Abstract
Background
Although there have been several studies on concordance of different assays testing programmed cell death ligand‐1 (PD‐L1) expression using surgical specimens, studies using real‐world biopsy specimens are scarce. However, many of the non‐small cell lung cancer (NSCLC) cases requiring immunotherapy and thus PD‐L1 testing are unresectable having to rely on small biopsy results. Therefore, we sought to assess the concordance of two diagnostic assays (22C3 and SP263) in evaluating PD‐L1 expression using specimens from CT‐guided transthoracic needle biopsy (TNB) specimens in a routine clinical setting.
Methods
A total of 202 NSCLC cases that underwent CT‐guided TNB from April 2017 to February 2018 were retrospectively reviewed. Biopsy specimens tested with both 22C3 and SP263 assays were included. Concordance of PD‐L1 expression levels determined by two assays was assessed using intraclass correlation coefficient, and the agreement of dichotomized values at various cutoffs (1%, 25%, and 50%) were assessed using Cohen's κ coefficient of agreement.
Results
A total of 80 patients (M:F = 47:33, mean age: 68.0 years) were included in the study. Concordance of PD‐L1 expression levels was high (intraclass coefficient: 0.892) between 22C3 and SP263 assays. Agreements at cutoff levels of 1%, 25%, and 50% were also good, with κ values of 0.878, 0.698, and 0.790, respectively. Positive percent agreement was 93.2%, 100.0%, and 95.2% for agreements at 1%, 25%, and 50%.
Conclusion
There is a high concordance of PD‐L1 expression evaluated with 22C3 and SP263 assays using CT‐guided TNB specimens.
Keywords: Image‐guided biopsy, immunohistochemistry, non‐small cell lung cancer, PD‐L1 protein
Introduction
With the advent of immunotherapies and the immune checkpoint inhibitors targeting programmed death 1/programmed death ligand 1 (PD‐L1) in the treatment of advanced non‐small cell lung cancer (NSCLC),1 PD‐L1 testing has also become an indispensable part of the evaluation of lung cancer patients. Pembrolizumab requires PD‐L1 testing to determine patient eligibility, and PD‐L1 testing is not necessary for other drugs, such as Nivolumab, Durvalumab, or Atezolizumab.2, 3 However, many clinical trials on these drugs have consistently demonstrated correlation between the drug response and the PD‐L1 expression levels, as measured by PD‐L1 immunohistochemistry (IHC), and PD‐L1 IHC has also been established as a complementary diagnostic for Nivolumab and Atezolizumab to determine NSCLC patient eligibility, respectively.3
There are several different PD‐L1 assays currently at use, from different companies and independently developed for different drugs. For practical purposes and to reduce confusion, there have been many studies on comparability and interchangeability of these assays.4, 5, 6, 7, 8, 9, 10, 11 Most of these studies use surgical specimens for analysis, citing tumor heterogeneity and discordance of PD‐L1 status between small biopsy samples and surgical specimens12 as the reason. In reality, however, many of the patients requiring immunotherapy have unresectable lung cancers and have to rely on small biopsy specimens for PD‐L1 testing. Many of these small biopsy specimens must be acquired using CT‐guided transthoracic needle biopsy (TNB), as a large portion of diagnosis of advanced lung cancer is made using CT‐guided TNB. However, there are not many studies assessing the comparability of PD‐L1 assays using biopsy samples, and to the best of our knowledge, no study has addressed the biopsy‐related characteristics of CT‐guided biopsy for PD‐L1 testing in a routine clinical setting. Phase 2 of Blueprint study did include biopsy specimens for the comparability analysis of four different assays,7 but the absolute number is very small (20 samples), core needle samples and mixed with bronchial biopsy samples, and lacks detailed information on how those biopsy samples were acquired. There is another study exploring the feasibility of PD‐L1 testing from samples obtained with CT‐guided TNB,13 but biopsies in that study were carried out exclusively for enrollment into the clinical trial (KEYNOTE‐001), which we believe to inadequately represent the biopsy setting in routine clinical practice. Therefore, the purpose of this study is to evaluate the concordance of two different PD‐L1 IHC assays specifically using CT‐guided biopsy samples and assess the CT‐guided‐biopsy‐related characteristics concerning PD‐L1 testing in a routine clinical setting.
Methods
Study population
We retrospectively reviewed 202 consecutive NSCLC patients who underwent CT‐guided TNB at Seoul St. Mary's Hospital from April 2017 to February 2018. As immunotherapy became more incorporated into the routine care of NSCLC patients, the clinicians at our institution started ordering 22C3 and SP263 tests for NSCLC patients. Some patients were tested with only one of either 22C3 or SP263 tests, while others were tested with both 22C3 and SP263 tests. A total of 80 patients whose biopsy specimens tested with both 22C3 and SP263 assays at our institution were included in this study.
CT‐guided TNB
All CT‐guided TNBs were performed using a multidetector CT scanner (Siemens Definition AS Plus, Siemens Healthcare, Erlangen, Germany) with no CT‐fluoroscopy function. All procedures were performed using coaxial technique by two chest radiologists with three years and one year of experience in CT‐guided TNB, respectively. Prior to biopsy, a pre‐procedural CT scan was initially performed to determine the most optimal and safest needle path to the target. Thereafter, an 18‐gauge coaxial introducer was inserted under intermittent CT guidance. After confirming the proper placement of the needle tip within the target, tissue biopsy was conducted with a 20‐gauge cutting needle (Stericut, TSK Laboratory, Tochigi, Japan). The outer cannula of the coaxial needle was not moved, but the direction of the biopsy needle was rotated at each biopsy, whenever feasible, in order to sample different regions of the tumor. After the removal of the coaxial introducer, post‐procedural CT was performed to identify immediate procedure‐related complications, such as pneumothorax or perilesional hemorrhage. An erect chest radiograph was routinely obtained three hours after biopsy to evaluate the presence of pneumothorax.
Data collection
Demographic and clinical features of the study population were collected from the electronic medical records of Seoul St. Mary's Hospital. Patient age, sex, cancer stage, and pathologic diagnosis of biopsy specimens were obtained. Several biopsy‐related features were also obtained by reviewing the procedural CT images and the operators’ reports in the radiology database. Name of the operator, tumor size (longest diameter), tumor location (lobe), presence of emphysema, position of the patient, pleura‐to‐target distance, number of core biopsy specimens obtained, whether it was first or repeat biopsy, and biopsy‐related complications at post‐procedural CT scan were documented.
Pathological examination process
Hematoxylin–eosin (HE) stained sections of formalin‐fixed paraffin‐embedded (FFPE) biopsy specimens were first analyzed for the presence, quantity, quality, and histological type of the tumor tissue. Immunohistochemistry with TTF1 antibody, P40, CK‐5/6, and CK‐7 was performed either when the primary or secondary nature of the tumor was unknown, at the discretion of the pathologist. When NSCLC is suspected, ALK FISH testing and gene mutation analysis by polymerase chain reaction‐based sequencing for EGFR exons 18–21 were performed on unstained FFPE tumor tissue sections. At the time of the study, the updated IASLC guidelines for molecular testing14 had not been published and testing for ROS1 was not routinely done. When PD‐L1 analyses were later requested by clinicians, unstained FFPE tumor tissue sections were cut from the remaining available biopsy specimens for IHC staining.
PD‐L1 analysis
IHC analysis was conducted with the PD‐L1 IHC 22C3 pharmDx and the Ventana PD‐L1 (SP263) assays on the DAKO Autostainer Link 48 and Ventana BenchMark platforms, respectively. Consecutive 4 μm thick sections cut from the same core specimen were pretreated and stained with the PD‐L1 antibody 22C3 mouse monoclonal primary antibody from pharmDx on a Dako Autostainer Link 48 with EnVision DAB Detection System (Agilent/Dako, Santa Clara, CA, USA) with negative control reagents and cell line run controls, as described in the PD‐L1 IHC 22C3 pharmDx, and PD‐L1 antibody SP263 rabbit monoclonal primary antibody from Ventana on a Ventana Benchmark Ultra with OptiView Universal DAB Detection Kit (Ventana Medical Systems, Tucson, AZ, USA) with a matched rabbit immunoglobulin G–negative control, in accordance with the manufacturers’ instructions, respectively. The detection and quantification of the percentage of immunoreactive tumor cells was performed according to the manufacturers’ recommendations. Briefly, neoplastic cells were considered positive when any cell membrane staining (partial or complete) was present, ignoring pure cytoplasmic immunoreaction. Staining on immune cells was also disregarded. Quantification of immunoreactive neoplastic cells was obtained by evaluating the ratio between stained carcinoma cells and all viable carcinoma cells.
Statistical analysis
Concordance of PD‐L1 expression levels determined by 22C3 and SP263 were was assessed using intraclass correlation coefficient, and the agreement of dichotomized values at various cutoffs (1%, 25%, and 50%) were assessed using Cohen's κ coefficient of agreement. Overall percent agreement (OPA), positive percent agreement (PPA), and negative percent agreement (NPA) were also calculated. All statistical analyses were performed using IBM SPSS statistics software, version 24 (IBM Corp., Armonk, NY), and R Studio (version 3.3.2) utilizing the R statistical language version 2.15.
Results
Patient and biopsy‐related characteristics
A total of 80 patients were included in this study, and their clinical and biopsy‐related characteristics are summarized in Table 1. There were 47 male and 33 female patients, with a mean age of 68 years. A majority of the patients had either adenocarcinoma (73.8%) or squamous cell carcinoma (20.0%) as histologic subtypes and either stage III (22.5%) or IV (57.5%) disease. A total of 67 patients had also undergone testing for EGFR mutation status, and there were 16 EGFR‐mutant cases: nine exon 19 deletion, six exon 21 L858R mutation, and one exon 18 p.G719 mutation. A total of 64 patients had undergone ALK FISH testing, and there were three positive cases.
Table 1.
Patient demographics and biopsy‐related factors
| Patient demographics (n = 80) | |
|---|---|
| Agea (years) | 68.0 ± 9.9 |
| Sex | |
| Male | 47 (58.8%) |
| Female | 33 (41.2%) |
| Histology | |
| Adenocarcinoma | 59 (73.8%) |
| Squamous cell carcinoma | 16 (20.0%) |
| Other NSCLC | 5 (6.2%) |
| Stage | |
| I | 8 (10.0%) |
| II | 8 (10.0%) |
| III | 18 (22.5%) |
| IV | 46 (57.5%) |
| Smoking history | |
| Current smoker | 6 (7.5%) |
| Former smoker | 42 (52.5%) |
| Never smoker | 32 (40.0%) |
| EGFR mutation status | |
| Testing not performed | 2 (2.5%) |
| Mutant Exon 19 deletion Exon 21 L858R mutation Exon 18 p.G719 mutation |
16 (20.0%) 9 (11.3%) 6 (7.5%) 1 (1.3%) |
| Wild‐type | 51 (63.8%) |
| ALK FISH | |
| Testing not performed | 16 (20.0%) |
| Positive | 3 (3.8%) |
| Negative | 61 (76.3%) |
| Repeat biopsy | |
| Yes | 9 (11.2%) |
| No | 71 (88.8%) |
| Emphysema | |
| Yes | 10 (12.5%) |
| No | 70 (87.5%) |
| Tumor sizeb (cm) | 4.0 (2.7, 5.8) |
| CT finding of the tumor | |
| Solid | 75 (93.8%) |
| Cavitary | 4 (5.0%) |
| Part‐solid | 1 (1.3%) |
| Pleura‐to‐target distanceb (cm) | 1.5 (0.1, 2.5) |
| Number of coresb | 5 (4, 5) |
| Biopsy location | |
| Upper lobes | 42 (52.5%) |
| Middle and lower lobes | 36 (45.0%) |
| Pleura or mediastinum | 2 (2.5%) |
| Biopsy position | |
| Supine | 41 (51.3%) |
| Prone | 39 (48.7%) |
| Pneumothorax | |
| Yes | 16 (20.0%) |
| No | 64 (80.0%) |
| Hemoptysis | |
| Yes | 3 (3.8%) |
| No | 77 (96.2%) |
Data are means ± standard deviations.
Data are median (with interquartile range in parenthesis).
There were 11 (13.8%) repeat biopsy cases and 69 (86.3%) initial biopsy cases. Median size of the biopsied lesion was 4.0 cm, with median pleura‐to‐target distance of 1.5 cm. Median number of cores obtained was five. A total of 16 patients (20.0%) and three patients (3.8%) had developed pneumothorax and hemoptysis, respectively, after biopsy; all patients with pneumothorax were asymptomatic and none required drainage procedure. No other immediate complications were observed after CT‐guided biopsy.
Comparison of PD‐L1 expression results based on 22C3 and SP263 assays
The PD‐L1 levels detected by the 22C3 and SP263 tests were compared, and the cases were also compared after classifying them according to various cutoff levels. Figure 1 shows a comparison of the TC scores assessed by 22C3 and SP263 for each case. The mean TC scores for 22C3 and SP263 were 32.5 ± 34.7% and 24.1 ± 31.8%, and their agreement was good, with an intraclass correlation coefficient of 0.892 (Table 2). Figure 2 shows a representative case stained with 22C3 and SP263 assays. Agreements at cutoff levels of 1%, 25%, and 50% were also good, with κ values of 0.878, 0.698, and 0.790 respectively. Positive percent agreement was 93.2%, 100.0%, and 95.2% for agreements at 1%, 25%, and 50%. κ values, overall percent agreement, positive percent agreement, and negative percent agreement at various cutoff levels are summarized in Table 3.
Figure 1.
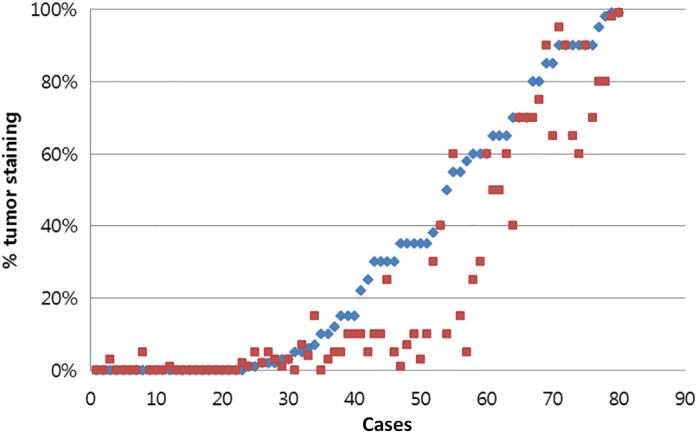
Comparison of tumor cell scores for programmed death ligand 1 assays 22C3 and SP263. ( ) 22C3 and (
) 22C3 and ( ) SP263.
) SP263.
Table 2.
Comparison of PD‐L1 status between 22C3 and SP263 using various cutoffs
| Mean TC% ± SD | TC staining ≥1% (%) | TC staining ≥25% (%) | TC staining ≥50% (%) | |
|---|---|---|---|---|
| 22C3 | 32.5 ± 34.7 | 55 (68.8%) | 39 (48.8%) | 26 (32.5%) |
| SP263 | 24.1 ± 31.8 | 59 (73.8%) | 27 (33.8%) | 21 (26.2%) |
PD‐L1, programmed cell death ligand‐1; SD, standard deviation; TC, tumor cells.
Figure 2.
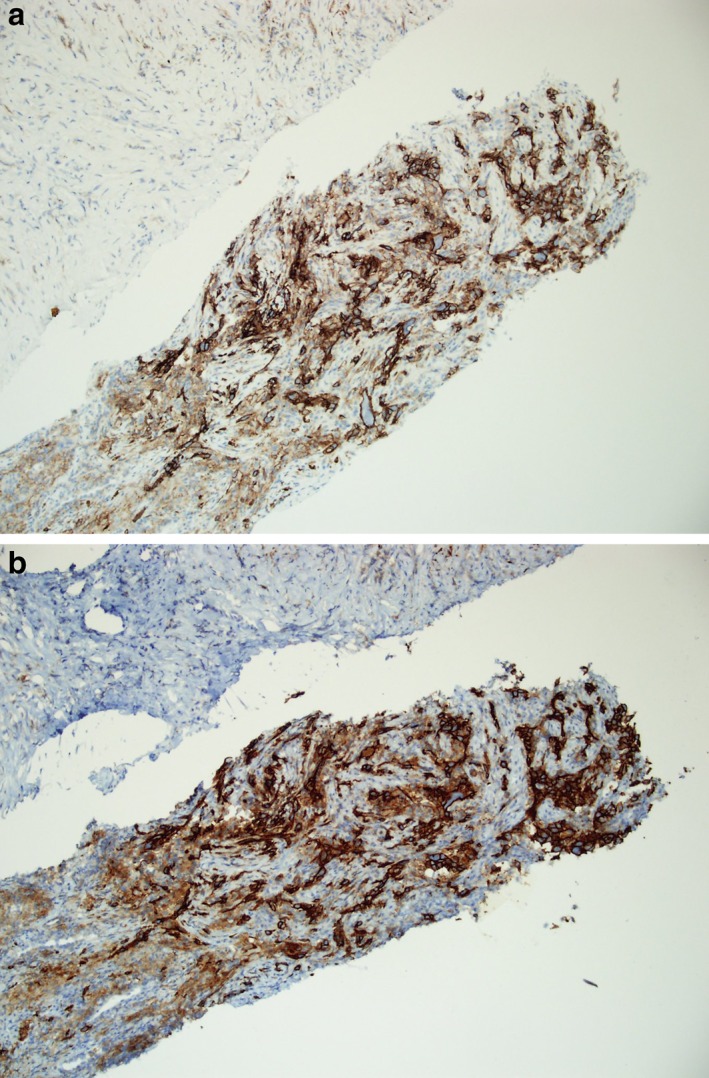
A representative case stained with the programmed death ligand 1 assays (a) 22C3 and (b) SP263 at a magnification of × 100.
Table 3.
Agreement between 22C3 and SP263 at various cutoffs
| Agreement (k) | OPA (%) | PPA (%) | NPA (%) | |
|---|---|---|---|---|
| Agreement at 1% | 0.878 | 95.0 | 93.2 | 100.0 |
| Agreement at 25% | 0.698 | 85.0 | 100.0 | 77.3 |
| Agreement at 50% | 0.790 | 91.3 | 95.2 | 89.8 |
NPA, negative percent agreement; OPA, overall percent agreement; PPA, positive percent agreement.
Discussion
This study has shown that staining of specimens acquired using CT‐guided TNB with 22C3 and SP263 assays in a routine clinical practice show comparable results. This is similar to other study results that have shown high concordance of 22C3 and SP263 assays when assessing PD‐L1 expression on tumor cell membrane.3
There are some studies which question the reliability of small biopsy samples for evaluation of PD‐L1 status; one study has demonstrated significant discordance of PD‐L1 status among small biopsy surrogate samples.15 The small biopsy surrogates were obtained randomly from diverse areas of the tumor for that study. However, whether that could represent practice in the real‐world is questionable; in reality, the site to perform biopsy is limited in order to choose the needle entry site that is accessible percutaneously (i.e., avoiding adjacent structures, such as the heart or pulmonary vessels). Our study has shown that despite concerns about tumor heterogeneity affecting the reliability of small biopsy samples, two different assays testing PD‐L1 status showed comparable results even in small biopsy samples acquired in a routine clinical setting. The phase 2 of the Blueprint project has made this conclusion with a smaller number of biopsy samples (n = 20),7 and our study has demonstrated this in a larger number of samples.
The same study mentioned above has shown that at least four cores are needed to reach an optimal correlation with the whole tumor for PD‐L1 status.15 Another study evaluating the feasibility of CT‐guided biopsy for evaluation of PD‐L1 status has acquired average of eight cores exclusively for the analysis of PD‐L1 status.13 Greater number of cores and lower gauge needles do result in greater amount of tumor tissue,16 and intuitively, having a larger amount of tissue is advantageous for undergoing various molecular studies. However, there is limited amount of time and resource when performing CT‐guided biopsy; in practice, there has to be a certain standard or a limit in the number of cores to be acquired. Acquiring as much tissue as possible in what limited time would allow, without compromising patient safety would be optimal when performing CT‐guided TNBs. Acquiring five cores with a 20‐gauage needle seems to be sufficient for the evaluation of PD‐L1 status even when using two different assays in addition to the diagnosis of NSCLC and evaluation of EGFR and ALK statuses.
The mean TC scores on 22C3 and SP263 in our study are 32.5% ± 34.7 and 24.1% ± 31.8, respectively. These scores are higher than the results from another comparative study by Hendry et al., 4 in which mean TC scores were 9.20% ± 24.5 and 12.05% ± 27.3 for 22C3 and SP263, respectively. The percentages of specimens to be positive with the 22C3 and SP263 tests were 68.8% and 73.8%, respectively, at cutoff of 1% or higher, respectively, 48.8% and 33.8%, respectively, at cutoff of 25% or higher, and 32.5% and 26.2%, respectively, at cutoff of 50% or higher. These results are remarkably higher than some of the studies that demonstrated the frequencies of specimens positive with the 22C3 and SP263 tests to be approximately 20–40% at cutoff of 1% or higher and 4–15% at cutoff of 50% or higher.4, 5, 9 All these studies used resection specimens, which would probably be mostly early stage cancers, for their studies, and this could have caused differences in PD‐L1 status compared to our study, which mainly involved stage 3 or 4 unresectable NSCLC. Another study, which also involved resected specimens, showed similar results with our study: the percentage of specimens positive with 22C3 test was about 65% at cutoff of 1% or higher and 20% at cutoff of 50% or higher.6 This variability even among resected specimens may be explained by the differences in the histology of the study specimens and interobserver variability. The results of the phase 2 of the Blueprint project, which involved a heterogeneous group of real‐world resection, biopsy, and lymph node specimens, were also similar to our results: the percentages of specimens positive with 22C3 and SP263 tests were 55% and 65%, respectively, at cutoff of 1% or higher, and 18% and 25%, respectively, at cutoff of 50% or higher.7 The PD‐L1 status results of various studies are summarized in Table 4. In most of these studies, SP263 was more sensitive than 22C3, and in the study by Kim et al., 22C3 is marginally more sensitive than SP263,5 although in our study, 22C3 was slightly more sensitive than SP263. One explanation for this would be interobserver variability. One study has shown that the interpathologist variability in scoring PD‐L1 is higher than interassay variability, and stated that interpathologist variability is an intrinsic source of error to be considered in PD‐L1 scores.17 Also, different practice patterns of pathology departments across different institutions may be another reason for these results. It is known that ability to detect PD‐L1 in tumor may be altered by tissue processing and storage,18 which would vary across different institutions. Different quantification methods, staining procedures, and different type of specimens used may all contribute to variabilities in PD‐L1 expression.19 Heterogeneity in patient populations, such as race and treatment background, is also known to affect PD‐L1 expression. Our patient population is entirely Korean, whereas other studies are from the United States or the European countries, without exact racial information of the patient population. Treatment backgrounds of the patients from those studies, which consist of mostly resected specimens, also largely differ from ours. We suspect that all these factors have contributed to the somewhat contradictory results of our study.
Table 4.
Comparison of PD‐L1 status using various cutoffs in different studies
| 22C3 | SP263 | ||||
|---|---|---|---|---|---|
| Study | TC staining ≥1% (%) | TC staining ≥50% (%) | TC staining ≥1% (%) | TC staining ≥50% (%) | Study population |
| Kim et al.5 | 35% | 12% | 34% | 1% | 97 resected NSCLCs |
| Hendry et al.4 | 23.9% | 10.1% | 34.6% | 11.8% | 368 resected lung malignancies |
| Marchetti et al.9 | 37.3% | 14.3% | 41.3% | 14.7% | 100 resected lung adenocarcinomas |
| Rimm et al.6 | 65% | 20% | N/A | N/A | 90 resected adenocarcinomas and squamous cell carcinomas |
| Tsao et al.7 | 55% | 18% | 65% | 25% | 71 NSCLCs (resection, core needle or bronchial biopsy, cytology, lymph node excision or biopsy samples) |
N/A, not available; NSCLC, non‐small cell carcinoma; TC, tumor cell.
The complication rates of our study – 20.0% pneumothorax and 3.4% hemoptysis – are lower than the pooled complication rates of CT‐guided transthoracic lung biopsy in a meta‐analysis study,20 which were 25.3% pneumothorax and 4.1% hemoptysis. The 20.0% pneumothorax rate of our study are also lower than 23% from the study by Tsai et al. that assessed the feasibility and safety of CT‐guided biopsy for evaluation of PD‐L1.13 In their study, in which an average of eight cores was obtained for evaluation of PD‐L1, 5 out of 25 patients who developed pneumothorax required intervention, whereas in our study with an average acquisition of five cores, none required intervention. Although direct comparison cannot be made between the studies, higher number of cores may be a factor for more serious pneumothorax, because it probably increases the chance of air seeping through the cannula.
Our study has limitations. First, it is a retrospective study done in a single tertiary care center, and the expertise and experience of both the radiologists performing the biopsy or the pathologists interpreting the IHC studies may vary. However, we believe such variations adequately reflect routine clinical practice in tertiary care centers, and our results can serve as a reference. Second, biopsies were not done exclusively for the evaluation of PD‐L1 status, and PD‐L1 status may have been underestimated and prove discordant with the status of the whole tumor, as we did not use whole five cores for evaluation of PD‐L1 status, when one study claimed that at least four cores are needed for an optimal correlation with the whole tumor.15 However, many of the patients did not undergo surgery and therefore we do not have whole tumor specimens to compare the results with. This limitation is inherent in the lung cancer population with advanced stages, and thus more accurately reflects the real‐world setting. Third, we did not include the bronchial biopsy samples in the study population. As bronchial biopsies are also routinely done along with CT‐guided TNB for workup of lung cancer, including bronchial biopsy specimens would have better reflected the “real world.” However, at our hospital, there are far more cases of peripheral lung cancers that undergo CT‐guided biopsies for tissue acquisition compared to central lung cancers undergoing transbronchial biopsies, so the absolute number of bronchial biopsy samples undergoing PD‐L1 testing is very small. Also, we wanted to analyze the biopsy‐related characteristics regarding CT‐guided needle biopsy and PD‐L1 testing; bronchial biopsy uses biopsy forceps, whereas CT‐guided biopsies are done using semi‐automatic core needles, which means the technical aspects of tissue acquisition are entirely different, so we thought combining these results were not suitable for this study. In short, the results of bronchoscopy biopsy are beyond the scope of this study, and future research dedicated to bronchoscopic biopsy would be enlightening. Fourth, since this was a retrospective study, we could not compare the concordance rates with those from different number of core specimens. Therefore we cannot be sure whether acquiring fewer or greater number of cores would still result in similar concordance rates.
In conclusion, there is a high concordance of PD‐L1 expression evaluated with 22C3 and SP263 assays using CT‐guided TNB specimens in a routine clinical setting. Five cores obtained with a 20‐gauge coaxial needle are adequate for analysis of PD‐L1 status in NSCLC.
Disclosure
All authors have nothing to disclose.
References
- 1. Gong J, Chehrazi‐Raffle A, Reddi S, Salgia R. Development of PD‐1 and PD‐L1 inhibitors as a form of cancer immunotherapy: A comprehensive review of registration trials and future considerations. J Immunother Cancer 2018; 6 (1): 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Assi HI, Kamphorst AO, Moukalled NM, Ramalingam SS. Immune checkpoint inhibitors in advanced non‐small cell lung cancer. Cancer 2018; 124 (2): 248–61. [DOI] [PubMed] [Google Scholar]
- 3. Buttner R, Gosney JR, Skov BG et al Programmed death‐ligand 1 immunohistochemistry testing: A review of analytical assays and clinical implementation in non‐small‐cell lung cancer. J Clin Oncol 2017; 35 (34): 3867–76. [DOI] [PubMed] [Google Scholar]
- 4. Hendry S, Byrne DJ, Wright GM et al Comparison of four PD‐L1 immunohistochemical assays in lung cancer. J Thorac Oncol 2018; 13 (3): 367–76. [DOI] [PubMed] [Google Scholar]
- 5. Kim H, Kwon HJ, Park SY, Park E, Chung JH. PD‐L1 immunohistochemical assays for assessment of therapeutic strategies involving immune checkpoint inhibitors in non‐small cell lung cancer: A comparative study. Oncotarget 2017; 8 (58): 98524–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rimm DL, Han G, Taube JM et al A prospective, multi‐institutional, pathologist‐based assessment of 4 immunohistochemistry assays for PD‐L1 expression in non‐small cell lung cancer. JAMA Oncol 2017; 3 (8): 1051–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tsao MS, Kerr KM, Kockx M et al PD‐L1 immunohistochemistry comparability study in real‐life clinical samples: Results of Blueprint Phase 2 Project. J Thorac Oncol 2018; 13 (9): 1302–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xu H, Lin G, Huang C et al Assessment of concordance between 22C3 and SP142 immunohistochemistry assays regarding PD‐L1 expression in non‐small cell lung cancer. Sci Rep 2017; 7 (1): 16956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marchetti A, Barberis M, Franco R et al Multicenter comparison of 22C3 PharmDx (Agilent) and SP263 (Ventana) assays to test PD‐L1 expression for NSCLC patients to be treated with immune checkpoint inhibitors. J Thorac Oncol 2017; 12 (11): 1654–63. [DOI] [PubMed] [Google Scholar]
- 10. Hirsch FR, McElhinny A, Stanforth D et al PD‐L1 immunohistochemistry assays for lung cancer: Results from phase 1 of the Blueprint PD‐L1 IHC Assay Comparison Project. J Thorac Oncol 2017; 12 (2): 208–22. [DOI] [PubMed] [Google Scholar]
- 11. Ratcliffe MJ, Sharpe A, Midha A et al Agreement between programmed cell death Ligand‐1 diagnostic assays across multiple protein expression cutoffs in non‐small cell lung cancer. Clin Cancer Res 2017; 23 (14): 3585–91. [DOI] [PubMed] [Google Scholar]
- 12. Ilie M, Long‐Mira E, Bence C et al Comparative study of the PD‐L1 status between surgically resected specimens and matched biopsies of NSCLC patients reveal major discordances: A potential issue for anti‐PD‐L1 therapeutic strategies. Ann Oncol 2016; 27 (1): 147–53. [DOI] [PubMed] [Google Scholar]
- 13. Tsai EB, Pomykala K, Ruchalski K et al Feasibility and safety of intrathoracic biopsy and repeat biopsy for evaluation of programmed cell death Ligand‐1 expression for immunotherapy in non‐small cell lung cancer. Radiology 2018; 287 (1): 326–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lindeman NI, Cagle PT, Aisner DL et al Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: Guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. J Thorac Oncol 2018; 13 (3): 323–58. [DOI] [PubMed] [Google Scholar]
- 15. Munari E, Zamboni G, Marconi M et al PD‐L1 expression heterogeneity in non‐small cell lung cancer: Evaluation of small biopsies reliability. Oncotarget 2017; 8 (52): 90123–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jamshidi N, Huang D, Abtin FG et al Genomic adequacy from solid tumor core needle biopsies of ex vivo tissue and in vivo lung masses: Prospective Study. Radiology 2017; 282 (3): 903–12. [DOI] [PubMed] [Google Scholar]
- 17. Brunnstrom H, Johansson A, Westbom‐Fremer S et al PD‐L1 immunohistochemistry in clinical diagnostics of lung cancer: Inter‐pathologist variability is higher than assay variability. Mod Pathol 2017; 30 (10): 1411–21. [DOI] [PubMed] [Google Scholar]
- 18. Yu H, Boyle TA, Zhou C, Rimm DL, Hirsch FR. PD‐L1 expression in lung cancer. J Thorac Oncol 2016; 11 (7): 964–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Parra ER, Villalobos P, Mino B, Rodriguez‐Canales J. Comparison of different antibody clones for immunohistochemistry detection of programmed cell death ligand 1 (PD‐L1) on non‐small cell lung carcinoma. Appl Immunohistochem Mol Morphol 2018; 26 (2): 83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heerink WJ, de Bock GH, de Jonge GJ, Groen HJ, Vliegenthart R, Oudkerk M. Complication rates of CT‐guided transthoracic lung biopsy: Meta‐analysis. Eur Radiol 2017; 27 (1): 138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]


