells and molecules into the brain which, in conjunction with the central nervous system (CNS) microenvironment, creates a certain degree of immune privilege.2 The BBB is known to become disrupted during and after an AIS. There is an early, reversible opening of the BBB during the hours after stroke which is followed by a second irreversible disruption of the BBB in the days after the stroke due to an inflammatory response.1 Clinically, this neuroinflammatory phase is seen as gadolinium enhancement on magnetic resonance imaging (MRI) when performed several days after the AIS.3
In this study we quantified BBB disruption on MRI as a biomarker for post-stroke neuroinflammation during the days after thrombolysis for AIS in order to investigate the role of this measure in functional outcome.
Methods
Population
All patients included in this study were enrolled in our IRB-approved National Institutes of Health (NIH) Natural History of Stroke (NHS) Study (identification number NCT00009243) which is an observational cohort study of stroke patients. Patients enrolled in the NHS study, whenever possible, have MRIs performed 24 hours after treatment and again approximately 5 days after their stroke. Patients enrolled during 2013 and 2014 who had an MRI scan prior to treatment with intravenous (IV) tPA and follow-up MRI imaging with gadolinium at the 24 hour and/or the 5-day time points after their AIS were included in this study if they had a unilateral supratentorial stroke. This study required comparison between the affected and unaffected hemispheres to perform the BBB analysis; thus, patients with bilateral strokes were excluded.
MRI Parameters
Images were acquired on a 1.5T GE Signa scanner (General Electric Medical Systems, Milwaukee, WI), a 3T Philips Achieva scanner (Philips Healthcare, Best, The Netherlands), or a 3T Siemens Skyra scanner (Siemens AG, Munich, Germany). Image sequences and typical parameter ranges were: Diffusion tensor imaging (TR 4461 – 10500msec, TE 61.6 – 91.3 msec, 3.5mm slice thickness, 40 slices) used to generate trace diffusion weighted images (DWI) using three orthogonal directions (b = 0 and 1000 s/mm2) and apparent diffusion coefficient (ADC) maps; dynamic susceptibility contrast (DSC) perfusion weighted imaging (PWI) (TR 1–1.5 sec, TE 25 – 45 msec, 7mm slice thickness, 20 slices, 40 – 80 dynamics), which was collected during the injection of a weight-based dose of gadolinium; FLAIR imaging (TR 9000 msec, TE 120 – 145 msec, 3.5 mm slice thickness, 40 slices); time-of-flight magnetic resonance angiography (MRA) images (TR 18 – 23 msec, TE 3.43–6.8 msec, 0.75 – 1.4 mm slice thickness, 73 – 95 slices); and gradient recall echo (GRE) images (TR 700 – 800 msec, TE 12 – 20.4 msec, 3.5 – 7mm slice thickness, 20 – 40 slices).
Image Processing
MRI scans were co-registered to a template atlas using a diffeomorphic registration algorithm.4 The template atlas was used to segment the left and right hemispheres and remove infratentorial structures. Blood-brain barrier (BBB) permeability maps were generated from the PWI source images using a method previously described.5–7 BBB permeability of the affected hemisphere was calculated relative to the unaffected hemisphere on a voxel-by-voxel basis, expressed as a percent increase over normal. Voxels in the affected hemisphere that demonstrated increased BBB permeability above the noise threshold of 1% were averaged to generated the average permeability of the hemisphere. The size of the pre-treatment perfusion deficit was calculated from the PWI using a time-to-peak threshold (TTP) of 4 seconds beyond normal tissue. TTP was used instead of time-to-maximum (Tmax) since it has been shown to be reasonably interchangeable with Tmax; it is more easily reproduced, and it is less susceptible to artifacts introduced by deconvolution.8, 9 The final infarct volume was calculated from the 24-hour MRI scan as per recommended guidlines10 using an ADC threshold of 600 μm2/sec. The 24-hour time point was used to calculate infarct volume to avoid the effects of vasogenic edema and normalization of ADC values that can occur at the 5-day time point.10 Image processing was performed in Matlab (Mathworks, Natick, MA). Image processing was done using an automated pipeline with minimal user interaction. The BBB calculations were completely automated and thus not influenced by an operator or other variables as described previously.11
Clinical and outcome measures
Stroke severity was determined at the 5-day time point based on the NIH Stroke Scale (NIHSS). If a patient was discharged prior to the 5-day time point, the NIHSS at the time of discharge was used as the 5-day value. Hemorrhagic transformation complications were assessed on the MRI at the 24-hour time point. New hemorrhage since the prethrombolysis MRI was graded according to ECASS criteria.12 Patients were assessed for any hemorrhage, parenchymal hematoma (PH), or symptomatic intracranial hemorrhage (sICH) based on SITS-MOST criteria.13 Large vessel occlusion (LVO) was defined as occlusion of the internal carotid artery or the first segment of the middle cerebral artery on the pretreatment MRA. Chronic cerebral small vessel disease was assessed by applying the Fazekas scale14 to the pretreatment FLAIR scan. Functional outcome was assessed based on the modified Rankin scale (mRS), evaluated by stroke researchers certified in performing this evaluation. Patients who returned for an MRI one month after their acute event had their one-month mRS assessed during that visit. Patients who could be contacted by phone three months after their event had their three-month mRS calculated based on that phone interview. Final mRS used for this study was based on the three-month mRS if that data point was available. In the absence of a three-month mRS, the one-month mRS was used as the final mRS. Patients without either a one-month or a three-month mRS were excluded from this study. Good functional outcome was defined as a final mRS of 0 or 1 since this is the standard definition used in studies of thrombolysis.15 Outcome assessments were conducted blinded to the results of the BBB analysis.
Statistical Analysis
Average permeability was treated as a continuous independent variable and compared with the continuous dependent variable mRS using linear regression. Average permeability was also treated as a continuous independent variable when compared with the binary dependent variable good functional outcome using logistic regression. A p-value less than 0.05 was considered significant. Receiver operator characteristic (ROC) analysis was used to compare the 24-hour and 5-day average BBB values to determine which was better at predicting a good functional outcome. To compare the degree of BBB disruption at 24 hours with the degree at 5 days in patients with a good functional outcome, a mean comparison t-test was used. Demographics, vascular risk factors, stroke severity, perfusion deficit and final infarct volume where included in a logistic regression multivariate analysis comparing average permeability with good functional outcome if they had a p-value less than 0.1 in univariate analysis. All statistical analysis was performed using STATA 13 (StataCorp LLC, College Station, TX).
Availability of Data and Materials
Data in this study is monitored by the NIH Office of Human Subjects Research Protections (OHSRP) and the Combined NeuroScience (CNS) IRB of NIH. Requests for access to the data may be possible if approved by these governing bodies. Please contact author for data requests.
Results
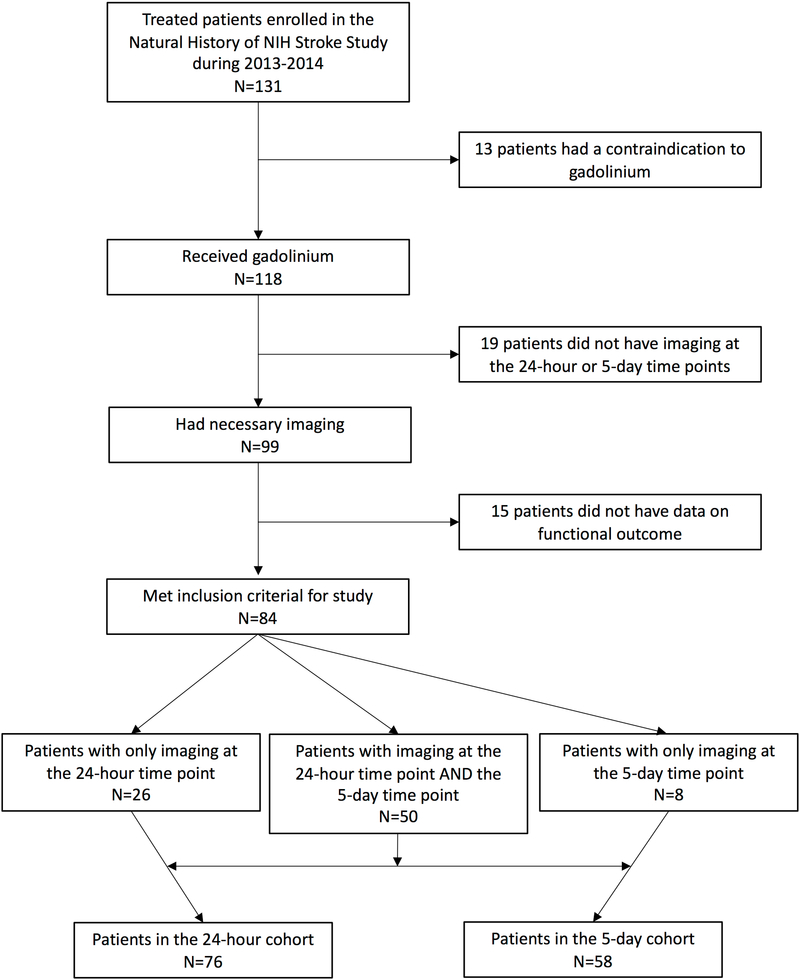
Of the 131 tPA-treated patients who were evaluated with MRI and enrolled during the study period, 76 patients met the inclusion criteria for this sub-study and had the necessary imaging at the 24-hour time point. For the 5-day time point, 58 patients had the necessary imaging. These two cohorts overlapped by 50 patients who had both timepoints. The breakdown for the population is shown diagrammatically in figure 1. Average permeability measured at 24 hours (p=0.012, r2=0.08) and 5 days (p<0.001, r2=0.19) was significantly associated with mRS one to three months later. However, the r2 terms suggest that the role of BBB disruption in determining outcome is twice as important at 5 days than at 24 hours.
Figure 1:
A flow chart shows how the original population of patients enrolled in the study were either included or excluded to arrive at the final cohort populations. Note the 50 patients who had both time points were included in both cohorts.
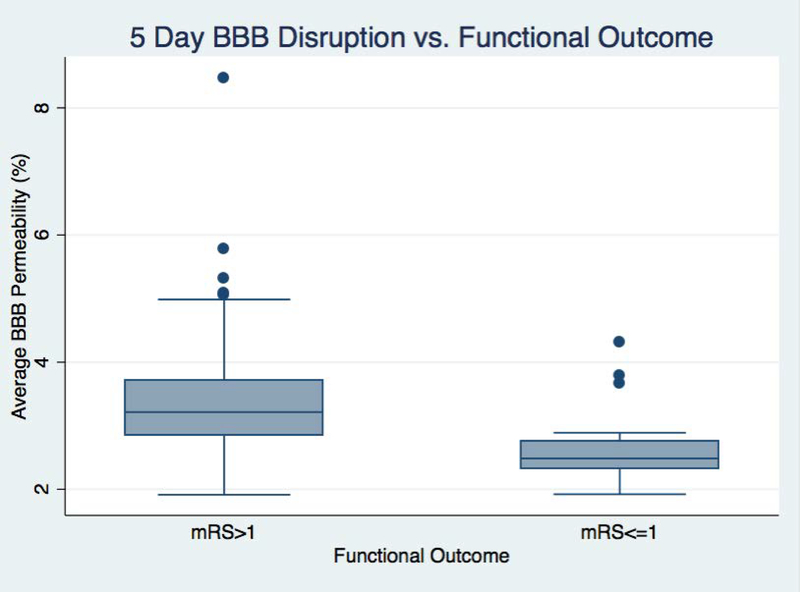
Looking at the binary outcome of good functional outcome (mRS of 0 or 1), average permeability was significant when measured at 24 hours (p=0.045, OR 0.57; CI 33:0.99) but even more so at 5 days (p=0.005, OR 0.24; CI 0.09:0.66). This indicates that for every one percent increase in the BBB permeability measured 5 days after stroke, the chance of achieving a good functional outcome decreases by 75%. A boxplot showing the difference in day 5 BBB disruption between the outcome groups is shown in figure 2.
Figure 2:
A box plot compares the blood-brain barrier (BBB) measured on day five between patients who had a good outcome (modified Rankin scale (mRS) <= 1) with patients who did not (mRS > 1).
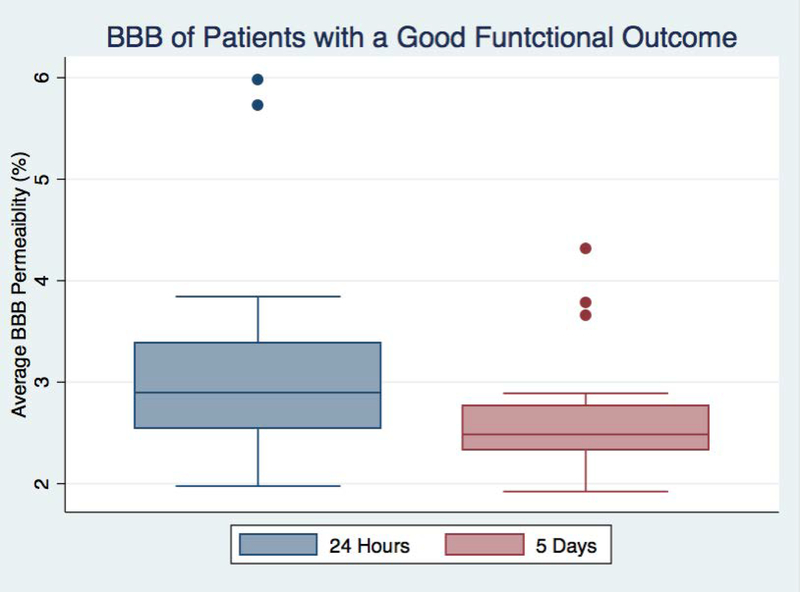
ROC analysis testing the predictive value of average permeability in determining if a patient will have a good outcome found an area under the curve (AUC) of 0.67 for the 24-hour BBB and AUC of 0.77 for the 5-day BBB. This confirms that 5-day BBB disruption plays a more substantial role in determining functional outcome than the BBB measured at 24-hours. Looking only at patients who had a good functional outcome, average BBB was significantly higher at 24 hours than at 5 days (p=0.04) as seen in figure 3. This suggests that some portion of the BBB disruption detected at the earlier time point was reversible in patients who did well, again supporting the notion that 5-day BBB disruption is a better predictor of outcome.
Figure 3:
A box plot compares the blood-brain barrier (BBB) measured 24 hours after stroke with the BBB measured five days after stroke for patients who had a good outcome (modified Rankin scale (mRS) <= 1).
Given the stronger association with 5-day BBB, the subsequent analysis focused only on the 5-day BBB measurements. Table 1 shows the characteristics of the 58 patients included in the 5-day BBB analysis separated by those who did, and did not, achieve a good functional outcome. Overall this cohort had predominantly mild strokes with a median NIHSS of 3 at the time of the 5-days scan and fairly good outcomes with a median mRS of 2. LVO was present in 14 patients, seven that had a good functional outcome and seven that did not. At the time of the study, the use of endovascular treatment had not been validated, however, three patients in this cohort did have endovascular treatment in addition to IV thrombolysis, and all had a good functional outcome.
Table 1:
The population of patients who had BBB measured five days after stroke is characterized and separated by outcome. The p-values reflect the significance of differences between the groups based on the listed characteristic. Sample size indicates the number of patients who had the necessary information to be part of the logistic regression.
| All patients (n=58) | GFO, mRS 0–1(n=24) | No GFO, mRS>1 (n=34) | p-value | Sample size | |
|---|---|---|---|---|---|
| Demographics: | |||||
| Age (median) | 75 | 68 | 80 | 0.003* | 58 |
| Sex (% female) | 51% | 42% | 59% | 0.2 | 58 |
| Risk Factors: | |||||
| Hypertension | 72% | 67% | 76% | 0.412 | 58 |
| Diabetes | 26% | 25% | 26% | 0.9 | 58 |
| Hyperlipidemia | 31% | 21% | 38% | 0.164 | 58 |
| Atrial Fibrillation | 34% | 38% | 32% | 0.685 | 58 |
| Fazekas Score (median) | 3 | 3 | 4 | 0.060 | 57 |
| Stroke Characteristics: | |||||
| LVO | 24% | 30% | 21% | 0.454 | 58 |
| 5-day NIHSS (median) | 3 | 1 | 5 | 0.043* | 57 |
| Acute Perfusion Volume (mean) | 57.7 mL | 51.2 mL | 62.3 mL | 0.600 | 53 |
| 24-hour Infarct Volume (mean) | 14.6 mL | 5.1 mL | 21.8 mL | 0.162 | 49 |
| Average 5-day Permeability | 3.17% | 2.64% | 3.53% | 0.005* | 58 |
| Hemorrhage: | |||||
| Any HT | 26% | 25% | 26% | 0.79 | 56 |
| PH | 7% | 4% | 9% | 0.47 | 56 |
| sICH | 4% | 0% | 6% | -- | 56 |
| Outcome: | |||||
| mRS (median) | 2 | 0 | 3.5 |
=significant difference
GFO=good functional outcome; LVO=large vessel occlusion; HT=hemorrhagic transformation; PH=parenchymal hematoma; sICH=symptomatic intracranial hemorrhage
Although strokes were on average larger in the poor outcome group, this did not reach significance. Good functional outcome was associated with younger age (p=0.003) and lower NIHSS at 5 days (p=0.043). Chronic small vessel disease trended toward a larger white matter lesion load in patients with a poor outcome (p=0.060). In multivariate analysis, 5-day average permeability remained independent of age (p=0.016, OR 0.33; CI 0.13:0.82), NIHSS at 5 days (p=0.019, OR 0.27; CI 0.09:0.80) or Fazekas score (p=0.008, OR 0.26; CI 0.10:0.71). None of the variables included in the multivariate analysis were identified as being collinear with 5-day average permeability.
Discussion
This study used a quantitative measure of gadolinium leakage through the BBB as a component measure of post-stroke inflammation. We identified a range of values across the population suggesting that post-stroke inflammation is variable between patients in a manner that is independent of stroke size or severity. Patients with more post-stroke inflammation five days after their ischemic event were more likely to have a poor outcome one to three months later. In our cohort, BBB permeability had a stronger relationship with outcome than final infarct volume. Final infarct volume reflects the injury that has already occurred, while BBB disruption presumably reflects ongoing injury and thus may be a better predictor of functional outcome over the subsequent months.
The use of BBB disruption as a marker for post-stroke inflammation performed better at the five-day time point than the 24-hour time point which is consistent with the known time course of BBB disruption and its relationship to post-stroke inflammation. BBB permeability has been shown to be dynamic in animal models of stroke16 and also in humans.7 BBB disruption early after stroke has been attributed to hypoxia induced activation of latent matrix metalloproteinase 2 (MMP-2) and is reversible.1 However a second phase of BBB disruption can occur in the days after a stroke mediated by MMP-3, MMP-9, and cyclooxygenases 2 (COX-2).17 This inflammatory opening of the BBB leads to neutrophil infiltration, cerebral edema, microglial activation and the production of neuroinflammatory mediators that exacerbate the cerebral injury.16
Cerebral ischemia-related inflammation engages both the innate and adaptive immune systems with innate immunity being activated in the first 24–48 hours, and adaptive responses occurring several days later.18 In animal models of ischemia-reperfusion, peak inflammation was observed three to seven days after the ischemic event.19 This inflammation peak was associated with a high infiltration rate of not only neutrophils but also adaptive immune system cells which were represented by dendritic cells, T-cells and NK cells.19
This peak of inflammation occurs during the second phase of BBB which allows invasion of immune cells into the infarcted brain. These cells, upon encountering novel brain antigens, initiate an autoimmune response which leads to further destruction of neurological tissue.20 Pathological studies in humans have shown an inflammatory infiltrate persists after the stroke between days 3–37 and is characterized by the presence of polymorphonuclear leucocytes and mononuclear cells in 84% and 41% of cases respectively.21 Given the existing evidence that the second phase of BBB disruption on day 3–7 coincides with the peak of proinflammatory cell infiltration in infarcted tissue, it is likely that inflammatory immune reactions, characterized by BBB disruption, are responsible for the unfavorable stroke outcome seen in our study.
If proinflammatory immune response and BBB disruption are associated with unfavorable stroke outcome, could anti-inflammatory interventions improve stroke outcome? Thus far, there is limited clinical evidence that anti-inflammatory therapy improves stroke outcome. A phase II, randomized, double-blind, placebo-controlled clinical trial of recombinant human IL-1ra in patients with acute stroke showed that, among patients with cortical infarcts, clinical outcomes at three months in the rhIL-1ra treated group were better than in the placebo group.22 Minocycline, the antibiotic with anti-inflammatory and protease inhibitor properties, has been shown to be safe when administered together with IV t-PA.23 In another open-label study, minocycline appeared to improve NIHSS, mRS, and Barthel Index.24 However it is also possible that the BBB disruption itself is facilitating the ongoing injury leading to poor outcome. Thus, treatments targeting BBB stabilization may also play a role. One approach is to identify agents that inhibit MMP activity.25
Natalizumab, which is anti-α4-integrin monoclonal antibody, was tested in a phase II, randomized, placebo-controlled clinical trial in acute ischemic stroke26 and was found to be associated with better functional outcome in the treatment group compared to placebo. Natalizumab is known, in multiple sclerosis, to prevent white blood cells from crossing the blood-brain barrier and attacking the CNS.27 It is possible that favorable clinical outcome in the stroke trial was achieved through the modulation of late proinflammatory effects associated with the prevention of T-cell traffic through disrupted BBB. A follow-up study of natalizumab in stroke was reportedly negative. However, measurement of BBB disruption prior to enrollment has not been part of any clinical trial testing anti-inflammatory medications in stroke patients. The present study indicates that measurement of BBB leakage may provide a useful biomarker for future trials of anti-inflammatory agents as promoters of recovery from stroke.
MRI has previously been used to measure post-stroke gadolinium leakage in stroke patients.28 This previous study also found variable levels of BBB disruption across the population that appeared to peak in the timeframe of 6–48 hours after stroke; however that study did not have serial imaging, nor the temporal resolution, to identify the fluctuations seen in the current study. Our study used a method for measuring BBB disruption that involved post processing of an MRI sequence that is routinely acquired as part of a clinical stroke evaluation.5, 29, 30 Thus, in identifying a link between post-stroke BBB disruption and functional outcome, a biomarker for identification of patients who may benefit from a therapeutic intervention may also have been identified.
We previously reported increased BBB permeability in patients with chronic small vessel disease.11 This form of BBB disruption is also thought to be inflammatory in nature.1 In the current study we controlled for chronic white matter hyperintensities and found that elevated BBB permeability at 5 days was associated with poor outcome independent of the baseline burden of white matter lesions. However, there may be a link between acute and chronic BBB disruption in cerebrovascular disease, and further studies are needed. Increased BBB permeability is a known risk factor for sICH5, and idiopathic ICH itself causes opening of the BBB. sICH was rare in our cohort and only occurred in the poor outcome group; thus, we are not able to separate the role of sICH versus the 5-day BBB measurement in functional outcome. However, removing the two patients with sICH from the logistic regression did not impact the relationship between 5-day BBB and functional outcome (p=0.006).
There are several limitations to this study; the sample size was modest and varied based on the available data. However, this did not lead to marginal significance or a small effect size with regard to the primary hypothesis. There may be selection bias since only patients who signed informed consent to be part of our observational study were included, and, among those who participated, only those with adequate data were included in the analysis. Because of this, it is also possible, that patients with more severe strokes were not included in the study. Since patients with poor renal function are unable to received gadolinium, they were excluded from this study. As such, these results may not generalize to them. All patients in this study received IV tPA which may have affected the BBB, thus it is not known if these results would apply to an untreated population. Also, despite the significant circumstantial support presented above, we do not know definitively that the BBB measured is a true reflection of the inflammatory response. Similarly, we do not know that treatment of the measured BBB disruption would change outcome. Future studies should test the effect of anti-inflammatory drugs on post-stroke BBB disruption to further characterize these points.
Conclusions
This study found that higher degrees of BBB disruption, measured on MRI five days after thrombolytic therapy for acute stroke, was associated with worse outcome despite prior treatment with acute reperfusion therapy and independent of stroke size or severity. Thus, BBB leakage as a biomarker for post-stroke inflammation may constitute a useful therapeutic index for treatment in future studies of ischemic stroke.
Acknowledgements
This research was possible because of contributions from the NIH Natural History of Stroke Investigators including: Richard T. Benson, Amie W. Hsia, Lawrence L. Latour, Richard Leigh, Marie Luby, John K. Lynch, and Zurab Nadareishvili.
Sources of funding:
Funding for this study came from the National Institute of Neurological Disorders and Stroke (NINDS) Intramural Research Program of the NIH.
Footnotes
Disclosures:
The authors have no conflicts of interest to declare.
Statement of Ethics:
This study was approved by the Combined NeuroScience (CNS) Institutional Review Board (IRB) of the National Institutes of Health (NIH). All patients, or their legally authorized representative, signed informed consent.
References
- 1.Yang Y, Rosenberg GA. Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke. 2011;42:3323–3328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galea I, Bechmann I, Perry VH. What is immune privilege (not)? Trends in immunology. 2007;28:12–18 [DOI] [PubMed] [Google Scholar]
- 3.Karonen JO, Partanen PL, Vanninen RL, Vainio PA, Aronen HJ. Evolution of mr contrast enhancement patterns during the first week after acute ischemic stroke. AJNR. American journal of neuroradiology. 2001;22:103–111 [PMC free article] [PubMed] [Google Scholar]
- 4.Oishi K, Faria A, Jiang H, Li X, Akhter K, Zhang J, et al. Atlas-based whole brain white matter analysis using large deformation diffeomorphic metric mapping: Application to normal elderly and alzheimer’s disease participantstlas. Neuroimage. 2009;46:486–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leigh R, Jen SS, Hillis AE, Krakauer JW, Barker PB, Stir, et al. Pretreatment blood-brain barrier damage and post-treatment intracranial hemorrhage in patients receiving intravenous tissue-type plasminogen activator. Stroke. 2014;45:2030–2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leigh R, Christensen S, Campbell BC, Marks MP, Albers GW, Lansberg MG, et al. Pretreatment blood-brain barrier disruption and post-endovascular intracranial hemorrhage. Neurology. 2016;87:263–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simpkins AN, Dias C, Leigh R, National Institutes of Health Natural History of Stroke I. Identification of reversible disruption of the human blood-brain barrier following acute ischemia. Stroke. 2016;47:2405–2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaro-Weber O, Moeller-Hartmann W, Heiss WD, Sobesky J. Maps of time to maximum and time to peak for mismatch definition in clinical stroke studies validated with positron emission tomography. Stroke. 2010;41:2817–2821 [DOI] [PubMed] [Google Scholar]
- 9.Wouters A, Christensen S, Straka M, Mlynash M, Liggins J, Bammer R, et al. A comparison of relative time to peak and tmax for mismatch-based patient selection. Frontiers in neurology. 2017;8:539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warach SJ, Luby M, Albers GW, Bammer R, Bivard A, Campbell BC, et al. Acute stroke imaging research roadmap iii imaging selection and outcomes in acute stroke reperfusion clinical trials: Consensus recommendations and further research priorities. Stroke. 2016;47:1389–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arba F, Leigh R, Inzitari D, Warach SJ, Luby M, Lees KR, et al. Blood-brain barrier leakage increases with small vessel disease in acute ischemic stroke. Neurology. 2017;89:2143–2150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hacke W, Kaste M, Fieschi C, von KR, Davalos A, Meier D, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ecass ii). Second european-australasian acute stroke study investigators. Lancet. 1998;352:1245–1251 [DOI] [PubMed] [Google Scholar]
- 13.Wahlgren N, Ahmed N, Davalos A, Ford GA, Grond M, Hacke W, et al. Thrombolysis with alteplase for acute ischaemic stroke in the safe implementation of thrombolysis in stroke-monitoring study (sits-most): An observational study. Lancet. 2007;369:275–282 [DOI] [PubMed] [Google Scholar]
- 14.Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. Mr signal abnormalities at 1.5 t in alzheimer’s dementia and normal aging. AJR. American journal of roentgenology. 1987;149:351–356 [DOI] [PubMed] [Google Scholar]
- 15.Tissue plasminogen activator for acute ischemic stroke. The national institute of neurological disorders and stroke rt-pa stroke study group. N Engl J Med. 1995;333:1581–1587 [DOI] [PubMed] [Google Scholar]
- 16.Sandoval KE, Witt KA. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiology of disease. 2008;32:200–219 [DOI] [PubMed] [Google Scholar]
- 17.Candelario-Jalil E, Gonzalez-Falcon A, Garcia-Cabrera M, Leon OS, Fiebich BL. Post-ischaemic treatment with the cyclooxygenase-2 inhibitor nimesulide reduces blood-brain barrier disruption and leukocyte infiltration following transient focal cerebral ischaemia in rats. J Neurochem. 2007;100:1108–1120 [DOI] [PubMed] [Google Scholar]
- 18.Iadecola C, Anrather J. The immunology of stroke: From mechanisms to translation. Nat Med. 2011;17:796–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gelderblom M, Leypoldt F, Steinbach K, Behrens D, Choe CU, Siler DA, et al. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. 2009;40:1849–1857 [DOI] [PubMed] [Google Scholar]
- 20.Meisel C, Schwab JM, Prass K, Meisel A, Dirnagl U. Central nervous system injury-induced immune deficiency syndrome. Nat Rev Neurosci. 2005;6:775–786 [DOI] [PubMed] [Google Scholar]
- 21.Mena H, Cadavid D, Rushing EJ. Human cerebral infarct: A proposed histopathologic classification based on 137 cases. Acta neuropathologica. 2004;108:524–530 [DOI] [PubMed] [Google Scholar]
- 22.Emsley HC, Smith CJ, Georgiou RF, Vail A, Hopkins SJ, Rothwell NJ, et al. A randomised phase ii study of interleukin-1 receptor antagonist in acute stroke patients. Journal of neurology, neurosurgery, and psychiatry. 2005;76:1366–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fagan SC, Waller JL, Nichols FT, Edwards DJ, Pettigrew LC, Clark WM, et al. Minocycline to improve neurologic outcome in stroke (minos): A dose-finding study. Stroke. 2010;41:2283–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lampl Y, Boaz M, Gilad R, Lorberboym M, Dabby R, Rapoport A, et al. Minocycline treatment in acute stroke: An open-label, evaluator-blinded study. Neurology. 2007;69:1404–1410 [DOI] [PubMed] [Google Scholar]
- 25.Yang Y, Rosenberg GA. Matrix metalloproteinases as therapeutic targets for stroke. Brain research. 2015;1623:30–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elkins J, Veltkamp R, Montaner J, Johnston SC, Singhal AB, Becker K, et al. Safety and efficacy of natalizumab in patients with acute ischaemic stroke (action): A randomised, placebo-controlled, double-blind phase 2 trial. Lancet Neurol. 2017;16:217–226 [DOI] [PubMed] [Google Scholar]
- 27.Bauer M, Brakebusch C, Coisne C, Sixt M, Wekerle H, Engelhardt B, et al. Beta1 integrins differentially control extravasation of inflammatory cell subsets into the cns during autoimmunity. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1920–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merali Z, Huang K, Mikulis D, Silver F, Kassner A. Evolution of blood-brain-barrier permeability after acute ischemic stroke. PLoS One. 2017;12:e0171558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leigh R, Jen SS, Varma DD, Hillis AE, Barker PB. Arrival time correction for dynamic susceptibility contrast mr permeability imaging in stroke patients. PLoS.One. 2012;7:e52656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leigh R, Krakauer JW. Mri-guided selection of patients for treatment of acute ischemic stroke. Curr Opin Neurol. 2014;27:425–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data in this study is monitored by the NIH Office of Human Subjects Research Protections (OHSRP) and the Combined NeuroScience (CNS) IRB of NIH. Requests for access to the data may be possible if approved by these governing bodies. Please contact author for data requests.