Abstract
Background:
Setting a positive end-expiratory pressure (PEEP) on patients with acute respiratory distress syndrome (ARDS) receiving mechanical ventilation has been an issue of great contention. Therefore, we aimed to determine effects of lung recruitment maneuver (RM) and titrated PEEP versus low PEEP on adult patients with moderate–severe ARDS.
Methods:
Data sources and study selection proceeded as follows: PubMed, Ovid, EBSCO, and Cochrane Library databases were searched from 2003 to May 2018. Original clinical randomized controlled trials which met the eligibility criteria were included. To compare the prognosis between the titrated PEEP and low PEEP groups on patients with moderate–severe ARDS (PaO2/FiO2 < 200 mmHg). Heterogeneity was quantified through the I2 statistic. Egger’s test and funnel plots were used to assess publication bias.
Results:
No difference was found in 28-day mortality and ICU mortality (OR = 0.97, 95% CI (0.61–1.52), p = 0.88; OR = 1.14, 95% CI (0.91–1.43), p = 0.26, respectively). Only ventilator-free days, length of stay in the ICU, length of stay in hospital, and incidence of barotrauma could be systematically reviewed owing to bias and extensive heterogeneity.
Conclusion:
No difference was observed in the RM between the titrated PEEP and the low PEEP in 28-day mortality and ICU mortality on patients with moderate–severe ARDS.
Keywords: acute respiratory distress syndrome, meta-analysis, mortality, positive end-expiratory pressure, systematic review
Background
Acute respiratory distress syndrome (ARDS) is a common life-threatening complication in the intensive care unit (ICU) because of the diffuse alveolar damage and the subsequent hyaline–membrane formation, epithelial cell hyperplasia, and interstitial edema. Its mortality rate is 33–52%.1,2 Since Uzawa et al. found that continuous positive-pressure breathing could improve oxygenation on patients with acute hemorrhagic pulmonary edema in 1969, respiratory support has become a powerful defense for patients with ARDS.3–5
Although mechanical ventilation with small tidal volumes can improve prognosis, how to adjust the positive end-expiratory pressure (PEEP) remains controversial. Recent findings showed that PEEP was associated with static stress of the respiratory system and ventilator-induced lung injury (VILI).6–8 Low PEEP may cause atelectasis and atelectrauma because of the shear force induced by the repetitive opening and closing of the alveoli. High PEEP overinflates the normal lung tissue, thus increasing the risk of VILI and reducing the effective circulating blood volume.9 Theoretically, the setting of PEEP should be based on the static pressure volume curve (P-V curve), dynamic respiratory compliance, static or dynamic compliance of the lung, maximal oxygenation, collapsing pressure, and so on. PEEP is the pressure adding a 2 cm H2O level at the low inflection point in the inspiratory phase,10–13 but the P-V curve is not always plotted clinically. Traditionally, PEEP selection is always based on the best respiratory system compliance or the maximum oxygenation.14–16 Multiple studies found that recruitment maneuver (RM) and PEEP titration have been an effective therapy for ARDS patients with refractory hypoxemia since 2003.10–17 However, recently, a new study reported that the lung recruitment and titrated PEEP compared with the low PEEP increased the 28-day mortality in moderate–severe ARDS patients.6 RM is the process that increases trans-pulmonary pressure to open nonaerated or poorly aerated alveoli to improve oxygenation and lung compliance.4,5,14 However, the temporary reopening of nonaerated alveoli in the inspiration phase is far from adequate. The alveoli will eventually collapse in the end-expiration, and thus appropriate PEEP is required to prevent shear force-induced VILI caused by the repetitive opening and closing of the alveoli.4–8,10,13–18 Thus, we performed a systematic review and meta-analysis to verify which maneuver is suitable for patients with moderate–severe ARDS.
Methods
Search strategy
The complete meta-analysis protocol was constructed and adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses standards.19 PubMed, Ovid, EBSCO, and Cochrane Library databases were searched from 2003 to May 2018 by two investigators (Xi Zheng and Yijia Jiang) independently. The keywords or medical terms that were used included ‘lung recruitment,’ ‘PEEP setting,’ ‘recruitment ARDS,’ ‘titrated PEEP,’ ‘PEEP titration,’ ‘low PEEP,’ ‘protective lung strategy,’ ‘high PEEP,’ and ‘ARDS.’
Trial selection
The inclusion criteria were as follows. (1) The study must be a randomized controlled trial (RCT). (2) The study included at least the titrated PEEP and low PEEP groups. The former is the strategy that processes RM and decreases pressure stepwise or measures static compliance to find the PEEP, and the latter is the strategy proposed by the ARDS Network (ARDSnet) that sets the PEEP based on a table according to the inspired fraction of oxygen (FiO2) and oxygenation target.20 (3) English was the only language used. (4) The patients had moderate–severe ARDS (PaO2/FiO2 < 200 mmHg). (5) The outcomes included at least mortality and hospital stay.
The exclusion criteria were as follows: (1) the ventilator procedures of titrated PEEP and low PEEP were not clarified; (2) the studies were animal experiments, reviews, correspondences, case reports, expert opinions, or editorials; (3) the study included patients under the age of 15 years; and (4) the articles were in languages other than English.
Two authors independently screened the titles of studies as the search terms. The duplicates were removed. Irrelevant articles were eliminated through the titles. Then, we examined the abstracts of the remaining studies. The final number of included studies was assessed by the full-text reading. Any disagreement was resolved by a discussion between the two authors. If a consensus could not be reached, further decision was adjudicated by the third author.
Data extraction
The analyzed data were extracted from the included studies by the two co-authors independently.
The following information was extracted: (1) first author, (2) year of publication, (3) case number of each group, (4) methods of randomization and masking, (5) ventilation strategy of the titrated PEEP, (6) ventilation strategy of the low PEEP, and (7) outcomes of mortality, length of stay in the ICU, length of stay in hospital, cases of barotrauma, and cases of rescue therapy. The detailed information was obtained by telephone or by e-mailing the authors if a number was not provided.
Quality assessment
We assessed all the included studies according to the Cochrane Collaboration’s tool for assessing risk of bias. This tool includes seven questions about selection bias, performance bias, detection bias, attrition bias, reporting bias, and other biases.
Statistics analysis
Review manager 5.3 (Review Manager (RevMan): [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) was used for statistical analyses and STATA (STATA: StataCorp. 2011. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP) was used for the analysis of publication bias and influence. We used the chi-squared test to analyze the heterogeneity among studies. Heterogeneity was quantified through the I2 statistic. If the I2 values were less than 30%, which indicates low heterogeneity, we used the fixed effects model to pool data; otherwise, the random effects model was used. A p value less than 0.05 was considered statistically significant.
Results
Characteristics and quality of the included studies
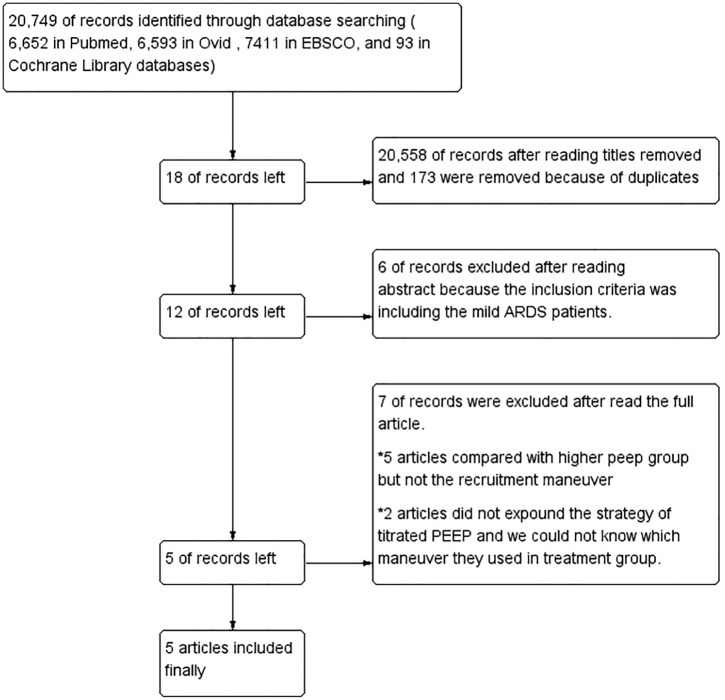
A flow diagram summarizing the study selection process is presented in Figure 1. A total of 20,749 articles were searched though the databases: 6652 in PubMed, 6593 in Ovid, 7411 in EBSCO, and 93 in Cochrane Library databases (CML). In total, 20,558 articles were removed after Huimiao Jia and Wenliang Ma read the titles, respectively, and 173 were removed because of duplicates. Eighteen articles were left, but six articles were then excluded after reading the abstract because the inclusion criteria included mild ARDS patients. Twelve articles remained, but then seven were excluded after reading the full article. Five of these articles compared the higher PEEP group but not the RM, and two articles did not expound on the strategy of titrated PEEP; thus, we had no way of knowing what maneuver they used in the treatment group. Five articles finally remained.6,10–13 The data extracted from the five studies are summarized in Table 1. The level of risk of bias for each included study is shown in Figure 2.
Figure 1.
Summary of the evidence search and selection.
Table 1.
Characteristics of the included studies.
| First author/year | n (titrated PEEP group) | n (low PEEP group) | The ventilation strategy of titrated PEEP | The ventilation strategy of low PEEP | Prone ventilation | Primary diseases on included patients |
|---|---|---|---|---|---|---|
| Hodgson CL, 2011 | 10 | 10 | The high pressure was set to 15 cm H2O above the PEEP, PEEP was increased in a stepwise manner to 20, then 30 and then 40 cm H2O every two minutes, and then reduced to 25, then 22.5, then 20, then 17.5 or then an absolute minimum of 15 cm H2O every three minutes until a decrease in SaO2 ⩾1% from maximum SaO2 was observed. | The low-PEEP strategy proposed by the Acute Respiratory Distress Syndrome Network (ARDS Net) | Not available | 11 ARDS of pulmonary origin (ARDSp) (55.0%) 9 ARDS of extra-pulmonary origin (ARDSexp) (45.0%) |
| Cavalcanti AB, 2017 | 501 | 509 | Using lung recruitment maneuver and PEEP titrated according to the best respiratory-system compliance. | The low-PEEP strategy proposed by the Acute Respiratory Distress Syndrome Network (ARDS Net) | 30 and 31 patients in low PEEP and titrated PEEP group used prone ventilation. (The baseline data were collected on prone position from the 407th patient onward.) | 626 ARDS of pulmonary origin (ARDSp) (62.0%) 384 ARDS of extra-pulmonary origin (ARDSexp) (38.0%). |
| Jin WH, 2009 | 30 | 27 | PEEP level was considered the alveolar collapsing pressure and optimal PEEP after the ARM was set 2 cmH2O above this pressure. | The low-PEEP strategy proposed by the Acute Respiratory Distress Syndrome Network (ARDS Net) | Not available | 38 ARDS of pulmonary origin (ARDSp) (66.7%) 19 ARDS of extra-pulmonary origin (ARDSexp) (33.3%). |
| Kacmarek RM, 2016 | 99 | 101 | First, found the maximum compliance PEEP. Then made the lung again to recruit and PEEP set at the maximum compliance PEEP + 3 cm H2O | The low-PEEP strategy proposed by the Acute Respiratory Distress Syndrome Network (ARDS Net) | Not available | Not available |
| Pintado MC, 2013 | 34 | 36 | Static compliance was calculated dividing tidal volume by the pressure difference at end of inflation hold (2 seconds) and PEEP was increased at steps of 2 cm H2O beginning at 5 cmH2O, without an upper PEEP titration limit (RM was not mentioned in the article but the method of RM was used). | PEEP level was set based on the patient’s FiO2, as applied in the ARDS Net study. | Not available | Not available |
Figure 2.
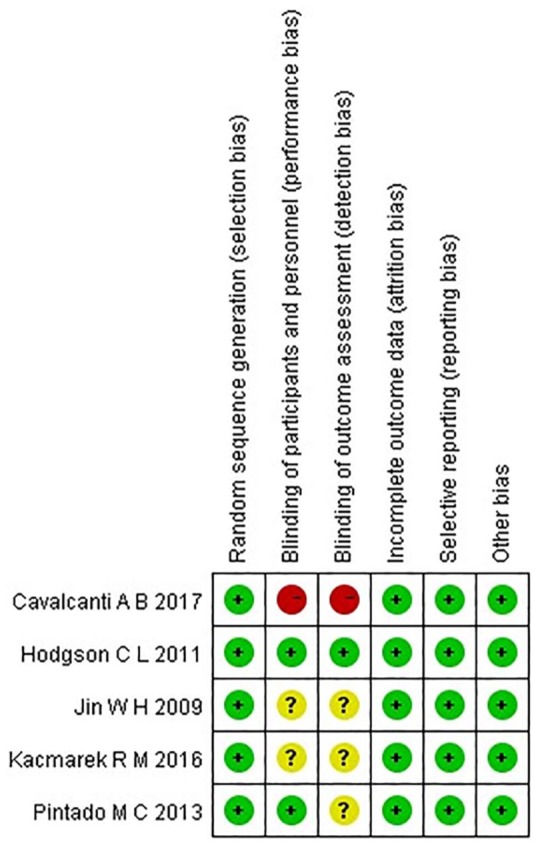
Risk of bias summary. Green, yellow, and red indicate low, moderate, and high risk of bias, respectively.
Mortality
28-day mortality
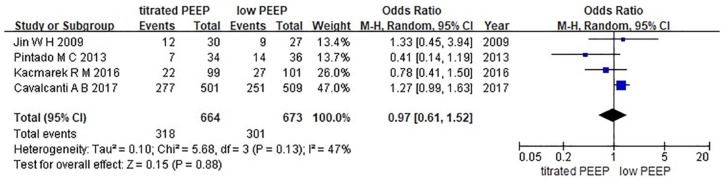
Four studies were included in this analysis because Hodgson reported hospital mortality only.6,10–13 We used Egger’s test to analyze the publication bias. As shown in Figure 3, the bias found had no statistical difference (p = 0.27). The funnel plot is illustrated in Figure 4. We chose the random effects model to analyze the I2 = 47%. The result showed that p = 0.88 represented no statistical difference between the titrated PEEP and the low PEEP in moderate–severe ARDS patients during the first 28 days [OR = 0.97, 95% CI (0.61–1.52)]. The low PEEP could not improve survival in the early phase in moderate–severe ARDS (Figure 5).
Figure 3.
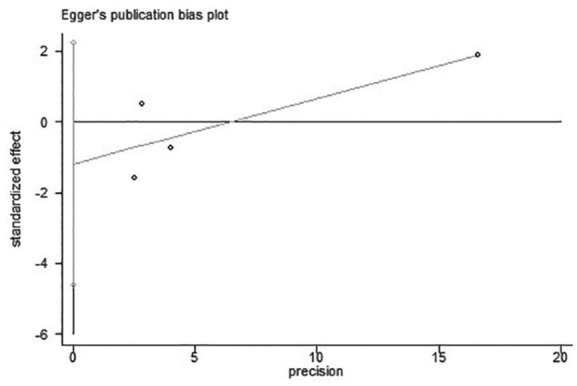
Result of Egger’s test on the four studies for 28-day mortality.
Figure 4.
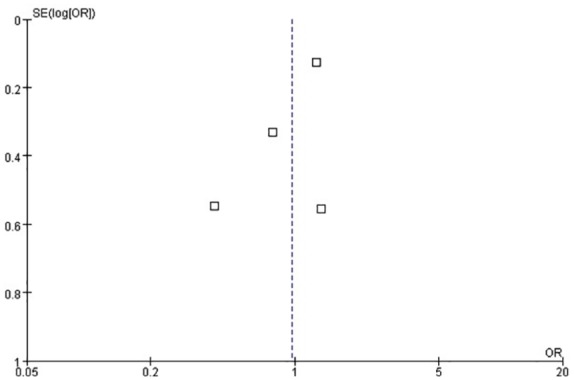
Funnel plot for the identification of potential publication bias in the four studies for 28-day mortality.
Figure 5.
Forest map of 28-day mortality between the two groups.
ICU mortality
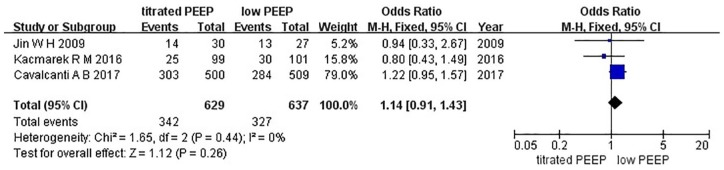
Three studies were included in this analysis.6,11,12 We used Egger’s test to analyze the publication bias. As shown in Figure 6, the bias found had no statistical difference (p = 0.39). The funnel plot is presented in Figure 7. As indicated by the result of the fixed effects model, no statistical difference was found between the titrated PEEP and the low PEEP in moderate–severe ARDS patients in the ICU [OR = 1.14, 95% CI (0.91–1.43), p = 0.26], as shown in Figure 8.
Figure 6.
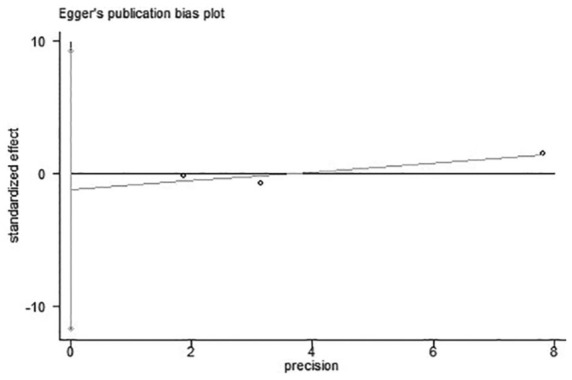
Result of Egger’s test on the three studies for ICU mortality.
Figure 7.
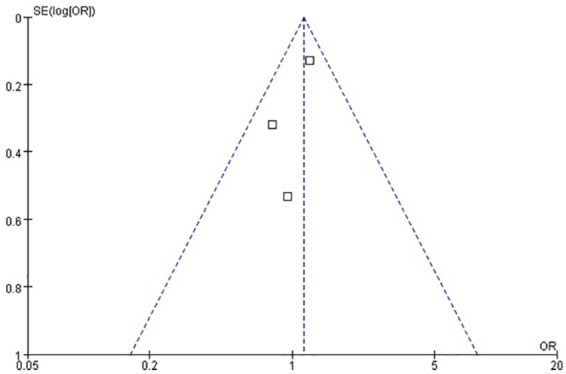
Funnel plot for the identification of potential publication bias in the three studies for ICU mortality.
Figure 8.
Forest map of ICU mortality between the two groups.
Length of stay
Length of stay in the ICU
The included studies recorded the length of stay in the ICU to evaluate prognosis6,10–13 (Table 2). In three of the five studies, the provided data represented the skewed distribution but could not calculate the standard deviation.10,12,13 Although the data of the two studies formed a normal distribution, they produced extensive heterogeneity (I2 = 80%) that could not be examined by meta-analysis. Nevertheless, we found that each included study showed no statistically significant difference between the low PEEP and the titrated PEEP in the length of stay in the ICU.
Table 2.
Length of stay in the ICU.
| Study | Distribution of the data | Low-PEEP group (days) | Titrated PEEP group (days) | p value |
|---|---|---|---|---|
| Hodgson et al.10 | Skewed distribution | 16.0 (8.1–19.3) | 9.9 (5.6–14.8) | 0.19 |
| Cavalcanti et al.6 | Gaussian distribution | 19.2 ± 25.9 | 18.2 ± 22.4 | 0.51 |
| Jin et al.11 | Gaussian distribution | 21.4 ± 5.3 | 25.1 ± 5.6 | 0.643 |
| Kacmarek et al.12 | Skewed distribution | 16 (11–28) | 18 (10–28) | 0.79 |
| Pintado et al.13 | Skewed distribution | 20 (12–29) | 21 (15–46) | 0.24 |
Length of stay in hospital
Four of the included studies recorded the length of stay in hospital to evaluate the prognosis6,10,12,13 (Table 3). In two of the four studies, the provided data represented the skewed distribution but could not calculate the standard deviation.10,12 Although the data of two studies formed a normal distribution, they produced extensive heterogeneity (I2 = 99%).6,13 We found that the patients in the titrated PEEP group were obviously worse than those in the low PEEP group. The data could not be examined by meta-analysis. No statistically significant difference was found between the two groups for length of stay in hospital in each of the four studies.
Table 3.
Length of stay in hospital.
| Study | Distribution of the data | Low-PEEP group (days) | Titrated PEEP group (days) | p value |
|---|---|---|---|---|
| Hodgson et al.10 | Skewed distribution | 24.7 (20.5–39.8) | 17.9 (13.7–34.5) | 0.16 |
| Cavalcanti et al.6 | Gaussian distribution | 26.2 ± 31.7 | 25.5 ± 32.3 | 0.74 |
| Kacmarek et al.12 | Skewed distribution | 23 (14–41) | 27 (16–46) | 0.49 |
| Pintado et al.13 | Gaussian distribution | 32 ± 3 | 55 ± 7 | <0.01 |
Ventilator-free days
Five studies showed the results of ventilator-free days.6,10–13 However, no standard deviation was given in three of the five studies.10,12,13 The remaining studies had extensive heterogeneity (I2 = 98%) that could not be examined by meta-analysis. Four studies showed no difference in the ventilator-free days between the titrated PEEP and the low PEEP (p = 0.53, p = 0.16, p = 0.38, and p = 0.13),10–13 and one article based on a large sample size showed a significant difference (p = 0.03).6
Barotrauma
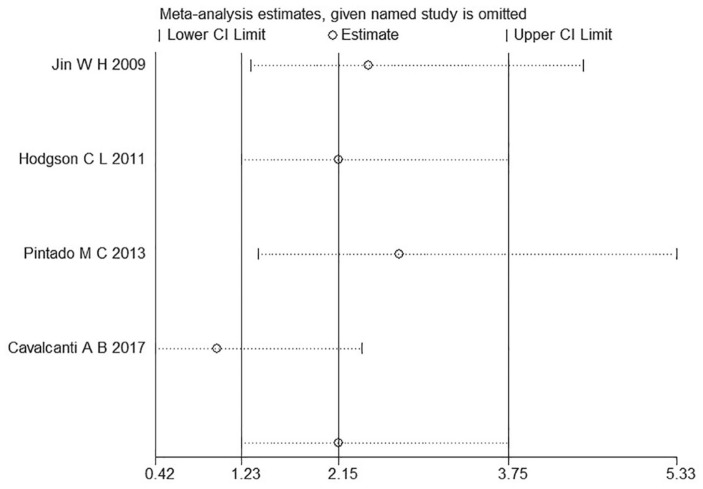
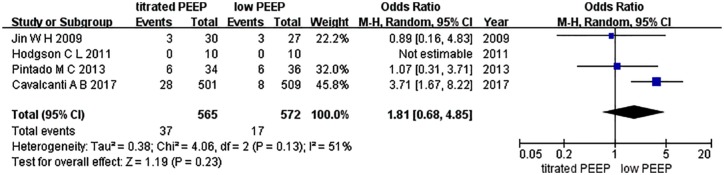
VILI is defined as severe permeability pulmonary edema and diffusive alveolar damage by inappropriate tidal volume mechanical ventilation in pathophysiology. Barotrauma is one form of VILI expression in the clinic, defined as persistent pneumothorax or expanding subcutaneous emphysema, and so on. Four studies were included in this analysis.6,10,11,13 Hodgson et al. reported a barotrauma incidence of 0, and thus Egger’s test was not conducted in this study.10 The funnel plot is shown in Figure 9. We conducted an influence analysis, as illustrated in Figure 10. The study of Cavalcanti et al. caused a publication bias but publication bias is anticipated for small studies. This study is three times the size of the other studies combined and thus we did not exclude this study.6 No statistically significant difference was found between the two groups for barotrauma incidence [OR = 1.81, 95% CI (0.68–4.85), p = 0.23] (Figure 11).
Figure 9.
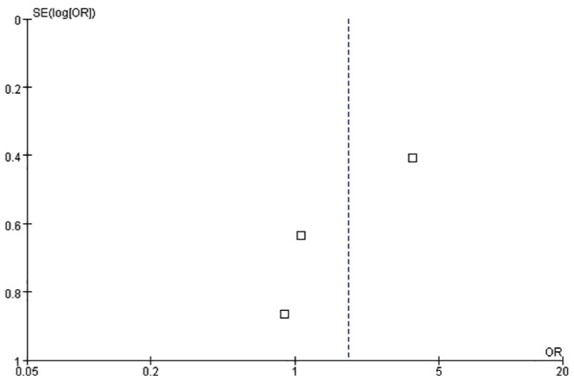
Funnel plot for the identification of potential publication bias in the three studies about barotrauma.
Figure 10.
Result of the influence analysis on the four studies about barotrauma.
Figure 11.
Forest map of barotrauma between the two groups.
Discussion
Five RCTs studying patients with moderate–severe ARDS (PaO2/FiO2 < 200 mmHg) were finally included in the systematic review and meta-analysis.6,10–13 The results showed no statistical difference between the RM and the titrated PEEP and low PEEP in 28-day mortality and ICU mortality. Unfortunately, ventilator-free days, length of stay in the ICU, the length of stay in hospital, and incidence of barotrauma could not be examined by the meta-analysis because of bias and extensive heterogeneity.
The low-PEEP strategy was proposed by ARDSnet in 2000 to control plateau pressures less than 28–30 cm H2O while delivering tidal volumes of less than 6–8 ml/kg ideal body weight, and it used a table-based PEEP setting to obtain a lower PEEP level compatible with the oxygenation target.20 Not long after the ARDSnet study was published, RM and PEEP titration were proposed to improve oxygenation and lung compliance in succession.6,10–13,17
However, RM and titrated PEEP have different strategies with diverse maximum plateau pressures and driving pressures not only in the included five studies but also in the current clinical practice.6,10–13 In Cavalcanti et al.’s study on 1013 patients,6 the levels of PEEP were at 25, 30, and 35 cm H2O in the first phase. The maximum plateau pressure changed to 50 cm H2O. The decremental PEEP trial in the second phase was shorter, with each PEEP step lasting for 3 min. Jin et al.11 reported that in the first phase, PEEP was added from the baseline to 15, 20, and 25 cm H2O and every 30 s from the baseline PEEP until 25 cm H2O. The second phase was performed with the decremental PEEP stepwise in increments of 1 cm H2O every 30 s from 20 cm H2O to the baseline. Hodgson et al.10 used the base pressure set to 15 cm H2O that increased in 20, 30, and 40 cm H2O every 2 min in the first phase. In the second phase, the PEEP was reduced to 25, 22.5, 20, 17.5, and the absolute minimum of 15 cm H2O every 3 min until a decrease in SaO2 of equal to or greater than percentage from the maximum SaO2 was observed.6,10–13
The previously mentioned studies have some limitations. First, the main cause of ARDS must be considered. Primary diseases greatly affect the prognosis of patients, and they should not be confused with ARDS of pulmonary origin (ARDSp) and ARDS of extrapulmonary origin (ARDSexp) according to different pathogenesis. Some pulmonary regions with severe consolidation cannot be opened no matter how high the selected PEEP was. The high PEEP mainly served the normal lung regions and caused more stress. Thus, the selected RM and PEEP titration were blind without evaluating the capacity of the lung to be recruited.4,5,8 Several studies showed that the patients with early ARDSexp could benefit from RM and titration PEEP because of their recruitability.6,10–15 Therefore, the potential recruitability of collapsed lung parenchyma should be evaluated before RM and titrated PEEP are performed. Second, none of these studies made a subgroup analysis to differentiate the patients with moderate ARDS from those with severe ARDS, although the severity of disease affected the outcome.21–24 For moderate ARDS, low or moderate PEEP may be sufficient to prevent the repetitive opening and closing of alveoli in the nonaerated or poorly aerated lung regions, but RM and high PEEP may bring the risk of VILI because of high stress.4,5,8,23–26 Third, the PEEP selection after RM was usually based on the maximum oxygenation or the best respiratory system compliance; avoiding bias by the manipulator is difficult using this approach. The targets of the PEEP setting are also controversial. Electrical impedance tomography and ultrasound were considered new methods for measuring recruitable lung volume and homogeneity in recent studies. These new methods seem to be objective and accurate to help physicians adjust the parameters and reduce the risk of VILI.27–29 Fourth, prone ventilation may be one of significant methods to improve prognosis because of improving lung ventilatory functions. Unfortunately, only Cavalcanti et al.’s study reported prone ventilation from the 407th patient onward, and we cannot evaluate how effective the prone ventilation will prove. RM and prone ventilation may be a research orientation to improve patient outcomes. Finally, the maximum plateau pressure and driving pressure during RM must be limited and individualized in different ARDS patients to reduce the risk of VILI. In sum, the role of RM and titrated PEEP in severe ARDS patients with potential recruitability needs further study.
Conclusion
The results show no difference between the RM and the titrated PEEP and low PEEP in 28-day mortality and ICU mortality on patients with moderate–severe ARDS. RM and titrated PEEP based on reduced VILI may be preferable in severe ARDS patients with potential recruitability. A large number of RCT studies are still demanded.
Footnotes
Author contributions: Xi Zheng, Yijia Jiang, and Huimiao Jia collected the study data; Wenliang Ma and Yue Han analyzed the data; Xi Zheng wrote the article; and Wenxiong revised the article. All the authors read and approved the final manuscript.
Availability of data and material: Please contact the author for data requests.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Wenxiong Li  https://orcid.org/0000-0001-7074-1136
https://orcid.org/0000-0001-7074-1136
Contributor Information
Xi Zheng, Surgical Intensive Care Unit, Beijing Chao-Yang Hospital, Capital Medical University, Beijing, China.
Yijia Jiang, Surgical Intensive Care Unit, Beijing Chao-Yang Hospital, Capital Medical University, Beijing, China.
Huimiao Jia, Surgical Intensive Care Unit, Beijing Chao-Yang Hospital, Capital Medical University, Beijing, China.
Wenliang Ma, Surgical Intensive Care Unit, Beijing Chao-Yang Hospital, Capital Medical University, Beijing, China.
Yue Han, Surgical Intensive Care Unit, Beijing Chao-Yang Hospital, Capital Medical University, Beijing, China.
Wenxiong Li, Surgical Intensive Care Unit, Beijing Chao-Yang Hospital, Capital Medical University, 8 Gongren Tiyuchang Nanlu, Chaoyang District, Beijing, 100020, China.
References
- 1. Erickson SE, Martin GS, Davis JL, et al. Recent trends in acute lung injury mortality: 1996–2005. Crit Care Med 2009; 37: 1574–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Standiford TJ, Ward PA. Therapeutic targeting of acute lung injury and acute respiratory distress syndrome. Transl Res 2015; 167: 183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Uzawa T, Ashbaugh DG. Continuous positive-pressure breathing in acute hemorrhagic pulmonary edema. J Appl Physiol 1969; 26: 427–432. [DOI] [PubMed] [Google Scholar]
- 4. Suzumura EA, Amato MB, Cavalcanti AB. Understanding recruitment maneuvers. Intensive Care Med 2016; 42: 908–911. [DOI] [PubMed] [Google Scholar]
- 5. Nieman GF, Gatto LA, Habashi NM. The impact of mechanical ventilation on the pathophysiology of progressive acute lung injury. J Appl Physiol 2015; 119: 1245–1261. [DOI] [PubMed] [Google Scholar]
- 6. Cavalcanti AB, Suzumura ÉA, Laranjeira LN, et al. Effect of lung recruitment and titrated positive end-expiratory pressure (PEEP) vs low PEEP on mortality in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA 2017; 318: 1335–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kida Y, Ohshimo S, Shime N. Optimal cutoff value for lung injury prediction score and potential confounders for identifying the risk of developing acute respiratory distress syndrome. Crit Care Med 2017; 45: e624–e625. [DOI] [PubMed] [Google Scholar]
- 8. Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med 2013; 369: 2126–2136. [DOI] [PubMed] [Google Scholar]
- 9. Cannon JW, Gutsche JT, Brodie D. Optimal strategies for severe acute respiratory distress syndrome. Crit Care Clin 2017; 33: 259–275. [DOI] [PubMed] [Google Scholar]
- 10. Hodgson CL, Tuxen DV, Davies AR, et al. A randomised controlled trial of an open lung strategy with staircase recruitment, titrated PEEP and targeted low airway pressures in patients with acute respiratory distress syndrome. Crit Care 2011; 15: R133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jin WH, Jung H, Choi HS, et al. Efficacy of positive end-expiratory pressure titration after the alveolar recruitment manoeuvre in patients with acute respiratory distress syndrome. Crit Care 2009; 13: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kacmarek RM, Villar J, Sulemanji D, et al. Open lung approach for the acute respiratory distress syndrome: a pilot, randomized controlled trial. Crit Care Med 2016; 44: 32–42. [DOI] [PubMed] [Google Scholar]
- 13. Pintado MC, Pablo RD, Trascasa M, et al. Individualized positive end-expiratory pressure setting in patients with acute respiratory distress syndrome. A randomized controlled pilot study. Resp Care 2013; 62: 1–31. [Google Scholar]
- 14. Hess DR. Recruitment maneuvers and PEEP titration. Resp Care 2015; 60: 1688–1704. [DOI] [PubMed] [Google Scholar]
- 15. Ramnath VR, Hess DR, Thompson BT. Conventional mechanical ventilation in acute lung injury and acute respiratory distress syndrome. Clin Chest Med 2006; 27: 601–613. [DOI] [PubMed] [Google Scholar]
- 16. Sahetya SK, Brower RG. Lung recruitment and titrated PEEP in moderate to severe ARDS: is the door closing on the open lung? JAMA 2017; 318: 1327–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barbas CS, de Matos GF, Okamoto V, et al. Lung recruitment maneuvers in acute respiratory distress syndrome. Resp Care Clin 2003; 9: 401–418. [DOI] [PubMed] [Google Scholar]
- 18. Burns KEA, Adhikari NKJ, Slutsky AS, et al. Pressure and volume limited ventilation for the ventilatory management of patients with acute lung injury: a systematic review and meta-analysis. PLoS One 2011; 6: e14623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Knobloch K, Yoon U, Vogt PM. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J Cranio Maxill Sur 2011; 39: 91–92. [DOI] [PubMed] [Google Scholar]
- 20. Brower RG, Matthay MA, Morris A, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000; 343: 813–814. [DOI] [PubMed] [Google Scholar]
- 21. Soto GJ, Kor DJ, Park PK, et al. Lung injury prediction score in hospitalized patients at risk of acute respiratory distress syndrome. Crit Care Med 2016; 44: 2182–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schmidt M, Stewart C, Bailey M, et al. Mechanical ventilation management during extracorporeal membrane oxygenation for acute respiratory distress syndrome: a retrospective international multicenter study. Crit Care Med 2015; 43: 654–664. [DOI] [PubMed] [Google Scholar]
- 23. Brochard L, Slutsky A, Pesenti A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Resp Crit Care 2017; 195: 438–442. [DOI] [PubMed] [Google Scholar]
- 24. Dreyfuss D, Ricard JD, Gaudry S. Did studies on HFOV fail to improve ARDS survival because they did not decrease VILI? On the potential validity of a physiological concept enounced several decades ago. Intensive Care Med 2015; 41: 2076–2086. [DOI] [PubMed] [Google Scholar]
- 25. Wrigge H, Zinserling J, Muders T, et al. Electrical impedance tomography compared with thoracic computed tomography during a slow inflation maneuver in experimental models of lung injury. Crit Care Med 2008; 36: 903–909. [DOI] [PubMed] [Google Scholar]
- 26. Grasso S, Stripoli T, Sacchi M, et al. Inhomogeneity of lung parenchyma during the open lung strategy: a computed tomography scan study. Am J Resp Crit Care 2009; 180: 415–423. [DOI] [PubMed] [Google Scholar]
- 27. Wong JJ, Phan HP, Phumeetham S, et al. Risk stratification in pediatric acute respiratory distress syndrome: a multicenter observational study. Crit Care Med 2017; 45: 1820–1828. [DOI] [PubMed] [Google Scholar]
- 28. Bouhemad B, Brisson H, Le-Guen M, et al. Bedside ultrasound assessment of positive end-expiratory pressure-induced lung recruitment. Am J Resp Crit Care 2011; 183: 341–347. [DOI] [PubMed] [Google Scholar]
- 29. Bikker I G, Leonhardt S, Miranda DR, et al. Bedside measurement of changes in lung impedance to monitor alveolar ventilation in dependent and non-dependent parts by electrical impedance tomography during a positive end-expiratory pressure trial in mechanically ventilated intensive care unit patients. Crit Care 2010; 14: R100. [DOI] [PMC free article] [PubMed] [Google Scholar]