Abstract
Background
Rodents are important reservoirs for zoonotic vector-borne agents. Thus, the distribution of rodents and their vicinity to humans and companion animals may have an important impact on human and animal health. However, the reservoir potential of some rodent genera, e.g. Microtus, has not yet been precisely examined concerning tick-borne pathogens in Central Europe. Therefore, we examined small mammals from Germany and the Czech Republic for the following vector-borne pathogens: Babesia spp., Bartonella spp., Anaplasma phagocytophilum, “Candidatus Neoehrlichia mikurensis” (CNM) and Coxiella burnetii. Spleen DNA from 321 small mammals belonging to four genera, Myodes (n = 78), Apodemus (n = 56), Microtus (n = 149), Sorex (n = 38), collected during 2014 in Germany and the Czech Republic were available for this study. DNA samples were examined for the presence of Babesia and Bartonella DNA by conventional PCR targeting the 18S rRNA gene and the 16S–23S rRNA intergenic spacer region, respectively. For the detection of CNM, A. phagocytophilum and C. burnetii real-time PCR assays were performed.
Results
Bartonella spp. DNA was detected in 216 specimens (67.3%) with 102/174 (58.6%) positive in Germany and 114/147 (77.6%) in the Czech Republic. The prevalence in each genus was 44.9% for Myodes, 63.2% for Sorex, 77.2% for Microtus and 75% for Apodemus. Four Bartonella species, i.e. Bartonella sp. N40, B. grahamii, B. taylorii and B. doshiae, as well as uncultured bartonellae, were detected. The Bartonella species diversity was higher in rodents than in shrews. In total, 27/321 (8.4%) small mammals were positive for CNM and 3/321 (0.9%) for A. phagocytophilum (S. coronatus and M. glareolus). All samples were negative for Babesia spp. and Coxiella spp.
Conclusions
While the detected high prevalence for Bartonella in Apodemus and Myodes spp. is confirmatory with previous findings, the prevalence in Microtus spp. was unexpectedly high. This indicates that individuals belonging to this genus may be regarded as potential reservoirs. Interestingly, only Sorex spp. and M. glareolus were positive for A. phagocytophilum in the present study, suggesting a possible importance of the latter for the maintenance of certain A. phagocytophilum strains in nature.
Electronic supplementary material
The online version of this article (10.1186/s13071-019-3576-7) contains supplementary material, which is available to authorized users.
Keywords: Anaplasma, Apodemus, Babesia, Bartonella, “Candidatus Neoehrlichia mikurensis”, Coxiella burnetii, Microtus, Myodes, Rodent, Shrew
Background
Rodents and other small mammals are important reservoir hosts for a range of pathogenic and non-pathogenic viral, bacterial and parasitic agents [1]. They are of importance for the development of subadult tick stages and contribute in the natural life-cycle of several tick-borne bacterial and parasitic pathogens [2]. Thus, the distribution of rodents and their close contact to humans and companion animals may have impact on the health status of the latter. Bartonellae are known to infect endothelial cells and erythrocytes of mammals and humans [3]. The most common causative agent for bartonellosis in humans, Bartonella henselae, is mainly harboured by wild and domestic cats [4]. However, rodents are known to be the main reservoirs for the majority of over 22 species and subspecies of the already described bartonellae [5]. Nevertheless, although zoonotic bartonellae are confirmed to be harboured by rodents, the pathogenic potential is still unknown for most of them [5]. In Europe, Bartonella spp. were thus far reported in different vole and mice species from Austria, Finland, Germany and Poland [6–9].
“Candidatus Neoehrlichia mikurensis” (CNM) as well as Anaplasma phagocytophilum are both tick-borne alpha-proteobacteria [10]. While A. phagocytophilum has zoonotic potential and is responsible for a broad spectrum of symptoms in humans as well as in companion animals, CNM seems to be a health risk mainly in immunosuppressed humans as well as in dogs, causing mostly mild symptoms [11, 12]. In Europe, mainly rodents belonging to the genera Myodes and Apodemus are regarded as reservoirs for CNM. Specimens belonging to the genus Microtus have tested positive, but thus far they have only been examined in small sample sizes (n < 24) [13–15]. In central Europe, most rodent species are regarded as accidental hosts for A. phagocytophilum [16]. Nevertheless, it is yet not known whether rodents belonging to the genus Microtus are potential reservoirs [17].
Coxiella burnetii, the causative agent of Q fever, is a coccoid, obligate intracellular pathogen belonging to the order Legionellales and the family Coxiellaceae. Ticks may transfer C. burnetii to humans and mammals. The causative agent of Q fever may persist in endemic areas in reservoir hosts such as small mammals [18].
Several small mammal species of the genera Myodes, Apodemus and Microtus are supposed to be reservoirs for the tick-borne protozoan Babesia microti (order Piroplasmida, family Babesiidae) in Europe [17, 19]. Nonetheless, human babesiosis caused by B. microti, displaying various symptoms, has been rarely reported in Europe [20]. As data on the aforementioned vector-borne pathogens in small mammals from central Europe are scarce, the aims of this study were: (i) to evaluate the presence of Bartonella spp., CNM, A. phagocytophilum, Babesia spp. and Coxiella burnetii in small mammals captured in Germany and the Czech Republic; and (ii) to compare and analyse differences in the prevalence of these pathogens between small mammal species in connection with weight and age in order to evaluate the respective potential reservoir roles.
Methods
Collection of small mammal samples
A total of 321 small mammals belonging to nine different species [Apodemus agrarius (n = 2); A. flavicollis (n = 48); A. sylvaticus (n = 6); Microtus agrestis (n = 1); M. arvalis (n = 148); Myodes glareolus (n = 78); Sorex araneus (n = 30); S. coronatus (n = 7); and S. minutus (n = 1)] were collected for a previous study [21] (Table 1). Out of 148 M. arvalis, 147 individuals were collected according to standard protocols during late fall 2014 at three grassland grids close to Brno, the second largest city of the Czech Republic, located in the south-east. A further 174 individuals of different species were collected during spring, summer and fall in 2014 at grassland and forest grids at three sites in Germany [21]. The age of Microtus spp. was categorized in three classes according to the animals’ body weight: (1) < 14 g (less than 1.5 months-old); (2) 14–19 g (1.5 to 2.5 months old); and (3) > 19 g (2.5 months and older). Accordingly, the age categories in relation to the body weight for Apodemus spp. were classified as follows: (1) < 20 g (less than 3.5 months-old); (2) 20–30 g (3.5 to 7 months-old); and (3) > 30 g (7 months and older). For M. glareolus they were: (1) < 15 g (less than 1.5 months-old); (2) 15–19.5 g (1.5 to 2.5 months-old); and (3) > 19.5 g (2.5 months and older) [22]. Individuals belonging to body weight classes 1 and 2 were considered as sub-adults and individuals belonging to class 3 as adults. For S. araneus and S. coronatus, 2 categories were determined: (1) weight class < 8 g as sub-adult, and (2) weight class > 8 g as adult [23].
Table 1.
Bartonella spp., Anaplasma phagocytophilum and “Candidatus Neoehrlichia mikurensis” in small mammals from Germany and the Czech Republic
| Small mammal family/species | No. of analysed small mammals | No. of small mammals positive (%; 95% CIa) for | ||||
|---|---|---|---|---|---|---|
| Total | Females | Males | Bartonella spp. | A. phagocytophilum | CNMb | |
| Muridaec | 56 | 26 | 30 | 42 (75; 62.2–84.6) | 0 (0) | 8 (14.3; 7.2–26.0) |
| Apodemus agrarius | 2 | 1 | 1 | 2 (100; 29.0–100) | 0 (0) | 0 (0) |
| Apodemus flavicollis | 48 | 23 | 25 | 36 (75; 61.1–85.2) | 0 (0) | 6 (12.5; 5.5–25.1) |
| Apodemus sylvaticus | 6 | 2 | 4 | 4 (66.7; 29.6–90.8) | 0 (0) | 2 (33.3; 9.3–70.4) |
| Cricetidae | 227d | 118d | 108d | 150 (66.1; 59.7–71.9) | 1 (0.4; 0–2.7) | 19 (8.4; 5.4–12.8) |
| Microtus agrestis c | 1 | 1 | 0 | 0 (0) | 0 (0) | 0 (0) |
| Microtus arvalis e | 148d | 77d | 70d | 115 (77.7; 70.3–83.7) | 0 (0) | 7 (4.7; 2.1–9.6) |
| Myodes glareolus c | 78 | 40 | 38 | 35 (44.9; 34.3–55.9) | 1 (1.3; 0–7.6) | 13 (16.7; 9.9–26.6) |
| Soricidaec | 38 | 17 | 21 | 24 (63.2; 47.3–76.7) | 2 (5.3; 0.5–18.2) | 0 (0) |
| Sorex araneus | 30 | 12 | 18 | 19 (63.3; 45.5–78.2) | 0 (0) | 0 (0) |
| Sorex coronatus | 7 | 4 | 3 | 5 (71.4; 35.2–92.4) | 2 (28.6; 7.6–64.8) | 0 (0) |
| Sorex minutus | 1 | 1 | 0 | 0 (0) | 0 (0) | 0 (0) |
| Total | 321 | 161 | 159 | 216 (67.3; 62.0–72.2) | 3 (0.9; 0.2–2.8) | 27 (8.4; 5.8–12.0) |
a95% CI, 95% confidence interval
b“Candidatus Neoehrlichia mikurensis”
cAll derived from Germany
dSex could not be determined for one individual
e147 individual derived from the Czech Republic, 1 individual from Germany
Preparation of spleen DNA samples for molecular biological examination
Spleen-derived DNA samples of each individual were isolated separately as described previously [21] and were determined in terms of quantity and quality by the use of a spectrophotometer (Nano Drop ND-1000; PeqLab, Erlangen, Germany). As erythrocytes are the target cells of invasion and replication for Bartonella spp., spleen was chosen as the target tissue due to its important role with regard to removing old erythrocytes, and may thereby hold a reserve of erythrocytes that are highly infected by non-replicating bartonellae [24]. DNA samples with a concentration > 40 ng/µl were diluted with water (bioscience grade, nuclease-free) using different dilution steps in order to receive approximately equal DNA amounts between 20 and 40 ng/µl for further usage in PCR.
Detection of Bartonella spp., Babesia spp., “Candidatus Neoehrlichia mikurensis”, Coxiella burnetii and Anaplasma phagocytophilum via real-time and conventional PCR
For the detection of Bartonella spp., a conventional PCR targeting a fragment of the 16S–23S rRNA ITS region [453–780 base pairs (bp)] was performed as described [25, 26]. A conventional PCR targeting the 18S rRNA gene (411–452 bp) was performed for the detection of Babesia spp. [27] with slight modifications [25]. Obtained amplicons for both pathogens were separated by electrophoresis in 2% agarose gels, and visualized with HDGreen Plus DNA Stain (Intas Science Imaging Instruments GmbH, Göttingen, Germany) under UV-light. PCR products were purified using the NucleoSpin® Gel and PCR clean-up kit (Macherey-Nagel GmbH & Co. KG, Düren, Germany) according to the manufacturer’s instructions, and sequenced commercially (Sanger method) with forward and reverse primers (Interdisziplinäres Zentrum für Klinische Forschung, Leipzig, Germany). Sequences were analysed with BioNumerics v.7.6 (Applied Maths NV, Austin, TX, USA) and aligned to sequences obtained in GenBank using BLASTn (National Center for Biotechnology Information, Bethesda, MD, USA). A speciation cut-off was set at 98%. A selection of sequences (n = 50) was uploaded to GenBank under following accession numbers: MN056364-MN056413.
To detect CNM, a real-time PCR was performed targeting a 99-bp-sized fragment of the groEL gene [14] with modifications as described [16]. For detection of A. phagocytophilum a real-time PCR was performed targeting the msp2 gene (77 bp) [28, 29]. Presence of C. burnetii was evaluated via real-time PCR targeting the single copy icd gene as described previously [30]. Briefly, DNA samples were tested and compared to icd plasmid standards ranging from 10 to 106 copies/µl. All samples with > 10 copies/µl (detection limit) were considered positive. Details on primers are given in Additional file 1: Table S1.
Statistical analysis
Confidence intervals (95% CI) for prevalences of the various pathogens were determined by the Clopper and Pearson method using Graph Pad Prism Software v. 4.0. (Graph Pad Software Inc., San Diego, CA, USA).
Host specificity was modelled using a generalized linear model (GLM using package lme4) with binomial error distribution where individual infection probability depended on the respective species. To estimate species-specific infection probabilities, estimated marginal means were obtained from the emmeans-package. After back transformation from logit scale-based on the reference GLM, the resulting infection probabilities were used to visualize host specificity. Only species with more than 10 trapped individuals were incorporated into the analysis. Similarly, binomial GLM’s were used to identify if certain demographic groups were particularly prone to infection. Here, sex (binary) and weight (continuous; used as surrogate for age) were used to predict individual infection status. Backwards parameter selection was performed using the drop1 function. All analyses were performed using R [31].
Results
PCR results and sequence analysis for Bartonella spp. in small mammals
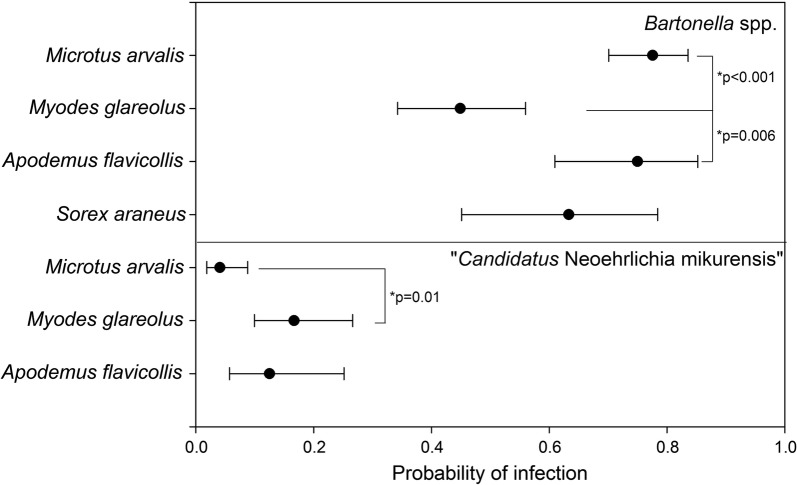
In total, 216 out of 321 individuals (67.3%; 95% CI: 62.0–72.2%) were positive for Bartonella spp. DNA, with 102/174 (58.62%; 95% CI: 51.19–65.68%) from Germany and 114/147 individuals (77.6%; 95% CI: 69.94–84.02%) from the Czech Republic (Table 1). The prevalence also differed between host genera (χ2 = 27.536, df = 8, P = 0.000571; Table 1). Microtus arvalis and A. flavicollis had a significantly higher infection probability compared to M. glareolus (Fig. 1). There were, however, no significant effects of sex or age on individual infection probability in any of the small mammal species (Table 2).
Fig. 1.
Results of generalized linear models for species specific infection probabilities for Bartonella spp. and CNM infections. P-values were obtained from post-hoc analysis (Tukey’s test)
Table 2.
Results of a generalized linear model with binominal error distribution on individual demographic factors (sex, weight) on the probability of infection with CNM
| Species | Source of variation | Coef. | SE | z-value | P-value |
|---|---|---|---|---|---|
| M. arvalis | Intercept | − 10.47 | 2.74 | − 3.82 | < 0.001 |
| Sex (male) | 1.81 | 1.07 | 1.68 | 0.092 | |
| Weight | 0.30 | 0.10 | 2.96 | 0.003 | |
| M. glareolus | Intercept | − 6.27 | 2.06 | − 3.05 | 0.002 |
| Sex (male) | 1.60 | 0.82 | 1.95 | 0.051 | |
| Weight | 0.16 | 0.07 | 2.32 | 0.020 | |
| A. flavicollis | Intercept | − 3.09 | 1.02 | − 3.02 | 0.002 |
| Sex (male) | 1.71 | 1.14 | 1.50 | 0.134 | |
| Weight | – | – | – | – |
Notes: Reference category for sex is female, weight was used a continuous variable. Significant factors are marked in bold; – indicates that parameter was removed during the selection process
Abbreviation: SE, standard error
Bartonella strain characterization by sequence analysis
A representative number of 84 out of 216 (41.2%) Bartonella-positive samples were further processed via sequencing. A randomized algorithm was conducted to receive sequences from 35–50% of Bartonella-positive individuals per small mammal species, sex and country. Four Bartonella species, i.e. Bartonella sp. N40, B. grahamii, B. taylorii and B. doshiae, as well as uncultured Bartonella strains, were detected in small mammals (Table 3). Most samples yielded sequences with 98–100% similarity to uncultured Bartonella strains (n = 35) (Table 3). While M. glareolus were negative for uncultured Bartonella strain, A. flavicollis and M. arvalis yielded three different Bartonella uncultured strains [GenBank: MF039571 (M. arvalis: n = 24; A. flavicollis: n = 4); MF039555 (n = 1, A. flavicollis only); KU886454 (M. arvalis: n = 5; A. flavicollis: n = 1)]. In total, 21 samples showed 97–100% similarity to B. taylorii [GenBank: AJ269788 (M. glareolus: n = 6; M. arvalis: n = 4); AJ269784 (S. araneus: n = 5; S. coronatus: n = 4; A. flavicollis: n = 2)], 11 samples showed 100% identity with B. grahamii (GenBank: CP001562), and ten with a similarity of 99–100% to B. doshiae [GenBank: AJ269786 (n = 9); AF442954 (n = 1), all M. arvalis]. Seven samples showed 99–100% similarity to Bartonella sp. N40 [GenBank: AJ269787 (A. flavicollis: n = 2; A. agrarius: n = 2; M. glareolus: n = 1; M. arvalis: n = 1); AJ269791 (n = 1, M. arvalis only)] (Table 3). The highest diversity of Bartonella species was detected in M. arvalis, followed by A. flavicollis and M. glareolus. The diversity of Bartonella strains was higher in rodents (at least 4 Bartonella species per host species) than in shrews (only B. taylorii). Interestingly, B. grahamii was detected exclusively in M. arvalis originating in Germany and B. doshiae exclusively in M. arvalis from the Czech Republic.
Table 3.
Bartonella species in small mammals from Germany and the Czech Republic
| Small mammal family/ speciesa | No. of Bartonella DNA positive samples (%) | No. of Bartonella-positive samples selected for sequencing | No. of Bartonella species-positive samples | ||||
|---|---|---|---|---|---|---|---|
| B. sp. N40 | B. grahamii | B. taylorii | B. doshiae | B. uncultured | |||
| Muridae | |||||||
| Apodemus agrarius | 2 (100) | 2 | 2 | – | – | – | – |
| Apodemus flavicollis | 36 (75.0) | 13 | 2 | 3 | 2 | - | 6 |
| Apodemus sylvaticus | 4 (66.7) | 3 | – | 3 | – | – | – |
| Cricetidaea | |||||||
| Microtus arvalis | 115 (74.7) | 46 | 2 | 1b | 4 | 10 | 29 |
| Myodes glareolus | 35 (44.9) | 11 | 1 | 4 | 6 | – | – |
| Soricidaea | |||||||
| Sorex araneus | 19 (63.3) | 5 | – | – | 5 | – | – |
| Sorex coronatus | 5 (71.4) | 4 | – | – | 4 | – | – |
| Total | 216 (66.1) | 84 | 7 | 11 | 21 | 10 | 35 |
aMicrotus agrestis (n = 1) and Sorex minutus (n = 1) were negative for Bartonella spp. DNA (see Table 1) and therefore not included in this table
bDetected only in 1 out of 1 individual from Germany
PCR results for A. phagocytophilum, CNM, C. burnetii, and Babesia spp. in small mammals
In total, 27 out of 321 (8.4%; 95% CI: 5.8–12.0%) small mammals were positive for CNM (Table 1). Samples sizes only permitted a GLM analysis for three small mammal species. Figure 1 shows that M. glareolus had a significantly higher probability for CNM infection compared to M. arvalis, but not compared to A. flavicollis. The two species belonging to the family Cricetidae showed an effect of weight on infection probability (Table 2). Heavier (=older) individuals were significantly more likely to be infected with CNM. Although not formally significant, sex remained in the final model and there was a trend that males were more likely to be infected compared to females. For A. flavicollis, only the category “sex” remained in the final model. In total, 3 out of 321 (0.9%; 95% CI: 0.2–2.8%) small mammals tested positive for A. phagocytophilum (S. coronatus, n = 2; M. glareolus, n = 1) (Table 1). All investigated small mammals were negative for Babesia spp. and Coxiella spp. DNA (0%; 95% CI: 0–1.4%). Regarding co-infections, double infections of Bartonella spp. and CNM were most frequently detected (n = 18; 7× in M. glareolus, 6× in A. flavicollis, 4× in M. arvalis, 1× in A. sylvaticus). Co-infections with A. phagocytophilum and Bartonella spp. occurred less often (n = 2; 1× in M. glareolus, 1× in S. araneus).
Discussion
This study presents the examination of arthropod-borne pathogens such as Bartonella spp., A. phagocytophilum, CNM, Babesia spp. and C. burnetii in different small mammal species from the Czech Republic and Germany. The study was focussed on small mammals from Germany and on Microtus spp. from Czech Republic, which mainly inhabit pastured areas and have been neglected so far regarding their reservoir competence for arthropod-borne bacterial pathogens in central Europe. Bartonellae are zoonotic pathogens currently arranged in different phylogenetic clades with respect to their main reservoir host species. The rodent-associated bartonellae clade is by far the most diverse regarding host and Bartonella species [32]. The prevalence (8.1%) as well as the species variety of bartonellae in black rats (Rattus rattus) as well as in Norway rats (Rattus norvegicus) (only either B. tribocorum or B. coopersplainsensis, respectively) is observed to be low to moderate in Europe [33]. In previous European studies, Bartonella spp. were reported with high prevalences (16–70.6%) in Apodemus and Myodes from Sweden, Germany and Poland [6, 34, 35]. The prevalences for both rodent genera fall in line with findings from the present study. The prevalence in M. glareolus is expected to be lower because bank voles are known to have an immune-mediated clearance of the infection within a few months [35]. This is why it is not surprising that the prevalence in M. glareolus was significantly lower than in Apodemus and Microtus in the present study. Thus far, prevalences in Microtus voles from Poland and Austria have ranged between 14–18%; however, only a low number of individuals were tested [7, 9]. In the present study, a very high prevalence (74.7%) was detected in Microtus spp. which is line with recent studies from Poland and Spain (47–66.8%) [36, 37]. Individuals belonging to the genus Microtus were thus far not examined for immunity or the ability to resolve Bartonella infections. However, regarding the prevalence from the present study it seems highly unlikely that they have the ability to resolve an infection with Bartonella or the duration of resolving the infections seems rather long. The Bartonella species found in this study were likewise present in small mammals from a former study on small mammals [6]. Most Bartonella-positive samples yielded similarity to uncultured Bartonella spp. with unknown pathogenicity. This observation is in line with previous findings in other small mammals from Germany [6]. In our study, the species variety of Bartonella spp. was higher in rodents than in shrews. However, B. taylorii was found in all examined small mammal genera. This Bartonella species is known to be strongly associated with rodent hosts and fleas adapted to rodents such as Ctenophthalmus nobilis [5]. Closely related B. taylorii-associated strains which form in a cluster were found earlier in Sorex shrews from Sweden [34]. Additionally, a moderate prevalence (14.5%) for these B. taylorii-associated strains was detected in S. araneus from the UK [38]. Our study supports this hypothesis of host-specificity of B. taylorii-strains adapted to Sorex spp. as the collected specimens were solely positive for B. taylorii. Bartonella grahamii is the only Bartonella species of proven human-pathogenicity [3] found in rodents from the present study. Although only a small number of Microtus spp. originated in Germany, B. doshiae could exclusively be detected in these individuals, hinting that B. doshiae may have a rather focal distribution pattern in comparison to all other Bartonella species which were detected likewise in voles of both examined countries. Sex and age could not be confirmed as significant demographic factors determining individual infection status with Bartonella sp., which is in contrast to previous studies [35, 39].
CNM was exclusively detected in rodents, and in none of the insectivores here or in previous studies. Earlier studies showed moderate to high prevalences in M. glareolus and A. flavicollis from the Netherlands, Germany, France and Slovakia (1.8–52.7%) [14, 16, 40, 41]. Individuals belonging to the genus Microtus were also previously analysed for the presence of CNM in Germany, Russia, Slovakia and Sweden [10, 13, 15, 42]. However, sample sizes ranged from only two up to 24 individuals per study with a prevalence range of 0–100%. The present study shows a moderate prevalence of 4.6% in Microtus spp. with a more representative number of individuals (n = 149). Individuals belonging to the family Soricidae are assumed not to maintain CNM in the natural life-cycle [15]. As none of the examined Sorex spp. in our study was positive, this suggestion may be confirmed. Previous studies have reported approximately equally high prevalences of CNM in both A. flavicollis and M. glareolus [14, 16]. Moreover, our study showed that males tended to be more often infected with CNM than females. This sex-biased result has already been previously observed in M. glareolus and A. flavicollis and was explained by a higher chance of encountering CNM through a higher stress level in males as well as their higher activity radius and fights due to territorial behaviour [13]. However, another study from Slovakia could not confirm this observation [42]. Moreover, there are reports of male rodents having also higher I. ricinus burdens than females, which was explained by higher levels of testosterone reducing the resistance to tick infestation [43].
Interestingly, only Sorex spp. (5.3%) and M. glareolus (1.3%) were positive for A. phagocytophilum in the present study. High prevalences in Sorex spp. and M. glareolus have previously been reported in studies from Romania, the UK and Switzerland (9.09–19.2%) [2, 44, 45]. In particular, Bown et al. [45] emphasized the importance of S. araneus for the maintenance of certain A. phagocytophilum strains in nature. In this regard, future studies should focus on a more thorough investigation of Sorex spp. as potential reservoirs, as our study also found high prevalences in Sorex spp. In contrast, all other captured small mammal species from the present study presumably only play a minor or no role in the maintenance of A. phagocytophilum in its natural life-cycle in central Europe.
In the present study, neither Babesia nor C. burnetii were found in the small mammals leading to the conclusion that the captured small mammal species may play only a subordinate role in their transmission life-cycle. Pluta et al. [18] also reported a lack of C. burnetii in small mammals from endemic areas in southern Germany. Nonetheless, DNA of C. burnetii was detected by low prevalence rates in brown and in black rats at livestock farms from the Netherlands [46]. In Spain, C. burnetii was further found in a few small mammals collected from a sheep farm with reported Q fever outbreaks [47]. However, these rodents may have acquired the infection via indirect contact with infected sheep rather than through tick bites. In earlier studies, Babesia was found in Microtus and other small mammal species with a low to moderately high prevalence in Switzerland, Germany and Poland (0.4–14.17%) [48–50]. Still, a lack of Babesia spp. has also been reported in A. flavicollis and M. glareolus from Poland [51] which is in line with our findings. Moreover, the overall prevalence for B. microti in Ixodes ricinus ticks from central and eastern Europe is also known to be rather low (0.5–13%) [52].
Conclusions
To our knowledge, this study shows for the first time a very high prevalence of Bartonella in M. arvalis from the Czech Republic. The prevalence for flea-borne bartonellae was higher than for tick-borne pathogens in M. arvalis in contrast to other tested common rodent species such as M. glareolus. The reason may be that Microtus spp. are more likely to live in grassland and agricultural areas than in urban or sylvatic regions where ticks are more prevalent. The species diversity of Bartonella spp. was higher in rodents than in shrews. Sorex spp. seem to be only relevant for the maintenance of non-pathogenic B. taylorii. Interestingly, only Sorex spp. and M. glareolus were positive for A. phagocytophilum in the present study, suggesting their potential importance for the maintenance of certain A. phagocytophilum strains in nature.
Additional file
Additional file 1: Table S1. Details on primers and PCR assays used for the detection of vector-borne pathogens in rodents from Germany and the Czech Republic. Abbreviations: C, conventional PCR; RT, real-time PCR; ITS, intergenic spacer.
Acknowledgements
The authors wish to thank Kerstin Cerncic, Dana Rüster, Viola Haring, Anja Hackbart, Byrgit Hofmann and Dörte Kaufmann for their excellent technical assistance and Klaus Henning for his continuous support. Publication of this paper has been sponsored by Bayer Animal Health in the framework of the 14th CVBD World Forum Symposium.
Abbreviations
- BLASTn
Basic Local Alignment Search Tool nucleotide
- CI
confidence interval
- CNM
“Candidatus Neoehrlichia mikurensis”
- CVBD
Canine vector-borne diseases
- df
degrees of freedom
- GLM
Generalized linear model
- icd
Isocitrate dehydrogenase
- PCR
polymerase chain reaction
Authors’ contributions
MP, AO, RGU, HT and KMS organized and planned the study. JS, MH, KJ and SF provided the samples. AO, NK, MA, KJ, SF, HT and KMS prepared the samples in the laboratory. AO, NK, KMS and MA tested the samples for the presence of Babesia spp., A. phagocytophilum, CNM, Bartonella spp. and C. burnetii. AO performed the sequence analysis. AO and CI performed the statistical analysis. AO, RGU, CI and MP drafted the manuscript and wrote the final version. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
The data supporting the conclusions of this article are included within the article and its additional file. The raw data used and/or analysed during the present study are available from the corresponding author upon reasonable request.
Ethics approval and consent to participate
The present study used DNA samples which were previously obtained for another study. The terms of ethics approval are therefore already mentioned in Jeske et al. [21].
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Anna Obiegala, Email: Anna.Obiegala@vetmed.uni-leipzig.de.
Kathrin Jeske, Email: Kathrin.Jeske@fli.de.
Marie Augustin, Email: marie_augustin@web.de.
Nina Król, Email: nina.krol@vetmed.uni-leipzig.de.
Stefan Fischer, Email: Stefan.Fischer@fli.de.
Katja Mertens-Scholz, Email: Katja.Mertens-Scholz@fli.de.
Christian Imholt, Email: christian.imholt@julius-kuehn.de.
Josef Suchomel, Email: suchomel@mendelu.cz.
Marta Heroldova, Email: heroldova@ivb.cz.
Herbert Tomaso, Email: Herbert.Tomaso@fli.de.
Rainer G. Ulrich, Email: rainer.ulrich@fli.de
Martin Pfeffer, Email: Pfeffer@vetmed.uni-leipzig.de.
References
- 1.Karesh WB, Dobson A, Lloyd-Smith JO, Lubroth J, Dixon MA, Bennett M, et al. Ecology of zoonoses: natural and unnatural histories. Lancet. 2012;380:1936–1945. doi: 10.1016/S0140-6736(12)61678-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liz JS, Anderes L, Sumner JW, Massung RF, Gern L, Rutti B, et al. PCR detection of granulocytic ehrlichiae in Ixodes ricinus ticks and wild small mammals in western Switzerland. J Clin Microbiol. 2000;38:1002–1007. doi: 10.1128/jcm.38.3.1002-1007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breitschwerdt EB. Bartonellosis: one health perspectives for an emerging infectious disease. ILAR J. 2014;55:46–58. doi: 10.1093/ilar/ilu015. [DOI] [PubMed] [Google Scholar]
- 4.Chomel BB, Boulouis HJ, Maruyama S, Breitschwerdt EB. Bartonella spp. in pets and effect on human health. Emerg Infect Dis. 2006;12:389. doi: 10.3201/eid1203.050931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gutiérrez R, Krasnov B, Morick D, Gottlieb Y, Khokhlova IS, Harrus S. Bartonella infection in rodents and their flea ectoparasites: an overview. Vector Borne Zoonotic Dis. 2015;15:27–39. doi: 10.1089/vbz.2014.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silaghi C, Pfeffer M, Kiefer D, Kiefer M, Obiegala A. Bartonella, rodents, fleas and ticks: a molecular field study on host–vector–pathogen associations in Saxony, Eastern Germany. Microb Ecol. 2016;72:965–974. doi: 10.1007/s00248-016-0787-8. [DOI] [PubMed] [Google Scholar]
- 7.Welc-Falęciak R, Bajer A, Behnke JM, Siński E. The ecology of Bartonella spp. infections in two rodent communities in the Mazury Lake District region of Poland. Parasitology. 2010;137:1069–1077. doi: 10.1017/S0031182009992058. [DOI] [PubMed] [Google Scholar]
- 8.Huitu O, Aaltonen K, Henttonen H, Hirvelä-Koski V, Forbes K, Perez-Vera C, et al. Field voles Microtus agrestis as reservoirs of Bartonella spp. Genes, ecosystems and risk of infection (GERI), 21–23 April 2015, Heraklion, Crete, Greece. Abstracts.
- 9.Schmidt S, Essbauer SS, Mayer-Scholl A, Poppert S, Schmidt-Chanasit J, Klempa B, et al. Multiple infections of rodents with zoonotic pathogens in Austria. Vector Borne Zoonotic Dis. 2014;14:467–475. doi: 10.1089/vbz.2013.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rar V, Golovljova I. Anaplasma, Ehrlichia, and “Candidatus Neoehrlichia” bacteria: pathogenicity, biodiversity, and molecular genetic characteristics, a review. Infect Genet Evol. 2011;11:1842–1861. doi: 10.1016/j.meegid.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 11.Obiegala A, Silaghi C. Candidatus Neoehrlichia mikurensis—recent insights and future perspectives on clinical cases, vectors, and reservoirs in Europe. Curr Clin Microbiol Rep. 2018;5:1–9. doi: 10.1007/s40588-018-0085-y. [DOI] [Google Scholar]
- 12.Dumler JS, Choi KS, Garcia-Garcia JC, Barat NS, Scorpio DG, Garyu JW, et al. Human granulocytic anaplasmosis and Anaplasma phagocytophilum. Emerg Infect Dis. 2005;11:1828. doi: 10.3201/eid1112.050898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Obiegala A, Pfeffer M, Pfister K, Tiedemann T, Thiel C, Balling A, et al. Candidatus Neoehrlichia mikurensis and Anaplasma phagocytophilum: prevalences and investigations on a new transmission path in small mammals and ixodid ticks. Parasites Vectors. 2014;7:563. doi: 10.1186/s13071-014-0563-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jahfari S, Fonville M, Hengeveld P, Reusken C, Scholte EJ, Takken W, et al. Prevalence of Neoehrlichia mikurensis in ticks and rodents from north-west Europe. Parasites Vectors. 2012;5:74. doi: 10.1186/1756-3305-5-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andersson M, Råberg L. Wild rodents and novel human pathogen Candidatus Neoehrlichia mikurensis, southern Sweden. Emerg Infect Dis. 2011;17:1716. doi: 10.3201/eid1709.101058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silaghi C, Woll D, Hamel D, Pfister K, Mahling M, Pfeffer M. Babesia spp. and Anaplasma phagocytophilum in questing ticks, ticks parasitizing rodents and the parasitized rodents—analyzing the host–pathogen–vector interface in a metropolitan area. Parasites Vectors. 2012;5:191. doi: 10.1186/1756-3305-5-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bown KJ, Lambin X, Ogden NH, Begon M, Telford G, Woldehiwet Z, et al. Delineating Anaplasma phagocytophilum ecotypes in coexisting, discrete enzootic cycles. Emerg Infect Dis. 2009;15:1948. doi: 10.3201/eid1512.090178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pluta S, Hartelt K, Oehme R, Mackenstedt U, Kimmig P. Prevalence of Coxiella burnetii and Rickettsia spp. in ticks and rodents in southern Germany. Ticks Tick Borne Dis. 2010;1:145–147. doi: 10.1016/j.ttbdis.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Gray J, Von Stedingk LV, Gürtelschmid M, Granström M. Transmission studies of Babesia microti in Ixodes ricinus ticks and gerbils. J Clin Microbiol. 2002;40:1259–1263. doi: 10.1128/JCM.40.4.1259-1263.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hildebrandt A, Hunfeld KP, Baier M, Krumbholz A, Sachse S, Lorenzen T, et al. First confirmed autochthonous case of human Babesia microti infection in Europe. Eur J Clin Microbiol Infect Dis. 2007;26:595–601. doi: 10.1007/s10096-007-0333-1. [DOI] [PubMed] [Google Scholar]
- 21.Jeske K, Tomaso H, Imholt C, Schulz J, Beerli O, Suchomel J, et al. Detection of Francisella tularensis in three vole species in central Europe. Transbound Emerg Dis. 2018 doi: 10.1111/tbed.13078. [DOI] [PubMed] [Google Scholar]
- 22.Bajer A, Bednarska M, Pawełczyk A, Behnke JM, Gilbert FS, Sinski E. Prevalence and abundance of Cryptosporidium parvum and Giardia spp. in wild rural rodents from the Mazury Lake District region of Poland. Parasitology. 2002;125:21–34. doi: 10.1017/S0031182002001865. [DOI] [PubMed] [Google Scholar]
- 23.Baláž I, Ambros M. Shrews (Sorex spp.) somatometry and reproduction in Slovakia. Biologia. 2006;61:611–620. doi: 10.2478/s11756-006-0098-5. [DOI] [Google Scholar]
- 24.Kosoy M, McKee C, Albayrak L, Fofanov Y. Genotyping of Bartonella bacteria and their animal hosts: current status and perspectives. Parasitology. 2018;145:543–562. doi: 10.1017/S0031182017001263. [DOI] [PubMed] [Google Scholar]
- 25.Schorn S, Pfister K, Reulen H, Mahling M, Silaghi C. Occurrence of Babesia spp., Rickettsia spp. and Bartonella spp. in Ixodes ricinus in Bavarian public parks, Germany. Parasites Vectors. 2011;4:135. doi: 10.1186/1756-3305-4-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maggi RG, Diniz PP, Cadenas MB, Breitschwerdt EB. The use of molecular diagnostic techniques to detect Anaplasma, Bartonella and Ehrlichia species in arthropods or patients. In: The international canine vector-borne disease symposium, April 18th–20th 2006, Billesley, Alcester, UK. p. 9–14.
- 27.Casati S, Sager H, Gern L, Piffaretti JC. Presence of potentially pathogenic Babesia sp. for human in Ixodes ricinus in Switzerland. Ann Agric Environ Med. 2006;13:65–70. [PubMed] [Google Scholar]
- 28.Silaghi C, Kauffmann M, Passos LM, Pfister K, Zweygarth E. Isolation, propagation and preliminary characterisation of Anaplasma phagocytophilum from roe deer (Capreolus capreolus) in the tick cell line IDE8. Ticks Tick Borne Dis. 2011;2:204–208. doi: 10.1016/j.ttbdis.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Courtney JW, Kostelnik LM, Zeidner NS, Massung RF. Multiplex real-time PCR for detection of Anaplasma phagocytophilum and Borrelia burgdorferi. J Clin Microbiol. 2004;42:3164–3168. doi: 10.1128/JCM.42.7.3164-3168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klee SR, Tyczka J, Ellerbrok H, Franz T, Linke S, Baljer G, et al. Highly sensitive real-time PCR for specific detection and quantification of Coxiella burnetii. BMC Microbiol. 2006;6:2. doi: 10.1186/1471-2180-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.R Core Team . R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2016. [Google Scholar]
- 32.Harms A, Dehio C. Intruders below the radar: molecular pathogenesis of Bartonella spp. Clin Microbiol Rev. 2012;25:42–78. doi: 10.1128/CMR.05009-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Obiegala A, Heuser E, Ryll R, Imholt C, Fürst J, Prautsch L-M, et al. Norway and black rats in Europe: potential reservoirs for zoonotic arthropod-borne pathogens? Pest Manag Sci. 2019 doi: 10.1002/ps.5323. [DOI] [PubMed] [Google Scholar]
- 34.Holmberg M, Mills JN, McGill S, Benjamin G, Ellis BA. Bartonella infection in sylvatic small mammals of central Sweden. Epidemiol Infect. 2003;130:149–157. doi: 10.1017/S0950268802008075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paziewska A, Harris PD, Zwolińska L, Bajer A, Siński E. Differences in the ecology of Bartonella infections of Apodemus flavicollis and Myodes glareolus in a boreal forest. Parasitology. 2012;139:881–893. doi: 10.1017/S0031182012000170. [DOI] [PubMed] [Google Scholar]
- 36.Rodríguez-Pastor R, Escudero R, Lambin X, Vidal MD, Gil H, Jado I, et al. Zoonotic pathogens in fluctuating common vole (Microtus arvalis) populations: occurrence and dynamics. Parasitology. 2019;146:389–398. doi: 10.1017/S0031182018001543. [DOI] [PubMed] [Google Scholar]
- 37.Tołkacz K, Alsarraf M, Kowalec M, Dwużnik D, Grzybek M, Behnke JM, Bajer A. Bartonella infections in three species of Microtus: prevalence and genetic diversity, vertical transmission and the effect of concurrent Babesia microti infection on its success. Parasites Vectors. 2018;11:491. doi: 10.1186/s13071-018-3047-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bray DP, Bown KJ, Stockley P, Hurst JL, Bennett M, Birtles RJ. Haemoparasites of common shrews (Sorex araneus) in northwest England. Parasitology. 2007;134:819–826. doi: 10.1017/S0031182007002302. [DOI] [PubMed] [Google Scholar]
- 39.Kosoy M, Bai Y, Sheff K, Morway C, Baggett H, Maloney SA, et al. Identification of Bartonella infections in febrile human patients from Thailand and their potential animal reservoirs. Am J Trop Med Hyg. 2010;82:1140–1145. doi: 10.4269/ajtmh.2010.09-0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Víchová B, Majláthová V, Nováková M, Stanko M, Hviščová I, Pangrácová L, et al. Anaplasma infections in ticks and reservoir host from Slovakia. Infect Genet Evol. 2014;22:265–272. doi: 10.1016/j.meegid.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 41.Vayssier-Taussat M, Le Rhun D, Buffet JP, Maaoui N, Galan M, Guivier E, et al. Candidatus Neoehrlichia mikurensis in bank voles, France. Emerg Infect Dis. 2012;18:2063. doi: 10.3201/eid1812.120846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Svitálková ZH, Haruštiaková D, Mahríková L, Mojšová M, Berthová L, Slovák M, et al. Candidatus Neoehrlichia mikurensis in ticks and rodents from urban and natural habitats of south-western Slovakia. Parasites Vectors. 2016;9:2. doi: 10.1186/s13071-015-1287-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hughes VL, Randolph SE. Testosterone increases the transmission potential of tick-borne parasites. Parasitology. 2001;123:365–371. doi: 10.1017/S0031182001008599. [DOI] [PubMed] [Google Scholar]
- 44.Matei IA, D’Amico G, Ionică AM, Kalmár Z, Corduneanu A, Sándor AD, et al. New records for Anaplasma phagocytophilum infection in small mammal species. Parasites Vectors. 2018;11:193. doi: 10.1186/s13071-018-2791-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bown KJ, Lambin X, Telford G, Heyder-Bruckner D, Ogden NH, Birtles RJ. The common shrew (Sorex araneus): a neglected host of tick-borne infections? Vector Borne Zoonotic Dis. 2011;11:947–953. doi: 10.1089/vbz.2010.0185. [DOI] [PubMed] [Google Scholar]
- 46.Reusken C, van der Plaats R, Opsteegh M, de Bruin A, Swart A. Coxiella burnetii (Q fever) in Rattus norvegicus and Rattus rattus at livestock farms and urban locations in the Netherlands; could Rattus spp. represent reservoirs for (re) introduction? Prev Vet Med. 2011;101:124–130. doi: 10.1016/j.prevetmed.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 47.Barandika JF, Hurtado A, García-Esteban C, Gil H, Escudero R, Barral M, et al. Tick-borne zoonotic bacteria in wild and domestic small mammals in northern Spain. Appl Environ Microbiol. 2007;73:6166–6171. doi: 10.1128/AEM.00590-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burri C, Dupasquier C, Bastic V, Gern L. Pathogens of emerging tick-borne diseases, Anaplasma phagocytophilum, Rickettsia spp., and Babesia spp., in Ixodes ticks collected from rodents at four sites in Switzerland (Canton of Bern) Vector Borne Zoonotic Dis. 2011;11:939–944. doi: 10.1089/vbz.2010.0215. [DOI] [PubMed] [Google Scholar]
- 49.Obiegala A, Pfeffer M, Pfister K, Karnath C, Silaghi C. Molecular examinations of Babesia microti in rodents and rodent-attached ticks from urban and sylvatic habitats in Germany. Ticks Tick Borne Dis. 2015;6:445–449. doi: 10.1016/j.ttbdis.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 50.Siński E, Bajer A, Welc R, Pawełczyk A, Ogrzewalska M, Behnke JM. Babesia microti: prevalence in wild rodents and Ixodes ricinus ticks from the Mazury Lakes District of north-eastern Poland. Int J Med Microbiol. 2006;296:137–143. doi: 10.1016/j.ijmm.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 51.Karbowiak G, Stanko M, Rychlik L, Nowakowski W, Siuda K. The new data about zoonotic reservoir of Babesia microti in small mammals in Poland. Acta Parasitol. 1999;44:142–144. [Google Scholar]
- 52.Rudolf I, Golovchenko M, Sikutová S, Rudenko N. Babesia microti (Piroplasmida: Babesiidae) in nymphal Ixodes ricinus (Acari: Ixodidae) in the Czech Republic. Folia Parasitol. 2005;52:274–276. doi: 10.14411/fp.2005.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Details on primers and PCR assays used for the detection of vector-borne pathogens in rodents from Germany and the Czech Republic. Abbreviations: C, conventional PCR; RT, real-time PCR; ITS, intergenic spacer.
Data Availability Statement
The data supporting the conclusions of this article are included within the article and its additional file. The raw data used and/or analysed during the present study are available from the corresponding author upon reasonable request.