Abstract
Background:
The environment surrounding Helicobacter pylori eradication treatment is dramatically changing. Recently, vonoprazan, a first-in-class potassium-competitive acid blocker (P-CAB), was introduced onto the market in 2015. The aging of Japan’s demographic structure is becoming pronounced. In this study, we examined the trend of the eradication rate of H. pylori in the metropolitan area and examined factors concerning successful eradication.
Methods:
We collected data from 20 hospitals in the Tokyo metropolitan area on patients who received first-line eradication therapy with a proton-pump inhibitor (PPI)/P-CAB, amoxicillin, and clarithromycin for 1 week and second-line eradication therapy with a PPI/P-CAB, amoxicillin, and metronidazole for 1 week from 2013 to 2018. The annual eradication rate and associated factors for successful eradication were analyzed.
Results:
We collected data of 4097 and 3572 patients in the first- and second-line eradication therapies, respectively. The eradication rate decreased from 2013 to 2014 and increased again from 2015 to 2018 with the first-line therapy [the eradication rates in 2013, 2014, 2015, 2016, 2017 and 2018 were 71.8%, 63.7%, 78.5%, 84.6%, 89.7 and 90.1%, respectively, in the per protocol (PP)]. The second-line eradication rates were 90.0%, 82.6%, 88.8%, 87.5%, 91.8% and 90.1% in 2013, 2014, 2015, 2016, 2017 and 2018, respectively, in PP. Vonoprazan was an independent factor for successful eradication in not only first-line, but also second-line eradication. Age over 75 years was an independent factor for eradication failure in both first- and second-line eradication therapies.
Conclusion:
The eradication rate improved from 2015 to 2018 with the first-line therapy because of the introduction of vonoprazan in the market. The eradication rates with first- and second-line regimens in elderly patients were lower than those in younger patients.
Keywords: amoxicillin, clarithromycin, Helicobacter pylori, metronidazole, vonoprazan
Introduction
Helicobacter pylori infection is associated with peptic ulcers, gastric-mucosa-associated lymphoid tissue lymphomas, and gastric cancer.1,2 Earlier, we have shown the trends with first- and second-line eradication therapies for H. pylori in Japan.3,4 However, the environment surrounding H. pylori eradication treatment is dramatically changing. In 2000, first-line eradication, a triple therapy based on a proton-pump inhibitor (PPI), amoxicillin (AMPC), and clarithromycin (CAM), was approved and covered by the national health insurance scheme for peptic ulcers in Japan.5 Moreover, second-line eradication, a triple therapy based on a PPI, AMPC, and metronidazole (MTZ), was also approved and covered by insurance in 2007.5 To prevent gastric carcinogenesis, the Japanese Ministry of Health, Labor and Welfare approved the insurance coverage of H. pylori eradication therapy in all patients with H. pylori-associated chronic gastritis in 2013.6–8 Moreover, vonoprazan (VPZ), a first-in-class potassium-competitive acid blocker (P-CAB), which exhibited more rapid, strong, and continuous gastric acid suppression compared with conventional PPIs, was introduced in the market in 2015.9,10 Meanwhile, the aging of Japan’s demographic structure is becoming pronounced. In 2015, 12.8% of population were over 75 years of age in Japan.11 The influence of aging on H. pylori eradication is less well understood.
Therefore, we examined the trend of eradication rate of H. pylori in the metropolitan area and examined factors that were associated with successful eradication.
Subjects and methods
Study population
A retrospective analysis of results of first- and second-line eradication therapies was conducted by the Tokyo Hp Study Group, which comprised staff members of 20 facilities in the Tokyo metropolitan area in Japan.
To investigate changes in the first- and second-line eradication rates, we retrospectively examined data of patients who received first-line eradication therapy with a PPI/P-CAB [rabeprazole (RPZ) 10 mg twice a day, lansoprazole (LPZ) 30 mg twice a day, omeprazole (OPZ) 20 mg twice a day, esomeprazole (EPZ) 20 mg twice a day, or VPZ 20 mg twice a day], AMPC (750 mg twice a day), and CAM (200 or 400 mg twice a day) for 1 week in a 4–6 month period each year from 2013 to 2018, and second-line eradication therapy with a PPI/P-CAB (RPZ 10 mg twice a day, LPZ 30 mg twice a day, OPZ 20 mg twice a day, EPZ 20 mg twice a day, or VPZ 20 mg twice a day), AMPC (750 mg twice a day), and MTZ (250 mg twice a day) for 1 week in a whole year from 2013 to 2018. The data were extracted from the medical records, of those patients who were prescribed the combination of drugs. Patients who were not treated for H. pylori eradication or who did not receive an upper gastrointestinal endoscopy before eradication were excluded. The patients were interviewed for treatment naïve or treatment experienced before the eradication treatment. The patients had undergone upper gastrointestinal endoscopy prior to H. pylori eradication therapy, and the findings of chronic atrophic gastritis were confirmed according to the Kyoto classifications, and background diseases were investigated.12,13 H. pylori infection was confirmed through at least one of the following methods before first-line treatment: the H. pylori stool antigen test (HpSA), urea breath test (UBT), rapid urease test, serology, culture, and histology. After a diagnosis of first-line treatment failure with UBT or HpSA, the patients were treated with second-line treatment. This study protocol was approved by the Committee for Medical Ethics of Keio University School of Medicine (no. 20160051; 22 June 2016) and was conducted in accordance with the tenets of the Declaration of Helsinki 1975, and its later amendment in 1983. Written informed consent was not obtained, and informed consent included an opt-out clause according to the Medical Ethics Committee. The study was observational and reported in accordance with the Strengthening Reporting of Observational Studies in Epidemiology guidelines.14
Study design
To investigate changes in the first- and second-line eradication rates, the annual eradication rate was compared with that in the base period, the eradication rate of 2013. Associated factors (age, sex, choice of PPIs or VPZ, the dosage of CAM and background diseases of the stomach) for successful eradication were analyzed. The elderly population is defined as people aged 75 years and over.15 At least 4 weeks after the end of eradication therapy, successful eradication was confirmed using UBT or HpSA.16–18 The cut-off value for a negative UBT was <2.5%.16 Values of UBT between 2.5 and 5.0% were considered as being in the gray zone.19
Associated factors of UBT gray zone in the patients with first- and second-line eradication failure (age, sex, choice of PPIs or VPZ, first- or second-line treatment, and dosage of CAM) were analyzed.
Statistical analysis
All patients were evaluated with the intention to treat (ITT), in which patients without a final H. pylori determination, or with protocol violations, were considered treatment failures. The per-protocol (PP) analysis included all patients who took at least 80% of the prescribed medication and completed the final H. pylori status assessment.20 Comparisons of the annual eradication rate and that of the base period were conducted using Fisher’s exact test in the ITT and PP analysis. Univariate logistic regression analysis was used to evaluate factors associated with successful eradication and UBT gray zone. Subsequently, multivariate logistic regression analysis was performed by adjusting for factors that showed a marginally significant association (p < 0.1) in the univariate analysis. Statistical analyses were performed using SPSS 25 for Windows (SPSS Inc., Chicago, IL, USA). Data are expressed as means ± standard deviation.
Results
Patient characteristics
A total of 4097 and 3572 were enrolled in this study (Tables 1 and 2). The proportions of PPI gradually decreased from 2013 to 2018 in both the first- and second-line regimens [Tables 1 and 2, Figure 1(c) and 2(c)]. The percentage of LPZ in the first-line regimen was rapidly decreasing from 2013 to 2018; it was 81.8% in 2013 and only 3.4% in 2018. The percentage of RPZ was also decreasing from 2014 to 2018, being 35.4% in 2013 and only 0.8% in 2018. As a second-line regimen, the percentage of LPZ in the first-line regimen was rapidly decreasing from 2013 to 2018, being 65.7% in 2013 and only 1.6% in 2018. The percentage of RPZ was also decreasing from 2014 to 2018, as it was 41.3% in 2013 and only 6.8% in 2018. In contrast, VPZ was introduced in 2015, and its proportions dramatically increased toward 2018 in both the first- and second-line regimens [Tables 1 and 2, Figure 1(c) and 2(c)]. In 2018, the proportions of VPZ were 95.5% and 90.2% in the first- and second-line regimens, respectively. Regarding background diseases of the stomach, the proportions of peptic ulcer disease gradually decreased, and those of chronic gastritis gradually increased from 2013 to 2018 in both first- and second-line regimens (Tables 1 and 2).
Table 1.
Patients’ demographics of first-line therapy.
| 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | |
|---|---|---|---|---|---|---|
| Total | 813 | 773 | 766 | 684 | 438 | 623 |
| Male/female | 412/401 | 409/364 | 412/354 | 359/325 | 228/210 | 336/287 |
| Age, mean ± SD | 60.6 ± 12.9 | 59.3 ± 13.3 | 58.6 ± 13.8 | 59.7 ± 13.7 | 58.1 ± 15.2 | 59.4 ± 13.9 |
| Rabeprazole (%) | 70 (8.6) | 274 (35.4) | 252 (32.9) | 92 (13.5) | 46 (10.5) | 5 (0.8) |
| Lansoprazole (%) | 665 (81.8) | 459 (59.4) | 155 (20.2) | 72 (10.5) | 13 (3.0) | 21 (3.4) |
| Omeprazole (%) | 0 (0) | 1 (0.1) | 0 (0) | 0 (0) | 1 (0.2) | 1 (0.2) |
| Esomeprazole (%) | 78 (9.6) | 39 (5.0) | 10 (1.3) | 7 (1.0) | 1 (0.2) | 1 (0.2) |
| Vonoprazan (%) | 0 (0) | 0 (0) | 349 (45.6) | 513 (75.0) | 377 (86.1) | 595 (95.5) |
| CAM 400 mg/800 mg per day | 450/363 | 551/222 | 528/238 | 509/175 | 343/95 | 481/142 |
| Peptic ulcer disease (%) | 196 (24.1) | 166 (21.5) | 150 (19.6) | 104 (15.2) | 72 (16.4) | 83 (13.3) |
| Chronic gastritis (%) | 561 (69.0) | 543 (70.2) | 594 (77.5) | 556 (81.3) | 336 (76.7) | 495 (79.5) |
| MALT lymphoma (%) | 1 (0.1) | 0 (0) | 0 (0) | 6 (0.9) | 1 (0.2) | 4 (0.6) |
| Gastric polyp (%) | 18 (2.2) | 23 (3.0) | 10 (1.3) | 6 (0.9) | 7 (1.6) | 10 (1.6) |
| Early gastric cancer (%) | 24 (3.0) | 13 (1.7) | 8 (1.0) | 10 (1.5) | 20 (4.6) | 23 (3.7) |
| Other disease (%) | 14 (1.7) | 28 (3.6) | 4 (0.5) | 2 (0.3) | 2 (0.5) | 8 (1.3) |
SD, standard deviation; CAM, clarithromycin; MALT, mucosa-associated lymphoid tissue.
Table 2.
Patients’ demographics of second-line therapy.
| 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | |
|---|---|---|---|---|---|---|
| Total | 744 | 804 | 742 | 598 | 315 | 369 |
| Male/female | 361/383 | 409/395 | 381/361 | 321/277 | 145/170 | 197/172 |
| Age, mean ± SD | 59.6 ± 13.7 | 59.4 ± 13.5 | 57.9 ± 13.4 | 59.4 ± 14.2 | 58.8 ± 13.5 | 59.1 ± 13.8 |
| Rabeprazole (%) | 196 (26.3) | 332 (41.3) | 260 (35.0) | 113 (18.9) | 40 (12.7) | 25 (6.8) |
| Lansoprazole (%) | 490 (65.9) | 416 (51.7) | 174 (23.5) | 70 (11.7) | 22 (7.0) | 6 (1.6) |
| Omeprazole (%) | 11 (1.5) | 2 (0.2) | 0 (0) | 1 (0.2) | 3 (1.0) | 0 (0) |
| Esomeprazole (%) | 47 (6.3) | 54 (6.7) | 5 (0.7) | 6 (1.0) | 2 (0.6) | 5 (1.0) |
| Vonoprazan (%) | 0 (0) | 0 (0) | 303 (40.8) | 408 (68.2) | 248 (78.7) | 333 (90.2) |
| Peptic ulcer disease (%) | 167 (22.4) | 151 (18.8) | 113 (15.2) | 86 (14.4) | 37 (11.7) | 44 (11.9) |
| Chronic gastritis (%) | 498 (66.9) | 562 (69.9) | 597 (80.5) | 472 (78.9) | 258 (81.9) | 300 (81.3) |
| MALT lymphoma (%) | 2 (0.3) | 1 (0.1) | 1 (0.1) | 1 (0.2) | 2 (0.6) | 0 (0) |
| Gastric polyp (%) | 33 (4.4) | 15 (1.9) | 10 (1.3) | 12 (2.0) | 8 (2.5) | 8 (2.2) |
| Early gastric cancer (%) | 31 (4.2) | 33 (4.1) | 17 (2.3) | 22 (3.7) | 9 (2.9) | 13 (3.5) |
| Other disease (%) | 13 (1.7) | 42 (5.2) | 4 (0.5) | 5 (0.8) | 1 (0.3) | 4 (1.1) |
SD, standard deviation; MALT, mucosa-associated lymphoid tissue.
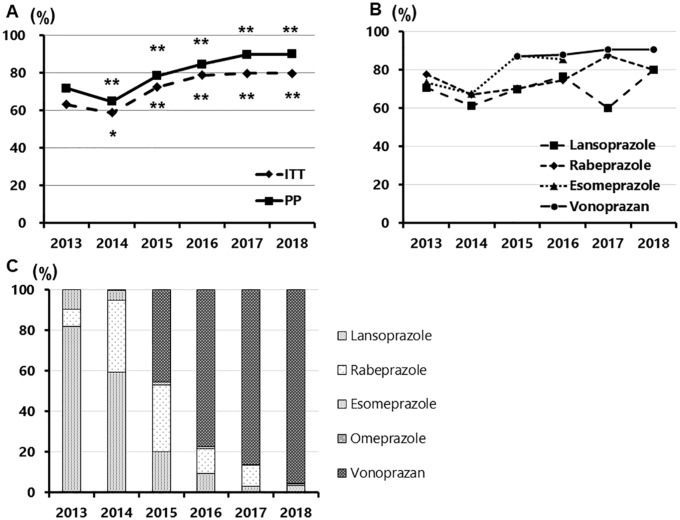
Figure 1.
Annual changes of the eradication rate of first-line therapy and the proportion of PPIs/P-CAB in the first-line regimen.
(a) Annual changes of the eradication rate of first-line therapy in ITT and PP; (b) annual changes of the eradication rate of first-line therapy divided into each PPIs/P-CAB; (c) the proportion of PPIs/P-CAB in the first-line regimen. (a) Once the eradication rate decreased from 2013 to 2014 (p = 0.02 and <0.01, ITT and PP, respectively), after that, the rate showed backward rising from 2015 to 2018 in the first-line eradication (p < 0.01, <0.01, <0.01, <0.01 and p < 0.01, <0.01, <0.01, <0.01, ITT and PP, respectively); (b) the eradication rates of VPZ were constantly over 87% from 2015 to 2018 (87.2%, 87.5%, 90.8 and 90.6% in PP, respectively). As PPI-containing regimens, the eradication rates of LPZ range from 60.0% to 80.0%, and that of RPZ range from 67.1% to 87.5%. Five and less in each item are not described in the graph; (c) VPZ was introduced in 2015, and its proportions dramatically increased toward 2018 in the first-line regimens. In 2018, the proportion of VPZ was 95.5%.
ITT, intention to treat; PP, per protocol; VPZ, vonoprazan; PPI, proton-pump inhibitor; LPZ, lansoprazole; RPZ, rabeprazole.
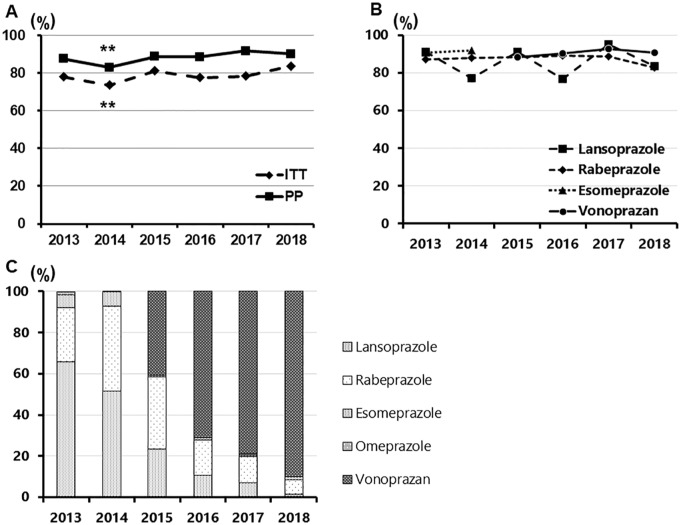
Figure 2.
Annual changes of the eradication rate of second-line therapy and the proportion of PPIs/P-CAB in the second-line regimen.
(a) Annual changes of the eradication rate of second-line therapy in ITT and PP; (b) annual changes of the eradication rate of second-line therapy divided into each PPIs/P-CAB; (c) the proportion of PPIs/P-CAB in the second-line regimen. (a) Once the eradication rate decreased from 2013 to 2014 (p < 0.01 and <0.01, ITT and PP, respectively), the rate generally continued to stabilize (p = 0.65, 0.07, 0.50, 0.17 and p = 0.48, 0.19, 0.46, 1.00, ITT and PP, respectively); (b) the eradication rates of VPZ were constantly over 88% from 2015 to 2018 (88.4%, 89.2%, 92.8 and 90.6% in PP, respectively). As PPI-containing regimens, the eradication rates of LPZ range from 76.7% to 95.0%, and that of RPZ range from 82.6% to 89.2%. Five and less in each item are not described in the graph; (c) VPZ was introduced in 2015, and its proportions dramatically increased toward 2018 in the second-line regimens. In 2018, the proportion of VPZ was 90.2%.
ITT, intention to treat; PP, per protocol; VPZ, vonoprazan; PPI, proton-pump inhibitor; LPZ, lansoprazole; RPZ, rabeprazole.
Annual changes of the eradication rate
The overall first-line eradication rates of PPI, AMPC and CAM triple therapy were 69.4% and 62.7% in PP and ITT, respectively, and those of VPZ, AMPC and CAM triple therapy were 89.1% and 81.4% in PP and ITT, respectively. The eradication rates of the first-line eradication from 2013 to 2018 were 64.6%, 58.7%, 72.5%, 77.3%, 79.9% and 79.8% in ITT and 71.8%, 63.7%, 78.5%, 84.6%, 89.7 and 90.1% in PP [Figure 1(a)]. After the eradication rate decreased from 2013 to 2014 (p = 0.02 and p < 0.01 for ITT and PP, respectively), the rate showed backward increase from 2015 to 2018 in the first-line eradication [p < 0.01, 0.01, 0.01, 0.01 and p < 0.01, 0.01, 0.01, 0.01 for ITT and PP, respectively; Figure 1(a)]. The eradication rates of VPZ were constantly over 87% from 2015 to 2018 [87.2%, 87.5%, 90.8 and 90.6% in PP, respectively; Figure 1(b)]. As PPI-containing regimens, the eradication rates of LPZ range from 60.0% to 80.0%, and that of RPZ range from 67.1% to 87.5% [Figure 1(b)].
The overall second-line eradication rates of PPIs, AMPC, and MTZ regimen were 86.6% and 77.6% in PP and ITT, respectively, and those of the VPZ, AMPC, and MTZ regimens were 90.1% and 80.0% in PP and ITT, respectively. The rate of the second-line eradication was 80.2%, 73.6%, 81.3%, 76.1%, 78.4% and 83.7% in ITT and 90.0%, 82.6%, 88.8%, 87.5%, 91.8% and 90.1% in PP [Figure 2(a)]. After the eradication rate decreased from 2013 to 2014 (p < 0.01 and p < 0.01 for ITT and PP, respectively), the rate generally continued to stabilize [p = 0.65, 0.07, 0.50, 0.17 and p = 0.48, 0.19, 0.46, 1.00 for ITT and PP, respectively; Figure 2(a)]. The eradication rates of VPZ were constantly over 88% from 2015 to 2018 [88.4%, 89.2%, 92.8 and 90.6% in PP, respectively; Figure 2(b)]. As PPI-containing regimens, the eradication rates of LPZ range from 76.7% to 95.0% and that of RPZ range from 82.6% to 89.2% [Figure 2(b)].
Associated factors for the eradication success in each regimen
Using univariate logistic regression analysis, sex [female, p = 0.03; odds ratio (OR) 0.84; 95% confidence interval (CI) 0.72–0.98], age of 75 years or older (p = 0.04; OR 0.79; 95% CI 0.64–0.99) and choices of PPIs /P-CAB (PPIs, p < 0.01; OR 0.28; 95% CI 0.23–0.33) were associated with the eradication rate in first-line therapy (Table 3). Age of 50 years or older (p = 0.67; OR 0.96; 95% CI 0.80–1.16), and the dose of CAM (CAM 800 mg, p = 0.41; OR 1.07; 95% CI 0.91–1.27) were not associated with successful eradication (Table 3). Age of 75 years or older (p = 0.01; OR 0.77; 95% CI 0.60–0.94) and choice of PPIs/P-CAB (PPIs, p < 0.01; OR 0.28; 95% CI 0.23–0.33) were independently associated with eradication failure in the multivariate logistic regression analysis (Table 3). In the second-line therapy, using univariate logistic regression analysis, age of 75 years or older (p < 0.01; OR 0.55; 95% CI 0.42–0.72) and choice of PPIs/P-CAB (PPIs, p < 0.01; OR 0.71; 95% CI 0.57–0.90) were associated with the eradication rate (Table 3). Sex and age of 50 years or older was not associated with successful eradication (female, p = 0.29; OR 1.12; 95% CI 0.91–1.39 and age of 50 years or older, p = 0.45; OR 0.91; 95% CI 0.71–1.17; Table 3). Age of 75 years or older (p < 0.01; OR 0.55; 95% CI 0.42–0.71) and choice of PPIs/P-CAB (PPIs, p < 0.01; OR, 0.71; 95% CI, 0.56–0.89) were independently associated with eradication failure in the multivariate logistic regression analysis (Table 3).
Table 3.
Factors associated with eradication success in each regimen.
| Eradicated | Not eradicated | Univariate analysis OR (95% CI) |
Multivariate analysis OR (95% CI)$ | |
|---|---|---|---|---|
| First-line eradication | n = 2911 | n = 808 | ||
| Dosage of CAM | ||||
| CAM 800 mg/day | 843 | 246 | 1.07 (0.91–1.27) | |
| CAM 400 mg/day | 2068 | 562 | ||
| Sex | ||||
| Female | 1546 | 393 | 0.84 (0.72–0.98)* | 0.88 (0.75–1.03) |
| Male | 1365 | 415 | ||
| Age | ||||
| ⩾50 years | 2208 | 618 | 0.96 (0.80–1.16) | |
| ⩾75 years | 385 | 130 | 0.79 (0.64–0.99)* | 0.75 (0.60–0.94)* |
| Choice of PPI/P-CAB | ||||
| PPIs | 1418 | 625 | 0.28 (0.23–0.33)** | 0.28 (0.23–0.33)** |
| P-CAB | 1493 | 183 | ||
| Second-line eradication | n = 2809 | n = 389 | ||
| Sex | ||||
| Female | 1417 | 185 | 1.12 (0.91–1.39) | |
| Male | 1392 | 204 | ||
| Age | ||||
| ⩾50 years | 2116 | 300 | 0.91 (0.71–1.17) | |
| ⩾75 years | 370 | 84 | 0.55 (0.42–0.72)** | 0.55 (0.42–0.71)** |
| Choice of PPI/P-CAB | ||||
| PPIs | 1776 | 275 | 0.71 (0.57–0.90)** | 0.71 (0.56–0.89)** |
| P-CAB | 1033 | 114 |
p < 0.05.
p < 0.01.
Multivariate logistic regression analysis was performed, adjusted by marginally associated factors (p < 0.1) in univariate analysis. Sex, age, and choice of PPIs/P-CAB in first-line eradication, and age and choice of PPIs/P-CAB in second-line eradication were included in the analyses.
CAM, clarithromycin; PPI, proton-pump inhibitor; P-CAB, potassium-competitive acid blocker; OR, odds ratio; CI, confidence interval.
Proportions of UBT gray zone in patients with eradication failure
A total of 1171 (761 and 410 in first- and second-line therapy, respectively) patients were diagnosed as eradication failure with UBT. The overall proportions of UBT gray zone in patients with eradication failure were 21.3% (250/1171). A total of 15.2% (118/761) and 32.2% (132/410) in the first- and second-line therapy were UBT gray zone, respectively. Comparing the elderly (aged 75 years or older) and younger populations (aged less than 75 years) in patients, 19.9% (192/964) and 28.0% (58/207) in the younger population and the elderly were UBT gray zone, respectively. Comparing male and female, 25.2% (146/580) and 17.6% (104/591) in male and female were UBT gray zone, respectively. Comparing P-CAB- and PPI-containing regimens, 27.8% (85/306) and 19.1% (165/865) in P-CAB and PPI were UBT gray zone, respectively. Associated factors for UBT gray zone in eradication failure population were analyzed with univariate and multivariate logistic regression analysis (Table 4). Using univariate logistic regression analysis, second-line therapy (p < 0.01; OR 2.59; 95% CI 1.95–3.44), male (p < 0.01; OR 1.58; 95% CI 1.19–2.09), age of 75 years or older (p = 0.01; OR 1.57; 95% CI 1.11–2.20), and choice of P-CAB (p < 0.01; OR 1.63; 95% CI 1.21–2.21) were associated with UBT gray zone, respectively (Table 4). Second-line therapy (p < 0.01; OR 2.49; 95% CI 1.86–3.31), male sex (p < 0.01; OR 1.54; 95% CI 1.13–2.10), age of 75 years or older (p = 0.02; OR 1.54; 95% CI 1.08–2.18), and choice of P-CAB (p < 0.01; OR 1.54; 95% CI 1.13–2.10) were independently associated with eradication failure in the multivariate logistic regression analysis (Table 4).
Table 4.
Associated factors for UBT gray zone in eradication failure population.
| UBT gray zone 2.5 to 4.9 (%) |
UBT high 5.0 < or = (%) |
Univariate analysis OR (95% CI) |
Multivariate analysis OR (95% CI)$ | |
|---|---|---|---|---|
| First- or second-line eradication | n = 250 | n = 921 | ||
| Second-line eradication | 132 | 278 | 2.59 (1.95–3.44)** | 2.49 (1.86–3.31)** |
| First-line eradication | 118 | 643 | ||
| Dosage of CAM | ||||
| CAM 800 mg/day | 74 | 447 | 1.36 (0.90–2.04) | |
| CAM 400 mg/day | 44 | 196 | ||
| Sex | ||||
| Male | 146 | 434 | 1.58 (1.19–2.09)** | 1.54 (1.13–2.10)** |
| Female | 104 | 487 | ||
| Age | ||||
| ⩾75 years | 58 | 149 | 1.57 (1.11–2.20)* | 1.54 (1.08–2.18)* |
| Choice of PPI/P-CAB | ||||
| P-CAB (vonoprazan) | 85 | 221 | 1.63 (1.21–2.21)** | 1.54 (1.13–2.10)** |
| PPIs | 165 | 700 |
p < 0.05.
p < 0.01.
Multivariate logistic regression analysis was performed adjusted by marginally associated factors (P<0.1) in univariate analysis. First- or second-line eradication, sex, age and choices of PPIs/P-CAB were included in the analyses.
UBT, urea breath test; CAM, clarithromycin; PPI, proton-pump inhibitor; P-CAB, potassium-competitive acid blocker.
Discussion
In this study, we showed changes in first- and second-line eradication rate from 2013 to 2018. We previously reported AMPC, CAM, and PPI triple therapy achieving 79.4% of successful eradication of PP in 2001.4 Thereafter, the eradication rate gradually decreased; the rate decreased to 63.7% in 2014 [Figure 1(a)]. An increase in CAM-resistant H. pylori has been a serious problem in Japan. There was an increase, from 6.2% in 1995 to 22.1% in 2000–2001, in the proportion of patients infected with CAM-resistant H. pylori.21 Furthermore, the resistance rate increased yearly; from 18.9% in 2002–2003 to 21.1% in 2003–2004, and 27.7% in 2004–2005.22 Murakami and colleagues reported that the eradication rate of CAM-resistant strains treated with LPZ, AMPC, and CAM triple therapy was 40.0%, and that of CAM-susceptible strains was 97.3%.9 Therefore, the proportion of CAM-resistant strains is one of the most important factors influencing successful eradication with PPIs, AMPC, and CAM triple therapy.
The multivariate logistic regression analysis highlighted that use of PPIs was an independent factor affecting eradication failure in first-line eradication therapy in this study (p < 0.01; Table 3). In our study, the overall eradication rate of PPI, AMPC, and CAM triple therapy was 69.4% in PP, and that of VPZ, AMPC, and CAM triple therapy was 89.1% in PP. Ample studies have demonstrated that efficacy of VPZ, AMPC, and CAM triple therapy was superior to that of PPIs, AMPC, and CAM triple therapy.9,10,23–26 In our study, the proportion of VPZ-containing therapy in first-line therapy started at 45.6% in 2015, sharply increasing to 95.5% in 2018. A rapid increase in the proportion of VPZ-containing therapy should reflect the backward increase of the successful eradication ratio from 2015 to 2018.
Regarding second-line eradication, after the eradication rate decreased from 2013 to 2014, the rate generally continued to stabilize until 2018. The overall eradication rate of the PPI, AMPC, and MTZ regimen was 86.6%, and that of the VPZ, AMPC, and MTZ regimen was 90.1%, in this study. The multivariate logistic regression analysis revealed that choice of VPZ was an independent factor affecting successful eradication in second-line therapy (Table 3). The proportions of VPZ dramatically increased toward 2018 in the second-line regimen [Table 2 and Figure 2(c)]; however, secular changes in the eradication rate of each year remained only in a small range [Figure 2(a)]. MTZ resistance in Japan was reported as lower, and no change has been observed in the trend.21,22,27 The eradication rates of the PPI, AMPC, and MTZ regimen were reported to be high at 84–97% in Japan;3,10,27–30 hence, there are no previous reports regarding the superiority of the VPZ, AMPC, and MTZ regimen for second-line eradication.10,31 The present study, to our knowledge, is the first report showing that efficacy of the VPZ, AMPC and MTZ second-line regimen showed a significant difference compared with the PPI, AMPC, and MTZ regimen. Murakami and colleagues reported that the second-line eradication rate for RPZ, AMPC and MTZ in MTZ-sensitive strains was higher than that in MTZ-resistant strains (97% versus 82%).32 Our results suggest that the VPZ, AMPC, and MTZ regimen might be more effective than the PPI, AMPC, and MTZ regimen for MTZ-resistant strains. To confirm this hypothesis, a large-scale prospective study with evaluation of the relationship between MTZ resistance and the VPZ, AMPC, and MTZ regimen is warranted.
Our results showed that VPZ-containing triple regimens were more effective than PPI-containing regimens in first- and second-line treatments, and increasing frequency of use of VPZ, AMPC, and CAM triple therapy has clearly improved the eradication rate in the first-line treatment. However, considering increasing resistance rates to antibiotics worldwide, there is no doubt that it is better to use fewer numbers of antibiotics for the treatment of H. pylori. Furuta and colleagues showed that effectiveness between the dual therapy of AMPC and P-CAB and the VPZ, AMPC and CAM triple therapy was nearly the same with a small sample size.33 In future research, large-scale, prospective randomized trials are warranted to confirm the efficacy of the dual therapy of AMPC and P-CAB.
Age over 75 years was defined as an independent risk factor of eradication failure in both first- and second-line eradication (Table 3). The possibility that the false-positive results in the UBT gray zone may affect the low eradication rate in elderly patients (Table 4). Tokunaga and colleagues showed that the UBT has high sensitivity, specificity, and accuracy for diagnosis of H. pylori infection, with a cut-off value as 2.5%. However, the sensitivity was lower to diagnose the infection status of H. pylori after eradication. Then, they proposed that, to avoid false-positive results of the UBT, the gray zone of the UBT needs to be set at a level of 2.5–5.0%.34 Kwon and colleagues reported that 34% of patients who received eradication treatment and thereafter judged as UBT gray zone had a false-positive result.17 Moreover, they revealed a history of two or more previous H. pylori eradication therapies and moderate-to-severe gastric intestinal metaplasia were associated with a false-positive UBT result.19 Our findings that the proportions of UBT gray zone in the second-line therapy were higher than that in first-line therapy, and that in the elderly population was higher than that in the younger population are consistent with the report of Kwon et al. because the elderly population are likely to have moderate-to-severe gastric intestinal metaplasia.19,35 Urease-positive bacteria, such as Klebsiella pneumoniae and alpha-streptococcus, may increase the UBT level in the stomach with moderate-to-severe gastric intestinal metaplasia.36 From our findings, the UBT should not be used alone in elderly patients, and the other modalities such as HpSA for H. pylori catalase, are recommended for evaluation of successful eradication. To improve accuracy of the UBT, adding citric acid to the UBT may be useful.37,38 Noncompliance to prescribed medication in elderly patients could also affect the eradication failure.39
In contrast, age of 50 years or older was not associated with successful eradication in both first- and second-line eradication therapies in our study. Nishizawa and colleagues showed the eradication rate of the first-line therapy with PPIs, but not VPZ, was significantly lower for people aged below 50 years than those aged 50 years or older.10 They suggested that the cause for low eradication rate in those aged below 50 years was a high resistant rate of CAM in the young generation, especially in those aged below 30 years.10,40 In our study, the proportion of patients aged below 30 years was 1.6%, and among them, those treated with PP-containing therapy were only 0.9% in the first-line therapy. Moreover, the mean age of participants in this study was higher than that in Nishizawa’s report (59.4 ± 13.7 versus 52.3 ± 7.5). Thus, we cannot evaluate the influence of high resistant rate of CAM in the young generation in this study. Meanwhile, it was reported the elderly population had a higher resistance rate to CAM compared with the younger population.22,41,42 Our results are compatible with those in previous reports.
The major limitation of the study is lack of evaluation of antibiotic resistance in H. pylori. Antibiotic resistance in H. pylori is an important factor for eradication failure. Murakami et al. reported that the eradication rate of CAM-resistant strains treated with LPZ, AMPC, and CAM triple therapy was significantly lower than that treated with VPZ, AMPC, and CAM triple therapy (40% versus 82%, p < 0.0001);9 however, there are no reports about the superiority of the VPZ, AMPC, and MTZ triple regimen for MTZ-resistant strains. In order to determine the efficacy of choices of the PPI/P-CAB for H. pylori eradication regimen more precisely, evaluation of antibiotic resistance in H. pylori is mandatory. Second, the atrophic degree of gastritis was not investigated in this study. Third, this study was retrospective, and any adverse events were not available.
In conclusion, the eradication rate showed backward increase from 2015 to 2018 in the first-line therapy because of the introduction of VPZ onto the market and increasing frequency of VPZ use. The eradication rates of first- and second-line regimens in elderly patients were lower than those in younger patients. In the elderly, many post-therapy UBT results may be false positive due to the presence of achlorhydria and overgrowth of urease-containing bacteria other than H. pylori.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: During the last 3 years, HS received scholarship funds for the research from Daiichi-Sankyo Co., EA Pharma Co., Otsuka Pharmaceutical Co. Ltd., and Tsumura Co., and received service honoraria from Astellas Pharma Inc, AstraZeneca KK, Daiichi-Sankyo Co., EA Pharma Co., Otsuka Pharmaceutical Co. Ltd., Mylan EPD Co., Takeda Pharmaceutical Co., Ltd., Tsumura, Co., and Zeria Pharmaceutical Co. Ltd. AN received scholarship funds for the research from AstraZeneca KK, Daiichi-Sankyo Co., Otsuka Pharmaceutical Co. Ltd., Takeda Pharmaceutical Co., Ltd., EA Pharma Co., and received service honoraria from AstraZeneca KK, Daiichi-Sankyo Co., Otsuka Pharmaceutical Co. Ltd., and Takeda Pharmaceutical Co., Ltd. TM received service honoraria from Takeda Pharmaceutical Co., Ltd.
ORCID iDs: Hideki Mori  https://orcid.org/0000-0001-8830-4671
https://orcid.org/0000-0001-8830-4671
Hidekazu Suzuki  https://orcid.org/0000-0002-3855-3140
https://orcid.org/0000-0002-3855-3140
Contributor Information
Hideki Mori, Tokyo Hp Study Group, Tokyo, Japan; Department of Gastroenterology, National Hospital Organization Tokyo Medical Center, Tokyo, Japan.
Hidekazu Suzuki, Department of Gastroenterology and Hepatology, Tokai University School of Medicine, 143 Shimokasuya, Isehara, Kanagawa, 259-1193, Japan; Tokyo Hp Study Group Tokyo, Japan.
Fumio Omata, Tokyo Hp Study Group, Tokyo, Japan; Gastroenterology Division, St. Luke’s International Hospital, Tokyo, Japan.
Tatsuhiro Masaoka, Tokyo Hp Study Group, Tokyo, Japan; Division of Gastroenterology and Hepatology, Department of Internal Medicine, Keio University School of Medicine, Tokyo, Japan.
Daisuke Asaoka, Tokyo Hp Study Group, Tokyo, Japan; Department of Gastroenterology, Juntendo Tokyo Koto Geriatric Medical Center, Tokyo, Japan.
Kohei Kawakami, Tokyo Hp Study Group, Tokyo, Japan; Department of General Medicine and Primary Care, Tokyo Medical University Hospital, Tokyo, Japan.
Shigeaki Mizuno, Tokyo Hp Study Group, Tokyo, Japan; Mizuno Icho Clinic, Tokyo, Japan.
Naoto Kurihara, Tokyo Hp Study Group, Tokyo, Japan; Department of Surgery, Nerima General Hospital, Tokyo, Japan.
Akihito Nagahara, Tokyo Hp Study Group, Tokyo, Japan; Department of Gastroenterology, Juntendo University School of Medicine, Tokyo, Japan.
Nobuhiro Sakaki, Tokyo Hp Study Group, Tokyo, Japan; Foundation for Detection of Early Gastric Carcinoma, Tokyo, Japan.
Masayoshi Ito, Tokyo Hp Study Group, Tokyo, Japan; Department of Gastroenterology, Yotsuya Medical Cube, Tokyo, Japan.
Yo Kawamura, Tokyo Hp Study Group, Tokyo, Japan; Tokyo Daiya Clinic, Tokyo, Japan.
Masayuki Suzuki, Tokyo Hp Study Group, Tokyo, Japan; Department of Gastroenterology, National Hospital Organization Tokyo Medical Center, Tokyo, Japan.
Yuji Shimada, Tokyo Hp Study Group, Tokyo, Japan; Department of Gastroenterology, Juntendo Shizuoka Hospital, Shizuoka, Japan.
Hitoshi Sasaki, Tokyo Hp Study Group, Tokyo, Japan; Department of Gastroenterology, Juntendo Tokyo Koto Geriatric Medical Center, Tokyo, Japan.
Takeshi Matsuhisa, Tokyo Hp Study Group, Tokyo, Japan; Department of Gastroenterology, Tama-Nagayama University Hospital, Tokyo, Japan.
Akira Torii, Tokyo Hp Study Group, Tokyo, Japan; Torii Medical Clinic, Tokyo, Japan.
Toshihiro Nishizawa, Tokyo Hp Study Group, Tokyo, Japan; Digestive Disease Center, International University of Health and Welfare, Mita Hospital, Tokyo, Japan.
Tetsuya Mine, Tokyo Hp Study Group, Tokyo, Japan; Department of Gastroenterology and Hepatology, Tokai University, School of Medicine, Isehara, Japan.
Toshifumi Ohkusa, Tokyo Hp Study Group, Tokyo, Japan; Department of Gastroenterology and Hepatology, The Jikei University School of Medicine Kashiwa Hospital, Chiba, Japan.
Takashi Kawai, Tokyo Hp Study Group, Tokyo, Japan; Endoscopy Center, Tokyo Medical University Hospital, Tokyo, Japan.
Kengo Tokunaga, Tokyo Hp Study Group, Tokyo, Japan; Department of General Medicine, Kyorin University School of Medicine, Tokyo, Japan.
Shin’ichi Takahashi, Tokyo Hp Study Group, Tokyo, Japan; Department of Gastroenterology, Kosei Hospital, Tokyo, Japan.
References
- 1. Soll AH. Consensus conference. Medical treatment of peptic ulcer disease. Practice guidelines. Practice Parameters Committee of the American College of Gastroenterology JAMA 1996; 275: 622–629. [DOI] [PubMed] [Google Scholar]
- 2. Wotherspoon AC, Ortiz-Hidalgo C, Falzon MR, et al. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet 1991; 338: 1175–1176. [DOI] [PubMed] [Google Scholar]
- 3. Asaoka D, Nagahara A, Matsuhisa T, et al. Trends of second-line eradication therapy for Helicobacter pylori in Japan: a multicenter study in the Tokyo metropolitan area. Helicobacter 2013; 18: 468–472. [DOI] [PubMed] [Google Scholar]
- 4. Kawai T, Takahashi S, Suzuki H, et al. Changes in the first line Helicobacter pylori eradication rates using the triple therapy-a multicenter study in the Tokyo metropolitan area (Tokyo Helicobacter pylori study group). J Gastroenterol Hepatol 2014; 29(Suppl. 4): 29–32. [DOI] [PubMed] [Google Scholar]
- 5. Asaka M, Kato M, Takahashi S, et al. Guidelines for the management of Helicobacter pylori infection in Japan: 2009 revised edition. Helicobacter 2010; 15: 1–20. [DOI] [PubMed] [Google Scholar]
- 6. Shiota S, Murakawi K, Suzuki R, et al. Helicobacter pylori infection in Japan. Expert Rev Gastroenterol Hepatol 2013; 7: 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ito M, Takata S, Tatsugami M, et al. Clinical prevention of gastric cancer by Helicobacter pylori eradication therapy: a systematic review. J Gastroenterol 2009; 44: 365–371. [DOI] [PubMed] [Google Scholar]
- 8. Fukase K, Kato M, Kikuchi S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet 2008; 372: 392–397. [DOI] [PubMed] [Google Scholar]
- 9. Murakami K, Sakurai Y, Shiino M, et al. Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy for Helicobacter pylori eradication: a phase III, randomised, double-blind study. Gut 2016; 65: 1439–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nishizawa T, Suzuki H, Fujimoto A, et al. Effects of patient age and choice of antisecretory agent on success of eradication therapy for Helicobacter pylori infection. J Clin Biochem Nutr 2017; 60: 208–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bureau MoIAaCS. Chapter 2 Population. The Statistical Handbook of Japan 2018. Statistics Bureau/Publishing office: Japan Statistical Association. 2018, pp. 7–15. [Google Scholar]
- 12. Kato M. Endoscopic findings of H. pylori infection. In: Suzuki H, Warren R, Marshall B. (eds) Helicobacter pylori. Tokyo: Springer Japan, 2016, pp.157–167. [Google Scholar]
- 13. Nishizawa T, Sakitani K, Suzuki H, et al. A combination of serum anti-Helicobacter pylori antibody titer and Kyoto classification score could provide a more accurate diagnosis of H. pylori. United European Gastroenterol J 2019; 7: 343–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg (London, England) 2014; 12: 1495–1499. [DOI] [PubMed] [Google Scholar]
- 15. Ouchi Y, Rakugi H, Arai H, et al. Redefining the elderly as aged 75 years and older: proposal from the Joint Committee of Japan Gerontological Society and the Japan Geriatrics Society. Geriatr Gerontol Int 2017; 17: 1045–1047. [DOI] [PubMed] [Google Scholar]
- 16. Kato M, Asaka M, Ohara S, et al. Clinical studies of 13C-urea breath test in Japan. J Gastroenterol 1998; 33(Suppl. 10): 36–39. [PubMed] [Google Scholar]
- 17. Vaira D, Malfertheiner P, Megraud F, et al. Diagnosis of Helicobacter pylori infection with a new non-invasive antigen-based assay. HpSA European study group. Lancet 1999; 354: 30–33. [DOI] [PubMed] [Google Scholar]
- 18. Tanaka A, Watanabe K, Tokunaga K, et al. Evaluation of Helicobacter pylori stool antigen test before and after eradication therapy. J Gastroenterol Hepatol 2003; 18: 732–738. [DOI] [PubMed] [Google Scholar]
- 19. Kwon YH, Kim N, Lee JY, et al. The diagnostic validity of citric acid-free, high dose (13)C-urea breath test after Helicobacter pylori eradication in Korea. Helicobacter 2015; 20: 159–168. [DOI] [PubMed] [Google Scholar]
- 20. Leodolter A, Dominguez-Munoz JE, Von Arnim U, et al. Citric acid or orange juice for the 13C-urea breath test: the impact of pH and gastric emptying. Aliment Pharmacol Ther 1999; 13: 1057–1062. [DOI] [PubMed] [Google Scholar]
- 21. Rimbara E, Noguchi N, Tanabe M, et al. Susceptibilities to clarithromycin, amoxycillin and metronidazole of Helicobacter pylori isolates from the antrum and corpus in Tokyo, Japan, 1995–2001. Clin Microbiol Infect 2005; 11: 307–311. [DOI] [PubMed] [Google Scholar]
- 22. Kobayashi I, Murakami K, Kato M, et al. Changing antimicrobial susceptibility epidemiology of Helicobacter pylori strains in Japan between 2002 and 2005. J Clin Microbiol 2007; 45: 4006–4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Suzuki S, Gotoda T, Kusano C, et al. The efficacy and tolerability of a triple therapy containing a potassium-competitive acid blocker compared with a 7-day PPI-based low-dose clarithromycin triple therapy. Am J Gastroenterol 2016; 111: 949–956. [DOI] [PubMed] [Google Scholar]
- 24. Ozaki H, Harada S, Takeuchi T, et al. Vonoprazan, a novel potassium-competitive acid blocker, should be used for the Helicobacter pylori eradication therapy as first choice: a large sample study of vonoprazan in real world compared with our randomized control trial using second-generation proton pump inhibitors for helicobacter pylori eradication therapy. Digestion 2018; 97: 212–218. [DOI] [PubMed] [Google Scholar]
- 25. Takara Y, Endo H, Nakano R, et al. Smoking and drinking did not increase the failure of therapeutic Helicobacter pylori eradication by vonoprazan, clarithromycin, and amoxicillin. Digestion 2019: 99: 172–178. [DOI] [PubMed] [Google Scholar]
- 26. Tanabe H, Ando K, Sato K, et al. Efficacy of vonoprazan-based triple therapy for Helicobacter pylori eradication: a multicenter study and a review of the literature. Dig Dis Sci 2017; 62: 3069–3076. [DOI] [PubMed] [Google Scholar]
- 27. Suzuki H, Mori H. World trends for H. pylori eradication therapy and gastric cancer prevention strategy by H. pylori test-and-treat. J Gastroenterol 2018; 53: 354–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sasaki H, Nagahara A, Hojo M, et al. Ten-year trend of the cumulative Helicobacter pylori eradication rate for the ‘Japanese eradication strategy’. Digestion 2013; 88: 272–278. [DOI] [PubMed] [Google Scholar]
- 29. Suzuki H, Nishizawa T, Hibi T. Helicobacter pylori eradication therapy. Future Microbiol 2010; 5: 639–648. [DOI] [PubMed] [Google Scholar]
- 30. Fukuda S, Shimoyama T, Tanaka M, et al. Duration of the metronidazole-containing regimen for eradication of Helicobacter pylori infection in northern Japan. Jpn J Infect Dis 2006; 59: 367–369. [PubMed] [Google Scholar]
- 31. Sakurai K, Suda H, Ido Y, et al. Comparative study: vonoprazan and proton pump inhibitors in Helicobacter pylori eradication therapy. World J Gastroenterol 2017; 23: 668–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Murakami K, Sato R, Okimoto T, et al. Efficacy of triple therapy comprising rabeprazole, amoxicillin and metronidazole for second-line Helicobacter pylori eradication in Japan, and the influence of metronidazole resistance. Aliment Pharmacol Ther 2003; 17: 119–123. [DOI] [PubMed] [Google Scholar]
- 33. Furuta T, Yamade M, Uotani T, et al. Vonoprazan-based dual therapy with amoxicillin is as effective as the triple therapy for eradication of H. pylori. Am J Gastroenterol 2017; 112: S672. [Google Scholar]
- 34. Tokunaga K, Watanabe K, Tanaka A, et al. [Evaluation of 13C-urea breath test to confirm eradication of Helicobacter pylori]. Nihon Shokakibyo Gakkai Zasshi 2005; 102: 176–182. [PubMed] [Google Scholar]
- 35. Yamamichi N, Hirano C, Shimamoto T, et al. Associated factors of atrophic gastritis diagnosed by double-contrast upper gastrointestinal barium X-ray radiography: a cross-sectional study analyzing 6,901 healthy subjects in Japan. PloS One 2014; 9: e111359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Furuta T, Baba S, Yamade M, et al. High incidence of autoimmune gastritis in patients misdiagnosed with two or more failures of H. pylorieradication. Aliment Pharmacol Ther 2018; 48: 370–377. [DOI] [PubMed] [Google Scholar]
- 37. Leodolter A, Dominguez-Munoz JE, Von Arnim U, et al. Citric acid or orange juice for the 13C-urea breath test: the impact of pH and gastric emptying. Aliment Pharmacol Ther 1999; 13: 1057–1062. [DOI] [PubMed] [Google Scholar]
- 38. Gisbert JP, Pajares JM. Review article: 13C-urea breath test in the diagnosis of Helicobacter pylori infection – a critical review. Aliment Pharmacol Ther 2004; 20: 1001–1017. [DOI] [PubMed] [Google Scholar]
- 39. Salzman C. Medication compliance in the elderly. J Clin Psychiatry 1995; 56(Suppl. 1): 18–22; discussion 23. [PubMed] [Google Scholar]
- 40. Okamura T, Suga T, Nagaya T, et al. Antimicrobial resistance and characteristics of eradication therapy of Helicobacter pylori in Japan: a multi-generational comparison. Helicobacter 2014; 19: 214–220. [DOI] [PubMed] [Google Scholar]
- 41. Horiki N, Omata F, Uemura M, et al. Annual change of primary resistance to clarithromycin among Helicobacter pylori isolates from 1996 through 2008 in Japan. Helicobacter 2009; 14: 86–90. [DOI] [PubMed] [Google Scholar]
- 42. Pilotto A, Di Mario F, Franceschi M. Treatment of Helicobacter pylori infection in elderly subjects. Age Ageing 2000; 29: 103–109. [DOI] [PubMed] [Google Scholar]