Abstract
Inorganic arsenic (iAs) is an established environmental diabetogen. The link between iAs exposure and diabetes is supported by evidence from adult human cohorts and adult laboratory animals. The contribution of prenatal iAs exposure to the development of diabetes and underlying mechanisms are understudied. The role of factors that modulate iAs metabolism and toxicity in adults and their potential to influence diabetogenic effects of prenatal iAs exposure are also unclear. The goal of this study was to determine if prenatal exposure to iAs impairs glucose metabolism in mice and if maternal supplementation with folate and methylcobalamin (B12) can modify this outcome. C57BL/6J dams were exposed to iAs in drinking water (0, 100, and 1000 μg As/L) and fed a folate/B12 adequate or supplemented diet from before mating to birth of offspring. After birth, dams and offspring drank deionized water and were fed the folate/B12 adequate diet. The metabolic phenotype of offspring was assessed over the course of 14 weeks. Male offspring from iAs-exposed dams fed the folate/B12-adequate diet developed fasting hyperglycemia and insulin resistance. Maternal folate/B12 supplementation rescued this phenotype but had only marginal effects on iAs metabolism in dams. The diabetogenic effects of prenatal iAs exposure in male offspring were not associated with changes in global DNA methylation in the liver. Only minimal effects of prenatal iAs exposure or maternal supplementation were observed in female offspring. These results suggest that prenatal iAs exposure impairs glucose metabolism in a sex-specific manner and that maternal folate/B12 supplementation may improve the metabolic phenotype in offspring. Further studies are needed to identify the mechanisms underlying these effects.
Keywords: Arsenic, Prenatal exposure, Diabetes, Vitamin, Folate, Methylcobalamin, Dietary supplementation
Introduction
Globally, 9% of the human population has diabetes, a condition of chronic hyperglycemia (WHO 2016), which is associated with high morbidity and for which there is no cure. Type 2 diabetes (T2D) represents 90–95% of diabetes cases and is characterized by insulin resistance followed by beta cell dysfunction and impaired insulin secretion. The prevalence of T2D has almost doubled since 1980 (WHO 2016) and is expected to continue increasing (Rowley et al. 2017). While the increase in obesity is the main driver for the rise of T2D, particularly in children (Mayer-Davis et al. 2017), obesity alone does not explain the trends in T2D seen around the world. Exposures to environmental chemicals such as inorganic arsenic (iAs), bisphenol A, or various persistent organic pollutants may contribute (Thayer et al. 2012; Maull et al. 2012). Furthermore, a growing amount of data on environmentally induced T2D supports the developmental origins of health and disease (DOHaD) paradigm, in which adverse health and disease outcomes later in life are results of prenatal exposures to environmental chemicals (Heindel et al. 2015). This paradigm is also applicable to T2D associated with chronic exposure to iAs.
iAs is a common drinking water and food contaminant. The World Health Organization and US EPA limit for iAs concentration in drinking water is 10 parts per billion (ppb), or 10 μg As/L (WHO 2017; USEPA 2001). In many areas around the world, iAs levels in drinking water exceed this limit. Chronic exposure to iAs has been shown to be associated with increased incidence or prevalence of T2D in many geographical regions (Maull et al. 2012; Sung et al. 2015). In some of these regions, residents have been exposed to iAs only recently. For example, in Bangladesh, chronic exposure to iAs in drinking water began with the introduction of new wells drilled in the 1970s (Smith et al. 2000). However, in other regions, water contaminated with iAs has been used for drinking and cooking for generations (e.g., in Taiwan or Mexico); many residents in these regions have been exposed to iAs from preconception to adulthood. Thus, it is unclear how iAs exposure during different developmental windows (e.g., prenatal vs postnatal) contributes to the prevalence or incidence of T2D in these regions.
Data on the effects of prenatal iAs exposure on T2D development later in life is limited. In humans, cord blood As levels, a marker of in utero exposure, have been associated with changes in DNA methylation of genes that are implicated in T2D and with altered expression of microR-NAs that are involved in T2D-related dysfunctions (Martin et al. 2017a). In laboratory studies, rats and mice exposed to iAs prenatally or during both pre- and postnatal windows developed diabetic phenotypes (Dávila-Esqueda et al. 2011; Bonaventura et al. 2017; Ditzel et al. 2016; Sanchez-Soria et al. 2014; Rodriguez et al. 2016). However, different phenotypes were associated with different levels of iAs exposure and with different exposure windows. Taken together, human and animal data indicate that prenatal exposure alone, or combined with postnatal exposure, can result in T2D development later in life.
Mechanisms underlying the diabetogenic effects of iAs during the prenatal exposure window have never been systematically studied. The human data suggest that iAs exposure could disrupt DNA methylation during fetal development in utero, and that a competition between iAs and DNA methylation pathways for a methyl group donor could be an underlying mechanism (Martin et al. 2017a). In humans and many animal species, iAs is metabolized in a series of methylation steps to form monomethylarsenic (MAs), dimethylarsenic (DMAs), and in some species also trimethy-larsenic. Metabolism of iAs facilitates its excretion from the body. The methylation of iAs is catalyzed by arsenic (+ 3 oxidation state) methyltransferase which uses S-adenosyl-methionine (SAM) as a methyl group donor (Thomas et al. 2007). SAM is also the methyl donor for DNA methylation by DNA methyltransferases (Anderson et al. 2012). It has been hypothesized that an increased demand for SAM during iAs exposures could disrupt DNA methylation of genes that regulate insulin production and/or glucose metabolism, thus, affecting the susceptibility of adult offspring to T2D.
Several micronutrients, including the B vitamins, folate (B9) and methylcobalamin (B12), are required for the synthesis of SAM (Selhub 1999). In adult cohort studies, high folate intake has been associated with greater efficiency of iAs metabolism, typically understood as a lower proportion of urinary iAs and MAs and higher proportion of urinary DMAs, and with lower risk of iAs-associated urothelial cancers and skin lesions (Argos et al. 2010; Gamble et al. 2005, 2006; Hall et al. 2007; Hall and Gamble 2012; Huang et al. 2008). Similar effects have been reported for B12, but data are limited and inconsistent (Hall and Gamble 2012; Heck et al. 2007). Animal studies generally support the positive association between folate intake and efficiency of iAs metabolism (Wlodarczyk et al. 2012; Spiegelstein et al. 2003, 2005; Tsang et al. 2012), but few studies have investigated the role of B12. Combined folate and B12 supplementation has been found to alleviate iAs-induced apoptosis, oxidative stress, and hepatotoxicity (Acharyya et al. 2015; Mukherjee et al. 2006; Chattopadhyay et al. 2012), suggesting possible protective effects of these vitamins in iAs-associated diseases.
Increasing efficiency of iAs methylation (detoxification) by increasing folate and/or B12 intake may also improve health outcomes associated with in utero iAs exposures, although few studies have addressed this hypothesis. In a Bangladesh cohort, higher maternal folate and B12 levels were associated with lower concentrations of cord blood As (Hall et al. 2007). On the other hand, the increased efficiency of iAs metabolism throughout pregnancy was not associated with folate or B12 status in a different cohort of Bangladeshi women (Gardner et al. 2011; Li et al. 2008). Mice with impaired folate transport had higher rates of exen-cephaly than wild-type mice when exposed in utero to iAs. However, the deficiency in folate transport in these mice was not associated with altered iAs methylation efficiency (Wlodarczyk et al. 2001). In our studies, high dietary folate intake decreased iAs levels and increased ratios of DMAs/ MAs in livers of pregnant CD-1 mice exposed to iAs (Tsang et al. 2012). Exposure to 85 ppm iAs combined with folate supplementation had more profound effects on global DNA methylation in fetal livers than exposure to iAs or folate supplementation alone (Tsang et al. 2012), suggesting that epigenetic effects of prenatal iAs exposure in fetus can be modulated by maternal folate intake.
No studies to date have investigated the role of maternal dietary folate or B12 supplementation in modifying the risk of T2D in offspring exposed prenatally to iAs. The goal of the present study was to determine (1) if prenatal exposure to environmentally relevant levels of iAs results in an impaired metabolic phenotype (e.g. hyperglycemia, insulin resistance, glucose intolerance) in male and female offspring, and (2) if dietary supplementation with folate and B12 affects the metabolism of iAs in dams and the metabolic phenotype of the offspring. Our results suggest that prenatal iAs exposure impairs glucose homeostasis in offspring in a sex-dependent manner. Notably, maternal folate and B12 supplementation had a minimal effect on iAs metabolism in dams but appeared to alleviate some adverse metabolic phenotypes in the offspring.
Methods
Mice and diets
All procedures involving mice were approved by the University of North Carolina (UNC) Institutional Animal Care and Use Committee. Eight-week-old male and female C57BL/6J mice were obtained from Jackson Laboratories (Bar Harbor, ME, USA). Two types of purified AIN-93G diet (Dyets, Bethlehem, PA, USA) were used: (1) diet that contained 2 mg/kg folate, the level needed to support pregnancy and fetal health (Reeves et al. 1993), and 10 μg/kg B12 the level that represents adequate intake for adult mice (American Institute of Nutrition 1977), and (2) diet containing 6 mg/ kg folate and 50 μg/kg B12 (Dyets, Bethlehem, PA, USA). These diets are hereafter referred to as folate/B12 adequate and folate/B12 supplemented, respectively. A large amount of each diet from the same lot was purchased for this study to avoid biases associated with lot-to-lot differences in diet composition. After delivery to UNC, both male and female mice were fed the folate/B12 adequate diet and drank deionized water (DIW) for 1 week prior to exposure. Mice were housed under controlled conditions with 12-h light/dark cycle at 22 ± 1 °C and 50 ± 10% relative humidity. Food and water consumption was measured weekly.
Exposures and mating
After 1 week on the folate/B12 adequate diet and DIW, dams and sires were randomly divided into six treatment groups with different levels of iAs in drinking water and different levels of folate and B12 in the diet: (1) 0 ppb As and folate/B12 adequate diet, (2) 100 ppb As and folate/B12 adequate diet, (3) 1000 ppb As and folate/B12 adequate diet, (4) 0 ppb As, and folate/B12 supplemented diet, (5) 100 ppb As and folate/B12 supplemented diet, and (6) 1000 ppb As and folate/B12 supplemented diet. There were 6 dams in each treatment group except for the 0 ppb/folate/B12 supplemented group which had 9 dams. Drinking water (DIW) with iAs (sodium arsenite, ≥ 99% pure; Sigma–Aldrich, St. Louis, MO, USA) was prepared weekly to minimize oxidation of iAsIII to iAsV. After 1 week of treatment with iAs and feeding with either folate/B12 adequate or supplemented diet, dams and sires from the same treatment group were allowed to mate for 1 week (3 dams:1 sire). During mating, one dam in the 100 ppb/folate/B12 adequate group had to be euthanized because of significant loss of weight, leaving only 5 dams in this group. After mating, dams were housed 3 per cage and remained on the exposures and diets throughout gestation until parturition. After giving birth, the dams in all treatment groups were placed on the folate/B12 adequate diet and drank only pure DIW. All offspring were weaned at 3 weeks of age and maintained on the folate/B12 adequate diet and DIW for the remainder of the study. Experimental design is summarized in Supplemental Fig. 1 (Online resource 1).
Analysis of iAs and its metabolites in mouse diet
Arsenic species were measured in the adequate and folate/B12 supplemented diets using hydride-generation atomic absorption spectrometry coupled with a cryotrap (HG-CT-AAS) as previously described (Hernández-Zavala et al. 2008; Currier et al. 2011). The diets were pulverized and microwave digested in ultra-pure phosphoric acid (J. T. Baker, Phillipsburg, NJ, USA) prior to analysis (Currier et al. 2016).
Analyses carried out in dams
Analysis of iAs and its metabolites in urine HG-CT-AAS
was used to determine the concentrations of arsenic (As) species in spot urine samples collected from dams after the first week of exposure, just prior to mating. The HG-CT-AAS analysis determined concentrations of total iAs (iAsIII + iAsV), total MAs (MasIII + MAsV) and total DMAs (DMAsIII + DMAsV); no TMAs was detected. In this study, total As in urine is defined as the sum of all As species detected.
Plasma folate analysis
Plasma was isolated from blood collected from dams via tail bleed after 1 week of iAs exposure. Samples were diluted in Dulbecco’s phosphate-buffered saline (Mediatech, Manassas, VA, USA) and analyzed using ELISA kits following manufacturer’s protocol (Monobind Inc, Lake Forest, CA, USA).
Procedures involving offspring
Phenotyping
Body weight of weaned offspring was monitored weekly. Body composition (lean and fat mass) was determined at 14 weeks of age by magnetic resonance imaging (MRI) using EchoMRI Three-in-One Composition Analyzer and Labmaster version 3.2.2 software (Echo Medical Systems, Houston, TX, USA). Blood for measurement of fasting blood glucose (FBG) and fasting plasma insulin (FPI) was obtained via tail bleeds after a 6-h fast. FBG was measured by a OneTouch Ultra 2 glucometer (LifeScan, Milpitas, CA, USA) at 7, 8, 13, 14, and 18 weeks of age. FPI was measured at week 8 and 14. Plasma insulin levels were determined by ELISA (Millipore, Billerica, MA, USA) following the manufacturer’s protocol. FBG (mg/dL) and FPI (μU/mL) were used to calculate the homeostasis model assessment-insulin resistance (HOMA-IR) value:
Glucose tolerance tests were administered at 7 and 13 weeks of age. Fasted mice were injected intraperitoneally (i.p.) with 2 g/kg body weight of D-glucose (Sigma–Aldrich) dissolved in Dulbecco’s phosphate-buffered saline (Mediatech, Manassas, VA, USA). Blood glucose levels were measured at 15, 30, 60 and 120 min post-injection. To quantify glucose tolerance, the area under the curve (AUC) was calculated for a plot of blood glucose (mg/dL) versus time (minutes) using Prism 5 (GraphPad, La Jolla, CA, USA).
Global DNA methylation in liver of offspring
Liver collected during sacrifice were flash-frozen in liquid nitrogen, and stored at –80 °C. DNA was extracted using Allprep DNA/RNA extraction kit (Qiagen, Venlo, Netherlands). Global DNA methylation was assessed using the MethylFlash Methylated DNA Quantification kit (Epigentek, Farmingdale, NY). Percent 5-mC was calculated using the equation provided by the manufacturer:
where Abs = absorbance at 450 nm, NC=negative control (0% 5-mC), and PC = positive control (50% 5-mC).
Statistical analysis
Differences due to iAs exposure were assessed using ANOVA and Tukey’s post hoc tests. Differences due to sex or diet were assessed using two-tailed Student’s T tests between groups of interest (e.g., to determine significant effect of sex: male offspring from dams fed the folate/B12 adequate diet and exposed to 0 ppb iAs were compared to female offspring from dams fed the folate/B12 adequate diet and exposed to 0 ppb iAs animals). Three-way factorial ANOVAs were used to test for a significant effect of individual variables (sex, dam diet, dam iAs exposure) in most metabolic measures. This analysis also identified significant two-way and three-way interactions between the three variables. All statistical analyses were conducted using JMP 12 (SAS Institute, Cary, NC).
Results
Maternal measures
Dams were exposed to iAs in drinking water and fed the specified diets 1 week before and 1 week during the mating. This time period is sufficient to establish equilibrium of As (Hughes et al. 2003) and folate (Schmitz et al. 1994) in blood and tissues of mice. With limited data on B12, we assumed that this time was also sufficient to reach B12 equilibrium. The folate/B12 adequete and supplemented diets used in this study contained between 40 and 50 ppb As, all iAs. There were no differences in body weight at the start of exposure or at the beginning of mating (Suppl. Figure 2). Folate levels in plasma of the folate/B12 supplemented dams were significantly higher than in dams fed the folate/B12 adequate diet (Fig. 1). Notably, exposure to 100 ppb iAs, but not 1000 ppb iAs, significantly increased plasma folate in dams in both adequate and supplemented groups. Plasma B12 concentration could not be measured due to insufficient volume of blood collected from tail bleeds.
Fig. 1.
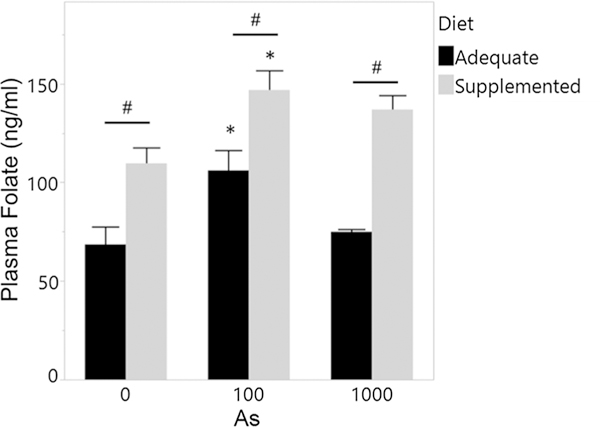
Plasma folate concentrations of dams fed folate/B12 adequate and supplemented diets and exposed to iAs. Plasma was isolated from blood collected via tail bleed from dams after exposure to iAs for 1 week, shortly before mating. Mean + SEM for N = 3 are shown. *p < 0.05 significantly different from controls (0 ppb iAs) within the same diet group; #p < 0.05 significantly different from dams fed the adequate diet
To determine effects of folate/B12 intake on iAs metabolism, we measured concentrations of iAs and its metabolites in spot urine samples collected from dams (Fig. 2). Total As concentrations were proportionally higher in urine of dams exposed to 100 and 1000 ppb iAs as compared to untreated controls. DMAs was the major urinary metabolite representing 85–94% and 79–93% of total As in urine of iAs-treated dams fed adequate diet and supplemented diet, respectively. There were no significant differences in %DMAs, %MAs, or %iAs in urine due to the exposure or supplementation (Suppl. Figure 3). Folate/B12 supplementation increased total As levels (i.e., sum of As species) in urine in both 100 and 1000 ppb exposure groups, but these effects were not statistically significant (Fig. 2D). Marginally significant effects of folate/B12 supplementation were found in the urine of dams exposed to 100 ppb iAs (Fig. 2B, C); here, MAs and DMAs concentrations were higher in urine of the supplemented dams (p = 0.11 and 0.079, respectively). The content of As in the folate/B12 adequate and supplemented diets ranged from 40 to 43 ppb; only iAs was detected.
Fig. 2.
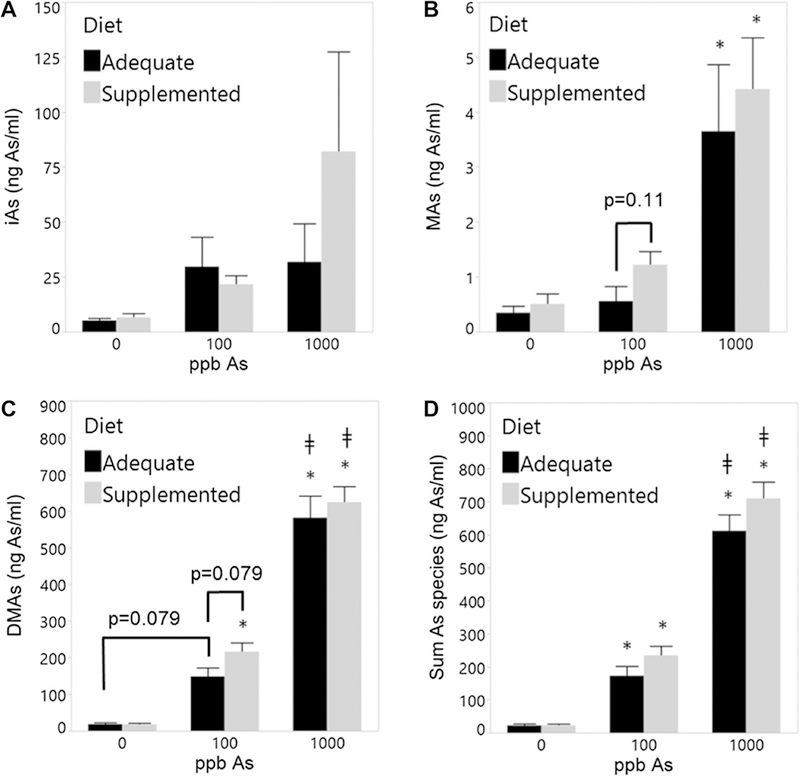
Arsenic metabolites in urine of dams fed a folate/B12-adequate or supplemented diet and exposed to 0, 100, or 1000 ppb iAs. A iAs, B MAs, C DMAs, and D sum of As. Spot urine samples were collected from dams exposed to iAs for 1 week, shortly before mating. Mean + SEM for N = 5–9 is shown. *p < 0.05 significantly different from respective control (0 ppb) within the same diet group; ǂp < 0.05 significantly different from 100 ppb iAs exposure
Number of offspring
Since dams were housed 3 per cage, the number of pups per litter could not be determined and assessed statistically. Instead, the total numbers of offspring in each treatment group were recorded (Suppl. Table 1).
Phenotypes of the offspring
Food and water consumption
There were no significant differences in food or water consumption due to prenatal iAs exposure or maternal supplementation throughout the course of the study (data not shown). Male offspring tended to eat and drink more than female offspring.
Body weight
Only minimal differences were observed in body weight between offspring of the same sex after weaning, regardless of treatment (Suppl. Figure 4). Males were heavier than females throughout the entire study. Few statistically significant differences were found between treatment groups and only at few time points. There were no significant differences in body weight at the end of the study, except that female offspring from supplemented dams exposed to 100 ppb iAs were heavier than controls at sacrifice.
Body composition
Body composition was assessed at 14 weeks of age. At this time point, male offspring had higher body weight and higher lean mass than female offspring, but had similar %fat (Fig. 3). Prenatal exposure to iAs had little or no effect on body composition of the offspring from either the folate/B12 adequate or supplemented dams. However, male offspring from dams fed the adequate diet and prenatally exposed to 1000 ppb iAs had higher %fat than the corresponding controls (p = 0.09) and males exposed prenatally to 100 ppb iAs (p = 0.018) (Fig. 3B). Notably, maternal supplementation significantly lowered %fat in male offspring exposed to 1000 ppb iAs to levels even lower than those found in controls (Fig. 3B). Maternal supplementation also increased body weight and lean mass in female controls, but had no statistically significant effect on %fat (Fig. 3).
Fig. 3.
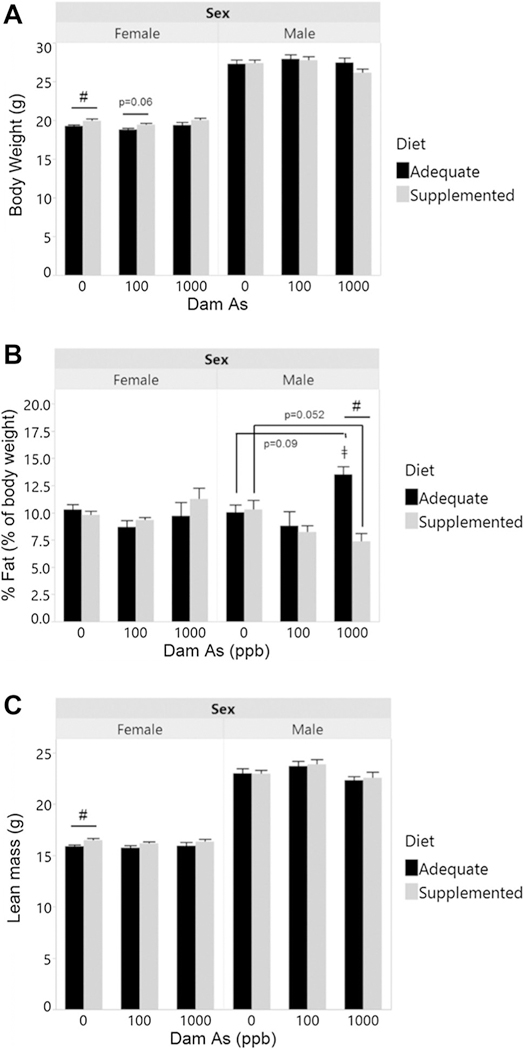
Body weight and composition of male and female offspring prenatally exposed to: A body weight, B fat mass (expressed as % of body weight), and C lean mass were determined in 14-week-old offspring from dams exposed to 0, 100, or 1000 ppb iAs in drinking water and fed either a folate/B12-adequate diet. Mean + SEM for N=7–16 is shown. ǂp < 0.05 significantly different from 100 ppb iAs exposure; #p < 0.05 significantly different from offspring from dams fed the adequate diet
Fasting blood glucose (FBG)
Blood glucose after a 6-h fast was measured at week 7, 8, 13, 14, and 18 of age (Fig. 4). In general, male offspring had higher FBG than female offspring, regardless of supplementation or exposure. Prenatal exposure to iAs had significant effects on FBG, but almost exclusively in male offspring from dams fed the folate/B12-adequate diet. Specifically, males exposed prenatally to iAs had higher FBG than control males at multiple time points (Fig. 4A). The trajectory of FBG levels during the 18 weeks of the study differed between males in different exposure groups. In males exposed prenatally to 100 ppb, FBG peaked between weeks 13 and 14 and then decreased to the control level at week 18; in males exposed to 1000 ppb iAs, FBG increased at week 14 and remained significantly higher than the control levels until the end of the study. In comparison, iAs exposure did not increase FBG in male offspring from folate/B12 supplemented dams, or the effects were not statistically significant. In contrast, prenatal iAs exposure or folate/B12 supplementation had little or no effects on FBG of female offspring (Fig. 4B). FBG was higher in 7-week-old females from adequate-diet dams exposed to 100 ppb iAs compared to respective controls, but no effects of iAs exposure were observed in other groups or time points.
Fig. 4.
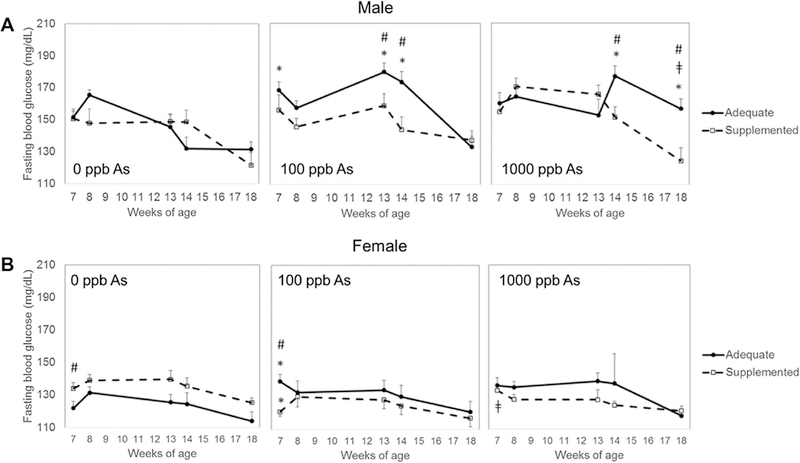
Fasting blood glucose of offspring prenatally exposed to iAs. Fasting blood glucose was measured in A male and B female offspring from dams exposed to 0, 100, or 1000 ppb iAs in drinking water and fed either a folate/B12-adequate (closed circles) or supplemented (open squares) diet. Blood was collected from offspring at age 7, 8, 13, 14 and 18 weeks. Mean + SEM of N = 5–16 is shown. *p < 0.05 significantly different from controls (0 ppb iAs) within the same diet goup; ǂp < 0.05 significantly different from 100 ppb iAs exposure; #p < 0.05 significantly different from offspring from dams fed the adequate diet
Maternal supplementation significantly decreased FBG in 7-week-old females prenatally exposed to 100 ppb iAs, but no statistically significant effects of the supplementation were found in this or the 1000 ppb iAs exposure group at other time points. Additionally, control females from supplemented dams had higher FBG than control females from adequate diet dams, but only at week 7; the effects of maternal supplementation at later time points were not statistically significant.
Glucose tolerance
Intraperitoneal glucose tolerance tests were conducted at week 7 and 13 (Fig. 5). Female offspring were generally more glucose tolerant than males, regardless of prenatal iAs exposure or maternal supplementation. Surprisingly, 7-week-old female offspring from supplemented dams in the control group were more glucose intolerant that females from control adequate dams (Fig. 5A). At this time point, glucose tolerance was significantly lower in female offspring from adequate-diet dams exposed to 100 ppb iAs as compared to the respective controls (Fig. 5A). There were no significant effects of prenatal iAs on glucose tolerance in 7-week-old male offspring. At week 13, there were no differences in glucose tolerance in females or males due to prenatal iAs exposure or maternal supplementation (Fig. 5B). In male offspring, supplementation improved glucose tolerance in animals prenatally exposed to 100 ppb iAs.
Fig. 5.
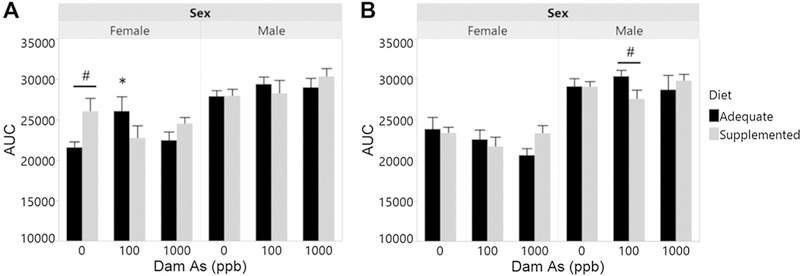
Glucose tolerance of offspring prenatally exposed to iAs. Intraperitoneal glucose tolerance tests were administered to offspring of dams exposed to 0, 100, or 1000 ppb iAs in drinking water and fed either a folate/B12-adequate or supplemented diet. Area under the curve for A 7-week-old and B 13-week-old offspring is shown. Mean + SEM of N = 7–16 is shown. *p < 0.05 significantly different from controls (0 ppb iAs) within the same diet group; #p < 0.05 significantly different from offspring from dams fed the adequate diet
Fasting plasma insulin (FPI) and HOMA-IR
FBG and FPI values were used to calculate HOMA-IR, a measure of insulin resistance, at weeks 8 and 14. FPI and HOMA-IR in all offspring were higher at week 14 compared to week 8 (Fig. 6). At week 8, there were no significant differences in FPI and HOMA-IR due to prenatal iAs exposure in either female or male offspring (Fig. 6A, B). Maternal supplementation lowered HOMA-IR in control male offspring and in female offspring exposed prenatally to 1000 ppb iAs, but these effects were only marginally significant. At week 14, there were no significant differences in FPI and HOMA-IR in female offspring related to iAs exposure or maternal diet. In contrast, in males from dams fed the adequate diet, prenatal iAs exposure increased HOMA-IR compared to the respective controls (Fig. 6D). This effect was statistically significant for 100 ppb exposure but not for 1000 ppb exposure (p = 0.21). However, no effects of iAs exposure on FPI were found. Notably, maternal supplementation decreased HOMA-IR in both 100 and 1000 ppb groups and decreased FPI with marginal significance.
Fig. 6.
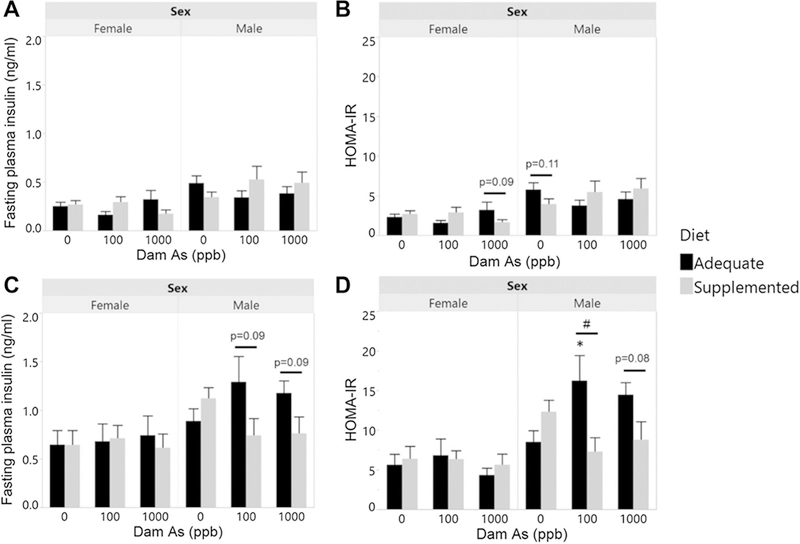
Fasting plasma insulin and HOMA-IR measures of offspring prenatally exposed to iAs: Fasting plasma insulin (A, C) and HOMA-IR (B, D) of 8-week-old offspring (A, B) (N = 7–16) and 14-week-old offspring (C, D) (N = 5–15) from dams exposed to 0, 100, or 1000 ppb iAs in drinking water and fed either a folate/B12-adequate or supplemented diet. Mean + SEM is shown. *p < 0.05 significantly different from controls (0 ppb iAs) within the same diet group; #p < 0.05 significantly different from offspring from dams fed the adequate-diet
Significant effects and interactions
Three-way factorial ANOVAs were conducted to assess overall significance of sex, maternal supplementation (dam diet), and prenatal iAs exposure as variables affecting the measurements of metabolic phenotype (e.g., to assess if sex is a significant variable in FBG measures, after averaging over all other variables). Sex of the offspring was a significant variable in most metabolic measures (Table 1). Dam diet and iAs exposure were significant variables only in some outcomes. The ANOVA analysis also identified significant interactive effects of these variables. The interaction of sex of the offspring with dam diet or iAs exposure were significant in some outcomes, indicating that the effect of dam diet or iAs exposure may be different in male compared to female offspring (Table 1).
Table 1.
p values from three-way ANOVA tests assessing the significance of sex, dam diet, or prenatal iAs exposure and the significance of interactions of these variables in measures of metabolic phenotype
| Outcomes | Effect |
||||||
|---|---|---|---|---|---|---|---|
| Sex | Dam diet | Prenatal iAs | Sex* Diet | Sex*iAs | Diet* iAs | Sex* iAs* Diet | |
| Week 7 measures | |||||||
| AUC | < 0.01 | 0.04 | 0.60 | 0.05 | 0.50 | 0.02 | 0.14 |
| FPI | 0.02 | 0.35 | 0.98 | 0.24 | 0.89 | 0.09 | 0.16 |
| HOMA-IR | < 0.01 | 0.33 | 0.81 | 0.14 | 0.92 | 0.14 | 0.10 |
| Week 14 measures | |||||||
| Body weight | < 0.0001 | 0.29 | 0.72 | 0.43 | 0.03 | 0.46 | 0.48 |
| Lean mass | < 0.0001 | 0.39 | 0.11 | 0.32 | 0.02 | 1.00 | 0.88 |
| %Fat | 0.86 | 0.85 | 0.01 | 0.60 | 0.85 | 0.12 | < 0.01 |
| AUC | < 0.0001 | 0.78 | 0.47 | 0.81 | 0.46 | 0.08 | 0.66 |
| FPI | 0.01 | 0.42 | 0.83 | 0.41 | 0.96 | 0.19 | 0.12 |
| HOMA-IR | 0.01 | 0.15 | 0.69 | 0.33 | 0.69 | 0.01 | 0.03 |
| Fasting blood glucose | |||||||
| Week 7 | < 0.0001 | 0.31 | 0.17 | 0.01 | 0.13 | 0.75 | 0.06 |
| Week 8 | < 0.0001 | 0.33 | 0.12 | 0.01 | 0.30 | 0.89 | 0.05 |
| Week 13 | 0.01 | 0.13 | 0.05 | 0.35 | 0.03 | 0.04 | 0.09 |
| Week 14 | 0.08 | 0.02 | 0.24 | 0.52 | < 0.01 | < 0.01 | 0.04 |
| Week 18 | 0.20 | 0.90 | 0.16 | 0.05 | 0.34 | 0.10 | 0.09 |
Significance of p values in bold (p < 0.05)
Global DNA methylation
DNA methylation was assessed only in livers of male offspring from folate/B12 adequate and supplemented dams exposed to 0 or 1000 ppb iAs, i.e., the offspring with different phenotypes at the time of sacrifice. Specifically, the male offspring from the folate/B12 adequate dams exposed to 1000 ppb iAs had significantly higher FBG levels and HOMA-IR than male offspring from the adequate control dams (Figs. 4, 6). Supplementation of dams with folate/B12 prevented this increase, suggesting that epigenetic mechanisms may underlie the effects of iAs exposure and/or folate/B12 supplementation. We found that the fraction of methylated DNA (%5-mC) was significantly higher in livers of offspring from both control and iAs-exposed dams fed the folate/B12 supplemented diet as compared to offspring from dams fed the adequate diet (Fig. 7). However, no significant effects of iAs exposure on DNA methylation were found.
Fig. 7.
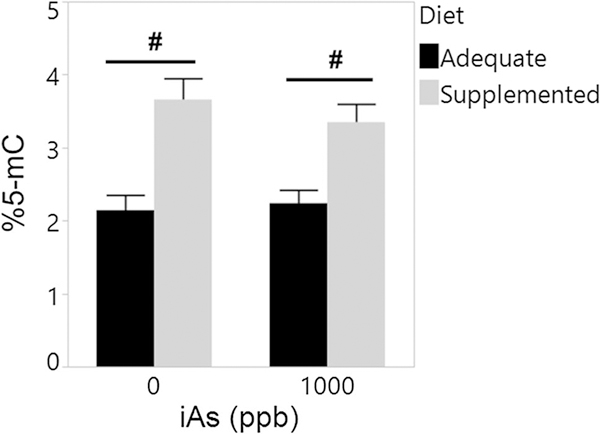
Percent global DNA methylation in livers of male offspring exposed prenatally to iAs. DNA methylation was assessed in livers of male offspring of dams exposed to 0 and 1000 ppb iAs in drinking water and fed a folate/B12-adequate or supplemented diet. Mean + SEM for N = 3–7 is shown. #p < 0.05 significantly different from offspring of adequate-diet dams
Discussion
Effect of prenatal iAs exposure
Numerous studies in adult human cohorts and in adult laboratory animals support the association between iAs exposure and T2D (Maull et al. 2012; Kuo et al. 2017). Less is known about the role of prenatal iAs exposure in the development of T2D, or metabolic disease in general, later in life. Previously published laboratory studies used rats or mice with either prenatal exposure or combined prenatal and postnatal exposures. In rats, the combined pre- and postnatal exposure to iAs (3–50 ppm) produced hyperglycemia, impaired glucose tolerance, and insulin resistance (increased HOMA-IR). Effects on plasma insulin levels were inconsistent (Bonaventura et al. 2017; Dávila-Esqueda et al. 2011). In a study using Sprague–Dawley rats, whole-life exposure to 50 ppm iAs increased HOMA-IR, but only in female offspring (Bonaventura et al. 2017). In another study, female CD-1 offspring exposed to a very low level of iAs in drinking water (10 ppb) during gestation accumulated more fat than unexposed controls and exhibited impaired glucose tolerance and fasting hyperinsulinemia; male mice were not studied (Rodriguez et al. 2016). In a study by Ditzel et al., Swiss Webster mice were exposed to 100 ppb iAs either prenatally, postnatally, or both pre- and postnatally, and fed a Western-style diet (2016). Here, only male mice with combined prenatal and postnatal exposures developed an adverse metabolic phenotype characterized by insulin resistance and increased plasma triglyceride levels. These males were also heavier than unexposed male controls. Thus, the published studies on prenatal iAs exposures and its effects on glucose metabolism include a variety of exposure periods and iAs doses, and report a variety of metabolic phenotypes, some of which are sex-dependent. Notably, levels of iAs in rodent diets used in these studies are usually not measured or reported. Analyses of several types of rodent chows in our lab and in other laboratories have found total As levels to range from ~ 10 to over 400 ppb, with iAs being a major As species in some of these diets (Douillet et al. 2016; Murko et al. 2018). Thus, exposure to iAs from chow diet could be a significant confounder in laboratory studies, particularly in studies examining effects of low-level iAs exposure in drinking water.
In this study, we used the C57BL/6J mice, a strain commonly used for laboratory study of obesity-related T2D and metabolic disease. To limit background exposure to iAs from diet, we fed the mice a purified diet that contained only ~ 40–50 ppb iAs. We exposed these mice to 100 or 1000 ppb As in the form of iAs, levels that are consistent with human environmental exposures. We found that mice exposed to iAs during preconception and gestation periods developed an adverse metabolic phenotype characterized mainly by fasting hyperglycemia, insulin resistance, and fat accumulation. These phenotypic characteristics have also been reported in some of the previous prenatal exposure studies (Ditzel et al. 2016; Dávila-Esqueda et al. 2011; Bonaventura et al. 2017; Sanchez-Soria et al. 2014). We did not observe significant differences in glucose tolerance, as had been reported by Rodriguez et al. (2016) and Bonaventura et al. (2017). Impaired glucose tolerance indicates a failure of the pancreas to adequately respond to a high glucose dose, while high FBG usually reflects increased activity of gluconeogenesis or glycogenolysis during fasting (Petersen et al. 2017). The lack of differences in glucose tolerance between exposed and control offspring in this study suggests that the pancreas was able to produce enough insulin to handle the glucose challenge during the glucose tolerance tests. Increased FBG levels, which appear to drive the increase in HOMA-IR, may point to the mechanisms regulating hepatic gluconeogenesis and/or glycogenolysis as a target of iAs exposure. In general, the effects on glucose homeostasis found in our study were not as dramatic or persistent as those found in some of the other studies, in which animals were exposed to iAs both pre- and postnatally, and which used higher iAs doses or did not control for the exposure to iAs from animal diet. It is possible that higher iAs doses and/or continued postnatal iAs exposure, in addition to the prenatal exposure, are necessary to observe more robust or persistent effects on metabolic outcomes.
In the present study, manifestation of the adverse metabolic phenotype depended strongly on sex, an important biological variable that has not been systematically examined in previous studies. We found that the male offspring were more susceptible than the female offspring to the adverse effects of prenatal iAs exposure. These results differ from findings reported in rats after whole-life (pre- and postnatal) exposure to much higher levels of iAs (50 ppm) where females were more susceptible to developing insulin resistance than males (Bonaventura et al. 2017). Sex-dependent responses to prenatal iAs exposure, specifically smaller fetal size and altered DNA methylation patterns in cord blood, have also been reported in human studies (Broberg et al. 2014; Pilsner et al. 2012). It has been suggested that these differences may be related to sexual dimorphisms in epigenetic pattering in the placenta, which could lead to differential transport of toxicants, nutrients, and signaling factors (Martin et al. 2017b). Inclusion of male and female offspring in future prenatal iAs exposure studies in both animals and humans should help characterize the extent and mechanisms of this differential susceptibility.
Effects of maternal folate and B12 supplementation
Low folate and/or vitamin B12 intake have been linked to low iAs methylation capacity in human studies (Argos et al. 2010; Gamble et al. 2005, 2006; Hall et al. 2007). It has been proposed that risk of iAs-associated disease could be lowered by stimulating iAs metabolism via increasing SAM availability through dietary supplementation with these B vitamins. In the present study, plasma folate levels were higher in the folate/B12-supplemented dams as compared to dams fed the adequate diet. However, no significant stimulation of iAs metabolism was observed. The concentrations of MAs and DMAs were higher in urine of the supplemented dams compared to dams fed the adequate diet, but because of small numbers of mice per treatment group, these differences were only marginally significant. No differences were found in urinary total As levels or in %DMAs, the indicator of iAs methylation efficiency. In comparison, folate supplementation in an iAs-exposed Bangladeshi population increased %DMAs in urine from 72 to 79% (Gamble et al. 2006). However, this population was folate-deficient while the mice in our study had adequate folate intake. This may explain why we did not find significant effects on iAs metabolism.
Notably, we found that exposure to 100 ppb iAs increased plasma folate levels in dams fed either the adequate or supplemented diet (Fig. 1). This finding has never been reported in human or laboratory studies. Chi and coworkers have recently reported that iAs exposure results in upregulation of microbial genes that regulate synthesis and metabolism of folate in mouse colon (Chi et al. 2017). Thus, the increase in plasma folate in iAs-exposed dams in the present study may be due to stimulation of folate synthesis in gut microbiome.
Despite only small effects on iAs metabolites in urine, maternal folate and B12 supplementation rescued the metabolic defects observed in male offspring exposed prenatally to iAs. These protective effects could be due to changes in the methylation of fetal DNA. Maternal folate and/or B12 supplementation has previously been reported to affect DNA methylation in offspring (Cooney et al. 2002; Water-land and Jirtle 2003; Yadav et al. 2018) and to alter body weight, glucose tolerance, insulin resistance, and expression of genes regulating food intake in rat offspring (Cho et al. 2015). Effects of gestational iAs exposure on DNA methylation and expression of genes in the fetus have also been reported. Specifically, gestational exposure to iAs was associated with altered DNA methylation and expression of genes in cord blood of newborns, including methylation of genes associated with T2D (Rojas et al. 2015). It is possible that maternal folate/B12 may prevent the reprograming of fetal T2D-associated genes by iAs exposure, conferring the protective effects observed in the present study. In this study, we did find that maternal supplementation with folate/ B12 increased global DNA methylation in the livers of male offspring; however, we did not assess DNA methylation of specific genes. Our future studies will focus on DNA methylation of T2D-associated genes as a potential mechanism underlying the adverse phenotype in offspring exposed prenatally to iAs and on the role of sex.
In conclusion, we found modest defects in the metabolic phenotype of C57B/6J mice after prenatal (combined preconception and in utero) exposure to environmentally relevant levels of iAs in drinking water. These defects included hyperglycemia and insulin resistance and were found almost exclusively in male offspring, indicating that sex plays a major role in T2D risk associated with this type of iAs exposure. Maternal folate and B12 supplementation alleviated the adverse effects of prenatal iAs exposure for some metabolic endpoints but did not increase urinary As excretion or significantly change the profiles of As species in urine. Tissue analyses of As metabolites may help to better understand the role these vitamins play in iAs metabolism in mice. Altered DNA methylation remains a potential mechanism of the adverse metabolic effects of prenatal iAs exposure and of the protective effects of maternal folate/B12 supplementation in the offspring. Further investigation into the methylation of specific T2D-related genes will be needed to probe this mechanism.
Supplementary Material
Acknowledgements
The authors would like to thank Dr. Zuzana Drobna (North Carolina State University, Raleigh, NC, USA) for her advice regarding prenatal folate and B12 supplementation. This work was funded by NIH 1R01ES022697 and R01 ES022697–03S1 Grants to M.S. and NIEHS F31ES027743 Grant to M.H. Additional support was provided by NIH Grant DK 056350 to the Nutrition Obesity Research Center at UNC.
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s00204–018-2206-z) contains supplementary material, which is available to authorized users.
References
- Acharyya N, Deb B, Chattopadhyay S, Maiti S (2015) Arsenic-induced antioxidant depletion, oxidative DNA breakage, and tissue damages are prevented by the combined action of folate and vitamin B12. Biol Trace Elem Res 168(1):122–132. 10.1007/s12011-015-0324-5 [DOI] [PubMed] [Google Scholar]
- American Institute of Nutrition (1977). AIN report of the AIN ad hoc committee on standards for nutritional studies. J Nutr 107:1340–1348 [DOI] [PubMed] [Google Scholar]
- Anderson OS, Sant KE, Dolinoy DC (2012) Nutrition and epigenetics: an interplay of dietary methyl donors, one-carbon metabolism, and DNA methylation. J Nutr Biochem 23:853–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argos M, Rathouz PJ, Pierce BL, Kalra T, Parvez F, Slavkovich V, Ahmed A, Chen Y, Ahsan H (2010) Dietary B vitamin intakes and urinary total arsenic concentration in the Health Effects of Arsenic Longitudinal Study (HEALS) cohort, Bangladesh. Eur J Nutr 49:473–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaventura MM, Bourguignon NS, Bizzozzero M, Rodriguez D, Ventura C, Cocca C, Libertun C, Lux-Lantos VA (2017) Arsenite in drinking water produces glucose intolerance in pregnant rats and their female offspring. Food Chem Toxicol 100:207–216 [DOI] [PubMed] [Google Scholar]
- Broberg K, Ahmed S, Engström K, Hossain MB, Mlakar SJ, Bottai M, Grandér M, Raqib R, Vahter M (2014) Arsenic exposure in early pregnancy alters genome-wide DNA methylation in cord blood, particularly in boys. J Dev Orig Health Dis 5:288–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S, Deb B, Maiti S (2012) Hepatoprotective role of vitamin B12 and folic acid in arsenic intoxicated rats. Drug Chem Toxicol 35:81–88 [DOI] [PubMed] [Google Scholar]
- Chi L, Bian X, Gao B, Tu P, Ru H, Lu K (2017) The effects of an environmentally relevant level of arsenic on the gut microbiome and its functional metagenome. Toxicol Sci 160:193–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho CE, Pannia E, Huot PSP, Sánchez-Hernández D, Kubant R, Dodington DW, Ward WE, Bazinet RP, Anderson GH (2015) Methyl vitamins contribute to obesogenic effects of a high multivitamin gestational diet and epigenetic alterations in hypothalamic feeding pathways in Wistar rat offspring. Mol Nutr Food Res 59:476–489 [DOI] [PubMed] [Google Scholar]
- Cooney CA, Dave AA, Wolff GL (2002) Maternal methyl supplements in mice affect epigenetic variation and DNA methylation of offspring. J Nutr 132:2393S–2400S [DOI] [PubMed] [Google Scholar]
- Currier JM, Svoboda M, Matoušek T, Dědina J, Stýblo M (2011) Direct analysis and stability of methylated trivalent arsenic metabolites in cells and tissues. Metallomics 3:1347–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currier JM, Douillet C, Drobná Z, Stýblo M (2016) Oxidation state specific analysis of arsenic species in tissues of wild-type and arsenic (+ 3 oxidation state) methyltransferase-knockout mice. J Environ Sci (China) 49:104–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dávila-Esqueda ME, Morales JMV, Jiménez-Capdeville ME, De la Cruz E, Falcón-Escobedo R, Chi-Ahumada E, Martin-Pérez S (2011) Low-level subchronic arsenic exposure from prenatal developmental stages to adult life results in an impaired glucose homeostasis. Exp Clin Endocrinol Diabetes 119:613–617 [DOI] [PubMed] [Google Scholar]
- Ditzel EJ, Nguyen T, Parker P, Camenisch TD (2016) Effects of arsenite exposure during fetal development on energy metabolism and susceptibility to diet-induced fatty liver disease in male mice. Environ Health Perspect 124:201–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douillet C, Huang MC, Saunders RJ, Dover EN, Zhang C, Stýblo M (2017) Knockout of arsenic (+ 3 oxidation state) methyltransferase is associated with adverse metabolic phenotype in mice: the role of sex and arsenic exposure. Arch Toxicol 91:2617–2627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble MV, Liu X, Ahsan H, Pilsner R, Ilievski V, Slavkovich V, Parvez F, Levy D, Factor-Litvak P, Graziano JH (2005) Folate, homocysteine, and arsenic metabolism in arsenic-exposed individuals in Bangladesh. Environ Health Perspect 113:1683–1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble MV, Liu X, Ahsan H, Pilsner JR, Ilievski V, Slavkovich V, Parvez F, Chen Y, Levy D, Factor-Litvak P et al. (2006) Folate and arsenic metabolism: a double-blind, placebo-controlled folic acid–supplementation trial in Bangladesh. Am J Clin Nutr 84:1093–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner RM, Nermell B, Kippler M, Grandér M, Li L, Ekström E-C, Rahman A, Lönnerdal B, Hoque AMW, Vahter M (2011) Arsenic methylation efficiency increases during the first trimester of pregnancy independent of folate status. Reprod Toxicol 31:210–218 [DOI] [PubMed] [Google Scholar]
- Hall MN, Gamble MV (2012) Nutritional manipulation of one-carbon metabolism: effects on arsenic methylation and toxicity. J Toxicol 2012:595307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M, Gamble M, Slavkovich V, Liu X, Levy D, Cheng Z, van Geen A, Yunus M, Rahman M, Pilsner JR et al. (2007) Determinants of arsenic metabolism: blood arsenic metabolites, plasma folate, cobalamin, and homocysteine concentrations in maternal-newborn pairs. Environ Health Perspect 115:1503–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck JE, Gamble MV, Chen Y, Graziano JH, Slavkovich V, Parvez F, Baron JA, Howe GR, Ahsan H (2007) Consumption of folate-related nutrients and metabolism of arsenic in Bangladesh. Am J Clin Nutr 85:1367–1374 [DOI] [PubMed] [Google Scholar]
- Heindel JJ, Newbold R, Schug TT (2015) Endocrine disruptors and obesity. Nat Rev Endocrinol 11:653–661 [DOI] [PubMed] [Google Scholar]
- Hernández-Zavala A, Matoušek T, Drobná Z, Paul DS, Walton F, Adair BM, Jiří D, Thomas DJ, Stýblo M (2008) Speciation analysis of arsenic in biological matrices by automated hydride generation-cryotrapping-atomic absorption spectrometry with multiple microflame quartz tube atomizer (multiatomizer). J Anal At Spectrom 23:342–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y-K, Pu Y- S, Chung C- J, Shiue H- S, Yang M- H, Chen C- J, Hsueh Y- M (2008) Plasma folate level, urinary arsenic methylation profiles, and urothelial carcinoma susceptibility. Food Chem Toxicol 46:929–938 [DOI] [PubMed] [Google Scholar]
- Hughes MF, Kenyon EM, Edwards BC, Mitchell CT, Razo LMD, Thomas DJ (2003) Accumulation and metabolism of arsenic in mice after repeated oral administration of arsenate. Toxicol Appl Pharmacol 191:202–210 [DOI] [PubMed] [Google Scholar]
- Kuo C-C, Moon KA, Wang S- L, Silbergeld E, Navas-Acien A (2017) The association of arsenic metabolism with cancer, cardiovascular disease, and diabetes: a systematic review of the epidemiological evidence. Environ Health Perspect 125:087001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Ekström E-C, Goessler W, Lönnerdal B, Nermell B, Yunus M, Rahman A, Arifeen SE, Persson L, Vahter M (2008) Nutritional status has marginal influence on the metabolism of inorganic arsenic in pregnant Bangladeshi women. Environ Health Perspect 116:315–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin EM, Stýblo M, Fry RC (2017a) Genetic and epigenetic mechanisms underlying arsenic-associated diabetes mellitus: a perspective of the current evidence. Epigenomics 9:701–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin E, Smeester L, Bommarito PA, Grace MR, Boggess K, Kuban K, Karagas MR, Marsit CJ, O’Shea TM, Fry RC (2017. b) Sexual epigenetic dimorphism in the human placenta: implications for susceptibility during the prenatal period. Epigenomics 9:267–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maull EA, Ahsan H, Edwards J, Longnecker MP, Navas-Acien A, Pi J, Silbergeld EK, Styblo M, Tseng C-H, Thayer KA et al. (2012) Evaluation of the association between arsenic and diabetes: a national toxicology program workshop review. Environ Health Perspect 120:1658–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer-Davis EJ, Lawrence JM, Dabelea D, Divers J, Isom S, Dolan L, Imperatore G, Linder B, Marcovina S, Pettitt DJ et al. (2017) Incidence trends of type 1 and type 2 diabetes among youths, 2002–2012. N Engl J Med 376:1419–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Das D, Mukherjee M, Das AS, Mitra C (2006) Synergistic effect of folic acid and vitamin B12 in ameliorating arsenic-induced oxidative damage in pancreatic tissue of rat. J Nutr Biochem 17:319–327 [DOI] [PubMed] [Google Scholar]
- Murko M, Elek B, Styblo M, Thomas D, Francesconi K (2018) Dose and diet-sources of arsenic intake in mouse in utero exposure scenarios. Chem Res Toxicol 31:156–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen MC, Vatner DF, Shulman GI (2017) Regulation of hepatic glucose metabolism in health and disease. Nat Rev Endocrinol 13:572–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilsner JR, Hall MN, Liu X, Ilievski V, Slavkovich V, Levy D, Factor-Litvak P, Yunus M, Rahman M, Graziano JH et al. (2012) Influence of prenatal arsenic exposure and newborn sex on global methylation of cord blood DNA. PLoS ONE 7:e37147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves PG, Nielsen FH, Fahey GC (1993) AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123:1939–1951 [DOI] [PubMed] [Google Scholar]
- Rodriguez KF, Ungewitter EK, Crespo-Mejias Y, Liu C, Nicol B, Kissling GE, Yao HH-C (2016) Effects of in utero exposure to arsenic during the second half of gestation on reproductive end points and metabolic parameters in female CD-1 mice. Environ Health Perspect 124:336–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas D, Rager JE, Smeester L, Bailey KA, Drobná Z, Rubio-Andrade M, Stýblo M, García-Vargas G, Fry RC (2015) Prenatal arsenic exposure and the epigenome: identifying sites of 5-methylcytosine alterations that predict functional changes in gene expression in newborn cord blood and subsequent birth outcomes. Toxicol Sci 143:97–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley WR, Bezold C, Arikan Y, Byrne E, Krohe S (2017) Diabetes 2030: insights from yesterday, today, and future trends. Popul Health Manag 20:6–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Soria P, Broka D, Quach S, Hardwick RN, Cherrington NJ, Camenisch TD (2014) Fetal exposure to arsenic results in hyperglycemia, hypercholesterolemia, and nonalcoholic fatty liver disease in adult mice. J Toxicol Health 1:1 [Google Scholar]
- Schmitz JC, Grindey GB, Schultz RM, Priest DG (1994) Impact of dietary folic acid on reduced folates in mouse plasma and tissues. Relationship to dideazatetrahydrofolate sensitivity. Biochem Pharmacol 48:319–325 [DOI] [PubMed] [Google Scholar]
- Selhub J (1999) Homocysteine metabolism. Annu Rev Nutr 19:217–246 [DOI] [PubMed] [Google Scholar]
- Smith AH, Lingas EO, Rahman M (2000) Contamination of drinking-water by arsenic in Bangladesh: a public health emergency. Bull World Health Organ 78:1093–1103 [PMC free article] [PubMed] [Google Scholar]
- Spiegelstein O, Lu X, Le XC, Troen A, Selhub J, Melnyk S, James SJ, Finnell RH (2003) Effects of dietary folate intake and folate binding protein-1 (Folbp1) on urinary speciation of sodium arsenate in mice. Toxicol Lett 145:167–174 [DOI] [PubMed] [Google Scholar]
- Spiegelstein O, Lu X, Le XC, Troen A, Selhub J, Melnyk S, James SJ, Finnell RH (2005) Effects of dietary folate intake and folate binding protein-2 (Folbp2) on urinary speciation of sodium arsenate in mice. Environ Toxicol Pharmacol 19:1–7 [DOI] [PubMed] [Google Scholar]
- Sung T-C, Huang J- W, Guo H- R (2015). Association between arsenic exposure and diabetes: a meta-analysis. Biomed Res Int 2015: 368087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer KA, Heindel JJ, Bucher JR, Gallo MA (2012) Role of environmental chemicals in diabetes and obesity: a national toxicology program workshop review. Environ Health Perspect 120:779–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DJ, Li J, Waters SB, Xing W, Adair BM, Drobna Z, Devesa V, Styblo M (2007) Arsenic (+ 3 oxidation state) methyltransferase and the methylation of arsenicals. Exp Biol Med (Maywood) 232:3–13 [PMC free article] [PubMed] [Google Scholar]
- Tsang V, Fry RC, Niculescu MD, Rager JE, Saunders J, Paul DS, Zeisel SH, Waalkes MP, Styblo M, Drobná Z (2012) The epigenetic effects of a high prenatal folate intake in male mouse fetuses exposed in utero to arsenic. Toxicol Appl Pharmacol 264:439–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Environmental Protection Agency (2001). Technical fact sheet: final rule of arsenic in drinking water. EPA 815-F-00–016 [Google Scholar]
- Waterland RA, Jirtle RL (2003) Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol 23:5293–5300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlodarczyk B, Spiegelstein O, Gelineau-van Waes J, Vorce RL, Lu X, Le CX, Finnell RH (2001) Arsenic-induced congenital malformations in genetically susceptible folate binding protein-2 knockout mice. Toxicol Appl Pharmacol 177:238–246 [DOI] [PubMed] [Google Scholar]
- Wlodarczyk B, Spiegelstein O, Hill D, Le XC, Finnell RH (2012) Arsenic urinary speciation in Mthfr deficient mice injected with sodium arsenate. Toxicol Lett 215:214–218 [DOI] [PubMed] [Google Scholar]
- World Health Organization (2016) Global report on diabetes. WHO Library cataloguing-in-publication data. http://apps.who.int/iris/bitstream/10665/204871/1/9789241565257_eng.pdf [Google Scholar]
- World Health Organization (2017) Guidelines for drinking-water quality: Fourth edition incorporating the first addendum. WHO library cataloguing-in-publication data. http://apps.who.int/iris/bitstream/10665/254637/1/9789241549950-eng.pdf?ua=1 [PubMed] [Google Scholar]
- Yadav DK, Shrestha S, Lillycrop KA, Joglekar CV, Pan H, Holbrook JD, Fall CH, Yajnik CS, Chandak GR (2018) Vitamin B12 supplementation influences methylation of genes associated with type 2 diabetes and its intermediate traits. Epigenomics 10:71–90 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


