Abstract
Pill aversion, defined as difficulty swallowing pills without identifiable medical cause, is a poorly-characterized barrier to sustained viral suppression for many HIV-infected persons. We aimed to quantify the frequency of self-reported pill aversion, characterize its symptoms, and measure the association between self-reported pill aversion and missing antiretroviral doses.
This is a prospective, observational, exploratory survey study of English-speaking persons living with HIV (PLHIV) at a single urban tertiary outpatient clinic. Participants completed anonymous questionnaires about their experiences of swallowing antiretroviral pills. The primary outcome was skipping pills due to pill aversion symptoms.
Of 384 participants, a quarter (25.5%) skipped pills due to pill aversion symptoms. Younger age, being Non-Hispanic Black or Hispanic, not being married or partnered, having public insurance, not being employed, having less than a college education, and having a mental health diagnosis were associated with skipping pills due to pill aversion. On multivariable regression analyses, PLHIV who skipped pills were more likely to report symptoms of gagging, nausea at the time of swallowing, and heavy feeling in the stomach, as well as being bothered by the taste, smell, and size of the pills. PLHIV who skipped pills were also more likely to report negative and fear-based emotions about pill-taking than PLHIV who did not skip pills due to pill aversion.
HIV-related pill aversion may represent a significant and frequent barrier to adherence in an adult HIV population.
Keywords: adherence, conditioning, human immunodeficiency virus, pill aversion, pill taking
INTRODUCTION
In the HIV Continuum of Care, the Centers for Disease Control and Prevention (CDC) identified 4 sequential steps that are critical to controlling this disease and its transmission: HIV testing (diagnosis), linkage to care, retention in care, and finally, viral suppression (1). The CDC called out a national alarm, noting that significant losses occur at each step, resulting in only 48% of persons living with HIV (PLHIV) in the US having achieved an undetectable viral load (VL) at their latest visit (1) despite the availability of highly effective medications. Verified by repeated serum levels, an undetectable VL provides a surrogate measure of a patient’s adequate antiretroviral (ARV) adherence (2–5); in contrast, a detectable VL is the primary marker of poor adherence. A long-standing detectable VL is associated with HIV morbidity and mortality as well as serving as the primary mechanism through which ARV resistance can develop (3–5). Improving our understanding of the obstacles to medication adherence in HIV is likely to increase the portion of identified PLHIV who reach the therapeutic goal of maintaining a suppressed VL.
There are many published studies documenting barriers to HIV medication adherence (e.g. HIV stigma, depression, pill burden, side effects of medication, competing priorities, substance abuse, heightened stress, and lack of disclosure) (6–12) as well as many effective interventions (13–17). To our knowledge, however, there is no focused discussion of pill swallowing difficulties in the absence of a medical or anatomic etiology among adults with HIV. Pill swallowing difficulties are clinically observed but not mentioned among factors contributing to poor adherence in the recent AIDSinfo Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV (18) or the existing HIV literature. Although the pharmaceutical industry has alleviated much of the problem of pill burden via combination oral drugs, smaller pills, and the development of injectable medications, still the vast majority of PLHIV must swallow one or more pills per day to successfully control their disease. This study examines the day-to-day emotions, physical sensations, and behavior of PLHIV during the daily process of swallowing pills in order to better understand this undescribed adherence barrier.
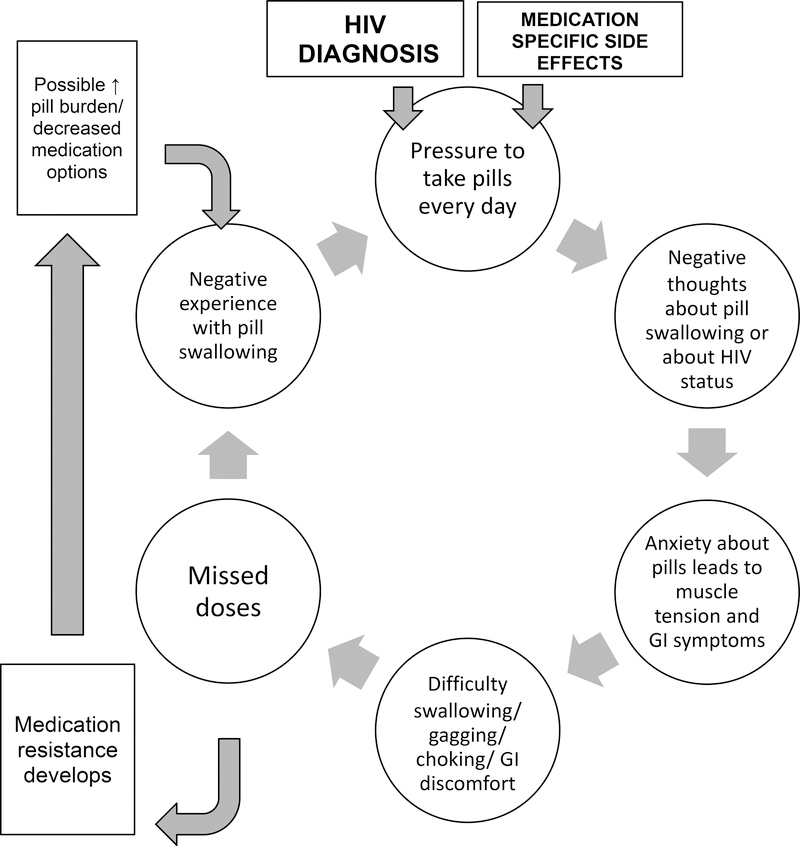
Herein, our group has identified pill aversion as a clinically observed yet previously un-characterized barrier to adherence to ARVs. Pill aversion is defined as difficulty swallowing pills without identifiable medical cause as well as anxiety and negative emotions associated with pill swallowing (19). Although there are distinct physical sensations for most individuals experiencing pill aversion, the etiology appears to be psychological and not due to ARV side effects; symptoms commonly begin prior to, during, or immediately after swallowing, before significant medication absorption has taken place. Due to the lack of empirical support for pill aversion, our group has applied different psychological theories to hypothesize the development and maintenance of pill aversion, primarily classical conditioning, operant conditioning, and cognitive theories (19). More specifically, the varying experiences of physical, cognitive, and emotional discomfort reinforce negative pill swallowing experiences and continue a cycle of poor medication adherence (Fig. 1). One model of pill aversion supported by the oncology treatment literature proposes classical conditioning and anxiety as two predominant contributing factors to anticipatory nausea and vomiting before chemotherapy (20). Following this model, PLHIV who experience pill aversion symptoms prior to taking their ARVs may be conditioned to experience physical symptoms based upon previous negative side effects. A different theoretical explanation is based upon the physical changes from the “fight or flight” response in reaction to a perceived (cognitive) threat of the pills (21). This can explain the sensation of throat tightening, acid reflux, nausea, and others at the time of swallowing or immediately after swallowing, yet before actual side effects could develop (22). In clinical practice we have observed that PLHIV vary greatly in their attitudes, reactions, and level of distaste for their pills as well as thoughts, emotions, and perceived stigma of HIV; these variations appear to be important in understanding and promoting adherence.
Figure 1.
Pill Aversion Conceptualization
It is well established that ARVs are less effective unless taken with consistency (23). Suboptimal adherence can lead to viral resistance (23) and when this occurs, those affected often require an increased pill burden and/or have fewer choices of effective medications. Thus, those who experience pill aversion chronically are at risk for intensifying problems with this behavioral issue over time. One clinical example helps to illustrate the complex psychological components of pill aversion (Fig 2).
Figure 2.
Pill Aversion Clinical Example
As pill aversion is not part of the US national guidelines or standard of care in assessing HIV medication adherence, we hypothesize that providers may fail to identify pill aversion symptoms and that this is a more prevalent barrier than they are aware. Thus, in this pilot study, the objective was to characterize HIV-related pill aversion in several dimensions. We aimed to quantify the frequency of pill aversion in a diverse US cohort, assess the symptoms reported by individuals with pill aversion, and determine emotional characteristics or characteristics of pills that affect skipping pills due to pill aversion.
METHODS
Setting and Recruitment
This was a prospective, observational, exploratory study of PLHIV at a single urban tertiary care center in Chicago, Illinois. Individuals receiving HIV care were invited to complete an anonymous questionnaire about their experience of swallowing antiretroviral pills. We chose the format of an anonymous survey to encourage as many patients as possible to participate in order to begin describing the presence of pill aversion symptoms. Patients of the Northwestern Memorial Hospital Division of Infectious Disease Clinic were asked to participate if they were at least 18 years old, English-speaking, able to read a questionnaire in English independently, living with HIV, and prescribed ARVs. All genders and pregnant women were included in this study. As this was an anonymous survey, the study was deemed exempt from the Northwestern University Institutional Review Board (IRB) and written consent was not required.
A convenience sample of outpatients was identified as eligible to participate in the survey by the patient care technician who placed each patient into an exam room and took vital signs prior to a physician or nurse practitioner visit. Each participant was invited to complete the survey privately during the wait time prior to their clinician visit. The technician followed a standardized script to offer the voluntary and anonymous survey, indicating that it was a study about “pill swallowing.” Further information about the study as required by the Institutional Review Board was written on the cover page of the survey. Participants completed the survey while in the exam room, placed it into an unmarked envelope, and handed it to the clinic scheduling staff upon leaving the clinic. Participants were also offered the opportunity to place the blank survey in the envelope to conceal their decision to participate or not, or to leave the survey in their exam room. The survey required approximately 10 minutes and was self-administered on paper. Survey responses were transcribed to Research Electronic Data Capture (REDCap; Vanderbilt University, Nashville, TN).
Surveys were offered over a 10-month period (March-December 2016). The sample size goal of at least 375 surveys was based on the a priori goal of obtaining 60% of eligible participants seen at this facility in the study period; as this was an exploratory study without a predetermined estimated prevalence of pill aversion, a power calculation was not performed. At the time of study initiation, approximately 1600 PLHIV received care at this facility, of whom approximately 70% were English-speaking adults able to consent. Approximately two-thirds of these individuals are seen in this outpatient setting at least twice per year, yielding a population of approximately 625 individuals likely to be accessed during a 10-month study period. The final sample of 384 participants was representative of the overall clinic population.
Survey design
As there are no prior reports describing pill aversion in a population of PLHIV and no existing validated assessment tool, our study team used a process of expert discussion and review to generate novel survey items. The content of survey items was based upon the clinical team’s repeated clinical assessment, expertise in the area of adherence issues facing those being treated for HIV, and close rapport with this particular population. Prior to use in the research setting, this pen-and-paper survey was tested with the clinical staff of the Infectious Disease and Maternal-Fetal Medicine clinics to establish face validity. Modifications were made based on feedback prior to investigation with patients.
The first portion of the survey consisted of basic demographic questions, including age, race, ethnicity, insurance status, employment, education, and marital status. Clinical information on the survey included year of HIV diagnosis, viral load, adherence, pill schedule, and comorbid mental health conditions. All clinical data were self-reported. We did not query regarding perinatal HIV diagnosis due to the small number of individuals in this clinical site who were infected via perinatal transmission and the subsequent risk of non-anonymous survey results. Adherence was queried using a common Adherence Self-Assessment Instrument, which uses a simple Visual Analog Scale (ranging from 0% from 100%) to ask, “Select the point below showing your best guess about how much of ALL of your HIV medications you have taken in the last MONTH. For example: 0% means you have taken none of your HIV medications; 50% means you have taken half of your HIV medications; 100% means you have taken every single dose of your HIV medications. Mark on the line which percent represents you” (24, 25). This question was embedded in the survey.
Next, the survey included specific questions about pill aversion-related symptoms generated by the clinical team based on experience caring for and discussing patients who have reported this experience. These questions included items about the emotional experience of pill taking which were rated using a 5-point Likert scale. Items asked questions about trouble swallowing pills, the presence of negative feelings and thoughts about pills, negative or scary images about pill swallowing (i.e., pills getting stuck in the throat). Participants were then asked to indicate if they have trouble swallowing pills due to various pill qualities (such as size, color, or smell of the pill) or physical symptoms (such as immediate vomiting, gagging, or feeling the pill is stuck). Given the novelty of this topic and lack of prior survey items, as well as the lack of a gold standard diagnostic tool for pill aversion, these measures were considered exploratory.
The primary outcome of the study was skipping pills due to pill aversion symptoms. Although we believe that not all individuals with pill aversion symptoms skip pills due to their pill aversion (i.e., some may have developed effective coping mechanisms that promote pill-taking despite these symptoms or emotions), we chose this primary outcome for this exploratory analysis as it represents the most extreme and detrimental phenotype of pill aversion, and thus was thought to be both clinically relevant as well as an important area for future investigations and interventions. Skipping pills due to pill aversion was queried in three different questions in the survey to offer improved triangulation for this concept. Participants were characterized as skipping pills due to pill aversion if they responded affirmatively to any of the questions about skipping pills due to feeling uncomfortable taking them (“I sometimes skip my pills because it is uncomfortable to take them”), problems swallowing them (“How often do you skip your HIV pills because of these problems?”), or pill qualities (“How often do you skip your HIV pills because of these qualities?”). Thus, responding affirmatively to any one of these three questions was considered “skipping pills” per the definition of the primary outcome. Skipping pills for any reason other than pill-related swallowing discomfort was not considered skipping pills due to pill aversion for this analysis.
Analytic Plan
Descriptive statistics regarding both population characteristics and frequency and features of pill aversion were performed. Demographic and clinical characteristics of the cohort were examined using chi-square and Mann-Whitney U tests, as appropriate. A multivariable logistic regression model was used to determine demographic factors independently associated with skipping pills due to pill aversion.
Next, we examined the emotions and pill-associated problems and symptoms associated with skipping pills. Items asked via 5-point Likert scales were dichotomized as affirmative versus negative symptoms; responses of “neutral” were deemed negative for that symptom. This approach was to ensure that we were capturing only the most extreme examples of pill aversion symptoms. These dichotomized pill-taking emotions and problems/symptoms were characterized by pill-skipping status using chi-square tests. Finally, to investigate the independent relationship between each of these pill aversion emotions or symptoms/problems, we performed a series of multivariable logistic regression models controlling for demographic or clinical factors with p<0.05 on bivariable analyses (race and ethnicity, gender, age, marital status, education, employment status, insurance, and mental health diagnosis). All tests were two-sided and we utilized a p<0.05 to designate statistical significance. Analyses were performed using Stata v.14 (StataCorp, College Station, TX).
RESULTS
Of 384 total participants (mean age 47.8 years), a majority were male (76.1%) and identified as non-Hispanic white (46.2%) or non-Hispanic black (NHB) (42.0%) (Table I). A quarter of participants (25.5%) skipped pills due to pill aversion symptoms. Younger age, being NHB or Hispanic compared to white, not being married or partnered, having less than a college education, not being employed, and having public insurance were associated with skipping pills due to pill aversion. Depression (28.4%) and anxiety (20.6%) were common and associated with pill aversion. Individuals who skipped pills were more likely to report a detectable VL (28.7% vs. 9.8%, p=<0.001) and have a lower self-reported adherence (79.5% vs. 95.3%, p<0.001) than those who do not skip pills due to pill aversion. Characteristics that were not associated with skipping pills due to pill aversion symptoms included the number of years since receiving an HIV diagnosis and having been born outside of the US (Table I). In multivariable analysis to identify demographic factors associated with skipping pills due to pill aversion, NHB and Hispanic race/ethnicity (aOR 7.08, 95% CI 3.37–14.90 and aOR 5.71, 95% CI 1.25–25.96, respectively), younger age (aOR 0.97, 95% CI 0.95–0.99), and public insurance (aOR 2.51, 95% CI 1.08–5.79) remained significantly associated with skipping pills due to pill aversion.
Table I:
Clinical and demographic characteristics of persons living with HIV who do and do not skip pills due to aversion symptoms
| Skip pills N=94 (25.5) | Do not skip pills N=275 (74.5) | p-value | |
|---|---|---|---|
| Gender | <0.001 | ||
| Male | 58 (62.4) | 223 (82.0) | |
| Female | 35 (37.6) | 49 (18.0) | |
| Race and ethnicity | <0.001 | ||
| Non-Hispanic white | 13 (14.0) | 156 (57.6) | |
| Non-Hispanic black | 72 (77.4) | 79 (29.2) | |
| Hispanic | 7 (7.9) | 36 (13.4) | |
| Other | 8 (8.6) | 36 (13.4) | |
| Age (years) | 44.2 | 48.7 | 0.003 |
| Country of birth | 0.910 | ||
| United States | 82 (88.2) | 241 (88.6) | |
| Out of the United States | 11 (11.8) | 31 (11.4) | |
| Married/Partnered | 23 (24.5) | 107 (39.2) | 0.010 |
| Public insurance | 70 (76.9) | 119 (43.8) | <0.001 |
| Years since HIV diagnosis | 15.2 | 15.7 | 0.748 |
| Some college education or greater | 32 (34.0) | 153 (56.3) | <0.001 |
| Employed for wages | 38 (40.1) | 171 (62.6) | 0.002 |
| History of mental illness diagnosis | 51 (54.3) | 93 (33.8) | <0.001 |
| Anxiety | 31 (33.0) | 47 (17.1) | 0.001 |
| Depression | 39 (41.5) | 66 (24.0) | 0.001 |
| Other diagnosis | 11 (11.7) | 25 (9.1) | 0.461 |
| New HIV diagnosis (<3 years) | 7 (7.8) | 36 (13.6) | 0.142 |
| Self-reported detectable viral load | 27 (28.7) | 27 (9.8) | <0.001 |
| Number of daily HIV pills | 2.5 (1,5) | 2 (1,3)1.5 | 0.002 |
| Self-reported adherence | 79.5 (27.3) | 95.3 (12.7) | <0.001 |
Data displayed as N(%), mean (standard deviation), or median (interquartile range).
Regarding pill aversion symptoms, participants who skipped pills were more likely to report sensations of gagging (16% vs 5.5%, p=0.001), choking (8.5% vs 2.6%, p=0.011), pills getting stuck in the throat (22.3% vs 9.8%, p=0.002), nausea (24.5% vs 1.8%, p<0.001), and a heavy feeling in the stomach (18.1% vs 1.5%, p<0.001) at the time of swallowing pills; additionally, those who skipped pills due to pill aversion symptoms reported more of these physical sensations than those who do not skip (p<0.001). On multivariable analyses, sensations that remained significantly associated with skipping pills due to pill aversion included gagging, nausea at the time of swallowing, and a heavy feeling in the stomach (Table II).
Table II:
Sensations and pill qualities noted by persons living with HIV who do and do not skip pills due to aversion symptoms
| Bivariable analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|
| Skip pills N=94 (25.5) | Do not skip pills N=275 (74.5) | p-value | Adjusted Odds Ratio* | 95% CI | |
| Sensations | |||||
| Gagging | 15 (16.0) | 15 (5.5) | 0.001 | 2.61 | 1.00–6.85 |
| Choking | 8 (8.5) | 7 (2.6) | 0.011 | 1.77 | 0.49–6.35 |
| Vomiting | 9 (9.6) | 3 (1.1) | <0.001 | 4.51 | 0.84–24.16 |
| Feeling of pill getting stuck | 21 (22.3) | 27 (9.8) | 0.002 | 1.91 | 0.88–4.14 |
| Nausea | 23 (24.5) | 5 (1.8) | <0.001 | 28.11 | 7.48–105.62 |
| Heavy feeling in stomach | 17 (18.1) | 4 (1.5) | <0.001 | 11.12 | 3.08—40.16 |
| Total number of the above problems | 1.1 (1.6) | 0.2 (0.7) | <0.001 | ||
| Pill characteristics | |||||
| Taste | 25 (26.6) | 15 (5.5) | <0.001 | 3.22 | 1.41–7.33 |
| Smell | 18 (19.2) | 4 (1.5) | <0.001 | 9.46 | 2.59–34.54 |
| Size | 34 (36.2) | 36 (13.1) | <0.001 | 3.93 | 1.97–7.84 |
| Color | 3 (3.2) | 2 (0.7) | 0.074 | 5.39 | 0.64–45.34 |
| Texture | 6 (6.4) | 5 (1.8) | 0.025 | 3.03 | 0.75–12.23 |
| Shape | 7 (7.5) | 5 (1.8) | 0.008 | 3.45 | 0.80–14.79 |
| Total number of above pill qualities | 1.0 (1.4) | 0.27 (0.6) | <0.001 | ||
Data displayed as N(%) or mean (standard deviation).
Adjusted for race/ethnicity, gender, age, marital status, education, employment status, public insurance, and mental health diagnosis.
Next, qualities of the pills themselves were also important factors in skipping pills. Participants who skipped pills were more likely to report being bothered by the taste (26.6% vs 5.5%), the smell (19.2% vs 1.5%), and the size (36.2% vs 31.1%) of their pills (all p<0.001). Also significant was the texture of the pills (6.4% vs 1.8%, p=0.025). Pill color was not significantly associated with skipping pills. Individuals who skipped pills due to qualities of the pills were also more likely to report more bothersome pill qualities (p<0.001). On multivariable analyses, pill qualities that remained significantly associated with skipping pills due to pill aversion included pill taste, smell, and size (Table II).
Finally, regarding emotional experiences of pill-taking, participants who reported they skip pills due to discomfort with swallowing were more likely to report negative or fear-based emotions about their pills (Table III). One third of the participants who reported pill-skipping indicated it is upsetting to swallow pills (i.e., have negative cognitions about HIV while taking pills) (33.0% vs 8.5%, p<0.001) or that they have negative feelings about swallowing pills (i.e., feeling badly about oneself or one’s health) (27.7% vs 5.5%, p<0.001). Individuals who endorsed skipping pills due to pill aversion symptoms were also more likely to indicate that the negative emotions directly lead to not wanting to take their HIV pills, that they were nervous about swallowing pills, or had scary images related to swallowing pills, such as images of the pill going down “sideways” or getting stuck part of the way down. Some participants indicated that taking their pills made them feel badly for having HIV, and this was true more often for those who admitted skipping pills than who did not (35.5% vs 18.4%, p=0.001). A quarter of the individuals who admitted skipping pills due to pill aversion symptoms indicated that they had difficulty swallowing pills and negative feelings about swallowing pills before having been diagnosed with HIV. On multivariable regression analyses, several negative emotions remained statistically significantly associated with skipping pills due to pill aversion (Table III).
Table III:
Feelings about taking pills of those who do and do not skip pills due to aversion symptoms
| Bivariable analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|
| Emotional experience of pill taking | Skip pills N=94 | Do not skip pills N=275 | p-value | Adjusted Odds Ratio* | 95% CI |
| I have trouble swallowing pills | 26 (27.7) | 23 (8.4) | <0.001 | 2.96 | 1.36–6.44 |
| I find it upsetting to take pills | 30 (33.0) | 23 (8.5) | <0.001 | 4.49 | 2.10–9.62 |
| I have negative feelings about swallowing pills | 26 (27.7) | 15 (5.5) | <0.001 | 4.67 | 1.96–11.14 |
| I feel nervous about swallowing pills | 14 (14.9) | 12 (4.4) | 0.001 | 2.06 | 0.73–5.81 |
| I feel it is harder to swallow HIV pills than other pills | 21 (22.3) | 33 (12.1) | 0.016 | 1.76 | 0.84–3.68 |
| Taking my HIV pills make me feel badly about having HIV | 33 (35.5) | 50 (18.4) | 0.001 | 1.60 | 0.82–3.12 |
| Negative emotions make me not want to take my HIV medications | 18 (19.2) | 9 (3.3) | <0.001 | 5.15 | 1.82–14.51 |
| I have upsetting or scary images about swallowing pills | 16 (17.2) | 12 (4.4) | <0.001 | 2.56 | 0.99–6.65 |
| I would like some help to swallow pills more easily | 16 (17.2) | 15 (5.6) | 0.001 | 1.81 | 0.74–4.46 |
| I had difficulty swallowing pills before I was diagnosed with HIV | 23 (24.7) | 23 (8.4) | <0.001 | 2.81 | 1.26–6.26 |
| I had negative feelings about swallowing pills before I was diagnosed with HIV | 22 (23.4) | 12 (4.4) | <0.001 | 4.73 | 1.84–12.17 |
Data displayed as N(%).
Adjusted for race/ethnicity, gender, age, marital status, education, employment status, public insurance, and mental health diagnosis.
DISCUSSION
Adherence to ARVs is the cornerstone of treatment for PLHIV. Although much has been studied about the social, environmental, psychiatric, medical, and community barriers to HIV treatment adherence, as well as the side effects and toxicity of medications, there remain unexplored issues regarding the psychological experience of pill swallowing among PLHIV. In this exploration of pill aversion in a diverse US population of PLHIV, pill aversion resulting in skipped pills was surprisingly frequent, reported by approximately a quarter of participants. Moreover, there appear to be many factors that contribute to pill aversion symptoms including physical sensations, qualities of the pills themselves, negative attitudes and thoughts about HIV, negative feelings about pills, and previous negative experiences with pills. Importantly, although some pill qualities such as taste and size were associated with skipping pills due to pill aversion, pill aversion symptoms appear to not be fully explained by these qualities, as participants who skipped pills due to pill aversion also were more likely to report negative fear- or shame-based emotions associated with HIV or their ARVs than those who did not skip pills due to pill aversion. This indicates that the issue is unlikely to be solved by pharmaceutical companies altering the shape, size, or texture of pills during manufacturing. The psychological issues that interfere with pill swallowing (i.e., fear, anxiety, shame-based emotions/stigma) would likely persist for many individuals regardless of physical qualities of the pills.
Our findings suggest that psychological factors, including stigma, may contribute to the experience of pill aversion. The CDC reports that internalized HIV-related stigma is reported by almost 8 of 10 adult patients, independent of ethnicity, age, or gender (26). A meta-analysis of ten articles discussing HIV stigma suggests that this issue affects 42% to 83% of individuals on ARVs, which can lead to low self-worth related to their HIV status (27). Consistent with this report we have observed in our clinical setting that some PLHIV presenting with pill aversion symptoms report that their ARVs are a regular and disturbing representation of this stigmatizing, life-long, incurable chronic illness. Our findings corroborate this clinical observation, as it appears that the negative experience of pill taking is associated with cognitive, emotional, and physical discomfort regarding pill swallowing. These findings suggest pill aversion has a large psychological component that can develop both in the context of simple pill regimens with low pill burden and exacerbated and reinforced by increased pill burden. We propose that HIV-related stigma may be one area to explore in the understanding of these psychological underpinnings. Uncomfortable side effects, negative experiences with the pills or with swallowing, discouragement about virologic failure, and resulting low self-efficacy can continue to reinforce this problematic cycle of anxiety about pills and ultimately pill aversion (Fig 1).
Our findings are important not only due to the unexpectedly high prevalence of pill aversion symptoms at our center but also because we find many with this presentation show improved adherence when we apply cognitive behavioral therapy strategies (19). Again, analogous to the research with anticipatory nausea and vomiting in oncology treatment, the effective interventions are behavioral and include relaxation training and systematic desensitization (20). Additionally, cognitive interventions, including cognitive restructuring, tend to be the hallmark interventions for depression and adherence in order to reframe and modify negative thoughts about HIV and medications (13). We propose that the same interventions would be relevant to prevent the fear, shame, and stress of the HIV diagnosis from interfering with the actual act of pill swallowing. We believe that if we can identify the phenomenon of pill aversion more readily in a clinical setting, we can use these types of cognitive behavioral interventions to address the symptoms and therefore contribute towards the goal of more people living with HIV having an undetectable VL. This investigation of pill aversion serves as a preliminary, critical step towards developing and/or applying effective and early interventions for persons with these symptoms and presents an additional target for future research in HIV outcomes.
Adherence is an exceedingly complex issue in HIV treatment as so many different barriers exist and many people face variable and often multiple barriers. Pill aversion may be one factor that contributes to poor adherence and may actually be a “moving target” that varies over an individual’s life course. For example, perhaps pill aversion symptoms develop by classical conditioning when paired with a negative side effect, but then cease when the regimen is changed. As a second example, some pregnant women who start ARVs during the first trimester may become conditioned to associate pregnancy’s nausea and vomiting with the act of taking ARVs, resulting in the development of pill aversion that persists even after first trimester nausea resolves. In contrast, we observe women who have historically experienced pill aversion symptoms but whose symptoms disappear during pregnancy due to motivation to protect her fetus; for such women, pill aversion symptoms may even recur once the enhanced motivation of pregnancy has ended. We consider also the role of stigma in negative cognitions about oneself and HIV. As an individual works through the adjustment to diagnosis, disclosure, and finding an accepting community, perhaps the negative cognitions decrease and pill aversion symptoms correspondingly decrease. Or conversely, a negative message from a partner increases negative thoughts and subsequently pill aversion symptoms. We see pill aversion exacerbations as an important area for future longitudinal study as well as a subject of ongoing reassessment for individuals in clinical care.
We believe that a primary strength of this study is the attention to this previously under-reported adherence barrier, as well as the exploration of these findings in a large, diverse outpatient US cohort. These findings deepen our understanding of how pill aversion may contribute to non-sustained ARV adherence. Notably, if pill aversion symptoms negatively affect adherence in a large minority of patients taking ARVs but are not appreciated by the health care team, we are potentially missing a substantial opportunity to provide focused support, validation, and intervention for this barrier to adherence.
Our study does, however, have several limitations and should serve as a hypothesis-generating investigation to spark future work. First, this was an exploratory single-site study; further investigation to characterize pill aversion and establish its generalizability and prevalence is essential. Second, to enhance survey participation, we intentionally designed this as a self- reported, anonymous survey. Future investigations are needed to correlate patient emotions and reports of pill taking with their clinical history and serial VL measurements. Third, survey data capture responses to pre-determined questions, but these questions may not fully capture the breadth and depth of experiences about pill swallowing in this cohort. As there are no validated instruments to measure pill aversion and no “gold standard” by which to test new instruments, the survey was designed based on expert opinion and clinical experience. Finally, the primary outcome was skipping pills due to pill aversion symptoms, which is likely the most extreme phenotype in a wide spectrum of symptoms. This outcome captures only those who were not always able or willing to swallow pills despite discomfort and not those who have developed pill aversion symptoms but are able to take their medications anyway.
This hypothesis-generating study serves as a foundation for further investigations regarding pill aversion. A critical next step is to undertake in depth qualitative interviewing in order to develop a fuller understanding of pill aversion. Interview data can then be used to refine and subsequently improve upon survey instruments as we move toward creating a validated, clinically useful screening tool. Following survey refinement and inclusion of new concepts generated from qualitative investigations, additional validation steps will be critical. Additionally, we noted potential gender and racial/ethnic disparities in the frequency of skipping pills due to pill aversion symptoms, and we propose that better understanding these potential differences and their underlying etiologies may be essential to developing therapeutic interventions. In addition, future research is warranted to understand the continuum of pill aversion symptoms and factors that mediate the relationship between pill aversion symptoms and skipping pills. Also, we plan to investigate how pill aversion might vary over the lifespan or vary based on various special circumstances such as pregnancy, depression, or other critical life events. Finally, future research should seek to develop and test the efficacy of behavioral interventions tailored to reduce or resolve pill aversion’s impact upon ARV adherence and upon the lives of PLHIV.
ACKNOWLEDGMENTS
FUNDING: LMY is supported by the NICHD K12 HD050121-11. RMD is supported by the Northwestern Memorial Foundation Evergreen Invitational Women’s Health Initiative.
Footnotes
DISCLOSURES: The authors report no conflicts of interest.
REFERENCES
- 1).Centers for Disease Control and Prevention. HIV Continuum of Care, U.S., 2014, Overall and by Age, Race/Ethnicity, Transmission Route and Sex. Press release July 27, 2017 [Accessed 28 November 2017] Available: https://www.cdc.gov/nchhstp/newsroom/2017/HIV-Continuum-of-Care.html
- 2).Paterson DL, Swindells S. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Annals of Internal Medicine. 2000; 133(Suppl. 1): 21–30. [DOI] [PubMed] [Google Scholar]
- 3).Mannheimer S, Friedland G, Matts J, Child C, Chesney M. The consistency of adherence to antiretroviral therapy predicts biologic outcomes for human immunodeficiency virus-infected persons in clinical trials. Clin Infect Dis. 2002; 34:1115–21. [DOI] [PubMed] [Google Scholar]
- 4).McNabb J, Ross JW, Abriola K, Turley C, Nightingale CH, Nicola DP. Adherence to highly active antiretroviral therapy predicts virologic outcome at an inner-city human immunodeficiency virus clinic. Clin Infect Dis. 2001; 33:700–5. [DOI] [PubMed] [Google Scholar]
- 5).Ickovics JR, Cameron A, Zackin R et al. Consequences and determinants of adherence to antiretroviral medication: Results from Adult AIDS Clinical Trials Group protocol 370. Antiviral Therapy. 2002; 7:185–93. [DOI] [PubMed] [Google Scholar]
- 6).Johnson MO, Catz SL, Remien RH, et al. Theory guided, empirically supported avenues for intervention on HIV medication nonadherence: Findings from the Healthy Living Project. AIDS Patient Care STDS. 2003; 17(12): 645–565. [DOI] [PubMed] [Google Scholar]
- 7).Malta M, Strathdee SA, Magnanini MM, Bastos FI. Adherence to antiretroviral therapy for human immunodeficiency virus/acquired immune deficiency syndrome among drug users: a systematic review. Addiction. 2008; 103(8): 1242–1257. [DOI] [PubMed] [Google Scholar]
- 8).Simoni JM, Amico KR, Pearson CR, & Malow R. Strategies for promoting adherence to antiretroviral therapy: A review of the literature. Current Infectious Disease Reports. 2008; 10(6): 515–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Ammassari A, Trotta MP, Murri R, et al. Correlates and predictors of adherence to highly active antiretroviral therapy: Overview of published literature. Journal of Acquired Immune Deficiency Syndromes. 2002; 31(Suppl 3): S123–S127. [DOI] [PubMed] [Google Scholar]
- 10).Do AN, Rosenberg ES, Sullivan PS, et al. Excess burden of depression among HIV-infected persons receiving medical care in the United States: Data from the Medical Monitoring Project and the Behavioral Risk Factor Surveillance System. Plos One. 2014; 9(3): e92842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Martin L, Kagee A. Lifetime and HIV-Related PTSD among persons recently diagnosed with HIV. AIDS and Behavior. 2011; 15: 125–131. [DOI] [PubMed] [Google Scholar]
- 12).Nightingale VR, Sher TG, Hansen NB. The impact of receiving an HIV diagnosis and cognitive processing on psychological distress and posttraumatic growth. Journal of Traumatic Stress. 2010; 23(4): 452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Safren SA, O’Cleirigh C, Tan JY, et al. A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected individuals. Health Psychology. 2009; 28(1): 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Crepaz N, Passin WF, Herbst JH, et al. Meta-analysis of cognitive behavioral interventions on HIV-positive persons’ mental health and immune functioning. Health Psychology. 2008; 27(1): 4–14. [DOI] [PubMed] [Google Scholar]
- 15).Kennard BD, Brown LT, Hawkins L, et al. Development and implementation of health and wellness CBT for individuals with depression and HIV. Cognitive and Behavioral Practice. 2014; 21: 237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Mbuagbaw L, Ye C, Thabane L. Motivational interviewing for improving outcomes in youth living with HIV. Cochrane Database of Systematic Reviews. 2012; 9 Art. No.: CD009748. [DOI] [PubMed] [Google Scholar]
- 17).Kalichman SC, Cherry C, Kalichman MO, et al. Integrated behavioral intervention to improve HIV/AIDS treatment adherence and reduce HIV transmission. American Journal of Public Health. 2011; 101(3): 531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. [Accessed 28 November 2017]. Available: https://aidsinfo.nih.gov/guidelines
- 19).Dorman RM, Yee LM, Sutton, SH. Pill aversion in HIV-infected pregnant women: Theory to practice. Journal of Perinatology. 2017; 37: 215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Kamen C, Tejani MA, Chandwani K, et al. Anticipatory nausea and vomiting due to chemotherapy. European Journal of Pharmacology. 2014; 722: 172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Cannon W Wisdom of the Body. United States: W.W. Norton & Company, 1932. [Google Scholar]
- 22).Gleitman H, Fridlund AJ, Reisberg D. Psychology (6 ed.). W.W. Norton & Company, 2004. [Google Scholar]
- 23).Johnston V, Cohen K, Wiesner L, et al. Viral suppression following switch to second-line antiretroviral therapy: Associations with nucleoside reverse transcriptase inhibitor resistance and subtherapeutic drug concentrations prior to switch. J Infect Dis. 2014; 209(5): 711–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Walsh JC, Gazzard BG. Responses to a 1 month self-report on adherence to antiretroviral therapy are consistent with electronic data and virological treatment outcome. AIDS. 2002; 16(2): 269–277. [DOI] [PubMed] [Google Scholar]
- 25).Machtinger EL, Bangsberg DR. Adherence to HIV Antiretroviral Therapy In: Coffey S, Volberding PA, eds. HIV InSite Knowledge Base [textbook online]; San Francisco: UCSF Center for HIV Information; 2005. Available: http://hivinsite.ucsf.edu/InSite?page=kb-03-02-09#S5.2X [Accessed 23 May 2017]. [Google Scholar]
- 26).Centers for Disease Control and Prevention. Internalized HIV-related Stigma. February, 2018. [Accessed 9 September, 2018] Available: https://www.cdc.gov/hiv/pdf/statistics/mmp/cdc-hiv-internalized-stigma.pdf
- 27).Lowther K, Selman L, Harding R, Higginson IJ. Experience of persistent psychological symptoms and perceived stigma among people with HIV on antiretroviral therapy (ART): A systematic review. International Journal of Nursing Studies. 2014; 51: 1171–1189. [DOI] [PubMed] [Google Scholar]