Abstract
Background
Subclinical hypothyroidism is defined as an elevated serum thyroid‐stimulating hormone (TSH) level with normal free thyroid hormones values. The prevalence of subclinical hypothyroidism is 4% to 8% in the general population, and up to 15% to 18% in women who are over 60 years of age. There is considerable controversy regarding the morbidity, the clinical significance of subclinical hypothyroidism and if these patients should be treated.
Objectives
To assess the effects of thyroid hormone replacement for subclinical hypothyroidism.
Search methods
We searched The Cochrane Library, MEDLINE, EMBASE and LILACS. Ongoing trials databases, reference lists and abstracts of congresses were scrutinized as well.
Selection criteria
All studies had to be randomised controlled trials comparing thyroid hormone replacement with placebo or no treatment in adults with subclinical hypothyroidism. Minimum duration of follow‐up was one month.
Data collection and analysis
Two authors independently assessed trial quality and extracted data. We contacted study authors for missing or additional information.
Main results
Twelve trials of six to 14 months duration involving 350 people were included. Eleven trials investigated levothyroxine replacement with placebo, one study compared levothyroxine replacement with no treatment. We did not identify any trial that assessed (cardiovascular) mortality or morbidity. Seven studies evaluated symptoms, mood and quality of life with no statistically significant improvement. One study showed a statistically significant improvement in cognitive function. Six studies assessed serum lipids, there was a trend for reduction in some parameters following levothyroxine replacement. Some echocardiographic parameters improved after levothyroxine replacement therapy, like myocardial relaxation, as indicated by a significant prolongation of the isovolumic relaxation time as well as diastolic dysfunction. Only four studies reported adverse events with no statistically significant differences between groups.
Authors' conclusions
In current RCTs, levothyroxine replacement therapy for subclinical hypothyroidism did not result in improved survival or decreased cardiovascular morbidity. Data on health‐related quality of life and symptoms did not demonstrate significant differences between intervention groups. Some evidence indicates that levothyroxine replacement improves some parameters of lipid profiles and left ventricular function.
Keywords: Adult, Humans, Hormone Replacement Therapy, Hormone Replacement Therapy/adverse effects, Cardiovascular System, Cardiovascular System/drug effects, Cholesterol, Cholesterol/blood, Hypothyroidism, Hypothyroidism/blood, Hypothyroidism/drug therapy, Randomized Controlled Trials as Topic, Thyroxine, Thyroxine/adverse effects, Thyroxine/therapeutic use
Thyroid hormone replacement for subclinical hypothyroidism
Subclinical hypothyroidism is a condition where some laboratory findings point at a thyroid gland not working properly. Patients with subclinical hypothyroidism may have vague, non‐specific symptoms of actual hypothyroidism (for example dry skin, cold skin or feeling colder, constipation, slower thinking, poor memory) but these thyroid‐related symptoms are not specific, that is why the diagnosis is based on test results. The fundamental question regarding people with subclinical hypothyroidism is whether they should be treated with thyroid hormones. To answer this question twelve studies of six to 14 months duration involving 350 people were analysed. Thyroid hormone therapy for subclinical hypothyroidism did not result in improved survival or decreased cardiovascular morbidity (for example less heart attacks or strokes). Data on health‐related quality of life and symptoms did not demonstrate significant differences between placebo and thyroid hormone therapy. Some evidence indicated that thyroid hormone had some effects on blood lipids and technical measurements of heart function. Adverse effects were inadequately addressed in most of the included studies and have to be urgently investigated in future studies, especially in older patients.
Background
Description of the condition
The introduction of sensitive assays to determine serum thyroid‐stimulating hormone (TSH) concentrations in the last two decades increased the number of newly diagnosed cases of subclinical thyroid disease. Subclinical hypothyroidism, defined by the presence of an elevated serum TSH concentration, in association with normal values of serum free thyroxine (FT4), is the most common condition found during thyroid function screening (Ayala 2000; Evered 1973; Tunbridge 1977).
The prevalence of subclinical hypothyroidism is variable and can occur in 8% of women and 3% of men (Vanderpump 1995), but this prevalence may increase to 15% to 18% in women who are over 60 years of age (Canaris 2000; Tunbridge 1977) or decrease to 3% in both women and men in populations whose iodine intake is low (Alghini‐Lombardi1999; Knudsen 1999; Laurberg 2000; Szabolcs 1997). The term 'subclinical hypothyroidism' may not be strictly correct, since some of these patients have mild clinical symptoms (Monzani 1993; Monzani 1997). Moreover, recent studies have shown that undetected subclinical hypothyroidism during pregnancy is associated with impaired neuropsychological development of the child (Haddow 1999).
Complications of subclinical hypothyroidism
The condition may progress to overt hypothyroidism. The rate of progression can occur in 2% to 3% of patients per year but can increase to 4% to 5% per year when thyroid auto‐antibodies are present. According to Engler, the patients with subclinical hypothyroidism can be divided into a 'low‐risk' and a 'high‐risk' group for overt hypothyroidism according to TSH levels and antibody status (Engler 1992). In the Wickham survey study, during 20 years of follow up, a higher risk of overt hypothyroidism was found in women who had both elevated serum levels of TSH levels and antithyroid antibodies at baseline (4.3% per year, or 38 times that of women who had normal serum TSH levels and no antithyroid antibodies) (Vanderpump 1995). The importance of thyroid antibodies is also evident from a Dutch study (Geul 1993). Spontaneous return of increased TSH values to the normal range occurs in 5.5% of patients after one year (Parle 1993).
Subclinical hypothyroidism may be associated with increased morbidity, particularly for cardiovascular disease, and subtly decreased myocardial contractility (Rigway 1981). The effects of subclinical hypothyroidism on serum lipid levels remains controversial. Danese in a meta‐analysis of 13 studies, mostly of non‐randomised design, of subclinical hypothyroidism found that thyroxine (T4) therapy resulted in a significant reduction of serum total cholesterol of 7.9 mg/dl or 5%, and serum LDL‐cholesterol concentration decreased by 10 mg/dl (Danese 2000). Furthermore, there were no significant effects of T4 on serum HDL‐cholesterol or triglycerides concentration. These results differed to the findings of another non‐systematic review with a reduction in serum total cholesterol of 15 mg/dl (Tanis 1996).
An inverse correlation between TSH levels and flow‐mediated endothelium‐dependent vasodilatation of peripheral arteries was reported by Lekakis, and this dysfunction may be the first stage of developing atherosclerosis (Lekakis 1997). In addition, a possible increased peripheral vascular resistance and negative effects on lipid levels may be related to an increased risk of developing atherosclerosis and coronary heart disease (Althaus 1988; Caron 1990). Perk, in a coronary angiographic study reported greater coronary atherosclerosis progression in patients with hypothyroidism and TSH levels in the range seen in subclinical hypothyroidism compared to patients whose TSH levels were maintained in the normal range (Perk 1997). Hak reported an association between aortic atherosclerosis and myocardial infarction in women who had subclinical hypothyroidism and positive antithyroid antibodies (Hak 2000).
Haggerty suggests that subclinical hypothyroidism may lower the threshold for the occurrence of depression and may be a risk indicator for depression (Haggerty 1993). In addition, people with subclinical hypothyroidism show significant alterations in memory and mood, including anxiety and somatic symptoms (Monzani 1993). Patients with major depression also have a poorer response to antidepressive drugs if they are subclinically hypothyroid (Toffe 1992).
Description of the intervention
Thyroid substitution could prevent progression to overt hypothyroidism, improve neuropsychiatric symptoms and somatic symptoms, mood disorders, cognitive dysfunction, atypical responses to standard psychiatric therapeutic interventions, and minimize deleterious effects on cardiovascular function and lipid levels. However, there may also be adverse effects from replacement doses of thyroxine, and these include nervousness, palpitations, atrial fibrillation, and exacerbation of angina pectoris. Reduction in bone density of the spine, femoral neck, distal radius, and femoral trochanter can also occur. The over‐treatment with thyroxine probably contributes to the development of osteoporosis in postmenopausal women, and an undetectable TSH level suggests over‐treatment (Nyström 1988; Sawin 1994; Uzzan 1996).
A 1998 American College of Physicians position paper questioned whether sufficient data exist to recommend treatment for all patients with subclinical hypothyroidism (Helfand 1998). The authors recommended screening with a sensitive TSH assay in women older than 50 years of age. For TSH levels between 5 to 10 mU/L, decisions are usually individualized treating the patients if symptoms exist or doing a repeat measurement of serum TSH every year. Furthermore, the recognition of hypothyroidism in pregnant women argues in favour of routine screening at the prenatal visit. Levothyroxine therapy to decrease the serum TSH into the normal range in patients with positive thyroid peroxidase (TPO) antibodies is recommended. If the TPO antibodies are negative, therapy is recommended if symptoms or elevated lipid levels are present. It also appears reasonable to treat subclinical hypothyroidism in pregnant women. When both symptoms and TPO antibodies are negative, levothyroxine therapy should not be advised but careful monitoring of the serum TSH should be done (Braverman 1999; Tunbridge 2000; Cooper 2001).
Costs of the intervention
Danese performed a cost‐effectiveness analysis of screening 35 year old patients for mild thyroid failure with a serum TSH assay every five years and reported a $9223 (6844 Euro) per QALY (quality‐adjusted life‐year) for women, $22.595 (16.766 Euro) per QALY for men, and $5000 (3710 Euro) per QALY for women over the age of 65. The cost‐effectiveness of screening for subclinical hypothyroidism was comparable with the cost of other commonly performed preventive and therapeutic health practices such as breast cancer screening and hypertension screening (Danese 1996). Consequently, the American Thyroid Association has recommended screening of both women and men beginning at age 35 years and every five years thereafter (Landerson 2000).
Why it is important to do this review
Subclinical hypothyroidism is an extremely common condition that is already being widely identified and treated without compelling evidence that treatment is worthwhile. This systematic review will try to establish if treatment of subclinical hypothyroidism is beneficial and safe.
Objectives
To assess the effects of thyroid hormone replacement for subclinical hypothyroidism.
Methods
Criteria for considering studies for this review
Types of studies
All studies had to be randomised controlled trials, irrespective of publication status, date of publication or language. Trials were only included if the treatment was given for a minimum of one month.
Types of participants
Only adult patients with subclinical hypothyroidism, receiving triiodothyronine (T3) or thyroxine (T4) or a combination thereof as a replacement treatment.
Subclinical hypothyroidism was defined by a thyroid‐stimulating hormone (TSH) level above the upper limit of the reference value for each method used to determine TSH with normal values of total T4 or free T4 (FT4), with or without T3 or free T3 (FT3) measurements. Only outpatients with no severe illness were included (O'Reilly 2000). Children and adolescents were excluded.
Types of interventions
T3 or T4 or a combination of T3 and T4 replacement therapy compared to placebo or no treatment. The length of treatment had to be at least one month and the minimum length of follow‐up was three months.
Types of outcome measures
Primary outcomes
cardiovascular mortality or morbidity;
improvement in symptoms and signs (for example prolonged ankle reflex, dry skin, paraesthesia, cold skin or feeling colder, constipation, slower thinking, poor memory);
health‐related quality of life (ideally investigated using a validated instrument).
Secondary outcomes
all‐cause mortality;
normalisation of TSH levels (according to the reference value for each method used to determine TSH);
lipid levels (for example total cholesterol, low‐density lipoprotein (LDL) cholesterol, high‐density lipoprotein (HDL) cholesterol, triglycerides);
systolic and diastolic heart function;
adverse effects (for example hyperthyroidism symptoms like nervousness, palpitations; cardiac arrhythmia).
Timing of outcome measurement
Medium (3 to 12 months) and long‐term (more than 12 months) outcome measurements will be assessed.
Search methods for identification of studies
Electronic searches
We used the following sources for the identification of trials:
The Cochrane Library (issue 1, 2006);
MEDLINE (until May 2006);
EMBASE (until May 2006);
CINAHL (until May 2006);
LILACS (until May 2006 ).
The Cochrane sensitive search strategy for identification of controlled clinical trials described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2005) was used for the MEDLINE and EMBASE search, in combination with terms specific to subclinical hypothyroidism and substitution therapy. For the LILACS search we used the search strategy for controlled clinical trials described by Castro et al (Castro 1997), employing the same terms as those used for the MEDLINE search. The described search strategy (see for a detailed search strategy under Appendix 1) was used for MEDLINE. For use with the other databases this strategy was slightly adapted.
We also searched databases of ongoing trials: Current Controlled Trials (www.controlled‐trials.com ‐ with links to other databases of ongoing trials).
Additional key words of relevance were not identified during any of the electronic or other searches. If this had been the case, electronic search strategies would have been modified to incorporate these terms.
Searching other resources
We tried to detect additional studies by searching the reference lists of included trials and (systematic) reviews identified.
Authors of identified clinical trials, other experts and pharmaceutical companies were contacted to search additional and unpublished randomised controlled trials.
Data collection and analysis
Selection of studies
Two reviewers (HV and HS) independently assessed the titles and abstracts of all reports of trials identified by the electronic search. Full text hard copies were obtained for studies that appeared to fulfil the selection criteria and for studies where there was any doubt. Interrater agreement for study selection was measured using the kappa statistic (Fleiss 1981). In case of discrepancy, the opinion of a third reviewer was sought in order to reach a consensus.
Data extraction and management
The reviewers were not masked to the authors and results of the trial. Data were independently extracted by the reviewers and cross‐checked. A standard data extraction form was used, collecting the following key data:
METHODS: Research question; randomisation process, allocation concealment, length of post‐interventional follow‐up period, blinded assessment of primary outcomes, baseline assessment of primary outcomes, reliable main outcome measure(s), protection against contamination. Setting (location of care, academic status, country), unit of randomisation, unit of analysis, power calculation, sample representativeness, quality indicators.
PARTICIPANTS: Inclusion criteria, exclusion criteria, etiology of disease, gender, severity of outcome, presence of antithyroid antibodies, confounding variables as smoking status, use of contraceptives and other co‐morbidities. Proportion of eligible patients (or allocation units), number of patients included in the study, reasons for withdrawal from the study.
INTERVENTIONS: Replacement with triiodothyronine (T3) or thyroxine (T4) or both, dose, duration of treatment and any co‐interventions.
OUTCOMES: Primary and secondary outcome(s) as specified above, any other outcomes assessed, other events, length of follow‐up, quality of reporting of outcomes.
RESULTS: For outcomes and time of assessment (including a measure of variation), if necessary converted to measures of effect specified below; intention‐to‐treat analysis.
NOTES: Sources of funding, ethical approval, conflict of interest of the authors.
Assessment of risk of bias in included studies
For evaluation of methodological quality the Jadad scale (Jadad 1996) and the Schulz (Schulz 1995) criteria were applied. The methodological quality assessment was performed by two reviewers. Interrater agreement was measured using the kappa statistic (Fleiss 1981). If a discrepancy in opinion occurred, a third reviewer was asked for his opinion in order to reach consensus.
The Jadad scale consists of the following questions:
Was the trial described as randomised (this includes the use of the words such as random, randomly and randomisation)? (1 point).
Is the method of randomisation appropriate? (1 point is added if the methods to generate the sequence of randomisation are described) and it is appropriate (e.g., table of random numbers, computer generated). (0 points if the method of randomisation is not described).
Was the study described as double blind? (1 point if yes, 0 points if no).
Is the method of blinding used appropriate? One point is added if the method of masking is described and appropriate (e.g., identical placebo). (1 point is deducted if the method of masking is inappropriate (comparison of tablet versus injection with no double dummy), 0 points if the method of masking is not described).
Is there a description of withdrawals and dropouts? (1 point is given if the numbers and reasons for withdrawal in each group are stated. If there are no withdrawals, the report must say so. If there is no statement on withdrawals, this item is given no points).
The scale awards one to five points to randomised controlled trials (RCTs). RCTs with one and two points are considered low quality and RCTs with three to five points are considered high quality (Moher 1998).
The criteria specified by Schulz consist of the following questions: A. Generation of allocation sequence:
Adequate sequence of generation is reported using a computer random number generator or random number tables.
Does not report on one of the adequate forms of generation of allocation concealment mentioned above, but mentions the randomisation method.
Other methods of allocation that appear to be unbiased (e.g., minimisation).
B. Allocation concealment:
Adequate measures taken to conceal allocations such as central randomisation; serially numbered, opaque, sealed envelopes; or other description that contains elements convincing of adequate concealment.
Vaguely concealed trials, in which the authors either do not report an allocation concealment approach at all, or report an approach that does not fall into one of the categories.
Inadequately concealed trials, in which the method of allocation was not concealed, such as alternation methods or use of case record numbers (quasi‐randomisation) ‐ such trials are going to be excluded in the present systematic review.
C. Inclusion of all randomised participants:
Trials in which an intention‐to‐treat analysis is possible with only a few losses to follow‐up.
Trials, which report exclusions as listed above, but exclusions are less than 10%.
No reporting on exclusions, or exclusions greater then 10%, or wide differences in exclusions between groups.
D. Blinded assessment:
Trials in which the double‐blind or double masked technique is used.
Trials trying to control information bias (i.e., bias originating from withdrawals, co‐interventions, assessment) by other methods (e.g., single blinded trials).
Trials in which reduction of information bias is not employed.
Studies will be subdivided into the following three categories : A ‐ all quality criteria met: low risk of bias. B ‐ one or more of the quality criteria only partly met: moderate risk of bias. C ‐ one or more criteria not met: high risk of bias.
Quality assessment was used for sensitivity analysis and not as an exclusion criterion.
Dealing with missing data
In the case of duplicate publications and companion papers of a primary study, we tried to maximise yield of information by simultaneous evaluation of all available data. In cases of doubt, the original publication (usually the oldest version) obtained priority. Principal investigators were asked to check the data and to supply missing information.
Assessment of heterogeneity
In the event of substantial clinical or methodological or statistical heterogeneity, study results were not planned to be combined in a meta‐analysis. Heterogeneity was identified by visual inspection of the forest plots, by using a standard χ2‐test and a significance level of α = 0.1, in view of the low power of such tests. Quantification of heterogeneity was also examined with I2, ranging from 0% to 100% including its 95% confidence interval (Higgins 2002). I2 demonstrates the percentage of total variation across studies due to heterogeneity and was used to judge the consistency of evidence. I2 values of 50% and more indicate a substantial level of heterogeneity (Higgins 2003). When heterogeneity was found, we attempted to determine potential reasons for it by examining individual study characteristics and those of subgroups of the main body of evidence.
Data synthesis
Data were summarised statistically if they were available, of sufficient quality and sufficiently similar. Dichotomous data were expressed as odds ratio (OR). Continuous data were expressed as weighted mean differences (WMD) and an overall WMD was calculated. Overall results were calculated based on the fixed and random effects model. Possible sources of heterogeneity were assessed by subgroup and sensitivity analyses as described below (Egger 1995).
Subgroup analysis and investigation of heterogeneity
If the results for the primary outcomes were significant, subgroup analyses were planned in order to explore effect size differences according to:
age: 18 to 49 years, older than 50 years;
antithyroid antibodies: present or not,
prior thyroid disease;
thyroid‐stimulating hormone (TSH) level before replacement: equal or less than 10 mU/L or more than 10 mU/L;
triiodothyronine (T3) or thyroxine (T4) or a combination of T3 and T4;
normal or elevated lipid levels: Total cholesterol less than 200 mg/dl, LDL‐cholesterol less than 100 mg/dl , HDL‐cholesterol more than 40 mg/dl, triglycerides less than 150 mg/dl;
medium (3 to 12 months) and longer term (more than 12 months) outcome measurement.
Sensitivity analysis
The following strategies were planned to be used for the sensitivity analyses:
excluding unpublished RCTs;
excluding RCTs published as abstracts;
excluding RCTs of lower quality score as assessed by Jadad's scale (score one and two);
excluding studies using the following filters: diagnostic criteria, language of publication, source of funding (industry versus other), and country;
excluding cross‐over studies.
The robustness of the results was also to be tested by repeating the analysis using different measures of effects size (risk difference, odds ratio, etc.) and different statistical models (fixed and random effects models).
Results
Description of studies
Results of the search
Of the 2513 studies identified, 20 were retrieved for further scrutiny with 12 publications meeting our inclusion criteria (Caraccio 2002; Caraccio 2005; Cooper 1984; Jaeschke 1996; Jorde 2006; Kong 2002; Meier 2001; Monzani 2001; Monzani 2004; Nystrom 1988; Ross 1993; Yazici 2004). One publications needs additional information from authors ( Fadeyev 2006). Eight publications were excluded: Four studies were duplicates of one included study (Meier 2001) and excluded for the following reasons: Outcome beyond scope of review (Christ‐Crain 2003; Christ‐Crain 2005; Meier 2003), insufficient data presented (Meier 2004). Four studies were not RCTs (Biondi 1999; Biondi 2002; Jensovsky 2002; Pinnes 1999).
Included studies
Interventions
One study compared levothyroxine (LT4) with no treatment (Ross 1993) and all of the others studies compared LT4 with placebo. The dose was titrated, until suppression of the thyroid‐stimulating hormone (TSH) level below the upper limit of range occurred and the mean final replacement dose was 65 mcg daily (Caraccio 2005; Caraccio 2002; Monzani 2001; Yazici 2004), 68 mcg daily (Jaeschke 1996), 72 mcg daily (Cooper 1984; Monzani 2001; Monzani 2004; Ross 1993) or 86 mcg daily (Meier 2001). The dose was titrated to 50 or 100 mcg daily if TSH levels were greater than 6.0 mIU/L in the Kong study (Kong 2002). In the Nystrom study (Nystrom 1988) the daily LT4 dose was 50 mcg for two weeks, then 100 mcg for two weeks, thereafter 150 mcg daily.
Participants
Five studies included persons without known history of thyroid disease (Jaeschke 1996; Jorde 2006; Kong 2002; Nystrom 1988; Ross 1993). All patients in the Cooper study (Cooper 1984) had previously been treated for hyperthyroidism. Four studies (Cooper 1984; Meier 2001; Monzani 2004; Yazici 2004) included patients that had autoimmune thyroiditis, previously treated Graves's disease and goiter; these patients were treated with antithyroid drugs, surgery or radioiodine. In the Monzani and Caraccio study (Caraccio 2005; Monzani 2001) the etiology of subclinical hypothyroidism was Hashimoto's thyroiditis in all patients. Most of the patients were females older than 18 years and the average age was nearly 35 years old, except in three studies (Jaeschke 1996; Meier 2001; Ross 1993) where participants were older than 50 years. In these studies none patient was taken medications that interfered with thyroid function test results. The TSH levels was less than 12 mIU/L in three studies (Monzani 2001; Ross 1993; Yazici 2004, ), less than 15 mIU/L in three studies (Caraccio 2002; Monzani 2004; Nystrom 1988) and less than 10 mIU/L in three studies (Caraccio 2005; Jorde 2006; Kong 2002). TSH was as high as 32 or 55 mIU/L in three studies (Cooper 1984; Jaeschke 1996; Meier 2001).
Duration of studies
Study duration was between six to 14 months.
Outcomes
Seven studies analysed outcomes related to symptoms improvement, neuropsychological function or quality of life (Caraccio 2005; Cooper 1984; Jaeschke 1996; Jorde 2006; Kong 2002; Meier 2001; Nystrom 1988), seven studies analysed lipids (Caraccio 2002; Cooper 1984; Jaeschke 1996; Kong 2002; Meier 2001; Monzani 200; Nystrom 1988) and four evaluated cardiac function ( Cooper 1984; Monzani 2001; Nystrom 1988; Yazici 2004). Bone density was investigated in two studies (Jaeschke 1996; Ross 1993), carotis intima‐media thickness in one study (Monzani 2004).
Risk of bias in included studies
Study design
All studies refer to be randomised and controlled but three of them did not describe the generation of allocation sequence and allocation concealment was unclear (Cooper 1984; Jorde 2006; Nystrom 1988). One study was of crossover design (Nystrom 1988). Blinding was attempted in all studies (patients and investigators involved in assessment or care) except in two studies (Caraccio 2002; Ross 1993). In these studies placebo was described as identical to levothyroxine tablets.Randomisation methodAll studies were randomised controlled trials. Eight studies had an adequate allocation concealment and this information was described in the publication (Kong 2002; Meier 2001), or some information about allocation concealment was provided by authors (Caraccio 2002; Caraccio 2005; Jaeschke 1996; Monzani 2001; Monzani 2004; Ross 1993). Four studies (Cooper 1984; Jorde 2006; Nystrom 1988; Yazici 2004) did not provide information about randomisation procedures or allocation concealment.Blinding methodsTen studies described double‐blind methods (Caraccio 2005; Cooper 1984; Jaeschke 1996; Jorde 2006; Kong 2002; Meier 2001; Monzani 2001; Monzani 2004; Nystrom 1988; Yazici 2004). Two studies provided no information about blinding procedures (Caraccio 2002; Ross 1993).Attrition ratesThere were no drop‐outs in five studies (Caraccio 2002; Caraccio 2005; Monzani 2001; Monzani 2004; Yazici 2004). Attrition rates of less than 10% were observed in four studies (Cooper 1984; Meier 2001; Ross 1993; Yazici 2004) and high drop‐out rates were reported in three studies (Jaeschke 1996; Kong 2002; Nystrom 1988).Additional considerationsAuthors of included studies were tried to be contacted and the majority of them responded (Caraccio 2002; Cooper 1984; Jaeschke 1996; Monzani 2001; Monzani 2004; Ross 1993). The quality of the evaluated studies according to the Schulz criteria and the Jadad scale is described under Appendix 2.
Effects of interventions
Primary outcomes
Cardiovascular mortality or morbidity was not analysed in any of the included studies.
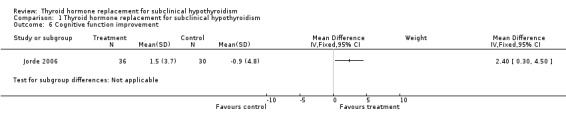
Seven studies assessed symptoms, signs and health‐related quality of life using a variety of different scales (for details see 'Characteristics of included studies' and 'Figures' ). No study demonstrated statistically significant differences between intervention groups in health‐related quality of life. One study showed a statistically significant improvement in cognitive function (Jorde 2006).
Secondary outcomes
All‐cause mortality was not investigated in any of the included studies.
Lipids
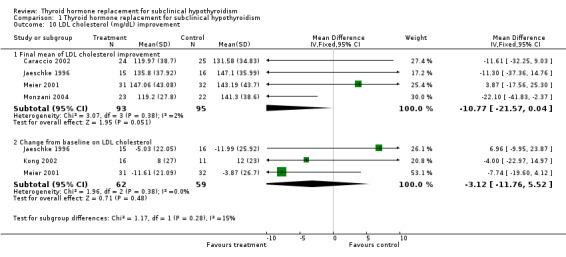
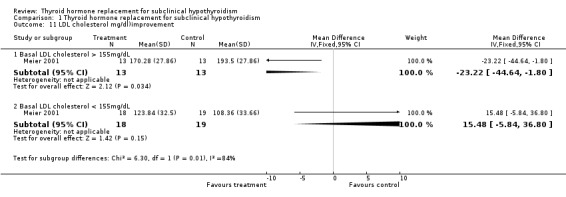
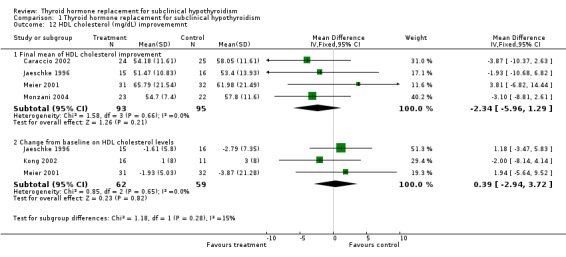
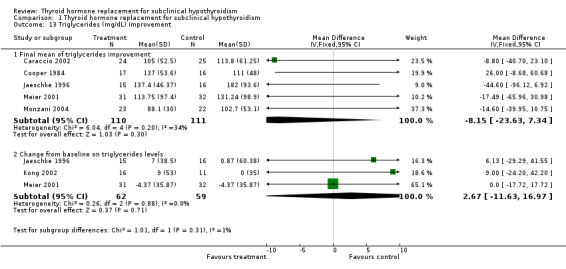
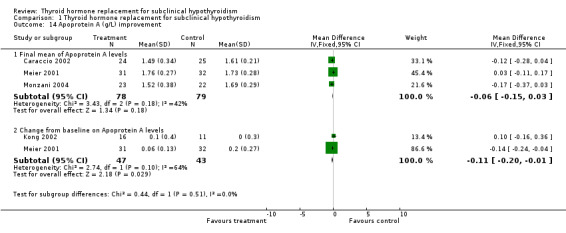
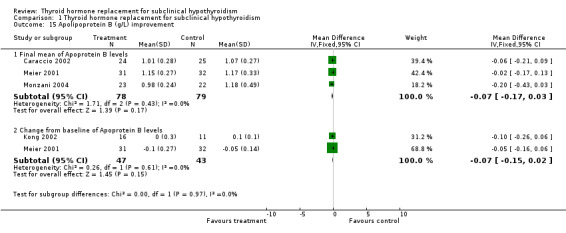
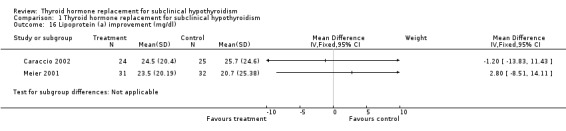
Total cholesterol levels were not significantly improved by thyroid hormone replacement therapy (though a trend was noted in favour of levothyroxine treatment). HDL‐ as well as LDL‐cholesterol also did not reveal significant effects of levothyroxine treatment. A subgroup with LDL values greater than 155 mg/dl showed significant effects, but this result should be regarded as hypothesis‐generating only. Moreover, no significant differences between intervention groups were seen in the outcomes triglycerides, apolipoprotein A and B and lipoprotein A.
Systolic and diastolic heart function
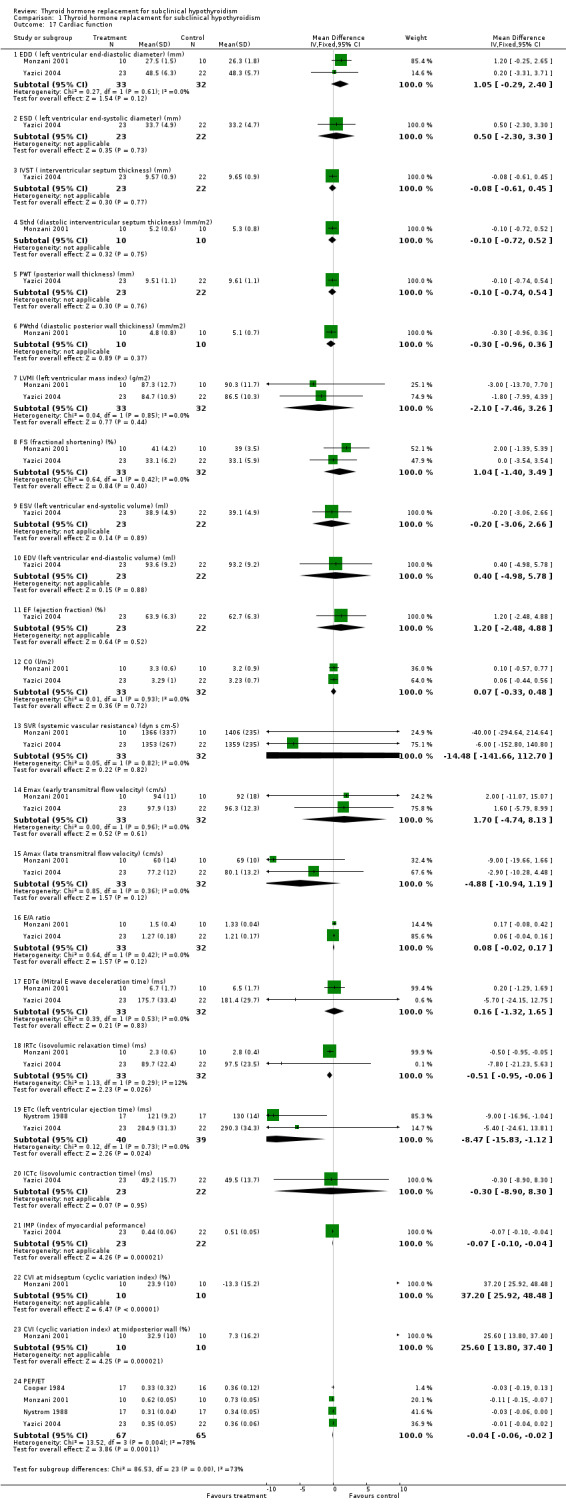
A great variety of parameters for systolic and diastolic heart function were investigated in some of the included studies. Only some indices were associated with treatment related significant differences, like isovolumic relaxation time, index of myocardial performance and cyclic variation index. Two studies in 79 participants investigated left ventricular ejection time revealing a shortening of ‐8.5 ms in favour of the levothyroxine treated group (WMD ‐8.5 ms, 95% confidence interval (CI) ‐15.8 to ‐1.1).
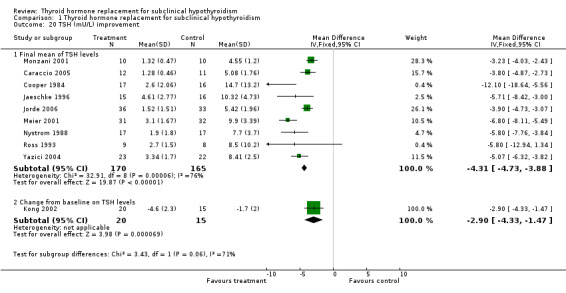
Normalisation of thyroid‐stimulating hormone (TSH) levels
Nine studies with 335 participants (170 on treatment and 165 on placebo) reported findings. In the group treated with levothyroxine, TSH levels were significantly reduced (WMD ‐4.3 mU/L; 95% CI ‐4.8 to ‐3.9). There was a significant heterogeneity between trials (I2 = 75.7%) but mean differences of all comparisons were in favour of levothyroxine treatment.
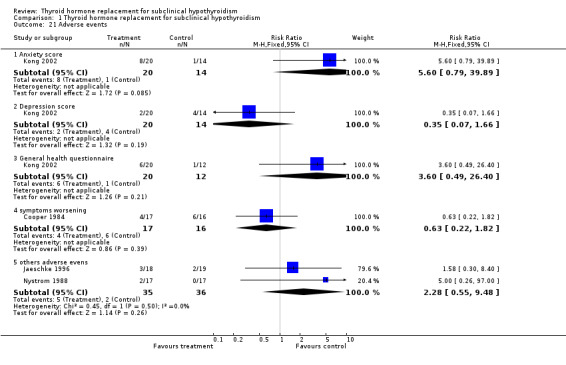
Adverse effects
Only four studies with 138 participants on treatment reported investigation of adverse events (anxiety, depression, general health, symptoms score and other adverse events). No statistically significant differences between the groups were noted.
Discussion
There is considerable controversy regarding the clinical significance of subclinical hypothyroidism, its morbidity and whether these patients should be treated at all. This review tried to provide an overview of studies associated with minimum risk of bias, that is randomised controlled clinical trials of levothyroxine (LT4) therapy in subclinical hypothyroidism.
Summary of main results
Mortality and cardiovascular risk
We did not identify any RCT that assessed the outcomes cardiovascular morbidity or mortality. Previous studies have suggested that subclinical hypothyroidism is associated with alterations of the cardiovascular systems (Hak 2000; Imaizumi 2004; Walsh 2005) or no effect (Parle 2001; Vanderpump 1995). One study showed a decreased cardiovascular and all‐cause mortality (Gussekloo 2004). Recently, Cappola, in a 12 year cohort study of people older than 65 years, reported no significant differences in the risk of coronary heart disease, cerebrovascular disease, cardiovascular death or all‐cause death between the groups euthyroidism and subclinical or overt hypothyroidism (Cappola 2006). Rodondi, studied 2730 men, aged 70 to 79 years in a cohort of four years follow up to investigate cardiovascular events (Rodondi 2005). The rate of congestive heart failure increased in participants whose thyroid‐stimulating hormone (TSH) was greater than 7.0 mU/L (HR 2.49, 95% confidence interval (CI) 1.20 to 5.18) and in participants with previous congestive heart failure with a risk for recurrence (HR 7.62, 95% CI 2.25 to 25.77). The rate of coronary heart disease events, stroke, peripheral arterial disease, and mortality did not significantly differ by different TSH levels. On the other side, older patients with subclinical hypothyroidism are associated with greater longevity: In a four year cohort with people aged 85, increasing levels of TSH were associated with a decreased risk of both cardiovascular and non cardiovascular mortality (HR 0.66, 95% CI 0.48 to 0.98 and HR 0.84, 95% CI 0.66 to 1.07, respectively). The reason for this is not clear, but it may be that subclinical hypothyroidism has different implications in very old people compared with the general population, or because very old patients may be biologically different from elderly patients (Gussekloo 2004). Can LT4 therapy be justified, and is it cost‐effective? Unfortunately, this systematic review was not able to definitely determine the efficacy and safety of LT4 replacement in subclinical hypothyroidism.
Symptoms, mood and quality of life
In one study the percentage of people reporting symptoms associated with hypothyroidism was slightly but significantly higher than in the euthyroid group (13.7% vs 12.1%) (Canaris 2000). Other studies did not find the same results (Lindeman 1999; Zulewski 1997), including patients of 85 years (Gussekloo 2004). We found seven placebo‐controlled blinded studies that assessed these outcomes, and there were no statistically significant improvements. One study showed statistically significant improvement in cognitive function (Jorde 2006). TSH levels were less than 15 mU/L at the beginning of the study and patients had no previous thyroid disease. The Meier (Meier 2001) study met our inclusion criteria, but the sub‐group analysis was not included in the meta‐analysis because it related information on symptoms only for treated group. In the meta‐analysis evaluating health‐related quality of life, three studies were included (Kong 2002; Jaeschke 1996; Jorde 2006) and we did not find improvements, in contrast to the Bianchi and Gulseren studies (Bianchi 2004; Gulseren 2006).
Improvement in thyroid‐stimulating hormone (TSH)
There was a statistically significant difference between the LT4 and placebo groups in all eleven trials that were included. The daily average dose required to normalize TSH in the active group varied from 67.5 to 85.5 mcg (range 50 to 125 mcg). Subclinical hyperthyroidism at the end of study was reported in two studies (Jorde 2006; Ross 1993). TSH levels normalized spontaneously in 42%, 25% and 24% of patients in the placebo groups (Jaeschke 1996; Kong 2002; Ross 1993, ). These studies included patients without thyroid disease.
Hyperlipidaemia
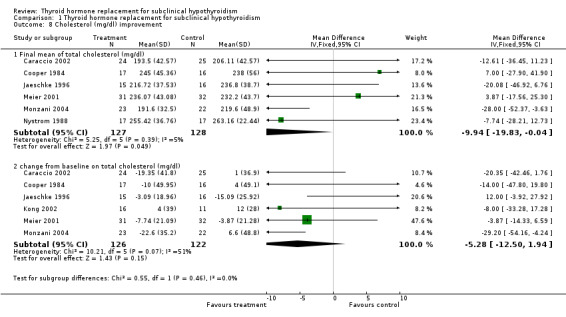
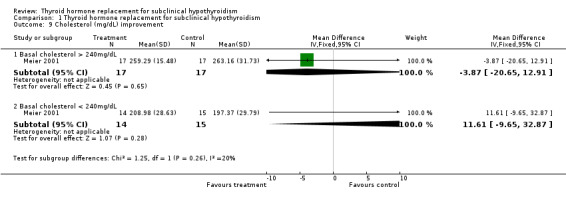
The association between total plasma cholesterol (or LDL‐cholesterol) and cardiovascular disease is well established. It has been estimated from large trials that cardiovascular disease is reduced by 15% for each 10% reduction in plasma LDL‐cholesterol or 25% by a 38 mg/dL reduction in plasma total cholesterol (Isles 2000). In addition, as confirmed by a meta‐analysis of cholesterol‐lowering, a 10% reduction in serum levels of cholesterol may reduce the risk of cardiovascular mortality by 20% (La Rosa 1999). The effect of LT4 therapy on cholesterol levels and others lipids, and on cardiovascular disease, is controversial. A meta‐analysis of both observational and randomised studies concluded that cholesterol levels decreased by 0.20 mmol/L (7.9 mg/dL) or 5% in LT4 group, LDL‐cholesterol decreased by 0.26 nmol/L (10 mg/dL), and no change in triglycerides concentrations was noted (Danese 2000). The main problem with this review is that it included studies of poor quality, of observational design, many before‐after studies with small sample sizes, and a heterogeneous population with previous thyroid diseases. In our systematic review, we founded six studies that assessed serum lipids. One difficulty here was the great difference in basal levels of cholesterol between the LT4 and placebo groups in small trials. As an example, in one study the means of basal cholesterol levels in LT4 and placebo groups were 251.9 mg/dL and 219.8 mg/dL, respectively and the results were reported in changes from baseline and as final values (Jaeschke 1996). When analysed using the differences of the means between LT4 and placebo post‐treatment values, there was a decrease in the cholesterol levels of 20 mg/dL, favouring treatment. When analysed using changes from baseline (that is the mean difference between pre‐ and post‐intervention measurements), we could observe a decrease of 12 mg/dL in cholesterol levels favouring placebo (‐15 mg/dL compared to the LT4 group: ‐3.1 mg/dL). According to techniques described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2005), we imputed standard deviation for the changes, when this information was not available in the studies (Caraccio 2002; Cooper 1984; Monzani 2004). Imputed standard deviations should not be used for a majority of studies in a meta‐analysis, but may be reasonable for a small proportion of studies comprising a small proportion of the data if it enables them to be combined with other studies for which full data are available. However, different sensitivity analyses showed that the significant treatment effect of cholesterol reduction after LT4 treatment was sensitive to various parameters.
Cardiac function
Subclinical hypothyroidism causes changes in cardiac function similar to, but less marked than, those that occur in patients with overt hypothyroidism. Overt hypothyroidism may exacerbate underlying cardiac disease, but cardiomyopathy severe enough to cause heart failure is rare (Kahaly 2005). Impaired myocardial relaxation and decreased myocardial contractility have been postulated as possible mechanisms leading to cardiac dysfunction in those with overt hypothyroidism. The T3 (the active cellular form of thyroid hormone) status influences cardiac action because it exerts a direct effect on cardiac myocytes influencing cardiac gene expression involved in the regulation of intracellular calcium handling, which is an important event in systolic contraction and diastolic relaxation; in addition, T3 may influence the sensitivity of the sympathetic system, and leads to hemodynamic alterations in the periphery resulting in increased cardiac filling and modifications of cardiac contraction. T3 also decreases systemic vascular resistance by promoting relaxation in vascular smooth muscle cells (Kahaly 2000; Klein 2001). In subclinical hypothyroidism, impaired left ventricular function is described and these patients have resting left ventricular diastolic dysfunction, evidenced by delayed relaxation and impaired systolic dysfunction on effort that results in poor exercise capacity. In our systematic review we identified three trials that assessed cardiac function by colour‐Doppler echocardiograms (Cooper 1984; Monzani 2001; Yazici 2004,) and videodensitometry (Yazici 2004). Analysing these three studies we found statistically significant differences of improvement of diastolic function in some parameters like isovolumic relaxation time, and systolic function by pre‐ejection period to ejection time ratio and left ventricular ejection time. The abnormalities of myocardial relaxation, as indicated by significant prolongation of the isovolumic relaxation time, showed an improvement after LT4 replacement, also, an improvement of diastolic dysfunction occurred. In addition, a normalization of the CVI was obtained in the Monzani study (Monzani 2001) as well as a normalization of index of myocardial performance, an integrated index expressing global (diastolic to systolic) ventricular performance was found in the Yazici study (Yazici 2004), after LT4 replacement. In conclusion, subclinical hypothyroidism clearly appears to be in the spectrum and part of the continuum from overt hypothyroidism to euthyroidism as far as cardiovascular changes are regarded. Both diastolic and systolic cardiac functions are impaired in patients with subclinical hypothyroidism, and restoration of euthyroidism by T4 seems to improve or normalise most of the alterations of myocardial performance. However, these studies included individuals with high serum TSH, with previous thyroid disease and without further stratification. It remains to be determined whether a continuum of cardiac dysfunction can be expected across the spectrum of subclinical hypothyroidism and which is the clinical significance of these alterations
Adverse events
Most of the studies did not report adverse events in these patients. Kong (Kong 2002) and Cooper (Cooper 1984) reported that some patients felt worse with regards to symptoms. In addition, Jaeschke reported three adverse events in the LT4 group ( worsening of angina, new‐onset atrial fibrillation, one episode of gout) and in the placebo group (two strokes) but no statistically significant differences were found (Jaeschke 1996). In the Nystrom study two patients (LT4 group) felt worse with palpitations and left the study ( Nystrom 1988).
Authors' conclusions
Our systematic review could not demonstrate improved survival or reduced cardiovascular morbidity following levothyroxine replacement therapy for asymptomatic subclinical hypothyroidism. Data on health‐related quality of life and symptoms did not demonstrate significant differences between intervention groups. Some evidence indicates that levothyroxine replacement improves some parameters of lipid profiles and left ventricular function. Until better data are available, clinical judgment and patients' preferences remain the best manner to decide.
Further controlled, randomised studies in larger groups are necessary. These studies should analyse subgroups with and without previous thyroid disease, initial TSH levels greater than 10 mU/L or not and with a longer follow up to assess cardiovascular events. In addition, these RCTs should use the Consort statement as a model for reporting RCTs and if possible, report changes from baseline with standard deviation for continuous measures, and number of events and total patients analysed for dichotomous measures. These studies should also analyse patients older than 85 years of age whose safety might be compromised with thyroid hormone replacement.
Acknowledgements
We thank authors Caraccio et al, Cooper et al, Jaeschke et al, Monzani et al and Ross et al for providing additional information about their studies.
Appendices
Appendix 1. Search strategy
| Search terms |
| Unless otherwise stated, search terms are free text terms; MeSH = Medical subject heading (Medline medical index term); exp = exploded MeSH; the dollar sign ($) stands for any character(s); the question mark (?) = to substitute for one or no characters; tw = text word; pt = publication type; sh = MeSH; adj = adjacent. 1. Hypothyroidism/ [MeSH term, all subterms and sub trees included] 2. hypothyr* or hypo‐thyr* [in abstract or title] 3. thyroid deficien* [in abstract or title] 4. thyroid insufficien* [in abstract or title] 5. 1 or 2 or 3 or 4 6. (mild or compens* or subclinic* or sub‐clinic* or moderat* or short‐term) [in abstract or title] 7. 5 and 6 8. Thyroid hormones/ [MeSH term, all subterms and sub trees included] 9. thyroid hormon* [in abstract or title] 10. (thyronin* or thyroxin* or tyroxin*) [in abstract or title] 11. (L‐thyroxin* or L‐triiod?thyronin* or L‐thyronin*) [in abstract or title] 12. (L‐T3 or L‐T4 or FT3 or FT4) [in abstract or title] 13. (t3 hormon* or t 3 hormon* or t4 hormon* or t 4 hormon* or T3 or T4) [in abstract or title] 14. substitution therap* [in abstract or title] 15. 8 or 9 or 10 or 11 or 12 or 13 or 14 16. 7 and 15 Search strategy for systematic reviews: 1. meta‐analysis [publication type] 2. meta‐anal* [text word] 3. metaanal* [text word] 4. (quantitativ* OR cuantitativ*) AND (review* OR overview* OR revis* OR evaluation*) [text word] 5. (systematic* OR sistemat*) AND (review* OR overview* OR revis* OR evaluation*) [text word] 6. (methodologic* OR metodolog*) AND (review* OR overview* OR revis* OR evaluation*) [text word] 7. #1 OR #3 OR #3 OR #4 OR #5 OR #6 |
Appendix 2. Quality of included studies
| Study ID | Jadad scale | G A | A C | B A |
| Generation of allocation | Allocation concealment | Blinded assessment | ||
| Yazici 2004 | 4 | B | B | A |
| Ross 1993 | 3 | A | A | C |
| Nystrom 1988 | 3 | B | B | A |
| Monzani 2004 | 5 | A | A | A |
| Monzani 2001 | 5 | A | A | A |
| Meier 2001 | 4 | A | A | A |
| Kong 2002 | 4 | A | A | A |
| Jorde 2006 | 4 | B | B | A |
| Jaeschke 1996 | 4 | A | A | A |
| Cooper 1984 | 4 | B | B | A |
| Caraccio 2002 | 3 | A | A | C |
| Caraccio 2005 | 5 | A | A | A |
Data and analyses
Comparison 1.
Thyroid hormone replacement for subclinical hypothyroidism
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 symptoms improvement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 Symptoms score improvement | 4 | 155 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.62, 0.02] |
| 2.1 Questionnaire modified of Hypothyroidism Diagnostic Index of Billewicz | 2 | 69 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.36 [‐0.84, 0.13] |
| 2.2 Billewicz score (points) | 1 | 63 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.59, 0.40] |
| 2.3 neuromuscular symptoms score | 1 | 23 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.68 [‐1.53, 0.17] |
| 3 Change on symptoms score | 3 | 164 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.24 [‐0.54, 0.07] |
| 3.1 Change of Billewscz score | 1 | 63 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.26 [‐0.76, 0.23] |
| 3.2 Change of symptoms ‐ Cooper questionnaire | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.67, 0.72] |
| 3.3 Change on symptoms score | 1 | 69 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐0.81, 0.14] |
| 4 Quality of life improvement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Anxiety score improvement | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.51, 1.98] |
| 4.2 Depression score improvement | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.61, 1.68] |
| 4.3 General Health Questionary improvement | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.49, 1.20] |
| 5 Change on Health‐related quality of life (HRQL) | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
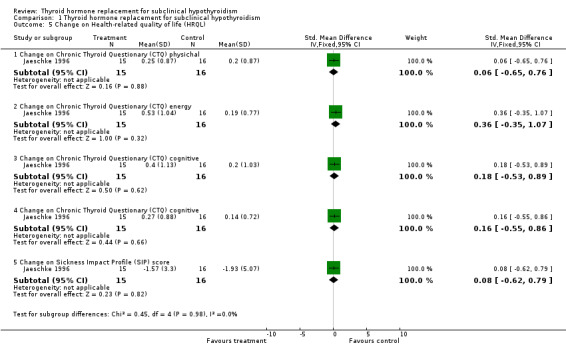
| 5.1 Change on Chronic Thyroid Questionary (CTQ) physichal | 1 | 31 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.65, 0.76] |
| 5.2 Change on Chronic Thyroid Questionary (CTQ) energy | 1 | 31 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.36 [‐0.35, 1.07] |
| 5.3 Change on Chronic Thyroid Questionary (CTQ) cognitive | 1 | 31 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.18 [‐0.53, 0.89] |
| 5.4 Change on Chronic Thyroid Questionary (CTQ) cognitive | 1 | 31 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.16 [‐0.55, 0.86] |
| 5.5 Change on Sickness Impact Profile (SIP) score | 1 | 31 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.62, 0.79] |
| 6 Cognitive function improvement | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
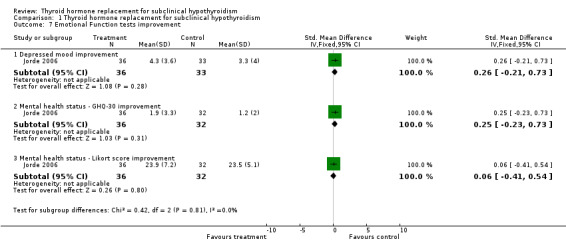
| 7 Emotional Function tests improvement | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Depressed mood improvement | 1 | 69 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.26 [‐0.21, 0.73] |
| 7.2 Mental health status ‐ GHQ‐30 improvement | 1 | 68 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.25 [‐0.23, 0.73] |
| 7.3 Mental health status ‐ Likort score improvement | 1 | 68 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.41, 0.54] |
| 8 Cholesterol (mg/dl) improvement | 7 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Final mean of total cholesterol (mg/dl) | 6 | 255 | Mean Difference (IV, Fixed, 95% CI) | ‐9.94 [‐19.83, ‐0.04] |
| 8.2 change from baseline on total cholesterol (mg/dl) | 6 | 248 | Mean Difference (IV, Fixed, 95% CI) | ‐5.28 [‐12.50, 1.94] |
| 9 Cholesterol (mg/dL) improvement | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 Basal cholesterol > 240mg/dL | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐3.87 [‐20.65, 12.91] |
| 9.2 Basal cholesterol < 240mg/dL | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 11.61 [‐9.65, 32.87] |
| 10 LDL cholesterol (mg/dL) improvement | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 Final mean of LDL cholesterol improvement | 4 | 188 | Mean Difference (IV, Fixed, 95% CI) | ‐10.77 [‐21.57, 0.04] |
| 10.2 Change from baseline on LDL cholesterol | 3 | 121 | Mean Difference (IV, Fixed, 95% CI) | ‐3.12 [‐11.76, 5.52] |
| 11 LDL cholesterol mg/dl)improvement | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 Basal LDL cholesterol > 155mg/dL | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐23.22 [‐44.64, ‐1.80] |
| 11.2 Basal LDL cholesterol < 155mg/dL | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 15.48 [‐5.84, 36.80] |
| 12 HDL cholesterol (mg/dL) improvememnt | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12.1 Final mean of HDL cholesterol improvement | 4 | 188 | Mean Difference (IV, Fixed, 95% CI) | ‐2.34 [‐5.96, 1.29] |
| 12.2 Change from baseline on HDL cholesterol levels | 3 | 121 | Mean Difference (IV, Fixed, 95% CI) | 0.39 [‐2.94, 3.72] |
| 13 Triglycerides (mg/dL) improvement | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 13.1 Final mean of triglycerides improvement | 5 | 221 | Mean Difference (IV, Fixed, 95% CI) | ‐8.15 [‐23.63, 7.34] |
| 13.2 Change from baseline on triglycerides levels | 3 | 121 | Mean Difference (IV, Fixed, 95% CI) | 2.67 [‐11.63, 16.97] |
| 14 Apoprotein A (g/L) improvement | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 14.1 Final mean of Apoprotein A levels | 3 | 157 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.15, 0.03] |
| 14.2 Change from baseline on Apoprotein A levels | 2 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.20, ‐0.01] |
| 15 Apolipoprotein B (g/L) improvement | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 15.1 Final mean of Apoprotein B levels | 3 | 157 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.17, 0.03] |
| 15.2 Change from baseline of Apoprotein B levels | 2 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.15, 0.02] |
| 16 Lipoprotein (a) improvement (mg/dl) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 17 Cardiac function | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 17.1 EDD ( left ventricular end‐diastolic diameter) (mm) | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | 1.05 [‐0.29, 2.40] |
| 17.2 ESD ( left ventricular end‐systolic diameter) (mm) | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐2.30, 3.30] |
| 17.3 IVST ( interventricular septum thickness) (mm) | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.61, 0.45] |
| 17.4 Sthd (diastolic interventricular septum thickness) (mm/m2) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.72, 0.52] |
| 17.5 PWT (posterior wall thickness) (mm) | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.74, 0.54] |
| 17.6 PWthd (diastolic posterior wall thickiness) (mm/m2) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.96, 0.36] |
| 17.7 LVMI (left ventricular mass index) (g/m2) | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐2.10 [‐7.46, 3.26] |
| 17.8 FS (fractional shortening) (%) | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | 1.04 [‐1.40, 3.49] |
| 17.9 ESV (left ventricular end‐systolic volume) (ml) | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐3.06, 2.66] |
| 17.10 EDV (left ventricular end‐diastolic volume) (ml) | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐4.98, 5.78] |
| 17.11 EF (ejection fraction) (%) | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [‐2.48, 4.88] |
| 17.12 CO (l/m2) | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.33, 0.48] |
| 17.13 SVR (systemic vascular resistance) (dyn s cm‐5) | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐14.48 [‐141.66, 112.70] |
| 17.14 Emax (early transmitral flow velocity) (cm/s) | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | 1.70 [‐4.74, 8.13] |
| 17.15 Amax (late transmitral flow velocity) (cm/s) | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐4.88 [‐10.94, 1.19] |
| 17.16 E/A ratio | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.02, 0.17] |
| 17.17 EDTe (Mitral E wave deceleration time) (ms) | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | 0.16 [‐1.32, 1.65] |
| 17.18 IRTc (isovolumic relaxation time) (ms) | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐0.51 [‐0.95, ‐0.06] |
| 17.19 ETc (left ventricular ejection time) (ms) | 2 | 79 | Mean Difference (IV, Fixed, 95% CI) | ‐8.47 [‐15.83, ‐1.12] |
| 17.20 ICTc (isovolumic contraction time) (ms) | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐8.90, 8.30] |
| 17.21 IMP (index of myocardial peformance) | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.10, ‐0.04] |
| 17.22 CVI at midseptum (cyclic variation index) (%) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 37.2 [25.92, 48.48] |
| 17.23 CVI (cyclic variation index) at midposterior wall (%) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 25.60 [13.80, 37.40] |
| 17.24 PEP/ET | 4 | 132 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.06, ‐0.02] |
| 18 Change on BMI (%) ‐ wrist | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
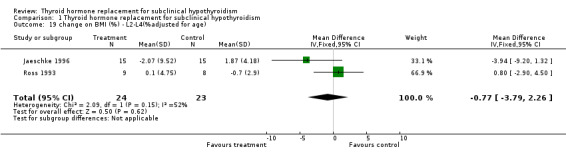
| 19 change on BMI (%) ‐ L2‐L4(%adjusted for age) | 2 | 47 | Mean Difference (IV, Fixed, 95% CI) | ‐0.77 [‐3.79, 2.26] |
| 20 TSH (mU/L) improvement | 10 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 20.1 Final mean of TSH levels | 9 | 335 | Mean Difference (IV, Fixed, 95% CI) | ‐4.31 [‐4.73, ‐3.88] |
| 20.2 Change from baseline on TSH levels | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐2.90 [‐4.33, ‐1.47] |
| 21 Adverse events | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 21.1 Anxiety score | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.6 [0.79, 39.89] |
| 21.2 Depression score | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.07, 1.66] |
| 21.3 General health questionnaire | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.6 [0.49, 26.40] |
| 21.4 symptoms worsening | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.22, 1.82] |
| 21.5 others adverse evens | 2 | 71 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.28 [0.55, 9.48] |
Analysis 1.1.
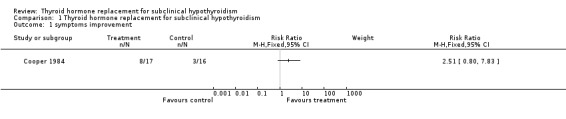
Comparison 1 Thyroid hormone replacement for subclinical hypothyroidism, Outcome 1 symptoms improvement.
Analysis 1.2.
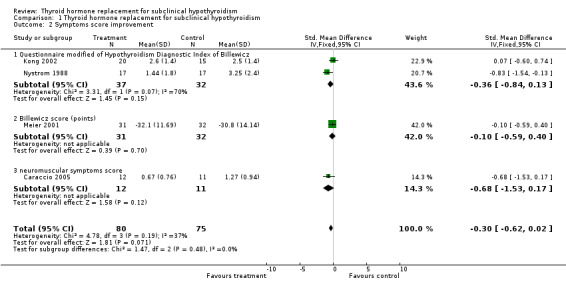
Comparison 1 Thyroid hormone replacement for subclinical hypothyroidism, Outcome 2 Symptoms score improvement.
Analysis 1.3.
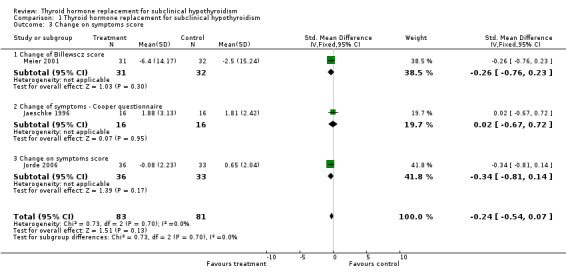
Comparison 1 Thyroid hormone replacement for subclinical hypothyroidism, Outcome 3 Change on symptoms score.
Analysis 1.4.
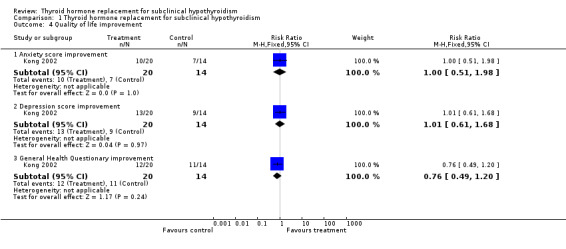
Comparison 1 Thyroid hormone replacement for subclinical hypothyroidism, Outcome 4 Quality of life improvement.
Analysis 1.5.
Comparison 1 Thyroid hormone replacement for subclinical hypothyroidism, Outcome 5 Change on Health‐related quality of life (HRQL).
Analysis 1.6.
Comparison 1 Thyroid hormone replacement for subclinical hypothyroidism, Outcome 6 Cognitive function improvement.
Analysis 1.7.
Comparison 1 Thyroid hormone replacement for subclinical hypothyroidism, Outcome 7 Emotional Function tests improvement.
Analysis 1.8.
Comparison 1 Thyroid hormone replacement for subclinical hypothyroidism, Outcome 8 Cholesterol (mg/dl) improvement.
Analysis 1.9.
Comparison 1 Thyroid hormone replacement for subclinical hypothyroidism, Outcome 9 Cholesterol (mg/dL) improvement.
Analysis 1.10.
Comparison 1 Thyroid hormone replacement for subclinical hypothyroidism, Outcome 10 LDL cholesterol (mg/dL) improvement.
Analysis 1.11.
Comparison 1 Thyroid hormone replacement for subclinical hypothyroidism, Outcome 11 LDL cholesterol mg/dl)improvement.
Analysis 1.12.
Comparison 1 Thyroid hormone replacement for subclinical hypothyroidism, Outcome 12 HDL cholesterol (mg/dL) improvememnt.
Analysis 1.13.
Comparison 1 Thyroid hormone replacement for subclinical hypothyroidism, Outcome 13 Triglycerides (mg/dL) improvement.
Analysis 1.14.
Comparison 1 Thyroid hormone replacement for subclinical hypothyroidism, Outcome 14 Apoprotein A (g/L) improvement.
Analysis 1.15.
Comparison 1 Thyroid hormone replacement for subclinical hypothyroidism, Outcome 15 Apolipoprotein B (g/L) improvement.
Analysis 1.16.
Comparison 1 Thyroid hormone replacement for subclinical hypothyroidism, Outcome 16 Lipoprotein (a) improvement (mg/dl).
Analysis 1.17.
Comparison 1 Thyroid hormone replacement for subclinical hypothyroidism, Outcome 17 Cardiac function.
Analysis 1.18.
Comparison 1 Thyroid hormone replacement for subclinical hypothyroidism, Outcome 18 Change on BMI (%) ‐ wrist.
Analysis 1.19.
Comparison 1 Thyroid hormone replacement for subclinical hypothyroidism, Outcome 19 change on BMI (%) ‐ L2‐L4(%adjusted for age).
Analysis 1.20.
Comparison 1 Thyroid hormone replacement for subclinical hypothyroidism, Outcome 20 TSH (mU/L) improvement.
Analysis 1.21.
Comparison 1 Thyroid hormone replacement for subclinical hypothyroidism, Outcome 21 Adverse events.
What's new
| Date | Event | Description |
|---|---|---|
| 1 November 2008 | Amended | Converted to new review format. |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | 1. Generation of allocation sequence: List of randomization obtained using the software Procplan SAS 2. Allocation concealment: In a blinded manner 3. Blinding: No description 4. Sample size calculation: No description 5. Loss to follow‐up: No losses 6. Intention‐to‐treat analysis: No description 7. Similarity between groups: Yes | |
| Participants | 1. Inclusion criteria: SCH (TSH>3.6 mIU/L, and normal free T4) patients with history of previous thyroid disease 2. Exclusion criteria: BMI > 30kg/m2, smokers, dyslipidemia, diabetes mellitus, renal and hepatic failure,systemic diseases 3. Characteristics: (Age (mean (SD)), gender, ethnicity, other) 7 men and 42 women with 35 +/‐ 9yr, 48 had Hashimoto's thyroiditis and one had Graves's disease and radiodine therapy. | |
| Interventions | 1. Intervention in experimental group (including number of patients, dosage, mode of administration, duration of treatment): Thyroxine replacement averaged 67,5 mcg/d, VO, during 6‐15 months. N= 24 2. Intervention in control group 1 (including number of patients, dosage, mode of administration, duration of treatment): Placebo tablets, N= 25, 6 months | |
| Outcomes | 1. Thyroid hormones and antibodies: FT4 (measured by RIA) TSH
(measured by ultrasensitive immunoradiometric assay ‐ IRMA), FT3
(measured by RIA), anti‐Tg antibodies (measured by IRMA assay),
anti‐TPO antibodies (measured by RIA) 2. Fasting lipid
profiles: Cholesterol, triglycerides, HDL, LDL, apolipoprotein
A1 and B, lipoprotein A, cholesterol/HDL, LDL/HDL, LDL/ApoB
3. BMI Duration of follow‐up: Patients treated with L‐T4 were re‐evaluated after 6 months of stable euthyroidism (mean 11months; range 6‐15), whereas patients taking placebo were restudied after 6 months of treatment. |
|
| Notes | 1. Setting: Outpatients clinic of Department of Internal Medicine of University of Pisa, Italy 2. Funding source: Ministero dellÚniversità e della Ricerca Scientifica e Tecnologica. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | 1. Generation of allocation sequence: List of randomization obtained using the software Procplan SAS 2. Allocation Concealment: In a blinded manner 3. Blinding: Yes 4. Sample size calculation: No description 5. Loss to follow‐up: No losses 6. Intention‐to‐treat analysis: No description 7. Similarity between groups: Yes | |
| Participants | 1. Inclusion criteria: SCH (TSH > 3.6mIU/L and free T4 levels within the normal range) patients with Hashimoto thyroiditis and positive TPO‐Ab. 2. Exclusion criteria: Neurological, cardiovascular, respiratory, and other systemic diseases. 3. Characteristics: (Age (mean (SD)), gender, ethnicity, other): 2 men and 21 women with 32 +/‐2 yr, all with Hashimoto's thyroiditis. | |
| Interventions | 1. Intervention in experimental group (including number of patients, dosage, mode of administration, duration of treatment): Thyroxine replacement averaged 67,5 mcg/d, VO, during 6 ‐15 months. N= 12 2. Intervention in control group 1 (including number of patients, dosage, mode of administration, duration of treatment): Placebo tablets, N= 11, 6 months | |
| Outcomes | 1. Thyroid hormones: FT3 and FT4 (measured by RIA), TSH
(measured by ultrasensitive immunoradiometric assay ‐ IRMA),
anti‐Tg antibodies (measured by IRMA assay), anti‐TPO
antibodies (measured by RIA) 2. Questionnaire on neuromuscular
symptoms (paresthesias, muscle cramps, fatigue, and muscle weakness).
3. BMI (body mass index), 4. LBM(kg)‐ lean
body mass, FM (kg) ‐ fat mass, 5. AUC glucose
(mol/lL/h), AUC insulin (nmol/lL/h), maximal power output (W), resting HR
(bpm), resting VO2 (ml/min/kg). Duration of follow‐up: Patients treated with L‐T4 were re‐evaluated after 6 months of stable euthyroidism (mean 11months; range 6‐15), whereas patients taking placebo were restudied after 6 months of treatment. |
|
| Notes | 1. Setting: Outpatients Clinic of Departament of Internal Medicine of University of Pisa, Italy 2. Funding source: Ministero Istruzione, Università e Ricerca, Rome, and Bracco S.p.A., Milan, Italy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | 1. Generation of allocation sequence: No description 2. Allocation concealment: No description 3. Blinding: Double‐blinding 4. Placebo: Identical L‐thyroxine tablets 4. Sample size calculation: No description 5. Loss to follow‐up: 2 6. Intention‐to‐treat analysis: No description 7. Similarity between groups: Yes | |
| Participants | 1. Inclusion criteria: Subclinical hypothyroidism ‐ normal serum levels of T3, T4, FT4; elevated levels of thyrotrophin. Prior thyroid disease. 2. Exclusion criteria: Use of L‐thyroxine 3. Characteristics: (Age (mean (SD)), gender, ethnicity, other: 32 women and 1 man (age median = 58.2 +/‐ 2.3 yr thyroxine group and 50.2 +/‐ 2.8 yr placebo group), Grave's disease (17 in thyroxine group, 15 in placebo group); Hashimoto's thyroiditis (1 in placebo group), previous thyroid treatment with radioiodine (12 in thyroxine group, 9 in placebo group), antithyroid drugs (1 in thyroxine group and 3 in placebo group), surgery (2 in thyroxine group, 3 in placebo group), radioiodine plus surgery (2 in thyroxine group, 3 in placebo group) | |
| Interventions | 1. Intervention in experimental group (including number of patients, dosage, mode of administration, duration of treatment): L‐thyroxine 25 mcg tablets ‐ 71.2 +/‐7 mcg/d (range 50‐125), VO, during 12 months, N = 17 2. Intervention in control group 1 (including number of patients, dosage, mode of administration, duration of treatment): Placebo ‐ 2 or 3 tablets daily, VO, during 12 month, N = 16. | |
| Outcomes | 1. Thyroid hormones: T4 ‐ measured by RIA, TSH ‐
measured by RIA, peak TSH after TRH test, antithyroid antibodies measured by
fluorescence 2. Basal metabolic rate: Measured
with a Benedict‐Roth closed‐circuit apparatus 3.
Cardiac function: Systolic time intervals ‐ measured by
eletrocardiogram, phonocardiogram, and carotid pulse tracing. Total
electromechanical systole, left ventricular ejection time (LVET).
pre‐ejection period (PEP), index of myocardial contractility measured
by PEP/LVET.QKd interval 4. Hypothyroidism:
Symptoms index of Billewiscz ‐ muscle cramps, dry skin,
cold intolerance, constipation, poor energy, easy fatigue. 5.
Prolactin: Basal PRL measured by RIA, peak prolactin after TRH
test. Duration of follow‐up: 12 months |
|
| Notes | 1. Setting: Thyroid Clinic of the Massachusetts General Hospital, Boston, EUA 2. Funding source: Grant AM‐16791 from the U.S. Public Health Service and Grant RR 1066 from the Clinical Research Center. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | 1. Generation of allocation sequence: Blind to treatment allocation, but they did not reported which method was used to allocation concealment 2. Allocation concealment: Yes 3. Blinding: Double‐blinding 4. Sample size calculation: No description 5. Loss to follow‐up: 3 patients in thyroxine group, 3 patients in placebo group 6. Intention‐to‐treat analysis: No description 7. Similarity between groups: Yes 8. Placebo characteristics: The placebo tablets were identical in appearance, taste, color, and consistency | |
| Participants | 1. Inclusion criteria: Adults > 55 yr , TSH >6.0 mIU/, LFT4 and TBG in normal range 2. Exclusion criteria: Patients with replacement estrogen (possible contaminating effects on total thyroid hormone level), narcotics, perphenazine, phenytoin, glucocorticoids, propanolol and amiodarone (drugs that may also spuriously change measured total T4 levels). Ethanol or drug abuse, Clinical instability as indicated by hospitalization in the 1 month prior to entry, Unable to complete HRQL questionare, refused participation or declined to give informed consent. 3. Characteristics (Age (mean (SD)), gender, ethnicity, other: N= 37, adults (age, median = 68 +/‐ 9.4 yr thyroxine group and 68 +/‐ 6.4 yr placebo group) | |
| Interventions | 1. Intervention in experimental group (including number of patients, dosage, mode of administration, duration of treatment): Thyroxine tablets ( 50‐100 mcg/d), mean dose of 68 +/‐ 21mcg/d, VO, during 10 months. N=18. 2. Intervention in control group 1 (including number of patients, dosage, mode of administration, duration of treatment): Placebo tablets, VO, during 10 months, N= 19 | |
| Outcomes | 1. CTQ physical 2. CTQ mood 3. CTQ energy
4. CTQ cognitive 5. SIP total score
6. Psychomotor speed composite score 7. Cooper
‐ total score 8. Memory composite score 9.
Bone mineral density ‐ lumbar spine dual photon absorptiometry (DPA)
10. Thyroid Hormones: TSH ‐ measured by
IRMA, T4 ‐ measured by RIA, T3 ‐ measured by RIA, TBG ‐
measured by IRMA 11. Fasting lipid profiles:
Cholesterol, HDL‐cholesterol, LDL‐cholesterol Duration of follow‐up: 10 months |
|
| Notes | 1. Setting: Hamilton, Canada 2. Funding source: Supported by a grant from Ontario Ministry of Health and by a grant from Boots Pharmaceuticals. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | 1. Generation of allocation sequence: No description 2. Allocation concealment: No description 3. Blinding: Double‐blinding 4. Placebo characteristics: The placebo tablets were packed and prepared in an identical manner as L‐T4 tablets 5. Sample size calculation: No description. 6. Loss to follow‐up: 1 loss. 1 participant in the placebo group dropped out after 6 months because of serious disease unrelated to thyroid function and was excluded from the analyses 7. Intention‐to‐treat analysis: No description. 8. Similarity between groups : Yes. | |
| Participants | 1. Inclusion criteria : Men and women > 29 yr and < 80 yr, no prior history of coronary infarction, angina pectoris, or stroke; no use of thyroid medication or those participating in other follow‐up studies. 2. Exclusion criteria: Serious illness, heart disease, stroke or diabetes mellitus. 3. Characteristics (Age (mean (SD)), gender, ethnicity, other): 33 female and 36 males; thyroxine group n= 19F/17M (61.6 +/‐ 11.5 yr), placebo group n= 18F/15M (63 +/‐ 12.4yr); smokers/nonsmokers in thyroxine group n= 6/30, in placebo group n= 3/30. | |
| Interventions | 1. Intervention in experimental group (including number of patients, dosage, mode of administration, duration of treatment): L‐thyroxine 25, 50,75,100 mcg tablets ‐ each participant was to take three tablets every day, and a combination T4 and placebo tablets, daily doses of T4 could be 25, 50, 75, 100, 125, 150 or 175 mcg, VO, during 12 months, N = 36. The T4 dose was given according to the TSH levels, aiming at a level between 0.5 and 1.5 mU/L. 2. Intervention in control group 1 (including number of patients, dosage, mode of administration, duration of treatment): Placebo tablets were identical to T4 tablets. Each participant was to take three tablets every day, VO, during 12 months, N = 33. | |
| Outcomes | 1. TSH levels (mU/L) ‐ were analyzed with with AxSYM (Abbott, IL),
reference range 0.2‐4.2mU/L. 2. Free T4 ‐ was
analyzed with AxSYM (Abbott, IL). 3. Free T3 ‐ was
analyzed with AxSYM (Abbott, IL). 4. Tests of cognitive
function: For attention, sustained attention, and working
memory ( digit span forward and backward test; and the Seashore Rhythm test
from the Halstead Reitan test battery). For
psychomotor/cognitive speed ‐ Trail Making test, part A; Stroop
Color‐Word test, parts 1 and 2 and Digit Simbol test.
For memory ‐ the verbal and visual paired associates
immediate and 30 min delayd recall (from Wechster Memory
Scale‐Revised) and verbal recall test, and a word list consisting of
12 words, a subtest from California Verbal Learning Test. For
language/word fluency ‐ ontrolled Oral Word association test.
For cognitive flexibility/executive function ‐ Trail
Making test, and Stroop Color‐Word test. For speed of
information processing ‐ California Computadorized Assessment Package
(CalCap). For intelligence ‐ subtest Vocabulary from
Wechsler Adult Intelligence Scale (WAIS). A composite score for cognitive function ‐ adding together the Z‐score for the seven tests: Digit Span forward, Digit Span backward, Stroop test part 1 and 2, Digit Symbol test, verball recall, visual recall, and Stroop test part 3. 5. Tests of emotional function: For Depressed mood‐ Beck Depression Inventary (BD). For Menthal health status ( or psychological distress) ‐ General Health Questionnaire (GHQ‐30) 6. Symptoms score: A questionnaire containing questions related to hypothyroidism symptoms. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | 1. Generation of allocation sequence: Computer‐generated randomization code 2. Allocation concealment: Not clearly description 3. Blinding: Double‐blinding 4. Sample size calculation: A sample size of 18 per group would allow the detection of a 10% change in resting energy expenditure (assuming a standard deviation of 10%), as well as of a reduction in the prevalence of an impaired anxiety score from a predicted 43% (the prevalence in medical outpatients) to 11% (the prevalence in the nonselected adult population). 5. Loss to follow‐up: 5 women (4 in the placebo group and 1 in thyroxine group) withdrew before any baseline measurements had been taken. Withdrawn during treatment period = 5 women (3 in the thyroxine group and 2 in the placebo group). Total = 10 /45 6. Intention‐to‐treat analysis: Yes (in the statistical analysis section, intention to treat analysis was mentioned, authors excluded 5 patients after randomization and before these received treatment). 7. Similarity between groups: Yes, just for age. They did not report data on similarity on other variables. 8. Number of randomized patients: 45 ( 24 thyroxine and 21 placebo) | |
| Participants | 1. Inclusion criteria: Normal serum free thyroxine (FT4) 0.8 ‐ 16 ng/dL and TSH levels between 5 ‐ 10 nU/mL 2. Exclusion criteria: History of previous thyroid disease, psychiatric disorder or anticipated pregnancy. 3. Characteristics: (Age (mean (SD)), gender, ethnicity, other): Women > 18 years (53 +/‐ 3 [T4], 45 +/‐ 4 [placebo]. | |
| Interventions | 1. Intervention in experimental group (including number of patients, dosage, mode of administration, duration of treatment): Thyroxine 50 ‐ 100 mcg daily, VO, N =23, 6 months | |
| Outcomes | 1. Quality of life: Hospital Anxiety and Depression Scale
‐ score of 8 General Health Questionares ‐ score of 4
2. Symptoms score: Fatigue, poor concentration,
weight gain, reduced appetite, dry/puffy skin, cold intolerance,
constipation. One point was given for each symptom.
3. Anthropometry: Body mass index, bioimpedance,
resting energy expenditure ‐ calorimetry 4. Thyroid
hormones ( mean difference from baseline) and antibodies: FT4
(measured by competitive immunoassay), TSH (measured by chemiluminescent
technology), FT3 (measured by competitive immunoassay), thyroid
antimicrossomal antibodies (measured by autoparticle agglutination)
5. Fating lipid profiles: Cholesterol,
triglycerides, HDL, LDL, sialic acid, Apolipoprotein A and B, mevalonic
acid Duration of follow‐up: 6 months |
|
| Notes | 1. Setting: London, United Kingdom. 2. Funding source: No description. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | 1. Generation of allocation sequence: Predefined randomization list 2. Allocation concealment: No description 3. Blinding: Double‐blinding 4. Placebo characteristics: The placebo tablets were packed and prepared in an identical manner as the L‐T4 tablets 4. Sample size calculation: No description 5. Loss to follow‐up: 3 losses. 2 participants due to previously unknown serious medical comorbidities (endometrial cancer ‐ T4‐group, malignant astrocytoma ‐ placebo group) and 1 participants to rapid progression to clinically overt hypothyroidism ‐ T4 group. 6. Intention‐to‐treat analysis: Yes, unless otherwise specified. 7. Similarity between groups: Yes | |
| Participants | 1. Inclusion criteria: Women 18 ‐ 75 yr, TSH > 5.0mIU/L, exaggerated TSH response > 35 mIU/L after oral TRH stimulation, FT4 in normal range, good health 2. Exclusion criteria: Coronary heart disease, pituitary/hypothalamic disorders, other nonthyroidal illnesses, thyroid hormone medication up to 3 months before enrollment, lipid‐lowering agents within 6 months before enrollement, poor compliance. 3. Characteristics (Age (mean (SD)), gender, ethnicity, other): 66 women (mean age, 58 +/‐ 1.3 yr), 49 women had a postmenopausal hormone status. The underlying thyroid disorders leading to SCH were autoimmune thyroiditis (n=33), Graves's disease (n=22; treated with radiodine or carbimazole ), toxic multinodular goiter (n=1; treated with radiodine), surgically resected goiter (n=6), and idiopathic SCH (n=4). | |
| Interventions | 1. Intervention in experimental group (including number of patients, dosage, mode of administration, duration of treatment): Tablets with 25,50,75,100 or 125 mcg thyroxine , VO, 48 weeks, n = 33 2. Intervention in control group 1 (including number of patients, dosage, mode of administration, duration of treatment): Placebo tablets, VO, 48 weeks, n = 33 | |
| Outcomes | 1. Thyroid hormones and antibodies, FT4, TSH, T3 2. Fasting
lipid profiles: Cholesterol, triglycerides, HDL‐C,
LDL‐C, C/HDL ratio, apolipoprotein A, apolipoprotein B, lipoprotein A
3. Billewicz score (points) 4. Zullewski score
(points) Duration of follow‐up: 50 weeks. 2 weeks without treatment and 48 weeks with treatment |
|
| Notes | 1. Setting: Thyroid Research Unit of the Division of Endocrinology, Department of Medicine, Iniversity Hospital Basel, Switzerland 2. Funding source: Supported by grants from the Swiss Research Foundation and unconditional research grants from Henning Berlin, Sandoz Research, and Roche Research Foundations. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | 1. Generation of allocation sequence: List of randomization obtained using the software Procplan SAS 2. Allocation concealment: Blind manner 3. Blinding: Double‐blinding 4. Placebo: Identical to L‐thyroxine tablets 5. Sample size calculation: No description 6. Loss to follow‐up: No losses 7. Intention‐to‐treat analysis: No description 8. Similarity between groups: Yes | |
| Participants | 1. Inclusion criteria: Patients with stable elevated serum TSH and normal thyroid hormone levels for at least 1 yr before study. TSH > 3.6 mIU/L (range 3.8 ‐ 12). Normal serum levels of FT3 and FT4. 2. Exclusion criteria: Cardiovascular and respiratory diseases 3. Characteristics (Age (mean (SD)), gender, ethnicity, other): Patients with Hashimoto's thyroiditis, 18 women and 2 men; mean age 32.6 +/‐ 12.1yr (34.3 +/‐ 12.3 in L‐thyroxine group, and 29.2 +/‐ 9.4 in placebo group). | |
| Interventions | 1. Intervention in experimental group (including number of patients, dosage, mode of administration, duration of treatment): L‐thyroxine 25 mcg tablets (mean = 65mcg/d; 50 ‐ 75), VO, during 6 months after serum TSH level had become normal, N = 10 2. Intervention in control group 1 (including number of patients, dosage, mode of administration, duration of treatment): Placebo tablets (2 ‐ 3/d), during 6 months. N = 10 | |
| Outcomes | 1. BMI (kg/m2) 2. Body surface area (m2) 3.
Cardiac function: SBP (systolic blood pressure) ‐ mm
HgDBP (diastolic blood pressure) ‐ mm HgMBP (mean blood pressure)
‐ mm HgHR (heart rate) ‐ beats/min 4. Thyroid
hormones: FT3 ‐ measured by RIA, FT4 ‐ measured
by RIA, TSH ‐ measured by ultrasensitive IRMA 5.
Echo‐Doppler parameters: EDD (end‐diastolic
diameter), FS (fractional shortening), Sthd (diastolic interventricular
septum thickness), PWthd (diastolic posterior wall thickness), LVM (left
ventricular mass index), CO (cardiac output), SVR (systemic vascular
resistence), PEP (pre‐ejection period), ET (ejection time ), PEP/ET
peak E (early transmitral flow velocity), peak A (late transmitral flow
velocity), E/A MAT (mitral acceleration time), MDT (mitral deceleration
time), IVRT (isovolumic relaxation time) 6. Ultrasonic
parameters: video‐densitometry, MGLed (mean gray level‐end
diastole), MGLes (mean gray level‐end systole), CVI (cyclic variation
index), (MGLed‐MGLes)/MGLes Duration of follow‐up: 6 or 12 months? |
|
| Notes | 1. Setting: Pisa, Italy 2. Funding source: No description | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | 1. Generation of allocation sequence: List of randomization obtained using the software Procplan SAS 2. Allocation concealment: In a blinded manner, 3. Blinding: Double‐blinding 4. Sample size calculation: No clear description 5. Loss to follow‐up: None 6. Intention‐to‐treat analysis: Not cleary described 7. Similarity between groups: Yes, in age and others, except in serum basal TSH, FT4, CT, LDLc and IMT. 8. Number of randomized patients: 45 ( 23 thyroxine and 22 placebo) | |
| Participants | 1. Inclusion criteria: Normal serum free thyroxine (FT4) 5,6 ‐ 13pg/mL and TSH levels between 3,65 ‐ 15mIU/lL (normal = 0,3 ‐ 3,6 mIU/mL); with history of previous radioiodine therapy for toxic adenoma or multinodular toxic goiter, thyroid disease or Hashimoto's thyroiditis. 2. Exclusion criteria: Individuals over age > 55yr, obese (BMI > 30 kg/m2) individuals; individuals with hypertention, diabetes mellitus, renal and hepatic failure, and postmenopausal women. 3. Characteristics (Age (mean (SD)), gender, ethnicity, other): Age 37 +/‐11 yr; female = 37 and male = 8; BMI = 24,7 +/‐ 11,5 kg/m2. | |
| Interventions | 1. Intervention in experimental group (including number of patients, dosage, mode of administration, duration of treatment): N=23, thyroxine median = 70mcg/d. Six months after the normalization of serum TSH levels (median 10.5 months, range 9 ‐ 15 from the beginning of the study). 2. Intervention in control group 1 (including number of patients, dosage, mode of administration, duration of treatment): N=22, placebo not described. Six months after the normalization of serum TSH levels (median 10.5 months, range 9 ‐ 12 from the beginning of the study). | |
| Outcomes | 1. Total cholesterol (TC), enzymatic methods (Roche
Diagnostics, Mannheim, Germany). 2. Triglycerides (TG),
enzymatic methods (Roche Diagnostics, Mannheim, Germany).
3. High‐density lipoprotein cholesterol (HDLc),
Mg2+‐dextran (Roche Diagnostics). 4. LDL
cholesterol (LDLc), Mg2+‐dextran (Roche Diagnostics). 5.
Apolipoprotein A1 (ApoA1), immunochemically (Nephelometer,
Behring Diagnostics, Marburg, Germany). 6. Apolipoprotein B
(ApoB), immunochemically (Nephelometer, Behring Diagnostics,
Marburg, Germany). 7. Lp(a), nephelometry (N latex Lp(a)
reagent, Behring Diagnostics). 8. Total homocysteine
tHcy was measured by HPLC 9. Ac. folico specific
RIA (ICN Pharmaceuticals, Costa Mesa, CA). 10. Vit. B12,
specific RIA (ICN Pharmaceuticals, Costa Mesa, CA). 11.
Carotide artery intima‐media thickness (IMT) ‐ maximal IMT
(mm), median IMT (mm) with high‐resolution ultrasonography (SONOS
2500, Hewlett‐Packard, Andover, MA) and a 7.5‐MHz linear
transducer (Hewlett‐Packard). 12. FT4 specific RIA
(Techno‐Genetics Recordati, Milan, Italy). 13. TSH
ultrasensitive immunoradiometric assay (IRMA) method (Cis Diagnostici,
Tronzano Vercellese, Italy). 14. BMI 15. Blood
pressure Duration of follow‐up: Six months after the normalization of serum TSH levels (median 10.5 months, range 9 ‐ 15 from the beginning of the study). |
|
| Notes | 1. Setting: Outpatient Clinic of the Department of Internal Medicine of the University of Pisa, Italy. 2. Funding source: None mentioned. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | 1. Generation of allocation sequence: No description 2. Allocation concealment: No description 3. Blinding: Double‐blinding 4. Sample size calculation: No description 5. Loss to follow‐up: 3 (during the first study period) 6. Intention‐to‐treat analysis: No description 7. Similarity between groups: Yes | |
| Participants | 1. Inclusion criteria: Women with SH ‐ FT4, T3, T4 normal serum levels; TSH response (delta TSH > 30 mU/L) upon TRH administration. 2. Exclusion criteria: Cardiovascular disease 3. Characteristics (Age (mean (SD)), gender, ethnicity, other): Women, aged 51‐73 years | |
| Interventions | 1. Intervention in experimental group (including number of patients, dosage, mode of administration, duration of treatment): L‐thyroxine tablets 50 mcg daily for the first 2 weeks, 100 mcg daily for the next 2 weeks, and 150 mcg daily for the rest of the study period. 2. Intervention in control group 1 (including number of patients, dosage, mode of administration, duration of treatment): Placebo tablets | |
| Outcomes | 1. Symptoms: Physical tiredness, mental lethargy, dry skin, cold intolerance, dry hair and constipation. 2. Clinical neurological examination: Psychometric tests 1‐ Identical forms test according to Thurstone 2 ‐ Bingley's memory test 3 ‐ Reaction time 3. Cardiac function: Systolic time intervals LVET, PEP, PEP/LVET, pulse rate 4. Creatine kinase activity 5. Procollagen‐III‐peptide 6. Sex‐hormone binding globulin 7. Thyroid Hormones 8. Cholesterol | |
| Notes | 1. Setting: Göteborg, Sweden 2. Funding source: Supported by grants from "Torsten Söderbergs och Ragnar Söderbergs stiftelse" and the Swedish National Association against Heart and Chest Disease. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | 1. Generation of allocation sequence: Using a computer generated program, numbered envelopes, when a patient was enrolled the next consecutive envelope was opened assigning the patient to control or treatment. 2. Allocation concealment: Yes 3. Blinding: No blinding 4. Sample size calculation: No description 5. Loss to follow‐up: 2 patients (no description the group) 6. Intention‐to‐treat analysis: No 7. Similarity between groups: Yes | |
| Participants | 1. Inclusion criteria: Women > 50 yr, no prior history of thyroid disease, at least 5 years postmenopausal, no use of estrogens or other drugs known to affect bone density. 2. Exclusion criteria: No description 3. Characteristics (Age (mean (SD)), gender, ethnicity, other): 19 women, thyroxine group n= 9 (68 +/‐ 6 yr), placebo group n = 10 (60 +/‐ 5 yr). | |
| Interventions | 1. Intervention in experimental group (including number of patients, dosage, mode of administration, duration of treatment): Thyroxine (50 ‐ 125 mcg/d) ,VO, during 14 +/‐1months, N = 9 2. Intervention in control group 1 (including number of patients, dosage, mode of administration, duration of treatment): Placebo, VO, N= 10, during 14 +/‐ 1 months | |
| Outcomes | 1. Thyroid hormones and antibodies: FT4 index, TSH‐
measured in a third‐generation chemiluminometric assay.
2. Bone density measurements: Lumbar spine
(L1‐L4), wrist at the junction of the proximal two thirds and the
distal one third of the nondominant radius 3. Duration of follow‐up: 14 +/‐1 months |
|
| Notes | 1. Setting: Massachusetts General Hospital, Boston, EUA 2. Funding source: Grant support: by Grant AM‐16791 from U.S.Public Health Service and Grant RR 1066 from the Clinical Research Center. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | 1. Generation of allocation sequence: Not cleary described 2. Allocation concealment: Patients were randomly assigned to receive either L‐T4 (as two 0.025 mg tablets), or two identical placebo tablets in a blinded manner. 3. Blinding: Double‐blinding 4. Sample size calculation: Not cleary described 5. Loss to follow‐up: None 6. Intention‐to‐treat analysis: Not cleary described 7. Similarity between groups: Yes | |
| Participants | 1. Inclusion criteria: Patients with TSH level greater than 4.0 mU/L in the presence of normal free thyroxine level (0.9 ‐ 1.9 ng/l). The etiology of SHT was Hashimoto's thyroiditis in patients (37/45). Only eight patients had been previously treated for hyperthyroidism (five by subtotal thyroidectomy, three with radioiodine). Euthyroidism was defined as a normal TSH level (0.4 ‐ 4.0 mU/L) with normal FT4. 2. Exclusion criteria: Neither the patients nor the members of the control group displayed signs of cardiovascular disease in their medical histories, physical examinations or electrocardiographies. Routine laboratory chemistry (plasma fasting glucose, blood urine nitrogen, creatinine, ALT, AST) was normal in all participants, and none were taking any medication. 3. Characteristics (Age (mean (SD)), gender, ethnicity, other): N=45, 38 female and 7 male, age 39.9 +/‐ 7.9. | |
| Interventions | 1. Intervention in experimental group (including number of patients, dosage, mode of administration, duration of treatment): L‐T4 (as two 0.025 mg tablets. A laboratory technician, who was in our study team, had access to the treatment code and the dose of L‐throxine was increased by 0.025 mg if the TSH level was still greater than 4.0 mcg/ml. This process continued until euthyroidism was achieved, with a mean dose of 0.064 mg daily. 2. Intervention in control group 1 (including number of patients, dosage, mode of administration, duration of treatment): Patients taking two identical placebo tablets in a blinded manner; some were given additional placebo tablets to maintain the blindness of the study. | |
| Outcomes | 1. BSA‐ body surface area 2. BMI‐ body mass
index 3. DBP‐ diastolic blood pressure 4.
SBP‐ systolic blood pressure 5. HR‐ hear rate
6. FT4 7. TSH 8. Conventional and
doppler echocardiographic measurements: FS (fractional
shortening), EF (ejection fraction), ICT (isovolumic contraction time), PEP
(pre‐ejection period), ET (ejection time), CO (cardiac output),
PEP/ET ratio. IRT (isovolumic relaxation time), Amax (late
transmitral flow velocity), E/A ratio, Emax (early transmitral flow
velocity), EDT (deceleration time), IRT (isovolumic relaxation time),
IMT (index of myocardial performance) EDD (left
ventricular end‐diastolic diameter), ESD (left ventricular end
systolic diameter), IVST (interventricular septum thickness), PWT (posterior
wall thickness), LVMI (left ventricular mass index), ESV(left ventricular
end‐systolic volume), EDV (left ventricular end‐diastolic
volume), SVR(systemic vascular resistance), EDT(mitral wave deceleration
time), ETC (left ventricular ejection time), Duration of follow‐up: 6 months |
|
| Notes | 1.Setting: Thoracic and Cardiovascular Surgery Center, Department of Cardiology, Istanbul, Turkey 2. Funding source: None. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
BMI = body mass index; SCH = subclinical hypothyroidism
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Biondi 1999 | not a randomized controlled study |
| Biondi 2002 | not a randomized controlled study |
| Christ‐Crain 2003 | duplicate of Meier 2001, outcomes beyond scope of review |
| Christ‐Crain 2005 | duplicate of Meier 2001, outcomes beyond scope of review |
| Jensovsky 2002 | not a randomized controlled study |
| Meier 2003 | duplicate of Meier 2001, outcomes beyond scope of review |
| Meier 2004 | duplicate of Meier 2001, insufficient outcome data |
| Pinnes 1999 | not a randomized controlled study |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Thyroid dysfunction in the elderly: Quantifying the association between sub‐clinical disease and (i) atrial fibrillation (and thus stroke) (ii) depression and (iii) cognitive dysfunction. A community based survey and clinical trial of thyroxine therapy |
| Methods | |
| Participants | Phase 1: Aged 65+ Phase 2a: Patients identified in Phase 1 to be suffering from sub‐clinical hyperthyroidism Phase 2b: Patients identified in Phase 1 to be suffering from sub‐clinical hypothyroidism |
| Interventions | Intervention: Thyroxine replacement therapy. Dose to be
increased by 25 mcg steps during 6 monthly period of monthly monitoring of
thyroid function Control: As for intervention group but placebo |
| Outcomes | Not available |
| Starting date | 12 September 2002 |
| Contact information | Professor James V Parle ISRCTN23090699 |
| Notes |
| Trial name or title | Subclinical hypothyroidism (SCH), cardiovascular risk and quality of life |
| Methods | |
| Participants | Participants from primary care (general practices), aged 18‐80 years of age |
| Interventions | Double blind randomised controlled crossover study using thyroxine (100 mcg/day) versus placebo |
| Outcomes | Brachial artery flow mediated dilatation (FMD), changes in health status, quality of life, body mass index, waist circumference and waist hip ratio, blood pressure, triglyceride, high density lipoprotein (HDL) cholesterol, low density lipoprotein (LDL) cholesterol, apolipoprotein A1 and apolipoprotein B, serum markers of endothelial function (e‐selectin, soluble adhesion molecule 1, tissue plasminogen activator and plasminogen activator inhibitor 1) and markers of inflammation high sensitive C‐reactive protein |
| Starting date | 01 February 2003 |
| Contact information | Dr Jolanta Weaver ISRCTN35570362 |
| Notes |
Contributions of authors
HELOISA CCE VILLAR: Protocol development, literature searching, study selection, data extraction, statistical analysis, drafting of written submissions, development of final review.
HUMBERTO SACONATO: Protocol development, literature searching, study selection, data extraction, statistical analysis, drafting of written submissions, development of final review.
ORSINE VALENTE: Protocol development, study selection, data extraction, drafting of written submissions, development of final review.
ALVARO NAGIB ATALLAH: Protocol development, study selection, drafting of written submissions, development of final review.
Sources of support
Internal sources
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior ‐ UNIFESP, Brazil.
Faculdade Estadual de Medicina de Marília, Brazil.
External sources
No sources of support supplied
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
- Caraccio N, Ferrannini E, Monzani F. Lipoprotein profile in subclinical hypothyroidism: response to levothyroxine replacement, a rndomized placebo‐controlled study. The Journal of Clinical Endocrinology and Metabolism 2002;87:1533‐8. [DOI] [PubMed] [Google Scholar]
- Caraccio N, Natali A, Sironi A, Baldi S, Frascerra S, Dardano A, et al. Muscle metabolism and exercise tolerance in subclinical hypothyroidism: a controlled trial of levothyroxine. The Journal of Clinical endocrinology and Metabolism 2005;90:4057‐62. [DOI] [PubMed] [Google Scholar]
- Cooper DS, Halpern R, Wood L, Levin A, Ridgway C. L‐Thyroxine therapy in subclinical hypothyroidism. Annals of Internal Medicine 1984;101:18‐24. [DOI] [PubMed] [Google Scholar]
- Jaeschke R, Guyatt G, Gerstein H, Patterson C, Molloy W, Cook D, et al. Does treatment with L‐thyroxine influence health status in middle‐aged and older adults with subclinical hypothyroidism?. Journal of General Internal Medicine 1996;11:744‐9. [DOI] [PubMed] [Google Scholar]
- Jorde R, Waterloo K, Storhaug I, Nyrnes A, Sundsfjrd J, Jenssen G. Neuropsychological function and symptoms in subjects with subclinical hypothyroidism and the effect of thyroxine treatment. The Journal of Clinical Endocrinology and Metabolism 2006;91:145‐53. [DOI] [PubMed] [Google Scholar]
- Kong WM, Chir MBB, Sheikh MH, Lumb PJ, Freedman DB, Crook M. A 6‐month randomized trial of thyroxine treatment in women with mild subclinical hypothyroidism. The American journal of medicine 2002;112(5):348‐54. [DOI] [PubMed] [Google Scholar]
- Meier C, Staub JJ, Roth CB, Guglielmetti M, Kunz M, Miserez AR, et al. TSH‐controlled L‐thyroxine theraphy reduces cholesterol levels and clinical symptoms in subclinical hypohtyroidism: a double blind, placebo‐controlled trial (Basel Thryroid Study). The Journal of Clinical Endocrinology and Metabolism 2001;86:4860‐6. [DOI] [PubMed] [Google Scholar]
- Monzani F, Bello V, Caraccio N, Bertini A, Giorgi D, Giusti C, et al. Effect of levothyroxine on cardiac function and structure in subclinical hypothyroidism: a double‐blind, placebo‐controlled study. The Journal of Clinical Endocrinology and Metabolism 2001;86:1110‐5. [DOI] [PubMed] [Google Scholar]
- Monzani F, Caraccio N, Kozakowa M, Dardano A, Vittone F, Virdis A. Effect of levothyroxine on lipid profile and intima‐media thickness in subclinical hypothyroidism: a duble‐blind, placebo‐controlled study. The Journal of Clinical Endocrinology and Metabolism 2004;89:2099‐106. [DOI] [PubMed] [Google Scholar]
- Niström E, Caidahi K, Fager G, Wikkelso C, Lundeberg PA, Lindestedt G. A double‐blind cross‐over 12‐month study of l‐thyroxine treatment of women with "subclinical" hypothyroidism. Clinical Endocrinology(Oxf) 1988;29:63‐76. [DOI] [PubMed] [Google Scholar]
- Ross, D. Bone density is not reduced during the short‐term administration of levothyroxine to postmenopausal women with subclinical hypothyroidism: a randomized, prospective study. The American Journal of Medicine 1993;95:385‐8. [DOI] [PubMed] [Google Scholar]
- Yazici M, Gorgulu S, Sertbas Y, Erbilen E, Albayarak S, Yildiz O. Effects of thyroxine therapy on cardiac function i patients with subclinical hypothyroidism: index of myocardial performance in the evaluation of left ventricular function. International Journal of Cardiology 2004;95:135‐43. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- EMPTY
- Biondi B, Palmieri EA, Lombardi G, Fazio S. Effect of subclinical hypothyroidism dysfunction in the heart. Annals of Internal Medicine 2002;137:904‐14. [DOI] [PubMed] [Google Scholar]
- Christ‐Crain M, Meier C, Guglielmetti M, Huber PR, Riese W, Staub JJ, Muller B. Elevated c‐reactive protein and homocysteine values: cardiovascular risk factors in hypothyroidism? A cross‐sectional and a double‐blind, placebo‐controlled trial. Atherosclerosis 2003;166:379‐86. [DOI] [PubMed] [Google Scholar]
- Christ‐Crain M, Morgenthaler NG, Meier C, Muller C, Nussbaumer C, Bergmann A, et al. Pro‐A‐type and N‐terminal pro‐B‐type natriuretic peptides in different thyroid function states. Swiss Medical Weekly 2005;135:549‐54. [DOI] [PubMed] [Google Scholar]
- Jensovsky J, Ruzicka E, Spackova N, Heidukova B. Changes of event related potential and cognitive process in patients with subclinical hypothyroidism after thyroxine treatment. Endocrine regulations 2002;36:115‐22. [PubMed] [Google Scholar]
- Meier C, Christ‐Crain M, Guglielmetti M, Huber P, Staub JJ, Muller B. Prolactin dysregulation in women with subclinical hypothyroidism: effect of levothyroxine replacement therapy. Thyroid 2003;13:979‐85. [DOI] [PubMed] [Google Scholar]
- Meier C, Beat M, Guglielmetti M, Christ‐Crain M, Staub JJ, Kraenzlin M. Restoration of euthyroidism accelerates bone turnover in patients with subclinical hypothyroidism: a randomized controlled trial. Osteoporosis International 2004;15:209‐16. [DOI] [PubMed] [Google Scholar]
- EMPTY
References to studies awaiting assessment
- Fadeyev VV, Sytch J, Kalashnikov V, Rojtman A, Syrkin A, Melnichenko G. Levothyroxine replacement therapy in patients with subclinical hypothyroidisd coronary artery disease. Endocrine Practice 2006;12(1):5‐17. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
- Thyroid dysfunction in the elderly: Quantifying the association between sub‐clinical disease and (i) atrial fibrillation (and thus stroke) (ii) depression and (iii) cognitive dysfunction. A community based survey and clinical trial of thyroxine therapy. Ongoing study 12 September 2002.
- Subclinical hypothyroidism (SCH), cardiovascular risk and quality of life. Ongoing study 01 February 2003.
Additional references
- Alghini‐Lombardi F, Antonangelli L, Martino E, Vitti P, Maccherini FL, Leoli F, et al. The spectrum of thyroid disorders in an iodine‐deficient community: The Pescopagano Survey. The Journal Clinical of Endocrinology and Metabolism 1999;84:561. [DOI] [PubMed] [Google Scholar]
- Althaus BU, Staub JJ, Ryff‐De Leche A, Oberhansli A, Stahelin HB. LDL/HDL‐changes in subclinical hypothyroidism; possible risk factors for coronary heart disease. Clinical Endocrinology 1988;28:157‐63. [DOI] [PubMed] [Google Scholar]
- Ayala AR, Danese M, Landerson PW. When to treat mild hypothyroidism. Endocrinology and Metabolism Clinics of North America 2000;29(2):399‐415. [DOI] [PubMed] [Google Scholar]
- Bianchi GP, Zccheroni V, Solaroli E, Vescini F, Cerutti R, Zoli M, et al. Health‐related quality of life in patients with thyroid disorders. Quality of Life Research 2004;13:45‐54. [DOI] [PubMed] [Google Scholar]
- Biondi B, Fazio S, Palmieri EA, Carella C, Panza N, Cittadini A, et al. Left ventricular diastolic dysfunction in patients with subclinical hypothyroidism. The Journal of Clinical Endocrinology and Metabolism 1999;84:2064‐7. [DOI] [PubMed] [Google Scholar]
- Braverman LE. Subclinical hypothyroidism and hyperthyroidism in elderly subjects: Should they be treated?. Journal of Endocrinological Investigation 1999;22(Suppl. to no.10):1‐3. [PubMed] [Google Scholar]
- Canaris GJ, Manowitz NR, Mayor G, Ridgway C. The Colorado thyroid disease prevalence study. Archives of Internal Medicine 2000;160:526‐34. [DOI] [PubMed] [Google Scholar]
- Cappola AR, Fried L, Arnold A, Danese MD, Kuller LH, Burke GL, et al. Thyroid status, cardiovascular risk, and mortality in older adults. JAMA 2006;295:1033‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron PH, Calazel C, Parra HJ, Hoff M, Louvert JP. Decreased HDL cholesterol in subclinical hypothyroidism: the effect of L‐thyroxine therapy. Clinical Endocrinology 1990;33:519‐23. [DOI] [PubMed] [Google Scholar]
- Castro AA, Clark OAC, Atallah A. Optimal search strategy for clinical trials in the Latin American and Caribbean Health Science Literature Database (LILACS). São Paulo Medical Journal 1997;115:1423‐6. [DOI] [PubMed] [Google Scholar]
- Cooper DS. Subclinical Hypothyroidism. New England Journal of Medicine 2001;345(4):260‐5. [DOI] [PubMed] [Google Scholar]
- Danese MD, Powe NR, Sawin CT, Laderson P. Screening for mild thyroid failure at the periodic health examination. A decision and cost‐effectiveness analysis. JAMA 1996;276(4):285‐92. [PubMed] [Google Scholar]
- Danese M, Landerson P, Meinert CL, Powe N. Effect of thyroxine on serum lipoproteins in patients with mild failure: A quantitative review of the literature. The Journal of Clinical Endocrinology and Metabolism 2000;85(9):2993‐3001. [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G. Misleading meta‐analysis. Lessons from an "effective, safe, simple" intervention that wasn´t. BMJ 1995;310:752‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler H, Staub JJ, Kunz M, Althaus B, Ryff A, Viollier E, et al. Does isolated TSH elevation need treatment? Study of risk factors for the development of manifest hypothyroidism. Schweizerische Medizinische Wochenschrift 1992;122(3):66‐9. [PubMed] [Google Scholar]
- Evered DC, Ormston BJ, Smith PA, Hall R, Bird T. Grades of hypothyroidism. BMJ 1973;1:657‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleiss JL. In: Statistical Methods for Rates and Proportions. 2nd Edition. New York, 1981:217‐34. [Google Scholar]
- Geul KW, Sluisveld IL, Grobbee DE, Docter R, Bruyn AM, Hooykaas H, et al. The importance of thyroid microsomal antibodies in the development of elevated serum TSH in middle‐aged women: associations with serum lipids. Clinical Endocrinology (Oxf) 1993;39(3):275‐80. [DOI] [PubMed] [Google Scholar]
- Gulseren S, Gulseren L, Hekimsoy Z, Cefinay P, Ozen C, Tokatlioglu B. Depression, anxiety, health‐related quality of life, and disability in patients with overt and subclinical thyroid dysfunction. Archives of Medical Resarch 2006;37:133‐9. [DOI] [PubMed] [Google Scholar]
- Gussekloo J, Exel E, Craen AJM, Meinders AE, Frolich M, Westendorp R. Thyroid status, disability and cognetive fuction, and survival in old age. JAMA 2004;292:2591‐9. [DOI] [PubMed] [Google Scholar]
- Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. New England Journal of Medicine 1999;341(8):549‐55. [DOI] [PubMed] [Google Scholar]
- Haggerty JJ, Stern RA, Mason GA, Beckwith MSN, Morey BS, Prange AJ. Subclinical hypothyroidism: a modifiable risk factor for depression?. American Journal of Psychiatry 1993;150:508‐10. [DOI] [PubMed] [Google Scholar]
- Hak AE, Pols HAP, Visser TJ, Drexhane HA, Hofman A, Witteman JCM. Subclinical hypothyroidism is a independent risk for atherosclerosis and myocardial infarction in elderly women: the Rotterdam study. Annals of Internal Medicine 2000;132:270‐8. [DOI] [PubMed] [Google Scholar]
- Helfand M, Redfern CC. Screening for thyroid disease: An update. Annals of Internal Medicine 1998;129:144‐8. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in medicine 2002;21:1539‐58. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 [updated May 2005]. In: The Cochrane Library, Issue 3, 2005. Chichester, UK: John Wiley & Sons, Ltd.
- Imaizumi M, Akahoshi M, Ichimaru S, Nakashima E, Hida A, Soda M, et al. Risk for ischemic heart disease and all‐cause mortality in subclinical hypothyroidism. The Journal of Clinical Endocrinology and Metabolism 2004;89:3365‐70. [DOI] [PubMed] [Google Scholar]
- Isles CG, Paterson JR. Identifying patients at risk for coronary heart disease: implications from trials of lipid‐lowering drug therapy. Quarterly Journal of Medicine 2000;93:567‐74. [DOI] [PubMed] [Google Scholar]
- Jadad AR, Moore RA, Carrol D, Jenkinson C, Reynolds JM, Gavaghan DJ, et al. Assesing the quality of reports of randomized clinical trials: Is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
- Kahaly GJ. Cardiovascular and atherogenic aspects of subclinical hypothyroidism. Thyroid 2000;10:665‐79. [DOI] [PubMed] [Google Scholar]
- Kahaly GJ, Dillmann WH. Thyroid hormone action in the heart. Endocrine Reviews 2005;26:704‐28. [DOI] [PubMed] [Google Scholar]
- Klein I, Ojamaa K. Mechanisms of disease: Thyroid hormone and the cardiovascular system. The New England Journal of Medicine 2001;334:501‐9. [DOI] [PubMed] [Google Scholar]
- Knudsen N, Jorgensen T, Rasmussen S, Christiansen E, Perrild H. The prevalence of thyroid dysfunction in a population with boderline iodine deficiency. Clinical Endocrinology (Oxf) 1999;51(3):361‐7. [DOI] [PubMed] [Google Scholar]
- Rosa JC, He J, Vupputuri S. Effects of statins on risk of coronary artery disease. JAMA 1999;282:2340‐6. [DOI] [PubMed] [Google Scholar]
- Landerson PW, Singer PA, Kenneth BA, Nandalal B, Bigos T, Levy EG, et al. American Thyroid Association Guidelines for Detection of Thyroid Dysfunction. Archives of Internal Medicine 2000;160(12):1573‐5. [DOI] [PubMed] [Google Scholar]
- Laurberg P, Nohr SB, Pedersen KM, Hreidarsson AB, Andersen S, Bulow Pedersen I, et al. Thyroid disorders in mild iodine deficiency. Thyroid 2000;10(11):951‐63. [DOI] [PubMed] [Google Scholar]
- Lekakis J, Papamichael C, Alevizaki M, Piperingos G, Marafelia P, Mantzos J, et al. Flow‐mediated, endothelium‐dependent vasodilation is impaired in subjects with hypothyroidism, boderline hypothyroidism, and high‐normal serum (TSH) values. Thyroid 1997;7:411‐4. [DOI] [PubMed] [Google Scholar]
- Lindeman RD, Schade DS, Rue A, et al. Subclinical Hypothyroidism in a biethinic, urban community. Journal of The American Geriatrics Society 1999;47:703‐9. [DOI] [PubMed] [Google Scholar]
- Moher D, Pham B, Jones A, Cook DJ, Jadad A, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analysis?. Lancet 1998;352:609‐13. [DOI] [PubMed] [Google Scholar]
- Monzani F, Guerra P, Caraccio N, Pruneti CA, Pucci E, Luisi M, et al. Subclinical hypothyroidism: neurobehavioral features and beneficial effect of l‐thyroxine treatment. Clinical Investigator 1993;71:367‐71. [DOI] [PubMed] [Google Scholar]
- Monzani F, Caraccio N, Siciliano G, Manca L, Murri L, Ferrannini E. Clinical and biochimical features of muscle dysfunction in subclinical hypothyroidism. The Journal of Clinical Endocrinology and Metabolism 1997;82:3315‐8. [DOI] [PubMed] [Google Scholar]
- Niström E, Caidahi K, Fager G, Wikkelso C, Lundeberg PA, Lindestedt G. A double‐blind cross‐over 12‐month study of l‐thyroxine treatment of women with "subclinical" hypothyroidism. Clinical Endocrinology(Oxf) 1988;29:63‐75. [DOI] [PubMed] [Google Scholar]
- O´Reilly D. Thyroid function tests ‐ time for a ressessment. BMJ 2000;320(13):1332‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parle JV, Franklyn JA, Cross KW, Jones SC, Sheppard MC. Prevalence and follow‐up of abnormal thyrotropin (TSH) concentrations in the elderly in the United Kingdom. Clinical Endocrinology 1993;34:77‐83. [DOI] [PubMed] [Google Scholar]
- Parle JV, Maisonneuve P, Sheppard MC, Boyle P, Franklyn JA. Prediction of all‐cause and cardiovascular mortality in elderly people from one low serum thyrotropin result: a 10‐year cohort study. Lancet 2001;358:861‐5. [DOI] [PubMed] [Google Scholar]
- Perk M, O´Neill BJ. The effect of thyroid hormone therapy on angiographic coronary arteri disease progression. Canadian Journal of Cardiology 1997;13:273‐6. [PubMed] [Google Scholar]
- Rigway EC, Cooper DS, Walker H, Rodbard D, Maloof F. Peripheral responses to thyroid hormone before and after l‐thyroxine therapy in patients with subclinical hypothyroidism. The Journal Clinical of Endocrinology and Metabolism 1981;53:1238‐42. [DOI] [PubMed] [Google Scholar]
- Rodondi N, Newman AB, Vittinghoff E, Rekeneire N, Satterfield S, Harris TB, et al. Subclinical hypothyroidism and the risk of heart failure, other cardiovascular events and death. Archives of Internal Medicine 2005;165:2460‐6. [DOI] [PubMed] [Google Scholar]
- Sawin CT, Geller A, Wolf PA, Belanger AJ, Baker E, Bacharach P, et al. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. New England Journal of Medicine 1994;331:1249‐52. [DOI] [PubMed] [Google Scholar]
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical Evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
- Szabolcs I, Podoba J, Feldkamp J, Dohan O, Farkas I, Sajgo M, et al. Comparative screening for thyroid disorders in old age in areas of iodine deficiency, long‐term iodine prophylaxis and abundant iodine intake. Clinical Endocrinology (Oxf) 1997;47(1):87‐92. [DOI] [PubMed] [Google Scholar]
- Tanis BC, Westendorp RGJ, Smelt AHM. Effect of thyroid substitution on hypercholesterolaemia in patients with subclinical hypothyroidism: a reanalysis of quality criteria. Clinical Endocrinology 1996;44(6):643‐9. [DOI] [PubMed] [Google Scholar]
- Toffe RJ, Levitt AJ. Major depression and subclinical (grade 2) hypothyroidism. Psychoneuroendocrinology 1992;17:215‐21. [DOI] [PubMed] [Google Scholar]
- Tunbridge WM, Evered DC, Hall R, Appleton D, Brewis M, Clark F, et al. The spectrum of thyroid disease in a community: the Whickham survey. Clinical Endocrinology (Oxf) 1977;7:481‐93. [DOI] [PubMed] [Google Scholar]
- Tunbridge WMG, Vanderpump MPJ. Population screening for autoimmune thyroid disease. Endocrinology and Metabolism Clinics of North America 2000;29(2):239‐53. [DOI] [PubMed] [Google Scholar]
- Uzzan B, Campos J, Cucherat M, Nony P, Boisel JP, Perret GY. Effects on bone mass of long term treatment with thyroid hormones: a meta‐analysis. The Journal Clinical of Endocrinology and Metabolism 1996;81:4278‐89. [DOI] [PubMed] [Google Scholar]
- Vanderpump MP, Tunbridge WM, French JM, Appleton D, Bates D, Clark F, et al. The incidence of thyroid disorders in the community: a twenty‐year follow‐up of the Whickham survey. Clinical Endocrinology (Oxf) 1995;43:55‐68. [DOI] [PubMed] [Google Scholar]
- Walsh JP, Bremner AP, Bulsara MK, O'Leary P, Leedman PJ, Feddema P, et al. Subclinical thyroid dysfunction as a risk factor for a cardiovascular disease. Archives of Internal Medicine 2005;165:2467‐72. [DOI] [PubMed] [Google Scholar]
- Zullewski H, Muller B, Miserez AR, Staub JJ. Estimation of tissue hypothyroidism by a new clinical score: evaluation of patients with various grades of hypothyroidism and controls. The Journal of Clinical and Endocrinology and Metabolism 1997;82:771‐6. [DOI] [PubMed] [Google Scholar]


