Abstract
Background
Lacunar stroke, a frequent clinical manifestation of small vessel disease (SVD), differs pathologically from other ischaemic stroke subtypes and has no specific long-term secondary prevention. Licenced drugs, isosorbide mononitrate (ISMN) and cilostazol, have relevant actions to prevent SVD progression.
Methods
We recruited independent patients with clinically confirmed lacunar ischaemic stroke without cognitive impairment to a prospective randomised clinical trial, LACunar Intervention-1 (LACI-1). We randomised patients using a central web-based system, 1:1:1:1 with minimisation, to masked ISMN 25 mg bd, cilostazol 100 mg bd, both ISMN and cilostazol started immediately, or both with start delayed. We escalated doses to target over two weeks, sustained for eight weeks. Primary outcome was the proportion achieving target dose. Secondary outcomes included symptoms, safety (haemorrhage, recurrent vascular events), cognition, haematology, vascular function, and neuroimaging. LACI-1 was powered (80%, alpha 0.05) to detect 35% (90% versus 55%) difference between the proportion reaching target dose on one versus both drugs at 55 patients. Registration ISRCTN12580546.
Findings
LACI-1 enrolled 57 participants between March 2016 and August 2017: 18 (32%) females, mean age 66 (SD 11, range 40–85) years, onset-randomisation 203 (range 6–920) days. Most achieved full (64%) or over half (87%) dose, with no difference between cilostazol vs ISMN, single vs dual drugs. Headache and palpitations increased initially then declined similarly with dual versus single drugs. There was no between-group difference in BP, pulse-wave velocity, haemoglobin or platelet function, but pulse rate was higher (mean difference, MD, 6.4, 95%CI 1.2–11.7, p = 0.02), platelet count higher (MD 35.7, 95%CI 2.8, 68.7, p = 0.03) and white matter hyperintensities reduced more (Chi-square p = 0.007) with cilostazol versus no cilostazol.
Interpretation
Cilostazol and ISMN are well tolerated when the dose is escalated, without safety concerns, in patients with lacunar stroke. Larger trials with longer term follow-up are justified.
Funding
Alzheimer's Society (AS-PG-14-033).
Keywords: Lacunar stroke, Small vessel disease, Randomised controlled trial, Cilostazol, Isosorbide mononitrate, Endothelium, Blood–brain barrier, White matter hyperintensities, Cerebrovascular reactivity
Research in context
Evidence before this study
A quarter of all strokes are lacunar in type with a high risk of cognitive decline, yet there are few trials of secondary prevention. Conventional secondary ischaemic stroke prevention after lacunar ischaemic stroke had limited benefit in preventing recurrent stroke, small vessel disease progression or cognitive decline. Licenced drugs which improve endothelial function, cilostazol or isosorbide mononitrate, may be effective but lack data in Western populations. In Asia-Pacific countries, cilostazol reduced recurrent stroke (five trials, 4780 patients, many with lacunar stroke, OR 0.64, 95%CI 0.51–0.79) and incident dementia (population registry analysis, 9148 dementia-free subjects, adjusted HR 0.75, 95%CI 0.61–0.92, p for dose trend, 0.001). There are no data on nitrates in secondary stroke prevention despite their widespread use in cardiovascular disease.
Added value of this study
Cilostazol and isosorbide mononitrate are well tolerated individually and together in patients with lacunar stroke, in addition to conventional secondary stroke prevention, may improve vascular function, cognitive and neuroimaging secondary outcomes.
Implications of all the available evidence
LACI-1 supports wider testing of cilostazol and isosorbide mononitrate to prevent worsening of cerebral small vessel disease, a common cause of stroke, cognitive decline and dementia.
Alt-text: Unlabelled Box
1. Introduction
Cerebral small vessel disease (SVD) is a common cause of ischaemic (‘lacunar’) and haemorrhagic stroke, vascular and mixed dementias [1]. SVD is most commonly due to an intrinsic disorder of the brain's small perforating arterioles with endothelial dysfunction, blood–brain barrier leakage [2], impaired vasoreactivity and inflammation [1].
There is currently no specific secondary prevention for lacunar ischaemic stroke, or SVD-associated cognitive decline [3]. Dual antiplatelet therapy increased bleeding, and intensive antihypertensive treatment did not reduce recurrence [4], [5]. We identified two oral agents with potential modifying effects on the endothelial dysfunctions mentioned above, isosorbide mononitrate (ISMN) and cilostazol, from a detailed systematic literature analysis [3].
ISMN is a nitric oxide (NO) donor commonly used in angina. NO levels are reduced in acute and chronic stroke including lacunar stroke [6]. Glyceryl trinitrate (GTN), an NO donor, improved cognitive test scores at 90 days if given within 6 h of stroke [7]. NO has multiple potential effects which may benefit SVD, including improved blood–brain barrier integrity, vasodilation, reducing inflammation, and neuroprotection [3], [8]. However, data on ISMN in lacunar stroke are sparse since its main therapeutic indication, ischaemic heart disease, is uncommon in these patients [9].
Cilostazol is a phosphodiesterase 3′ inhibitor [3] used for peripheral vascular disease in Europe and North America [10], and stroke prevention in Asia-Pacific countries where there are data from trials including over 6000 patients showing reduction in recurrent stroke [11]. Cilostazol also has multiple potential benefits relevant to SVD, including improving blood–brain barrier integrity, vasodilation, reducing inflammation, and neuroprotection [3], [12]. In a large national health registry in Taiwan, prescription of cilostazol was associated with reduced incidence of dementia [13].
There is little experience of cilostazol in lacunar stroke outside the Asia-Pacific Region, of ISMN in lacunar stroke anywhere, or of the drugs in combination, yet the effects are potentially synergistic [3]. The aims of the LACunar Intervention Trial-1 (LACI-1) trial were to test ISMN and cilostazol, alone and combined, for tolerability including symptoms, safety, and signals for efficacy (cognitive, systemic, cerebrovascular and haematological endpoints) after lacunar ischaemic stroke. LACI-1 tested short-term drug administration including dose escalation to prepare for a larger trial of longer-term drug administration with safety and efficacy outcomes.
2. Materials and Methods
LACI-1 was a Phase IIa, partial factorial, dose-escalation, prospective, randomised, open-label, blinded endpoint (PROBE) trial conducted in two large UK stroke centres (Edinburgh and Nottingham). The trial was approved by the Scotland A Research Ethics Committee (Ref 15/SS/0154), the Medicines and Healthcare Regulatory Agency (Ref 01384/0244/001-0001) and NHS R + D (Ref 2015/0354/TMF) and all participants gave written informed consent. There were no changes to the methods after trial commencement.
The trial was registered: ISRCTN-12580546, ClinicalTrials.gov (NCT02481323), EudraCT (2015-001953-33). The Protocol and Statistical Analysis Plan are published [14]. LACI-1 reporting follows the CONSORT statement.
The first participant was recruited and randomised on 16th March 2016, the final patient on 29th August 2017 and final follow-up was competed on 31 October 2017.
2.1. Patient Selection
We included patients with: clinical lacunar stroke in the past four years with brain magnetic resonance imaging (MRI) or CT scanning obtained at the presentation with stroke showing a symptomatic small subcortical (lacunar) infarct (< 20 mm), or (if no recent relevant infarct was visible), that excluded other causes of symptoms (e.g. acute cortical infarct, tumour); age ≥ 35 years; were independent in activities of daily living (modified Rankin Scale of ≤ 2); and had capacity to give informed consent [14]. An acute small subcortical infarct was defined on CT as a < 20 mm diameter hypoattenuated area (relative to the brain, but not as hypoattenuated as CSF) in white or deep grey matter, in a brain region corresponding with the stroke symptoms, not present on neuroimaging (if available) prior to the stroke.
We excluded patients with: other active neurological illness since the incident stroke; cognitive impairment (Montreal Cognitive Assessment [MoCA] score below 20); active cardiac disease; symptomatic carotid artery stenosis > 50% requiring urgent treatment; definite contraindication to or indication for either trial drug; taking prohibited medications that could not be changed (anticoagulants, phosphodiesterase 5′ inhibitors, macrolides, ketoconazole, itraconazole, omeprazole); creatinine clearance < 25 ml/min; bleeding tendency or a history of intracranial haemorrhage; or inability to swallow [14].
2.2. Data Collection at Baseline
We collected baseline demographic, clinical and cognitive characteristics, including minimisation variables of age ≤/>70 years, local investigator-determined SVD severity on brain scanning (SVD score ≤/>2) [15], systolic blood pressure ≤/>140 mm Hg and time after stroke ≤/>100 days, prior to randomisation.
2.3. Randomisation and Masking
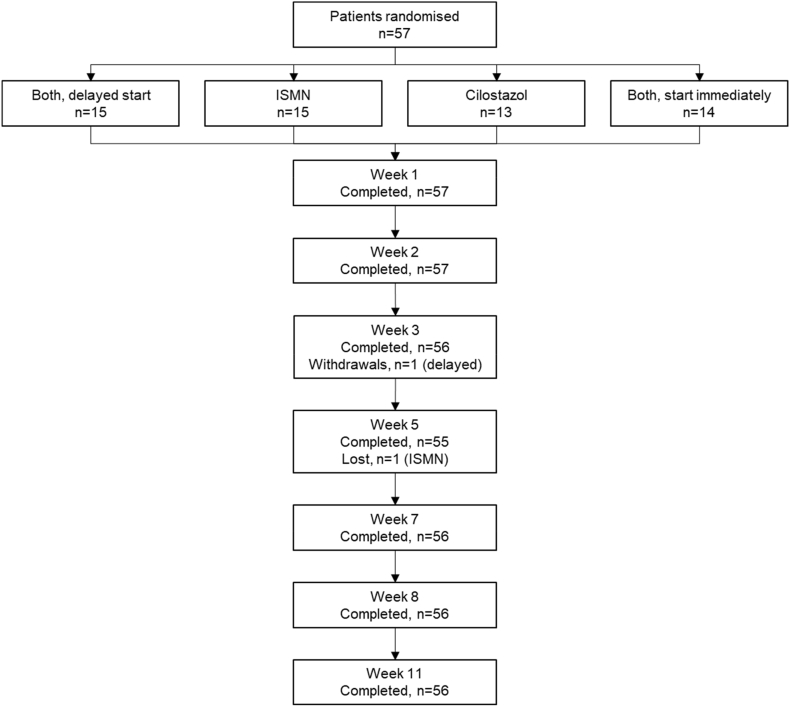
We used a secure web-based randomisation system, hosted at the University of Nottingham, to randomise participants 1:1:1:1 into four groups (CONSORT Diagram, Fig. 1). We used minimisation on age, SVD score, systolic BP, time since onset to maintain balance in baseline variables between the groups, and switching 5% of randomisations randomly to an alternate treatment group, to reduce predictability:
-
1)
cilostazol alone, 50 mg twice daily, increasing to 100 mg twice daily;
-
2)
ISMN alone, 25 mg once daily increasing to 25 mg twice daily;
-
3)
cilostazol and ISMN combined, started immediately, ISMN given first; and.
-
4)
cilostazol and ISMN combined, start delayed for three weeks, cilostazol given first.
Fig. 1.
CONSORT diagram.
ISMN = isosorbide mononitrate.
Participants allocated to both drugs aimed to attain the same target doses as for either drug alone. Group four (delayed start, both drugs) provided a ‘no drug’ comparison during the first three weeks of the trial while they received no drug.
To maintain blinding of participants, prior to the start of the trial, the Investigational Supplies Group (ISG), an independent arm of the Research and Development Office, NHS Lothian, removed study medications from their original blister packs and placed them in bottles labelled ‘Drug A’ or ‘Drug B’, a process which was approved by the Medicines and Healthcare Regulatory Agency. The labelled bottles were distributed to the hospital pharmacies at the City Hospital, Nottingham and the Western General Hospital, Edinburgh for storage and dispensing to participants following randomisation.
The randomisation program provided a prescription detailing the number of bottles of ‘Drug A’ and/or ‘Drug B’, which was dispensed by the hospital pharmacy with instructions about dose escalation and a diary for the participant to record their actual doses. The instructions referred to ‘Drug A’ or ‘Drug B’, not to either drug by name.
Trial medication was taken for nine weeks, starting at low dose and increasing gradually over 2–3 weeks, as tolerated, to full dose, sustained until eight weeks post-randomisation, then decreased and stopped over one week, with a final follow-up after two further weeks without trial tablets [14]. We designed the escalating dose to reduce potential adverse effects during cilostazol initiation (recommended where cilostazol is more widely used in Asia-Pacific countries), and escalation is standard for ISMN. Nine weeks allowed time for dose escalation and a period on sustained dose to evaluate tolerability since data were insufficient to move directly to a trial with longer term administration. Patients returned unused tablets to pharmacy for counting and destruction. Participants continued to take their prescribed medications, including conventional stroke prevention drugs, during the trial.
2.4. Outcomes
The primary outcome was the proportion of participants achieving target dose by the end of the eight-week trial period, assessed by structured questionnaire, by staff masked to allocated group. Secondary outcomes, in all participants, also by staff masked to allocated group, were: symptoms (headache, nausea, diarrhoea, vomiting, bleeding) recorded by structured questionnaire on alternate weeks; safety, (systemic or intracranial bleeding, recurrent vascular events, death); blood pressure, measured sitting and standing at study visits; heart rate; Trails A and B tests for cognitive function; systemic arterial stiffness (pulse wave analysis using the SphygmoCor tonometry device [Atcor Medical]); and platelet function (P-selectin flow cytometry) [16]. Participants recruited in Edinburgh also had MR brain imaging at randomisation using the Blood Oxygen Level Dependent (BOLD) method to assess cerebrovascular reactivity (CVR) to a challenge of 6% CO2 in inspired air in white and deep grey matter, using a validated technique [17] (full details of CVR acquisition and analysis are in the Supplementary material). The CVR examination was repeated after three weeks of no drug in the delayed start group, and at the end of the eight-week treatment period in the other three groups, to assess if the trial treatments altered CVR. The repeat structural scanning also allowed us to test for alterations in white matter lesions (WML), lacunes or microbleeds between baseline and the end of the trial.
We performed central blinded reading of the diagnostic CT and MRI imaging to score the index infarct, SVD features and total SVD score [15], using structured, validated tools [18]. We also assessed the structural MR brain images, performed as part of the CVR examination, for change in SVD lesions, new infarcts or haemorrhages occurring during the trial [18].
2.5. Statistical Analysis and Power
We powered the LACI-1 trial to test the hypothesis that patients would tolerate one drug better than both drugs, setting the sample size to detect a difference of 90% versus 55% (i.e. an absolute difference of 35%) between those reaching target (full) dose on one drug versus both drugs. For 80% power, significance 0.05, a sample size of 55 was needed; therefore, we aimed to recruit 60 participants (for sample size estimation for the CVR substudy, please see the Supplementary material p3) [14].
In primary and secondary analyses, we compared outcomes by treatment allocation to test cilostazol versus no cilostazol, ISMN versus no ISMN, and both drugs versus one drug with the delayed start group providing a drug-free control period. We calculated odds ratios (ORs) for tablet compliance by allocated group using binary logistic regression with adjustment for minimisation factors (age, SVD score, systolic blood pressure and time from stroke to randomisation in days). We assessed secondary outcomes of symptoms, safety, cognitive and physiological measures in all, and change in CVR and in SVD lesions in patients recruited in Edinburgh, using binary logistic regression for binary variables, multiple linear regression for continuous variables, Kruskal–Wallis test for between group differences in continuous variables since the variables were not normally distributed, and refer to mean differences (MD) where relevant. We also examined time trends over the 11 weeks of the trial in repeated measures analysis comparing delayed versus immediate start of both drugs. All analyses were on intention to treat, except we performed an additional secondary analysis of change in CVR by proportion of target dose achieved.
3. Role of the Funding Source
The trial was designed, conducted, analysed, interpreted, the paper written and submitted independently of the funders.
4. Results
Recruitment into LACI-1 began on 16th March 2016 and ended on 29th August 2017, and we randomised 57 patients, 29 in Nottingham and 28 in Edinburgh (Fig. 1), rate 1.64 patients/centre/month (Supplementary Fig. S1). The mean age was 66.1 (SD 11.1) years, 18 were female (32%), the median time from index stroke to randomisation was 203 days, 42 (74%) had hypertension, and 26 (46%) were current smokers (Table 1). Most participants (55, 97%) were taking clopidogrel for secondary stroke prevention and two (3%) were taking aspirin. Most participants (80%) had a visible index infarct on their diagnostic imaging (MRI in 44 (77%) and CT in 13 (23%) participants, some had both), and 25 (44%) had moderate to severe WML.
Table 1.
Baseline characteristics of patients randomised in LACI-1.
| All | Both delayed | ISMN | Cilostazol | Both immediate | |
|---|---|---|---|---|---|
| Patients (number) | 57 | 15 | 15 | 13 | 14 |
| Age (years)⁎ | 66.1 (11.1) | 63.4 (11.5) | 62.2 (11.0) | 75.8 (8.7) | 64.4 (8.1) |
| Sex, female | 18 (31.6%) | 6 (40.0%) | 4 (26.7%) | 5 (38.5%) | 3 (21.4%) |
| Onset to randomisation (days) | 202.6 (256.2) | 153.3 (180.4) | 137.5 (214.2) | 279.2 (324.7) | 254.0 (290.7) |
| Systolic BP (mm Hg) | 147.3 (20.5) | 141.8 (19.6) | 150.9 (19.0) | 146.1 (21.3) | 150.4 (22.9) |
| Diastolic BP (mm Hg) | 83.3 (12.6) | 82.9 (14.3) | 86.6 (11.5) | 79.3 (12.0) | 83.7 (12.6) |
| Past medical history (%) | |||||
| Treated hypertension | 42 (73.7%) | 11 (73.3%) | 11 (73.3%) | 12 (92.3%) | 8 (57.1%) |
| Treated hyperlipidaemia | 48 (84.2%) | 11 (73.3%) | 12 (80.0%) | 13 (100.0%) | 12 (85.7%) |
| Diabetes | 11 (19.3%) | 3 (20.0%) | 3 (20.0%) | 2 (15.4%) | 3 (21.4%) |
| Atrial fibrillation | 0 | 0 | 0 | 0 | 0 |
| Ipsilateral carotid stenosis > 50% | 1 (3.1%) | 0 | 0 | 1 (14.3%) | 0 |
| Myocardial infarction or angina | 3 (5.3%) | 2 (13.4%) | 0 | 1 (7.7%) | 0 |
| Previous stroke or TIA | 8 (14.0%) | 2 (13.4%) | 2 (13.3%) | 2 (15.4%) | 2 (14.3%) |
| Smoking | 26 (45.6%) | 10 (66.7%) | 6 (40.0%) | 6 (46.2%) | 4 (28.6%) |
| Alcohol intake [units per week] | 1.0 [0.0, 7.0] | 1.0 [0.0, 5.0] | 1.0 [0.0, 7.0] | 1.0 [0.0, 9.0] | 3.5 [0.0, 10.0] |
| Patient status | |||||
| mRS, baseline [/6] | 1.0 [0.0, 1.0] | 1.0 [0.0, 2.0] | 1.0 [1.0, 1.0] | 1.0 [1.0, 1.0] | 1.0 [0.0, 1.0] |
| Current NIHSS [/42] | 0.0 [0.0, 1.0] | 0.0 [0.0, 1.0] | 0.0 [0.0, 1.0] | 0.0 [0.0, 1.0] | 0.5 [0.0, 1.0] |
| Cognition | |||||
| MoCA (/30) | 25.8 (2.4) | 25.5 (2.9) | 27.0 (1.7) | 25.2 (2.2) | 25.4 (2.6) |
| TMT part B (secs) | 101.1 (74.7) | 84.9 (44.2) | 77.0 (32.3) | 141.1(101.5) | 107.3 (93.3) |
| TMT part B (points)⁎ | 23.5 (4.1) | 24.7 (0.7) | 24.3 (0.9) | 21.2 (6.9) | 23.4 (4.5) |
| NART (error score) | 18.0 (12.7) | 16.8 (9.2) | 14.3 (9.7) | 20.4 (16.1) | 21.0 (15.2) |
| Investigator reported scan information | |||||
| SVD score MRI (/4) | 1.2 (1.0) | 1.6 (1.3) | 0.9 (0.6) | 1.0 (0.0) | 1.3 (1.3) |
| SVD score CT (/2) | 0.9 (0.8) | 0.7 (0.8) | 0.9 (0.7) | 1.3 (0.9) | 0.8 (0.8) |
| Centrally adjudicated scan information | |||||
| Scan type, MRI | 44 (77.2%) | 13 (86.7%) | 14 (93.3%) | 7 (53.8%) | 10 (71.4%) |
| Visible acute stroke lesion MRI | 36 (81.8%) | 9 (69.2%) | 11 (78.6%) | 6 (85.7%) | 10 (100.0%) |
| SVD score MRI (/4) | 1.8 (1.2) | 1.9 (1.2) | 1.4 (1.2) | 2.1 (0.7) | 2.1 (1.5) |
| ≥ 1 lacune | 27 (61.4%) | 10 (76.9%) | 6 (42.9%) | 4 (57.1%) | 7 (70.0%) |
| ≥ 1 microbleeds | 7 (17.9%) | 2 (18.2%) | 1 (7.7%) | 1 (16.7%) | 3 (33.3%) |
| Perivascular spaces | 26 (59.1%) | 7 (53.8%) | 8 (57.1%) | 5 (71.4%) | 6 (60.0%) |
| WML Fazekas MRI | 19 (43.2%) | 6 (46.2%) | 4 (28.6%) | 4 (57.1%) | 5 (50.0%) |
| Scan type, CT | 13 (22.8%) | 2 (13.3%) | 1 (6.7%) | 6 (46.2%) | 4 (28.6%) |
| Visible acute stroke lesion CT | 10 (76.9%) | 2 (100.0%) | 1 (100.0%) | 4 (66.7%) | 3 (75.0%) |
| Side of brain, left | 5 (50.0%) | 1 (50.0%) | 1 (100.0%) | 1 (25.0%) | 2 (66.7%) |
| SVD score CT (/2) | 0.9 (0.9) | 0.5 (0.7) | 1.0 (–) | 1.5 (0.8) | 0.3 (0.5) |
| ≥ 1 lacune | 6 (46.2%) | 0 | 1 (100.0%) | 4 (66.7%) | 1 (25.0%) |
| WML Fazekas CT⁎ | 6 (46.2%) | 1 (50.0%) | 0 | 5 (83.3%) | 0 |
Note: If a participant had both a CT and MRI centrally reviewed then the results of the MRI are used for analysis. ISMN = isosorbide mononitrate; BP = blood pressure; TIA = transient ischaemic stack; mRS = modified Rankin Scale; NIHSS = National Institutes of Health Stroke Scale; MoCA = Montreal Cognitive Assessment; NART = National Adult Reading Test; TMT = trail making test; SVD = small vessel disease; MRI = magnetic resonance imaging; WML = white matter hyperintensities.
Significant difference between the treatment groups (p < 0.05), comparisons done using Chi-square and Kruskal–Wallis tests.
The groups were well balanced for baseline characteristics except for the participants allocated to cilostazol who were older, had higher Fazekas scores indicating more WML, and had lower Trails B scores respectively (Table 1). One participant withdrew at week three (delayed start dual drugs, prior to receiving any trial drug) and did not complete that follow-up or any after; one participant missed a follow-up in week five (ISMN group) but completed all prior and subsequent follow-ups.
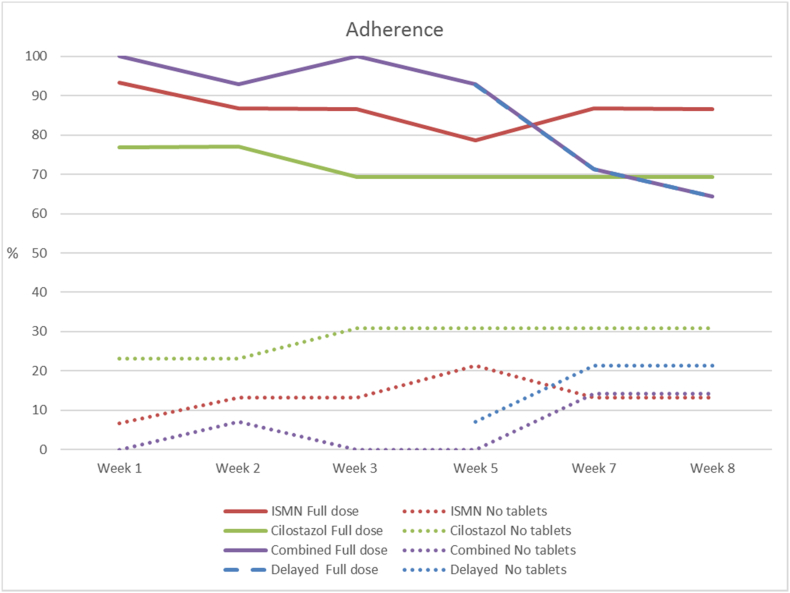
4.1. Tablet Compliance
For the primary outcome, 40 (72%) were taking full (no doses missed) or partial doses (includes missing between one and up to 50% of allocated dose) of the allocated tablets by the end of the eight-week treatment period, with no difference between groups (Table 2, Fig. 2; partial dose details, Supplementary Fig. S2). Most patients who achieved ‘partial dose’ only missed one or two doses in any assessment period. ISMN may be tolerated better than cilostazol: 13/15 allocated ISMN alone (87%) achieved full or partial ≥ 50% dose by week eight versus 9/13 (69%) allocated cilostazol alone (any ISMN versus no ISMN achieving full dose, OR 3.77, 95%CI 0.98–14.46, p = 0.053; any cilostazol versus no cilostazol achieving full dose, OR 0.39, 95%CI 0.11–1.34, p = 0.14). Dual drugs were tolerated similarly to either individual drug: ISMN plus cilostazol versus one or other drug alone achieving full dose, OR 0.84, 95%CI 0.27–2.64, p = 0.77 (Table 2). Combining the full and partial doses versus no tablets gave similar results (data not shown). In the dual drug groups, there was no evidence that starting with one drug produced different symptoms or tolerance than starting with the other drug, or that those who ceased to take tablets did so because of more symptoms.
Table 2.
Outcomes at week 8. All analyses are adjusted for baseline age, mean SBP, time from onset to randomisation and SVD total. Adjustment for other baseline covariates is included where stated. For SVD total, the central scan readings were used and MRI was chosen over CT if both were available.
| Both delayed N = 14 |
ISMN N = 15 |
Cil N = 13 |
Both immediate N = 14 |
Cilostazol vs. no Cil (2 + 3 v 1 + 4a) |
ISMN vs. no ISMN (1 + 3 v 2 + 4a) |
Cil + ISMN vs. Cil alone or ISMN alone (3 + 4 v 1 or 2) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Week 3 (4a) |
Week 8 (4) |
Week 8 (1) |
Week 8 (2) |
Week 8 (3) |
OR/MD (95%CI) |
p-Value | OR/MD (95%CI) |
p-Value | OR/MD (95%CI) |
p-Value | |
| Primary outcome | |||||||||||
| On target dose week 8 (%) | NA | 5 (35.7%) | 8 (53.3%) | 3 (23.1%) | 5 (35.7%) | 0.39 (0.11, 1.34) | 0.136 | 3.77 (0.98, 14.46) | 0.053 | 0.84 (0.27, 2.64) | 0.769 |
| On partial dose (≥ 1/2 tablets) week 8 (%) | NA | 4 (28.6%) | 5 (33.3%) | 6 (46.2%) | 4 (28.6%) | 1.68 (0.48, 5.83) | 0.414 | 0.49 (0.13, 1.79) | 0.280 | 0.51 (0.15, 1.71) | 0.277 |
| On partial dose (< 1/2 tablets) week 8 (%) | NA | 2 (14.3%) | 0 | 0 | 3 (21.4%) | 2.37 (0.31, 18.33) | 0.408 | 1.40 (0.18, 11.00) | 0.748 | NC | NC |
| Taking no tablets on week 8 (%) | NA | 3 (21.4%) | 2 (13.3%) | 4 (30.8%) | 2 (14.3%) | 1.00 (0.19, 5.22) | 0.997 | 0.26 (0.04, 1.46) | 0.125 | 0.86 (0.19, 3.92) | 0.847 |
| Secondary outcomes | |||||||||||
| Patients with symptom | |||||||||||
| Headache (%) | 3 (21.4%) | 4 (28.6%) | 4 (26.7%) | 1 (7.7%) | 1 (7.1%) | 0.29 (0.05, 1.76) | 0.178 | 0.93 (0.18, 4.81) | 0.928 | 1.26 (0.26, 6.18) | 0.774 |
| Nausea (%) | 1 (7.1%) | 3 (21.4%) | 1 (6.7%) | 2 (15.4%) | 3 (21.4%) | 5.07 (0.58, 44.42) | 0.143 | 1.24 (0.18, 8.74) | 0.828 | 2.81 (0.51, 15.34) | 0.234 |
| Loose stools (%) | 5 (35.7%) | 4 (28.6%) | 0 | 2 (15.4%) | 4 (28.6%) | 1.43 (0.35, 5.88) | 0.623 | 0.39 (0.08, 1.90) | 0.242 | 7.07 (1.07, 46.66) | 0.042 |
| Bleeding (%) | 2 (14.3%) | 0 | 3 (20.0%) | 2 (15.4%) | 2 (14.3%) | 1.05 (0.18, 6.26) | 0.959 | 0.36 (0.06, 2.29) | 0.281 | 0.25 (0.03, 1.98) | 0.189 |
| Peripheral haemodynamics, adjusted for baseline | |||||||||||
| Systolic (mm Hg), mean (SD) | 134.8 (12.4) | 131.4 (19.0) | 144.1 (16.5) | 146.7 (19.4) | 139.9 (20.7) | − 1.1 (− 8.4, 6.2) | 0.77 | − 1.1 (− 8.5, 6.4) | 0.78 | − 4.9 (− 11.7, 1.9) | 0.16 |
| Average of systolic & diastolic (mm Hg), mean (SD) | 107.6 (10.6) | 104.7 (14.0) | 114.5 (11.1) | 114.0 (13.2) | 109.9 (14.7) | − 1.1 (− 6.3, 4.1) | 0.67 | − 0.7 (− 6.0, 4.6) | 0.79 | − 3.6 (− 8.4, 1.2) | 0.14 |
| Diastolic (mm Hg), mean (SD) | 80.4 (9.7) | 78.0 (10.8) | 84.9 (7.6) | 81.3 (11.2) | 79.8 (10.5) | − 1.6 (− 5.8, 2.6) | 0.45 | − 0.7 (− 5.0, 3.7) | 0.77 | − 2.6 (− 6.5, 1.4) | 0.20 |
| Heart rate (bpm), mean (SD) | 74.3 (9.3) | 79.5 (11.2) | 74.7 (12.5) | 80.6 (15.0) | 84.8 (15.1) | 6.4 (1.2, 11.7) | 0.02 | 0.1 (− 5.5, 5.6) | 0.98 | 3.1 (− 1.9, 8.2) | 0.23 |
| Platelet function, adjusted for baseline | |||||||||||
| ADP median fluorescence, mean (SD) | 519.3 (234.5) | 502.1 (242.8) | 454.9 (238.8) | 518.9 (179.5) | 451.7 (176.5) | − 1.3 (− 77.6, 75.0) | 0.97 | − 0.5 (− 80.1, 79.1) | 0.99 | 11.9 (− 60.2, 84.1) | 0.75 |
| AA median fluorescence, mean (SD) | 741.0 (395.0) | 775.4 (474.4) | 675.2 (364.1) | 636.4 (272.4) | 679.6 (376.6) | − 83.6 (− 256.5, 89.4) | 0.34 | − 14.1 (− 192.5, 164.2) | 0.88 | 109.7 (− 54.0, 273.5) | 0.19 |
| Unstimulated median fluorescence, mean (SD) | 87.0 (29.0) | 80.4 (29.1) | 86.1 (29.0) | 75.9 (27.3) | 83.7 (33.8) | − 2.9 (− 15.1, 9.3) | 0.64 | − 8.2 (− 20.7, 4.2) | 0.20 | 7.5 (− 4.0, 19.0) | 0.20 |
| Hb (g/L), week 8, adjusted for baseline, mean (SD) | 133.0 (12.2) | 136.2 (13.5) | 146.9 (12.0) | 130.8 (9.5) | 137.1 (14.6) | − 3.7 (− 8.4, 1.0) | 0.12 | − 1.0 (− 6.9, 4.4) | 0.73 | − 2.4 (− 6.8, 2.1) | 0.30 |
| Platelet count, week8, adjusted for baseline, mean (SD) | 260.3 (47.1) | 258.4 (56.1) | 238.6 (45.5) | 289.5 (61.4) | 284.3 (57.8) | 35.7 (2.8, 68.7) | 0.03 | 3.9 (− 32.4, 40.2) | 0.83 | − 7.4 (− 36.8, 21.9) | 0.62 |
| Central arterial pressure waveform analysis, adjusted for baseline | |||||||||||
| Pulse wave velocity m/s, mean (SD) | 16.8 (7.5) | 23.2 (11.9) | 18.6 (11.3) | 17.9 (8.1) | 18.6 (8.5) | − 0.4 (− 5.1, 4.4) | 0.89 | 1.1 (− 3.7, 5.9) | 0.66 | − 0.8 (− 5.3, 3.6) | 0.72 |
| Pulse wave analysis | |||||||||||
| Augmentation index 75 (%), mean (SD) | 24.0 (10.6) | 16.2 (12.4) | 17.5 (12.9) | 23.0 (10.6) | 14.7 (10.5) | − 4.8 (− 9.7, 0.2) | 0.06 | − 4.7 (− 9.8, 0.3) | 0.07 | 0.1 (− 4.8, 4.9) | 0.97 |
| Central blood pressure, mean (SD) | 99.0 (14.6) | 95.4 (12.7) | 101.2 (12.4) | 103.1 (11.8) | 98.8 (11.7) | − 2.0 (− 8.2, 4.2) | 0.52 | − 4.7 (− 10.7, 1.2) | 0.12 | − 3.4 (− 9.1, 2.4) | 0.25 |
| Buckberg viability ratio, mean (SD) | 154.4 (21.5) | 164.8 (22.2) | 166.6 (25.2) | 152.2 (31.4) | 132.9 (22.7) | − 10.8 (− 21.2, − 0.5) | 0.04 | 6.8 (− 4.0, 17.1) | 0.20 | − 11.4 (− 21.0, − 1.7) | 0.02 |
| Cognition (TMT), adjusted for baseline | |||||||||||
| TMT A — time, mean (SD) | 39.5a (15.1) | 37.8 (16.8) | 33.5 (10.3) | 51.1 (33.5) | 31.9 (9.2) | − 4.0 (− 12.7, 4.7) | 0.37 | − 4.2 (− 12.8, 4.4) | 0.34 | − 5.6 (− 13.4, 2.3) | 0.17 |
| TMT A — points, mean (SD) | 24.9a (0.4) | 23.1 (6.7) | 25.0 (0.0) | 21.2 (9.4) | 24.9 (0.3) | − 1.1 (− 3.6, 1.5) | 0.41 | 2.6 (0.1, 5.1) | 0.05 | 0.3 (− 2.1, 2.7) | 0.81 |
| TMT B — time, mean (SD) | 84.9a (44.2) | 79.9 (34.4) | 76.9 (39.4) | 114.3 (63.1) | 84.3 (50.4) | − 3.4 (− 22.7, 16.0) | 0.73 | − 3.1 (− 22.4, 16.3) | 0.76 | − 2.3 (− 20.2, 15.6) | 0.80 |
| TMT B — points, mean (SD) | 24.7a (0.7) | 22.9 (6.6) | 24.7 (0.8) | 19.4 (9.3) | 22.9 (4.1) | − 2.2 (− 5.1, 0.7) | 0.14 | 2.3 (− 0.7, 5.3) | 0.13 | − 0.2 (− 3.0, 2.60) | 0.89 |
SVD = small vessel disease; MRI = magnetic resonance imaging; ISMN = isosorbide mononitrate; Cil = cilostazol; OR = odds ratio; HR = Hazard ratio; MD = mean difference; CI = confidence interval; NA = not applicable; SD = standard deviation; ADP = adenosine diphosphate; AA = arachidonic acid; Hb = haemoglobin; TMT = trail making test.
Week 0 values.
Fig. 2.
Tablet compliance: Proportion of participants who were taking full dose combined with the proportion taking ≥ 1/2 tablets into one group, (solid lines, labelled ‘full dose’) noting that missing even one dose was counted as ≥ 1/2 tablets). The combined and delayed groups from week 5 onwards had very close numbers and so the lines appear on top of each other. The dotted lines show those who had taken no tablets at all that week. More details of compliance by number of missed doses is given in Fig. S2. ISMN = isosorbide mononitrate.
4.2. Symptoms Potentially Related to Trial Medication
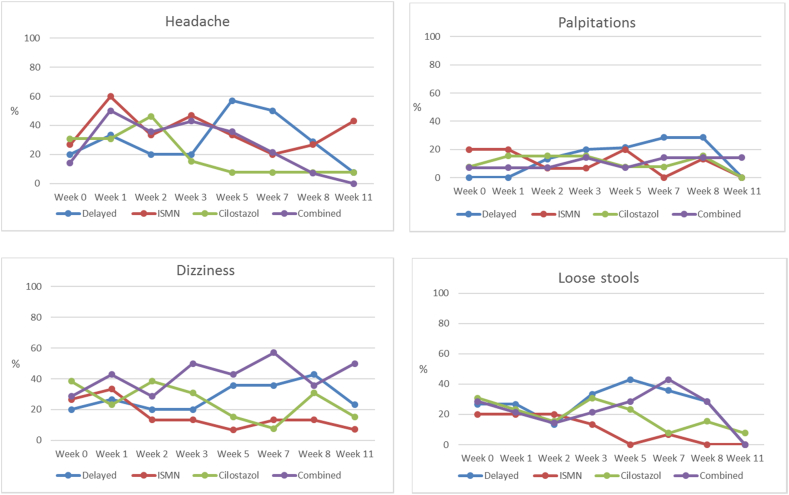
Symptoms were common without trial medication. In the week prior to randomisation, the following symptoms were experienced: headache 30%; palpitations 20%; dizziness 40%; loose stools 30%; nausea 15%; dyspepsia 45%; bruising 20%; bleeding from mucous membranes 15%; and rash 9%. Following drug initiation, there was a slight increase in headache, palpitations, dizziness, loose stools, nausea, followed by a return to baseline levels with continued trial drug (Fig. 3; Table 2; Supplementary Fig. S3). There was no increase in dyspepsia, bruising, or bleeding (Supplementary Fig. S3). Neither cilostazol nor ISMN were associated with more symptoms and patients allocated dual drugs did not report more symptoms than those allocated one drug (Fig. 3; Table 2; Supplementary Fig. S3).
Fig. 3.
Symptoms of headache, palpitations, dizziness, loose stools, and nausea. ISMN = isosorbide mononitrate.
4.3. Secondary Outcomes, All Participants
Cognition: The points achieved on Trails A increased in the group allocated to ISMN compared with no ISMN (MD 2.6, 95%CI 0.1, 5.1, p = 0.045), with no effect on Trails B points (MD 2.3, 95%CI − 0.7, 5.3, p = 0.126) after adjustment for baseline Trails (Table 2). Participants took less time to complete the tests in groups allocated cilostazol versus no cilostazol, ISMN versus no ISMN, and both versus one or other drug, although these differences did not reach significance.
Blood pressure (BP) reduced during drug initiation, but with no significant difference between groups by week eight (Table 2). Heart rate at week eight was higher in those allocated cilostazol versus no cilostazol (MD 6.4, 95%CI 1.2–11.7, p = 0.017). Buckberg Index (subendocardial perfusion) was reduced at week eight with cilostazol (OR − 10.87, 95%CI − 21.2, − 0.5, p = 0.04) and combined cilostazol and ISMN (OR − 11.4, 95%CI − 21.0, − 1.7, p = 0.02). There were no sustained changes in pulse wave velocity or Augmentation Index.
Haematology: There was no difference in haemoglobin or platelet function between groups at the end of the treatment period (Table 2). However, platelet count increased in those on cilostazol vs no cilostazol (MD 35.7 95%CI 2.8, 68.7, p = 0.033).
4.4. Adverse Events, All Participants
There were ten Serious Adverse Events (SAEs), six mild and four of moderate severity, three possibly and the rest definitely or probably not related to trial drugs (Supplementary Tables S1 and S2). There was one recurrent ischaemic stroke in the ‘dual drugs immediate’ group and one TIA in the ISMN group. There were no deaths or major haemorrhages and no SAEs in the cilostazol group.
4.5. Cerebrovascular Reactivity and SVD Lesion Change, Participants in Edinburgh
Of 28 participants randomised in Edinburgh, one chose not to participate in the MRI sub-study, one did not complete MRI due to claustrophobia and two received insufficient CO2 challenge due to problems with gas delivery, leaving 24 participants with complete CVR data to assess change. The groups were well balanced for demographic and neuroimaging characteristics (Supplementary Table S3), with mean age 67.5 (range 53–83) and 13/24 were male (56%).
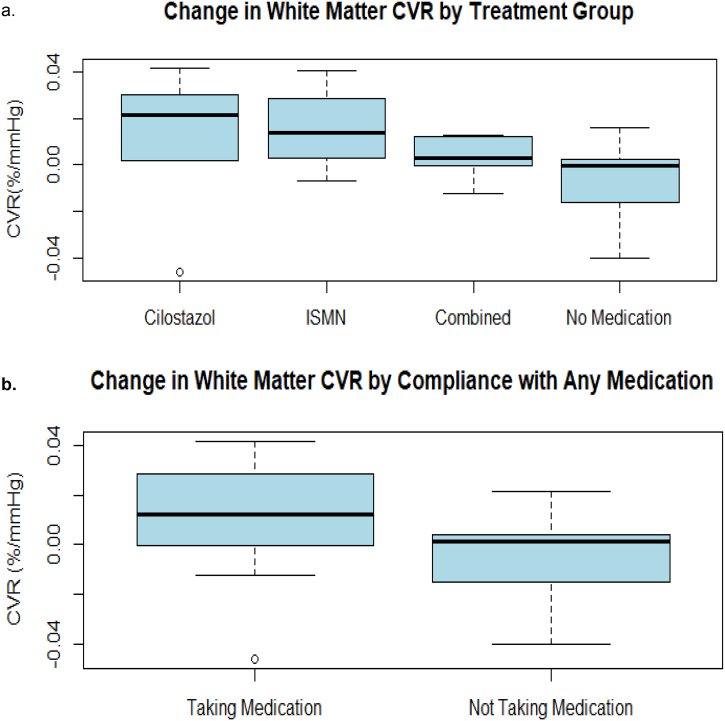
CVR increased more between baseline and follow-up in those allocated cilostazol or ISMN compared with those allocated delayed start (who were taking no drug at the follow-up CVR examination) although the difference was not significant (Kruskal–Wallis, p = 0.18), Fig. 4a. Final CVR values were higher in participants taking any versus no trial drug (Fig. 4b) although the difference was not significant (Kruskal–Wallis, p = 0.16).
Fig. 4.
Cerebrovascular reactivity (CVR). a) Change in white matter CVR by allocated treatment group (difference between groups, Kruskal–Wallis, p = 0.18). b) Change in white matter CVR in participants taking half target dose or greater of any allocated treatment versus those taking no or less than half dose (difference between groups, Kruskal–Wallis, p = 0.16).
Compared with baseline, the T2/FLAIR structural MRI at follow-up showed a decline in WML in participants allocated cilostazol (WML reduced in 5/12 participants) versus no cilostazol (no change), Chi-square p = 0.007. In participants allocated to ISMN, WML reduced in 3/13 versus in 2/13 in participants allocated to no ISMN, Chi-square p = 0.62. In addition, one microbleed disappeared in a participant allocated to ISMN and two lacunes appeared, both in participants allocated ‘dual drugs immediate start’.
5. Discussion
LACI-1 tested short-term administration of cilostazol and ISMN, alone and in combination, in patients with lacunar ischaemic stroke. The drugs and doses were well-tolerated and dose escalation was feasible. Most participants, including those in the dual drug groups, were taking target, or near to target dose, by the end of the treatment period. Symptoms such as headache, palpitations, nausea were common both off and on, either or both, trial medications, with no evidence of sustained increase in symptoms on target dose, or of any difference between groups allocated single or dual drugs. There were no drug-related adverse events or bleeding complications, despite all participants also taking prescribed antiplatelet drugs. The nitrate donor ISMN and the phosphodiesterase 3′ inhibitor cilostazol act on relevant target mechanisms [3] based on current knowledge of human sporadic SVD [1], and are licenced agents with established safety profiles. LACI-1 showed that both drugs affect systemic haemodynamic function, and may improve vasoreactivity in white matter, reduce WML, and improve cognitive performance, all of which require confirmation in larger trials. LACI-1 demonstrates drug tolerability and supports testing of ISMN and cilostazol in larger trials with clinical endpoints.
Data on nitrates in patients with lacunar stroke, or other SVD manifestations, are limited [9]. In the Efficacy of Nitric Oxide in Stroke (ENOS) Trial, seven days of GTN improved cognitive test scores at 90 days if started within 6 h of acute stroke [7], lowered BP and its variability in acute lacunar syndromes, and improved early neurological outcome in those with a confirmed acute lacunar infarct. NO donors could improve vasoreactivity (which is impaired in lacunar stroke [19]), are neuroprotective and anti-inflammatory [3], all relevant targets in SVD [1]. Such effects could explain improvements in WML, cognition, systemic haemodynamics and cerebral vasoreactivity suggested in LACI-1 and are worth testing in larger trials. Alternatively, the reduction in WML seen in some patients could reflect the modest blood pressure reduction seen in the trial, although whether such effects could occur in a few weeks is unknown. Data on other vasoactive drugs in SVD are limited. In post-hoc analyses, patients who experienced headache soon after starting dipyridamole for secondary stroke prevention had fewer recurrent strokes than those without headache [20], suggesting that headache may be a useful indicator of improved cerebrovascular reactivity which may help prevent further stroke.
Trials testing cilostazol for secondary stroke prevention include over 6000 patients in Korea, Japan or China, but focused on short-term stroke prevention after ischaemic stroke [11], with limited data on symptoms or tolerance rates and only one small study on platelet function [21]. Cilostazol has weak antiplatelet effects and therefore low bleeding risk [22]. Trials testing cilostazol to prevent cognitive decline are ongoing [23], and routinely prescribed cilostazol was associated with fewer incident dementias in a large Taiwanese health data registry, in a ‘dose response’ manner [13]. Short-term cilostazol improved cerebrovascular pulsatility [24]. There are no data on tolerance or stroke prevention in Western populations and limited data in peripheral vascular disease [10]. Differences in SVD prevalence, diet, lifestyle and risk factor exposures preclude direct generalisation from Asia-Pacific to Western populations [25]. In experimental models, cilostazol unblocks endothelial-dysfunction induced oligodendrocyte precursor cell maturation, thus improving myelin formation and repair and sustaining axons [26], reduces oxidative stress, attenuates microglial activation and WML formation [27], [28], and improves amyloid-β clearance [29]. These pleiotropic effects would be beneficial if translated to human sporadic SVD, and could explain improvements in cognition, cerebrovascular reactivity and reduction in WML suggested in LACI-1 and should be tested in future trials.
5.1. Limitations
LACI-1 limitations include its small size, short-term drug administration and limited clinical endpoints. The size limited analysis of whether drug effects differed by lacunar clinical syndrome, but could be tested in future trials. We used the Trails A and B tests of cognitive function which may be prone to practice effects but this will affect all uses of these standard tests. The minimisation algorithm was imperfect since there was a baseline imbalance in age, reflected in the WML and cognitive scores. The combined drugs were better tolerated than expected. There was no formal ‘no drug’ control group throughout the trial, although there was the ‘drug-free period’ in the ‘dual drug, delayed start’ group. There were no previous data available on initiation of both drugs together, therefore for dual administration, we integrated the dose escalation usually used for each drug. Slow dose escalation of dipyridamole and aspirin did not reduce headache, in comparison with standard dose escalation, although may have improved adherence [30]. Since the doses for licenced indications are somewhat arbitrary and wide ranging, it is reasonable to think that half dose would have some effect on the target mechanisms, and can be tested a priori in larger trials. Patients could enter the trial with CT or MRI brain imaging, thus achieving faster recruitment at lower expense than just using MRI, while ensuring generalisability. We estimate that the trial would have taken eight months (50%) longer and cost £85,000 (33%) extra with mandatory MRI. The flexible imaging entry criteria did not allow assessment of causes of lacunar stroke such as branch atheromatous disease; this could be assessed in future trials, but requirement for detailed MRI might reduce recruitment.
5.2. Conclusions
LACI-1 suggests that patients can be randomised to ISMN and cilostazol in addition to guideline secondary stroke prevention including single antiplatelet agents. LACI-1 indicates that LACI-2 (ISRCTN14911850), a partial-factorial prospective randomised open-label blinded endpoint trial, aiming to randomise 400 patients, should proceed, to assess these medications' effects on stroke recurrence, cognitive function, neuropsychiatric symptoms, progression of SVD and safety in patients with lacunar stroke, and feasibility for large definitive trial.
Data Sharing
The data are available from the authors.
Funding
LACI-1 is funded by the Alzheimer's Society Ref: 252 (AS-PG-14-033). Additional support is provided by the European Union Horizon 2020 project No 666881, ‘SVDs@Target’ (GWB, MS), the Stroke Association Princess Margaret Research Development Fellowship scheme (GWB), the Stroke Association Garfield Weston Foundation Senior Clinical Lectureship (FND), NHS Research Scotland (FND), the China Scholarship Council/University of Edinburgh (YS), NHS Lothian Research and Development Office (MJT), the Row Fogo Charitable Trust, the Scottish Funding Council through the Scottish Imaging Network, A Platform for Scientific Excellence (SINAPSE) Collaboration, the Fondation Leducq (ref no. 16 CVD 05) and Edinburgh and Lothians Health Foundation. JPA is supported, in part, by the National Institute for Health Research (NIHR) HTA TARDIS and BHF RIGHT-2 trials. ZKL and KF are supported, in part, by the NIHR HTA TICH-2 trial. RD is supported, in part, by the HTA TARDIS trial. CR is supported through the NIHR Clinical Research network (CRN) East Midlands. PMB is Stroke Association Professor of Stroke Medicine, and is a NIHR Senior Investigator. LACI-1 was adopted by the NIHR CRN.
Acknowledgments
Acknowledgements
We thank the patients and their families for participating in LACI-1, and the funders: The Alzheimer's Society (AS-PG-14-033), UK Stroke Association, NHS Research Scotland, Edinburgh and Lothians Health Foundation, China Scholarship Council, NHS Lothian Research and Development Office, Scottish Funding Council, European Union Horizon 2020 (666881, ‘SVDs@Target’), Fondation Leducq (16 CVD 05), British Heart Foundation, National Institute for Health Research (NIHR) HTA Programme, and NIHR Clinical Research network (CRN) East Midlands. The funders had no role in the study design, data collection, data analysis, interpretation, writing of the report or decision to submit for publication. We thank the other members of the LACI-1 Trial Steering Committee (Dr John Bamford, Independent Chair; Euan Haig, Sandra Duggan, Kieran Hanna, Mair Graham, participant representatives; and the Trial Sponsor) and the LACI-1 Data Monitoring Committee (Professor Colin Baigent, Chair, Oxford University; Professor Alison D Murray, University of Aberdeen; Professor Gary Ford, Oxford University; Dr. Jonathan Emberson, Oxford University).
Author Declaration of Interests
JMW, PB, NS, FD, GB, KF, RD, MJT, IG, JB received funding from the Alzheimer's Society (AS-PG-14-033);
FD, PB, NS, JMW report funding from the UK Stroke Association;
FD also reports funding from NHS Research Scotland;
GB also received funding from Edinburgh and Lothians Health Foundation and the Stroke Association Princess Margaret Research Development Fellowship scheme;
YS received funding from the China Scholarship Council;
MJT also received funding from NHS Lothian Research and Development Office;
JMW also received funding from the Scottish Funding Council;
JMW, FD, MJT, MS and GB also received funding from European Union Horizon 2020 (666881, ‘SVDs@Target’) and the Fondation Leducq (16 CVD 05);
NS, PB, JMW, JPA also received funding from the British Heart Foundation;
PB, NS, JPA, also received funding from the National Institute for Health Research (NIHR) HTA Programme;
PB, NS, CR also received funding from the NIHR Clinical Research network (CRN) East Midlands;
PB is a NIHR Senior Investigator, and is on advisory boards of Sanofi, Nestle, DiaMedica, Moleac, Phagenesis, ReNeuron and is a Non-executive Director (unpaid) of Platelet Solutions.
All other authors have nothing to disclose.
Author Contribution
Gordon W Blair, Jason P Appleton, Zhe Kang Law — recruitment, data collection, analysis, manuscript editing.
Katie Flaherty — statistical analysis, manuscript preparation and editing.
Richard Dooley — programming, web-based data collection forms, manuscript editing.
Kirsten Shuler, Julia Boyd — trial management, regulatory approvals, manuscript editing.
Carla Richardson — recruitment, data collection, manuscript editing.
Iona Hamilton, Yulu Shi, Michael Thrippleton, Michael S Stringer — data collection, manuscript editing.
Fergus Doubal, Nikola Sprigg — study design, supervision, study set up, data collection, SAE adjudication, manuscript editing.
Philip M Bath — trial conception, design, management, supervision, data collection and analysis, manuscript preparation and editing.
Joanna M Wardlaw — trial conception, design, funding, supervision, data collection, analysis, manuscript preparation and editing, overall guarantor.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.eclinm.2019.04.001.
Appendix A. Supplementary data
Supplementary material
References
- 1.Wardlaw J.M., Smith C., Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol. 2013;12:483–497. doi: 10.1016/S1474-4422(13)70060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wardlaw J.M., Makin S.J., Valdés Hernández M.C. Blood–brain barrier failure as a core mechanism in cerebral small vessel disease and dementia: evidence from a cohort study. Alzheimers Dement. 2017;13(6):634–643. [Google Scholar]
- 3.Bath P.M., Wardlaw J.M. Pharmacological treatment and prevention of cerebral small vessel disease: a review of potential interventions. Int J Stroke. 2015;10:469–478. doi: 10.1111/ijs.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The SPS3 Investigators Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817–825. doi: 10.1056/NEJMoa1204133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.SPS3 Study Group, Benavente O.R., Coffey C.S. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet. 2013;382:507–515. doi: 10.1016/S0140-6736(13)60852-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rashid P.A., Whitehurst A., Lawson N., Bath P.M. Plasma nitric oxide (nitrate/nitrite) levels in acute stroke and their relationship with severity and outcome. J Stroke Cerebrovasc Dis. 2003;12:82–87. doi: 10.1053/jscd.2003.9. [DOI] [PubMed] [Google Scholar]
- 7.Woodhouse L., Scutt P., Krishnan K. Effect of hyperacute administration (within 6 hours) of transdermal glyceryl trinitrate, a nitric oxide donor, on outcome after stroke: subgroup analysis of the Efficacy of Nitric Oxide in Stroke (ENOS) Trial. Stroke. 2015;46(11):3194–3201. doi: 10.1161/STROKEAHA.115.009647. [DOI] [PubMed] [Google Scholar]
- 8.Willmot M., Gray L., Gibson C., Murphy S., Bath P.M. A systematic review of nitric oxide donors and l-arginine in experimental stroke; effects on infarct size and cerebral blood flow. Nitric Oxide. 2005;12(3):141–149. doi: 10.1016/j.niox.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Jackson C.A., Hutchison A., Dennis M.S. Differing risk factor profiles of ischemic stroke subtypes: evidence for a distinct lacunar arteriopathy? Stroke. 2010;41:624–629. doi: 10.1161/STROKEAHA.109.558809. [DOI] [PubMed] [Google Scholar]
- 10.O'Donnell M.E., Badger S.A., Sharif M.A., Young I.S., Lee B., Soong C.V. The vascular and biochemical effects of cilostazol in patients with peripheral arterial disease. J Vasc Surg. 2009;49(5):1226–1234. doi: 10.1016/j.jvs.2008.11.098. [DOI] [PubMed] [Google Scholar]
- 11.Tan L., Margaret B., Zhang J.H. Efficacy and safety of cilostazol therapy in ischemic stroke: a meta-analysis. J Stroke Cerebrovasc Dis. 2015;24(5):930–938. doi: 10.1016/j.jstrokecerebrovasdis.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Miyamoto N., Pham L.D., Hayakawa K. Age-related decline in oligodendrogenesis retards white matter repair in mice. Stroke. 2013;44(9):2573–2578. doi: 10.1161/STROKEAHA.113.001530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tai S.Y., Chien C.Y., Chang Y.H., Yang Y.H. Cilostazol use is associated with reduced risk of dementia: a nationwide cohort study. Neurotherapeutics. 2017;14(3):784–791. doi: 10.1007/s13311-017-0512-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blair G.W., Appleton J.P., Law Z.K. Preventing cognitive decline and dementia from cerebral small vessel disease: the LACI-1 Trial. Protocol and statistical analysis plan of a phase IIa dose escalation trial testing tolerability, safety and effect on intermediary endpoints of isosorbide mononitrate and cilostazol, separately and in combination. Int J Stroke. 2018;13(5):530–538. doi: 10.1177/1747493017731947. [DOI] [PubMed] [Google Scholar]
- 15.Staals J., Makin S.D.J., Doubal F., Dennis M., Wardlaw J.M. Stroke subtype, vascular risk factors and total MRI brain small vessel disease burden. Neurology. 2014;83:1228–1234. doi: 10.1212/WNL.0000000000000837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fox S.C., May J.A., Shah A., Neubert U., Heptinstall S. Measurement of platelet P-selectin for remote testing of platelet function during treatment with clopidogrel and/or aspirin. Platelets. 2009;20(4):250–259. doi: 10.1080/09537100902912451. [DOI] [PubMed] [Google Scholar]
- 17.Thrippleton M.J., Shi Y., Blair G. Cerebrovascular reactivity measurement in cerebral small vessel disease: rationale and reproducibility of a protocol for MRI acquisition and image processing. Int J Stroke. 2018;13(2):195–206. doi: 10.1177/1747493017730740. [DOI] [PubMed] [Google Scholar]
- 18.Wardlaw J.M., Smith E.E., Biessels G.J. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration: a united approach. Lancet Neurol. 2013;12(8):822–838. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stevenson S.F., Doubal F.N., Shuler K., Wardlaw J.M. A systematic review of dynamic cerebral and peripheral endothelial function in lacunar stroke versus controls. Stroke. 2010;41 doi: 10.1161/STROKEAHA.109.569855. (e434-e42) [DOI] [PubMed] [Google Scholar]
- 20.Davidai G., Cotton D., Gorelick P. Dipyridamole-induced headache and lower recurrence risk in secondary prevention of ischaemic stroke: a post hoc analysis. Eur J Neurol. 2014;21(10):1311–1317. doi: 10.1111/ene.12484. [DOI] [PubMed] [Google Scholar]
- 21.Comerota A.J. Effect on platelet function of cilostazol, clopidogrel, and aspirin, each alone or in combination. Atheroscler Suppl. 2006;6:13–19. doi: 10.1016/j.atherosclerosissup.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Goto S. Cilostazol: potential mechanisms of action for antithrombotic effects accompanied by a low rate of bleeding. Atheroscler Suppl. 2006;6:3–11. doi: 10.1016/j.atherosclerosissup.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Saito S., Kojima S., Oishi N. A multicenter, randomized, placebo-controlled trial for cilostazol in patients with mild cognitive impairment: the COMCID study protocol. Alzheimers Dement (New York, N Y) 2016;2(4):250–257. doi: 10.1016/j.trci.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han S.W., Song T.J., Bushnell C.D. Cilostazol decreases cerebral arterial pulsatility in patients with mild white matter hyperintensities: subgroup analysis from the Effect of Cilostazol in Acute Lacunar Infarction Based on Pulsatility Index of Transcranial Doppler (ECLIPse) study. Cerebrovasc Dis. 2014;38(3):197–203. doi: 10.1159/000365840. [DOI] [PubMed] [Google Scholar]
- 25.Kim J.S., Kwon S.U., Uchiyama S. Cilostazol research in Asia: can it be applied to European and American patients? Int J Stroke. 2015;10(Suppl. 1):1–9. doi: 10.1111/ijs.12460. [DOI] [PubMed] [Google Scholar]
- 26.Rajani R.M., Quick S., Ruigrok S.R. Reversal of endothelial dysfunction reduces white matter vulnerability in cerebral small vessel disease in rats. Sci Transl Med. 2018;10(448) doi: 10.1126/scitranslmed.aam9507. [DOI] [PubMed] [Google Scholar]
- 27.Fujita Y., Lin J.X., Takahashi R., Tomimoto H. Cilostazol alleviates cerebral small-vessel pathology and white-matter lesions in stroke-prone spontaneously hypertensive rats. Brain Res. 2008;1203:170–176. doi: 10.1016/j.brainres.2008.01.103. [DOI] [PubMed] [Google Scholar]
- 28.Omote Y., Deguchi K., Tian F. Clinical and pathological improvement in stroke-prone spontaneous hypertensive rats related to the pleiotropic effect of cilostazol. Stroke. 2012;43(6):1639–1646. doi: 10.1161/STROKEAHA.111.643098. [DOI] [PubMed] [Google Scholar]
- 29.Maki T., Okamoto Y., Carare R.O. Phosphodiesterase III inhibitor promotes drainage of cerebrovascular beta-amyloid. Ann Clin Transl Neurol. 2014;1(8):519–533. doi: 10.1002/acn3.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Vos-Koppelaar N.C., Kerkhoff H., de Vogel E.M., Zock E., Dieleman H.G. The effect of a slower than standard dose escalation scheme for dipyridamole on headaches in secondary prevention therapy of strokes: a randomized, open-label trial (DOSE) Cerebrovasc Dis. 2014;37(4):285–289. doi: 10.1159/000360751. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material