Abstract
In this Chapter, we describe methods for functional genomics studies in mouse macrophages. In particular, we describe complementary methods for gene inhibition using RNA-interference (RNAi) and gene overexpression. These methods are readily amenable to medium- and high-throughput functional genomics investigations. These complementary loss-of-function and gain-of-function genomic approaches provide a rapid means of investigating the function of candidate genes prior to initiating more cumbersome studies in vivo.
Keywords: Macrophage, RAW264.7, RNAi, siRNA, overexpression
1. Introduction
While a genetic lesion is the gold standard to investigate gene function on a case by case basis [1], the ability to manipulate gene activity in medium- or high-throughput fashion remains a mainstay of biological investigation [2]. In this chapter, we outline methods for functional genomics studies in mouse macrophages. In particular, we describe (1) methods using RNA interference (RNAi) to inhibit the function of candidate genes and (2) gene overexpression approaches to activate candidate genes. These complementary loss-of-function and gain-of-function assays offer a powerful approach to test the function of novel gene candidates prior to the initiation of in vivo investigations.
Macrophages can be a challenging cell type in which to perform such experiments. Macrophages are difficult to transfect efficiently without affecting cell viability [3,4], and macrophages are somewhat resistant to RNAi compared to other cell types. These challenges are even more pronounced in primary macrophages. To overcome these issues, we have optimized transfection, RNAi using siRNAs for gene inhibition, and gene overexpression techniques in one of the most commonly used immortalized mouse macrophage cell lines, RAW264.7 [5–8]. While working with this cell line may not recapitulate all of the biology present in primary cells derived directly from mice, the ability to manipulate these cells is a positive trade-off.
First, we describe a method to efficiently deliver siRNA to RAW264.7 macrophages using an Amaxa electroporation system (see Note 1). We have optimized transfection with this system to obtain close to 100% transfection efficiency, excellent cell viability post-transfection, and good siRNA-mediated gene knockdown. Macrophages perform many critical immune functions [9], and methods to monitor some of these functions are described in other chapters in this volume. As an example of one possible immune assay, we describe the ability to test the effect of siRNA-mediated inhibition of candidate genes on lipopolysaccharide-induced inflammatory cytokine production.
Second, we describe a method to deliver plasmids engineered to overexpress candidate genes. With this optimized technique, we routinely obtain approximately 50% transfection efficiency. Because of imperfect transfection in macrophages, follow-up studies require approaches to identify transfected cells. These approaches include using plasmids engineered to express either drug resistance genes or fluorescent proteins. In the former, toxic agents (such as neomycin) are added to the transfected cells. Only those that have been successfully transfected can survive. In the latter, successfully transfected cells express fluorescent proteins and can be purified using flow cytometry with cell sorting. To illustrate an approach that we have found is amenable to high-throughput study, we describe the use of cytokine-luciferase reporters to investigate the immune effects of overexpression of candidate genes.
2. Materials
2.1. RNAi
RAW264.7 cells (immortalized mouse macrophage cell line)
Amaxa Nucleofector 96-well shuttle and associated equipment (Nucleofector 2 device and attached computer) (Lonza)
SF Cell Line 96-well Nucleofector Transfection Kit (Lonza). This kit contains the 96-well electroporation plate, the transfection solution, and the transfection supplement. The supplement solution should be added to the transfection solution prior to use.
Centrifuge
Macrophage culture medium: DMEM (containing glucose, L-glutamine, and sodium pyruvate) supplemented with 10% FBS
0.25% Trypsin/EDTA
PBS
RPMI-1640
siRNA(s) targeting gene(s) of interest
Negative and positive control siRNAs (see Note 2)
Fluorescently labeled siRNA (see Note 2)
Tissue culture treated dishes or flasks
96-well tissue culture plates
96-well round bottom plates
E. coli Lipopolysaccharide (LPS)
Mouse cytokine ELISA kit
2.2. Overexpression
Macrophage culture medium: DMEM (containing glucose, L-glutamine, and sodium pyruvate) supplemented with 10% FBS
0.25% Trypsin/EDTA
PBS
Fugene HD (Roche) (see Note 3)
Opti-Mem (Thermo Fisher Scientific)
Tissue culture treated dishes or flasks
24-well tissue culture plates
Plasmid(s) driving expression of candidate gene(s) (see Note 4)
Plasmid(s) driving expression of control gene(s) (see Note 4)
Plasmid driving expression of GFP (see Note 4)
Plasmid driving expression of firefly luciferase reporter (see Note 5)
Plasmid driving expression of Renilla luciferase internal control reporter (see Note 5)
Dual luciferase assay kit that can measure firefly and Renilla luciferase activity
3. Methods
3.1. RNAi: Setting up Amaxa System for macrophage transfection
Turn on the Amaxa 96-well shuttle, the Amaxa Nucleofector II device, and the attached laptop.
Start the Amaxa 96-well shuttle software. Choose new parameter file from the file menu.
Select the wells to be transfected on the 96-well schematic and apply program DS-136 to these wells (see Note 6).
Add the supplement solution to the Solution SF in the 96-well Nucleofector Solution SF kit prior to use. Allow this supplemented solution to equilibrate at room temperature.
3.2. RNAi: Preparation of macrophages for transfection
Maintain RAW264.7 cells in macrophage culture medium at 37°C in 5% CO2. Grow cells to approximately 70–80% confluence (see Note 7). Prepare enough cells to transfect the planned number of samples (200,000 cells per sample).
Pre-warm macrophage culture medium, Trypsin/EDTA, and RPMI-1640 to 37°C.
To collect macrophages for transfection, aspirate off culture medium from plates containing adherent RAW264.7 cells and wash with PBS. Aspirate the PBS wash and then add prewarmed Trypsin-EDTA to the cells (1 ml Trypsin-EDTA is sufficient to cover the cells on a 10 cm cell culture dish). Incubate cells for 2–3 minutes with occasional plate agitation. Do not over-trypsinize as this can damage the macrophages and diminish transfection efficiency. Once the cells are detached, add macrophage culture medium (9 ml for a 10 cm culture dish) and pipette up and down to finish detaching macrophages from the plate.
Count the macrophages using a hemacytometer. Calculate how many cells are needed for the experiment (200,000 cells/transfection well). Be sure to include a few extra wells worth of cells to allow for pipetting errors.
Centrifuge the calculated number of macrophages at 150 g for 10 min at room temperature (see Note 8).
Aspirate off the supernatant and resupend the pelleted macrophages in the appropriate volume (20 μl per 200,000 cells) of nucleofector SF transfection solution containing the transfection supplement. This solution is toxic to the cells, so once the cells are resupsended, additional transfection steps should be performed rapidly with the goal of returning the cells to macrophage culture medium as soon as feasible.
3.3. RNAi: Macrophage transfection and cell recovery
Aliquot 2 μl of siRNA to each well of a 96-well round-bottom plate (see Note 9 about siRNA dose). This step can be performed while the RAW264.7 cells are being centrifuged (Section 3.2, Step 5).
Add 20 μl of the resuspended cells to each well containing siRNA (this can be facilitated by using a multichannel or repeat pipettor). Mix gently by pipetting up and down. Transfer 20 μl of this mixture into the 96-well nucleofection plate. Avoid pipetting air bubbles to prevent electroporation errors.
Place covered nucleofection plate in the 96-well nucleofector shuttle, and press the upload and start button to initiate transfection. Note and censor any wells that have a transfection error (as reported by the software during transfection); if the solutions are prepared correctly and no bubbles are present, there should rarely if ever be errors.
Add 80 μl of pre-warmed RPMI-1640 to each well (see Note 10); be sure to add the liquid gently as the cells are very fragile after electroporation. Allow the cells to recover at 37°C in 5% CO2 for 2 minutes.
Transfer the cells to a fresh 96-well tissue culture plate, add an additional 100 μl of macrophage culture medium. Once the macrophages have attached to the plastic several hours later, the medium can be removed and fresh macrophage culture medium added (although in our experience, this is not necessary). Incubate macrophages in 96-well tissue culture plate until time of analysis (24–36 hours after transfection).
3.4. RNAi: Follow-up studies
As outlined in other chapters in this volume, macrophages perform numerous immune functions that can be monitored following siRNA delivery. In other cell types, such studies are typically performed 48–72 hours after siRNA delivery to allow time for the endogenous mRNA to be degraded. In our experience in mouse macrophages, we find that the optimal time follow-up study is much earlier, 24–36 hours post-transfection. This timing can be further optimized on a gene by gene basis.
A variety of controls should be performed to validate these RNAi studies. siRNA-mediated gene knockdown should be quantified at either the mRNA level (by isolating RNA from the adherent cells and monitoring gene knockdown by qPCR) or even better at the protein level (by western blot or flow cytometry). Knockdown varies from siRNA to siRNA and is not as robust as in other cell lines, but 70–90% knockdown for most genes should be achievable. A non-targeting siRNA or other negative control siRNA should be used as a control, because the electroporation can affect the cells. Transfection efficiency should be monitored using a fluorescently-labeled siRNA in conjunction with flow cytometry. We routinely obtain close to 100% transfection efficiency with this technique.
Cell viability should also be monitored for two reasons. First, improper transfection technique (in particular transfecting unhealthy cells or being too rough with the cells) can lead to significant cell death. Second, inhibition of essential target genes could alter cell viability. A variety of kits are available commercially to monitor cell viability.
Optimization considerations include modifying the dose of siRNA (we typically start with a high dose and then titrate the dose down if we identify siRNAs that induce a phenotype), timing after transfection for assay, and health and passage number of cells. To avoid off-target siRNA effects [10], various validation steps should be performed to confirm any siRNA-induced phenotypes observed. These include using multiple siRNA duplexes targeting the same gene to test if multiple duplexes induce the same phenotype, titrating the siRNA to the lowest dose that induces a phenotype, monitoring knockdown efficiency, and ultimately, rescuing the induced phenotype by re-introducing a transgene that cannot be targeted by the siRNA. Identifying a gene-overexpression-induced phenotype (see Section 3.5) that is opposite to that induced by RNAi also strengthens the argument that the effect of the siRNA is specific.
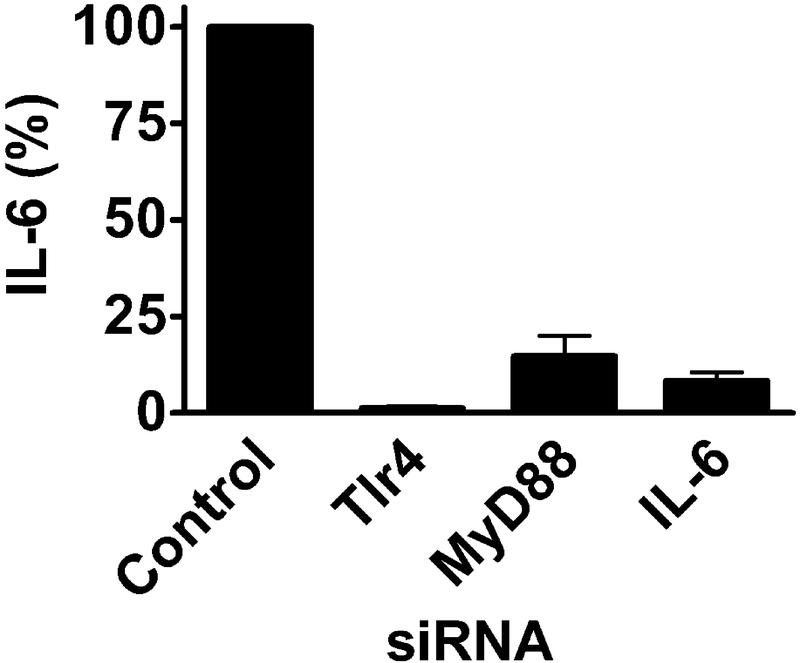
To illustrate one application of siRNA-mediated gene inhibition, we knocked down genes in the TLR4 signaling pathway that mediate the response to lipopolysaccharide (LPS) from Gram negative bacteria. In wild-type macrophages, LPS induces significant inflammatory cytokine production; mutation or inhibition of genes in the TLR4 signaling pathway greatly weakens this response. In this sample experiment, 24 hours after siRNA transfection the macrophage culture medium was replaced with macrophage culture medium containing 20 ng/ml LPS. Six hours later, the cell culture supernatant was collected for analysis of pro-inflammatory cytokine production by ELISA (Fig. 1).
Fig. 1. siRNA-mediated inhibition of genes in the TLR4 signaling pathway inhibits LPS-induced pro-inflammatory cytokine production.
Pools of 4 siRNA duplexes (Dharmacon) targeting the indicated genes or a pool of control siRNA duplexes that did not target any mouse gene (non-targetting control siRNA, Dharmacon) were transfected into RAW264.7 macrophages. After 24 hrs, 20 ng/ml LPS was added, and 6 hrs later, IL-6 release into the supernatant was monitored by ELISA. Inhibition of TLR4 (the LPS receptor), MyD88 (a downstream signaling adaptor), or IL-6 itself with siRNA all greatly weakened LPS-induced IL-6 production without altering cell viability (not shown).
3.5. Gene overexpression: macrophage transfection
Maintain RAW264.7 cells in macrophage culture medium at 37°C and 5% CO2. Grow cells to approximately 70–80% confluence. Prepare enough cells to transfect the planned number of wells (200,000 cells per well).
Pre-warm to 37°C macrophage culture medium and Trypsin/EDTA.
To collect macrophages for transfection, aspirate off macrophage culture medium from plates containing adherent RAW264.7 cells and wash with PBS. Aspirate the PBS wash and then add pre-warmed Trypsin-EDTA to the cells (1 ml Trypsin-EDTA is sufficient to cover the cells on a 10 cm cell culture dish). Incubate cells for 2–3 minutes with occasional plate agitation. Do not over-trypsinize as this can damage the macrophages and diminish transfection efficiency. Once the cells are detached, add macrophage culture medium (9 ml for a 10 cm culture dish) and pipette up and down to finish detaching macrophages from the plate.
Count the macrophages using a hemacytometer. Aliquot 200,000 cells/well of a 24-well plate and bring total volume of macrophage culture medium in each well to 1 ml. Incubate macrophages in 24-well format overnight at 37°C and 5% CO2.
Pre-warm cell OptiMem, macrophage culture medium, and Fugene HD to room temperature
Mix DNA (300 ng - 1 μg total), Fugene HD (3.75 μl) and OptiMem to a total volume of 100 μl. Incubate for 15 minutes at room temperature.
While incubating DNA and Fugene HD, wash the cells with 1 ml OptiMem and replenish with fresh macrophage culture media.
Add the entire mixture to one well of the 24-well plate containing RAW264.7 cells (see Note 11).
Incubate cells at 37°C in 5% CO2 for 24 hrs.
3.6. Gene overexpression: follow-up assays
The design of follow-up studies should take into account the imperfect transfection efficiency of macrophages. If the overexpression plasmid contains a suitable selectable marker, then the appropriate antibiotic can be added 24–48 hrs after transfection to select stable lines. Alternatively, if the plasmid expresses a fluorescent protein, then flow cytometry can be used to sort macrophages carrying the transfected plasmid.
We have also co-transfected cytokine-luciferase reporters to monitor the effect of gene overexpression on an inflammatory readout. In these studies, we used a high ratio of overexpression plasmid to luciferase reporters to maximize the likelihood that the reporter will be present in cells also containing overexpression plasmid. We outline this procedure below.
We transfect 600 ng of the candidate gene overexpression plasmid, 300 ng of Elam-firefly luciferase reporter (Elam-luc), and 100 ng of an internal control gene Renilla luciferase reporter (SV40-rluc). Elam-luc is an NFκB-dependent reporter; SV40-rluc was used to normalize/control for transfection efficiency between wells.
Transfect and recover cells as described in Section 3.5 (see Note 12).
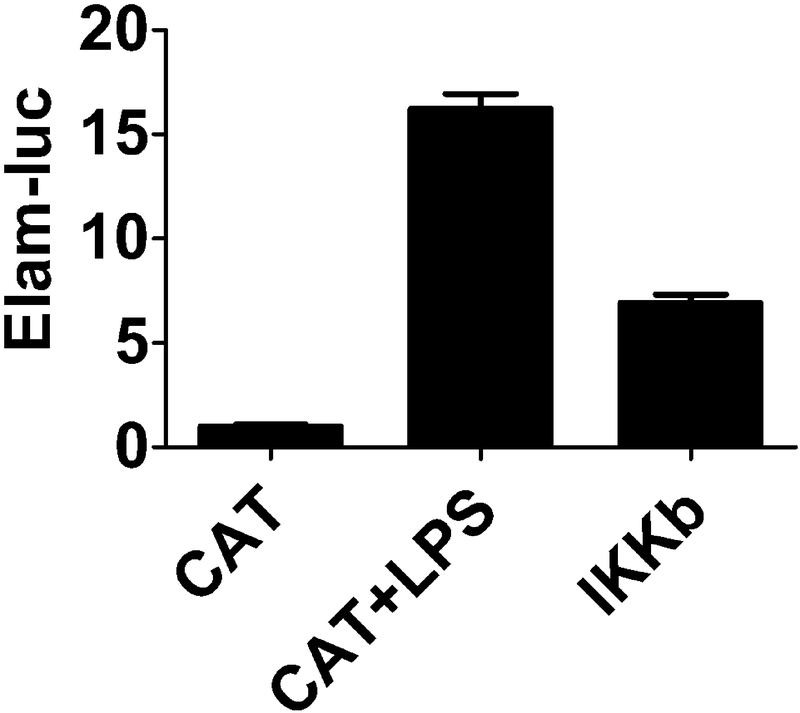
24 hours after transfection stimulate cells with 20 ng/ml LPS for 24 hrs and then monitor luciferase activity using a commercially available dual luciferase activity monitoring kit. We provide sample data for a gene that enhances NFκB-reporter activity in Fig. 2.
Fig. 2. IKKβ overexpression increases transcription of an NFκB-dependent luciferase reporter.
Three plasmids expressing 300 ng Elam-luc, 100 ng SV40-rluc, or 600 ng candidate gene overexpression were cotransfected into RAW264.7 macrophages in 24-well format. Elam-luciferase is an NFκB-dependent firefly luciferase reporter (gift of D. Golenbock). SV40-rluc is a Renilla luciferase reporter used for normalization to control for transfection efficiency (Promega). The candidate genes overexpressed include either the known NFκB activator HA-IKK2 (Addgene, [11]) or negative control Chloramphenicol Acetyltransferase (CAT) (Invitrogen). 24 hours after transfection, cells were stimulated with 20 ng/ml LPS for 24 hours or not as indicated. Luciferase activity was then monitored using the Dual Luciferace Assay Kit (Promega). LPS treatment or IKKβ overexpression stimulated transcription of the NFκB-dependent reporter.
4. Notes
A variety of approaches have been used to transfect macrophage cell lines. While lipid-mediated transfection can be used to deliver siRNA on a case-by-case basis, in our experience, electroporation is more consistently robust and more amenable to medium- and high-throughput approaches. The trade-off is that electroporation is more expensive than lipid-mediated transfection.
In addition to delivering siRNAs targeting candidate genes, several control siRNAs should be transfected in each experiment. These include fluorescently labeled siRNAs, which can be used to monitor transfection efficiency with flow cytometry or microscopy. Appropriate controls also include siRNAs that do not target any particular gene or that target a gene known to not affect the phenotype of interest. This serves as a negative control. It is important to compare the results with candidate siRNAs to these negative control siRNAs instead of (or at least in addition to) untransfected control cells, because the mere act of transfecting the cells does alter them biologically. Additionally, positive control siRNAs known to affect the phenotype of interest should also be included if available.
There are numerous chemical-transfection reagents available. In our experience, Fugene HD provides very efficient transfection of plasmids into macrophages (on the order of 50%). In contrast, we have not found Fugene HD to reliably transfect siRNAs into macrophages.
There are many commercial sources for plasmids driving cDNA expression for most mouse or human genes, all engineered with different promoters and selectable markers. We routinely use plasmids that drive gene expression with the strong CMV promoter but other promoter-driven plasmids are available. Control plasmids should be used in these overexpression studies, including negative control and positive control plasmids that express genes that either don’t or do affect the phenotype of interest, respectively. Transfection efficiency should also be monitored using plasmids expressing a fluorescent protein such as GFP in conjunction with flow cytometry or microscopy.
Transfection efficiencies above 50% or so are hard to obtain in macrophages. These luciferase reporters will be used to overcome this inefficiency.
We also have optimized siRNA-mediated transfection conditions for J774A.1, another commonly used mouse macrophage cell line. We treat this other macrophage cell line exactly the same as the RAW264.7 macrophages, except that program DS-130 should be used to transfect J774A.1 cells.
Key to obtaining good transfection is working with very healthy cells. Cells should not be allowed to overgrow, should be used at a relatively early passage number, should not be centrifuged too rapidly, and in general should be treated in a delicate fashion.
Don’t centrifuge too fast, as this can damage the cells.
The exact siRNA dose should be optimized for each siRNA. As a good starting point, we use a relatively high siRNA dose in macrophages, 2 μl of a 20 mM siRNA solution or 40 pmol of siRNA per transfection.
The cells are very fragile after electroporation, so treat them gently. To avoid introducing a calcium spike that can damage the cells at this stage, RPMI-1640 (which is low in calcium) rather than DMEM is added. Once the cells recover for a few minutes, DMEM can be added to the cells too.
The amount of DNA and Fugene HD used (and their relative ratio) may need to be titrated by the individual investigator.
LPS contamination of the plasmid preparations can confound these results. Be sure to use a miniprep kit for plasmid purification that removes LPS contamination and use a control plasmid prepared with the same kit.
Acknowledgements
This work was funded by NIH grant R01ES025161 and the Wendy Siegel Fund for Leukemia and Cancer Research. Thanks to D. Golenbock for the Elam-luc reporter construct.
Footnotes
This is a pre-copyedited version of an article published in Methods in Molecular Biology. The final authenticated version is available online at the Springer Website.
REFERENCES
- 1.Capecchi MR (2005) Gene targeting in mice: functional analysis of the mammalian genome for the twenty-first century. Nature reviews Genetics 6 (6):507–512. [DOI] [PubMed] [Google Scholar]
- 2.Taylor J, Woodcock S (2015) A Perspective on the Future of High-Throughput RNAi Screening: Will CRISPR Cut Out the Competition or Can RNAi Help Guide the Way? Journal of biomolecular screening 20 (8):1040–1051. [DOI] [PubMed] [Google Scholar]
- 3.Carralot JP, Kim TK, Lenseigne B, Boese AS, Sommer P, Genovesio A, Brodin P (2009) Automated high-throughput siRNA transfection in raw 264.7 macrophages: a case study for optimization procedure. Journal of biomolecular screening 14 (2):151–160. [DOI] [PubMed] [Google Scholar]
- 4.Lee G, Santat LA, Chang MS, Choi S (2009) RNAi methodologies for the functional study of signaling molecules. PloS one 4 (2):e4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Arras L, Alper S (2013) Limiting of the innate immune response by SF3A-dependent control of MyD88 alternative mRNA splicing. PLoS Genet 9 (10):e1003855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Arras L, Guthrie BS, Alper S (2014) Using RNA-interference to investigate the innate immune response in mouse macrophages. Journal of visualized experiments : JoVE (93):e51306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Arras L, Laws R, Leach SM, Pontis K, Freedman JH, Schwartz DA, Alper S (2014) Comparative genomics RNAi screen identifies Eftud2 as a novel regulator of innate immunity. Genetics 197 (2):485–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Arras L, Seng A, Lackford B, Keikhaee MR, Bowerman B, Freedman JH, Schwartz DA, Alper S (2013) An evolutionarily conserved innate immunity protein interaction network. The Journal of biological chemistry 288 (3):1967–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burke B, Lewis CE (2002) The macrophage. 2nd edn Oxford University Press, Oxford; New York [Google Scholar]
- 10.Editorial (2003) Whither RNAi? Nature cell biology 5 (6):489–490. [DOI] [PubMed] [Google Scholar]
- 11.Nakano H, Shindo M, Sakon S, Nishinaka S, Mihara M, Yagita H, Okumura K (1998) Differential regulation of IkappaB kinase alpha and beta by two upstream kinases, NF-kappaB-inducing kinase and mitogen-activated protein kinase/ERK kinase kinase-1. Proceedings of the National Academy of Sciences of the United States of America 95 (7):3537–3542. [DOI] [PMC free article] [PubMed] [Google Scholar]