Abstract
Background
Vaccination is a cost-effective strategy for reducing morbidity and mortality among children under 5 years old. To be fully protected from diseases such as tuberculosis, diphtheria, pertussis, and polio, children must receive all recommended vaccinations in a timely manner. In many countries, including Tanzania, high overall vaccination rates mask substantial regional variation in vaccination coverage and low rates of vaccination timeliness. This study evaluates the efficacy of mobile phone-based (mHealth) reminders and incentives for improving vaccination timeliness in the first year of life.
Methods
The study, conducted in Mtwara Region in Tanzania, includes 400 late-stage pregnant women enrolled from rural and urban health facilities and surrounding communities. The primary outcome is timeliness of vaccinations among their children at 6, 10, and 14 weeks after birth. Timeliness is defined as vaccination receipt within 28 days after the vaccination due date. The quasi-randomized controlled trial includes three arms: (1) standard of care (no reminders or incentives), (2) mobile phone-based reminders, and (3) mobile phone-based reminders and incentives in the form of conditional financial transfers. Assignment into study arms is based on scheduled vaccination dates. Reminder messages are sent to arms 2 and 3 participants via mobile phones 1 week and 1 day prior to each scheduled vaccination. For arm 3 participants, reminder messages offer an incentive that is provided in the form of a mobile phone airtime recharge voucher code for each timely vaccination. Vaccination dates are recorded via participant contact with an mHealth system, phone calls with mothers, and a review of government-issued vaccination cards during an end-line survey. Random effects logistic regression models will be used to estimate the effects of reminders and incentives on the timeliness of vaccinations.
Discussion
The results will inform implementation science research on the effectiveness of reminders and incentives as a means of improving vaccination timeliness.
Trial registration
ClinicalTrials.gov, NCT03252288. Registered on 17 August 2017 (retrospectively registered).
Electronic supplementary material
The online version of this article (10.1186/s13063-019-3430-4) contains supplementary material, which is available to authorized users.
Keywords: Vaccinations, Vaccination timeliness, Child health, Mobile phones, Targeted client communication, Transmit targeted alerts and reminders to clients, Short message service (SMS), Conditional financial transfers, Client financial transactions, Transmit or manage incentives to clients for health services, Tanzania, Sub-Saharan Africa
Background
Globally, childhood vaccinations are estimated to prevent 2.5 million deaths annually [1]. Yet, 19.7 million children under the age of 1 year have missed receiving basic vaccinations [1]. The majority of un- or under-vaccinated children reside in low- and middle-income countries (LMICs), with those from rural, remote, and poorer households being disproportionately less likely to receive all recommended vaccines in their first year of life [1–3]. In Tanzania, for example, 1 in 4 children has not received all recommended vaccines in the first year of life, compared to 1 in 10 globally [4, 5]. Significant regional variation in vaccination coverage has been reported in Tanzania, with several regions failing to meet the 80% coverage target set forth in the Global Vaccine Action Plan [5–7]. Furthermore, numerous reports indicate low timeliness of vaccinations in LMICs, including in Tanzania [8–10]. In a study in rural Tanzania, vaccination delays were reported in 69% of children for the third dose of the diphtheria-pertussis-tetanus (DPT) vaccine and in 46% of children for the measles-containing vaccine (MCV) [10]. In the quest for universal immunization, innovative strategies are needed to improve vaccination coverage for the “last mile” and to reduce vaccination delays that leave children susceptible to unnecessary morbidity and mortality from vaccine-preventable infections [2].
Barriers to timely immunization are multifaceted and include immunization system constraints (e.g., vaccine stock-outs, limited availability of health care providers), poor access to health facilities (e.g., transport cost, opportunity cost), lack of information (e.g., about the vaccination schedule or community mobilizations), and negative parental attitudes and inadequate knowledge (e.g., lack of trust in vaccinators, poor knowledge of vaccination benefits, fear of vaccination side effects) [3, 11, 12]. In recent years, many groups have leveraged the global pervasiveness of mobile phones for alleviating some of these barriers in order to improve vaccination coverage and timeliness in LMICs [13, 14]. Implementations of mobile phone-based (“mHealth”) strategies in the context of vaccinations include birth notification and newborn surveillance for early identification of children eligible for vaccination services [15–17]; development of digital vaccination registries to longitudinally track the vaccination status of children and rapidly identify those who are unvaccinated or under-vaccinated [17–23]; use of text messages (SMS) or simple voice communication to deliver health promotion messages and reminders about upcoming vaccination appointments [17, 24–26]; delivery of incentives for timely vaccinations [27–29]; digital aids to assist vaccinators in counseling parents [23], for work planning and scheduling [17, 18, 23], or for vaccine stock management [30]; and curation of performance indicators at the regional and national levels [31]. Despite these numerous instantiations, the evidence base on the efficacy of mHealth strategies for improving childhood vaccination coverage and timeliness is limited, with the need for rigorous evaluations identified in several systematic reviews [14, 32, 33].
With support from the National Immunization and Vaccines Development (IVD) program of the Tanzanian National Ministry of Health, Community Development, Gender, Elderly and Children, we are evaluating the efficacy of mobile phone-based vaccination reminders and incentives in the form of conditional financial transfers (CFTs) for offsetting demand-side barriers to timely vaccination. Per the recently released World Health Organization (WHO) classification of digital health interventions, the two key interventions in this study correspond to 1.1 Targeted client communication (specifically, 1.1.3 Transmit targeted alerts and reminders to clients) and 1.7 Client financial transactions (specifically, 1.7.3 Transmit or manage incentives to clients for health services) [34]. Our hypothesis is that reminders and incentives will increase timeliness of vaccinations relative to the status quo. The design and protocol of the evaluation are described in the following sections.
Methods/design
Study aim
The aim of the study is to assess the efficacy of (1) mobile phone-based reminders, and (2) mobile phone-based reminders combined with incentives in the form of CFTs, for improving vaccination timeliness among children at 6, 10, and 14 weeks after birth, compared to the standard of care.
Outcome measures
The recommended schedule for vaccinations by age 1 year is presented in Table 1. The primary study outcome is whether or not a child received an on-time vaccination; the outcome is measured for vaccinations scheduled at 6, 10, and 14 weeks after birth. Secondary outcomes include vaccination coverage, in aggregate and for each of the vaccinations due at 6, 10, and 14 weeks after birth. Timeliness is defined as vaccination within 0-28 days after the scheduled vaccination due date. Coverage is defined as the percentage of children vaccinated.
Table 1.
Recommended vaccinations by age 1 year in Tanzania
| Target age | Recommended vaccines |
|---|---|
| At or soon after birth | OPV(0), BCG |
| 6 weeks | OPV(1), Penta(1), PCV(1), Rota(1) |
| 10 weeks | OPV(2), Penta(2), PCV(2), Rota(2) |
| 14 weeks | OPV(3), Penta(3), PCV(3) |
| 9 months | MR |
Abbreviations: OPV oral polio vaccine, BCG Bacillus Calmette-Guerin vaccine; Penta pentavalent vaccine (diphtheria, pertussis, tetanus, Haemophilus influenzae type b, hepatitis B), PCV pneumococcal vaccine, Rota Rotarix (vaccine against rotavirus), MR Measles-rubella vaccine
Study design
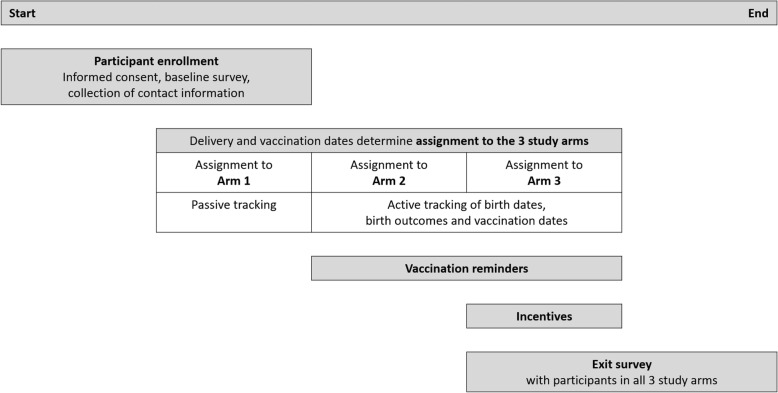
The study design represents a quasi-randomized controlled trial that includes three phases, each corresponding to one study arm: (1) standard of care (no reminders or incentives), (2) mobile phone-based reminders, and (3) mobile phone-based reminders combined with incentives in the form of CFTs. Arm 1 serves as the control arm; arms 2 and 3 are intervention arms. The trial is enfolded by baseline and end-line assessments (Fig. 1).
Fig. 1.
Sequence of events for the quasi-randomized controlled trial
Assignment to study arms and transitions between arms
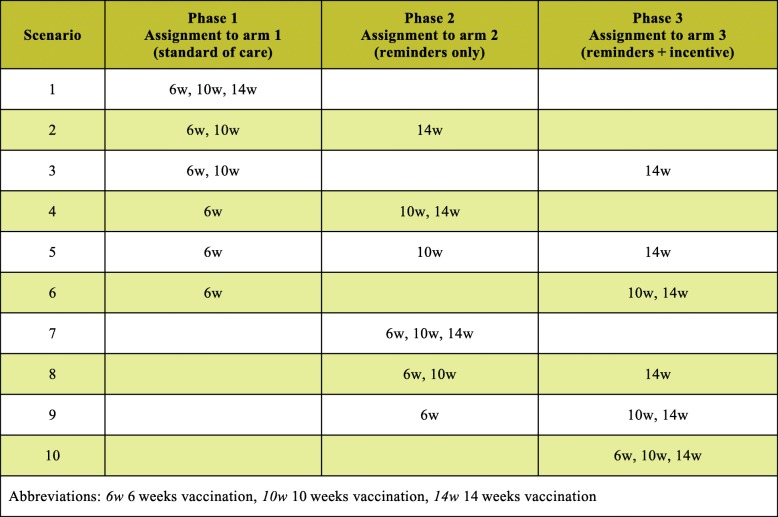
Assignment to study arms is implemented at the level of the vaccination event, with all vaccination events scheduled during a phase assigned to the corresponding arm. T1, the beginning of Phase 1, denotes the time of participants’ enrollment into the control arm. At time T2, the beginning of Phase 2, all participants are rolled over to the arm with reminders; at time T3, the beginning of Phase 3, all participants are rolled over to the arm with reminders combined with incentives. While T2 and T3 denote fixed points in calendar time, variation in delivery dates across participants results in between- and within-individual variation in the assignment of vaccination events across the three arms. Table 2 shows all possible combinations of vaccination event assignments to study arms at the level of the participant. With dates of birth exogenously determining children’s vaccination schedules, shifts in T2 and T3 directly affect the number of vaccination events that may be observed in each study phase. Based on the distributions of expected delivery and vaccination due dates, T2 and T3 are chosen such that the expected number of vaccination events is approximately equal across the three arms. With T2 and T3 being random in relation to mothers’ delivery dates, and thus children’s vaccination schedules, the assignment of vaccination events across arms may be considered quasi-random. Loss to follow-up and other reasons for non-delivery of vaccination reminders result in exposure indistinguishable from the control condition.
Table 2.
Possible combinations of vaccination assignments to study arms at the level of the participant
The assignment to study arms based on scheduled vaccination dates and the simultaneous transition of all participants between study arms address concerns that the individual randomization into, and co-existence of, incentive and non-incentive arms are not considered viable within closely knit communities. While cluster randomization was considered as an alternative approach, the heterogeneity of clusters suggests that this approach would confound the effects of settings with the effects of reminders and incentives.
Study setting
The study is conducted in two adjacent districts, one urban district (Mtwara Municipality) and one rural district (Mtwara District Council), in Mtwara Region in Southeastern Tanzania. In 2017, Mtwara Municipality had an estimated population of 115,132; Mtwara District Council had an estimated population of 130,757 [35]. Owing to logistical considerations, the study area in the rural district is limited to a 30-km radius around the urban district.
Study participants
Eligible study participants are women, ages 16 or older, who are in the third trimester of pregnancy and have access to a mobile phone. The target enrollment is 400 women.
Recruitment
Participants are recruited from at least four urban and at least eight rural health facilities and the surrounding communities. Eligible health facilities regularly provide childhood vaccinations. A combination of purposive and snowball sampling strategies is used for recruitment as follows: eligible women presenting for antenatal care at participating facilities are approached by trained study personnel and offered enrollment in the study. To minimize biases from facility-based enrollment, participating women and local community leaders (balozis) are asked to identify other pregnant women in their community, who, if eligible, are also offered enrollment in the study.
Enrollment and informed consent
All individuals contacted for participation in the study are informed of the study purpose and procedures, as well as the risks and benefits, during the informed consent process by study staff. Women are approached in person and are provided study information orally and in writing using an informed consent script in Kiswahili. Only consenting women are included in the study. Some women may access mobile phones via relatives, friends, or other designees: these persons are considered authorized agents for the purpose of this study. Authorized agents are not considered research subjects and, thus, are not independently consented. By designating authorized agents, participating women are considered to be providing implied consent for these agents to receive information on their behalf.
Since the study includes participants ages 16 years and older, the Institutional Review Board (IRB) granted a waiver of parental consent for pregnant women ages 16 and 17 years old. Tanzanian national policy indicates that adolescents under age 18 who are sexually active are considered “mature minors”; they have the right to access reproductive health services without parental or spousal consent, and they can make associated medical decisions on their own behalf [36]. Given that sexually active adolescents have this “adult” right to medical decision-making, it was determined that pregnant adolescents ages 16 and 17 years are also able to consent to research participation for themselves without parental or spousal consent.
No blinding
The nature of the interventions, mobile phone-based reminders and incentives in the form of CFTs, precludes blinding of study participants.
Intervention components and implementation
For the delivery of the study interventions, we developed an mHealth system comprising several components, as described in the following subsections.
Scheduling system
Upon enrollment, a participant’s name, contact information, and expected delivery date (EDD) are stored in a database, and participants are added to a process queue. For each participant, the process awaits inputs (e.g., date of birth of the child, vaccination dates) and executes tasks according to a prespecified schedule. Tasks include sending reminder SMS messages, tracking SMS delivery, and alerting study staff to call participants when follow-up action is required. When event-related inputs (e.g., dates of completed or rescheduled vaccinations) are received by the system, the database and the schedule of tasks are updated. For example, a delay in one vaccination shifts the remaining schedule by the length of the delay.
Unstructured Supplementary Service Data (USSD) notification system
Participants can notify the scheduling system of events (e.g., birth of child, vaccinations received) using an Unstructured Supplementary Service Data (USSD) dialog. USSD dialogs, which can be accessed free of charge by dialing a short code from any mobile phone, allow for a two-way exchange of data. In this case, the USSD dialog is implemented analogously to a short survey. Participants are guided through text-based menus which prompt them for information related to the event they are reporting. The information is passed to the scheduling system, which updates the schedule of upcoming tasks.
Mobile phone reminder system
Participants in arms 2 and 3 receive reminders for upcoming vaccination appointments 1 week and 1 day before the vaccination due date. No reminders are associated with the EDD or the vaccination due at birth. Reminders are issued in the form of text messages to the mobile phone numbers of study participants or their designees. Prior to study implementation, messages were tested for comprehension using a structured template, and revised based on feedback received. The timing of messages is based on findings from a cross-sectional survey of 134 Tanzanian mothers of children ages 12–23 months. In that survey, 54% of women who indicated any mobile phone use wanted to receive reminders 1 week before the due date, 40% 1 day before the due date, and 6% on the due date. Data on comprehension are collected in the end-line survey to inform future implementations.
Incentive disbursement system
For each timely vaccination for which a reminder with an incentive offer was sent to a mobile phone, a payment in the form of a mobile phone airtime recharge voucher code (worth either TSH 1000 or TSH 2000) is sent to a mobile phone number specified by the participant. The two-tiered incentive amount, which was chosen ad hoc by the study team as appropriate to “nudge” participants toward timely vaccinations without significantly changing their economic incentives [37], is conditional upon the timing of vaccinations (see the following sections).
Technical implementation
The scheduling and sending of SMS messages and incentives is implemented via a custom-built task scheduling, execution, and event tracking application called mParis (“mobile phone assisted reminder and incentive system”). mParis, which was developed for another National Institutes of Health (NIH)-funded study (R01MH106388), is built using Flowable, a flexible, open source, Java-based, workflow engine (https://www.flowable.org) [38]. The system is based at the Kilimanjaro Clinical Research Institute, in Moshi, Tanzania.
Study activities
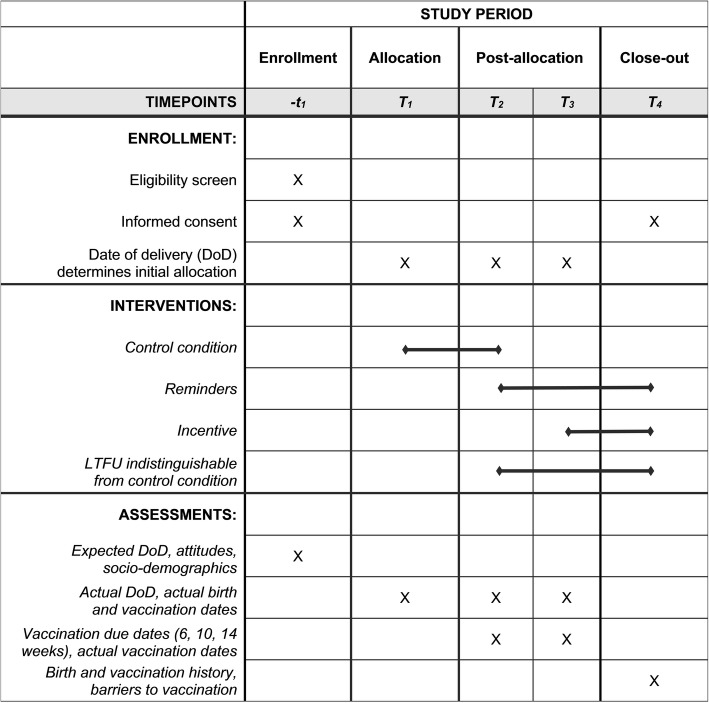
Key events and related study activities are summarized in Figs. 1 and 2 and Table 3.
Fig. 2.
SPIRIT figure depicting schedule of enrollment, interventions, and assessments
Table 3.
Study events and activities
| Arm 1 (standard of care) |
Arm 2 (reminders only) |
Arm 3 (reminders + incentive) |
|
|---|---|---|---|
| Pregnancy | Recruitment and enrollment during the last trimester (EDD within 3 months). In-person baseline survey | ||
| Birth | Phone-based documentation of birth date and birth outcome within 14 days of EDD | ||
|
Vaccination V0 At birth (t 0 ) BCG OPV(0) |
Phone-based confirmation of V0 visit at t0+14d. If not vaccinated, follow up every 14 days Updates to t6w, t10w, and t14w as needed |
||
|
Full amount if V0 visit by t0+14d Half amount if V0 visit between t0+14d and t0+28d None after t0+28d |
|||
|
Vaccination V1 6 weeks (t6w) OPV(1) Penta(1) PCV(1) Rota(1) |
Reminders at t6w-7d and t6w-1d Phone-based confirmation of V1 visit at t6w+14d. If not vaccinated, follow up every 14 days Updates to t10w and t14w as needed |
||
|
Full amount if V1 visit by t6w+14d Half amount if V1 visit between t6w+14d and t6w+28d None after t6w+28d |
|||
|
Vaccination V2 10 weeks (t 10w ) OPV(2) Penta(2) PCV(2) Rota(2) |
Reminders at t10w-7d and t10w-1d Phone based confirmation of V2 visit at t10w+14d. If not vaccinated, follow up every 14 days. Updates to t14w as needed |
||
|
Full amount if V2 visit by t10w+14d Half amount if V2 visit between t10w+14d and t10w+28d None after t10w+28d |
|||
|
Vaccination V3 14 weeks (t14w) OPV(3) Penta(3) PCV(3) |
Reminders at t14w-7d and t14w-1d Phone based confirmation of V3 visit at t14w+14d. If not vaccinated, follow up every 14 days. |
||
|
Full amount if V3 visit by t14w+14 days Half amount if V3 visit between t10w+14d and t10w+28d None after t14w+28d |
|||
| Exit survey | In-person survey to assess barriers to vaccinations, acceptability of reminders (arms 2 and 3) and incentives (arm 3), and document vaccination coverage and timeliness using government-issued vaccination cards | ||
Abbreviations: EDD = expected date of delivery; w = weeks; d = days; t = time; BCG, OPV, Penta, PCV, Rota: see Table 1
Women providing informed consent are enrolled in the study. At the time of enrollment, a baseline survey is conducted with all participants to assess socio-demographic characteristics, mobile phone ownership and use, reproductive history, EDD for the current pregnancy, vaccination knowledge, history of vaccinations in a prior child, if applicable, and previously experienced and/or anticipated barriers to vaccinations. Mobile phone numbers are obtained for mothers and any authorized agents. Surveys are implemented electronically on tablet devices and conducted by study staff at health facilities, participants’ homes, or other mutually agreed-upon locations.
Toward the end of Phase 1, all women are contacted by phone to ascertain birth dates and outcomes, and information on past and scheduled vaccinations. The information is used to generate child-specific vaccination schedules. In Phases 2 and 3, reminder messages are sent to registered mobile phone numbers 1 week and 1 day prior to each scheduled vaccination, and, if no vaccination confirmation is received, 2 weeks after the vaccination due date. Uniquely numbered cards, given to participants at each completed or rescheduled vaccination, allow participants to report vaccination dates via the USSD system. When no vaccination report is received via the USSD system, SMS, or phone calls from participants, study staff contact the participant by phone to verify whether and when a vaccination was received and to ascertain the due date for the next vaccination, if applicable. Recorded vaccination dates are used to iteratively update vaccination schedules and to reschedule reminders. Additional file 1: Table S1 shows the content of SMS reminders.
During Phase 3, reminder messages offer an incentive for each timely vaccination. The full incentive amount (TSH 2000; approximately $0.90) is paid when a vaccination is received within 2 weeks (14 days) of the due date; half the incentive is paid if the vaccination is completed more than 2 weeks but within 4 weeks (28 days) after the vaccination due date. Incentive amounts are paid if the mother, despite going to the health facility as reminded, is asked to return for a vaccination at a later date. Payments for timely vaccinations are made via an SMS message that includes a network-specific mobile phone airtime recharge voucher code and is sent to a phone number specified by the mother. An in-person end-line survey, conducted 12 months after enrollment, assesses barriers to vaccinations and receipt and acceptability of reminders (arms 2 and 3) and incentives (arm 3), and records vaccination dates from participants’ government-issued vaccination cards, allowing for comparisons with the data recorded in the mHealth system.
Data management
Password-protected tablet devices are used to collect survey data from participants. Data transfer agreements (DTAs), signed by all participating institutions, guide the secure sharing of data between Tanzania and foreign collaborators. To ensure confidentiality, identifying information is collected, transferred, and stored separately from survey data. On-site study monitoring, performed by the Principal Investigators or their designees, is used to verify compliance with human subjects and other research regulations and guidelines, assess adherence to the study protocol, and confirm the quality and accuracy of information collected and entered into the study database. All collaborators have access to the final data.
Statistical analysis
For each participant, outcomes are observed at up to three time points (corresponding to timely vaccinations at 6, 10, and 14 weeks, respectively). The probability of timely vaccinations and its association with reminders and incentives will be analyzed using a random effects logistic regression model. The outcome variable is 1 if the vaccination was received on time, and 0 if early, delayed, or not received at all. The primary covariates of interest are indicator variables for the receipt of reminders only (reminder) and the receipt of reminders with an incentive offer (reminder + incentive). For arm 1 the value of both covariates is 0 for all three time points. For outcomes in arm 2, the value for reminder (and for arm 3 the value for reminder + incentive) is 1 if the mHealth system confirmed delivery of the reminder, and 0 for all other observations. Additional covariates (e.g., mother’s education, rural vs. urban residence, recruitment from health facilities vs. the community) will control for differences in participant characteristics between study arms and their association with vaccination timeliness. Interactions of reminder and reminder + incentive with these covariates describe systematic variation in the effects of reminders and incentives on vaccination timeliness. Models will control for the number of phone-based contacts prior to or within the timeliness window for any given vaccination, and standard errors will be adjusted to account for clustering at the level of the health facility catchment area.
Statistical power
Across 400 participants we expect up to 1200 data points on vaccination timeliness. Assuming (1) that these data points are distributed equally across the 3 study arms (standard of care; reminders only; reminders and incentive); (2) a 10% attrition rate; (3) equal cluster sizes of 20 women per health facility catchment area, with an intra-cluster correlation of 0.02; (4) 3 observations per child, with a within-child correlation of 0.2; (5) a baseline rate of vaccination timeliness of 65% in the control condition; and (6) a 10 (15) percentage point difference associated with reminders (reminders and incentives), the power (α = 0.05, two-tailed) to detect such a difference is 72% (98%).
Dissemination
Results will be shared with stakeholders at the regional and national levels, presented at national and international conferences, and published in high-impact academic journals.
Data sharing
Efforts will be made to develop a data repository for the storage of de-identified study data. Investigators wishing to use study data to answer new research questions may submit data analysis concept proposals for consideration by the Principal Investigators. The Principal Investigators will review the proposals and will provide those submitting scientifically rigorous and promising proposals access to the data repository to address their research questions.
Discussion
This study examines the efficacy of mobile phone-based reminders and incentives in the form of conditional financial transfers for offsetting demand-side barriers to timely childhood vaccinations in Tanzania. Our implementation strategy has several unique components, including the use of a USSD-based birth and vaccination event notification system, an adaptive schedule for vaccination reminders which is derived from the date of the preceding event, a tiered incentive system, and the semi-automated disbursement of mobile phone credit for eligible timely vaccinations.
The study is subject to several limitations. First, feasibility considerations limit the study area to include only health facilities and their catchment areas within approximately 30 km of Mtwara Municipality. More remote populations may face additional barriers that cannot be evaluated in this study. Second, only women with access to mobile phones are included in the study. To minimize selection bias, women could designate anyone with a mobile phone to be their study contact. However, for women relying on such authorized agents, receipt of messages and payments for timely vaccinations could remain a challenge. Third, reminder schedules and payments for timely vaccinations are based on client-reported birth and vaccination dates. The dependence on client reports may introduce delays, affect schedule accuracy, and motivate inaccurate reporting of vaccination dates. Comprehensive quality control, including automated alerts to study staff for participant follow-up, algorithms that identify inconsistencies in near real time, and an in-person end-line survey with a review of government-issued vaccination cards, will be used to assess and/or mitigate these concerns. Fourth, it is plausible that follow-up phone calls by study staff to ascertain birth outcomes, the timing of past vaccinations, and/or the timing of the next scheduled vaccination, may themselves function as an intervention. Fifth, temporal changes that coincide with the points of transition between study arms could influence results. Finally, while the primary focus of the study is on demand-side barriers to timely vaccinations, participating health facilities may encounter vaccine stock-outs and power outages, resulting in vaccination activities being deferred until vaccine stocks are received or power is restored. We plan to monitor these events along with mHealth system and mobile network metrics (e.g., message transmission errors, calls dropped) during the study period. By characterizing, and if possible, addressing the limitations of our study, we will provide critical information for the feasibility of scaling up reminders and/or incentives to the regional or national levels in low-resource settings such as Tanzania.
With the continuing diffusion of mobile phones and the ongoing development of mHealth technologies, adaptive programming in the form of dynamic reminder systems and variable incentive structures has become a feasible reality even in low-resource settings. Our study sheds light on the demand-side barriers to vaccinations and the synergistic potential of combining mHealth reminders and incentives for improving vaccination coverage and timeliness. The results have implications for the design and potential efficacy of other subsidy or incentive programs aimed at improving health and health-seeking behaviors.
Trial status
The protocol was approved by the Institutional Review Boards at Duke University, the University of South Carolina, and the National Institute for Medical Research in Tanzania and registered in ClinicalTrials.gov (Protocol NCT03252288, September 28, 2017) on August 17, 2017 (retrospectively registered).
Recruitment was ongoing at the time of manuscript submission. At the time of manuscript revision, end-line surveys were completed for 325 of 412 participants enrolled in the study.
Additional files
Table S1. Content of SMS reminders. (PDF 65 kb)
Table S2. WHO Trial Registration Data Set. (PDF 66 kb)
Table S3. SPIRIT 2013 checklist: recommended items to address in a clinical trial protocol and related documents. (PDF 92 kb)
Acknowledgements
The authors thank the women who participated in this study. The authors acknowledge Marco van Zwetselaar (integrated technology (IT) specialist, Kilimanjaro Clinical Research Institute, Tanzania) and Ayubu Masasi (IT specialist, Muhimbili Research Centre, National Institute for Medical Research, Tanzania) for the development of the mHealth system and inputs on algorithms and their implementation. The authors are grateful to Sara Moses (Project manager, Muhimbili Research Centre, National Institute for Medical Research, Tanzania) and the study research assistants for input on study recruitment and implementation procedures. The authors would like to thank John Gallis (Duke Global Health Institute (DGHI) Research Design & Analysis Core) for his help with the power and sample size calculations. Finally, the authors acknowledge Dr. Dafrossa Lyimo (Program manager, Integrated Vaccine Disease unit, Tanzanian National Immunization and Vaccine Development (IVD) Program), Dr. Wedson Sichawle (Regional Medical Officer, Mtwara), members of the Mtwara regional and district administrations, and the health providers in Mtwara Municipality and Mtwara District Council for their feedback on study procedures, advice for selection of health facilities, and support of study implementation.
Standards of reporting
This manuscript was prepared in accordance with the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) statement. The completed checklist is provided as Additional file 3, and the SPIRIT schedule for the trial is shown in Fig. 2.
Abbreviations
- BCG
Bacillus Calmette-Guerin
- CFT
Conditional financial transfer
- DPT
Diphtheria-pertussis-tetanus
- EDD
Expected delivery date
- IRB
Institutional Review Board
- IVD
Immunizations and Vaccine Development (program)
- km
Kilometer
- LMIC
Low- and middle-income country
- MCV
Measles-containing vaccine
- mHealth
Mobile health
- MR
Measles-rubella
- OPV(x)
Oral polio vaccine; x indicates the first, second, or third dose in vaccine series. Zero indicates dose due at birth
- PCV(x)
Pneumococcal vaccine; x indicates the first, second, or third dose in the vaccine series
- Penta(x)
Pentavalent vaccine (DPT-Haemophilus influenzae B-Hepatitis B); x indicates the first, second, or third dose in the vaccine series
- Rota(x)
Vaccine for rotavirus infection; x indicates the first or second dose in the vaccine series
- SMS
Short message service (text messages)
- TSH
Tanzania Shilling
- USSD
Unstructured Supplementary Service Data
Authors’ contributions
JO and LV conceptualized the study. All authors contributed to the development of the study protocol. JO and LV contributed equally to the development of this manuscript, wrote the first draft of the manuscript, and led subsequent revisions. JNB, EN, and SGM read the manuscript and provided critical input. All authors read and approved the final manuscript and are accountable for all aspects of the accuracy and integrity of the manuscript in accordance with International Committee of Medical Journal Editors (ICMJE) criteria.
Funding
This study is supported by grants from the Fogarty International Center of the National Institutes of Health under Award Number R21TW010262 to Duke University, and the Maternal, Adolescent and Child Health (MACH) working group of the DGHI. LV is funded by the National Center for Advancing Translational Sciences of the NIH under Award Number 1KL2TR002554. mParis was developed and is being maintained using funding from the National Institutes of Mental Health of the NIH under Award Number R01MH106388. The funding bodies have no role in the design of the study, the collection, analysis, and interpretation of data, or writing of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
The protocol was registered in ClinicalTrials.gov (Protocol NCT03252288) on August 17, 2017. The protocol was approved by the Institutional Review Boards at Duke University (Protocol 2017-0591) and University of South Carolina (facilitated review, Pro00051213), USA, and the National Institute for Medical Research (NIMR) in Tanzania (NIMR/HQ/R.8a/Vol. IX/2194). Informed consent is obtained from all study participants. Any changes are communicated to the Institutional Review Boards via a protocol amendment. The WHO trial registration data set is listed in Additional file 2: Table S2.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jan Ostermann and Lavanya Vasudevan contributed equally to this work.
Contributor Information
Jan Ostermann, Email: jano@mailbox.sc.edu.
Lavanya Vasudevan, Email: lavanya.vasudevan@duke.edu.
Joy Noel Baumgartner, Email: joy.baumgartner@duke.edu.
Esther Ngadaya, Email: engadaya@yahoo.com.
Sayoki Godfrey Mfinanga, Email: gsmfinanga@yahoo.com.
References
- 1.World Health Organization. Immunization coverage. September 2016. http://www.who.int/mediacentre/factsheets/fs378/en/. Accessed 18 June 2019.
- 2.World Health Organization . Global Vaccine Action Plan 2011–2020. Geneva: World Health Organization; 2013. [Google Scholar]
- 3.Global Immunization Division, Centers of Disease Control and Prevention, and World Health Organization . Epidemiology of the unimmunized child: findings from the peer-reviewed published literature, 1999-2009. 2009. [Google Scholar]
- 4.World Health Organization. Immunization, vaccines and biologicals. 2017. http://www.who.int/immunization/en/. Accessed 18 June 2019.
- 5.The DHS Program. Demographic and Health Survey, and Malaria Indicator Survey, Tanzania. 2015-16. https://dhsprogram.com/pubs/pdf/FR321/FR321.pdf. Accessed 18 June 2019.
- 6.Kruger C, et al. Immunisation coverage and its associations in rural Tanzanian infants. Rural Remote Health. 2013;13(4):2457. [PubMed] [Google Scholar]
- 7.Semali IA. Trends in immunization completion and disparities in the context of health reforms: the case study of Tanzania. BMC Health Serv Res. 2010;10:299. doi: 10.1186/1472-6963-10-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akmatov MK, Mikolajczyk RT. Timeliness of childhood vaccinations in 31 low and middle-income countries. J Epidemiol Community Health. 2012;66(7):e14. doi: 10.1136/jech.2010.124651. [DOI] [PubMed] [Google Scholar]
- 9.Clark A, Sanderson C. Timing of children's vaccinations in 45 low-income and middle-income countries: an analysis of survey data. Lancet. 2009;373(9674):1543–1549. doi: 10.1016/S0140-6736(09)60317-2. [DOI] [PubMed] [Google Scholar]
- 10.Le Polain de Waroux O, et al. Timeliness and completeness of vaccination and risk factors for low and late vaccine uptake in young children living in rural southern Tanzania. Int Health. 2013;5(2):139–147. doi: 10.1093/inthealth/iht006. [DOI] [PubMed] [Google Scholar]
- 11.Sridhar S, et al. A systematic literature review of missed opportunities for immunization in low- and middle-income countries. Vaccine. 2014;32(51):6870–6879. doi: 10.1016/j.vaccine.2014.10.063. [DOI] [PubMed] [Google Scholar]
- 12.Cobos Munoz D, Monzon Llamas L, Bosch-Capblanch X. Exposing concerns about vaccination in low- and middle-income countries: a systematic review. Int J Public Health. 2015;60(7):767–780. doi: 10.1007/s00038-015-0715-6. [DOI] [PubMed] [Google Scholar]
- 13.International Telecommunications Union . ICT Facts and Figures. Evanston: Benton Foundation; 2016. [Google Scholar]
- 14.Oliver-Williams C, et al. Using mobile phones to improve vaccination uptake in 21 low- and middle-income countries: systematic review. JMIR Mhealth Uhealth. 2017;5(10):e148. doi: 10.2196/mhealth.7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xeuatvongsa A, et al. Improving hepatitis B birth dose in rural Lao People's Democratic Republic through the use of mobile phones to facilitate communication. Vaccine. 2016;34(47):5777–5784. doi: 10.1016/j.vaccine.2016.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Government of India, Health and Family Welfare Department, and National Rural Health Mission, Mother and Child Tracking System. New Delhi: Government of India; 2016.
- 17.Uddin MJ, et al. Use of mobile phones for improving vaccination coverage among children living in rural hard-to-reach areas and urban streets of Bangladesh. Vaccine. 2016;34(2):276–283. doi: 10.1016/j.vaccine.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.THRIVE consortium. Open Smart Register Platform (OpenSRP). 2017. http://smartregister.org/index.html. Accessed 18 June 2019.
- 19.Nguyen NT, et al. Digital immunization registry: evidence for the impact of mHealth on enhancing the immunization system and improving immunization coverage for children under one year old in Vietnam. Mhealth. 2017;3:26. doi: 10.21037/mhealth.2017.06.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.PATH. Better Immunization Data (BID) Initiative. 2017. https://bidinitiative.org/. Accessed 18 June 2019.
- 21.Jandee K, et al. Effectiveness of using mobile phone image capture for collecting secondary data: a case study on immunization history data among children in remote areas of Thailand. JMIR Mhealth Uhealth. 2015;3(3):e75. doi: 10.2196/mhealth.4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaewkungwal J, et al. Application of mobile technology for improving expanded program on immunization among highland minority and stateless populations in northern Thailand border. JMIR Mhealth Uhealth. 2015;3(1):e4. doi: 10.2196/mhealth.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen L, et al. Effectiveness of a smart phone app on improving immunization of children in rural Sichuan Province, China: study protocol for a paired cluster randomized controlled trial. BMC Public Health. 2014;14:262. doi: 10.1186/1471-2458-14-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rand CM, et al. Effects of phone and text message reminders on completion of the human papillomavirus vaccine series. J Adolesc Health. 2017;60(1):113–119. doi: 10.1016/j.jadohealth.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 25.Department of Health and Republic of South Africa. MomConnect. 2014. http://www.health.gov.za/index.php/mom-connect. Accessed 18 June 2019.
- 26.Health Compass. Wazazi Nipendeni — Love Me, Parents. June 16, 2016. https://www.thecompassforsbc.org/sbcc-spotlights/wazazi-nipendeni-love-me-parents. Accessed 18 June 2019.
- 27.Gibson DG, et al. Mobile phone-delivered reminders and incentives to improve childhood immunisation coverage and timeliness in Kenya (M-SIMU): a cluster randomised controlled trial. Lancet Glob Health. 2017;5(4):e428–e438. doi: 10.1016/S2214-109X(17)30072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wakadha H, et al. The feasibility of using mobile-phone based SMS reminders and conditional cash transfers to improve timely immunization in rural Kenya. Vaccine. 2013;31(6):987–993. doi: 10.1016/j.vaccine.2012.11.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chandir S, et al. Evaluation of small amount mobile conditional cash transfers (mCCTs) to improve immunization coverage and timeliness. IPROC. 2017;3(1):e47. doi: 10.2196/iproc.8560. [DOI] [Google Scholar]
- 30.VaxTrac. What is VaxTrac. https://www.ehealthafrica.org/vaxtrac. Accessed 18 June 2019.
- 31.World Health Organization . Assisting community health workers in Rwanda: MOH's RapidSMS and mUbuzima. Geneva: World Health Organization; 2013. [Google Scholar]
- 32.Watterson JL, Walsh J, Madeka I. Using mHealth to improve usage of antenatal care, postnatal care, and immunization: a systematic review of the literature. Biomed Res Int. 2015;2015:153402. doi: 10.1155/2015/153402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee SH, et al. Effectiveness of mHealth interventions for maternal, newborn and child health in low- and middle-income countries: systematic review and meta-analysis. J Glob Health. 2016;6(1):010401. doi: 10.7189/jogh.06.010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization. Classification of digital health interventions v1.0: a shared language to describe the uses of digital technology for health. 2018. https://www.who.int/reproductivehealth/publications/mhealth/classification-digital-health-interventions/en/. Accessed 18 June 2019.
- 35.Tanzania National Bureau of Statistics. Tanzania Total Population by District - Regions -2016-2017. 2018. https://www.nbs.go.tz/index.php/en/census-surveys/population-and-housing-census/178-tanzania-total-population-by-district-regions-2016-2017. Accessed 18 June 2019.
- 36.Fox K, et al. Adolescent consent to HIV testing: a review of current policies and issues in sub-Saharan Africa. In: HIV and adolescents: guidance for HIV testing and counselling and care for adolescents living with HIV. Geneva: World Health Organization; 2013. [Google Scholar]
- 37.Thaler RH, Sunstein CR. Nudge: improving decisions about health, wealth, and happiness. New Haven: Yale University Press; 2008. [Google Scholar]
- 38.Ostermann J. Mobile Phone Assisted Reminder and Incentive System (mParis): Combining mHealth reminders and conditional cash transfers to improve the timeliness of vaccinations in Tanzania, in National Institutes of Health mHealth Technology Showcase. Bethesda: Natcher Auditorium and Atrium, National Institutes of Health Campus; 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Content of SMS reminders. (PDF 65 kb)
Table S2. WHO Trial Registration Data Set. (PDF 66 kb)
Table S3. SPIRIT 2013 checklist: recommended items to address in a clinical trial protocol and related documents. (PDF 92 kb)
Data Availability Statement
Not applicable.