Abstract
In flowering plants, the evolution of females is widely hypothesized to be the first step in the evolutionary pathway to separate male and female sexes, or dioecy. Natural enemies have the potential to drive this evolution if they preferentially attack hermaphrodites over females. We studied sex-based differences in exposure to anther-smut (Microbotryum), a sterilizing pollinator-transmitted disease, in Dianthus pavonius, a gynodioecious perennial herb. We found that within a heavily diseased population, females consistently had lower levels of Microbotryum spore deposition relative to hermaphrodites and that this difference was driven by rapid floral closing in females following successful pollination. We further show that this protective closing behavior is frequency-dependent; females close faster when they are rare. These results indicate that anther-smut disease is an important source of selection for females, especially since we found in a common garden experiment no evidence that females have any inherent fecundity advantages over hermaphrodites. Finally, we show that among populations, those where anther-smut is present have a significantly higher frequency of females than those where the disease is absent. Taken together our results indicate that anther-smut disease is likely an important biotic factor driving the evolution and maintenance of females in this gynodioecious species.
INTRODUCTION
The evolution of females from hermaphroditic populations (gynodioecy) is widely hypothesized to be an important pathway for the evolution of separate males and females (dioecy: Charlesworth and Charlesworth 1978; Barrett 2002; Dufay et al. 2014). As females increase in frequency, hermaphrodites gradually gain more fitness through male function, setting the selective conditions necessary for the evolution of true males (Charlesworth and Charlesworth 1978). Theory shows that for females to increase within populations, they must have some fitness advantage over hermaphrodites, with the strength of this advantage depending on the genetic basis of sex (Charlesworth 1981; Gouyon et al. 1991). The majority of work has focused on selection arising from inherent female fertility advantages that result from reallocation of resources (Ashman 2001; Delph and Carroll 2001; Dufay and Billard 2012), and avoidance of inbreeding depression (Charlesworth and Charlesworth 1978; Sakai et al. 1997). However, these explanations are not always sufficient (Alonso and Herrera 2001; Marshall and Ganders 2001), and there has been a growing awareness that biotic interactions, particularly natural enemies, can play an important role in selection for females (Ashman 2002; Steets et al. 2007).
A plurality of studies have now found that herbivores, particularly florivores (Ashman et al. 2004; Tsuji and Sota 2010) and pre-dispersal seed predators (Marshall and Ganders 2001; Collin and Shykoff 2010; Clarke and Brody 2015; Miyake et al. 2018) preferentially favor hermaphrodites over females. Indeed, in some cases, natural enemies may provide the best explanation for the maintenance of females. For example, Marshall and Ganders (2001) found that the maintenance of females in natural populations of Sidalcea hendersonii, cannot be fully explained without taking into account differential predation by seed-eating weevils. However, there are three critical conceptual gaps in our knowledge about the role of natural enemies in the evolution and maintenance of females. First, we still know very little about the effect of infectious disease. The vast majority of studies have focused on herbivores while only a few studies have examined sex-specific effects of disease (reviewed in Vega-Frutis et al. 2013), and these have generally shown little to no difference in exposure and infection between females and hermaphrodites (Shykoff et al. 1997; Marr and Delph 2005).
Second, we have limited knowledge of the plant traits driving these sex-specific differences in herbivory. The few previous studies that have investigated traits have largely focused on the effects of smaller flower-size in females. For example, Ashman et al. (2004) showed that the larger flower size of hermaphrodites was an important driver of increased herbivory in wild strawberry. Similar effects of floral size were also reported by Doubleday and Adler (2017) for Silene vulgaris. In contrast, Tsuji and Sota (2010) found sex-specific differences in Eurya japonica were driven by differences in the concentration of defensive phenolic compounds. One trait that has not yet been investigated is floral longevity. Female flowers only need to stay open long enough to receive pollen, while hermaphrodite flowers must both disperse pollen and receive pollen. For species with sequential hermaphroditism where male phase preempts female phase (protandry), hermaphrodite flowers typically remain open significantly longer than female flowers (Collin et al. 2002). This longer flowering duration should lead to increased risk of exposure to natural enemies that target flowers, such as florivores or pollinator-vectored diseases. Moreover, since female flower closure is dependent on pollen receipt, reduced exposure to natural enemies is predicted to be frequency dependent, with females having the greatest advantage over hermaphrodites when they are rare. This is important, because theory shows that if female advantage is strongly frequency-dependent, this can limit their ability to drive the evolution of dioecy (Maurice and Fleming 1995).
Third, very few studies have shown that the frequency of females in natural populations covaries with the presence and abundance of enemies (Marr and Delph 2005; Caruso and Case 2007). This last point is critical, because even if natural enemies provide selection for females, they may not necessarily drive an increase in female frequency, an essential step in the evolution of separate sexes (Charlesworth and Charlesworth 1978; Spigler and Ashman 2012; Miller and Bruns 2016). For example, if selection for females from natural enemies is relatively weak, their impact could be swamped by other sources of selection or high stochasticity. In addition, if sex-determination is cyto-nuclear, selection for females does not always translate into an increase in female frequency (Miller and Bruns 2016 p. 201).
We studied floral longevity, and its impact on exposure to a pollinator-vectored disease (Microbotryum) in Dianthus pavonius, the alpine carnation. The carnation family (Caryophyllaceae), and especially the genera Silene and Dianthus, have become a model system for understanding mating system evolution (Shykoff et al. 2003; Bernasconi et al. 2009). While dioecy is relatively rare across angiosperms, occurring in just 5–6% of species (Renner 2014) it has evolved independently at least twice within Silene (Desfeux et al. 1996). In addition, rates of gynodioecy within these genera are high (24 species; Jurgens et al. 2002; Shykoff et al. 2003). Silene and Dianthus species are also commonly and persistently infected with anther-smut disease, caused by fungi in the genus Microbotryum (Hood et al. 2010; Kemler et al. 2013). Infected plants are sterilized and produce fungal spores in place of pollen. The disease is transmitted via insect pollinators creating a conflict similar to that in animal sexually-transmitted diseases where increased mating through more pollinator contacts also increases disease exposure (Antonovics 2005). Female plants can still transmit the disease because the pathogen induces anther production (Uchida et al. 2003); however sex-specific differences in floral traits that impact pollinator behavior could affect disease exposure rates.
Dianthus pavonius can experience high levels of anther-smut prevalence (ca. 40%; (Bruns et al. 2017), and can have populations with exceedingly high female frequencies (up to 60%). The stigma of female flowers is receptive as soon as the flower opens, and closes following pollination. In contrast hermaphrodite flowers are protandrous, producing pollen first, and only developing a receptive stigma after all the pollen has dehisced. We therefore hypothesized that hermaphrodite flowers would remain open longer than female flowers, and experience higher rates of Microbotryum spore deposition. Since female flowers only close following pollination, we further hypothesized that sex-specific differences in spore deposition would be frequency-dependent: females should have the lowest levels of spore deposition when hermaphrodites are common and pollen deposition more likely.
To test these hypotheses, we tracked floral closing in naturally occurring individual hermaphrodite and female flowers and quantified total spore deposition. We then used a manipulative field experiment to determine the effect of sex-ratio on female floral closure rates. To test the prediction that sex-specific differences in disease exposure may have selected for females, we surveyed 72 D. pavonius populations in 14 mountain valleys in the Maritime Alps (approx. 200 km2 area) and asked whether there was a positive correlation between disease incidence and female frequency. Finally, to contextualize our results, we examined other potential sources of selection for females including female fertility advantage and inbreeding depression in a common garden experiment.
METHODS
Overview
We first carried out a series of observational and experimental field studies within a single large D. pavonius population to determine whether there were sex-specific differences in disease exposure, and investigated the mechanisms for these differences. Then, we surveyed D. pavonius populations across a broad spatial scale to determine correlations between disease and female frequency. Lastly, we used common garden experiments to investigate two other potential sources of selection for females, inherent fertility advantages and inbreeding depression.
Natural history of anther-smut on Dianthus pavonius
Dianthus pavonius (alpine carnation, or peacock pink) is a perennial, gynodioecious species endemic to the Maritime Alps of Italy and France. It is abundant in meadow habitats above 1600m, and flowers in mid-summer. Individual plants produce either hermaphrodite or female flowers (Figure 1A, B); anthers are still present on female flowers but they are extremely reduced and sterile. Very rarely a hermaphrodite plant will produce a few female flowers. Census surveys across the Maritime Alps show that the sex-ratio within D. pavonius populations is highly variable, ranging from 0 to 61%. Crossing experiments have shown that sex-determination is cyto-nuclear since reciprocal crosses between hermaphrodites can lead to significantly different progeny sex ratios (Bruns, unpublished data).
Figure 1.

Sexual morphs of Dianthus pavonius. A) Healthy hermaphrodite, B) Healthy female, C) Diseased plant (sex unknown).
Microbotryum causes anther-smut disease, a sterilizing disease that infects many plants in the Caryophyllaceae (Hood et al. 2010), including several gynodioecious (Collin et al. 2002; López‐Villavicencio et al. 2005) and dioecious (Alexander and Antonovics 1988; Carlsson-Graner 1997) species. Individual species of Microbotryum tend to be highly specialized, with each species typically only found on one host species (Le Gac et al. 2007; Refrégier et al. 2008), although recent work has shown D. pavonius is infected by several genetic lineages of Microbotryum (Petit et al. 2017).
Infected individuals produce spores in place of pollen, and the ovary is sterilized. In female plants, the fungus induces the production of anthers (Figure 1C), making it impossible to distinguish the sex of diseased plants. In D. pavonius, the disease is systemic, affecting all flowers on the plant, and recovery is extremely rare (Bruns et al. 2017). Pollinators are the main route for spore dispersal although localized aerial dispersal also occurs (Bruns et al. 2017). We have observed a wide range of insects on D. pavonius flowers including bees, skipper butterflies, syrphid flies, bee-flies and small anthophilid flies.
Study area
All of the within-population studies were conducted near Rifugio Garelli in the Parco naturale del Marguareis in Italy (44°18’98.4” N, 7°69’15.0” E). The habitat is a high alpine meadow (ca. 2000m) where both the host and pathogen occur in high abundance. We have been formally monitoring the population and disease dynamics in this area since 2005. Overall disease prevalence in this area has been close to 40% for the entire time (Bruns et al. 2017), but there is significant heterogeneity in host and pathogen density at a more fine-scale local level (Antonovics et al, unpublished). While there are several lineages of Microbotryum that infect D. pavonius (Petit et al 2017), we have only ever found a single lineage at this location. Moreover D. pavonius is the only Dianthus species present at this site.
I. Floral closing and disease exposure
Local spatial and phenological variation in sex ratio
We quantified spatial and phenological variation in sex ratio and disease in four local sites (referred to as BG, MV, RF, and UP) near Rifugio Garelli. Sites were located approximately 50m apart and varied in disease frequency from 3 to 68%. Within each site, we demarcated a rectangular plot that ranged in size from 7×10m, to 10×10m (Figure S1). We divided each plot into square-meter quadrats and counted all the flowering plants (hermaphrodite, female, and diseased) and the total number of flowers within each quadrat. We censused each site three times over the course of a three-week period on July 28, Aug 5, and Aug 13 2014. Within each site and time point, we calculated disease prevalence as the frequency of infected plants out of the total number of flowering plants. We calculated the sex ratio as the frequency of females out of the total number of healthy flowering plants.
To determine if sex ratio or disease prevalence changed significantly over the three-week period, we used a generalized linear model with a logit link function (binomial glm) and site and time as fixed effects. We also asked if there was a significant spatial correlation between disease prevalence and female frequency within plots. We used a similar binomial glm approach to ask whether disease prevalence at a local scale (1 or 2m2) was a significant predictor of female frequency at that scale.
Floral longevity and spore deposition on naturally occurring plants
To determine floral longevity, we marked unopened buds of female and hermaphrodite flowers within each site (total of 49 females, and 72 hermaphrodites) during the second week of the study. We recorded the opening and closing dates every two days. We collected these flowers eight days later in coin envelopes for spore deposition counts. From each marked plant we also collected a flower that was beginning to set fruit to assess ovule number and fertilization success.
To determine whether differences in floral longevity between hermaphrodites and females were driven by earlier pollination of females we marked 40 (20 hermaphrodite, 20 female) plants in the vicinity of Rifugio Garelli. We marked 2 unopened buds on each plant with jewel tags. One bud was left open and the other was enclosed with a light netting to prevent pollinator visitation. We recorded the dates that each flower opened and closed.
To quantify spore deposition, dried flowers were softened in 1mL of Pohl’s solution (25% Methanol, 1.2% Aerosol OT), vortexed at high speed for 15 seconds, and allowed to sit for 24 hours. To concentrate the samples, a well-mixed 700μL aliquot of spore suspension was centrifuged for 2 minutes and 640μL of supernatant were removed. Concentrated samples were counted with a hemocytometer at 20X magnification. Each sample was counted twice. We used ANOVA to determine the overall effect of sex, site and sex*site on floral longevity and spore deposition (log transformed). We ran a second ANCOVA analysis on the spore deposition data, that included floral longevity and disease density as covariates in the model. The significance of each term in the model was assessed with type III sums of squares.
Floral longevity in experimental arrays
Longevity of female flowers should depend on the availability of pollen. Females should have the fastest closing rate when they are rare compared to hermaphrodites. To test this hypothesis, we set up cut-flower arrays in the field that varied sex ratio and quantified floral closure rates. Cut D. pavonius flowers can be maintained for up to a week in water and will open and close normally. We used floral tubes (10.2 cm tall, 1.6cm diameter) to set up a series of 15 cut flower arrays in the field. Each array was one square meter and contained 24 healthy flowers and 5 diseased flowers in an even grid 0.25 m apart (Figure S2). One of the diseased flowers was placed in the middle of the grid, while the remaining four were placed outside the grid, one on each side. We tested six different sex-ratio treatments, expressed as the ratio of hermaphrodites to females (with the number of replicate arrays in parentheses): 24/0 (2), 20/4 (4), 12/12 (3), 4/20 (4), 0/24 (2). Treatments with highly skewed sex-ratios were replicated at higher rates since there were fewer individual flowers of a particular sex to measure closure rates on. Flowers were collected from local plants as un-opened buds one day before the experiment was set up and kept inside, away from pollinators overnight. Only open flowers were placed in the arrays. This insured that all flowers in the experiment were the same age and had not previously received any pollinator visitations.
Arrays were set up on 26 July 2016, and monitored for floral closure. Flowers were collected as soon as they closed for spore deposition analysis. Nine hermaphrodite flowers closed prior to the end of the experiment and these were replaced to maintain the local frequency of pollen bearing flowers. Floral closure rates were analyzed with a Cox proportional hazards regression (Cox 1992) using the R package “survival” (Therneau and Lumly 2017).
Flower size.
To determine whether the two sexes differed in flower size, we measured the diameter of 40 hermaphrodite and 40 female flowers (all from separate plants). To determine if differences in size had a genetic basis, we carried out a common garden experiment in the greenhouse. Seeds from 20 maternal families were collected and planted in the 1” cone-tainers filled with Promix BX in greenhouse. Individuals were grown to flowering and we recorded sex, total inflorescence number, and diameter of the first flower. These field measurements were carried out in July 2005, and the greenhouse experiment was carried out in 2009.
II. Spatial variation in sex-ratio and disease
To determine if there is a positive correlation between disease prevalence and female-frequency, we surveyed D. pavonius populations throughout the broader Maritime Alps region. These surveys were part of an ongoing project to map host and pathogen distribution with respect to range margins (Bruns et al. 2018). Populations were surveyed every 50m in elevation along 13 transects in the Maritime Alps (Figure S3). We surveyed the first population within each 50m section by counting all hermaphrodite, female, and diseased plants within a 100m2 area. If the first population encountered within an elevational section was healthy, we also surveyed the first diseased population encountered. Since slope gradients varied considerably within and among valleys, the physical distance among sampled populations also varied, however, the median distance between populations was >200m. In the field surveys, populations were defined as having 5 or more plants within a 100 m2, however, for the purposes of this study we only included populations in the analysis if there were at least 10 healthy plants for which sex could be determined. The median population size was 49, and the distribution was strongly skewed right by 9 populations with densities over 100 (Figure S4). For populations with <100 plants (N=63), the distribution of population size was normal (Figure S4).
We used a weighted linear regression with an arcsine square root transformation to determine if there was a significant difference in the sex ratio between diseased and healthy populations (determined by the presence or absence of disease within the population), with elevation (m) included as a covariate. We used the same approach to determine if disease-prevalence (the frequency of disease within a population) was a significant predictor of female frequency within diseased populations. Disease prevalence was only calculated for populations with at least 1 diseased individual: we corrected for ascertainment bias by subtracting 1 from both the numerator and the denominator.
III. Additional sources of selection for females
Fecundity of female and hermaphrodite plants
To quantify sex-specific differences in inflorescence production in the field, we analyzed flowering data from a long-term census study of the Garelli population (Bruns et al. 2017). In July 2015, all the flowering plants in a 10×20m plot were counted, and the total number of flowering stems (regardless of whether they were in bud, flower, or fruit stage) for each plant was recorded. We also analyzed inflorescence data from the greenhouse common garden experiment (described above) to determine whether any sex-specific differences were genetically based.
To determine if there were differences in seed production per fruit in the field, we collected a single maturing fruit from each marked plant within the four phenology plots. Fruits were stored at 4°C before being dissected to count ovules and developing seeds. Ovules were categorically scored for viability and size, which we used as a proxy for fertilization (Figure S5). Inviable ovules were small and shriveled and likely represent non-fertilized ovules or aborted seeds. We assumed ovules in the large size class represented fertilized, developing seeds, while those in the small size class had not been fertilized. We used ANOVA to determine the effect of site and sex on the total number of ovules and the number of fertilized, viable ovules.
To determine whether there are genetically based differences in seed set between hermaphrodite and female plants, we hand-pollinated greenhouse-raised plants and measured seed set per fruit. All plants came from field-collected seeds from three valleys in the broader Maritime Alps: Garelli (the focal study site: 44°18’98.4” N, 7°69’15.0” E), Vallone di Nasta (44°16’44” N, 7°27’22” E), and Valle Santa Anna di Vinadio (44°23’30.5” N, 7°10’26.0” E). Seeds were collected in September 2011, and planted in the greenhouse in the spring of 2013. The plants flowered in March 2015. To measure seed set, we randomly crossed all available receptive flowers with one of three sources: selfed, or sympatric (a mix of pollen from plants within the same population) or allopatric (a mix of pollen from allopatric populations). Only hermaphrodite plants received the self-treatment. Hermaphrodite flowers were emasculated 1–2 days prior to pollination. We recorded fruit set and seed set for all pollinated flowers.
Inbreeding depression
Female flowers can only produce outcrossed seeds, while hermaphrodites have the potential to self-fertilize. To determine whether there was evidence of inbreeding depression, we planted a subset of the selfed (N=166), sympatric outcrossed (N=205) and allopatric outcrossed (N=126) seeds from the seed set experiment (see above). Seeds were planted on December 3, 2015 in agar plates, transplanted into the greenhouse on December 10, 2015, and measured for survival in April, 2016. In October 2016, we moved the plants outside to experience winter conditions. Plants were brought back inside March 2017 and we recorded survival and inflorescence number.
We analyzed the effect of cross type and population on five fitness components: proportion germination, survival to four months, survival to one year, probability of flowering given survival to one year, and number of inflorescences (given flowering). We used ‘aster’ models (Shaw et al. 2008) to integrate the survival and flowering fitness components into a single unified measure of fitness (total inflorescence production at one year), and tested for the effects of cross type and population. The germination stage was excluded from the aster model as this is the most likely to be influenced by maternal effects. Since we found no significant difference in fitness between progeny from sympatric vs allopatric crosses (Dev=0.22675, df=1, p=0.6339), we combined these two groups and contrasted the ‘outcrossed’ vs. ‘selfed’ treatments.
RESULTS
I. Floral closing and disease exposure
Spatial and temporal variation in sex ratio
The four local sites at Rifugio Garelli differed in plant density (F3,7= 3.980, p=0.0603) and disease prevalence (F3,7= 265.86, p<0.0001) with the largest plant density and highest level of disease in the upper plateau (UP) site (Figure 2: A, B). The frequency of females among plants with open flowers declined significantly over the three-week period in all four plots (F1,7= 49.991, p=0.0002; Figure 2C), but was fairly consistent among sites. This phenological decline in female frequency was largely driven by a decrease in the number of open females (presumably as females closed). In the MV and UP populations, hermaphrodites also initiated flowering later (Figure S6). Local disease prevalence (assessed at either at 1 or 2m2 scale) was not a significant predictor of female frequency for any time period within any of the sites (Results not shown).
Figure 2.
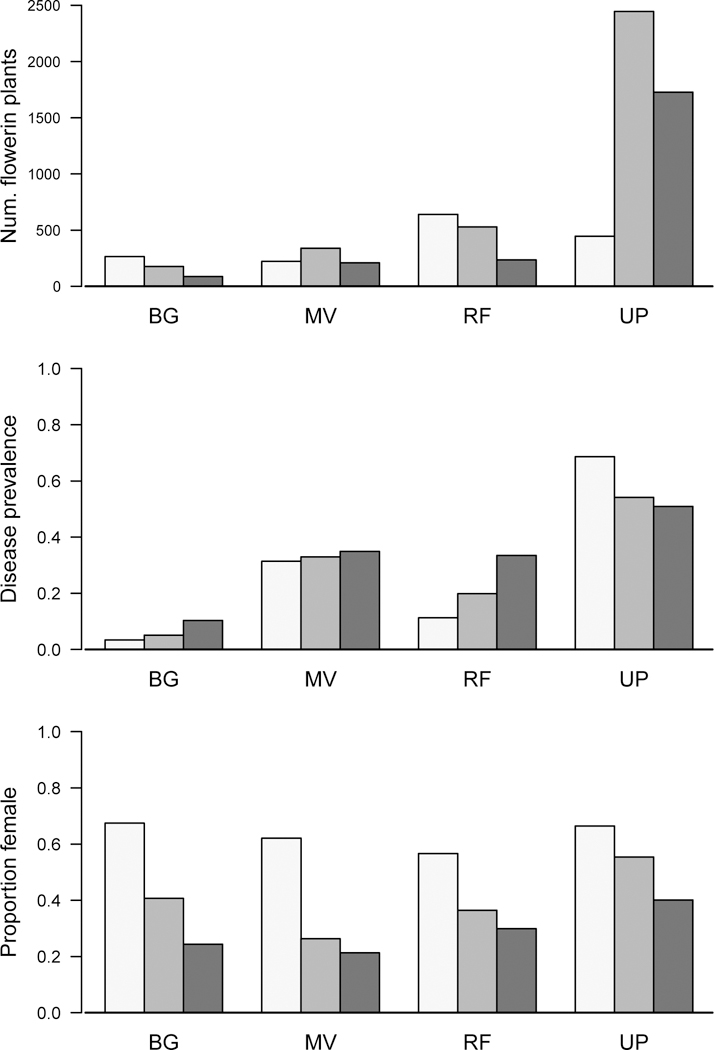
Phenological change in A) number of flowering plants, B) disease prevalence, and C) frequency of females among the healthy plants over a three week period. Within each site bars represent measurements at early, mid, and late time points. The size of the plots were as follows: BG: 7x10m, MV 8x8m, RF and UP: 10x10m.
Floral longevity and spore deposition of naturally occurring plants
Hermaphrodite flowers were open for significantly longer than female flowers in all four sites (Figure 3A). Floral longevity varied significantly among sites and there was a significant sex*site interaction effect (Table 1). Females stayed open for longer in the upper plateau, the site that had the highest level of disease.
Figure 3.
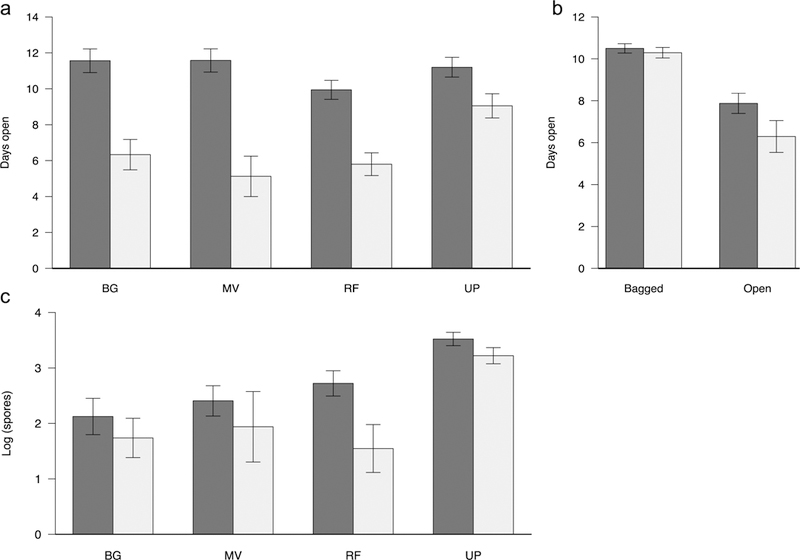
A) Mean longevity of marked hermaphrodite (dark bars) and female flowers (light bars) across four sites. B) Mean longevity of bagged and non-bagged flowers. C) Average spore deposition. Error bars are 1SEM.
Table 1.
ANOVA showing the effect of site and sex on floral longevity.
| Source | Type II SS | Df | F | p |
|---|---|---|---|---|
| Site | 81.45 | 3 | 3.870 | 0.0112 |
| Sex | 497.98 | 1 | 70.979 | <0.0001 |
| Site*Sex | 76.92 | 3 | 3.655 | 0.0145 |
| Residuals | 792.8 | 113 | ||
Bagging flowers to prevent pollination resulted in a significant increase in floral longevity for both sexes (Figure 3B). Only 36% of bagged flowers closed during the 10-day period (compared to 67% of un-bagged flowers) and there was no difference in longevity between hermaphrodites and females in the bagged treatment. In the un-bagged treatment, females tended to close earlier than hermaphrodites (t=1.75, df=24, p=0.09) and had greater variance in closing time than hermaphrodites (Fligner-Killeen test: X2=4.74, df = 1, p-value = 0.030).
Females had lower levels of spore deposition than hermaphrodites across all four sites (F1,102= 6.663, p=0.0113) (Figure 3C). Spore deposition was highest in the highly diseased upper plateau site. There was no significant sex*site interaction (F3,99= 0.853, p= 0.4682). Covariate analysis found a significant sex*longevity interaction effect (Table 2). Floral longevity was a significant positive predictor of spore deposition in females, but not in hermaphrodites (Figure S7). The difference occurs because there were many females that were only open for a short duration (2–4 days) and had correspondingly fewer spores, driving the positive correlation. In contrast, the minimum flowering duration for hermaphrodites was 6 days. Local disease frequency (within one-square meter) also had a significant, positive effect on spore production (Table 2). We found no detectable effect of local hermaphrodite frequency on spore deposition (F1,98 = 0.779, p= 0.3796).
Table 2.
ANCOVA showing the effect of floral longevity and local disease frequency on spore deposition within four different sites.
| Source | Type III SS | Df | F | p |
|---|---|---|---|---|
| Site | 7.01 | 3 | 2.627 | 0.055 |
| Sex | 14.24 | 1 | 16.019 | <0.0001 |
| Disease frequency (1m) | 4.56 | 1 | 5.128 | 0.0257 |
| Days open | 4.10 | 1 | 4.607 | 0.0343 |
| Sex*Days open | 10.46 | 1 | 11.765 | 0.0009 |
| Residuals | 88.00 | 99 | ||
Floral longevity in experimental arrays
Sex ratio had a significant effect on the closing time of female flowers in the floral array experiment (Fig. 4). The frequency of hermaphroditic flowers in the experimental arrays was associated with an increased probability (“risk”) of female flower closure (coefficient=0.92, Wald test Z=2.76, p=.006). The probability of hermaphroditic flower closure was not significantly associated with the frequency of hermaphroditic flowers in experimental arrays (Wald test Z=−0.716, p=0.47). Over the course of the entire experiment, many more female flowers (121/180) than hermaphroditic flowers (10/180) closed.
Figure 4.
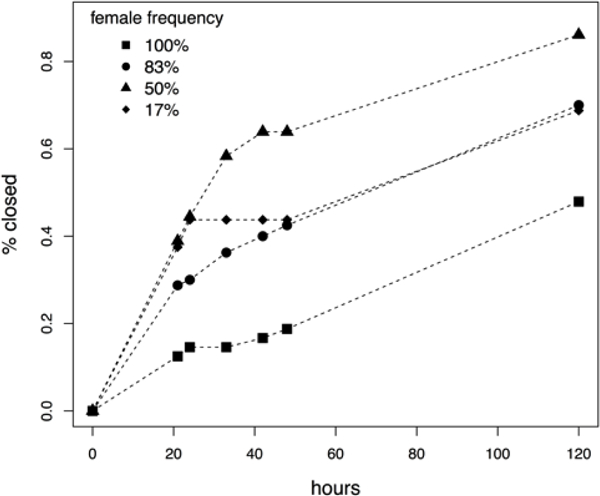
Average closure rates for female flowers in experimental arrays. Shapes indicate the frequency of females within each array.
Flower size
In the field, the average diameter of female flowers was 22% smaller than hermaphrodite flowers (mean H: 23.775mm, mean F: 18.450mm, t= 8.334, df = 77.3, p<0.0001). In greenhouse-reared plants, hermaphrodites had an average floral diameter of 32.7mm, while females only had an average diameter of 26.5mm (t = 10.22, df = 146.1, p<0.0001). A mixed model (package ‘nlme’ in R) showed that the pattern of smaller females was significant within seed families that produced progeny of both sexes (F1,15 =80.727, p <0.0001).
II. Spatial variation in sex-ratio and disease
Disease was present in 43 of the 72 surveyed D. pavonius populations (60%). Both disease presence (F1,68=10.313, p=0.002) and elevation (F1,68=29.768, p<0.0001) were significant predictors of female frequency. There was no significant interaction effect (F1,68=2.401, p= 0.12592). Diseased populations had an average female frequency of 35.5% (±3%, SE), while healthy populations had an average female frequency 30.7% (± 3%, SE). Female frequency increased with increasing elevation (Figure 5). Considering only diseased populations, we found no significant relationship between disease-prevalence and female frequency (p=0.9961).
Figure 5.
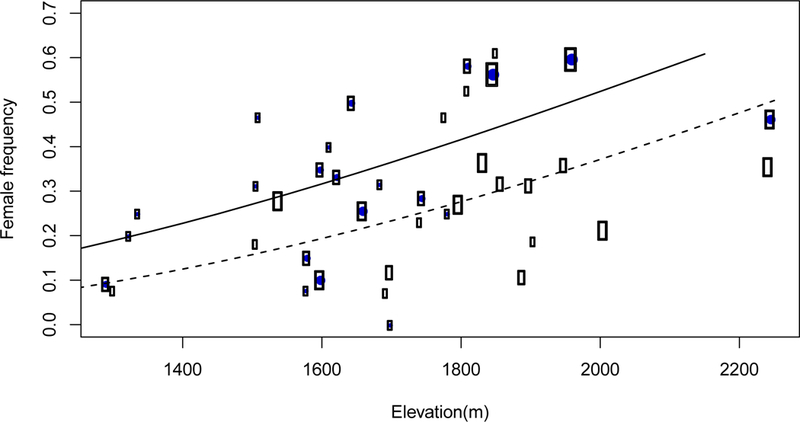
Effect of elevation and disease status on female frequency. Each circle represents a single D. pavonius population, with circle size indicating the population size. Healthy populations are shown with unfilled circles and a dashed line, diseased populations are shown with filled circles and a solid line. Trend lines are from an additive model of the effect of disease status and elevation on female frequency (arcsine square-root transformed) and weighted by population size.
III. Other sources of selection for females
Inflorescence production and seed set
There was no significant difference in inflorescence number between hermaphrodite and female plants in the field (t=0.616, df=551.3, p=0.538; Fig 6a), or in the greenhouse common garden experiment (t = 0.6002, df = 133.9, p = 0.5494; Fig 6b).
Figure 6.
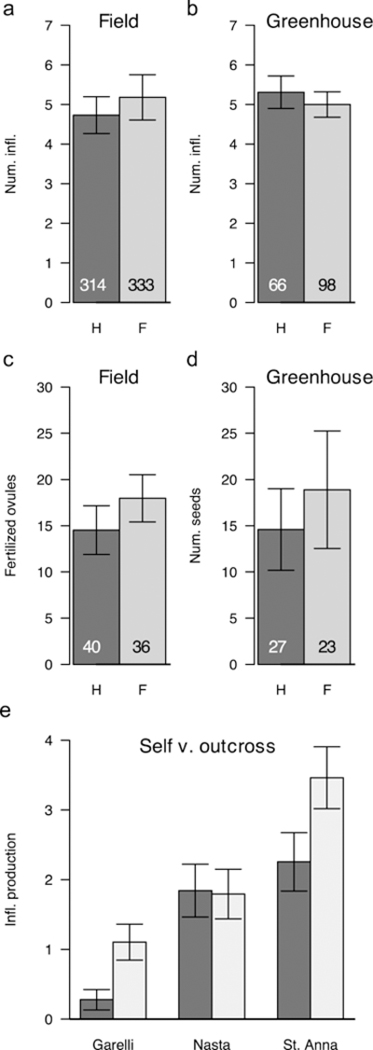
Average inflorescence number (a-b), fertilized ovules (c) and seed production (d) for hermaphrodite and female plants in the field and greenhouse. Sample size is given in the text. E) Expected inflorescence production predicted from aster model for selfed (dark bars) and out-crossed (light-bars) progeny from three populations. Error bars represent 1 SEM.
Hermaphrodite and female plants in the field did not differ significantly in total ovule number (F1,63=2.230, p=0.1413), in the number of viable ovules (F1,63= 2.000, p=0.0882), or in the number of putatively fertilized (large) ovules (F1,63=1.044, p=0.31081; Fig 6C). There were also no significant differences in seed set per capsule between the two sexes when plants were grown in a common garden and fertilized with either sympatric (F1,73 = 0.001, p= 0.977; Fig 6D) or allopatric (F1,46 = 2.216, p=0.143) pollen.
Inbreeding depression
There was no significant difference in germination between selfed and outcrossed progeny (Dev = 2.6253, df=1, p=0.461) or among valley populations (Dev=4.8921, df=2, p=0.6027). However, expected total inflorescence production, estimated through aster modeling, was significantly affected by cross type, valley, and their interaction (Table 3). The magnitude of inbreeding depression was greatest in the Garelli population (the focal population for the local studies) where the estimated fitness of the selfed progeny was 75% lower than that of the outcrossed (Figure 6E). In the Santa Anna population, fitness of the selfed progeny was 35% lower than that of the outcrossed. There was no significant inbreeding depression in the Nasta population. Lower survival of the selfed progeny, rather than reduced fecundity, was the main cause of inbreeding depression (Figure S8).
Table 3.
Effect of cross type (self-vs outcross) and valley of origin on predicted cumulative fitness estimated through Aster model.
| Source | Df | Dev | p |
|---|---|---|---|
| cross type | 1 | 5.0544 | 0.0246 |
| valley | 2 | 53.568 | <0.0001 |
| cross*valley | 2 | 8.7353 | 0.0127 |
DISCUSSION
Our results indicate that anther-smut disease and avoidance of self-fertilization are important sources of selection for females in Dianthus pavonius. We found that female flowers received significantly fewer Microbotryum spores than hermaphrodite flowers, and that this difference was driven by a shorter flowering duration in females, and smaller flowers. We also found a higher average frequency of females in populations where disease was present than in populations where disease was absent. Avoidance of inbreeding is also likely to favor females since we found strong evidence of inbreeding depression in 2 out of 3 studied populations. Finally, we found no evidence that female plants have any inherent fertility advantage over hermaphrodites in this species, either in production of flowers or seed set per flower, suggesting that differential exposure to disease, and outcrossing may be major factors maintaining females in this system.
Infection with Microbotryum results in a significant loss of fitness in D. pavonius. Our long-term demographic studies in the focal population found that over 93% of infected individuals were completely sterilized, and the recovery rate was less than 5% (Bruns et al. 2017). Reduced spore exposure is therefore likely to confer a significant fitness advantage to females, but the response to selection will depend on the relationship between spore load and infection probability. In Silene latifolia, Roche et al. (1995) showed a saturating relationship: infection probability increased rapidly with increasing spore loads at low doses but leveled off around 20,000 spores. Such measures are currently lacking for the Dianthus system, but if there is a similarly saturating relationship, it could mean that strength of selection for females is strongest in populations with intermediate prevalence, where the average spore dose is close to or below the threshold. A non-linear relationship between spore dose and infection probability could also explain why we saw a relationship between female frequency and the presence of disease in the landscape study, but no relationship with disease prevalence. Future studies that quantify the dose-response of Microbotryum on D. pavonius could clarify the level of disease prevalence that drives the strongest selection.
Even if there is not a direct relationship with infection probability, differential exposure to disease could still drive selection for females since Microbotryum spores have been shown to reduce seed set in several host species even if infection does not occur ( Carlsson-Graner et al. 1998; Alexander 1989; Marr 1997; López‐Villavicencio et al. 2005). In Silene acaulis, the presence of Microbotryum spores reduced pollen germination in both sexes (Marr 1998). In Gypsophila repens, spores reduced seed set in hermaphrodites but not females (López‐Villavicencio et al. 2005). In D. pavonius, we have found that flowers treated with spores and pollen stay open longer and have lower seed set than flowers treated with pollen only (Batzel and Bruns, in prep). Thus, the lower spore deposition rates we observed on females is likely to provide a fecundity advantage for females even if it does not result in decreased infection rates.
Our results add to a growing body of literature showing that natural enemies can play an important role in the evolution of separate sexes (Ashman 2002; Steets et al. 2007; Vega-Frutis et al. 2013). However, the majority of this work has focused on the impacts of herbivores (Marshall and Ganders 2001; Ashman et al. 2004; Clarke and Brody 2015) and we know far less about the effects of disease (reviewed in Vega-Frutis et al. 2013). Indeed, this is one of the first studies to show strong sex-specific differences in disease exposure favoring females in a gynodioecious species, since previous studies of anther-smut on other gynodioecious species have found limited or no evidence of sex-specific effects. For example, Shykoff et al. (1997) found no difference in spore deposition on females and hermaphrodites in a D. sylvestris population in Switzerland, but Collin et al. (2002) found that female D. sylvestris flowers in a population near Milan had higher spore deposition on their stigmas than hermaphrodite flowers. However, in both studies, flowers were randomly collected from the field without accounting for differences in flowering duration, which we found was a major driver of spore deposition rates. In addition, Collin et al. (2002) quantified spore deposition on the stigma which is typically longer in females (Shykoff 1997) rather than at the whole flower level.
While reduced damage to females from natural enemies is predicted to drive the evolution of higher female frequencies (Steets et al. 2007; Miller and Bruns 2016), very few studies have tested this predicted response to selection by comparing female frequencies in populations with high and low herbivore or disease pressure. In a landscape level study of 72 naturally occurring D. pavonius populations, we found that diseased populations had significantly higher female frequencies than healthy populations. While we do not know how long each population has been in its current disease status, a temporal demographic study of the Garelli population showed that disease levels have been maintained at a steady 40% for over 10 years (Bruns et al. 2017), indicating selection pressure is relatively constant from year to year. Moreover, we have revisited several of the populations included in the study during subsequent years and have found little change in plant population size or disease prevalence. The average lifespan of D. pavonius is estimated to be around ca. 5 years (Bruns et al. 2015), making the evolution of sex-ratio in response to long-lasting disease epidemics more than possible.
The few other studies that have examined broad-scale spatial correlation between female frequencies and enemies in nature have found mixed results. Marr and Delph (2005) found that Silene acaulis populations in the Rocky Mountains with a high prevalence of anther-smut disease had more females than populations with low disease prevalence. However, within populations they found no difference in the sex ratio of healthy and diseased plants. In Lobelia siphilitica, Caruso and Case (2007) found no significant correlation between female frequency and the average number of hermaphrodite plants attacked by a weevil seed-predator, although there was a negative correlation between the number of female plants attacked and female frequency.
Floral traits affecting disease exposure
Hermaphrodite flowers were significantly larger and remained open for longer than female flowers. Both these floral traits are likely to increase total pollinator visits to hermaphrodites, and by extension disease risk. Indeed, we found the spore deposition was positively correlated with floral longevity in the marked plant study. While we do not have any direct measures of the effect of flower size on spore deposition, the higher spore deposition on hermaphrodites was not fully explained by accounting for floral longevity suggesting that additional dimorphic traits contributed. Larger hermaphrodite flower size has been found in several other species (reviewed in Shykoff et al. 2003), and has been linked with increased florivory in wild strawberry (Ashman et al. 2004) and increased seed predation in Silene vulgaris (Doubleday and Adler 2017). Additionally, other factors, such as pollinator preference for pollen may also have contributed to the higher hermaphrodite spore loads. Recently, we have found that syrphid flies which frequently visit D. pavonius and are primarily pollen feeders, have strong preferences for hermaphrodites (Laura Pierce unpublished data).
Floral longevity has not previously been examined with respect to natural enemies, but it is likely to be important in other species with protandry. The difference in longevity occurs because female flowers are receptive to pollen as soon as they open but hermaphrodite flowers must dehisce pollen before they are capable of closing in response to pollination. Moreover, our floral array experiment demonstrated that closure in females is frequency-dependent. Female flowers closed rapidly in arrays where they were rare, and stayed open longer in arrays where they were common. While negative frequency-dependent selection for females is predicted (Maurice and Fleming 1995; McCauley and Taylor 1997) and has been empirically demonstrated in the context of pollen-limitation (McCauley and Brock 1998; De Cauwer et al. 2010; Rivkin et al. 2015), this is the first example of a frequency-dependent trait driving differential exposure to natural enemies. Moreover, we would predict stronger frequency-dependence in disease exposure than pollen limitation in this system, since it is the rate of pollination rather than the eventual outcome that drives closing response. For example, in our high-female frequency floral array treatment, over 60% of the flowers had closed by the end of the experiment, indicating successful pollination. However, closure in these high female-frequency arrays occurred more slowly than in arrays where females were rare, leaving females in the high-frequency arrays more vulnerable to spore deposition.
We also detected sex-specific phenological differences in flowering. Female plants in all four sites near Rifugio Garelli flowered earlier than hermaphrodites. This led to a decrease in the proportion of female flowers relative to hermaphrodites as the season progressed. In contrast, disease prevalence was roughly constant. At present it is unclear if or how this sex-specific difference in phenology affects disease exposure. On the one hand, our floral array experiments predict that females that flower later in the season when females are relatively rare, should close more rapidly than those flowering earlier in the season, reducing spore deposition. However, this prediction depends on pollinator abundance (and daily visitation rate) remaining constant across the three-week period, something we did not quantify in this study.
Identifying the plant traits driving differential exposure to disease is essential to predicting the current, historic, and future role of disease in the evolution of mating systems. For example, while any dimorphic trait that reduces female disease exposure relative to hermaphrodites can help maintain females in extant populations, only traits that arose through pleiotropy could have played a role in the incipient evolution of females. Developmental integration of floral whorls (Krisek & Fletcher 2005) makes it likely that dimorphism in many floral traits is driven by pleiotropy (Schultz 2003; Barr and Fishman 2011). Indeed, size dimorphism is widely observed in gynodioecious species (Shykoff et al. 2003) and pleiotropy with a male-sterility gene has been demonstrated in Mimulus nasutus (Barr & Fishman 2011). Closing behavior is strongly dimorphic due to the loss of the male phase: since females do not have a male phase they close as soon as they receive pollen. It therefore seems plausible that disease could have played a role in the incipient evolution of females in this species. However, the strong frequency-dependence of floral longevity may limit the ability of disease to drive the evolution of dioecy. Maurice and Flemming (1995) showed that if selection for females is strongly frequency-dependent, it can prevent the evolution of high female frequencies that are necessary for the evolution of males, essentially ‘trapping’ populations in permanent gynodioecy.
Other sources of selection for females
Theory has shown that females can only be maintained in gynodioecious populations if they have a fitness advantage relative to hermaphrodites (Charlesworth 1981; Gouyon et al. 1991). Differential exposure to Microbotryum may account for a significant proportion of this expected fitness difference, since we found no evidence that females produce more inflorescences or more seeds per flower, either in the field or in common garden experiments. However, female frequency is also likely to be affected by the degree of selfing that occurs among hermaphrodites since we found evidence of significant inbreeding depression. In two of the three studied populations, selfed progeny had significantly lower fitness, measured as expected inflorescence production. We currently do not know the extent to which inbreeding occurs in the field. Although hermaphrodites are protandrous and the temporal separation male and female phase tends to prevent selfing within a flower (autogamy), we have observed autogamous seed set on a small number of plants in the greenhouse (Bruns, unpublished data). In the field, selfing could also occur between different flowers on the same plant (geitonogamy). Studies that quantify the frequency of selfing within populations would help evaluate the relative contribution of disease and inbreeding depression to the maintenance of females.
The genetic basis of sex is also critically important to the evolution and maintenance of females. Species where sex is determined by interactions between cytoplasmic male-sterility (CMS) genes and nuclear restorers (cyto-nuclear sex-determination) can maintain higher frequencies of females with lower selective advantages than species with nuclear sex determination (Gouyon et al. 1991). Sex-determination in Dianthus pavonius appears to be cyto-nuclear with multiple CMS cytotypes since a reciprocal crossing experiment between hermaphrodites from the Garelli populations found asymmetric sex-ratios in the progeny, and differential restoration (Bruns, unpublished). Similar systems of multiple CMS cytotypes have been described for Silene vulgaris (Charlesworth and Laporte 1998) and S. nutans (Garraud et al. 2011). Thus, even if reduced spore exposure only results in moderate fitness benefits for females, this benefit could still be enough to maintain females. Another intriguing possibility is that sex-specific disease exposure could be driving cryptic evolution of CMS cytotypes within populations. Miller and Bruns (2016) showed that when sex-determination is cyto-nuclear, increased selection for females by the addition of a disease can result in an increase in CMS frequency with little change in female frequency, with the response depending on the initial frequency of the CMS cytotype, and the cost of restoration. Future studies that compare CMS frequency and disease prevalence within population might therefore find a stronger correlation than we found between disease prevalence and female frequency.
CONCLUSIONS
Anther-smut disease likely plays a significant role in the evolution and maintenance of females in the gynodioecious Dianthus pavonius. Not only did we find that females received significantly fewer spores than hermaphrodites, but we also found that populations where disease was present had more females than populations where disease was absent. While infectious disease has been widely studied in the context of the evolution of sex as a means of producing variable offspring (Lively et al. 1990; Busch et al. 2004; Koskella and Lively 2009), our results demonstrate that disease can also play a key role in the evolution of separate sexes.
Supplementary Material
Footnotes
COMPETING INTERESTS
The authors declare no competing interests
DATA ACCESSABILTIY
If the manuscript is accepted for publication, we will archive the associated data in Dryad.
REFERENCES
- Alexander HM 1989. An experimental field study of anther-smut disease of Silene alba caused by Ustilago violacea: Genotypic variation and disease incidence. Evolution 43:14. [DOI] [PubMed] [Google Scholar]
- Alexander HM, and Antonovics J. 1988. Disease spread and population dynamics of anther-smut infection of Silene alba caused by the fungus Ustilago violacea. Journal of Ecology 76:91. [Google Scholar]
- Alonso C, and Herrera CM. 2001. Neither vegetative nor reproductive advantages account for high frequency of male‐steriles in southern Spanish gynodioecious Daphne laureola (Thymelaeaceae). American Journal of Botany 88:1016–1024. [PubMed] [Google Scholar]
- Antonovics J 2005. Plant venereal diseases: insights from a messy metaphor. New Phytologist 165:71–80. [DOI] [PubMed] [Google Scholar]
- Ashman T-L 2001. Determinants of sex allocation in a gynodioecious wild strawberry: implications for the evolution of dioecy and sexual dimorphism. Journal of Evolutionary Biology 12:648–661. [Google Scholar]
- Ashman T-L 2002. The role of herbivores in the evolution of separate sexes from hermaphroditism. Ecology 83:1175–1184. [Google Scholar]
- Ashman T-L, Cole DH, and Bradburn M. 2004. Sex‐differential resistance and tolerance to herbivory in a gynodioecious wild strawberry. Ecology 85:2550–2559. [Google Scholar]
- Barr CM, and Fishman L. 2011. Cytoplasmic male sterility in Mimulus hybrids has pleiotropic effects on corolla and pistil traits. Heredity 106:886–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett SCH 2002. Evolution of sex: The evolution of plant sexual diversity. Nature Reviews Genetics 3:274–284. [DOI] [PubMed] [Google Scholar]
- Bateman AJ 1948. Intra-sexual selection in Drosophila. Heredity 2:349–368. [DOI] [PubMed] [Google Scholar]
- Bernasconi G, Antonovics J, Biere A, Charlesworth D, Delph LF, Filatov D, Giraud T, Hood ME, Marais GAB, McCauley D, Pannell JR, Shykoff JA, Vyskot B, Wolfe LM, and Widmer A. 2009. Silene as a model system in ecology and evolution. Heredity 103:5–14. [DOI] [PubMed] [Google Scholar]
- Bruns EL, Antonovics J, Carasso V, and Hood M. 2017. Transmission and temporal dynamics of anther-smut disease (Microbotryum) on alpine carnation (Dianthus pavonius). Journal of Ecology 105:1413–1424. [Google Scholar]
- Bruns EL, Antonovics J, and Hood M. 2018. Is there a disease-free halo at species range limits? The co-distribution of anther-smut disease and its host species. Journal of Ecology DOI: 10.1111/1365-2745.13009 [DOI] [Google Scholar]
- Busch JW, Neiman M, Koslow JM, and Kalisz S. 2004. Evidence for maintenance of sex by pathogens in plants. Evolution 58:2584–2590. [DOI] [PubMed] [Google Scholar]
- Carlsson-Graner U 1997. Anther-smut disease in Silene dioica: variation in susceptibility among genotypes and populations, and patterns of disease within populations. Evolution 51:1416. [DOI] [PubMed] [Google Scholar]
- Caruso CM, and Case AL. 2007. Sex ratio variation in gynodioecious Lobelia siphilitica: effects of population size and geographic location. Journal of Evolutionary Biology 20:1396–1405. [DOI] [PubMed] [Google Scholar]
- Charlesworth B, and Charlesworth D. 1978. A Model for the evolution of dioecy and gynodioecy. The American Naturalist 112:975–997. [Google Scholar]
- Charlesworth D 1981. A further study of the problem of the maintenance of females in gynodioecious species. Heredity 46:27–39. [Google Scholar]
- Charlesworth D, and Laporte V. 1998. The male-sterility polymorphism of Silene vulgaris: analysis of genetic data from two populations and comparison with Thymus vulgaris. Genetics 150:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke GL, and Brody AK. 2015. Gender inequality in predispersal seed predation contributes to female seed set advantage in a gynodioecious species. Ecology 96:1309–1317. [DOI] [PubMed] [Google Scholar]
- Collin CL, and Shykoff JA. 2010. Flowering phenology and female fitness: impact of a pre-dispersal seed predator on a sexually polymorphic species. Plant Ecology 206:1–13. [Google Scholar]
- Collin C, Pennings P, Rueffler C, Widmer A, and Shykoff J. 2002. Natural enemies and sex: how seed predators and pathogens contribute to sex-differential reproductive success in a gynodioecious plant. Oecologia 131:94–102. [DOI] [PubMed] [Google Scholar]
- Cox DR (1992). Regression models and life-tables. In Breakthroughs in statistics (pp. 527–541). Springer, New York, NY. [Google Scholar]
- De Cauwer I, Arnaud J-F, Schmitt E, and Dufay M. 2010. Pollen limitation of female reproductive success at fine spatial scale in a gynodioecious and wind-pollinated species, Beta vulgaris ssp. maritima: Frequency-dependent female fitness in a gynodioecious species. Journal of Evolutionary Biology 23:2636–2647. [DOI] [PubMed] [Google Scholar]
- Delph LF, and Carroll SB. 2001. Factors affecting relative seed fitness and female frequency in a gynodioecious species, Silene acaulis. Evolutionary Ecology Research 3:465–476. [Google Scholar]
- Desfeux C, Maurice S, Henry J, Lejeune B, and Gouyon P. 1996. Evolution of reproductive systems in the genus Silene. Proceedings of the Royal Society B 263:409–414. [DOI] [PubMed] [Google Scholar]
- Doubleday LAD, and Adler LS. 2017. Sex-biased oviposition by a nursery pollinator on a gynodioecious host plant: Implications for breeding system evolution and evolution of mutualism. Ecology and Evolution 7:4694–4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufay M, and Billard E. 2012. How much better are females? The occurrence of female advantage, its proximal causes and its variation within and among gynodioecious species. Ann Bot 109:505–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufay M, Champelovier P, Käfer J, Henry JP, Mousset S, and Marais G. a. B.. 2014. An angiosperm-wide analysis of the gynodioecy–dioecy pathway. Annals of Botany 114:539–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garraud C, Brachi B, Dufay M, Touzet P, and Shykoff JA. 2011. Genetic determination of male sterility in gynodioecious Silene nutans. Heredity 106:757–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouyon PH, Vichot F, and Van Damme JM. 1991. Nuclear-cytoplasmic male sterility: single-point equilibria versus limit cycles. The American Naturalist 137:498–514. [Google Scholar]
- Hood ME, Mena-Alí JI, Gibson AK, Oxelman B, Giraud T, Yockteng R, Arroyo MTK, Conti F, Pedersen AB, Gladieux P, and Antonovics J. 2010. Distribution of the anther-smut pathogen Microbotryum on species of the Caryophyllaceae. New Phytologist 187:217–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurgens A, Witt T, and Gottsberger G. 2002. Pollen grain numbers, ovule numbers and pollen-ovule ratios in Caryophylloideae: correlation with breeding system, pollination, life form, style number, and sexual system. Sex Plant Reprod 14:279–289. [Google Scholar]
- Kemler M, Martín MP, Telleria MT, Schäfer AM, Yurkov A, and Begerow D. 2013. Contrasting phylogenetic patterns of anther smuts (Pucciniomycotina: Microbotryum) reflect phylogenetic patterns of their caryophyllaceous hosts. Organisms Diversity & Evolution 13:111–126. [Google Scholar]
- Koskella B, and Lively CM. 2009. Evidence for negative frequency-dependent selection during experimental coevolution of a freshwater snail and a sterilizing trematode. Evolution 63:2213–2221. [DOI] [PubMed] [Google Scholar]
- Krizek BA, and Fletcher JC. 2005. Molecular mechanisms of flower development: an armchair guide. Nature Reviews Genetics 6:688–698. [DOI] [PubMed] [Google Scholar]
- Le Gac M, Hood ME, Fournier E, and Giraud T. 2007. Phylogenetic evidence of host-specific cryptic species in the anther smut fungus. Evolution 61:15–26. [DOI] [PubMed] [Google Scholar]
- Lively CM, Craddock C, and Vrijenhoek RC. 1990. Red Queen hypothesis supported by parasitism in sexual and clonal fish. Nature 344:864–866. [Google Scholar]
- López‐Villavicencio M, Branca A, Giraud T, and Shykoff JA. 2005. Sex-specific effect of Microbotryum violaceum (Uredinales) spores on healthy plants of the gynodioecious Gypsophila repens (Caryophyllaceae). American Journal of Botany 92:896–900. [DOI] [PubMed] [Google Scholar]
- Marr DL 1997. Impact of a pollinator-transmitted disease on reproduction in healthy Silene acaulis. Ecology 78:10. [Google Scholar]
- Marr DL 1998. The effect of Microbotryum violaceum spores on pollen germination in Silene acaulis. International Journal of Plant Sciences 159:221–227. [Google Scholar]
- Marr DL, and Delph LF. 2005. Spatial and temporal pattern of a pollinator-transmitted pathogen in a long-lived perennial, Silene acaulis. Evolutionary Ecology Research 7:18. [Google Scholar]
- Marshall M, and Ganders FR. 2001. Sex‐biased seed predation and the maintenance of females in a gynodioecious plant. American Journal of Botany 88:1437–1443. [PubMed] [Google Scholar]
- Maurice S, and Fleming TH. 1995. The effect of pollen limitation on plant reproductive systems and the maintenance of sexual polymorphisms. Oikos 74:55–60. [Google Scholar]
- McCauley DE, and Brock MT. 1998. Frequency-dependence in Silene vulgaris, a gynodioecious plant. Evolution 52:30–36. [DOI] [PubMed] [Google Scholar]
- McCauley DE, and Taylor DR. 1997. Local population structure and sex ratio: evolution in gynodioecious plants. The American Naturalist 150:406–419. [DOI] [PubMed] [Google Scholar]
- Miller I, and Bruns E. 2016. The effect of disease on the evolution of females and the genetic basis of sex in populations with cytoplasmic male sterility. Proceedings of the Royal Society B 283:20153035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake T, Satake I, and Miyake K. 2018. Sex-biased seed predation in gynodioecious Dianthus superbus var. longicalycinus (Capryophyllaceae) and differential influence of two seed predator species on the floral traits. Plant Species Biology 33:42–50. [Google Scholar]
- Petit E, Silver C, Cornille A, Gladieux P, Rosenthal L, Bruns E, Yee S, Antonovics J, Giraud T, and Hood ME. 2017. Co-occurrence and hybridization of anther-smut pathogens specialized on Dianthus hosts. Molecular Ecology 26:1877–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refrégier G, Le Gac M, Jabbour F, Widmer A, Shykoff JA, Yockteng R, Hood ME, and Giraud T. 2008. Cophylogeny of the anther smut fungi and their caryophyllaceous hosts: Prevalence of host shifts and importance of delimiting parasite species for inferring cospeciation. BMC Evolutionary Biology 8:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner SS 2014. The relative and absolute frequencies of angiosperm sexual systems: Dioecy, monoecy, gynodioecy, and an updated online database. American Journal of Botany 101:1588–1596. [DOI] [PubMed] [Google Scholar]
- Rivkin LR, Case AL, and Caruso CM. 2015. Frequency-dependent fitness in gynodioecious Lobelia siphilitica. Evolution 69:1232–1243. [DOI] [PubMed] [Google Scholar]
- Roche BM, Alexander HM, and Maltby AD. 1995. Dispersal and disease gradients of anther-smut infection of Silene alba at different life stages. Ecology 76:1863–1871. [Google Scholar]
- Sakai AK, Weller SG, Chen M-L, Chou S-Y, and Tasanont C. 1997. Evolution of gynodioecy and maintenance of females: the role of inbreeding depression, outcrossing rates, and resource allocation in Schiedea Adamantis (Caryophyllaceae). Evolution 51:724–736. [DOI] [PubMed] [Google Scholar]
- Schultz ST 2003. Sexual dimorphism in gynodioecious Sidalcea hirtipes (Malvaceae). II. Floral traits. International Journal of Plant Sciences 164:175–180. [Google Scholar]
- Shaw RG, Geyer CJ, Wagenius S, Hangelbroek HH, and Etterson JR. 2008. Unifying life‐history analyses for inference of fitness and population growth. The American Naturalist 172:E35–E47. [DOI] [PubMed] [Google Scholar]
- Shykoff JA, Bucheli E, and Kaltz O. 1997. Anther smut disease in Dianthus silvester (caryophyllaceae): Natural Selection on Floral Traits. Evolution 51:383. [DOI] [PubMed] [Google Scholar]
- Shykoff JA, Kolokotronis S-O, Collin CL, and López-Villavicencio M. 2003. Effects of male sterility on reproductive traits in gynodioecious plants: a meta-analysis. Oecologia 135:1–9. [DOI] [PubMed] [Google Scholar]
- Spigler RB, and Ashman T-L. 2012. Gynodioecy to dioecy: are we there yet? Annals of Botany 109:531–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steets JA, Wolf DE, Auld J, and Ashman T-L. 2007. The role of natural enemies in the expression and evolution of mixed mating in hermaphroditic plants and animals. Evolution 61:2043–2055. [DOI] [PubMed] [Google Scholar]
- Therneau TM, & Lumley T (2017). Package ‘survival’. R package version, 2–41. [Google Scholar]
- Tsuji K, and Sota T. 2010. Sexual differences in flower defense and correlated male-biased florivory in a plant-florivore system. Oikos 119:1848–1853. [Google Scholar]
- Carlsson-Graner U, Elmqvist T, Agren J, Gardfjell H, and Ingvarsson P. 1998. Floral sex ratios, disease and seed set in dioecious Silene dioica. Journal of Ecology 86:79–91. [Google Scholar]
- Uchida W, Matsunaga S, Sugiyama R, Kazama Y, and Kawano S. 2003. Morphological development of anthers induced by the dimorphic smut fungus Microbotryum violaceum in female flowers of the dioecious plant Silene latifolia. Planta 218:240–248. [DOI] [PubMed] [Google Scholar]
- Vega-Frutis R, Munguía-Rosas MA, Varga S, and Kytöviita M-M. 2013. Sex-specific patterns of antagonistic and mutualistic biotic interactions in dioecious and gynodioecious plants. Perspectives in Plant Ecology, Evolution and Systematics 15:45–55. [Google Scholar]
- Zuk M, and McKean KA. 1996. Sex differences in parasite infections: patterns and processes. International Journal for Parasitology 26:15. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


