Abstract
Background
Acute undifferentiated febrile illness (AUFI) is caused by a multitude of diverse pathogens, with significant morbidity and mortality in the developing world. The objective of this review was to characterise the diversity and relative importance of common infectious aetiologies of AUFI in South and Southeast Asia.
Methods
We conducted a comprehensive literature review to identify common aetiologies of AUFI in Asian countries. Four medical and life sciences databases including PubMed, Medline, Embase and Cochrane Central, and Google Scholar were searched for articles published from January 1998 to March 2019.
Results
Forty-three studies met the inclusion criteria. Among AUFI cases, viral aetiologies at 18.5% (14888) were more common than bacterial aetiologies (12.9% [10384]). From 80,554 cases, dengue fever was the most common aetiology (11.8%, 9511), followed by leptospirosis (4.4%, 3549), typhoid (4.0%, 3258), scrub typhus (4.0%, 3243) and influenza other than H1N1 (3.1%, 2514). In both adults and children: dengue fever was the leading cause of AUFI with 16.6% (1928) and 18.7% (1281) of the total cases. In admitted patients, dengue fever was the main cause of AUFI at 16.4% (2377), however leptospirosis at 13.9% (2090) was the main cause of AUFI for outpatients. In South Asia, dengue fever was the main cause of AUFI, causing 12.0% (6821) of cases, whereas in Southeast Asia, leptospirosis was the main diagnosis, causing 12.1% (2861) of cases.
Conclusions
In this study the most common causes of AUFI were viral, followed by bacterial and protozoal (malaria) infections. Dengue was the commonest virus that caused AUFI while leptospirosis and typhoid were important bacterial infectious causes. Therefore, it is imperative to maintain a sound epidemiological knowledge of AUFI so that evidence-based diagnostic criteria and treatment guidelines can be developed.
Electronic supplementary material
The online version of this article (10.1186/s12879-019-4185-y) contains supplementary material, which is available to authorized users.
Keywords: Acute undifferentiated febrile illness, Asia, Infection
Background
During the past 20 years, there has been a dramatic emergence and re-emergence of viruses, bacteria and parasitic infections, including novel pathogens as well as those previously believed to be under control. Many of these pathogens cause acute undifferentiated febrile illness (AUFI, or acute febrile illness, AFI). The common causes of AUFI include malaria, dengue fever, enteric fever, leptospirosis, rickettsiosis, hantavirus and Japanese encephalitis [1–3]. AUFI contributes to substantial morbidity and death among children and adults worldwide [4, 5]. Many preventable deaths occur because of incorrect or delayed diagnosis, largely due to limited access to medical care and laboratory diagnostic facilities in the developing countries [6–9]. The majority of patients present with non-specific symptoms such as low-grade fever, general malaise, headache, arthralgia, myalgia, and rash; and usually without a focal point of infection. The symptoms and differential diagnoses of these diseases are similar, making accurate clinical diagnosis difficult without laboratory confirmation [10–12].
In recent decades, dengue has rapidly emerged as a major cause of AUFI in tropical Asia particularly in the World Health Organization (WHO) Southeast Asia (SEA) region [13, 14]. However, many other infectious diseases can cause a dengue-like illness with thrombocytopaenia, including scrub typhus, chikungunya, infectious mononucleosis, malaria, typhoid fever, leptospirosis and acute human immuno-deficiency virus conversion disease [15]. Presumptive diagnosis and reporting of AUFI with thrombocytopaenia as dengue infection would lead to over-reporting of this infection and under-reporting of other illnesses.
Evidence-based decision-making in health requires the availability of sound data, but good quality information on the occurrence of infectious diseases is unavailable for most countries in Asia [16]. The provision of accurate epidemiological data for common pathogens will enable identification of changing patterns of disease aetiology and burden, allowing informed priority setting, and optimal allocation of resources to key areas. Understanding the common causes of AUFI in resource-poor settings in tropical and subtropical countries will help improve case management. In areas where there is limited access to laboratory diagnosis, the local epidemiology of AUFI and validated clinical predictors may help guide presumptive diagnosis and therapeutic interventions. Such information is also crucial for developing appropriate diagnostic tests and guidelines, and informing resource mobilization and public health interventions. Therefore, the objective of this review was to synthesise information on the diversity and relative importance of common infectious aetiologies of AUFI in recent history in South and Southeast Asia given it is a melting point of tropical infectious diseases and a hotspot for disease emergence [14, 17, 18].
Methods
Search strategy and inclusion criteria
A systematic literature review was undertaken in four medical and life sciences databases including PubMed, Medline, Embase and Cochrane Central, and Google Scholar search machine was also used. Publications from the last 21 years (January 1998–March 2019) were included because laboratory tests and diseases patterns have changed during recent decades in many parts of South and Southeast Asia. Articles were obtained electronically or in paper form. The search words included: i) aetiology OR etiology OR causes AND ii) acute febrile illnesses OR iii) undifferentiated fevers AND Asia OR Thailand OR Malaysia OR Singapore OR India OR Sri Lanka OR Nepal OR Bangladesh OR Pakistan OR Vietnam OR Laos OR Cambodia OR Indonesia OR Myanmar OR Timor-Leste OR Bhutan OR Maldives OR Philippines. The review included articles published in English only.
We did not limit our search by study design or patient age. Data were derived from studies on inpatients as well as outpatients with AUFI with no focus of infections identified after taking a detailed history and clinical examination. Inclusion criteria were: a) primary articles, published in peer review journals on AFI/AUFI in South Asia (Bhutan, Bangladesh, India, Nepal, and Sri Lanka) and Southeast Asia (Cambodia, Laos, Indonesia, Malaysia, Myanmar, Philippines, Singapore, Thailand, and Vietnam); b) published reports between January 1998 and March 2019 (to improve the reliability of laboratory confirmation and to reflect the distribution of more recent disease patterns) and c) published in English. Exclusion criteria included: a) studies carried out in other parts of Asia (Middle East and central Asia); b) studies conducted before 1998; c) articles such as preliminary reports, and case reports; d) editorials, opinions, review articles, vaccine and drug trials; and e) case reports and fever associated with a travel history (Additional file 1: Table S1). Titles and abstracts were screened for compliance with the inclusion criteria and then full papers were reviewed.
Data analysis
The selection of citations by title and abstract was carried out independently by two researchers (KW and SKK). The selected studies underwent a full-text review for all potentially relevant studies. Data from the 43 included studies were independently extracted in a spreadsheet by KW and SKK. Information from each paper was extracted and entered in to a Microsoft Excel (2010 version) spread sheet. Descriptive data included study location, study period, type of patients (inpatients/ outpatients/ both), age range and duration of fever. Quantitative data recorded included number of patients, pathogens isolated, and common presenting signs and symptoms. Paediatric data were defined as those that included patients younger than 16 years. Studies with non-segregated data for adults and children were analysed separately. Data for pathogens isolated in each study were compiled and analysed in aggregate to compare common aetiologies of AUFI. The proportion of fevers confirmed through laboratory diagnosis in each study were recorded as the main outcome measure.
Risk of bias assessment
The risk of bias (ROB) of the included studies was assessed using a modified checklist used previously [19]. The studies were assessed using eight questions with a possible maximum count of eight safe-guards (Additional file 1: Table S2), with three questions to assess external validity, and five questions for internal validity. We did not assess the ROB for the sampling methodology of populations with acute febrile illness, as these were defined populations presenting to a health facility with acute infection and no population-based sampling was used to capture these populations.
Results
Identification of studies
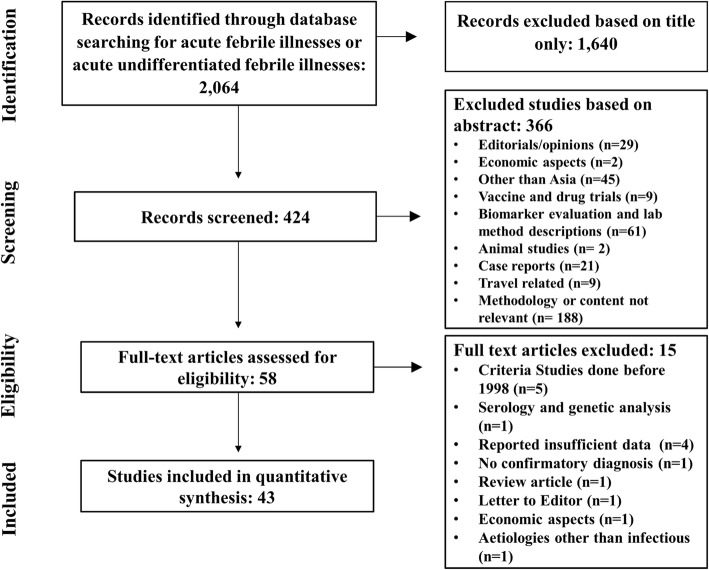
Using the key words in the search, 2064 articles were identified from four life science data base (PubMed, Medline, Web of Science, Embase) and Google Scholar. The titles and abstracts of all studies identified by the search strategy were screened for their relevance to this review and 1640 records that were not relevant for fever with infectious aetiologies were discarded. Four hundred fifteen (424) reports were screened further and from those records, 366 were excluded after reviewing the abstracts as they did not meet the inclusion criteria. All remaining 58 full text articles were reviewed using pre-determined criteria. A full-text review led to the exclusion of a further 15 papers, including five studies with potentially relevant data that were excluded as three of them were carried out in 1991, one study in 1994 and the other study from 1994 to 1999. The remaining 43 studies were from 11 countries in South and Southeast Asia, including a multicentre study, were then analysed (Fig. 1).
Fig. 1.
Study selection
Characteristics of included studies
Twenty-eight studies were from South Asia, of which 20 were from India. In the South East Asia, there were 15 studies and Thailand reported the highest number of studies in the region with nine studies (Fig. 2). There was one multi-centre study carried out in Indonesia, Malaysia, Philippines, Thailand and Vietnam. Most were prospective studies (n = 31) and six studies were retrospective; three were cross-sectional; one cohort study and two active fever surveillance (Table 1).
Fig. 2.
Summary of the studies by countries and regions. The size of the circles indicates the relative number of patients and light colour is the proportion of study subjects with unidentified aetiology. Countries with blue colour are in South East Asia and green colour are in South Asia
Table 1.
Summary of studies included in the analysis
| First author, year and reference | Country | Design study | Study duration | In patients/ outpatients | Number of patients | Age range | Adult/Children only | Sex | Duration of fever (mean duration) |
|---|---|---|---|---|---|---|---|---|---|
| Abhilash et al., 2016 [20] | India | Prospective observational study | 12 months | OPD + ED | 1258 | > 15 years | Adults + children |
M = 680 F = 568 |
3–14 days |
| Ahmad et al., 2016 [44] | India | Retrospective observational study | 12 months | IP | 298 | > 2 years | Adults + children | Not specified | AUFI |
| Andrews et al., 2014 [45] | India | Retrospective and Patient admitted prospective study | 2 months | IP | 369 | > 13 years | Adults |
F = 118 M = 251 |
AUFI |
| Arora et al., 2014 [46] | India | Retrospective study | 24 months | IP + OPD | 38,635 | All ages groups | Adult + Children | Not specified | AUF |
| Capeding et al., 2013 [11] | Indonesia, Malaysia, Philippines, Thailand and Vietnam | Active fever surveillance, cohort study | 9.8 months | Community based | 289 | 2–14 years | Children | Not specified | < 14 days |
| Chheng et al., 2013 [42] | Cambodia | Prospective study | 12 months | IP | 1225 | < 16 years | Children |
M = 668 F = 557 |
< 28 days |
| Chikkaveerariah et al., 2016 [47] | India | Prospective observational study | 24 months | IP | 150 | > 14 years | Adults |
F = 69 M = 81 |
AUFI |
| Chrispal et al., 2010 [1] | India | Prospective observational study | 12 months | IP | 398 |
> 16 years Mean 39.5 |
Adults |
M = 239 F = 159 |
5–21 days |
| Das et al., 2015 [48] | India | Cross-sectional study | 6 months | IP | 205 | All ages | Adults + Children |
F = 89 M = 116 |
Acute febrile illness |
| Ellis et al., 2006 [49] | Thailand | Prospective study | 33 months | IP + OPD | 613 | 20–87 years | Adults |
M = 325 F = 288 |
Fever over previous 48 h and fever longer than 48 h cause of fever not yet known |
| Gopalakrishnan et al., 2013 [21] | India | Prospective observational | 18 months | IP | 403 | > 16 years | Adults |
M = 264 F = 139 |
5–14 days |
| Joshi et al., 2008 [22] | India | Retrospective review of electronic discharge summaries | 6 months | IP | 1197 | > 12 years | Adults |
M = 640 F = 557 |
< 14 days |
| Kammili et al., 2013 [23] | India | Prospective descriptive hospital based study | 2 months | IP | 100 | All ages | Both children and adults | Not specified | > 24 h |
| Kashinkunti et al., 2013 [37] | India | Prospective observational | Not specified | IP | 100 | > 16 years | Adults |
M = 58 F = 42 |
< 15 days |
| Kasper et al., 2012 [24] | Cambodia | Fever surveillance study | Not specified | OPD | 9997 |
> 2 years Mean 19.6 Median 16.9 |
Adults and children |
M = 5398 F = 4599 |
< 10 days |
| Kumar et al., 2008 [39] | India | Prospective study | 12 months | OPD | 298 | 6 months −12 years | Children |
M = 117 F = 181 |
< 15 days |
| Laoprasopwattana et al., 2012 [25] | Thailand | Prospective cohort study | 4 months | IP + OPD | 50 | 1 month −15 years | Children | Not specified | < 7 days |
| Leelarasamee et at., 2004 [2] | Thailand | Prospective epidemiological study | 36 months | OPD | 1137 | > 2 years | Adults + Children | Not specified | < 1 day |
| Mayxay et al., 2013 [26] | Laos | Prospective study | 30 months | IP + OPD | 1938 |
5–49 median 19 |
Adults/children |
M = 1124 F = 814 |
< 8 days |
| McGready et al., 2010 [50] | Thailand | Prospective cohort study | 28 months | IP | 409 | > 15 years | Pregnant females only |
M = 467 F = 409 |
Any fever |
| Mittal et al., 2015 [51] | India | Retrospective observational study | 12 months | IP + OPD | 2547 | > 18 years | Adults |
F = 884 M = 1663 |
AUFI |
| Murdoch et al., 2004 [36] | Nepal | Prospective study | 3 months | IP + OPD | 876 |
> 14 years Median 27 |
Adults | F = 409 | 24 h |
| Oishr et al., 2006 [27] | Philippines | Prospective study | 24 months | IP | 503 | 2–17 years | Children |
M = 298 F = 205 |
< 5 days |
| Phuong et al., 2006 [28] | Vietnam | Prospective study | 12 months | OPD | 2096 | All ages | Both children and adults |
M = 1229 F = 865 |
< 14 days |
| Pradutkanchana et al., 2003 [7] | Thailand | Prospective study | 1 month | IP | 180 | Less than 15 years | Children | Not specified | < 21 days |
| Punjabi et al., 2012 [43] | Indonesia | Prospective study | 27 months | IP | 226 | 1–80 years | Adults and children |
M = 127 F = 99 |
1–30 days |
| Rafizah et al., 2012 [52] | Malaysia | Hospital-based cross sectional study | 6 months | IP | 999 | > 18 years | Adults |
F = 543 M = 456 |
Acute fever |
| Rani et al., 2016 [53] | India | Retrospective study | 6 Months | IP + OPD | 200 | All ages | Adults + children |
F = 82 M = 118 |
Acute febrile illness |
| Ray et al., 2012 [29] | India | Prospective descriptive study | 12 months | IP + OPD | 540 | All age groups | Both children and adults |
M = 329 F = 211 |
< 7 days |
| Reller et al., 2011 [31] | Sri Lanka | Prospective study | 8 months | IP + OPD | 773 | > 2 years | Both children and adults |
M = 463 F = 310 |
< 7 days |
| Reller et al., 2012 [30] | Sri Lanka | Prospective study | 8 months | IP + OPD | 859 | > 2 years | Both children and adults |
M = 526 F = 333 |
< 7 days |
| Sabchareon et al., 2012 [54] | Thailand | Prospective cohort study | 48 months | Community based + IP + OPD | 3401 | 3–15 years | Children |
M = 1733 F = 1668 |
All documented fever with school absenteeism |
| Suttinont et al., 2006 [12] | Thailand | Prospective observational study in 5 hospitals | 12 months | IP | 845 |
> 15 years Median 38 |
Adults |
M = 661 F = 184 |
< 15 days |
| Thompson et al., 2015 [56] | Nepal | Prospective study | 38 months | IP + OPD | 627 | > 2 years | Adults + children | Not specified | UFI |
| Zaki et al., 2010 [41] | India | Prospective observational study | 4 months | IP | 602 | 1 month −12 years | Children | Not specified | < 21 days |
| Kingston et al., 2018 [38] | Bangladesh | Prospective study | 12 months | IP | 416 | ≥12 years | Adults | Not specified | < 21 days |
| Raina et al., 2018 [32] | India | Cohort study | 2 months | IP | 1164 | > 18 years | Adults | Not specified | ≤14 days |
| Shelke et al., 2017 [33] | India | Prospective cross-sectional | 18 months | IP | 270 | All ages | Adults + children |
M = 138 F = 132 |
< 14 days |
| Gautam et al., 2019 [57] | Nepal | Cross-sectional study | 12 months | IP | 1585 | > 1 year | Adult + children |
M = 728 F = 757 |
> 4 days |
| Bodinayake et al., 2018 [35] | Sri Lanka | Prospective study | 12 months | IP | 976 | ≥1 years | Adults + children |
M = 628 F = 348 |
≤3 days |
| Salagre et al., 2017 [55] | India | Prospective observational study | 2 months | IP | 276 | > 13 years | Adults |
M = 187 F = 89 |
AFI |
| Andrews et al., 2018 [34] | India | Prospective observational study | 12 months | IP | 1324 | > 13 years | Adults |
M = 837 F = 487 |
< 14 days |
| Wangrangsimakul et al., 2018 [40] | Thailand | Prospective observational study | 27 months | IP | 200 | ≥15 years | Adults |
M = 114 F = 86 |
< 21 days |
ED Emergency department, IP Inpatients, OPD Outpatient department, M Males, F Females
Definition of fever duration in the acute febrile illnesses described in these studies varied widely, from 1 to 30 days. Of the 43 included studies, 19 included duration of fever of < 14 days [2, 11, 20–36], seven studies had fever duration of < 21 days [7, 12, 37–41], three studies reported fever with the duration of < 30 days [1, 42, 43], 13 studies did not define any specific duration [44–56] and one study recruited patients with acute fever of more than 4 days [57]. Similarly, the temperature threshold used to define fever varied from 37.5–38.5°Celsius. Sixteen studies included adult (> 16 years) patients [1, 12, 21, 22, 32, 36–38, 40, 45, 47, 49–52, 55], eight studies included children (< 16 years) [7, 11, 25, 27, 39, 41, 42, 54] and 19 studies included patients of all ages [2, 20, 23, 24, 26, 28–31, 33–35, 43, 44, 46, 48, 53, 56, 57]. The number of patients involved in each study varied from 50 to 38,635. Data on a total of 80,554 patients were included in the analysis of which 14.4% (11706) were adults, 8.5% (7840) were children and 77.1% (62068) were not specified either as adults or children and 39 cases could not be assigned to any age groups [35]. Among adults, mean age varied from 27 to 39.5 years and among children from 2 to 9.5 years. There were 19,030 males, 14,625 females (with a male to female ratio of 1.3:1), and gender was not reported for 46,899 patients.
Twenty-five studies included patients admitted to hospital [1, 7, 12, 21–23, 27, 32–35, 37, 38, 40–45, 47, 48, 50, 52, 55, 57] making up 18.7% (14420) of the total study sample (80554). Six studies included patients attending outpatients department corresponding to 18.7% (15075) of study sample [2, 11, 20, 24, 28, 39]. Twelve studies included patients attending outpatient departments (OPD) and those admitted to hospital wards representing 63.4% (51059) of total study sample [25, 26, 29–31, 36, 46, 49, 51, 53, 54, 56]. Common presenting symptoms were given in 24 studies, corresponding to 30,397 patients. Amongst these 23 studies, the most common presenting symptom was headache 39.7% (12072) followed by cough 29.7% (9035) and chills 20.5% (6241) (Additional file 1: Table S3).
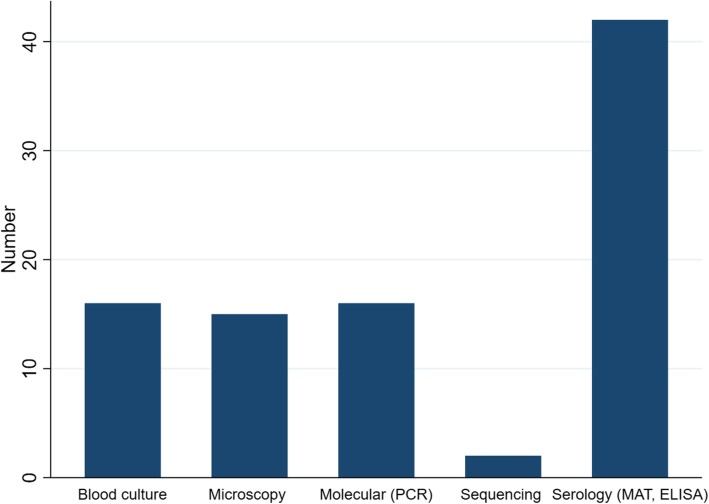
In all studies excepting one [34], diagnoses were made according to interpretation of antibody titres. Pathogen-specific IgM titres were determined by using IgM-capture enzyme-linked immunosorbent assay (ELISA) kits, which are commercially available. Molecular testing (using polymerase chain reaction [PCR] was carried out in 16 studies [24–27, 29, 30, 35, 38–40, 42, 49, 50, 54–56]. Serological diagnoses were confirmed by blood cultures in 16 studies out of 43 [2, 20–22, 24, 26, 32, 37, 40, 42, 43, 45, 47, 48, 50, 51]. Microscopy was used for the diagnosis in 15 studies [20–22, 24, 26, 32, 37, 43, 44, 46, 47, 49–51, 55]. Nucleotide sequencing was done in two studies [29, 35] (Fig. 3 and Additional file 1: Table S4).
Fig. 3.
Summary of different diagnostic methods. (MAT- microscopic agglutination test; ELISA- enzyme-linked immunosorbent assay; PCR- polymerase chain reaction)
Aetiology of AUFI was identified in 37.7% (30333) of patients: with viral aetiologies in 18.5% (14888) being the most common, followed by bacterial and protozoal aetiologies with 12.9% (10384), and 2.8% (2281) respectively. The underlying diagnosis could not be ascertained in 64.6% of patients (52003). Twenty studies reported 378 deaths in patients with AUFI [1, 2, 20–22, 25–27, 31, 34, 36, 38, 41–45, 48–50]. Co-infections were reported in 1.2% (981) of total cases, the most common co-infections with two organisms 0.9% (740).
Aetiology of AUFI by age group
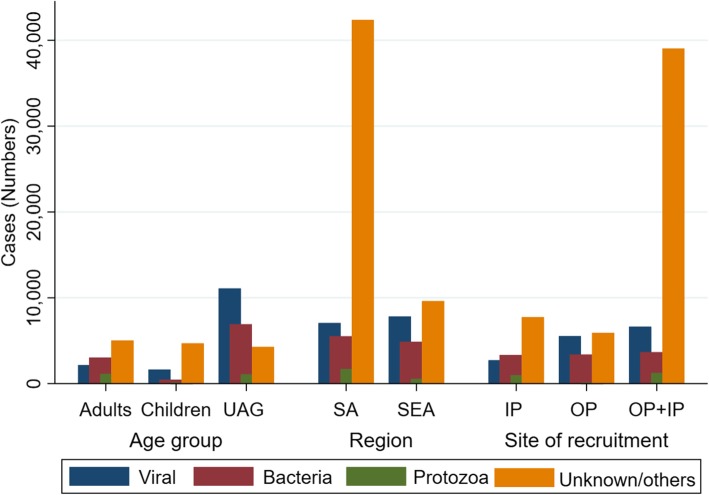
In adults, the commonest infection was from bacterial causes at 26.1% (3037), followed by viral aetiologies 18.6% (2169). The most common aetiologies of AUFI in adults were dengue fever 16.6% (1928), scrub typhus 10.7% (1244), malaria 9.8% (1139), leptospirosis 6.3% (732) and typhoid 6.0% (696). On the other hand, viral infection was the commonest cause of fever among children corresponding to 23.8% (1625) of the diagnosed cases, followed by bacterial aetiologies and malaria (corresponding to 6.4% (435) and 0.8% (57) of diagnosed cases, respectively). Dengue fever, chikungunya, and typhoid were the commonest cause of AUFI in children representing 18.7% (1281), 1.7% (114), and 1.6% (107) respectively. In the unspecified age group (UAG), dengue fever was the commonest cause of AUFI with 10.2% (6302) of total cases; leptospirosis was the second commonest cause with 4.4% (2729); typhoid and malaria contributed 4.0% (2455) and 1.7% (1085) of total cases (Table 2 and Fig. 4).
Table 2.
Common aetiologies of AUFI stratified by age
| Organism | Adults (n; %) | Children (n; %) | UAG (n; %) | Total⁑ (n; %) |
|---|---|---|---|---|
| Viral aetiologies | 2169 (18.6) | 1625 (23.8) | 11,094 (17.9) | 14,888 (18.5) |
| Dengue | 1928 (16.6) | 1281 (18.7) | 6302 (10.2) | 9511 (11.8) |
| JE*** | 5 (0.0) | 71 (1.0) | 233 (0.4) | 309 (0.4) |
| Influenza** | 180 (1.5) | 48 (0.7) | 2286 (3.7) | 2514 (3.1) |
| H1N1 | 5 (0.0) | 1 (0.0) | 513 (0.8) | 519 (0.6) |
| Chikungunya | 15 (0.1) | 114 (1.7) | 326 (0.5) | 455 (0.6) |
| Hepatitis A | 8 (0.1) | 7 (0.1) | 58 (0.1) | 73 (0.1) |
| Hepatitis B | 5 (0.0) | 0 (0.0) | 267 (0.4) | 272 (0.3) |
| Hepatitis E | 2 (0.0) | 0 (0.0) | 1038 (1.7) | 1040 (1.3) |
| Flavi virus | 0 (0.0) | 65 (1.0) | 0 (0.0) | 65 (0.1) |
| Para influenza 1 | 0 (0.0) | 10 (0.1) | 0 (0.0) | 10 (0.0) |
| Para influenza 3 | 0 (0.0) | 28 (0.4) | 0 (0.0) | 28 (0.0) |
| Hanta virus | 2 (0.0) | 0 (0.0) | 71 (0.1) | 73 (0.1) |
| HIV | 19 (0.2) | 0 (0.0) | 0 (0.0) | 19 (0.0) |
| Bacterial aetiologies | 3037 (26.1) | 435 (6.4) | 6912 (11.1) | 10,384 (12.9) |
| Leptospirosis | 732 (6.3) | 88 (1.3) | 2729 (4.4) | 3549 (4.4) |
| Typhoid | 696 (6.0) | 107 (1.6) | 2455 (4.0) | 3258 (4.0) |
| Paratyphoid | 57 (0.5) | 0 (0.0) | 0 (0.0) | 57 (0.1) |
| Rickettsiosis diseases | 1449 (12.5) | 140 (2.0) | 1654 (2.7) | 3243 (4.0) |
| Scrub typhus | 1244 (10.7) | 103 (1.5) | 1512 (2.4) | 2859 (3.5) |
| Murine typhus | 171 (1.5) | 0 (0.0) | 101 (0.2) | 272 (0.3) |
| Spotted fever | 34 (0.3) | 37 (0.5) | 41 (0.1) | 112 (0.1) |
| Q fever | 7 (0.1) | 0 (0.0) | 0 (0.0) | 7 (0.0) |
| E coli | 11 (0.1) | 21 (0.3) | 26 (0.0) | 58 (0.1) |
| Burkholderia pseudomallei | 3 (0.0) | 14 (0.2) | 6 (0.0) | 23 (0.0) |
| Tuberculosis | 29 (0.2) | 6 (0.1) | 8 (0.0) | 43 (0.1) |
| Klebsiella pneumoniae | 1 (0.0) | 0 (0.0) | 2 (0.0) | 3 (0.0) |
| Haemophilus influenza | 0 (0.0) | 0 (0.0) | 9 (0.0) | 9 (0.0) |
| Staph aureus | 0 (0.0) | 37 (0.5) | 12 (0.0) | 49 (0.1) |
| Strep pneumoniae | 51 (0.4) | 18 (0.3) | 6 (0.0) | 75 (0.1) |
| Strep Gr A | 0 (0.0) | 0 (0.0) | 2 (0.0) | 2 (0.0) |
| Strep Gr C | 0 (0.0) | 0 (0.0) | 1 (0.0) | 1 (0.0) |
| Neisseria meningitis | 1 (0.0) | 4 (0.1) | 2 (0.0) | 7 (0.0) |
| Protozoa | 1139 (9.8) | 57 (0.8) | 1085 (1.7) | 2281 (2.8) |
| Malaria | 1139 (9.8) | 57 (0.8) | 1085 (1.7) | 2281 (2.8) |
| Fungal aetiologies | 0 (0.0) | 0 (0.0) | 3 (0.0) | 3 (0.0) |
| Yeast non-Cryptococci | 0 (0.0) | 0 (0.0) | 2 (0.0) | 2 (0.0) |
| Cryptococcus neoformans | 0 (0.0) | 0 (0.0) | 1 (0.0) | 1 (0.0) |
| Co infections | 251 (2.2) | 30 (0.4) | 700 (1.1) | 981 (1.2) |
| Co infection* | 226 (1.9) | 30 (0.4) | 484 (0.8) | 740 (0.9) |
| Co infection† | 25 (0.2) | 0 (0.0) | 210 (0.3) | 235 (0.3) |
| Co infection‡ | 0 (0.0) | 0 (0.0) | 6 (0.0) | 6 (0.0) |
| Unknown/others | 5036 (43.3) | 4693 (68.6) | 42,274 (68.1) | 52,003 (64.6) |
| Deaths | 125 (33.1) | 81 (21.4) | 172 (45.5) | 378 (100.0) |
UAG Unknown age group, ***JE Japanese B Encephalitis; ⁑39 cases in the manuscript could not be assigned to any age group; **influenza other than H1N1; *co-infection with two organisms; †co-infection with three organisms; ‡co-infection with more than three organisms
The bold face shows the cumulative number of the stratified groups
Fig. 4.
Summary graph of main categories of AUFI across age group, region and site of patient recruitment in Asia. (UAG- unknown age group, IP-inpatient; OP-outpatient; SEA-Southeast Asia, SA- South Asia)
Aetiology of AUFI by site of patient recruitment
Among the 14,450 hospitalised patients, bacterial infection 23.1% (3340) was the leading cause of fever. However, the most common aetiology of AUFI was dengue fever 16.4% (2377), followed by scrub typhus 10.0% (1449), malaria 6.9% (990), and leptospirosis 6.8% (989). A total of 7053 representing 48.8% did not have a known diagnosis. Even though viral infections (36.7%, 5536) were the main cause for fever in outpatients, leptospirosis 13.9% (2090) was the commonest cause of AUFI followed by influenza other than HINI 13.8% (2077), dengue 8.5% (1277), and hepatitis E 6.9% (1038). Dengue was the commonest infection in patients recruited from both IP and OPD 11.5% (5857), followed by typhoid 3.8% (1940), malaria 2.4% (1234), scrub typhus 2.3% (1165), and leptospirosis 0.9% (470) respectively (Table 3 and Fig. 4).
Table 3.
Aetiology of AUFI by site of patient recruitment
| Organism | IP (n; %) | OP (n; %) | OP+IP (n; %) | Total (n; %) |
|---|---|---|---|---|
| Viral aetiologies | 2721 (18.8) | 5536 (36.7) | 6631 (13.0) | 14,888 (18.5) |
| Dengue | 2377 (16.4) | 1277 (8.5) | 5857 (11.5) | 9511 (11.8) |
| JE*** | 77 (0.5) | 7 (0.0) | 225 (0.4) | 309 (0.4) |
| Influenza** | 86 (0.6) | 2077 (13.8) | 351 (0.7) | 2514 (3.1) |
| HINI | 6 (0.0) | 513 (3.4) | 0 (0.0) | 519 (0.6) |
| Chikungunya | 54 (0.4) | 224 (1.5) | 177 (0.3) | 455 (0.6) |
| Hepatitis A | 0 (0.0) | 62 (0.4) | 11 (0.0) | 73 (0.1) |
| Hepatitis B | 4 (0.0) | 267 (1.8) | 1 (0.0) | 272 (0.3) |
| Hepatitis E | 2 (0.0) | 1038 (6.9) | 0 (0.0) | 1040 (1.3) |
| Flavi virus | 65 (0.4) | 0 (0.0) | 0 (0.0) | 65 (0.1) |
| Para influenza 1 | 10 (0.1) | 0 (0.0) | 0 (0.0) | 10 (0.0) |
| Para influenza 3 | 28 (0.2) | 0 (0.0) | 0 (0.0) | 28 (0.0) |
| Hanta virus | 1 (0.0) | 71 (0.5) | 1 (0.0) | 73 (0.1) |
| HIV | 11 (0.1) | 0 (0.0) | 8 (0.0) | 19 (0.0) |
| Bacterial aetiologies | 3340 (23.1) | 3383 (22.4) | 3662 (7.2) | 10,385 (12.9) |
| Leptospirosis | 989 (6.8) | 2090 (13.9) | 470 (0.9) | 3549 (4.4) |
| Typhoid | 406 (2.8) | 912 (6.0) | 1940 (3.8) | 3258 (4.0) |
| Paratyphoid | 57 (0.4) | 0 (0.0) | 0 (0.0) | 57 (0.1) |
| Rickettsial diseases | 1670 (11.6) | 341 (2.3) | 1232 (2.4) | 3243 (4.0) |
| Scrub typhus | 1449 (10.0) | 245 (1.6) | 1165 (2.3) | 2859 (3.5) |
| Murine typhus | 178 (1.2) | 65 (0.4) | 29 (0.1) | 272 (0.3) |
| Spotted fever | 43 (0.3) | 31 (0.2) | 38 (0.1) | 112 (0.1) |
| Q fever | 7 (0.0) | 0 (0.0) | 0 (0.0) | 7 (0.0) |
| E coli | 29 (0.2) | 25 (0.2) | 4 (0.0) | 58 (0.1) |
| Burkholderia pseudomallei | 17 (0.1) | 3 (0.0) | 3 (0.0) | 23 (0.0) |
| Tuberculosis | 36 (0.2) | 0 (0.0) | 7 (0.0) | 43 (0.1) |
| Klebsiella pneumonia | 2 (0.0) | 0 (0.0) | 2 (0.0) | 4 (0.0) |
| Haemophilus influenza | 9 (0.1) | 0 (0.0) | 0 (0.0) | 9 (0.0) |
| Staph aureus | 39 (0.3) | 8 (0.1) | 2 (0.0) | 49 (0.1) |
| Strep pneumonia | 73 (0.5) | 2 (0.0) | 0 (0.0) | 75 (0.1) |
| Strep Gr A | 1 (0.0) | 0 (0.0) | 1 (0.0) | 2 (0.0) |
| Strep Gr C | 0 (0.0) | 0 (0.0) | 1 (0.0) | 1 (0.0) |
| Neisseria meningitis | 5 (0.0) | 2 (0.0) | 0 (0.0) | 7 (0.0) |
| Protozoa | 990 (6.9) | 57 (0.4) | 1234 (2.4) | 2281 (2.8) |
| Malaria | 990 (6.9) | 57 (0.4) | 1234 (2.4) | 2281 (2.8) |
| Fungal aetiologies | 3 (0.0) | 0 (0.0) | 0 (0.0) | 3 (0.0) |
| Yeast non-Cryptococci | 2 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.0) |
| Cryptococcus neoformans | 1 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.0) |
| Co infections | 343 (2.4) | 176 (1.2) | 496 (1.0) | 1015 (1.3) |
| Co infection* | 331 (2.3) | 169 (1.1) | 270 (0.5) | 770 (1.0) |
| Co infection† | 12 (0.1) | 7 (0.0) | 220 (0.4) | 239 (0.3) |
| Co infection‡ | 0 (0.0) | 0 (0.0) | 6 (0.0) | 6 (0.0) |
| Unknown | 7053 (48.8) | 5923 (39.3) | 39,036 (76.5) | 52,012 (64.5) |
| Deaths | 270 (71.4) | 30 (7.9) | 78 (20.6) | 378 (100.0) |
IP Inpatients, OP Outpatients; ***JE- Japanese B Encephalitis, HAV Hepatitis A virus; HBV Hepatitis E virus, HEV Hepatitis E virus,
**Influenza other than H1N1; *co-infection with two organisms; †co-infection with three organisms; ‡co-infection with more than three organisms
The bold face shows the cumulative number of the stratified groups
Aetiology of AUFI by region
In both the regions, viral aetiologies were the leading cause of AUFI with 33.0% (7828) and 12.4% (7060) for SEA and South Asia, respectively. However, there was significant differences in the burden of AUFI when stratified by individual aetiologies. In South Asia, the commonest cause of fever was dengue fever 12.0% (6821) followed by typhoid 4.3% (2449), and malaria 3.0% (1722). While Leptospirosis was the leading infection 12.1% (2861) in SEA followed by dengue fever 11.4% (2690), influenza other than H1N1 10.6% (2511), and hepatitis E 4.4% (1038) (Table 4 and Fig. 4).
Table 4.
Aetiology by region (Southeast Asia and South Asia)
| Organism | SEA (n; %) | South Asia (n; %) | Total (n; %) |
|---|---|---|---|
| Viral aetiologies | 7828 (33.0) | 7060 (12.4) | 14,888 (18.5) |
| Dengue | 2690 (11.4) | 6821 (12.0) | 9511 (11.8) |
| JE*** | 309 (1.3) | 0 (0.0) | 309 (0.4) |
| Influenza** | 2511 (10.6) | 3 (0.0) | 2514 (3.1) |
| HINI | 514 (2.2) | 5 (0.0) | 519 (0.6) |
| Chikungunya | 256 (1.1) | 199 (0.3) | 455 (0.6) |
| Hepatitis A | 62 (0.3) | 11 (0.0) | 73 (0.1) |
| Hepatitis B | 267 (1.1) | 5 (0.0) | 272 (0.3) |
| Hepatitis E | 1038 (4.4) | 2 (0.0) | 1040 (1.3) |
| Flavi virus | 65 (0.3) | 0 (0.0) | 65 (0.1) |
| Para influenza 1 | 10 (0.0) | 0 (0.0) | 10 (0.0) |
| Para influenza 3 | 28 (0.1) | 0 (0.0) | 28 (0.0) |
| Hanta virus | 71 (0.3) | 2 (0.0) | 73 (0.1) |
| HIV | 7 (0.0) | 12 (0.0) | 19 (0.0) |
| Bacterial aetiologies | 4873 (20.6) | 5512 (9.7) | 10,385 (12.9) |
| Leptospirosis | 2861 (12.1) | 688 (1.2) | 3549 (4.4) |
| Typhoid | 809 (3.4) | 2449 (4.3) | 3258 (4.0) |
| Paratyphoid | 0 (0.0) | 57 (0.1) | 57 (0.1) |
| Rickettsial diseases | 1009 (4.3) | 2234 (3.9) | 3243 (4.0) |
| Scrub typhus | 764 (3.2) | 2095 (3.7) | 2859 (3.5) |
| Murine typhus | 146 (0.6) | 126 (0.2) | 272 (0.3) |
| Spotted fever | 99 (0.4) | 13 (0.0) | 112 (0.1) |
| Q fever | 7 (0.0) | 0 (0.0) | 7 (0.0) |
| E coli | 49 (0.2) | 9 (0.0) | 58 (0.1) |
| Burkholderia pseudomallei | 23 (0.1) | 0 (0.0) | 23 (0.0) |
| Tuberculosis | 21 (0.1) | 22 (0.0) | 43 (0.1) |
| Klebsiella pneumoniae | 3 (0.0) | 1 (0.0) | 4 (0.0) |
| Haemophilus influenza | 9 (0.0) | 0 (0.0) | 9 (0.0) |
| Staph aureus | 49 (0.2) | 0 (0.0) | 49 (0.1) |
| Strep pneumoniae | 24 (0.1) | 51 (0.1) | 75 (0.1) |
| Strep Gr A | 2 (0.0) | 0 (0.0) | 2 (0.0) |
| Strep Gr C | 1 (0.0) | 0 (0.0) | 1 (0.0) |
| Neisseria meningitides | 6 (0.0) | 1 (0.0) | 7 (0.0) |
| Protozoa | 559 (2.4) | 1722 (3.0) | 2281 (2.8) |
| Malaria | 559 (2.4) | 1722 (3.0) | 2281 (2.8) |
| Fungal aetiologies | 3 (0.0) | 0 (0.0) | 3 (0.0) |
| Yeast non Cryptococci | 2 (0.0) | 0 (0.0) | 2 (0.0) |
| Cryptococcus neoformans | 1 (0.0) | 0 (0.0) | 1 (0.0) |
| Co infections | 815 (3.4) | 196 (0.3) | 1011 (1.3) |
| Co infection* | 592 (2.5) | 178 (0.3) | 770 (1.0) |
| Co infection† | 217 (0.9) | 18 (0.0) | 235 (0.3) |
| Co infection‡ | 6 (0.0) | 0 (0.0) | 6 (0.0) |
| Unknown | 9621 (40.6) | 42,389 (74.5) | 52,010 (64.5) |
| Deaths | 114 (30.2) | 264 (69.8) | 378 (100.0) |
South Asian countries included: India, Bhutan, Bangladesh, Sri Lanka, and Nepal
Southeast Asian (SEA) countries included: Thailand, Indonesia, Malaysia, Laos, Philippines, Cambodia and Vietnam ***JE- Japanese B encephalitis;
**influenza other than H1N1; *co-infection with two organisms; †co-infection with three organisms; ‡co-infection with more than three organisms
The bold face shows the cumulative number of the stratified groups
Case fatalities
A total of 378 deaths were reported across 20 studies corresponding to a case fatality rate (CFR) of 0.5%. There were 114 deaths in the SEA region with a CFR of 0.5%. In South Asia, the CFR was 0.5% with 264 deaths. More than half (172) of the deaths were in patents whose age was unknown, with a case fatality of 0.3%, followed by children with 81 deaths (CFR of 1.2%), and adults with 112 deaths (CFR 1.3%). Most of the deaths occurred in hospitalised patients 270 (CFR 1.9%) followed by both inpatients and outpatients 78 (CFR 0.2%).
Risk of bias
The quality of the studies including types of study, randomization and other characteristics was assessed through eight safeguards against bias as outlined in the Additional file 1: Table S2. The ranges of score were 4–8. The most common safeguard missing was study’s target population. Only 15 studies recruited patients of all ages presenting with AUFI. The other studies restricted study population either to children or adults. All studies had study instrument that had validity and reliability (Additional file 1: Table S5).
Discussion
The findings of this review illustrate that in tropical and subtropical South and Southeast Asian countries, the most common causes of AUFI were viruses, followed by bacteria and malaria. Generally, dengue fever was the commonest cause followed by leptospirosis and typhoid. Consistent with our findings, the decline in malaria cases in Asia and Africa has resulted in a relative increase in non-malarial AUFIs in these continents [58]. Non-malarial fever was responsible for 20–50% of all fevers in Asia and Africa in children over 5 years of age and adults [59]. While dengue was mostly frequently reported febrile illness in Latin America [60].
Leptospirosis was the leading cause of AUFI in the Southeast region similar to other reported studies from that region [61–65], in agricultural workers [66, 67] and mostly in males [68]. The ability of all countries in the region to accurately report and monitor leptospirosis hinges strongly on their respective capacity to provide accurate and reliable laboratory diagnosis, and robust reporting and surveillance systems [69, 70]. While the microscopic agglutination test (MAT) is considered to be the gold standard serological test [71], there are limitations to the test including a need for live cultures of Leptospira of different serogroups, cross-reactions between serogroups and serovars, poor sensitivity in the first week of illness, and persistence of high titres for many years after an infection. Conversely, treatment with antibiotics can blunt the immune response in leptospirosis, reducing the number of cases detectable by serology [72]. Hence, the number of leptospirosis patients reported in this review could be under or overestimated.
Dengue was the commonest cause of AUFI in South Asia contrary to Southeast Asia. It is generally a childhood disease and our results are consistent with that trend because it was the commonest cause of AUFI among children [73–75]. In the past, dengue cases were mostly hospitalized irrespective of the severity of the disease. However, with the new admission criteria which includes clinical, laboratory, and dengue haemorrhage fever (DHF) predictive parameters [76], only severe cases of dengue: DHF and dengue shock syndrome (DSS) are admitted. The admission criteria were not clear in our study since most of the cases were from both OPD and IPD.
This review confirms that influenza is also an important cause of AUFI in the region, being the fourth commonest cause. Persistence of influenza virus especially in Southeast Asia is thought to be mediated by domestic ducks and large live poultry markets acting as a virus reservoir [77, 78]. Seasonal influenza is a highly transmissible, abrupt, and usually a self-limiting febrile infection of the respiratory tract and the majority of patients would present to outpatient departments [79, 80]. In many countries, the disease burden from influenza is underestimated because many cases are undiagnosed.
Typhoid fever was also identified as one of the major causes of AUFI. Previous reports have indicated that children are most at risk of developing typhoid fever [81]. The disease remains an important public health problem in developing countries. Similarly, rickettsial diseases including scrub typhus (Orientia tsutsugamushi) and murine typhus (Rickettsia typhi) were responsible for a small fraction of AUFI in this review. However, it is important to note that rickettsial diseases are an important cause of febrile illness worldwide but are often undiagnosed, sometimes leading to life-threatening conditions [82–85]. Given rickettsial infections are treatable causes of AUFI, greater recognition of scrub typhus and murine typhus is important to increase the index of suspicion amongst the physicians so that cases are not missed.
Protozoal infection particularly malaria was responsible for 3.7% of all AUFI cases. This figure is likely to have been an underestimate because four studies excluded malaria patients in their analysis as their inclusion criteria were non-malarial patients. Of the 11 member countries in the WHO SEA Region, 10 are endemic for malaria. Six countries (Bhutan, Democratic People’s Republic of Korea, Indonesia, Nepal, Sri Lanka, and Thailand) are aiming for malaria elimination as a longer-term goal [86], and Sri Lanka has already eliminated malaria [87].
We found that 1.0% of AUFIs were associated with co-infections, the majority being in inpatients. Patients not responding to treatment for a particular infection or those in whom the presentation was atypical or severe should be suspected of harbouring a second infectious agent. The possibility of co-infections of leptospirosis with hepatitis E virus (HEV) [88] has been described as water is the vehicle of transmission for both pathogens. The under-diagnosis of mixed infections is very likely due to the overlapping clinical spectrum [89]. The relatively high morbidity and mortality in mixed infections underscores the need for greater awareness of the possibility of mixed infections as well as the need for optimal use of microbiological laboratory services to reach a specific diagnosis [88].
Causes of fever remained unknown in more than half of patients with AUFI in this review. Similar findings have been reported in other studies, including a review of AUFI in South Asian countries [90]. A lack of an established diagnosis could be partly due to the fact that laboratory confirmations were not done in many studies of acute self-limiting viral infections. In addition, commercial serological rapid diagnostic tests used are semi-quantitative ELISAs that detect antibodies and are not conclusive of the present or past infection [91, 92]. For some pathogens, definitive diagnosis requires demonstration of a serial rise in antibody titres over a specific time period. Noncompliance of patients to report for repeat serological tests following improvement of the illness remains a major drawback in serology-based diagnostics [10, 93]. Moreover, ELISAs have poor specification and cross reactions are common [94]. Antigen-based or PCR-based diagnostics have been increasingly introduced to overcome these problems. However, their availability and affordability in resource-poor countries are limited, and the fact that they are not freely available in most government-run health institutions means that accessibility to such tests is limited to those in the private sector who can afford to pay from their own pocket [93].
This review has several limitations. Interpretation of data in this study should take into consideration the heterogeneity of the reviewed studies including study design, patient sampling and diagnostic testing. In addition, many of these studies were descriptive studies. Furthermore, there is no reliable way to judge the quality of heterogeneous descriptive studies included in this review. Some articles failed to report duration of fever and definition of AUFI varied widely between the studies. Aetiologies of AUFI of less than one-week duration would likely differ from those of a minimum of three weeks. Therefore, adherence to a common case definition between studies is important to make comparisons more reliable. Seasonal variation of diseases such as influenza, changes in disease patterns due to economic development, urbanization, environmental changes and changes in population densities during the last 15 years could have affected observed aetiologies and disease patterns. In addition, data from some countries including Bhutan and Timor-Leste were not available and results were also dominated by studies from India and Thailand. Since English is not the primary language in most of these countries, restricting the studies included in this review to studies published in English may have affected the findings.
Algorithms for the management of fevers at the community level as well as for inpatients have been developed by WHO [59]. A lack of knowledge of the geographical heterogeneity in AUFI aetiology prevents local adaptation of generic protocols, and thus precludes better targeting of drugs and implementation of early, effective management [95]. Therefore, it is necessary that data on pathogen presence collected incidentally in various studies and data collected by surveillance mechanisms be analysed systematically and mapped to provide information on the distribution and prevalence of infectious aetiologies of AFIs. Clinical algorithms could then be adapted, greatly improving targeting of treatment. Strengthening of notification systems (including sentinel systems) and sharing of data between clinical research communities will be important to construct more comprehensive information on geographically specific aetiologies of AUFI.
Conclusion
In this study the most common causes of AUFI were viral, followed by bacterial and protozoal (malaria) infections. Dengue was the commonest virus that caused AUFI while leptospirosis and typhoid were important bacterial infectious causes. The challenges of unidentified causes of AUFI can be partly overcome by roll-out of affordable serological tests. It is imperative that data on pathogen presence collected incidentally in various studies and data collected by surveillance systems be analysed systematically and mapped to provide information on the distribution and prevalence of infectious aetiologies of AFIs for improving treatment and prevention programmes.
Additional file
Table S1. Inclusion and exclusion criteria. Table S2. Risk of bias questionnaire and scale. Table S3. Common presenting symptoms among patients. Table S4. Different diagnostic tools used in the studies. Table S5. Risk of bias scores of included studies. (DOCX 25 kb)
Acknowledgements
Not applicable.
Abbreviations
- AFI
acute febrile illness
- AUFI
acute undifferentiated febrile illness
- CFR
case fatality rate
- DF
dengue fever
- DHF
dengue haemorrhage fever
- DSS
dengue shock syndrome
- ED
emergency department
- ELISA
enzyme-linked immunosorbent assay
- HAV
Hepatitis A virus
- HBV
Hepatitis B virus
- HEV
Hepatitis E virus
- IP
inpatients
- JE
Japanese encephalitis
- MAT
microscopic agglutination test
- OPD
outpatient department
- PCR
polymerase chain reaction
- SA
South Asia
- SEA
Southeast Asia
- UAG
unspecified age group
- WHO
World Health Organization
Authors’ contributions
ACAC, DJG, and KK conceived the review. KW and KK undertook the literature search, data extraction, and drafted the report. ACAC and DJG assisted in interpretation of results and was involved in the critical revision of report. SVN and CLL assisted in revision of this paper. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
The data used for this analysis can be available upon request to the corresponding author (KW).
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Kinley Wangdi and Kaushalya Kasturiaratchi are contributed equally to this article.
Contributor Information
Kinley Wangdi, Email: kinley.wangdi@anu.edu.au.
Kaushalya Kasturiaratchi, kaushalyak@googlemail.com.
Susana Vaz Nery, Email: snery@kirby.unsw.edu.au.
Colleen L. Lau, Email: colleen.lau@anu.edu.au
Darren J. Gray, Email: darren.gray@anu.edu.au
Archie C. A. Clements, Email: archie.clements@curtin.edu.au
References
- 1.Chrispal A, Boorugu H, Gopinath KG, Chandy S, Prakash JA, Thomas EM, Abraham AM, Abraham OC, Thomas K. Acute undifferentiated febrile illness in adult hospitalized patients: the disease spectrum and diagnostic predictors - an experience from a tertiary care hospital in South India. Trop Dr. 2010;40(4):230–234. doi: 10.1258/td.2010.100132. [DOI] [PubMed] [Google Scholar]
- 2.Leelarasamee A, Chupaprawan C, Chenchittikul M, Udompanthurat S. Etiologies of acute undifferentiated febrile illness in Thailand. Journal of the Medical Association of Thailand = Chotmaihet thangphaet. 2004;87(5):464–472. [PubMed] [Google Scholar]
- 3.Acestor N, Cooksey R, Newton PN, Menard D, Guerin PJ, Nakagawa J, Christophel E, Gonzalez IJ, Bell D. Mapping the aetiology of non-malarial febrile illness in Southeast Asia through a systematic review--terra incognita impairing treatment policies. PLoS One. 2012;7(9):e44269. doi: 10.1371/journal.pone.0044269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, Rudan I, Campbell H, Cibulskis R, Li M, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet (London, England) 2012;379(9832):2151–2161. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 5.Bell M, Archibald LK, Nwanyanwu O, Dobbie H, Tokars J, Kazembe PN, Reller LB, Jarvis WR. Seasonal variation in the etiology of bloodstream infections in a febrile inpatient population in a developing country. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases. 2001;5(2):63–69. doi: 10.1016/s1201-9712(01)90027-x. [DOI] [PubMed] [Google Scholar]
- 6.Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, Lovett MA, Levett PN, Gilman RH, Willig MR, Gotuzzo E, et al. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis. 2003;3(12):757–771. doi: 10.1016/s1473-3099(03)00830-2. [DOI] [PubMed] [Google Scholar]
- 7.Pradutkanchana J, Pradutkanchana S, Kemapanmanus M, Wuthipum N, Silpapojakul K. The etiology of acute pyrexia of unknown origin in children after a flood. The Southeast Asian journal of tropical medicine and public health. 2003;34(1):175–178. [PubMed] [Google Scholar]
- 8.Bruce MG, Sanders EJ, Leake JA, Zaidel O, Bragg SL, Aye T, Shutt KA, Deseda CC, Rigau-Perez JG, Tappero JW, et al. Leptospirosis among patients presenting with dengue-like illness in Puerto Rico. Acta Trop. 2005;96(1):36–46. doi: 10.1016/j.actatropica.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Levett PN, Branch SL, Edwards CN. Detection of dengue infection in patients investigated for leptospirosis in Barbados. The American journal of tropical medicine and hygiene. 2000;62(1):112–114. doi: 10.4269/ajtmh.2000.62.112. [DOI] [PubMed] [Google Scholar]
- 10.Dhingra B, Mishra D. Early diagnosis of febrile illness: the need of the hour. Indian Pediatr. 2011;48(11):845–849. doi: 10.1007/s13312-011-0134-6. [DOI] [PubMed] [Google Scholar]
- 11.Capeding MR, Chua MN, Hadinegoro SR, Hussain II, Nallusamy R, Pitisuttithum P, Rusmil K, Thisyakorn U, Thomas SJ, Huu Tran N, et al. Dengue and other common causes of acute febrile illness in Asia: an active surveillance study in children. PLoS Negl Trop Dis. 2013;7(7):e2331. doi: 10.1371/journal.pntd.0002331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suttinont C, Losuwanaluk K, Niwatayakul K, Hoontrakul S, Intaranongpai W, Silpasakorn S, Suwancharoen D, Panlar P, Saisongkorh W, Rolain JM, et al. Causes of acute, undifferentiated, febrile illness in rural Thailand: results of a prospective observational study. Ann Trop Med Parasitol. 2006;100(4):363–370. doi: 10.1179/136485906X112158. [DOI] [PubMed] [Google Scholar]
- 13.Gibbons RV, Vaughn DW. Dengue: an escalating problem. BMJ (Clinical research ed) 2002;324(7353):1563–1566. doi: 10.1136/bmj.324.7353.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dash AP, Bhatia R, Sunyoto T, Mourya DT. Emerging and re-emerging arboviral diseases in Southeast Asia. Journal of vector borne diseases. 2013;50(2):77–84. [PubMed] [Google Scholar]
- 15.Vaughn DW, Green S, Kalayanarooj S, Innis BL, Nimmannitya S, Suntayakorn S, Endy TP, Raengsakulrach B, Rothman AL, Ennis FA, et al. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis. 2000;181(1):2–9. doi: 10.1086/315215. [DOI] [PubMed] [Google Scholar]
- 16.Zaidi AK, Awasthi S, deSilva HJ. Burden of infectious diseases in South Asia. BMJ (Clinical research ed) 2004;328(7443):811–815. doi: 10.1136/bmj.328.7443.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gubler DJ. The global emergence/resurgence of arboviral diseases as public health problems. Arch Med Res. 2002;33(4):330–342. doi: 10.1016/s0188-4409(02)00378-8. [DOI] [PubMed] [Google Scholar]
- 18.Coker RJ, Hunter BM, Rudge JW, Liverani M, Hanvoravongchai P. Emerging infectious diseases in Southeast Asia: regional challenges to control. Lancet (London, England) 2011;377(9765):599–609. doi: 10.1016/S0140-6736(10)62004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C, Baker P, Smith E, Buchbinder R. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65(9):934–939. doi: 10.1016/j.jclinepi.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 20.Abhilash KP, Jeevan JA, Mitra S, Paul N, Murugan TP, Rangaraj A, David S, Hansdak SG, Prakash JA, Abraham AM, et al. Acute undifferentiated febrile illness in patients presenting to a tertiary Care Hospital in South India: clinical Spectrum and outcome. J Global Infect Dis. 2016;8(4):147–154. doi: 10.4103/0974-777X.192966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gopalakrishnan S, Arumugam B, Kandasamy S, Rajendran S, Krishnan B. Acute undifferentiated febrile illness among adults – a hospital based observational study. J Evol Med Dent Sci. 2013;2(14):2305–2319. [Google Scholar]
- 22.Joshi R, Colford JM, Jr, Reingold AL, Kalantri S. Nonmalarial acute undifferentiated fever in a rural hospital in Central India: diagnostic uncertainty and overtreatment with antimalarial agents. The American journal of tropical medicine and hygiene. 2008;78(3):393–399. [PubMed] [Google Scholar]
- 23.Kammili N, Swathi A, Devara SM, Anuradha pR. Prevalence of scrub typhus among acute undifferentiated febrile illness cases provisionally diagnosed as dengue fever. J Evol Med Dent Sci. 2013;2(16):2662–2664. [Google Scholar]
- 24.Kasper MR, Blair PJ, Touch S, Sokhal B, Yasuda CY, Williams M, Richards AL, Burgess TH, Wierzba TF, Putnam SD. Infectious etiologies of acute febrile illness among patients seeking health care in south-Central Cambodia. The American journal of tropical medicine and hygiene. 2012;86(2):246–253. doi: 10.4269/ajtmh.2012.11-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laoprasopwattana K, Kaewjungwad L, Jarumanokul R, Geater A. Differential diagnosis of chikungunya, dengue viral infection and other acute febrile illnesses in children. Pediatr Infect Dis J. 2012;31(5):459–463. doi: 10.1097/INF.0b013e31824bb06d. [DOI] [PubMed] [Google Scholar]
- 26.Mayxay M, Castonguay-Vanier J, Chansamouth V, Dubot-Pérès A, Paris DH, Phetsouvanh R, Tangkhabuanbutra J, Douangdala P, Inthalath S, Souvannasing P, et al. Causes of non-malarial fever in Laos: a prospective study. Lancet Glob Health. 2013;1(3):e46–e54. doi: 10.1016/S2214-109X(13)70008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oishr K, A Maputa C, Carlos CC, Cinco-Abanes MT, Saoto M, Inoue S, Morita K, Natividad FF: Dengue and other Febric illnesses among Children in the Philippines. 2006.
- 28.Phuong HL, de Vries PJ, Nga TT, Giao PT, Hung le Q, Binh TQ, Nam NV, Nagelkerke N, Kager PA. Dengue as a cause of acute undifferentiated fever in Vietnam. BMC Infect Dis. 2006;6:123. doi: 10.1186/1471-2334-6-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ray P, Ratagiri VH, Kabra SK, Lodha R, Sharma S, Sharma BS, Kalaivani M, Wig N. Chikungunya infection in India: results of a prospective hospital based multi-centric study. PLoS One. 2012;7(2):e30025. doi: 10.1371/journal.pone.0030025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reller ME, Bodinayake C, Nagahawatte A, Devasiri V, Kodikara-Arachichi W, Strouse JJ, Broadwater A, Ostbye T, de Silva A, Woods CW. Unsuspected dengue and acute febrile illness in rural and semi-urban southern Sri Lanka. Emerg Infect Dis. 2012;18(2):256–263. doi: 10.3201/eid1802.110962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reller ME, Bodinayake C, Nagahawatte A, Devasiri V, Kodikara-Arachichi W, Strouse JJ, Flom JE, Dumler JS, Woods CW. Leptospirosis as frequent cause of acute febrile illness in southern Sri Lanka. Emerg Infect Dis. 2011;17(9):1678–1684. doi: 10.3201/eid1709.100915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raina S, Raina RK, Agarwala N, Raina SK, Sharma R. Coinfections as an aetiology of acute undifferentiated febrile illness among adult patients in the sub-Himalayan region of North India. Journal of vector borne diseases. 2018;55(2):130–136. doi: 10.4103/0972-9062.242560. [DOI] [PubMed] [Google Scholar]
- 33.Shelke YP, Deotale VS, Maraskolhe DL. Spectrum of infections in acute febrile illness in Central India. Indian J Med Microbiol. 2017;35(4):480–484. doi: 10.4103/ijmm.IJMM_17_33. [DOI] [PubMed] [Google Scholar]
- 34.Andrews MA, Ittyachen AM. Aetiology of acute febrile illness: a multicentre study from the province of Kerala in southern India. Trop Dr. 2018;48(4):322–325. doi: 10.1177/0049475518794572. [DOI] [PubMed] [Google Scholar]
- 35.Bodinayake CK, Tillekeratne LG, Nagahawatte A, Devasiri V, Kodikara Arachchi W, Strouse JJ, Sessions OM, Kurukulasooriya R, Uehara A, Howe S, et al. Evaluation of the WHO 2009 classification for diagnosis of acute dengue in a large cohort of adults and children in Sri Lanka during a dengue-1 epidemic. PLoS Negl Trop Dis. 2018;12(2):e0006258. doi: 10.1371/journal.pntd.0006258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murdoch DR, Woods CW, Zimmerman MD, Dull PM, Belbase RH, Keenan AJ, Scott RM, Basnyat B, Archibald LK, Reller LB. The etiology of febrile illness in adults presenting to Patan hospital in Kathmandu, Nepal. The American journal of tropical medicine and hygiene. 2004;70(6):670–675. [PubMed] [Google Scholar]
- 37.Kashinkunti MD, Gundikeri SK, Dhananjaya M. Acute undifferentiated febrile illness- clinical spectrum and outcome from a tertiary care teaching hospital of North Karnataka. Int J Biol Med Res. 2013;4(2):3399–3402. [Google Scholar]
- 38.Kingston HW, Hossain M, Leopold S, Anantatat T, Tanganuchitcharnchai A, Sinha I, Plewes K, Maude RJ, Chowdhury MAH, Paul S, et al. Rickettsial illnesses as important causes of febrile illness in Chittagong, Bangladesh. Emerg Infect Dis. 2018;24(4). [DOI] [PMC free article] [PubMed]
- 39.Kumar R, Tripathi P, Tripathi S, Kanodia A, Pant S, Venkatesh V. Prevalence and clinical differentiation of dengue fever in children in northern India. Infection. 2008;36(5):444–449. doi: 10.1007/s15010-008-7172-6. [DOI] [PubMed] [Google Scholar]
- 40.Wangrangsimakul T, Althaus T, Mukaka M, Kantipong P, Wuthiekanun V, Chierakul W, Blacksell SD, Day NP, Laongnualpanich A, Paris DH. Causes of acute undifferentiated fever and the utility of biomarkers in Chiangrai, northern Thailand. PLoS Negl Trop Dis. 2018;12(5):e0006477. doi: 10.1371/journal.pntd.0006477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zaki SA, Shanbag P. Clinical manifestations of dengue and leptospirosis in children in Mumbai: an observational study. Infection. 2010;38(4):285–291. doi: 10.1007/s15010-010-0030-3. [DOI] [PubMed] [Google Scholar]
- 42.Chheng K, Carter MJ, Emary K, Chanpheaktra N, Moore CE, Stoesser N, Putchhat H, Sona S, Reaksmey S, Kitsutani P, et al. A prospective study of the causes of febrile illness requiring hospitalization in children in Cambodia. PLoS One. 2013;8(4):e60634. doi: 10.1371/journal.pone.0060634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Punjabi NH, Taylor WR, Murphy GS, Purwaningsih S, Picarima H, Sisson J, Olson JG, Baso S, Wangsasaputra F, Lesmana M, et al. Etiology of acute, non-malaria, febrile illnesses in Jayapura, northeastern Papua, Indonesia. The American journal of tropical medicine and hygiene. 2012;86(1):46–51. doi: 10.4269/ajtmh.2012.10-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahmad S, Dhar M, Mittal G, Bhat NK, Shirazi N, Kalra V, Sati HC, Gupta V. A comparative hospital-based observational study of mono- and co-infections of malaria, dengue virus and scrub typhus causing acute undifferentiated fever. European journal of clinical microbiology & infectious diseases : official publication of the. European Society of Clinical Microbiology. 2016;35(4):705–711. doi: 10.1007/s10096-016-2590-3. [DOI] [PubMed] [Google Scholar]
- 45.Andrews M, Elizabeth A, Kuttichira P. Clinical profile of acute undifferentiated febrile illness in patients admitted to a teaching hospital in Kerala. Health Sciences. 2014;(3):1.
- 46.Arora BS, Matlani M, Saigal K, Biswal I, Rajan S, Padmanandan A, Singh S: major aetiologies of acute undifferentiated fever in 2013 and 2014: an experience in retrospect. 2017 2017, 4(2):5.
- 47.Chikkaveeraiah SK, Bhograj A, Reddy R, Kumar A. Evaluating the etiology and disease specific clinical profiles of acute undifferentiated febrile illness. International Journal of Scientific Study. 2016(12):3.
- 48.Das D, Das B, Roy AD, Singh T. Common infectious etiologies of acute febrile illness in a remote geographical location: could scrub typhus be the Most common cause? British Journal of Medicine & Medical Research. 2015;10(10):1–10. [Google Scholar]
- 49.Ellis RD, Fukuda MM, McDaniel P, Welch K, Nisalak A, Murray CK, Gray MR, Uthaimongkol N, Buathong N, Sriwichai S, et al. Causes of fever in adults on the Thai-Myanmar border. The American journal of tropical medicine and hygiene. 2006;74(1):108–113. [PubMed] [Google Scholar]
- 50.McGready R, Ashley EA, Wuthiekanun V, Tan SO, Pimanpanarak M, Viladpai-Nguen SJ, Jesadapanpong W, Blacksell SD, Peacock SJ, Paris DH, et al. Arthropod borne disease: the leading cause of fever in pregnancy on the Thai-Burmese border. PLoS Negl Trop Dis. 2010;4(11):e888. doi: 10.1371/journal.pntd.0000888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mittal G, Ahmad S, Agarwal RK, Dhar M, Mittal M, Sharma S. Aetiologies of acute undifferentiated febrile illness in adult patients - an experience from a tertiary care hospital in northern India. Journal of clinical and diagnostic research : JCDR. 2015;9(12):Dc22–Dc24. doi: 10.7860/JCDR/2015/11168.6990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rafizah AAN, Aziah BD, Azwany YN, Imran MK, Rusli AM, Nazri M, Nabilah I, Zahirunddin WM, Zaliha I. Leptospirosis in northeastern Malaysia: misdiagnosed or coinfection? International Journal of Collaborative Research on Internal Medicine & Public Health. 2012;4(7):1420–1427. [Google Scholar]
- 53.Rani RV, Sundararajan T, Rajesh S, Jeyamurugan T. A study on common etiologies of acute febrile illness detectable by microbiological tests in a tertiary care hospital. Int J Curr Microbiol App Sci. 2016;5(7):670–674. [Google Scholar]
- 54.Sabchareon A, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, Jiwariyavej V, Dulyachai W, Pengsaa K, Margolis HS, Letson GW. Dengue infection in children in Ratchaburi, Thailand: a cohort study. I. Epidemiology of symptomatic acute dengue infection in children, 2006–2009. PLoS Negl Trop Dis. 2012;6(7):e1732. doi: 10.1371/journal.pntd.0001732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Salagre KD, Sahay RN, Pazare AR, Dubey A, Marathe KK. A study of clinical profile of patients presenting with complications of acute febrile illnesses during monsoon. J Assoc Physicians India. 2017;65(9):37–42. [PubMed] [Google Scholar]
- 56.Thompson CN, Blacksell SD, Paris DH, Arjyal A, Karkey A, Dongol S, Giri A, Dolecek C, Day N, Baker S, et al. Undifferentiated febrile illness in Kathmandu, Nepal. The American journal of tropical medicine and hygiene. 2015;92(4):875–878. doi: 10.4269/ajtmh.14-0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gautam Rajendra, Parajuli Keshab, Sherchand Jeevan Bahadur. Epidemiology, Risk Factors and Seasonal Variation of Scrub Typhus Fever in Central Nepal. Tropical Medicine and Infectious Disease. 2019;4(1):27. doi: 10.3390/tropicalmed4010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Prasad N, Murdoch DR, Reyburn H, Crump JA. Etiology of severe febrile illness in low- and middle-income countries: a systematic review. PLoS One. 2015;10(6):e0127962. doi: 10.1371/journal.pone.0127962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.WHO: WHO informal consultation on fever management in peripheral health care settings: a global review of evidence and practice. In. Geneva, Switzerland; 2013.
- 60.Moreira J, Bressan CS, Brasil P, Siqueira AM. Epidemiology of acute febrile illness in Latin America. Clinical microbiology and infection : the official publication of the European Society of Clinical. Microbiology and Infectious Diseases. 2018;24(8):827–835. doi: 10.1016/j.cmi.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Laras K, Cao BV, Bounlu K, Nguyen TK, Olson JG, Thongchanh S, Tran NV, Hoang KL, Punjabi N, Ha BK, et al. The importance of leptospirosis in Southeast Asia. The American journal of tropical medicine and hygiene. 2002;67(3):278–286. doi: 10.4269/ajtmh.2002.67.278. [DOI] [PubMed] [Google Scholar]
- 62.Sehgal SC, Sugunan AP, Vijayachari P. Leptospirosis disease burden estimation and surveillance networking in India. The Southeast Asian journal of tropical medicine and public health. 2003;34(2):170–177. [PubMed] [Google Scholar]
- 63.Tangkanakul W, Smits HL, Jatanasen S, Ashford DA. Leptospirosis: an emerging health problem in Thailand. The Southeast Asian journal of tropical medicine and public health. 2005;36(2):281–288. [PubMed] [Google Scholar]
- 64.LaRocque RC, Breiman RF, Ari MD, Morey RE, Janan FA, Hayes JM, Hossain MA, Brooks WA, Levett PN. Leptospirosis during dengue outbreak, Bangladesh. Emerg Infect Dis. 2005;11(5):766–769. doi: 10.3201/eid1105.041212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Costa F, Hagan JE, Calcagno J, Kane M, Torgerson P, Martinez-Silveira MS, Stein C, Abela-Ridder B, Ko AI. Global morbidity and mortality of leptospirosis: a systematic review. PLoS Negl Trop Dis. 2015;9(9):e0003898. doi: 10.1371/journal.pntd.0003898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tangkanakul W, Tharmaphornpil P, Plikaytis BD, Bragg S, Poonsuksombat D, Choomkasien P, Kingnate D, Ashford DA. Risk factors associated with leptospirosis in northeastern Thailand, 1998. The American journal of tropical medicine and hygiene. 2000;63(3–4):204–208. doi: 10.4269/ajtmh.2000.63.204. [DOI] [PubMed] [Google Scholar]
- 67.Kamath R, Swain S, Pattanshetty S, Nair NS. Studying risk factors associated with human leptospirosis. J Global Infect Dis. 2014;6(1):3–9. doi: 10.4103/0974-777X.127941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Torgerson PR, Hagan JE, Costa F, Calcagno J, Kane M, Martinez-Silveira MS, Goris MG, Stein C, Ko AI, Abela-Ridder B. Global burden of leptospirosis: estimated in terms of disability adjusted life years. PLoS Negl Trop Dis. 2015;9(10):e0004122. doi: 10.1371/journal.pntd.0004122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Victoriano AF, Smythe LD, Gloriani-Barzaga N, Cavinta LL, Kasai T, Limpakarnjanarat K, Ong BL, Gongal G, Hall J, Coulombe CA, et al. Leptospirosis in the Asia Pacific region. BMC Infect Dis. 2009;9:147. doi: 10.1186/1471-2334-9-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Libraty DH, Myint KS, Murray CK, Gibbons RV, Mammen MP, Endy TP, Li W, Vaughn DW, Nisalak A, Kalayanarooj S, et al. A comparative study of leptospirosis and dengue in Thai children. PLoS Negl Trop Dis. 2007;1(3):e111. doi: 10.1371/journal.pntd.0000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Toyokawa T, Ohnishi M, Koizumi N. Diagnosis of acute leptospirosis. Expert Rev Anti-Infect Ther. 2011;9(1):111–121. doi: 10.1586/eri.10.151. [DOI] [PubMed] [Google Scholar]
- 72.Levett PN. Leptospirosis. Clin Microbiol Rev. 2001;14(2):296–326. doi: 10.1128/CMR.14.2.296-326.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Khan E, Kisat M, Khan N, Nasir A, Ayub S, Hasan R. Demographic and clinical features of dengue fever in Pakistan from 2003-2007: a retrospective cross-sectional study. PLoS One. 2010;5(9):e12505. doi: 10.1371/journal.pone.0012505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hoti SL, R. S, Rajendran G, Das LK, Ravi R, Das PK: Dengue and Dengue haemorrhagic fever outbreak in Pondicherry , South India, during 2003–2004, Emergence of DENV-3. Dengue Bulletin 2006, 30:42–50.
- 75.Teng AK, Singh S. Epidemiology and new initiatives in the prevention and control of dengue in Malaysia. Dengue Bulletin. 2001;25:7–14. [Google Scholar]
- 76.Lee LK, Earnest A, Carrasco LR, Thein TL, Gan VC, Lee VJ, Lye DC, Leo YS. Safety and cost savings of reducing adult dengue hospitalization in a tertiary care hospital in Singapore. Trans R Soc Trop Med Hyg. 2013;107(1):37–42. doi: 10.1093/trstmh/trs009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sturm-Ramirez KM, Hulse-Post DJ, Govorkova EA, Humberd J, Seiler P, Puthavathana P, Buranathai C, Nguyen TD, Chaisingh A, Long HT, et al. Are ducks contributing to the endemicity of highly pathogenic H5N1 influenza virus in Asia? J Virol. 2005;79(17):11269–11279. doi: 10.1128/JVI.79.17.11269-11279.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sims LD. Lessons learned from Asian H5N1 outbreak control. Avian Dis. 2007;51(1 Suppl):174–181. doi: 10.1637/7637-042806R.1. [DOI] [PubMed] [Google Scholar]
- 79.Forrest HL, Webster RG. Perspectives on influenza evolution and the role of research. Anim Health Res Rev. 2010;11(1):3–18. doi: 10.1017/S1466252310000071. [DOI] [PubMed] [Google Scholar]
- 80.Influenza (Seasonal) [http://www.who.int/mediacentre/factsheets/fs211/en/].
- 81.Ochiai RL, Acosta CJ, Danovaro-Holliday MC, Baiqing D, Bhattacharya SK, Agtini MD, Bhutta ZA, Canh DG, Ali M, Shin S, et al. A study of typhoid fever in five Asian countries: disease burden and implications for controls. Bull World Health Organ. 2008;86(4):260–268. doi: 10.2471/BLT.06.039818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chaudhry D, Goyal S. Scrub typhus-resurgence of a forgotten killer. Indian journal of anaesthesia. 2013;57(2):135–136. doi: 10.4103/0019-5049.111836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fan MY, Walker DH, Yu SR, Liu QH. Epidemiology and ecology of rickettsial diseases in the People's Republic of China. Rev Infect Dis. 1987;9(4):823–840. [PubMed] [Google Scholar]
- 84.Parola P, Miller RS, McDaniel P, Telford SR, 3rd, Rolain JM, Wongsrichanalai C, Raoult D. Emerging rickettsioses of the Thai-Myanmar border. Emerg Infect Dis. 2003;9(5):592–595. doi: 10.3201/eid0905.020511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kularatne SA, Edirisingha JS, Gawarammana IB, Urakami H, Chenchittikul M, Kaiho I. Emerging rickettsial infections in Sri Lanka: the pattern in the hilly Central Province. Tropical medicine & international health : TM & IH. 2003;8(9):803–811. doi: 10.1046/j.1365-3156.2003.01108.x. [DOI] [PubMed] [Google Scholar]
- 86.WHO: E2020: update on the e-2020 initiative of 21 malaria-eliminating countries. 2018.
- 87.WHO: world malaria report 2017. WHO Library Cataloguing-in-Publication Data 2017.
- 88.Behera B, Chaudhry R, Pandey A, Mohan A, Dar L, Premlatha MM, Gupta E, Broor S, Aggarwal P. Co-infections due to leptospira, dengue and hepatitis E: a diagnostic challenge. Journal of infection in developing countries. 2009;4(1):48–50. doi: 10.3855/jidc.535. [DOI] [PubMed] [Google Scholar]
- 89.Chaudhry R, Pandey A, Das A, Broor S. Infection potpourri: are we watching? Indian journal of pathology & microbiology. 2009;52(1):125. doi: 10.4103/0377-4929.44990. [DOI] [PubMed] [Google Scholar]
- 90.Peacock SJ, Newton PN. Public health impact of establishing the cause of bacterial infections in rural Asia. Trans R Soc Trop Med Hyg. 2008;102(1):5–6. doi: 10.1016/j.trstmh.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 91.Blacksell SD, Bryant NJ, Paris DH, Doust JA, Sakoda Y, Day NP. Scrub typhus serologic testing with the indirect immunofluorescence method as a diagnostic gold standard: a lack of consensus leads to a lot of confusion. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2007;44(3):391–401. doi: 10.1086/510585. [DOI] [PubMed] [Google Scholar]
- 92.Wagenaar JF, Falke TH, Nam NV, Binh TQ, Smits HL, Cobelens FG, de Vries PJ. Rapid serological assays for leptospirosis are of limited value in southern Vietnam. Ann Trop Med Parasitol. 2004;98(8):843–850. doi: 10.1179/000349804X3207. [DOI] [PubMed] [Google Scholar]
- 93.Premaratna R. Dealing with acute febrile illness in the resource poor tropics. Tropical Medicine & Surgery. 2013(1):1.
- 94.Lau CL, DePasquale JM: Leptospirosis, American Samoa. Emerg Infect Dis 2012, 18(12):2079–2081. [DOI] [PMC free article] [PubMed]
- 95.FIND: acute febrile syndrome Straegy. In.; 2012.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Inclusion and exclusion criteria. Table S2. Risk of bias questionnaire and scale. Table S3. Common presenting symptoms among patients. Table S4. Different diagnostic tools used in the studies. Table S5. Risk of bias scores of included studies. (DOCX 25 kb)
Data Availability Statement
The data used for this analysis can be available upon request to the corresponding author (KW).