Abstract
Few studies have examined functional connectivity (FC) patterns using functional magnetic resonance imaging (fMRI) to predict outcomes in late life depression. We hypothesized that FC within and between frontal and limbic regions would be associated with 12-week depression outcome in older depressed adults. Seventy-one subjects with major depression were enrolled in the study. A study geriatric psychiatrist performed a clinical interview and completed a Montgomery-Asberg Depression Rating Scale (MADRS). All study participants were free of medication at baseline and had a brain fMRI scan. Using a regions of interest (ROI) atlas (including 164 ROIs), we conducted ROI-to-ROI resting-state FC analyses for each participant. In terms of treatment participants were offered sertraline initially, although in this naturalistic study, other medications were also prescribed. Subjects were evaluated every two weeks up to twelve weeks by the study psychiatrist, who followed a flexible, clinically based medication dosing schedule. Multivariate regression analysis was used to examine correlation between change of MADRS score over 12 weeks and baseline FC between brain regions, controlling for age, gender, mean head motion, and baseline MADRS. We found greater FC between the left inferior frontal gyrus pars triangularis and the left frontal eye field and FC of these two regions with a number of brain regions related to reward, salience, and sensorimortor function were correlated with change in MADRS score over 12 weeks. Our results highlight the important role of between inner speech-reward, attention-salience, and attention-sensorimotor network synchronization in predicting acute treatment response in late-life depression.
Keywords: Depression, Elderly, Treatment, Functional Magnetic Resonance Imaging, Connectivity
Introduction
Major depression in older adults, or late life depression (LLD), is a common condition associated with functional impairment and increased risk of dementia and mortality. Magnetic resonance imaging (MRI) studies have elucidated the neurobiology of LLD, both its etiology and its outcomes. Structural MRI studies demonstrated that individuals with LLD have greater burden of white matter disease, smaller hippocampal volumes, and lower structural integrity of frontotemporal white matter tracts (Vu and Aizenstein, 2013). Moreover, white matter hyperintensity volume and hippocampal volume are associated with both mood and cognitive outcomes of LLD.
More recently, functional MRI (fMRI) has also been used to study LLD. Compared with elderly controls, older depressed adults showed increased intrinsic connectivity within the frontal parietal network and within the sensorimotor network (Cieri et al., 2017), and within the default mode network (DMN) including left precuneus, the subgenual anterior cingulate cortex (ACC), the ventromedial prefrontal cortex (vmPFC), and lateral parietal regions (Alexopoulos et al., 2012). Others found decreased functional connectivity (FC) between the regions within the reward network i.e., right nucleus accumbens and the right medial orbitofrontal cortex (MPFC), and between the regions within attention network, i.e., right rostral anterior cingulate cortex (ACC) and bilateral superior frontal gyrus (Tadayonnejad et al., 2014).
Few fMRI studies have examined LLD treatment outcomes. Remission of LLD after 12 weeks of treatment with escitalopram was associated with greater baseline FC of the cognitive control network (CCN) including FC between the left dorsolateral prefrontal cortex (DLPFC) and bilateral dorsal ACC, between left and right DLPFC, and between bilateral DLPFC and bilateral inferior parietal cortices (Alexopoulos et al., 2012). Overall, prior studies found higher baseline within CCN connectivity was associated with better 12-week treatment outcome (Alexopoulos et al., 2012) and that treatment reduced within DMN connectivity (Wu et al., 2011). However, the sample size of these studies was small, and only a couple of seed regions within the CCN and DMN were examined. A study covering whole-brain regions in a larger depressed sample is necessary to better understand how baseline FC predicts treatment outcomes in LLD. Of note, a systematic review of functional MRI studies in younger depressed adults found associations between response to antidepressant medications and high baseline FC between frontal and limbic brain regions (Wu et al., 2011). Based on these studies, in the present functional MRI study of older depressed adults, we hypothesized that FC within and between frontal and limbic regions would be associated with 12-week depression outcomes
Methods
Subjects
All subjects were enrolled in Neurobiology of Late-Life Depression (NBOLD), an NIMH funded study at the University of Connecticut Health Center (UCHC) and the Olin Neuropsychiatry Research Center (ONRC) at the Institute of Living of Hartford Hospital. The study was approved by the Institutional Review Boards of UCHC and Hartford Hospital. Subjects were provided information about the study, reviewed the consent form, and then provided written, informed consent to participate.
Subjects were recruited from clinic referrals, newspaper advertisements and community presentations. Inclusion criteria were age 60 or above, meeting criteria for major depression, single episode or recurrent, ability to read and write English, Mini-Mental State Examination score 25 or greater. Exclusion criteria for the study were: current or recent alcohol or drug dependence; conditions associated with MRI abnormalities, e.g., hydrocephalus, brain tumors, epilepsy, Parkinson’s disease, Huntington’s chorea, dementia, and demyelinating diseases; endocrine disorder other than diabetes mellitus; physical or intellectual disability that may affect completion of self-rating instruments; established clinical diagnosis of dementia; other DSM Axis 1 psychiatric disorders except generalized anxiety; any metal or pacemaker in the body or claustrophobia that might preclude MRI; and current treatment with fluoxetine given its long wash-out period.
Upon enrollment and completion of baseline assessments, each participant was paid $100 for their time completing the MRI, cognitive test battery and experimental computerized measures. The clinical assessment procedures have been reported previously (Steffens et al., 2015), and are summarized below.
Baseline Assessments
Trained clinical research assistants administered the Duke Depression Evaluation Schedule (DDES) to each participant via computer-assisted data entry. The DDES contains items covering demographic data, life events, social support and coping, activities of daily living, self-rated depression severity, age of depression onset, and the Diagnostic Interview Schedule (DIS) sections for depression, mania, generalized anxiety disorder, somatization symptoms, and alcohol use. A study psychiatrist then interviewed each subject to establish a clinical diagnosis of major depression. During the visit, the following assessments are completed: Montgomery-Ǻsberg Depression Rating Scale (MADRS), 17-item Hamilton Depression Rating Scale, the Clinical Global Impression severity scale, and the Cumulative Illness Rating Scale as modified for geriatric patients.
Neuroimaging
Following baseline clinical assessment, subjects were transported to the ONRC for a brain MRI scan.
MRI data acquisition. MRI data were acquired at the ONRC using a Siemens Skyra 3T scanner with 32 surface coils. Both functional MRI and structural MRI were acquired axially paralleled with the AC-PC line with a whole-brain coverage. A simultaneous multislice EPI sequence with factor of 8 was used for fMRI acquisition. Functional MRI parameters were: FOV=240mm, flip angle = 60⁰; TR/TE = 475/30 ms; 49 slices; image matrix= 80× 80 × 49, voxel size = 3 × 3 ×3mm3. During the seven-minute resting state fMRI scan, subjects were asked to fixate on a cross sign in the center of the screen and were told to relax and not think about anything in particular. Twenty dummy volumes were discarded. Five MPRAGE structural images were also acquired with TR=2200ms, TE=2.88ms, flip angle = 13⁰, matrix size of 220 × 320 ×208, voxel size =0.8×0.8×0.8mm3.
MRI preprocessing. We conducted standard preprocessing procedures including realignment, co-registration, normalization, smoothness with a 6mm kernel, and bandpass filtering using CONN) toolbox v17h. Individual structural images (average of the five T1-weighted MPRAGE images) were co-registered to the mean functional image after realignment. The transformed structural images were then segmented into GM, WM and CSF. To remove the nuisance signals, the Friston 24-parameter model was utilized to regress out head motion effects from the realigned data. The signals from WM and CSF were regressed out. In addition, linear and quadratic trends were also included as regressors since the BOLD signal exhibits low-frequency drifts. Temporal filtering (0.01 – 0.1 Hz) was then performed on the time series.
FC analysis. Using the regions of interest (ROI) atlas (including 164 ROIs) provided by CONN toolbox v17h, we conducted ROI-to-ROI resting-state functional connectivity analyses for each participant.
Treatment
All subjects were offered initial treatment with sertraline, with dosing titration every two weeks, depending on clinical status and toleration of medication. Patients were followed every two weeks by a study geriatric psychiatrist. Those that could not tolerate sertraline or who had persistent depression were offered either augmentation with bupropion or switch to desvenlafaxine. Those with a history of poor response or intolerability of sertraline were treated with other antidepressant medication as indicated by their study psychiatrist or by their own psychiatrist. The study psychiatrist completed a MADRS at each visit through 12 weeks. Details of the treatment protocol have been previously published (Steffens et al., 2018).
Statistical analyses
Multivariate regression analysis was used to examine correlation between change in MADRS score over 12 weeks and baseline FC between brain regions, controlling for age, gender, mean head motion, and baseline MADRS. The main effect of each ROI seed with all other ROIs was examined. A post-hoc test on each ROI-to-ROI FC correlation with MADRS changing score was also tested. Significant level was set at p<0.05 with False Discovery Rate (FDR) correction. A permutation test with 1000 iterations was also used to confirm the results
Results
Seventy-one subjects were enrolled in the study. Among them, They had a mean age of 71 ± 6.60 years, were 66.2.8% female, had a mean educational level of 16 ± 2.62, and had mean baseline MADRS score of 20 ± 5.81 and mean baseline MMSE score of 29 ± 1.30.
In terms of treatment, 52 depressed subjects initiated treatment with sertraline, 12 with desvenlafaxine, one on paroxetine, one on escitalopram, one on a combination of escitalopram and bupropion. Four were started on other medications by their private psychiatrist.
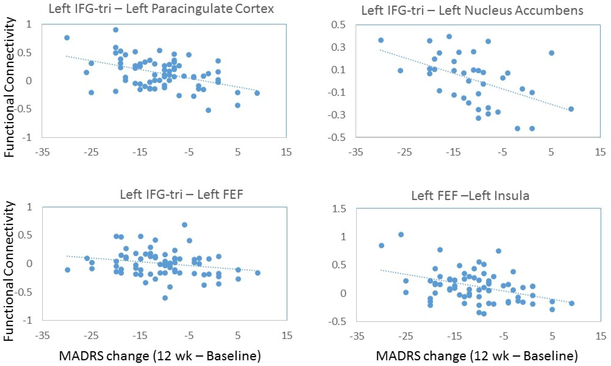
We calculated change in MADRS score from baseline to 12 weeks for each subject. The mean MADRS change score at 12 weeks was 8.7± 5.85. In analyses controlling for age, gender, and baseline depression severity, we did not find a significant main effect of correlation between MADRS change and FC between any ROI seed and all rest of ROIs. However, we found several pairs of regions whose baseline FC was significantly associated with change in MADRS score. As shown in Table 1 and Figure 1, greater FC between the left inferior frontal gyrus pars triangularis (IFG-tri) and the left frontal eye fields (FEF) and FC of these two regions with a number of brain regions were correlated with depression severity (measured by MADRS) reduction over 12 weeks. Specifically, other than IFG-tri – FEF FC, other pairs of regions include IFG-tri – paracingulate, IFG-tri – caudate, IFG-tri – nucleus accumbens, FEF – insula, FEF – precentral gyrus, FEF – sensorimotor cortex, FEF – central opercular cortex, and FEF – supramarginal gyrus. In addition, greater anterior cingulate (ACC) connectivity with cerebellar lobule II at baseline was also correlated with depression severity reduction. Of note, higher IFG-tri – sensorimotor cortex was positively correlated with MADRS change score, i.e., with increased MADRS score.
Table 1.
Regions with significant association between Functional Connectivity and change in MADRS score at 12 weeks (p<0.05, False Discovery Rate correction confirmed with permutation tests)
| Functional Connectivity | Statistic | p-unc | p-FDR |
|---|---|---|---|
| Anterior Cingulate Cortex – Right Cerebellar lobule-II | T(65) = −3.98 | 0.0002 | 0.0284 |
| Left IFG-tri – Left Paracingulate | T(65) = −3.51 | 0.0008 | 0.0330 |
| Left IFG-tri – Right Paracingulate | T(65) = −3.69 | 0.0005 | 0.0330 |
| Left IFG-tri – Right Caudate | T(65) = −3.93 | 0.0002 | 0.0330 |
| Left IFG-tri – Left Nucleus Accumbens | T(65) = −3.28 | 0.0017 | 0.0457 |
| Left IFG-tri – Right FEF | T(65) = −3.44 | 0.0010 | 0.0338 |
| Left IFG-tri – Left FEF | T(65) = −3.44 | 0.0010 | 0.0328 |
| Left IFG-tri – Left Sensorimotor | T(65) = 3.53 | 0.0008 | 0.0330 |
| Left FEF – Left Insular Cortex | T(65) = −3.47 | 0.0009 | 0.0328 |
| Left FEF – Left Precentral gyrus | T(65) = −3.65 | 0.0005 | 0.0328 |
| Left FEF – Right Precentral gyrus | T(65) = −3.35 | 0.0014 | 0.0328 |
| Left FEF – Left Sensorimotor | T(65) = −3.56 | 0.0007 | 0.0328 |
| Left FEF – Right Sensorimotor | T(65) = −3.34 | 0.0014 | 0.0328 |
| Left FEF – Right Central opercular cortex | T(65) = −3.43 | 0.0011 | 0.0328 |
| Left FEF – Right Supramarginal gyrus | T(65) = −3.15 | 0.0025 | 0.0451 |
p-unc = uncorrected p value; p-FDR = False Discovery Rate-corrected p value
IFG-tri = inferior frontal gyrus pars triangularis; FEF = Frontal Eye Fields
Figure 1.
Plots of correlations between functional connectivity with depression severity change over 12-weeks measured by MADRS. IFG-tri = inferior frontal gyrus pars triangularis, FEF= frontal eye field
Discussion
In this study, we found that those LLD patients with greater FC of the IFG-tri, FEF, and ACC with brain regions related to reward and sensorimotor networks had a better 12-week treatment outcome. This differs from a prior study of the cognitive control network (Alexopoulos et al., 2012).
Our finding of associations of treatment outcome and baseline FC between the left IFG-tri and several brain regions is interesting. The left IFG-tri has been associated with language processing and emotional processing. Studies of depression and non-depressed control subjects found that IFG-tri was one of four areas that contributed significantly to discriminating between the two groups (Yang et al., 2018), with greater cortical thinning in the left IFG-tri in the depressed group (Na et al., 2016), and volume reductions of the left IFG-tri in the depressed group were associated with a particular genetic polymorphism (Han et al., 2017). As left IFG-tri comprises the Broca’s area, our results also raise the possibility that inner speech or self-referential dialogue may play an important role in depression. Our findings that greater FC between IFG-tri and the nucleus accumbens, and between the caudate and the FEFs suggests that circuitry related to self-dialogue about reward and motivation may be important in depression outcome.
Also intriguing and initially counterintuitive is our finding of associations between treatment outcome and connectivities between the FEFs and multiple brain regions, as FEF is primarily related to eye movement. However, besides ocular motor control, an important function of the FEFs is visual attention. We found that greater FEF-sensorimotor and FEF-precentral gyrus FC was associated with better antidepressant effect. This might imply that circuitry related to attention and psychomotor activity might be related to treatment outcome. That high FC between the left FEF and left insular cortex was significantly correlated with reduced depression severity may indicate that the emotional salience network is also important for treatment outcome.
Our finding that FC between Left IFG-tri and Left Sensorimotor was associated with higher MADRS scores is interesting. A prior study noted positivity FC between the lateral prefrontal cortex and the somatosensory cortex in generalized anxiety disorder and an anxious subtype of depression (Drysdale et al., 2017). Thus, the positive correlation between IFG and somatosensory FC might be related to comorbid anxiety, which in turn is associated with poorer mood outcomes.
The study does have limitations that should be noted. Our sample size of 71 was moderate, but there remains a possibility of underdetection of FC between other regions related to treatment outcome. Also important to note is the variety of medications used in the study. Finally, as this was not a clinical trial, the lack of a placebo arm begs the question of whether our findings are specific to antidepressant effect or are just a non-specific marker of clinical improvement.
In conclusion, our results highlight the important role of between inner speech-reward, attention-salience, and attention-sensorimotor network synchronization, exemplified by IFG-tri – nucleus accumbens and FEF-insular coupling, in predicting acute treatment response in late-life depression.
Acknowledgments
Supported by NIH grants R01 MH096725 and R01 MH108578
Footnotes
Conflict of Interest
none
Description of authors’ roles:
D. Steffens designed the study, supervised the data collection and wrote the paper. L. Wang performed imaging analysis, was responsible for statistical analysis and assisted with writing the article. G. Pearlson supervised imaging data acquisition and assisted with the writing of the paper.
References
- Alexopoulos GS, Hoptman MJ, Kanellopoulos D, Murphy CF, Lim KO and Gunning FM (2012). Functional connectivity in the cognitive control network and the default mode network in late-life depression. Journal of Affective Disorders, 139, 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieri F, Esposito R, Cera N, Pieramico V, Tartaro A and Di Giannantonio M(2017). Late-Life Depression: Modifications of Brain Resting State Activity. Journal of Geriatric Psychiatry and Neurology, 30, 140–150. [DOI] [PubMed] [Google Scholar]
- Drysdale AT et al. (2017). Resting-state connectivity biomarkers define neurophysiological subtypes of depression, Nature Medicine, 23, 28–38, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han KM et al. (2017). Influence of FKBP5 polymorphism and DNA methylation on structural changes of the brain in major depressive disorder. Scientific Reports, 7, 42621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na KS et al. (2016). Brain-derived neurotrophic factor promoter methylation and cortical thickness in recurrent major depressive disorder. Scientific Reports, 6, 21089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens DC, Manning KJ, Wu R, Grady JJ, Fortinsky RH and Tennen HA (2015). Methodology and preliminary results from the neurobiology of late-life depression study. International Psychogeriatrics, 27, 1987–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens DC, Wu R, Grady JJ and Manning KJ (2018). Presence of neuroticism and antidepressant remission rates in late-life depression: results from the Neurobiology of Late-Life Depression (NBOLD) study. International Psychogeriatrics, 30, 1069–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadayonnejad R, Yang S, Kumar A and Ajilore O (2014). Multimodal brain connectivity analysis in unmedicated late-life depression. PLoS ONE, 9, e96033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu NQ and Aizenstein HJ (2013). Depression in the elderly: brain correlates, neuropsychological findings, and role of vascular lesion load. CUrrent Opinion in Neurology, 26, 656–61. [DOI] [PubMed] [Google Scholar]
- Wu M, Andreescu C, Butters MA, Tamburo R, Reynolds CF 3rd and Aizenstein H (2011). Default-mode network connectivity and white matter burden in late-life depression. Psychiatry Research, 194, 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J et al. (2018). Development and evaluation of a multimodal marker of major depressive disorder. Human Brain Mapping (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]