Abstract
In 2007, the nosology for HIV-1 associated neurocognitive disorders (HAND) was updated to a primarily neurocognitive disorder. However, currently available diagnostic tools lack the sensitivity and specificity needed for an accurate diagnosis for HAND. Scientists and clinicians, therefore, have been on a quest for an innovative biomarker to diagnose (i.e., diagnostic biomarker) and/or predict (i.e., prognostic biomarker) the progression of HAND in the post combination antiretroviral therapy (cART) era. The present review examined the utility and limitations of four proposed biomarkers, including neurofilament light (NFL) chain concentration, amyloid (i.e., sAPPα, sAPPβ, amyloid β) and tau proteins (i.e., total tau, phosphorylated tau), resting-state functional magnetic resonance imaging (fMRI), and prepulse inhibition (PPI). Although significant genotypic differences have been observed in NFL chain concentration, sAPPα, sAPPβ, amyloid β, total tau, phosphorylated tau and resting-state fMRI, inconsistencies and/or assessment limitations (e.g., invasive procedures, lack of disease specificity, cost) challenge their utility as a diagnostic and/or prognostic biomarker for milder forms of neurocognitive impairment (NCI) in the post-cART era. However, critical evaluation of the literature supports the utility of PPI as a powerful diagnostic biomarker with high accuracy (i.e., 86.7–97.1%), sensitivity (i.e., 89.3–100%) and specificity (i.e., 79.5–94.1%). Additionally, the inclusion of multiple CSF and/or plasma markers, rather than a single protein, may provide a more sensitive diagnostic biomarker for HAND, however, a critical need for additional research remains. Most notably, PPI may serve as a prognostic biomarker for milder forms of NCI, evidenced by its ability to predict later NCI in higher-order cognitive domains with regression coefficients (i.e., r) greater than 0.8. Thus, PPI heralds an opportunity for the development of a brief, non-invasive diagnostic and prognostic biomarker for milder forms of NCI in the post-cART era.
Keywords: Diagnostic Biomarker, Prognostic Biomarker, HIV-1 associated neurocognitive disorders, Prepulse Inhibition
INTRODUCTION
Human immunodeficiency virus type 1 (HIV-1) remains a human health pandemic, afflicting approximately 36.7 million individuals worldwide (UNAIDS, 2017), including approximately 4.2 million older individuals (>50 years of age; UNAIDS, 2014). By 2030, the prevalence of older individuals living with HIV-1 is expected to reach approximately 73% (Smit et al., 2015); a shift in the epidemiological features of HIV-1 resulting primarily from two phenomena (Justice, 2010). First, the advent of combination antiretroviral therapy (cART) in 1996 dramatically increased the life expectancy for HIV-1 seropositive individuals (e.g., Romley et al., 2014; Teeraananchai et al., 2017). Second, an increased number of older individuals are becoming infected with HIV-1. As a result, older individuals account for approximately 47% of all HIV-1 seropositive individuals in the United States, as well as 17% of new HIV-1 diagnoses (CDC, 2018).
In 2007, the nosology for HIV-1 associated neurocognitive disorders (HAND) was updated to a primarily neurocognitive disorder (Antinori et al., 2007). The gold-standard for the diagnosis of HAND in the post-cART era is a complete neurocognitive battery assessing at least five cognitive domains (i.e., executive function, speed of information processing, motor skills, etc.; Woods et al., 2009; Elbirt et al., 2015). A complete neurocognitive battery provides an opportunity to classify individuals into three categories (i.e., asymptomatic neurocognitive impairment, mild neurocognitive impairment, and HIV-1 associated dementia) based on their level of impairment (Antinori et al., 2007). In cases where detailed assessments were not readily accessible (Joska et al., 2016), standardized mental status examinations are recommended (e.g., Mini-Mental State Examination, Folstein et al., 1975; HIV Dementia Scale, Power et al., 1995; International HIV Dementia Scale, Sacktor et al., 2005). However, standardized mental status examinations currently lack the sensitivity and specificity needed for an accurate diagnosis of HAND (e.g., Haddow et al., 2013; Sakamoto et al., 2013; Zipursky et al., 2013). Thus, the development of an innovative biological marker (or biomarker) able to accurately diagnose milder forms of neurocognitive impairment (NCI), including HAND, and/or predict neurocognitive decline in aging HIV-1 seropositive individuals has the potential for great clinical significance (Chan & Brew, 2014; Zipursky et al., 2013).
At the broadest level, the term biomarker refers to an objective, measurable and reproducible subcategory of medical signs (Strimbu & Tavel, 2010). More precisely, the National Institutes of Health defines a biological marker as “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention” (Biomarkers Definitions Working Group, 2001); a definition that overlaps considerably with the one proposed by the World Health Organization (WHO, 2001). Biomarkers have a broad range of clinical utility, including diagnosing (i.e., diagnostic biomarker), monitoring (i.e., monitoring biomarker), and predicting disease progression (i.e., prognostic biomarker; Biomarkers Definitions Working Group, 2001; FDA-NIH Biomarker Working Group, 2016). The development of biomarkers, from identification to clinical validation and clinical use, however, is challenged by the criteria of both high specificity and high sensitivity. Additional stringent criteria must be employed in the development of a novel prognostic biomarker, including high test-retest reliability, a relationship to functional outcome, and practicality (Light & Swerdlow, 2015).
Scientists and clinicians have been on a quest for an innovative biomarker to diagnose and/or predict the progression of HAND in the post-cART era. Cerebrospinal fluid biomarkers (CSF) and neuroimaging biomarkers have been the focus of many clinical studies. However, neurophysiological biomarkers, including prepulse inhibition (PPI) of the auditory startle response (ASR), have also been proposed in preclinical assessments. PPI is a translational experimental paradigm that affords an opportunity for the assessment of temporal processing (McLaurin et al., 2018a); a deficit which may manifest prior to alterations in higher-order cognitive processes. Two stimuli, including a discrete prestimulus and startling stimulus (Hoffman & Ison, 1980), are presented in the PPI experimental paradigm. When the time interval, or interstimulus interval (ISI), between the two stimuli is between 30–500 msec, robust inhibition is observed (Hoffman & Ison, 1980). Given the well-established neural circuitry underlying PPI, the assessment of PPI may not only reveal deficits in temporal processing, but may also elucidate underlying mechanisms of more complex neurological processes (Hoffman & Ison, 1980). Thus, the present review examines the utility and limitations inherent in proposed biomarkers and the need to critically test the proposed biomarkers via statistical assessments, including discriminant function analyses (DFA) and receiver operator characteristic (ROC) curves.
CEREBROSPINAL FLUID (CSF) BIOMARKERS
Neurofilament Light Chain (NFL) Concentration
Within the central nervous system (CNS), neurofilament (NF) proteins, a class of intermediate filaments, are heteropolymers composed of four subunits, including neurofilament heavy (NFH; 190–210 kDa), neurofilament medium (NFM; 150 kDa), neurofilament light (NFL; 68 kDA) and α-internexin (Yuan et al., 2006; Yuan et al., 2012). NF proteins are a key structural component of neurons, commonly observed in perikarya, dendrites, and most abundantly, axons (Yuan et al., 2012). Emerging evidence also supports the structural and functional role of all four CNS NF subunits in synapses (Yuan et al., 2015; Yuan et al., 2016; Yuan et al., 2018). The four NF subunits form distinctive assemblies within synapses, with an increased abundance of synaptic NF proteins in the postsynaptic area (Yuan et al., 2015). Functionally, NF proteins exhibit subunit-specific modulation of synaptic function, including neurotransmission and synaptic plasticity (Yuan et al., 2016). More specifically, NFL modulates the N-methyl-D-aspartate receptor (NMDAR), glutamate-gated cation channels (Lau and Zukin, 2007), via its interaction with the GluN1 subunit (Yuan et al., 2018); a receptor (e.g., Haughey et al., 2001; Krogh et al., 2015) and neurotransmitter system (e.g., Patton et al., 2000; Wang et al., 2003; Gupta et al., 2010; Melendez et al., 2016) commonly altered by HIV-1 viral proteins.
NFL concentration, an indicator of CNS injury, is measurable in both CSF and blood plasma (e.g., Norgren et al., 2003; Rissin et al., 2010). CSF assessments of NFL concentration require a lumbar puncture, after which concentrations are measured using a sensitive enzyme-linked immunosorbent assay (ELISA; Norgren et al., 2003). Development of an ultrasensitive NFL concentration assay for plasma, and its validation in HIV-1 seropositive individuals (Gisslén et al., 2016), addresses the primary limitation of CSF measurements (i.e., lumbar puncture).
Significant increases in NFL concentration have been observed between HIV-1 seropositive individuals and HIV-1 seronegative controls, independent of measurement methodology (e.g., CSF: Abdulle et al., 2007, Peluso et al., 2013, Peterson et al., 2014; Jessen Krut et al., 2014; Gisslén et al., 2016; plasma: Gisslén et al., 2016; Anderson et al., 2018); differences which are further evaluated by conducting post-hoc analyses in individuals categorically classified based on their level of impairment (e.g.,; ‘primary’ HIV-infected, neuroasymptomatic, HAD; Abdulle et al., 2007; Peterson et al., 2014; Jessen Krut et al., 2014; Gisslén et al., 2016). Most notably, NFL concentration is sensitive to the initiation of antiretroviral therapy, leading to decreased concentrations in most HIV-1 seropositive individuals (i.e., 3 months: 10/21; 1 year: 12/16; Mellgren et al., 2007; 63% of patients; Jessen Krut et al., 2014). However, alterations are not disease specific, and have been observed in other neurodegenerative diseases, including Alzheimer’s disease (AD; e.g., Mattsson et al., 2017; Lewczuk et al., 2018), multiple sclerosis (e.g., Haghighi et al., 2004; Cai and Huang, 2018), and progressive supranuclear palsy (e.g., Donker Kaat et al., 2018).
Presence of a significant relationship between NFL concentration and NCI would support its potential utility as a diagnostic biomarker for milder forms of NCI in the post-cART era. Although a significant relationship between higher plasma NFL and increased NCI was observed in HIV-1 infected adults, the association appears to result primarily from individuals off cART (Anderson et al., 2018). In sharp contrast, an investigation of cART-naïve participants with primary HIV infection failed to observe a significant relationship between CSF NFL chain concentration and NCI (Peluso et al., 2013). Despite significant group differences in NFL concentration, further studies are needed to directly correlate NFL concentration and NCI, and thus systematically evaluate its utility as a diagnostic biomarker.
Although not yet systematically evaluated, CSF NFL chain concentration may also serve as a prognostic biomarker for milder forms of NCI observed in the post-cART era. A retrospective study in HIV-1 seropositive individuals in the pre-cART era revealed elevated CSF NFL concentrations during the two years before presentation of acquired immunodeficiency dementia complex (ADC); an increase rarely observed (i.e., 33%) in HIV-1-infected control subjects (Gisslén et al., 2007). Despite the small sample size (i.e., n=9 HIV-1 individuals with ADC; n=27 HIV-1-infected control subjects), results suggest a biomarker with acceptable sensitivity (i.e., 78%) and specificity (i.e., 67%; Gisslén et al., 2007). Relationships between baseline NFL levels and neuropsychological deterioration observed in other neurodegenerative disorders (e.g., Progressive Supranuclear Palsy: Rojas et al., 2016; Primary Progressive Aphasias: Steinacker et al., 2017; Huntington’s Disease: Byrne et al., 2017) support further longitudinal investigations of NFL proteins in HIV-1 seropositive individuals. However, statistical analyses must account for other comorbid conditions observed in HIV-1 seropositive individuals given the lack of disease specificity inherent in NFL chain concentration measurements.
Amyloid and Tau Proteins
Amyloid precursor protein (APP) is a type 1 transmembrane protein preferentially located in the synapses of neurons (De Strooper & Annaert, 2000). Functionally, APP plays an integral role in synaptogenesis (e.g., Moya et al., 1994; Priller et al., 2006), neuronal outgrowth (e.g., Ikin et al., 2007; Billnitzer et al., 2013) and dendritic spine morphology (e.g., Weyer et al., 2014; Zou et al., 2016). The proteolytic cleavage of APP by α-, β-, and/or γ-secretases leads to the generation of a vast number of different cleavage products (e.g., sAPPα, sAPPβ, amyloid β (Aβ)) via two primary pathways. Specifically, α-secretase cleavage via the non-amyloidogenic pathway releases soluble sAPPα from the cell surface (Chen et al., 2017); a proteolytic product that may be neuroprotective (e.g., Mattson et al., 1993; Goodman and Mattson, 1994). Along the amyloidogenic pathway, β-secretase cleavage generates sAPPβ. The cleavage of APP by β- and γ-secretases forms a peptide approximately 40-residues long commonly known as amyloid β (Aβ; Chen et al., 2017). Accumulating evidence supports important physiological roles for Aβ, including the modulation of synaptic activity (e.g., Kamenetz et al., 2003; Cirrito et al., 2008; Bero et al., 2011). However, excessive amounts of Aβ are deleterious, leading to aggregate formation of Aβ plaques in the extracellular space, a hallmark of AD, triggering synaptic deficits (e.g., Varga et al., 2015; Ulrich et al., 2015) and disruption of intracellular calcium (e.g., Kuchibhotla et al., 2008; Lim et al., 2013).
Tau is a microtubule-associated binding protein located preferentially in axons (Binder et al., 1985) and, albeit in small amounts, in dendrites and dendritic spines (e.g., Zempel et al., 2010; Kopeikina et al., 2013). Alternative mRNA splicing of a single gene leads to the formation of six tau isoforms in the adult human brain (Goedert et al. 1989a; Goedert et al., 1989b; Kosik et al., 1989). Historically, tau proteins have been recognized for their key regulatory role in microtubule stability (Weingarten et al., 1975). More recent evidence, however, supports the role of tau proteins in synaptic function (e.g., Ittner et al., 2010; Hunsberger et al., 2015). The phosphorylation of tau is developmentally regulated, decreasing with age and coinciding with the development of phosphatases (Yu et al., 2009). However, the hyperphosphorylation of tau can lead to the formation of neurofibrillary tangles, commonly observed in AD, subsequently leading to synaptic dysfunction (e.g., Merino-Serrais et al., 2013).
Postmortem assessment of Aβ and tau proteins in the brains of HIV-1 seropositive individuals has revealed distinct neuropathological changes. Neither AIDS individuals in the pre-HAART era nor HAART treated HIV-1 seropositive individuals exhibited significant extracellular Aβ plaques (Gelman & Schuenke, 2004; Anthony et al., 2006). However, intraneuronal accumulation of Aβ has been observed in HIV-1 seropositive individuals, with an increased prevalence in individuals with HIV-1 encephalitis (Achim et al., 2009). Additionally, elevated levels of hyperphosphorylated tau were observed in the hippocampus, evidenced by the presence of neuronal threads, pre-tangles, and tangles, in HIV-1 seropositive individuals relative to age-matched controls (Anthony et al., 2006).
The assessment of sAPPα, sAPPβ, Aβ, total tau (t-tau) and phosphorylated tau (p-tau) in CSF has further elucidated a unique pathology in HIV-1 seropositive individuals; a pathology which is largely dependent upon the categorical classification of individuals based on their level of NCI. Specifically, individuals with primary HIV-1 infection display elevated levels of p-tau and Aβ−42 (Peluso et al., 2013). Neuroasymptomatic HIV-1 seropositive individuals and individuals with HAND exhibited decreased levels of t-tau and p-tau relative to HIV-1 seronegative controls (Clifford et al., 2009). The most severe forms of NCI, including ADC and HAD, are characterized by decreased concentration of sAPPα and sAPPβ, as well as elevated levels of t-tau (Gisslén et al., 2009; Peterson et al., 2014). Relative to HIV-1 seropositive individuals, subjects with AD consistently exhibited increased levels of t-tau (Gisslén et al., 2009; Clifford et al., 2009; Krut et al., 2013; de Almeida et al., 2018), p-tau (Gisslén et al., 2009; Clifford et al., 2009; Krut et al., 2013; de Almeida et al., 2018), and sAPPα (Gisslén et al., 2009; Krut et al., 2013; de Almeida et al., 2018). Thus, both the neuropathology and CSF profile of HIV-1 appear distinct from AD.
CSF measurements are currently the only methodology available to systematically evaluate sAPPα, sAPPβ, Aβ, and p-tau. The need for a lumbar puncture, an invasive procedure necessary for CSF measurements, limits the broad applicability of these markers. To date, a plasma biomarker for either Aβ (Toledo et al., 2013) or p-tau remains elusive, constrained by biological and technical aspects. However, an ultrasensitive blood immunoassay for t-tau has been developed, assessed, and proposed as a diagnostic biomarker for AD (e.g., Chiu et al., 2013; Dage et al., 2016) suggesting its potential diagnostic utility for HAND.
Multiple statistical methodologies have been employed to evaluate the utility of sAPPα, sAPPβ, Aβ, t-tau and p-tau as a diagnostic biomarker for milder forms of NCI in the post-cART era. Correlational assessments suggest a selective diagnostic utility for CSF t-tau biomarkers. Deficits in both prospective and retrospective (i.e., episodic) memory have been implicated in HAND (e.g., Hinkin et al., 1996; Carey et al., 2006), however, a significant relationship was only observed between CSF t-tau and prospective memory in HIV-1 seropositive individuals (Woods et al., 2006). Additionally, the HIV-1 Dementia Scale, which has only limited success in diagnosing HAND (e.g., Haddow et al., 2013; Sakamoto et al., 2013), and the Mosaic Test, exclusively assessing visuoconstructive coordination, were significantly related to CSF t-tau (Steinbrink et al., 2013). A principal components analysis (PCA) utilized five CSF markers (i.e., sAPPα, sAPPβ, Aβ1–42, t-tau and p-tau) to separate individuals into three primary groups, including a group with HIV-1 seropositive individuals with ADC and CNS opportunistic infections, another group including neuroasymptomatic HIV-1 individuals and HIV-1 seronegative controls, and a final group with AD subjects (Gisslén et al., 2009). Failure to distinguish between neuroasymptomatic HIV-1 individuals and HIV-1 seronegative controls, despite the use of five markers, suggests an overall limited utility for amyloid and/or tau proteins as diagnostic biomarkers for milder forms of NCI.
Systematic evaluation of the utility of amyloid and/or tau proteins as a prognostic biomarker for HAND has not yet been conducted. However, studies evaluating the progression from mild cognitive impairment (MCI) to AD support its potential utility. Specifically, p-tau231 accurately predicted decline from mild cognitive impairment (MCI) to AD with a specificity of 80% (Brys et al., 2009). Use of two measurements, including CSF Aβ−42 and p-tau, for the calculation of a ratio predicted conversion from MCI to AD with 88% sensitivity and 90% specificity (Buchhave et al., 2012); a study which also suggests the benefit of including multiple measurements for the development of a biomarker.
NEUROIMAGING BIOMARKERS
Resting-State Functional Magnetic Resonance Imaging (fMRI)
Functional magnetic resonance imaging, or fMRI, was developed in 1990 (Ogawa et al., 1990) as an in vivo method to assess brain activity (Casey et al., 2002). Conventionally, fMRI experiments utilize Blood Oxygenation Level Dependent (BOLD) contrast, which assesses changes in oxygenation concentration as a measure of neural activity (e.g., Ogawa & Lee, 1990; Ogawa et al., 1990). The BOLD contrast results from magnetic differences in oxygenated and deoxygenated blood, whereby deoxygenated blood is highly paramagnetic (Thulborn et al., 1982). Thus, the activation of a specific brain region leads to increases in blood oxygenation, paralleling localized increases in blood flow, and decreases in deoxygenated blood; an effect which leads to larger magnetic resonance signals (Casey et al., 2002; Glover, 2011). Changes in magnetic resonance signals reflect neuronal mass activity, a primary limitation of the technology preventing differentiation between excitation and inhibition, as well as function-specific processing and neuromodulation (Logothetis, 2008).
fMRI assessments are a non-invasive procedure performed on a magnetic resonance (MR) imaging scanner. Specifically, for the assessment of brain activity in HIV-1 seropositive individuals, a resting-state analysis is commonly employed (e.g., Thomas et al., 2013; Guha et al., 2016; Ann et al., 2016; Chaganti et al., 2017). Use of resting-state fMRI allows for the examination of spontaneous BOLD fluctuations, an intrinsic property of the brain (Fox and Raichle, 2007). During resting-state fMRI scans, participants are typically instructed where to focus (eyes closed, focus on a point on the screen) and not to think about anything. In sharp contrast, task activation fMRI studies (not reviewed presently) may also be employed, manipulating stimuli (e.g., visual, auditory) to induce different neural states in the brain. Two measures that are commonly examined in fMRI include regional homogeneity, an assessment of functional coherence (Zang et al., 2004) and functional connectivity, providing insight into neuronal connections.
Resting-state fMRI studies have revealed significant differences in regional homogeneity and functional connectivity between HIV-1 seropositive individuals and controls at several ages. Adolescents perinatally infected with HIV-1 display increased regional homogeneity in brain regions associated with the central somatic motor-sensory cortex and decreased regional homogeneity in brain regions associated with the corticostriatal pathway (Wang P. et al., 2018). During primary HIV-1 infection (i.e., on average, less than 1 year), HIV-1 seropositive individuals displayed decreased connectivity strength within the lateral occipital network (Wang et al., 2011), a robust network associated with “cognition space” (Smith et al., 2009). In HIV-1 seropositive adults, decreased corticostriatal functional connectivity (Ortega et al., 2015), abnormalities in cerebro-cerebellar functional connectivity (Wang H. et al., 2018) and decreased synchronicity in salience (i.e., anterior insula and dorsal anterior cingulate cortex) and executive networks (i.e., dorsolateral prefrontal cortex; Changanti et al., 2017) have been observed relative to healthy controls. Studies have also conducted resting-state fMRIs in HIV-1 seropositive individuals with and without HAND, revealing decreased functional connectivity between the precuneus and prefrontal cortex (PFC) in HIV-1 seropositive individuals with HAND relative to individuals without HAND (Ann et al., 2016). Comparisons of HIV-1 seropositive individuals without HAND and healthy controls revealed increased efficiency in the posterior cingulate cortex (Ventura et al., 2018) and decreased functional connectivity in frontal areas, temporal areas and the cerebellum (Ann et al., 2016). In sharp contrast, other studies fail to observe significant genotypic differences in functional connectivity (Guha et al., 2016; Janssen et al., 2017; Abidin et al., 2018; Cole et al., 2018).
fMRI assessments have also been fundamental in assessing the independent and/or synergistic effects of HIV-1 and aging. Across studies, researchers failed to observe a significant HIV-1 x age interaction, after correcting for multiple comparisons, suggesting that HIV-1 and aging are independent processes (Ances et al., 2010; Thomas et al., 2013; Ebgert et al., 2018; Egbert et al., 2019). However, studies were conducted primarily in male HIV-1 seropositive individuals and controls and may not generalize to HIV-1 seropositive females. An extension of existing graph theory approaches to model functional connectivity, however, suggested that HIV and age were associated with divergent measures (i.e., HIV-1: centrality, an assessment of node importance; Age: diversity, an assessment of network disorder; Thomas et al., 2015). The development of new analytic approaches for resting-state fMRI, therefore, may more precisely capture the relationship between HIV-1 and aging.
Statistical techniques have been employed to examine the diagnostic utility of fMRI by examining the relationship between imaging metrics and neurocognitive performance. During primary HIV-1 infection, a relationship between the connectivity strength in the lateral occipital network and visual-motor coordination was observed (Wang et al., 2011). Connectivity alterations in brain regions within the PFC (e.g., posterior cingulate cortex, anterior cingulate cortex, medial prefrontal cortex) were associated with NCI in perinatally infected HIV-1 seropositive youth (Herting et al., 2015) and HIV-1 infected adults (de Plessis et al., 2017; Changanti et al., 2017; Ventura et al., 2018). NCI in HIV-1 seropositive individuals was also associated with functional connectivity in other brain regions and networks, including frontal areas (Ann et al., 2016), the basal ganglia (de Plessis et al., 2017), and somatosensory network (Changanti et al., 2017). Additionally, in HIV-1 seropositive adults, modularity and small-worldness, two measures representing components of the integrative connectivity of the brain, are both significantly associated with executive functioning, as well as overall NCI; a relationship dependent upon the methodology (i.e., non-linear mutual connectivity vs. correlation derived measures) employed to construct brain connectivity profiles (Abidin et al., 2018). Use of a ROC curve suggests the diagnostic utility of fMRI, distinguishing between HIV-1 seropositive individuals with HAND and controls with area under the curve measures greater than 0.8; discrimination which was enhanced with the use of a nonlinear approach (Dsouza et al., 2017). Other studies, however, have failed to find a significant relationship between resting-state fMRI measurements and degree of NCI (Thomas et al., 2013; Ortega et al., 2015; Guha et al., 2016; Wang P et al., 2018). Inconsistencies between studies may be due to a variety of experimental factors (e.g., differences in the time of infection, treatment with cART, neurocognitive assessments, and fMRI analysis methods), however, they likely reduce the utility of resting-state fMRI as a viable diagnostic biomarker for HAND.
To date, no study has systematically evaluated the utility of resting-state fMRI as a prognostic biomarker for milder forms of NCI observed in the post-cART era. However, assessments of HIV-1 seropositive individuals at baseline and after a two year follow-up, revealed no differences in the rates of change in resting-state fMRI relative to controls (Cole et al., 2018). Although further studies are needed to directly correlate neurocognitive progression and changes in resting-state fMRI, at this point, results suggest its utility may be limited.
NEUROPSYCHOLOGICAL BIOMARKERS
Prepulse Inhibition (PPI)
In a series of seminal papers (e.g., Hoffman & Searle, 1965, Ison & Hammond, 1971), Hoffman and Ison introduced and popularized the use of PPI of the ASR for the assessment of temporal processing; a construct analogous to speed of information processing in humans. Cross-modal PPI and gap prepulse inhibition (gap-PPI) are translational experimental paradigms that rely on the presentation of two stimuli: a discrete prestimulus or prepulse and a startle stimulus (Hoffman & Ison, 1980). Notably, cross-modal PPI relies upon the presentation of an added prestimulus (i.e., Visual: light, Auditory: tone, Tactile: air puff), while the salient prestimulus is removed in gap-PPI (i.e., gap in background light, noise, or air). Dramatic decreases in ASR are observed when the time interval, or interstimulus interval (ISI), between the discrete prestimulus and startle stimulus is 30–500 msec; an effect that is independent of sensory modality (Pickney, 1976; Hoffman & Ison, 1980; Campeau & Davis, 1995). Systematically manipulating the ISI (e.g., 0, 30, 50, 100, 200, 4000 msec) establishes the shape of the ISI function (e.g., a sharpening or flattening of the response amplitude curve, a broadening or narrowing of the response amplitude curve, shifts in the point of maximal inhibition), affording a critical opportunity to evaluate temporal processing (McLaurin et al., 2018a).
Prepulse inhibition is mediated through the cortico-striato-pallido-pontine (CSPP) circuit, which includes both sequential and parallel neural connections (Koch & Schnitzler, 1997). Broadly, sensory input is relayed to either the superior colliculus (auditory) or inferior colliculus (visual, tactile) and subsequently transmitted to the pedunculopontine tegmental nucleus (PPTg), triggering a cholinergic projection to the caudal pontine reticular nucleus eliciting a startle response (Fendt et al., 1994; Fendt et al., 2001; Koch et al., 1993; Koch & Schnitzler, 1997). Alterations in neurotransmitter system function, including the dopaminergic system, which is implicated in the pathogenesis of HAND (e.g., Kumar et al., 2009; Kumar et al., 2011), play a prominent role in the regulation of PPI. Specifically, the nucleus accumbens (NAc) receives dopaminergic afferents from the ventral tegmental area (VTA), which subsequently relays information to the PPTg; activity which may be mediated by glutamatergic projections from the medial prefrontal cortex (mPFC) to the VTA (Taber & Fibiger, 1995). Activation of the dopaminergic system via treatment with dopamine (DA) agonists leads to marked decreases in PPI (e.g., Mansbach et al., 1998; Zhang et al., 2000; Fitting et al., 2006;); an effect which is also observed following blockade of DA receptors in the mPFC, either via a large 6-OHDA lesion (Bubser et al., 1994) or local injections of dopamine antagonists (Ellenbroek et al., 1996). Alterations in PPI, therefore, may not only provide critical information on neurocognitive dysfunction, but may also elucidate underlying neural mechanisms in HAND.
The eyeblink reflex is one component of the startle response, providing a clinically relevant experimental paradigm for the assessment of PPI in humans. Although multiple methods are available for the assessment of eyeblink reflexes, the preferred method for clinical startle blink research utilizes surface electromyographic (EMG) recording electrodes (Blumenthal et al., 2005). EMG recording electrodes measure action potentials generated within the orbicularis oculi muscle (Blumenthal et al., 2005). Relative to other methodologies (e.g., magnetic search coils, vertical electrooculographic), EMG recording electrodes require less expensive equipment, have less obtrusive sensors, and exhibit increased sensitivity to blink activity (Flaten, 1993; Blumenthal et al., 2005). Most notably, the eyeblink startle paradigm displays characteristics that are critical for the development of a clinically relevant biomarker (Myers & Brown, 2006), including brevity (i.e., approximately 15–25 min; Fournier & Hebert, 2013; Minassian et al., 2013), test-retest reliability (Braff et al., 1978; Schwarzkopf et al., 1993) and ease of administration.
The assessment of cross-modal PPI and gap-PPI in clinical and preclinical studies has repeatedly revealed significant genotypic differences. HIV-1 seropositive individuals with HAND exhibited impairments in PPI relative to HIV-1 seropositive individuals without NCI; an impairment which was associated with alterations in higher-order cognitive processes, including attention/working memory (Minassian et al., 2013). Significant deficits in temporal processing were also observed across multiple animals systems utilized to model HAND, including the HIV-1 transgenic (Tg) rat (e.g., Moran et al., 2013; McLaurin et al., 2017a; McLaurin et al., 2017b), stereotaxic injections of HIV-1 viral proteins (e.g., Tat: Fitting et al., 2006a; Fitting et al., 2006b; gp120: Fitting et al., 2006c; Fitting et al., 2007), gp120 transgenic mice (Henry et al., 2014; Bachis et al., 2016) and Tat transgenic mice (Paris et al., 2015). Additionally, longitudinal studies in the HIV-1 Tg rat have revealed significant alterations in the progression of temporal processing in both cross-modal PPI (McLaurin et al., 2018b) and gap-PPI (McLaurin et al., 2016); an alteration that was more pronounced in female HIV-1 Tg rats (McLaurin et al., 2016; McLaurin et al., 2018b). Although alterations in PPI are not unique to HAND, having been observed in both schizophrenia (Braff et al., 1978) and attention deficit hyperactivity disorder (Castellanos et al., 1996), temporal processing deficits may demonstrate specificity relative to other neurodegenerative diseases (e.g., AD: Hejl et al., 2004; Ueki et al., 2006)
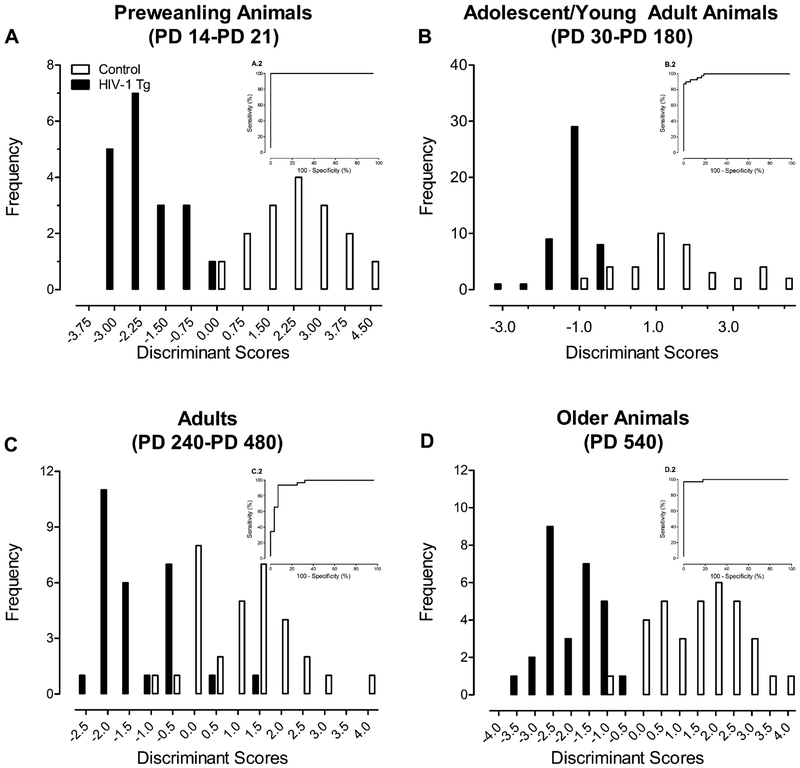
DFA and ROC curves have been conducted to assess the diagnostic utility of cross-modal PPI and gap-PPI. DFA and ROC curves are complementary statistical techniques that evaluate the diagnostic accuracy, sensitivity and specificity (Zweig & Campbell, 1993). In the HIV-1 Tg rat, cross-modal PPI and/or gap-PPI accurately diagnoses the presence of the HIV-1 transgene with both high sensitivity and high specificity across multiple ages (i.e., pre-weanling: McLaurin et al., 2017c (Figure 1A [DFA: 97.1% accuracy, 100% sensitivity, 93.8% specificity; ROC: Area 1.0]); adolescent/young adult animals: McLaurin et al., 2016 (Figure 1B [DFA: 90.8% accuracy, 100% sensitivity, 79.5% specificity; ROC: Area 0.986]); adult animals: (Figure 1C [DFA: 86.7% accuracy, 89.3% sensitivity, 84.4% specificity; ROC: Area 0.951]); older adult animals: (Figure 1D [DFA: 96.8% accuracy, 100% sensitivity, 94.1% specificity; ROC: Area 0.995])) and sensory modalities (McLaurin et al., 2017b). Thus, the diagnostic utility of temporal processing deficits, assessed using PPI, generalizes across two experimental paradigms, the functional lifespan, sensory modalities, and biological sex in the HIV-1 Tg rat, supporting a promising diagnostic biomarker for milder forms of NCI in the post-cART era.
FIGURE 1.
The diagnostic utility of measures of prepulse inhibition (PPI) and gap prepulse inhibition (gap-PPI) is illustrated as a function of genotype (i.e., HIV-1 transgenic (Tg) vs Control) using both discriminant function analyses (DFA) and receiver-operating curves (ROC; inset). In the HIV-1 Tg rat, cross-modal PPI and/or gap-PPI accurately diagnoses the presence of the HIV-1 transgene with high accuracy, high sensitivity and high specificity across multiple ages. ROC analyses were conducted based on the discriminant scores. ROC area under the curve (AUC) is utilized as a single measure of overall accuracy for the ROC. A: In pre-weanling animals (i.e., Postnatal Day 14–21), a DFA selected 6 PPI variables, exhibiting 97.1% accuracy, 100% sensitivity, and 93.8% specificity. An AUC of 1.0 was observed when an ROC analysis was conducted. Adapted from McLaurin et al., 2017c. B: In adolescent/young adult animals (i.e., PD 30-PD 180), 4 PPI variables best discriminated between HIV-1 Tg and control animals in a DFA, displaying 90.8% accuracy, 100% sensitivity, and 79.5% specificity. An AUC of 0.986 was observed when a ROC analysis was conducted. Adapted from McLaurin et al., 2016. C: In adult animals (i.e., PD 240-PD 480), a DFA selected 3 PPI variables, exhibiting 86.7% accuracy, 89.3% sensitivity, and 84.4% specificity. An AUC of 0.951 was observed when an ROC analysis was conducted. Reanalysis of data presented in McLaurin et al., 2018b. D: In older adult animals (i.e., PD 540), a 5 PPI variables best discriminated between HIV-1 Tg and control animals in a DFA, exhibiting 96.8% accuracy, 100% sensitivity and 94.1% specificity. An AUC of 0.995 was observed when an ROC analysis was conducted. Reanalysis of data presented in McLaurin et al. 2018d.
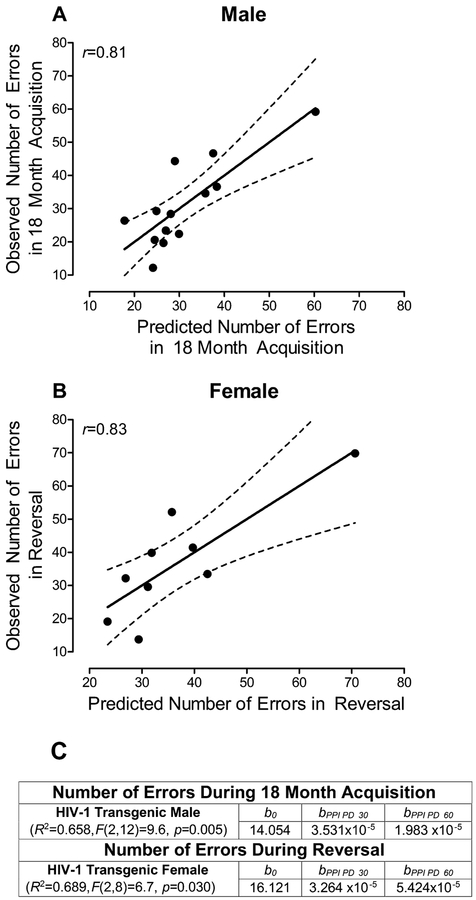
The prognostic utility of cross-modal PPI to predict disease progression in the HIV-1 Tg rat was examined using a longitudinal experimental design across the functional lifespan (Postnatal Day (PD) 30 to PD 600) revealing sex-dependent expression of NCI (McLaurin et al., 2018c). For HIV-1 Tg males, regressing auditory PPI at PD 30 and PD 60 on the number of errors in 18 month acquisition (i.e., a challenging signal detection task tapping sustained attention) revealed a regression coefficient (r) of 0.81. Therefore, in male HIV-1 Tg animals, auditory PPI explained 65.8% of the variance in the number of errors in 18 months acquisition (Figure 2A). For HIV-1 Tg females, regressing auditory PPI at PD 30 and PD 60 on the number of errors in an operant reversal task at 18 months of age, tapping flexibility and attention, exhibited a regression coefficient (r) of 0.83. Thus, in female HIV-1 Tg animals, auditory PPI explained 68.9% of the variance in the number of errors in a reversal task, tapping flexibility and inhibition, at 18 months of age (Figure 2B). Early alterations in temporal processing, assessed using PPI, therefore, are predictive of later NCI in executive functions.
FIGURE 2.
The utility of early (i.e., Postnatal Day (PD) 30 and PD 60)) prepulse inhibition (PPI) assessments to accurately predict later (i.e., PD 540) neurocognitive impairment in higher order cognitive processes (i.e., sustained attention, flexibility, and inhibition) was assessed in HIV-1 Tg animals. Regression assessments were conducted independently by sex due to sex-dependent expression of the HIV-1 transgene at an advanced age. Specifically, male HIV-1 Tg animals exhibited greater impairments in sustained attention (i.e., 18 Month Acquisition), while female HIV-1 Tg animals displayed greater impairments in flexibility and inhibition (i.e., Reversal; McLaurin et al., 2018c). A trimmed means regression analysis was conducted. A: For HIV-1 Tg males, regressing auditory PPI at PD 30 and PD 60 on the number of errors in 18 month acquisition (i.e., a challenging signal detection task tapping sustained attention) revealed a regression coefficient (r) of 0.81. Therefore, in male HIV-1 Tg animals, auditory PPI explained 65.8% of the variance in the number of errors in 18 months acquisition. B: For HIV-1 Tg females, regressing auditory PPI at PD 30 and PD 60 on the number of errors in an operant reversal task at 18 months of age, tapping flexibility and attention, exhibited a regression coefficient (r) of 0.83. Thus, in female HIV-1 Tg animals, auditory PPI explained 68.9% of the variance in the number of errors in a reversal task, tapping flexibility and inhibition, at 18 months of age. Reanalysis of data presented in McLaurin et al., 2018c. C: Regression Analysis.
CONCLUSIONS
Scientists and clinicians have been on a quest for an innovative biomarker to diagnose and/or predict the progression of HAND in the post-cART era. The present review examined the utility and limitations of four proposed biomarkers, including NFL chain concentration, amyloid and tau proteins, resting-state fMRI, and PPI. Although significant genotypic differences are observed in NFL chain concentration, amyloid and tau proteins, and resting-state fMRI, inconsistencies and/or limited research challenges their utility as a diagnostic and/or prognostic biomarker for milder forms of NCI in the post-cART era (reviewed in Table 1). Evaluation of the literature supports PPI as a powerful diagnostic biomarker for HAND (i.e., 86.7–97.1% accuracy, 89.3–100% sensitivity, 79.5–94.1% specificity), diagnosing presence of the HIV-1 transgene with high sensitivity and high specificity. The inclusion of multiple CSF and/or plasma markers, rather than a single protein, may provide a more accurate diagnostic biomarker for HAND, however, a critical need for additional research remains. Regarding a prognostic biomarker for milder forms of NCI, PPI notably predicts later NCI in higher-order cognitive domains with regression coefficients (i.e., r) greater than 0.8.
TABLE 1.
Comparison of Proposed Biomarkers
| Biomarker | Advantage & Utility | Disadvantage & Constraints | GOAL: Diagnostic Biomarker | GOAL: Prognostic Biomarker |
|---|---|---|---|---|
| NFL Chain Concentration | Plasma and CSF concentrations highly correlated; Sensitive to the initiation of cART | Alterations have been observed in other neurodegenerative diseases (e.g., AD, multiple sclerosis), and thus, it is not disease specific | Inconsistent relationship with NCI that may be driven primarily by cART | In the pre-cART era, increases in NFL concentration were associated with presentation of ADC |
| Amyloid and Tau Proteins | Distinct pathology from AD | Invasive procedure needed for CSF measurements | Selective relationships between NCI and protein markers; Unable to distinguish between milder forms of NCI and controls, despite the use of multiple markers | CSF Aβ−42 and p-tau have been utilized as a prognostic biomarker for the conversion from MCI to AD |
| Resting-State fMRI | Brief, non-invasive procedure | Currently unclear what changes in BOLD signaling indicate mechanistically; Inconsistencies between studies | Inconsistent relationship between fMRI measures and NCI | No changes in resting-state fMRI relative to controls were observed in a two-year follow-up |
| Prepulse Inhibition | Brief, non-invasive procedure; Clinically translational experimental paradigm | Alterations have been observed in other diseases, albeit evidence in other neurodegenerative disease (e.g., AD) is limited | Diagnostic utility generalizes across two experimental paradigms (i.e., cross-modal PPI, gap-PPI), the functional lifespan, sensory modalities, and biological sex; Overall high accuracy, sensitivity, and specificity | Early neurocognitive impairments in PPI predict later alterations in higher order cognitive functions (r≥0.81) |
NFL=Neurofilament Light; CSF=Cerebrospinal Fluid; cART=Combination Antiretroviral Therapy; AD=Alzheimer’s disease; NCI=Neurocognitive Impairments; ADC=AIDS Dementia Complex; Aβ−42=Amyloid β 1–42; MCI=Milder Cognitive Impairment; fMRI=Functional Magnetic Resonance Imaging; BOLD=Blood Oxygenation Level Dependent; PPI=Prepulse Inhibition; gap-PPI=Gap Prepulse Inhibition
All four proposed biomarkers examined in the present review are, at some level, related to synaptic dysfunction, functional connectivity, and/or neurotransmitter alterations; neural mechanisms which may underlie HAND. Specifically, synaptic dysfunction in HIV-1 is characterized by structural and chemical alterations in synapses, which have been observed in post-mortem HIV-1 seropositive individuals (Ellis et al., 2007; Gelman & Nguyen, 2010; Gelman et al., 2012). In the HIV-1 Tg rat, profound alterations in dendritic branching connectivity, neuronal morphology, and, to a lesser extent, dendritic spine morphology have been observed in medium spiny neurons of the NAc (Roscoe et al., 2014; McLaurin et al., 2018d), in pyramidal neurons of layers II-III of the mPFC (McLaurin et al., 2018c) and in pyramidal neurons of layers II-III of the mPFC following psychostimulant exposure (McLaurin et al., 2018e). Additionally, dopaminergic system dysfunction has been associated with NCI, evidenced by decreases in DA in the substantia nigra (Kumar et al., 2011), decreased CSF levels of homovanilic acid (Di Rocco et al., 2000) and decreased levels of dopamine transporter (Chang et al., 2008) in HIV-1 seropositive individuals. Thus, not only may these measurements (i.e., NFL chain concentration, amyloid and tau proteins, resting-state fMRI, and PPI) serve as potential biomarkers, but they may also enhance our understanding of the neural mechanisms underlying HAND.
Investigations for an innovative biomarker for HAND may be enhanced by addressing gaps in some of the current studies. First, many studies assessed a primarily, if not exclusively, male sample, limiting the generalizability of assessments. HIV-1 seropositive women are currently inadequately represented in both clinical and preclinical studies (Maki & Martin-Thormeyer, 2009), despite reports of profound sex differences in NCI (e.g., Royal et al., 2016; Maki et al., 2018; McLaurin et al., 2016; McLaurin et al., 2017d; Rowson et al., 2016; McLaurin et al., 2018c). Second, the arbitrary classification of HIV-1 seropositive individuals into three groups based on neurocognitive performance (i.e., ANI, MND, and HAD) naturally decreases variability, decreases power, and results in the loss of individual differences (Rucker et al., 2015). Utilizing the continuous neurocognitive performance scores ought to provide a stronger foundation for assessing the diagnostic and/or prognostic utility of a biomarker.
Use of a multiple biomarker approach may be one of the most critical gaps in the current studies. Despite reporting measurements for multiple CSF proteins or several fMRI metrics, diagnostic and/or prognostic utility was primarily evaluated using a single variable. One notable exception was presented in Gisslén et al. (2009), whereby a PCA was used to assess the ability of five CSF protein markers (i.e., sAPPα, sAPPβ, Aβ1–42, t-tau, p-tau) to separate individuals into arbitrarily classified groups. Although the PCA failed to distinguish between neuroasymptomatic HIV-1 individuals and HIV-1 seronegative controls, an important separation in the post-cART era, further assessments of multiple biomarkers are necessary. Inclusion of multiple variables may increase sensitivity and specificity, thereby increasing the likelihood of discovering an innovative and accurate diagnostic biomarker. The benefits of a multiple biomarker approach, however, extend beyond the development of an innovative biomarker for HAND in the post-cART era. For example, the pathogenesis of HAND is multidimensional, and may include neurotransmitter alterations (e.g., clinical: Kumar et al., 2011; HIV-1 Tg rat: Sinharay et al., 2017; Javadi-Paydar et al., 2017), synaptic dysfunction (e.g., clinical: Gelman et al., 2012; Desplats et al., 2013; HIV-1 Tg rat: Roscoe et al., 2014; McLaurin et al., 2018e), and neuroinflammation (e.g., clinical: Meier et al., 2009, Royal et al., 2016; HIV-1 Tg rat: Royal et al., 2012). Employing a DFA or multiple regression statistical approach on variables tapping multiple potential neural mechanisms may indicate both their utility as a diagnostic biomarker and their involvement (i.e., via variance accounted for) in the underlying pathogenesis of HAND.
Our review of potential biomarkers for milder forms of NCI in the post-cART era is not without limitation. First, the present review does not examine all biomarkers (e.g., cytokines, Yuan et al., 2013; structural magnetic resonance imaging, Steinbrink et al., 2013) that have been proposed for milder forms of NCI. Second, the scope of the present review could not cover all studies pertaining to the four biomarkers assessed. The integrative assessment of the diagnostic and prognostic utility of four biomarkers, however, may help identify key gaps in our knowledge, providing a foundation for further studies exploring these and other proposed biomarkers.
The development of an innovative biological marker (or biomarker) able to accurately diagnose milder forms of NCI, including HAND, and/or predict neurocognitive decline in aging HIV-1 seropositive individuals has the potential for great clinical significance (Chan & Brew, 2014; Zipursky et al., 2013). Analyses support PPI as a powerful diagnostic biomarker and promising prognostic biomarker. Thus, PPI heralds an opportunity for the development of a brief, non-invasive diagnostic and prognostic biomarker for milder forms of NCI in the post-cART era.
ACKNOWLEDGEMENTS
This work was supported in part by grants from NIH (National Institute on Drug Abuse, DA013137; National Institute of Child Health and Human Development, HD043680; National Institute of Mental Health, MH106392; National Institute of Neurological Disorders and Stroke, NS100624) and the interdisciplinary research training program supported by the University of South Carolina Behavioral-Biomedical Interface Program.
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
REFERENCES
- Abdulle S, Mellgren A, Brew BJ, Cinque P, Hagberg L, Price RW, Rosengren L, Gisslén M (2007) CSF neurofilament protein (NFL)—A marker of active HIV-related neurodegeneration. J Neurol 254:1026–1032. doi: 10.1007/s00415-006-0481-8 [DOI] [PubMed] [Google Scholar]
- Abidin AZ, Dsouza AM, Nagarajan MB, Wang L, Qiu X, Schifitto G, Wismuller A (2018) Alteration of brain network topology in HIV-associated neurocognitive disorder: A novel functional connectivity perspective. Neuroimage Clin 17:768–777. doi: 10.1016/j.nicl.2017.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achim CL, Adame A, Dumaop W, Everall IP, Masliah E, Neurobehavioral Research Center (2009) Increased accumulation of intraneuronal amyloid beta in HIV-infected patients. J Neuroimmune Pharmacol 4: 190–199. doi: 10.1007/s11481-009-9152-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ances BM, Vaida F, Yeh MJ, Liang CL, Buxton RB, Letendre S, McCutchan JA, Ellis RJ, the HIV Neurobehavioral Research (2010) HIV and aging independently affect brain function as measured by functional magnetic resonance imaging. J Infect Dis, 201: 336–340. doi: 10.1086/649899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AM, Easley KA, Kasher N, Franklin D, Heaton RK, Zetterberg H, Blennow K, Gisslén M, Letendre SL (2018) Neurofilament light chain in blood is negatively associated with neuropsychological performance in HIV-infected adults and declines with initiation of antiretroviral therapy. J Neurovirol [Epub ahead of print]. doi: 10.1007/s13365-018-0664-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ann HW, Jun S, Shin N, Han S, Ahn JY, Ahn MY, Jeon YD, Jung IY, Kim MH, Jeong WY, Ku NA, Kim JM, Smith DM, Choi JY (2016) Characteristics of resting-state functional connectivity in HIV-associated neurocognitive disorders. PLoS One 11: e0153493. doi: 10.1371/journal.pone.0153493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE (2006) Accelerated Tau deposition in the brains of individuals infected with human immunodeficiency virus-1 before and after the advent of highly active anti-retoviral therapy. Acta Neuropathol 111: 529–538. doi: 10.1007/s00401-006-0037-0 [DOI] [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslén M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE (2007) Updated research nosology for HIV-associated neurocognitive disorders. Neurology 69: 1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Forcelli P, Masliah E, Campbell L, Mocchetti I (2016) Expression of gp120 in mice evokes anxiety behavior: Co-occurrence with increased dendritic spines and brain-derived neurotrophic factor in the amygdala. Brain Behav Immun 54: 170–177. doi: 10.1016/j.bbi.2016.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bero AW, Yan P, Roh JH, Cirrito JR, Stewart FR, Raichle ME, Lee J-M, Holtzman DM (2011) Neuronal activity regulates the regional vulnerability to amyloid-b deposition. Nat. Neurosci 14: 750–756. doi: 10.1038/nn.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billnitzer AJ, Barskaya I, Yin C, Perez RG (2013) APP independent and dependent effects on neurite outgrowth are modulated by the receptor associated protein (RAP). J Neurochem 124: 123–132. doi: 10.1111/jnc.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder LI, Frankfurter A, Rebhun LI (1985) The distribution of tau in the mammalian central nervous system. J Cell Biol 101: 1371–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biomarkers Definitions Working Group (2001) Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin Pharmacol Ther 69:89–95. doi: 10.1067/mcp.2001.113989 [DOI] [PubMed] [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, van Boxtel A (2005) Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology 42: 1–15. doi: 10.1111/j.1469-8986.2005.00271.x [DOI] [PubMed] [Google Scholar]
- Braff D, Stone C, Callaway E, Geyer M, Glick I, Bali L (1978) Prestimulus effects on human startle reflex in normal and schizophrenics. Psychophysiology 15: 339–343. doi: 10.1111/j.1469-8986.1978.tb01390.x [DOI] [PubMed] [Google Scholar]
- Brys M, Pirraglia E, Rich K, Rolstad S, Mosconi L, Switalski R, Glodzik-Sobanska L, De Santi S, Zinkowski R, Mehta P, Pratico D, Saint Louis LA, Wallin A, Blennow K, de Leon MJ (2009) Prediction and longitudinal study of CSF biomarkers in mild cognitive impairment. Neurobiol Aging 30: 682–690. doi: 10.1016/j.neurobiolaging.2007.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubser M, Koch M (1994) Prepulse inhibition of the acoustic startle response of rats is reduced by 6-hydroxydopamine lesions of the medial prefrontal cortex. Psychopharmacology(Berl) 113: 487–492. [DOI] [PubMed] [Google Scholar]
- Buchhave P, Minthon L, Zetterberg H, Wallin AK, Blennow K, Hansson O (2012) Cerebrospinal fluid levels of β-amyloid 1–42, but not of tau, are fully changed already 5 to 10 years before the onset of Alzheimer dementia. Arch Gen Psychiatry 69: 98–106. doi: 10.1001/archgenpsychiatry.2011.155. [DOI] [PubMed] [Google Scholar]
- Byrne LM, Rodrigues FB, Blennow K, Durr A, Leavitt BR, Roos RAC, Scahill RI, Tabrizi SJ, Zetterberg H, Langbehn D, Wild EJ (2017) Neurofilament light protein in blood as a potential biomarker of neurodegeneration in Huntington’s disease: A retrospective cohort analysis. Lancet Neurol 16: 601–609. doi: 10.1016/S1474-4422(17)30124-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campeau S, Davis M (1995) Prepulse inhibition of the acoustic startle reflex usingvisual and auditory prepulses: disruption by apomorphine. Psychopharmacology (Berl) 17:267–274. [DOI] [PubMed] [Google Scholar]
- Cai L, Huang J (2018) Neurofilament light chain as a biological marker for multiple sclerosis: A meta-analysis study. Neuropsychiatr Dis Treat 14:2241–2254. doi: 10.2147/NDT.S173280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Rippeth JD, Heaton RK, Grant I, the HIV Neurobehavioral Research Center (HNRC) Group (2006) Prospective memory in HIV-1 infection J Clin Exp Neuropsychol 28: 536–548. doi: 10.1080/13803390590949494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Matthew D, Rosen B (2002) Functional magnetic resonance imaging: Basic principles of and application to developmental science. Dev Sci 5: 301–309. doi: 10.1111/1467-7687.00370 [DOI] [Google Scholar]
- Castellanos FX, Fine EJ, Kaysen D, Marsh WL, Rapoport JL, Hallett M (1996) Sensorimotor gating in boys with Tourette’s Syndrome and ADHD: Preliminary results. Biol Psychiatry 39:33–41. doi: 10.1016/0006-3223(95)00101-8. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2018) HIV Among People Aged 50 and Older. https://www.cdc.gov/hiv/group/age/olderamericans/index.html
- Chaganti JR, Heinecke A, Gates TM, Moffat KJ, Brew BJ (2017) Functional connectivity in virally suppressed patients with HIV-associated neurocognitive disorder: A resting-state analysis. AJNR Am J Neuroradiol 38:1623–1629. doi: 10.3174/ajnr.A5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan P, Brew BJ (2014) HIV associated neurocognitive disorders in the modern antiviral treatment era: prevalence, characteristics, biomarkers, and effects of treatment. Curr HIV/AIDS Rep 11: 317–324. doi: 10.1007/s11904-014-0221-0. [DOI] [PubMed] [Google Scholar]
- Chang L, Wang GJ, Volkow ND, Ernst T, Telang F, Logan J, Fowler JS (2008) Decreased brain dopamine transporters are related to cognitive deficits in HIV patients with or without cocaine abuse. Neuroimage 42: 869–878. doi: 10.1016/j.neuroimage.2008.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GF, Xu TH, Yan Y, Zhou YR, Jiang Y, Melcher K, Xu HE (2017) Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacol Sin 38:1205–1235. doi: 10.1038/aps.2017.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu MJ, Chen YF, Chen TF, Yang SY, Yang FPG, Tseng TW, Chieh JJ, Chen JCR, Tzen KY, Hua MS, Horng HE (2013) Plasma tau as a window to the brain—negative associations with brain volume and memory function in mild cognitive impairment and early alzheimer’s disease. Hum Brain Map 7: 3132–3142. doi: 10.1002/hbm.22390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito JR, Kang J-E, Lee J, Stewart FR, Verges DK, Silverio LM, Bu G, Mennerick S, Holtzman DM (2008) Endocytosis is required for synaptic activity-dependent release of amyloid-beta in vivo. Neuron 58: 42–51. doi: 10.1016/j.neuron.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford DB, Fagan AM, Holtzman DM, Morris JC, Teshome M, Shah AR, Kauwe JSK (2009) CSF biomarkers of Alzheimer’s disease in HIV-associated neurologic disease. Neurology 73: 1982–1987. doi: 10.1212/WNL.0b013e3181c5b445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JH, Caan MWA, Underwood J, De Francesco D, van Zoest RA, Wit FWNM, Mutsaerts HJMM, Leech R, Geurtsen GJ, Portegies P, Majoie CBLM, Schim van der Loeff MR, Sabin CA, Reiss P, Winston A, Sharp DJ, Comorbidity in Relations to AIDS (COBRA) Collaboration (2018) No evidence for accelerated aging-related brain pathology in treated human immunodeficiency virus: Longitudinal neuroimaging results from the comorbidity in relation to AIDS (COBRA) project. Clin Infect Dis 66:1899–1909. doi: 10.1093/cid/cix1124. [DOI] [PubMed] [Google Scholar]
- Dage JL, Wennberg AMV, Airey DC, Hagen CE, Knopman DS, Machulda MM, Roberts RO, Jack CR Jr, Petersen RC, Mielke MM (2016) Levels of tau protein in plasma are associated with neurodegeneration and cognitive function in a population-based elderly cohort. Alzheimers Dement 12: 1226–1234. doi: 10.1016/j.jalz.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Almeida SM, Ribeiro CE, Rotta I, Piovesan M, Tang B, Vaida F, Raboni SM, Letendre S, Potter M, Batistela Fernandes MS, Ellis RJ, HIV Neurobehavioral Research Center (HNRC) Group (2018) Biomarkers of neuronal injury and amyloid metabolism in the cerebrospinal fluid of patient infected with HIV-1 subtypes B and C. J Neurovirol 24: 28–40. doi: 10.1007/s13365-017-0591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desplats P, Dumaop W, Smith D, Adame A, Everall I, Letendre S, Ellis R, Cherner M, Grant I, Masliah E (2013) Molecular and pathologic insights from latent HIV-1 infection in the human brain. Neurology 80:1415–1423. doi: 10.1212/WNL.0b013e31828c2e9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Strooper B, Annaert W (2000) Proteolytic processing and cell biological functions of the amyloid precursor protein. J Cell Sci, 113:1857–1870. [DOI] [PubMed] [Google Scholar]
- Di Rocco A, Bottiglieri T, Dorfman D, Werner P, Morrison C, Simpson D (2000) Decreased homovanilic acid in cerebrospinal fluid correlates with impaired neuropsychologic function in HIV-1-infected patients. Clin Neuropharmacol 23:19–194. [DOI] [PubMed] [Google Scholar]
- Donker Kaat L, Meeter LH, Chiu WZ, Melhem S, Boon AJW, Blennow K, Zetterberg H, van Swieten JC (2018) Serum neurofilament ligh chain in progressive supranuclear palsy. Parkinsonism Ralat Disord [Epub Ahead of Print]. doi: 10.1016/j.parkreldis.2018.06.018. [DOI] [PubMed] [Google Scholar]
- Dsouza AM, Abidin AZ, Wismϋller A (2017) Investigating changes in resting-state connectivity from functional MRI data in patients with HIV associated neurocognitive disorder using MCA and machine learning. Proc SPIE Int Soc Opt Eng 10137:101371C. doi: 10.1117/12.2254189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Plessis L, Paul RH, Hoare J, Stein DJ, Taylor PA, Meintjes EM, Joska JA (2017) Resting-state functional magnetic resonance imaging in clade C HIV: Within-group association with neurocognitive function. J Neurovirol 23:875–885. doi: 10.1007/s13365-017-0581-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egbert AR, Biswal B, Karunakaran K, Gohel S, Pluta A, Wolak T, Szymanska B, Firlag-Burkacka E, Sobanska M, Gawron N, Bienkowski P, Sienkiewicz-Jarosz H, Scinska-Bienkowska A, Bornstein R, Rao S, Lojek E (2018) Age and HIV effects on resting state of the brain in relationship to neurocognitive functioning. Behav Brain Res 344: 20–27. doi: 10.1016/j.bbr.2018.02.007. [DOI] [PubMed] [Google Scholar]
- Egbert AR, Biswal B, Karunakaran KD, Pluta A, Wolak T, Rao S, Bornstein R, Szymanska B, Horban A, Firlag-Burkacka E, Sobanska M, Gawron N, Bienkowski P, Sienkiewicz-Jarosz H, Scinska-Bienkowska A, Lojek E (2019) HIV infection across aging: Synergistic effects on intrinsic functional connectivity of the brain. Prog Neuropsychopharmacol Biol Psychiatry 88:19–30. doi: 10.1016/j.pnpbp.2018.06.006. [DOI] [PubMed] [Google Scholar]
- Elbirt D, Mahlab-Guri K, Bezalel-Rosenberg S, Gills H, Attali M, Asher I (2015) HIV-associated neurocognitive disorders (HAND). Isr Med Assoc J 17:54–59. [PubMed] [Google Scholar]
- Ellenbroek BA, Budde S, Cools AR (1996) Prepulse inhibition and latent inhibition: the role of dopamine in the medial prefrontal cortex. Neuroscience 75: 535–542. [DOI] [PubMed] [Google Scholar]
- Ellis R, Langford D, Masliah E (2007) HIV and antiretroviral therapy in the brain: Neuronal injury and repair. Nat Rev Neurosci 8: 33–44. doi: 10.1038/nrn2040 [DOI] [PubMed] [Google Scholar]
- FDA-NIH Biomarker Working Group (2016) BEST (Biomarkers, EndpointS, and other Tools) Resource [Internet]. Silver Spring (MD): Food and Drug Administration (US) Available from: https://www.ncbi.nlm.nih.gov/books/NBK326791/ Co-published by National Institutes of Health (US), Bethesda (MD). [PubMed] [Google Scholar]
- Fendt M, Koch M, Schnitzler HU (1994) Sensorimotor gating deficit after lesions of the superior colliculus. NeuroReport 5:1725–1738. [DOI] [PubMed] [Google Scholar]
- Fendt M, Li L, Yeomans JS (2001) Brain stem circuits mediating prepulseinhibition of the startle reflex. Psychopharmacology (Berl) 156: 216–224. [DOI] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Mactutus CF (2006a) Neonatal intrahippocampal glycoprotein 120 injection: the role of dopaminergic alterations in prepulse inhibition in adult rats. J Pharmacol Exp Ther. 318:1352–1358. doi: 10.1124/jpet.106.105742. [DOI] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Mactutus CF (2006b) Neonatal hippocampal Tat injections: developmental effects on prepulse inhibition (PPI) of the auditory startle response. Int J Dev Neurosci 24: 275–283. doi: 10.1016/j.ijdevneu.2006.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Hasselrot U, Mactutus CF (2006c) Intrahippocampus injections of Tat: Effects on prepulse inhibition of the auditory startle response in adult male rats. Pharmacol Biochem Behav 84: 189–196. doi: 10.1016/j.pbb.2006.04.014 [DOI] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Mactutus CF (2007) Neonatal intrahippocampal gp120 injection: An examination early in development. Neurotoxicology 28:101–107. doi: 10.1016/j.neuro.2006.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaten MA (1993) A comparison of electromyographic and photoelectric techniques in the study of classical eyeblink conditioning and startle reflex modification. Journal of Psychophysiology, 7: 230–237. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198. [DOI] [PubMed] [Google Scholar]
- Fournier P, Hébert S (2013) Gap detection deficits in humans with tinnitus as assessed with the acoustic startle paradigm: does tinnitus fill in the gap? Hear. Res 295:16–23. doi: 10.1016/j.heares.2012.05.011 [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME (2007) Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Rev Neurosci 8: 700–711. doi: 10.1038/nrn2201 [DOI] [PubMed] [Google Scholar]
- Gelman BB, Schuenke K (2004) Brain aging in acquired immunodeficiency syndrome: Increased ubiquitin-protein conjugate is correlated with decreased synaptic protein but not amyloid plaque accumulation. J Neurovirol 10: 98–108. doi: 10.1080/13550280490279816 [DOI] [PubMed] [Google Scholar]
- Gelman BB, Nguyen TP (2010) Synaptic proteins linked to HIV-1 infection and immunoproteasome induction: proteomic analysis of human synaptosomes. J Neuroimmune Pharmacol 5: 92–102. doi: 10.1007/s11481-009-9168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman BB, Lisinicchia JG, Chen T, Johnson KM, Jennings K, Freeman DH Jr, Soukup VM (2012) Prefrontal dopaminergic and enkephalinergic synaptic accomodation in HIV-associated neurocognitive disorders and encephalitis. J Neuroimmune Pharmacol, 7: 686–700. doi: 10.1007/s11481-012-9345-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisslén M, Hagberg L, Brew BJ, Cinque P, Price RW, Rosengren L (2007) Elevated cerebrospinal fluid neurofilament light protein concentrations predict the development of AIDS dementia complex. J Infect Dis 195: 1774–1778. doi: 10.1086/518043 [DOI] [PubMed] [Google Scholar]
- Gisslén M, Krut J, Andreasson U, Blennow K, Cinque P, Brew BJ, Spudich S, Hagberg L, Rosengren L, Price RW, Zetterberg H (2009) Amyloid and tau cerebrospinal fluid biomarkers in HIV infection. BMC Neurol 9: 63. doi: 10.1186/1471-2377-9-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisslén M, Price RW, Andreasson U, Norgren N, Nilsson S, Hagberg L, Fuchs D, Spudich S, Blennow K, Zetterberg H (2016) Plasma concentration of the neurofilament light protein (NFL) is a biomarker of CNS injury in HIV infection: A cross-sectional study. EBioMedicine 3:135–140. doi: 10.1016/j.ebiom.2015.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover GH (2011) Overview of functional magnetic resonance imaging. Neurosurg Clin N Am 22:133–139. doi: 10.1016/j.nec.2010.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA (1989a) Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron 3: 519–526. [DOI] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG, Potier MC, Ulrich J, Crowther RA (1989b) Cloning and sequencing of the cDNA encoding an isoform of microtubule-associated protein tau containing four tandem repeats: differential expression of tau protein mRNAs in human brain. EMBO J 8: 393–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman Y, Mattson MP (1994) Secreted forms of beta-amyloid precursor protein protect hippocampal neurons against amyloid beta-peptide-induced oxidative injury. Exp Neurol 128:1–12. doi: 10.1006/exnr.1994.1107 [DOI] [PubMed] [Google Scholar]
- Guha A, Wang L, Tanenbaum A, Esmaeili-Firidouni P, Wedelken LA, Busovaca E, Clifford K, Desai A, Ances BM, Valcour V (2016) Intrinsic network connectivity abnormalities in HIV-infected individuals over age 60. J Neurovirol 22: 80–87. doi: 10.1007/s13365-015-0370-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Knight AG, Gupta S, Knapp PE, Hauser KF, Keller JN, Bruce-Keller AJ (2010) HIV-Tat elicits microglial glutamate release: Role of NAPDH oxidase and the cysteine-glutamate antiporter. Neurosci Lett 485:233–236. doi: 10.1016/j.neulet.2010.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddow LJ, Floyd S, Copas A, Gilson RJ (2013) A systematic review of the screening accuracy of the HIV Dementia Scale and International HIV Dementia Scale. PLoS One 8: e61826. doi: 10.1371/journal.pone.0061826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghighi S, Andersen O, Odén A, Rosengren L (2004) Cerebrospinal fluid markers in MS patients and their healthy siblings. Acta Neurol Scand 109:97–99. [DOI] [PubMed] [Google Scholar]
- Haughey NJ, Nath A, Mattson MP, Slevin JT, Geiger JD (2001) HIV-1 Tat through phosphorylation of NMDA receptors potentiates glutamate excitotoxicity. J Neurochem 78:457–467. [DOI] [PubMed] [Google Scholar]
- Hejl AM, Glenthoj B, Mackeprang T, Hemmingsen R, Waldemar G (2004) Prepulse inhibition in patients with Alzheimer’s disease. Neurobiol Aging 25: 1045–1050. doi: 10.1016/j.neurobiolaging.2003.11.005 [DOI] [PubMed] [Google Scholar]
- Henry BL, Geyer MA, Buell MR, Perry W, Young JW, Minassian A, Translational Methamphetamine AIDS Research Center (TMARC) Group (2014) Prepulse inhibition in HIV-1 gp120 transgenic mice after withdrawal from chronic methamphetamine. Behav Pharmacol 25:12–22. doi: 10.1097/FBP.0000000000000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Uban KA, Williams PL, Gautam P, Huo Y, Malee K, Yogev R, Csernansky J, Wang L, Nichols S, Van Dyke R, Sowell ER (2015) Default mode connectivity in youth with perinatally acquired HIV. Medicine (Baltimore) 94: e1417. doi: 10.1097/MD.0000000000001417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkin CH, van Gorp WG, Satz P, Marcotte T, Durvasula RS, Wood S, Campbell L, Baluda MR (1996) Actual versus self-reported cognitive dysfunction in HIV-1 infection: Memory-metamemory dissociations. J Clin Exp Neuropsychol 18: 431–443. doi: 10.1080/01688639608408999 [DOI] [PubMed] [Google Scholar]
- Hoffman HS, Searle JL (1965) Acoustic variables in modification of startle reaction in rat. J Comp Physiol Psychol 60:53–58. [DOI] [PubMed] [Google Scholar]
- Hoffman HS, Ison JR (1980) Reflex modification in the domain of startle: I. Some empirical findings and their implications for how the nervous system processes sensory input. Psychol Rev 87:175–189. [PubMed] [Google Scholar]
- Hunsberger HC, Rudy CC, Batten SR, Gerhard GA, Reed MN (2015) P301L tau expression affects glutamate release and clearance in the hippocampal trisynaptic pathway. J Neurochem 132:169–182. doi: 10.1111/jnc.12967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikin AF, Sabo SL, Lanier LM, Buxbaum JD (2007) A macromolecular complex involving the amyloid precursor protein (APP) and the cytosolic adapter FE65 is a negative regulator of axon branching. Mol Cell Neurosci 35:57–63. doi: 10.1016/j.mcn.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ison JR, Hammond GR (1971) Modification of startle reflex in rat by changes in auditory and visual environments. J Comp Physiol Psychol 75: 435–452. [DOI] [PubMed] [Google Scholar]
- Ittner LM, Ke YD, Delerue F, Bi M, Gladbach A, van Eersel J, Wolfing H, Chieng BC, Christie MJ, Napier IA, Eckert A, Staufenbiel M, Hardeman E, Gotz J (2010). Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer’s disease mouse models. Cell 142:387–397. doi: 10.1016/j.cell.2010.06.036 [DOI] [PubMed] [Google Scholar]
- Janssen MAM, Hinne M, Janssen RJ, van Gerven MA, Steens SC, Gόraj B, Koopmans PP, Kessels RPC (2017) Resting-state subcortical functional connectivity in HIV-infected patients on long-term cART. Brain Imaging Behav 11: 1555–1560. doi: 10.1007/s11682-016-9632-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javadi-Paydar M, Roscoe RF Jr, Denton AR, Mactutus CF, Booze RM (2017) HIV-1 and cocaine disrupt dopamine reuptake and medium spiny neurons in female rat striatum. PLoS One 12: e0188404. doi: 10.1371/journal.pone.0188404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen Krut J, Mellberg T, Price RW, Hagberg L, Fuchs D, Rosengren L, Nilsson S, Zetterberg H, Gisslén M (2014) Biomarker evidence of axonal injury in neuroasymptomatic HIV-1 patients. PLoS One 9:e88591. doi: 10.1371/journal.pone.0088591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joska JA, Witten J, Thomas KG, Robertson C, Casson-Crook M, Roosa H, Creighton J, Lyons J, McArthur J, Sacktor NC (2016) A comparison of five brief screening tools for HIV-associated neurocognitive disorders in the USA and South Africa. AIDS Behav 20:1621–1631. doi: 10.1007/s10461-016-1316-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice AC (2010) HIV and aging: Time for a new paradigm. Curr HIV/AIDS Rep 7: 69–76. doi: 10.1007/s11904-010-0041-9. [DOI] [PubMed] [Google Scholar]
- Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, Sisodia S, Malinow R (2003) APP processing and synaptic function. Neuron 37: 925–937. [DOI] [PubMed] [Google Scholar]
- Koch M, Kungel M, Herbert H (1993) Cholinergic neurons in the pedunculopontine tegmental nucleus are involved in the mediation of prepulse inhibition of the acoustic startle response in the rat. Exp Brain Res 97:71–82. [DOI] [PubMed] [Google Scholar]
- Koch M, Schnitzler HU (1997) The acoustic startle response in rats—Circuits mediating evocation, inhibition and potentiation. Behav Brain Res 89:35–49. [DOI] [PubMed] [Google Scholar]
- Kopeikina KJ, Polydoro M, Tai HC, Yaeger E, Carlson GA, Pitstick R, Hyman BT, Spires-Jones TL (2013) Synaptic alterations in the rTg4510 mouse model of tauopathy. J Comp Neurol 521:1334–1353. doi: 10.1002/cne.23234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krut JJ, Zetterberg H, Belnnow K, Cinque P, Hagberg L, Price RW, Studahl M, Gisslén M (2013) Cerebrospinal fluid Alzheimer’s biomarker profiles in CNS infections. J Neurol 260: 620–626. doi: 10.1007/s00415-012-6688-y. [DOI] [PubMed] [Google Scholar]
- Krogh KA, Lyddon E, Thayer SA (2015) HIV-1 Tat activates a RhoA signaling pathway to reduce NMDA-evoked calcium responses in hippocampal neurons via an actin-dependent mechanism. J Neurochem 132: 345–366. doi: 10.1111/jnc.12936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchibhotla KV, Goldman ST, Lattarulo CR, Wu HY, Hyman BT, Bacskai BJ (2008) Abeta plaques lead to abberant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks. Neuron 59:214–225. doi: 10.1016/j.neuron.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar AM, Fernandez JB, Singer EJ, Commins D, Waldrop-Valverde D, Ownby RL, Kumar M (2009) Human immunodeficiency virus type 1 in the central nervous system leads to decreased dopamine in difference regions of postmortem human brains. J Neurovirol 15: 257–274. doi: 10.1080/13550280902973952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar AM, Ownby RL, Waldrop-Valverde D, Fernandez B, Kumar M. (2011). Human immunodeficiency virus infection in the CNS and decreased dopamine availability: relationship with neuropsychological performance. J. Neurovirol 17: 26–40. doi: 10.1007/s13365-010-0003-4 [DOI] [PubMed] [Google Scholar]
- Lau CG, Zukin RS (2007) NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci 8:413–426. doi: 10.1038/nrn2153. [DOI] [PubMed] [Google Scholar]
- Lewczuk P, Ermann N, Andreasson U, Schultheis C, Podhorna J, Spitzer P, Maler JM, Kornhuber J, Blennow K, Zetterberg H (2018) Plasma neurofilament light as a potential biomarker of neurodegeneration in Alzheimer’s disease. Alzheimer’s Res Ther 10:71. doi: 10.1186/s13195-018-0404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light GA, Swerdlow NR (2015) Future clinical uses of neurophysiological biomarkers to predict and monitor treatment response for schizophrenia. Ann NY Acad Sci 1344:105–119. doi: 10.1111/nyas.12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim D, Iyer A, Ronco V, Grolla AA, Canonico PL, Aronica E, Genazzani AA (2013) Amyloid beta deregulates astroglia mGluR5-mediated calcium signaling via calcineurin and Nf-kB. Glia 61:1134–1145. doi: 10.1002/glia.22502. [DOI] [PubMed] [Google Scholar]
- Logothetis NK (2008) What we can do and what we cannot do with fMRI. Nature 453: 869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- Maki PM, Martin-Thormeyer E (2009) HIV, Cognition and Women. Neuropsychol Rev. 19: 204–214. doi: 10.1007/s11065-009-9093-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, Rubin LH, Springer G, Seaberg EC, Sacktor N, Miller EN, Valcour V, Young MA, Becker JT, Martin EM, Neuropsychology Working Groups of the Women’s Interagency HIV Study and the Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr 79:101–107. doi: 10.1097/QAI.0000000000001764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansbach RS, Geyer MA, Braff DL (1998) Dopaminergic stimulation disrupts sensorimotor gating in the rat. Psychopharmacology. 94:507–514. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Cheng B, Culwell AR, Esch FS, Lieberburg I, Rydel RE (1993) Evidence for excitoprotective and intraneuronal calcium-regulating roles for secreted forms of the beta-amyloid precursor protein. Neuron 10:243–254. [DOI] [PubMed] [Google Scholar]
- Mattsson N, Andreasson U, Zetterberg H, Blennow K, Alzheimer’s Disease Neuroimaging Initiative. (2017). Association of plasma neurofilament light with neurodegeneration in patients with Alzheimer disease. JAMA Neurol 74:557–566. doi: 10.1001/jamaneurol.2016.6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaurin KA, Booze RM, Mactutus CF (2016) Progression of temporal processing deficits in the HIV-1 transgenic rat. Sci Rep 6:32831. doi: 10.1038/srep32831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaurin KA, Moran LM, Li H, Booze RM, Mactutus CF (2017a) A gap in time: Extending our knowledge of temporal processing deficits in the HIV-1 transgenic rat. J Neuroimmune Pharmacol 12:171–179. doi: 10.1007/s11481-016-9711-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaurin KA, Booze RM, Mactutus CF (2017b) Temporal processing demands in the HIV-1 transgenic rat: Amodal gating and implications for diagnostics. Int J Dev Neurosci 57:12–20. doi: 10.1016/j.ijdevneu.2016.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaurin KA, Booze RM, Mactutus CF (2017c) Selective developmental alterations in the HIV-1 transgenic rat: opportunities for diagnosis of pediatric HIV-1. J Neurovirol 23:87–98. doi: 10.1007/s13365-016-0476-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaurin KA, Booze RM, Mactutus CF, Fairchild AJ (2017d) Sex matters: Robust sex differences in signal detection in the HIV-1 transgenic rat. Front Behav Neurosci 11:212. doi: 10.3389/fnbeh.2017.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaurin KA, Moran LM, Li H, Booze RM, Mactutus CF (2018a) The power of interstimulus interval for the assessment of temporal processing in rodents. J Vis Exp. [In press]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaurin KA, Booze RM, Mactutus CF (2018b) Evolution of the HIV-1 transgenic rat: Utility in assessing the progression of HIV-1-associated neurocognitive disorders. J Neurovirol 24:229–245. doi: 10.1007/s13365-017-0544-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaurin KA, Li H, Booze RM, Mactutus CF (2018c) Disruption of timing: NeuroHIV progression in the post-cART era. Under Review after Minor Revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaurin KA, Cook AK, Li H, League AF, Mactutus CF, Booze RM. (2018d) Synaptic connectivity in medium spiny neurons of the nucleus accumbens: A sex-dependent mechanism underlying apathy in the HIV-1 transgenic rat. Front Behav Neurosci [In press]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaurin KA, Li H, Booze RM, Fairchild AJ, Mactutus CF (2018e) Unraveling individual differences in the HIV-1 transgenic rat: Therapeutic efficacy of methylphenidate. Sci Rep 8:136. doi: 10.1038/s41598-017-18300-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier A, Chang JJ, Chan ES, Pollard RB, Sidhu HK, Kulkarni S, Wen TF, Lindsay RJ, Orellana L, Mildvan D, Bazner S, Streeck H, Alter G, Lifson JD, Carrington M, Bosch RJ, Robbins GK, Altfeld M (2009) Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV. Nat Med 15:955–959. doi: 10.1038/nm.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez RI, Roman C, Capo-Velez CM, Lasalde-Dominicci JA (2016). Decreased glial and synaptic glutamate uptake in the striatum of HIV-1 gp120 transgenic mice. J Neurovirol 22:358–365. doi: 10.1007/s13365-015-0403-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellgren A, Price RW, Hagberg L, Rosengren L, Brew BJ, Gisslén M (2007) Antiretroviral treatment reduces increased CSF neurofilament protein (NFL) in HIV-1 infection. Neurology, 69:1536–1541. doi: 10.1212/01.wnl.0000277635.05973.55. [DOI] [PubMed] [Google Scholar]
- Merino-Serrais P, Benavides-Piccione R, Blazquez-Llorca L, Kastanauskaite A, Rábano A, Avila J, DeFelipe J (2013) The influence of phopho-τ on dendritic spines of cortical pyramidal neurons in patients with Alzheimer’s disease. Brain 136:1913–1928. doi: 10.1093/brain/awt088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minassian A, Henry BL, Woods SP, Vaida F, Grant I, Geyer MA, Perry W (2013) Prepulse inhibition in HIV-associated neurocognitive disorders. J Int Neuropsychol Soc 19:709–717. doi: 10.1017/S1355617713000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran LM, Booze RM, Mactutus CF (2013a) Time and time again: temporal processing demands implicate perceptual and gating deficits in the HIV-1 transgenic rat. J Neuroimmune Pharmacol. 8:988–97. doi: 10.1007/s11481-013-9472-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moya KL, Benowitz LI, Schneider GE, Allinquant B (1994) The amyloid precursor protein is developmentally regulated and correlated with synaptogenesis. Dev Biol 161: 597–603. doi: 10.1006/dbio.1994.1055 [DOI] [PubMed] [Google Scholar]
- Myers CA, Brown PD (2006) Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. J Clin Oncol 24: 1305–1309. doi: 10.1200/JCO.2005.04.6086 [DOI] [PubMed] [Google Scholar]
- Norgren N, Rosengren L, Stigbrand T (2003) Elevated neurofilament levels in neurological diseases. Brain Res 987:25–31. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Kay AR, Tank DW (1990) Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA 87:9868–9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega M, Brier MR, Ances BM (2015) Effects of HIV and combination antiretroviral therapy on cortico-striatal functional connectivity. AIDS 29:703–712. doi: 10.1097/QAD.0000000000000611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris JJ, Singh HD, Carey AN, McLaughlin JP (2015) Exposure to HIV-1 Tat in brain impairs sensorimotor gating and activates microglia in limbic and extralimbic brain regions of male mice. Behav Brain Res 291:209–218. doi: 10.1016/j.bbr.2015.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton HK, Zhou ZH, Bubien JK, Benveniste EN, Benos DJ (2000) Gp120-induced alterations of human astrocyte function: Na(+)/H(+) exchange, K(+) conductance, and glutamate flux. Am J Physiol Cell Physiol 279: C700–C708. doi: 10.1152/ajpcell.2000.279.3.C700 [DOI] [PubMed] [Google Scholar]
- Peluso MJ, Meyerhoff DJ, Price RW, Peterson J, Lee E, Young AC, Walter R, Fuchs D, Brew BJ, Cinque P, Robertson K, Hagberg L, Zetterberg H, Gisslén M, Spudich S (2013) Cerebrospinal fluid and neuroimaging biomarker abnormalities suggest early neurological injury in a subset of individuals during primary HIV infection. J Infect Dis 207:1703–1712. doi: 10.1093/infdis/jit088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J, Gisslén M, Zetterberg H, Fuchs D, Shacklett BL, Hagberg L, Yiannoutsos CT, Spudich SS, Price RW (2014) Cerebrospinal fluid (CSF) neuronal biomarkers across the spectrum of HIV infection: Hierarchy of injury and detection. PLoS One 9:e116081. doi: 10.1371/journal.pone.0116081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickney LA (1976) Inhibition of the startle reflex in the rat by prior tactile stimulation. Anim Learn Behav 4:476–472. doi: 10.1093/jn/126.3.618 [DOI] [Google Scholar]
- Power C, Selnes OA, Grim JA, McArthur JC (1995) HIV Dementia Scale: A rapid screening test. J Acquir Immune Defic Syndr Hum Retrovirol 8:273–278. [DOI] [PubMed] [Google Scholar]
- Priller C, Bauer T, Mitteregger G, Krebs B, Kretzschmar HA, Herms J (2006) Synapse formation and function is modulated by the amyloid precursor protein. J Neurosci 26:7212–7221. doi: 10.1523/JNEUROSCI.1450-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissin DM, Kan CW, Campbell TG, Howes SC, Fournier DR, Song L, Patel PP, Chang L, Rivnak AJ, Ferrell EP, Randall JD, Provuncher GK, Walt DR, Duffy DC (2010) Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat Biotechnol 28:595–599. doi: 10.1038/nbt.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas JC, Karydas A, Bang J, Tsai RM, Blennow K, Liman V, Kramer JH, Rosen H, Miller BL, Zetterberg H, Boxer AL (2016) Plasma neurofilament light chain predicts progression in progressive supranuclear palsy. Ann Clin Transl Neurol 3: 216–225. doi: 10.1002/acn3.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romley JA, Juday T, Solomon MD, Seekins D, Brookmeyer R, Goldman DP (2014) Early HIV treatment led to life expectancy gains valued at $80 billion for people infected in 1996–2009. Health Aff (Millwood) 33:370–377. doi: 10.1377/hlthaff.2013.0623. [DOI] [PubMed] [Google Scholar]
- Roscoe RF Jr, Mactutus CF, Booze RM (2014) HIV-1 Transgenic female rat: synaptodendritic alterations of medium spiny neurons in the nucleus accumbens. J Neuroimmune Pharmacol. 9:642–653. doi: 10.1007/s11481-014-9555-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowson SA, Harrell CS, Bekhbat M, Gangavelli A, Wu MJ, Kelly SD, Reddy R, Neigh GN (2016) Neuroinflammation and behavior in HIV-1 transgenic rats exposed to chronic adolescent stress. Front. Psychiatry 7:102. doi: 10.3389/fpsyt.2016.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royal W 3rd, Zhang L, Guo M, Jones O, Davis H, Bryant JL (2012) Immune activation, viral gene product expression and neurotoxicity in the HIV-1 transgenic rat. J Neuroimmunol 247:16–24. doi: 10.1016/j.jneuroim.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royal W, Cherner M, Burdo TH, Umlauf A, Letendre SL, Jumare J, Abimiku A, Alabi P, Alkali N, Bwala S, Okwuasaba K, Eyzaguirre LM, Akolo C, Guo M, Williams KC, Blattner WA (2016) Associations between cognition, gender and monocyte activation among HIV infected individuals in Nigeria. PLoS One 11:e0147182.doi: 10.1371/journal.pone.0147182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucker DD, McShane BB, Preacher KJ (2015) A researcher’s guide to regression, dichotomization, and median splits of continuous variables. J Consumer Psychol 4: 666–678. doi: 10.1016/j.jcps.2015.04.004 [DOI] [Google Scholar]
- Sacktor NC, Wong M, Nakasujja N, Skolasky RL, Selnes OA, Musisi S, Robertson K, McArthur JC, Ronald A, Katabira E (2005) The International HIV Dementia Scale: A new rapid screening test for HIV dementia. AIDS 19:1367–1374. [PubMed] [Google Scholar]
- Sakamoto M, Marcotte TD, Umlauf A, Franklin D, Heaton RK, Ellis RJ, Letendre S, Alexander T, McCutchan JA, Morgan EE, Woods SP, Collier AC, Marra CM, Clifford DB, Gelman BB, McArthur JC, Morgello S, Simpson D, Grant I, CHARTER Group (2013) Concurrent classification accuracy of the HIV dementia scale for HIV-associated neurocognitive disorders in the CHARTER cohort. J Acquir Immune Defic Syndr 62: 36–42. doi: 10.1097/QAI.0b013e318278ffa4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzkopf SB, McCoy L, Smith DA, Boutros NN (1993) Test-retest reliability of prepulse inhibition of the acoustic startle response. Biol Psychiatry 34: 896–900. [DOI] [PubMed] [Google Scholar]
- Sinharay S, Lee D, Shah S, Muthusamy S, Papadakis GZ, Zhang X, Maric D, Reid WC, Hammoud DA (2017) Cross-sectional and longitudinal small animal PET shows pre and post-synaptic striatal dopaminergic deficits in an animal model of HIV. Nucl Med Biol 55: 27–33. doi: 10.1016/j.nucmedbio.2017.08.004. 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit M, Binkman K, Geerlings S, Smit C, Thyagarajan K, Sighem AV, de Wolf F, Hallett TB, ATHENA observational cohort (2015) Future challenges for clinical care of an ageing population infected with HIV: A modelling study. Lancet Infect Dis. 15:810–818. doi: 10.1016/S1473-3099(15)00056-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Wakins KE, Toro R, Laird AR, Beckmann CF (2009) Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci USA 106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinacker P, Semler E, Anderl-Straub S, Diehl-Schmid J, Schroeter ML, Uttner I, Foerstl H, Landwehrmeyer B, von Arnim CA, Kassubek J, Oeckl P, Huppertz HJ, Fassbender K, Fliessbach K, Prudlo J, Robmeier C, Kornhuber J, Schneider A, Volk AE, Lauer M, Danek A, Ludolph AC, Otto M, FTLDc Study Group (2017) Neurofilament as a blood marker for diagnosis and monitoring of primary progressive aphasias. Neurology 88: 961–969. doi: 10.1212/WNL.0000000000003688. [DOI] [PubMed] [Google Scholar]
- Steinbrink F, Evers S, Buerke B, Young P, Arendt G, Koutsilieri E, Reichelt D, Lohmann H, Husstedt IW, German Competence Network HIV/AIDS. (2013) Cognitive impairment in HIV infection is associated with MRI and CSF pattern of neurodegeneration. Eur J Neurol 20:420–428. doi: 10.1111/ene.12006. [DOI] [PubMed] [Google Scholar]
- Strimbu K, Tavel JA (2010) What are biomarkers? Curr Opin HIV AIDS 5:463–466. doi: 10.1097/COH.0b013e32833ed177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber MT, Fibiger HC (1995) Electrical stimulation of the prefrontal cortex increases dopamine release in the nucleus accumbens of the rat: Modulation by metabotropic glutamate receptors. J Neurosci 15:3896–3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teeraananchai S, Kerr SJ, Amin J, Ruxrungtham K, Law MG (2017) Life expectancy of HIV-positive people after starting combination antiretroviral therapy: A meta-analysis. HIV Med 18: 256–266. doi: 10.1111/hiv.12421. [DOI] [PubMed] [Google Scholar]
- Thomas JB, Brier MR, Snyder AZ, Vaida FF, Ances BM (2013) Pathways to neurodegeneration: Effects of HIV and aging on resting-state functional connectivity. Neurology 80:1186–1193. doi: 10.1212/WNL.0b013e318288792b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JB, Brier MR, Ortega M, Benzinger TL, Ances BM (2015) Weighted brain networks in disease: Centrality and entropy in human immunodeficiency virus and aging. Neurobiol Aging 36:401–412. doi: 10.1016/j.neurobiolaging.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thulborn KR, Waterton JC, Matthews PM, Radda GK (1982) Oxygenation dependence of the transverse relaxation time of water protons in whole blood at high field. Biochim Biophys Acta 714:265–270. [DOI] [PubMed] [Google Scholar]
- Toledo JB, Shaw LM, Trojanowski JQ (2013) Plasma amyloid beta measurements-a desired but elusive Alzheimer’s disease biomarker. Alzheimers Res Ther 5:8. doi: 10.1186/alzrt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki A, Goto K, Sato N, Iso H, Morita Y (2006) Prepulse inhibition of acoustic startle response in mild cognitive impairment and mild dementia of Alzheimer type. Psychiatry Clin Neurosci 60:55–62. DOI: 10.1111/j.1440-1819.2006.01460.x [DOI] [PubMed] [Google Scholar]
- Ulrich D (2015) Amyloid-β impairs synaptic inhibition via GABA(A) receptor endocytosis. J Neurosci 35:9205–9210. doi: 10.1523/JNEUROSCI.0950-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNAIDS People aged 50 years and older (2014) Available online at: http://www.unaids.org/sites/default/files/media_asset/12_Peopleaged50yearsandolder.pdf
- UNAIDS Fact Sheet (2017) Available online at: http://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf.
- Varga E, Juhász G, Bozsό Z, Penke B, Fϋlop L, Szegedi V (2015) Amyloid-β1–42 disrupts synaptic plasticity by altering glutamate recycling at the synapse. J Alzheimers Dis 45:449–456. doi: 10.3233/JAD-142367. [DOI] [PubMed] [Google Scholar]
- Ventura N, Douw L, Correa DG, Netto TM, Cabral RF, Lopes FCR, Gasparetto EL (2018) Increased posterior cingulate cortex efficiency may predict cognitive impairment in asymptomatic HIV patients. Neuroradiol J 31:372–378. doi: 10.1177/1971400918782327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Pekarskaya O, Bencheikh M, Chao W, Gelbard HA, Ghorpade A, Rothstein JD, Volsky DJ (2003) Reduced expression of glutamate transporter EAAT2 and impaired glutamate transport in human primary astrocytes exposed to HIV-1 or gp120. Virology 312: 60–73. [DOI] [PubMed] [Google Scholar]
- Wang X, Foryt P, Ochs R, Chung J, Wu Y, Parrish T, Ragin AB (2011) Abnormalities in resting-state functional connectivity in early human immunodeficiency virus infection. Brain Connect 1: 207–217. doi: 10.1089/brain.2011.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Li R, Zhou Y, Wang Y, Cui J, Nguchu BA, Qiu B, Wang X, Li H (2018) Altered cerebro-cerebellum resting-state functional connectivity in HIV-infected male patients. J Neurovirol [Epub ahead of print]. doi: 10.1007/s13365-018-0649-x. [DOI] [PubMed] [Google Scholar]
- Wang P, Li J, Wang X, Thapa D, Wu GY (2018) Asymptomatic human immunodeficiency virus vertical transmitted adolescents’ brain functional changes: Based on resting-state functional magnetic resonance imaging. AIDS Res Hum Retroviruses 34:699–704. doi: 10.1089/AID.2017.0267. [DOI] [PubMed] [Google Scholar]
- Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW (1975) A protein factor essential for microtubule assembly. Proc Natl Acad Sci U.S.A.72:1858–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyer SW, Zagrebelsky M, Herrmann U, Hick M, Ganss L, Gobbert J, Gruber M, Altmann C, Korte M, Deller T, Muller UC (2014) Comparative analysis of single and combined APP/APLP knockouts reveals reduced spine density in APP-KO mice that is prevented by APPsα expression. Acta Neuropathol Commun 2:36. doi: 10.1186/2051-5960-2-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO International Programme on Chemical Safety Biomarkers in Risk Assessment: Validity and Validation. 2001. Retrieved from http://www.inchem.org/documents/ehc/ehc/ehc222.htm.
- Woods SP, Morgan EE, Marquie-Beck J, Carey CL, Grant I, Letendre SL, The HIV Neurobehavioral Research Center Group (2006) Markers of macrophage activation and axonal injury are associated with prospective memory in HIV-1 disease. Cogn Behav Neurol 19: 217–221. DOI: 10.1097/01.wnn.0000213916.10514.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Moore DJ, Weber E, Grant I (2009) Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychol Rev 19:152–168. doi: 10.1007/s11065-009-9102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Run X, Liang Z, Li Y, Liu F, Liu Y, Iqbal K, Grundke-Iqbal I, Gong CX (2009) Developmental regulation of tau phosphorylation, tau kinases, and tau phosphatases. J Neurochem 108:1480–1494. doi: 10.1111/j.1471-4159.2009.05882.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan A, Rao MV, Sasaki T, Chen Y, Kumar A, Veeranna, Liem RK, Eyer J, Peterson AC, Julien JP, Nixon RA (2006) Alpha-internexin is structurally and functionally associated with the neurofilament triplet proteins in the mature CNS. J Neurosci 26:10006–10019. doi: 10.1523/JNEUROSCI.2580-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan A, Rao MV, Veeranna, Nixon RA (2012) Neurofilaments at a glance. J Cell Sci 125: 3257–3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Qiao L, Wei F, Yin J, Liu L, Ji Y, Smith D, Li N, Chen D (2013) Cytokines in CSF correlate with HIV-associated neurocognitive disorders in the post-HAART era in China. J Neurovirol 19:144–149. doi: 10.1007/s13365-013-0150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan A, Sershen H, Veeranna, Basavarajappa BS, Kumar A, Hashim A, Berg M, Lee J, Sato Y, Rao MV, Mohan PS, Dyakin V, Julien JP, Lee VM, Nixon RA (2015) Neurofilament subunits are integral components of synapses and modulate neurotransmission and behavior in vivo. Mol Psychiatry 20: 986–994. doi: 10.1038/mp.2015.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan A, Nixon RA (2016) Specialized roles of neurofilament proteins in synapses: Relevance to neuropsychiatric disorders. Brain Res Bull 126:334–346. doi: 10.1016/j.brainresbull.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan A, Veeranna, Sershen H, Basavarajappa BS, Smiley JF, Hashim A, Bleiwas C, Berg M, Guifoyle DN, Subbanna S, Darji S, Kumar A, Rao MV, Wilson DA, Julien J, Javitt DC, Nixon RA (2018) Neurofilament light interaction with GluN1 modulates neurotransmission and schizophrenia-associated behaviors. Transl Psychiatry 8:167. doi: 10.1038/s41398-018-0194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang Y, Jiang T, Lu Y, He Y, Tian L (2004) Regional homogeneity approach to fMRI data analysis. Neuroimage 22:394–400. doi: 10.1016/j.neuroimage.2003.12.030 [DOI] [PubMed] [Google Scholar]
- Zempel H, Thies E, Madelkow E, Mandelkow EM (2010) Abeta oligomers cause localized Ca(2+) elevation, missorting of endogenous Tau into dendrites, Tau phosphorylation, and destruction of microtubules and spines. J Neurosci 30:11938–11950. doi: 10.1523/JNEUROSCI.2357-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipursky AR, Gogolishvili D, Rueda S, Brunetta J, Carvalhal A, McCombe JA, Gill MJ, Rachlis A, Rosenes R, Arbess G, Marcotte T, Rourke SB (2013) Evaluation of brief screening tools for neurocognitive impairment in HIV/AIDS:a systematic review of the literature. AIDS 27: 2385–2401. doi: 10.1097/QAD.0b013e328363bf56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Forkstam C, Engel JA, Svensson L (2000) Role of dopamine in prepulse inhibition of acoustic startle. Psychopharmacology 149:181–188. [DOI] [PubMed] [Google Scholar]
- Zou C, Crux S, Marinesco S, Montagna E, Sgobio C, Shi Y, Shi S, Zhu K, Dorostkar MM, Muller UC, Herms J (2016) Amyloid precursor protein maintains constitutive and adaptive plasticity of dendritic spines in adult brain by regulating D-serine homeostasis. EMBO J 35:2213–2222. doi: 10.15252/embj.201694085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweig MH, Campbell G (1993) Receiver-operating characteristic (ROC) plots: A fundamental evaluation tool in clinical medicine. Clin Chem 39:561–577. [PubMed] [Google Scholar]