Abstract
Sulforaphane (SFN) is a phytochemical found in cruciferous vegetables. It has been shown to have many protective effects against many diseases, including multiple types of cancer. SFN is a potent activator of the nuclear factor erythroid 2-related factor 2 (Nrf2) antioxidant response element (ARE) genetic pathway. Upregulation of Nrf2-ARE increases the availability of multiple antioxidants. A substantial amount of preclinical research regarding the ability of SFN to protect the nervous system from many diseases and toxins has been done, but only a few small human trials have been completed. Preclinical data suggest that SFN protects the nervous system through multiple mechanisms and may help reduce the risk of many diseases and reduce the burden of symptoms in existing conditions. This review focuses on the literature regarding the protective effects of SFN on the nervous system. A discussion of neuroprotective mechanisms is followed by a discussion of the protective effects elicited by SFN administration in a multitude of neurological diseases and toxin exposures. SFN is a promising neuroprotective phytochemical which needs further human trials to evaluate its efficacy in preventing and decreasing the burden of many neurological diseases.
Keywords: Antioxidant, autism spectrum disorder, broccoli sprouts, epilepsy, isothiocyanate, neurodegenerative disease, nuclear factor erythroid 2-related factor 2, phytochemical, schizophrenia
Introduction
Sulforaphane (SFN) is a phytochemical whose precursor glucoraphanin is found in cruciferous vegetables, with the highest concentrations in broccoli sprouts.[1] SFN belongs to the group of plant-derived compounds called isothiocyanates. It is known for a being a powerful inducer of the nuclear factor erythroid 2-related factor 2 (Nrf2)-antioxidant response element (ARE) pathway which plays a major role in upregulating cellular defenses to oxidative stress.[2] SFN has been studied intensely regarding its ability to decrease the risk of various cancers and reduce the damage associated with varying forms of oxidative stress.[3]
More recently, a variety of preclinical research regarding the role of SFN in neuroprotection has been conducted with very promising results. Only a few human trials regarding the protective effects of SFN in the nervous system have been done; however, SFN has very strong antioxidant and anti-inflammatory properties which allow it to dramatically reduce cytotoxicity in the nervous system, with apparently very little toxicity of its own within the therapeutic range.[4] Animal studies suggest that SFN supplementation could be disease-modifying for many common, debilitating central nervous system (CNS) diseases including Alzheimer's disease, Parkinson's disease, epilepsy, stroke, and others.
To fully assess the research that has been completed regarding the neuroprotective effects of SFN, a literature search was done using MEDLINE for relevant articles published as of January 2019. The literature search included the following keywords: SFN, neuroprotection, neurodegeneration, nervous system, neuron, brain, neurogenesis, and Nrf2. Resulting articles were reviewed for relevance to the topic of neuroprotection. The dates of included publications range from 2004 to 2018. This review focuses on the research that has been completed regarding the neuroprotective properties of SFN in various disease states and toxin exposures. The mechanisms underlying SFN's protective properties will be discussed first, followed by the effects seen in various disease models.
Mechanisms of Neuroprotection
SFN is a well-known powerful inducer of the Nrf2-ARE pathway, which has been coined the “guardian of redox homeostasis.”[5,6] The activation of the Nrf2-ARE pathway leads to upregulation of many downstream products involved in protection against oxidative stress, including NAD(P)H quinone oxidoreductase 1, heme oxygenase 1, glutathione (GSH) peroxidase 1,[7] and gamma-glutamylcysteine synthetase, an important rate-limiting enzyme which controls the rate of GSH synthesis.[8] The adequate availability of reduced GSH is vital to avoid the damage induced by free radicals.[8] SFN increases GSH release by up to 2.4-fold in cultured astrocytes[8] and has been shown to reduce oxidative stress in multiple disease states in cultured cells and animal models.[9,10,11,12,13,14] A brief study in humans revealed that SFN increases the amount of GSH in the brain after 7 days of administration, which provides evidence that the antioxidant pathways activated by SFN are present in humans.[15] The Nrf2 pathway is vital for many of SFN's protective effects, as evidenced by a lack of neuroprotection from multiple toxins when SFN is given with an inhibitor of gamma-glutamylcysteine synthetase[16] or in Nrf2-knockout mice.[17,18] Nrf2-ARE plays a vital role in the protective effect of SFN against many diseases, including Parkinson's disease, neuropathy, Friedrich's ataxia, stroke, and Alzheimer's disease.[19,20,21,22,23]
Besides its promotion of antioxidant defenses, SFN also significantly lessens inflammatory responses to pathologic states, thus reducing the amount of damage done due to the body's immune response.[24] SFN reduces damage to neurons mediated by microglia by promoting polarization of microglia from the M1 to the anti-inflammatory M2 type,[25,26] thus down regulating the mRNA and proteins levels of multiple inflammatory mediators, including tumor necrosis factor-α, interleukin (IL)-1 β, IL-6, cyclooxygenase 2, and inducible nitric oxide synthetase.[5,11,24,27,28] Furthermore, SFN decreases the activation of multiple mitogen-activated protein kinases and other inflammatory mediators, including nuclear factor κB, RIPK3, and MLKL, resulting in reduced neuronal apoptosis and necrosis.[24] Interestingly, when the immune system is needed such as when cancer is present, however, SFN inhibits the ability of glioblastoma multiforme to create a supportive immunosuppressed environment by disallowing the transformation of monocytes into myeloid-derived suppressor cells.[29] SFN also reduces cleavage of caspase-1 and caspase-3,[12,30,31,32,33] increases the production of the anti-inflammatory cytokines IL-4 and IL-10,[25,34] and reduces the amount of gliosis, apoptosis, and necrosis in response to toxins.[11,17,23,27,31,35,36] The reduction of neuroinflammation plays a prominent role in protecting against many toxins, as well as neuronal damage associated with Alzheimer's disease, Parkinson's disease, epileptic seizures, cerebral infarction, hepatic encephalopathy, Huntington's disease, and spinal cord injury.[9,10,11,17,25,26,30,37,38] Oxidative stress and inflammation are major causes of cellular damage in a vast array of neurological diseases, and so by reducing both of these factors, SFN has major promise for helping protect against this damage.
Autophagy, a process used by cells to degrade damaged organelles and harmful proteins,[19] is also upregulated by SFN in neurons.[19,23,39,40] One study found that the promotion of autophagy by SFN is partially dependent on the Nrf2-ARE pathway, as indicated by Nrf2-knockout mice expressing fewer autophagy genes as well as the rescue of this expression by infection with an Nrf2-expressing lentivirus.[19] However, a separate study found that Nrf2 knockdown did not influence autophagy.[40] SFN produces oxidative stress itself, which is necessary for the upregulation of autophagy, evidenced by a lack of this upregulation when neurons are co-treated with SFN and the potent antioxidant N-acetyl-l-cysteine.[40] The upregulation of autophagy by SFN plays a role in its neuroprotection in many neurodegenerative diseases by increasing the breakdown of the harmful proteins that characterize these diseases, including Alzheimer's disease,[19] Parkinson's disease,[23] and prion diseases.[39]
SFN also protects mitochondrial function in neurons.[22,41,42,43,44] Neurons, which are highly metabolically active and rely on oxidative phosphorylation to keep up with energy demands, depend on healthy mitochondria.[43] The Nrf2-ARE pathway activates multiple genes which promote mitochondrial biogenesis, protect the function of mitochondrial complex I, II, and IV, and inhibit the decrease in adenosine triphosphate (ATP) generation caused by toxins.[42] The upregulation of antioxidant defenses by the Nrf2-ARE pathway also works to protect the mitochondria from damage.[43] Mitochondrial protection plays a role in reducing damage due to epileptic seizures,[44] chemotherapy-induced neuropathy,[5] models of Huntington's disease,[42] neurodegenerative diseases,[43] and carbon monoxide exposure.[41]
Neurogenesis, the production of new neurons from neural stem cells, is critically important for learning and memory, and it is dysregulated in many neurodegenerative diseases.[3] SFN increases neuronal expression of brain-derived neurotrophic factor, which promotes neuron generation[3] and upregulates Wnt signaling in neural stem cells, which then increases stem cell proliferation and their differentiation into neurons.[45]
Neuroprotection in Disease and Toxin Exposure
Table 1 provides a tabular view of the major evidence for the neuroprotective effects of SFN, categorized by disease.
Table 1.
Summary of major findings related to the neuroprotective effects of sulforaphane in various neurologic disease states
| Topic | Article | Model | Effect |
|---|---|---|---|
| Neurodegeneration | |||
| AD | Hou et al., 2018; Lee et al., 2018 | Mouse transgenic AD | Reduced amount of Aβ and phosphorylated tau and their aggregation in the brain; reduced memory deficits |
| Zhang et al., 2014 | Mouse aluminum and D-galactose-induced AD | Reduced cholinergic neuron loss in hippocampus and septum | |
| Angeloni et al., 2015 | Cultured neurons with methylglyoxal | Reduced cell death | |
| Park et al., 2009 | Cultured neurons with Aβ | Reduced cell death | |
| Memory | Wang et al., 2016 | Rat streptozotocin-induced DM | Reduced apoptosis of hippocampal neurons; reduced memory impairment |
| Sunkaria et al., 2018 | Mouse MG132 exposure | Protection against loss of spatial memory and memory consolidation | |
| Lee S et al., 2014 | Mouse scopolamine exposure | Protection against memory loss; increased level of ACh in hippocampus | |
| PD | Zhou et al., 2016 | Mouse rotenone-induced PD | Improved locomotor activity; reduced dopaminergic neuron loss in brain |
| Morroni et al., 2013 | Mouse 6-hydroxydopamine-induced PD | Improved motor coordination; reduced neuron apoptosis | |
| Morroni et al., 2018; Deng et al., 2012 | Mouse 6-hydroxydopamine-induced PD | Reduced dopaminergic neuron loss | |
| Vauzour et al., 2010 | Cultured cortical neurons with 5-S-cysteinyl-dopamine | Reduced neuron loss | |
| Jazwa et al., 2011 | Mouse MPTP-induced PD | Reduced loss of nigral dopaminergic neurons | |
| Siebert et al., 2009 | Nigrostriatal culture of rat brain exposed to 6-hydroxydopamine | Reduced neuron loss | |
| Prion diseases | Lee JH et al., 2014 | Human neuroblastoma cells exposed to PrP | Reduced cell death |
| HD | Luis-García et al., 2017 | Rat quinolinic-acid-induced HD | Reduced mitochondrial dysfunction |
| Jang et al., 2016 | Mouse 3-NP-induced HD | Improved neurological behavior; reduced animal death; reduced neuron loss | |
| Stroke and injury | Yu et al., 2017 | Rat 60 min occlusive injury | Improved neurological function scores; reduced infarct volume |
| Wu et al., 2012 | Cultured rat cortical neurons 1 h glucose-oxygen deprivation | Reduced cell death and injury | |
| Soane et al., 2010 | Cultured primary mouse immature hippocampal neurons exposed to oxygen-glucose deprivation | Reduced delayed neuronal death | |
| Soane et al., 2010 | Cultured primary mouse immature hippocampal neurons exposed to hemin | Reduced neuron loss | |
| Black et al., 2015 | Rat surgically-induced IUGR | Improved neurocognitive function in offspring; protection against loss of white matter and hippocampal neurons in offspring | |
| Yin et al., 2015 | Rat induced basal ganglia hemorrhage | Improved neurological function | |
| Zhao et al., 2009 | Mouse and rat induced ICH | Reduced neuron damage | |
| Mao et al., 2011 | Mouse compressive SCI | Improved locomotor function; reduced neuron loss | |
| Wang et al., 2012 | Rat mechanical SCI | Reduced contusion volume; improved motor coordination | |
| Benedict et al., 2012 | Rat contusive SCI | Improved locomotor function; increased 5-HT axons | |
| Hong et al., 2010 | Mouse and rat TBI | Improved neurological function; reduced contusion size; reduced neuron loss | |
| Epilepsy | Pauletti et al., 2017 | Rat electrically-induced epilepsy, co-treatment with N-acetylcysteine | Reduced frequency of seizures; reduced hippocampal neuron loss; improved cognitive function |
| Socała et al., 2017 | Mouse electrically-induced seizure | Potentiation of anti-convulsant effect of carbamazepine; at high concentrations, caused reduced seizure threshold | |
| Carrasco-Pozo et al., 2015 | Mouse epilepsy and SE models | Increased ATP production; anticonvulsant effect | |
| Diabetes and neuropathy | Negi et al., 2011 | Cultured peripheral neurons | Improved conduction velocity and blood flow |
| Negi et al., 2011 | Rat streptozocin-induced DM | Improved pain behavior | |
| Yang et al., 2018 | Mouse oxaliplatin-induced neuropathy | Improved pain sensation; improved mitochondrial function in DRG | |
| Di et al., 2016 | Rat nitroglycerin-induced hyperalgesia | Reduced tactile threshold | |
| Wang et al., 2016 | Rat streptozocin-induced DM | Reduced apoptosis of hippocampal neurons; reduced memory impairment | |
| Ren et al., 2018 | Mouse streptozocin-and high fat diet-induced DM-associated retinopathy | Improved ONL thickness; reduced retinal cell apoptosis | |
| Psychosis | Shirai et al., 2015 | Mouse PCP-induced model of schizophrenia | Improved cognitive function |
| Mas et al., 2012 | Human dopaminergic neuroblastoma cells exposed to antipsychotic medications and dopamine | Reduced cell death | |
| Shiina et al., 2015 | Human patients with schizophrenia | Improved accuracy component of one card learning task | |
| GBM | Kumar et al., 2017 | Cultured human monocytes in glioma-conditioned media | Increased mature dendritic cell development; reduced harmful monocyte transformation |
| Friedrich’s ataxia | Petrillo et al., 2017 | Cultured frataxin-deficient motor neurons | Increased neurite number and amount of extension |
| Hepatic encephalopathy | Hernandez-Rabaza et al., 2016 | Rat ammonia-induced encephalopathy | Improved learning; improved motor coordination |
| Hernandez-Rabaza et al., 2016 | Rat ammonia-induced encephalopathy | Improved spatial learning | |
| Herpes encephalitis | Schachtele et al., 2012 | Mouse HSV encephalitis | Reduced neuronal damage; reduced neuroinflammation |
| ASD | Singh et al., 2014 | Human men with ASD | Improved measures of aberrant behavior, social responsiveness, social interaction, and verbal communication |
| Bent et al., 2018 | Human children with ASD | Improved measures of social responsiveness | |
| Toxins | Bi et al., 2017 | Rat carbon monoxide exposure | Improved mitochondrial function; reduced hippocampal neuron damage |
| Innamorato et al., 2008 | Mouse LPS exposure | Reduced inflammatory markers in brain | |
| Townsend et al., 2017 | Mouse LPS exposure | Reduced inflammatory markers in hippocampus | |
| Dwivedi et al., 2016 | Rat okadaic acid exposure | Improved memory; reduced neuron apoptosis in cortex and hippocampus | |
| Wang et al., 2013 | Zebrafish larvae cadmium exposure | Reduced olfactory tissue damage | |
| Ishihara et al., 2012 | Cultured rat hippocampus exposed to TBT | Reduced cell death | |
| Chang et al., 2010 | Cultured rat spinal cord exposed to glutamate | Reduced glutamate-associated neuronal damage | |
| Shavali et al., 2008 | Human neuroblastoma cells exposed to arsenic and dopamine | Reduced cell death | |
| Pearson et al., 2016 | Cultured mouse neurons exposed to various neurotoxins | Reduced biochemical damage | |
Aβ: Amyloid β, AD: Alzheimer’s disease, ACh: Acetylcholine, ASD: Autism spectrum disorder, DM: Diabetes mellitus, GBM: Glioblastoma multiforme, HD: Huntington’s disease, HSV: Herpes simplex virus, ICH: Intracerebral hemorrhage, IUGR: Intrauterine growth restriction, LPS: Lipopolysaccharide, MPTP: Methyl-4-phenyl-1,2,3,6-tetrahydropyridine, ONL: Outer nuclear layer, PCP: Phencyclidine, PD: Parkinson’s disease, PrP: Prion protein, SCI: Spinal cord injury, SE: Status epilepticus, TBI: Traumatic brain injury, TBT: Tributyltin, 3-NP: 3-nitropropionic acid, DRG: Dorsal root ganglion, ATP: Adenosine triphosphate, 5-HT: 5-hydroxytryptamine (serotonin)
Neurodegenerative Diseases
SFN has many potential benefits in preventing and modifying the course and symptom burden of multiple neurodegenerative diseases. In the brains of transgenic mouse models of Alzheimer's disease, SFN reduces the amount of amyloid beta (Aβ) and phosphorylated tau proteins as well as their aggregation.[9,46] It also reduces memory deficits in mouse models.[9,46] The degradation of abnormal protein aggregates is likely promoted by the pro-autophagy pathways activated by SFN.[19] The oxidative stress that the aggregated proteins cause in Alzheimer's disease is also reduced with SFN supplementation.[9,46,47] SFN in a toxin-induced Alzheimer's mouse model led to sparing of cholinergic neuron loss in the hippocampus and medial septal region of the brain.[48] SFN also protects cultured neural cells from the toxicity of methylglyoxal, a precursor of advanced glycation end products (AGEs) which is associated with Alzheimer's disease.[12] Neural cell death due to Aβ exposure is also reduced with SFN supplementation by the activation of proteasomes.[49] Similarly, SFN may also prevent or slow the process of normal brain aging and memory problems.[50] Memory is protected by SFN when animals are exposed to various toxins, including streptozocin,[31] MG132 (an inhibitor of proteasomes),[50] and scopolamine.[51]
Parkinson's disease also benefits from SFN administration. In mouse models of Parkinson's disease induced by various neurotoxins, including rotenone,[23] 6-hydroxydopamine,[32,36,52] 5-S-cysteinyl dopamine,[53] and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine,[17] SFN administration protects neurons and reduces neuronal cell death of nigrostriatal dopaminergic neurons.[23,32,52] SFN also reduces motor deficits in toxin-induced animal models.[23,32] Brain slice culture of rat nigrostriatal area also shows protection from toxin-induced Parkinson's damage.[54] This protection is thought to be dependent on Nrf2 activation, with antioxidation and autophagy both participating in reducing damage.[23,32,55] SFN may also protect cells from damage in prion diseases by activating autophagy to degrade the misfolded proteins.[39] Furthermore, in animal models of Huntington's disease, SFN reduces striatal damage, decreases neuron death, and improves mitochondrial function.[27,42] SFN has a protective role in many neurodegenerative diseases that do not have any known cures, which makes it a quintessential agent for nervous system health. Research in humans is lacking, however, so its ability to decrease the risk of neurodegenerative diseases is unknown.
Stroke and Injury
Hypoxic-ischemic injury, hemorrhage, and traumatic spinal cord injury also cause great amounts of damage to the nervous system, including the primary event as well as the secondary damage due to the resulting inflammatory reaction and oxidative stress from reperfusion.[30] In hypoxic-ischemic injury such as infarction, SFN pre- and co-treatment reduces infarct volume and improves the neurological function in animals with induced infarcts.[30] Protection is also seen in cultured neurons exposed to ischemia.[33,56] The same appears to be true for immature, developing nervous systems in the hypoxic conditions resulting from chronic placental insufficiency as well as infarct, with SFN administration reducing the loss of white matter, improving neurological function,[57] and decreasing delayed neuronal cell death.[56] Reduction in multiple inflammatory markers and immune cell activation is also seen with SFN use in ischemic injury.[30,57,58] In the setting of intracerebral hemorrhage, SFN also improves neurological function[20] and decreases the amount of damage due to free hemoglobin by inducing haptoglobin production in the brain.[56,59]
SFN is also protective in spinal cord injury in multiple animal models, including traumatic, contusive, and compressive cord injury models.[38,60,61] Reductions in contusion volume, increased viable axons caudal to the lesion, and decreased neuronal cell death result from SFN administration.[38,60,61] Improvements in motor function and coordination are also seen with SFN use in spinal cord-injured animals.[38,60] The same is true in traumatic brain injury in mice and rats, with SFN administration leading to decreased neuronal cell death, decreased contusion volume, and improvements in neurological function.[18]
Epilepsy
Epileptic seizures damage neurons by inducing oxidative stress in seizure locations.[10] In animal models, SFN administration, when combined with the anti-oxidant N-acetylcysteine, reduces the frequency of seizures, improves cognitive function, and decreases hippocampal cell death.[10] Low-dose SFN alone also potentiates the anti-seizure effects of carbamazepine, thus increasing the seizure threshold.[62] SFN also raises the seizure threshold to protect against seizure occurrence.[44] High-dose SFN, however, can decrease the seizure threshold.[62] The administration of SFN in status epilepticus reduces lipid peroxidation in the hippocampus by protecting mitochondrial function and thus allowing the generation of more ATP in the energy-starved state induced by prolonged seizure.[44]
Diabetes and Neuropathy
AGEs are well-known neurotoxins that form due to high glucose concentrations as seen in diabetes mellitus (DM) and can cause peripheral neuropathy, cognitive dysfunction, and retinal damage.[12,28,63] In cultured peripheral neurons, SFN improves multiple parameters of AGE-induced neuronal damage, including the normalization of conduction velocity and blood flow.[28] In animal models of DM-induced neuropathy, pain behavior is lessened with SFN administration.[28] Protection from neuropathy due to other causes, such as the chemotherapeutic drug oxaliplatin- and nitroglycerin-induced trigeminal nerve pain, is also conferred by SFN.[22,64] In the CNS, SFN prevents AGE formation[65] and prevents memory dysfunction in DM animal models.[31] Retinal degeneration due to AGEs is also reduced with SFN administration.[63] Multiple mechanisms are at play in protection from AGE-induced damage, including the induction of thioredoxin,[63] increased generation of GSH,[12] decreased cleavage of caspase-3,[31] and induction of the detoxifying glyoxalase-1[12] which decreases AGE formation.[65]
Psychosis
The role of SFN in psychotic disorders is multifaceted and not yet fully elucidated. Both pre- and post-exposure administration of SFN in animals exposed to phencyclidine, a psychosis-inducing agent, reduces damage to the prefrontal cortex and improves cognitive dysfunction.[66] The Nrf2 gene also has a genetic association with cognitive impairments in schizophrenic patients, thus implying that the Nrf2 pathway and SFN may play a key role in psychosis in humans.[66] Furthermore, treatment with antipsychotic drugs including haloperidol, risperidone, and paliperidone causes neuronal damage due to the formation of oxidative stress.[14] SFN reduces this oxidative stress in dopaminergic neurons and thus may prevent some of the untoward effects associated with the treatment of psychotic disorders.[14] A small study of seven human patients with schizophrenia found that 8 weeks of SFN administration resulted in significant improvement in one of the three components of a test assessing working memory, but the study size may have limited its power to find other significant improvements.[1]
Other Diseases
As if the above protective effects are not enough, SFN also protects against neuronal damage in a variety of other diseases. Damage due to oxidative stress is reduced in models of Friedreich's ataxia, with SFN leading to an increased number of neurites, indicating increased plasticity.[21] As briefly discussed previously, SFN may have a role in upsetting the immunosuppressed environment that protects glioblastoma multiforme.[29] In hepatic encephalopathy in animals, SFN lowers the inflammatory response to hyperammonemia and normalizes cognitive function and coordination.[25,26] SFN administration also reduces neuronal damage induced by oxidative stress in mice with herpes encephalitis.[7] Research regarding SFN use in autism spectrum disorder (ASD) is one area where some human studies have been done. A randomized controlled trial in human men with ASD revealed that 18 weeks of SFN administration improves multiple types of behavior, including reducing aberrant behaviors by 34%, increasing social responsiveness by 17%, as well as improving social interaction and verbal communication behaviors.[67] A small study in children with ASD had similar results, with 12 weeks of SFN administration resulting in significantly improved social responsiveness, although this study found only a nonsignificant improvement in aberrant behavior.[68] With such a wide array of disease protection, SFN may be utilized in many ways to help reduce nervous system disease burden.
Toxins
Many neurotoxins exist with a variety of unique mechanisms of toxicity. The neuroprotection conferred by SFN appears to be quite broad, as evidenced by the reduction of neuronal damage in the setting of various toxin exposures. These include all the toxins used to induce models of disease mentioned above, as well as carbon monoxide,[41] lipopolysaccharide found in Gram-negative bacteria,[5,37] the memory-impairing chemical okadaic acid,[11] scopolamine,[51] cadmium,[69] the pesticide tributyltin,[13] the excitotoxicity-inducing agent threohydroxyaspartate,[70] arsenic,[35] and multiple fungicides associated with genetic changes seen in autism, aging, and neurodegeneration.[71] This broad scope of protection makes SFN a very useful tool to prevent or reduce neurotoxicity due to environmental or pharmaceutical toxin exposure.
Limitations and next steps
Several limitations are present in the currently available research. Nearly all SFN research regarding neuroprotection has been conducted with cultured neurons or animal models, apart from small trials regarding schizophrenia and ASD. While the results of this preclinical data are powerful, SFN use in humans with the diseases discussed will be crucial in understanding how well this animal research translates to human neurobiology. Without long-term prospective human cohort studies or controlled trials, it is difficult to assess whether the neuroprotection conferred by SFN will prevent the incidence of disease and burden of symptoms in preexisting disease. Another limitation is regarding the combination of SFN and antioxidants. Some researchers suggest that the concomitant use of SFN with antioxidants such as N-acetyl-l-cysteine reduces some the protective effects of SFN, specifically regarding the induction of autophagy.[40] However, other research suggests that the combination can be more beneficial than either alone.[10] This concept needs to be more fully elucidated to determine whether SFN with or without antioxidants will be most beneficial in each disease.
Further, only a few of the studies discussed mention possible neurotoxic effects of SFN, such as lowering the seizure threshold.[62] Some researchers have concluded that SFN is a goitrogen because it can reduce uptake of iodine into the thyroid, but a human safety trial did not show reductions in thyroid function after SFN administration.[4] Determining ideal dosages to maximize protection without causing detrimental effects will also be an important aspect of human trials.
Future research needs to address SFN use in humans with neurological diseases and disorders. This includes randomized controlled trials and longitudinal studies to assess the practical efficacy of SFN in neuroprotection. Research assessing the role of SFN as part of a multimodal treatment plan will also be important since SFN appears to have differential effects based on the concurrent treatments. Other research could look for even more efficacious Nrf2 activators or attempt to create them, like a melatonin-SFN hybrid molecule which may provide even further neuroprotection.[72] There are currently multiple clinical trials ongoing regarding the effects of SFN supplementation in patients with schizophrenia and patients with ASD.[73] Hopefully, more researchers find SFN to be a worthy compound to assess in other diseases as well.
Conclusion and Perspective
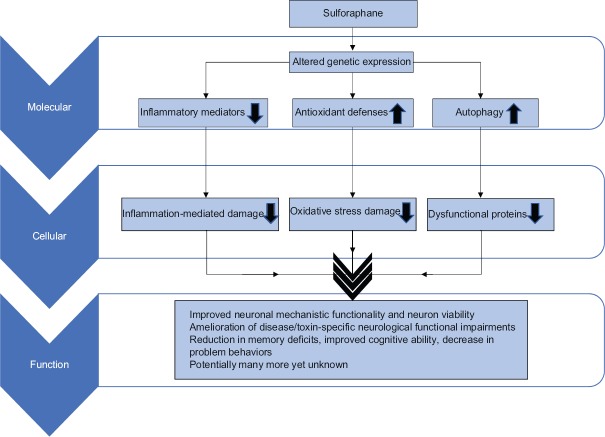
SFN is a powerful antioxidant and anti-inflammatory phytochemical with great promise in its ability to protect the nervous system from many diseases and toxins and reduce the symptomatic burden of multiple pervasive diseases [Figure 1]. Research regarding long-term use in humans and disease outcomes will be important to determine its clinical utility. SFN, found naturally in high concentrations in broccoli sprouts, is a powerful example of how important food is to our health, and we must remember that while simple things like broccoli do not seem as powerful as human-made pharmaceuticals, they can truly be as or more important. The area of phytochemical use in prevention and damage reduction of neurological diseases is blossoming and may well be an important next step in reducing the risk of the many diseases we have assumed inevitable or incurable.
Figure 1.
A schematic view of the effects of sulforaphane in the nervous system. Sulforaphane provides neuroprotective effects by altering genetic expression of various damaging or protective mediators, which reduces cellular damage and harmful protein accumulation, finally resulting in multiple functional neurological improvements in many neurological disease states and toxin exposures
Financial support and sponsorship
This work was partially supported by Merit Review Award (I01RX-001964-01) from the US Department of Veterans Affairs Rehabilitation R and D Service.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Shiina A, Kanahara N, Sasaki T, Oda Y, Hashimoto T, Hasegawa T, et al. An open study of sulforaphane-rich broccoli sprout extract in patients with schizophrenia. Clin Psychopharmacol Neurosci. 2015;13:62–7. doi: 10.9758/cpn.2015.13.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trio PZ, Fujisaki S, Tanigawa S, Hisanaga A, Sakao K, Hou DX. DNA microarray highlights Nrf2-mediated neuron protection targeted by wasabi-derived isothiocyanates in IMR-32 cells. Gene Regul Syst Bio. 2016;10:73–83. doi: 10.4137/GRSB.S39440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim J, Lee S, Choi BR, Yang H, Hwang Y, Park JH, et al. Sulforaphane epigenetically enhances neuronal BDNF expression and TrkB signaling pathways. Mol Nutr Food Res. 2017;61:1600194. doi: 10.1002/mnfr.201600194. [DOI] [PubMed] [Google Scholar]
- 4.Shapiro TA, Fahey JW, Dinkova-Kostova AT, Holtzclaw WD, Stephenson KK, Wade KL, et al. Safety, tolerance, and metabolism of broccoli sprout glucosinolates and isothiocyanates: A clinical phase I study. Nutr Cancer. 2006;55:53–62. doi: 10.1207/s15327914nc5501_7. [DOI] [PubMed] [Google Scholar]
- 5.Innamorato NG, Rojo AI, García-Yagüe AJ, Yamamoto M, de Ceballos ML, Cuadrado A. The transcription factor Nrf2 is a therapeutic target against brain inflammation. J Immunol. 2008;181:680–9. doi: 10.4049/jimmunol.181.1.680. [DOI] [PubMed] [Google Scholar]
- 6.Izumi Y, Kataoka H, Inose Y, Akaike A, Koyama Y, Kume T. Neuroprotective effect of an Nrf2-ARE activator identified from a chemical library on dopaminergic neurons. Eur J Pharmacol. 2018;818:470–9. doi: 10.1016/j.ejphar.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 7.Schachtele SJ, Hu S, Lokensgard JR. Modulation of experimental herpes encephalitis-associated neurotoxicity through sulforaphane treatment. PLoS One. 2012;7:e36216. doi: 10.1371/journal.pone.0036216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steele ML, Fuller S, Patel M, Kersaitis C, Ooi L, Münch G. Effect of Nrf2 activators on release of glutathione, cysteinylglycine and homocysteine by human U373 astroglial cells. Redox Biol. 2013;1:441–5. doi: 10.1016/j.redox.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hou TT, Yang HY, Wang W, Wu QQ, Tian YR, Jia JP. Sulforaphane inhibits the generation of amyloid-β oligomer and promotes spatial learning and memory in Alzheimer's disease (PS1V97L) transgenic mice. J Alzheimers Dis. 2018;62:1803–13. doi: 10.3233/JAD-171110. [DOI] [PubMed] [Google Scholar]
- 10.Pauletti A, Terrone G, Shekh-Ahmad T, Salamone A, Ravizza T, Rizzi M, et al. Targeting oxidative stress improves disease outcomes in a rat model of acquired epilepsy. Brain. 2017;140:1885–99. doi: 10.1093/brain/awx117. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Dwivedi S, Rajasekar N, Hanif K, Nath C, Shukla R. Sulforaphane ameliorates okadaic acid-induced memory impairment in rats by activating the Nrf2/HO-1 antioxidant pathway. Mol Neurobiol. 2016;53:5310–23. doi: 10.1007/s12035-015-9451-4. [DOI] [PubMed] [Google Scholar]
- 12.Angeloni C, Malaguti M, Rizzo B, Barbalace MC, Fabbri D, Hrelia S. Neuroprotective effect of sulforaphane against methylglyoxal cytotoxicity. Chem Res Toxicol. 2015;28:1234–45. doi: 10.1021/acs.chemrestox.5b00067. [DOI] [PubMed] [Google Scholar]
- 13.Ishihara Y, Kawami T, Ishida A, Yamazaki T. Tributyltin induces oxidative stress and neuronal injury by inhibiting glutathione S-transferase in rat organotypic hippocampal slice cultures. Neurochem Int. 2012;60:782–90. doi: 10.1016/j.neuint.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Mas S, Gassó P, Trias G, Bernardo M, Lafuente A. Sulforaphane protects SK-N-SH cells against antipsychotic-induced oxidative stress. Fundam Clin Pharmacol. 2012;26:712–21. doi: 10.1111/j.1472-8206.2011.00988.x. [DOI] [PubMed] [Google Scholar]
- 15.Sedlak TW, Nucifora LG, Koga M, Shaffer LS, Higgs C, Tanaka T, et al. Sulforaphane augments glutathione and influences brain metabolites in human subjects: A clinical pilot study. Mol Neuropsychiatry. 2018;3:214–22. doi: 10.1159/000487639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mizuno K, Kume T, Muto C, Takada-Takatori Y, Izumi Y, Sugimoto H, et al. Glutathione biosynthesis via activation of the nuclear factor E2-related factor 2 (Nrf2) – antioxidant-response element (ARE) pathway is essential for neuroprotective effects of sulforaphane and 6-(methylsulfinyl) hexyl isothiocyanate. J Pharmacol Sci. 2011;115:320–8. doi: 10.1254/jphs.10257fp. [DOI] [PubMed] [Google Scholar]
- 17.Jazwa A, Rojo AI, Innamorato NG, Hesse M, Fernández-Ruiz J, Cuadrado A, et al. Pharmacological targeting of the transcription factor Nrf2 at the basal ganglia provides disease modifying therapy for experimental Parkinsonism. Antioxid Redox Signal. 2011;14:2347–60. doi: 10.1089/ars.2010.3731. [DOI] [PubMed] [Google Scholar]
- 18.Hong Y, Yan W, Chen S, Sun CR, Zhang JM. The role of Nrf2 signaling in the regulation of antioxidants and detoxifying enzymes after traumatic brain injury in rats and mice. Acta Pharmacol Sin. 2010;31:1421–30. doi: 10.1038/aps.2010.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pajares M, Jiménez-Moreno N, García-Yagüe ÁJ, Escoll M, de Ceballos ML, Van Leuven F, et al. Transcription factor NFE2L2/NRF2 is a regulator of macroautophagy genes. Autophagy. 2016;12:1902–16. doi: 10.1080/15548627.2016.1208889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin XP, Chen ZY, Zhou J, Wu D, Bao B. Mechanisms underlying the perifocal neuroprotective effect of the Nrf2-ARE signaling pathway after intracranial hemorrhage. Drug Des Devel Ther. 2015;9:5973–86. doi: 10.2147/DDDT.S79399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petrillo S, Piermarini E, Pastore A, Vasco G, Schirinzi T, Carrozzo R, et al. Nrf2-inducers counteract neurodegeneration in Frataxin-silenced motor neurons: Disclosing new therapeutic targets for Friedreich's Ataxia. Int J Mol Sci. 2017;18:E2173. doi: 10.3390/ijms18102173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Y, Luo L, Cai X, Fang Y, Wang J, Chen G, et al. Nrf2 inhibits oxaliplatin-induced peripheral neuropathy via protection of mitochondrial function. Free Radic Biol Med. 2018;120:13–24. doi: 10.1016/j.freeradbiomed.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Zhou Q, Chen B, Wang X, Wu L, Yang Y, Cheng X, et al. Sulforaphane protects against rotenone-induced neurotoxicity in vivo: Involvement of the mTOR, Nrf2, and autophagy pathways. Sci Rep. 2016;6:32206. doi: 10.1038/srep32206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qin S, Yang C, Huang W, Du S, Mai H, Xiao J, et al. Sulforaphane attenuates microglia-mediated neuronal necroptosis through down-regulation of MAPK/NF-κB signaling pathways in LPS-activated BV-2 microglia. Pharmacol Res. 2018;133:218–35. doi: 10.1016/j.phrs.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 25.Hernandez-Rabaza V, Cabrera-Pastor A, Taoro-Gonzalez L, Gonzalez-Usano A, Agusti A, Balzano T, et al. Neuroinflammation increases GABAergic tone and impairs cognitive and motor function in hyperammonemia by increasing GAT-3 membrane expression. Reversal by sulforaphane by promoting M2 polarization of microglia. J Neuroinflammation. 2016;13:83. doi: 10.1186/s12974-016-0549-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hernández-Rabaza V, Cabrera-Pastor A, Taoro-González L, Malaguarnera M, Agustí A, Llansola M, et al. Hyperammonemia induces glial activation, neuroinflammation and alters neurotransmitter receptors in hippocampus, impairing spatial learning: Reversal by sulforaphane. J Neuroinflammation. 2016;13:41. doi: 10.1186/s12974-016-0505-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jang M, Cho IH. Sulforaphane ameliorates 3-nitropropionic acid-induced striatal toxicity by activating the keap1-Nrf2-ARE pathway and inhibiting the MAPKs and NF-κB pathways. Mol Neurobiol. 2016;53:2619–35. doi: 10.1007/s12035-015-9230-2. [DOI] [PubMed] [Google Scholar]
- 28.Negi G, Kumar A, Sharma SS. Nrf2 and NF-κB modulation by sulforaphane counteracts multiple manifestations of diabetic neuropathy in rats and high glucose-induced changes. Curr Neurovasc Res. 2011;8:294–304. doi: 10.2174/156720211798120972. [DOI] [PubMed] [Google Scholar]
- 29.Kumar R, de Mooij T, Peterson TE, Kaptzan T, Johnson AJ, Daniels DJ, et al. Modulating glioma-mediated myeloid-derived suppressor cell development with sulforaphane. PLoS One. 2017;12:e0179012. doi: 10.1371/journal.pone.0179012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu C, He Q, Zheng J, Li LY, Hou YH, Song FZ, et al. Sulforaphane improves outcomes and slows cerebral ischemic/reperfusion injury via inhibition of NLRP3 inflammasome activation in rats. Int Immunopharmacol. 2017;45:74–8. doi: 10.1016/j.intimp.2017.01.034. [DOI] [PubMed] [Google Scholar]
- 31.Wang G, Fang H, Zhen Y, Xu G, Tian J, Zhang Y, et al. Sulforaphane prevents neuronal apoptosis and memory impairment in diabetic rats. Cell Physiol Biochem. 2016;39:901–7. doi: 10.1159/000447799. [DOI] [PubMed] [Google Scholar]
- 32.Morroni F, Tarozzi A, Sita G, Bolondi C, Zolezzi Moraga JM, Cantelli-Forti G, et al. Neuroprotective effect of sulforaphane in 6-hydroxydopamine-lesioned mouse model of Parkinson's disease. Neurotoxicology. 2013;36:63–71. doi: 10.1016/j.neuro.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 33.Wu X, Zhao J, Yu S, Chen Y, Wu J, Zhao Y, et al. Sulforaphane protects primary cultures of cortical neurons against injury induced by oxygen-glucose deprivation/reoxygenation via antiapoptosis. Neurosci Bull. 2012;28:509–16. doi: 10.1007/s12264-012-1273-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maciel-Barón LÁ, Morales-Rosales SL, Silva-Palacios A, Rodríguez-Barrera RH, García-Álvarez JA, Luna-López A, et al. The secretory phenotype of senescent astrocytes isolated from wistar newborn rats changes with anti-inflammatory drugs, but does not have a short-term effect on neuronal mitochondrial potential. Biogerontology. 2018;19:415–33. doi: 10.1007/s10522-018-9767-3. [DOI] [PubMed] [Google Scholar]
- 35.Shavali S, Sens DA. Synergistic neurotoxic effects of arsenic and dopamine in human dopaminergic neuroblastoma SH-SY5Y cells. Toxicol Sci. 2008;102:254–61. doi: 10.1093/toxsci/kfm302. [DOI] [PubMed] [Google Scholar]
- 36.Morroni F, Sita G, Djemil A, D'Amico M, Pruccoli L, Cantelli-Forti G, et al. Comparison of adaptive neuroprotective mechanisms of sulforaphane and its interconversion product erucin in in vitro and in vivo models of Parkinson's disease. J Agric Food Chem. 2018;66:856–65. doi: 10.1021/acs.jafc.7b04641. [DOI] [PubMed] [Google Scholar]
- 37.Townsend BE, Johnson RW. Sulforaphane reduces lipopolysaccharide-induced proinflammatory markers in hippocampus and liver but does not improve sickness behavior. Nutr Neurosci. 2017;20:195–202. doi: 10.1080/1028415X.2015.1103463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mao L, Wang H, Wang X, Liao H, Zhao X. Transcription factor Nrf2 protects the spinal cord from inflammation produced by spinal cord injury. J Surg Res. 2011;170:e105–15. doi: 10.1016/j.jss.2011.05.049. [DOI] [PubMed] [Google Scholar]
- 39.Lee JH, Jeong JK, Park SY. Sulforaphane-induced autophagy flux prevents prion protein-mediated neurotoxicity through AMPK pathway. Neuroscience. 2014;278:31–9. doi: 10.1016/j.neuroscience.2014.07.072. [DOI] [PubMed] [Google Scholar]
- 40.Jo C, Kim S, Cho SJ, Choi KJ, Yun SM, Koh YH, et al. Sulforaphane induces autophagy through ERK activation in neuronal cells. FEBS Lett. 2014;588:3081–8. doi: 10.1016/j.febslet.2014.06.036. [DOI] [PubMed] [Google Scholar]
- 41.Bi M, Li Q, Guo D, Ding X, Bi W, Zhang Y, et al. Sulphoraphane improves neuronal mitochondrial function in brain tissue in acute carbon monoxide poisoning rats. Basic Clin Pharmacol Toxicol. 2017;120:541–9. doi: 10.1111/bcpt.12728. [DOI] [PubMed] [Google Scholar]
- 42.Luis-García ER, Limón-Pacheco JH, Serrano-García N, Hernández-Pérez AD, Pedraza-Chaverri J, Orozco-Ibarra M, et al. Sulforaphane prevents quinolinic acid-induced mitochondrial dysfunction in rat striatum. J Biochem Mol Toxicol. 2017;31:e21837. doi: 10.1002/jbt.21837. [DOI] [PubMed] [Google Scholar]
- 43.Denzer I, Münch G, Friedland K. Modulation of mitochondrial dysfunction in neurodegenerative diseases via activation of nuclear factor erythroid-2-related factor 2 by food-derived compounds. Pharmacol Res. 2016;103:80–94. doi: 10.1016/j.phrs.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 44.Carrasco-Pozo C, Tan KN, Borges K. Sulforaphane is anticonvulsant and improves mitochondrial function. J Neurochem. 2015;135:932–42. doi: 10.1111/jnc.13361. [DOI] [PubMed] [Google Scholar]
- 45.Han Z, Xu Q, Li C, Zhao H. Effects of sulforaphane on neural stem cell proliferation and differentiation. Genesis. 2017;55:e23022. doi: 10.1002/dvg.23022. [DOI] [PubMed] [Google Scholar]
- 46.Lee S, Choi BR, Kim J, LaFerla FM, Park JH, Han JS, et al. Sulforaphane upregulates the heat shock protein co-chaperone CHIP and clears amyloid-β and tau in a mouse model of Alzheimer's disease. Mol Nutr Food Res. 2018;62:e1800240. doi: 10.1002/mnfr.201800240. [DOI] [PubMed] [Google Scholar]
- 47.Kim HV, Kim HY, Ehrlich HY, Choi SY, Kim DJ, Kim Y, et al. Amelioration of Alzheimer's disease by neuroprotective effect of sulforaphane in animal model. Amyloid. 2013;20:7–12. doi: 10.3109/13506129.2012.751367. [DOI] [PubMed] [Google Scholar]
- 48.Zhang R, Zhang J, Fang L, Li X, Zhao Y, Shi W, et al. Neuroprotective effects of sulforaphane on cholinergic neurons in mice with Alzheimer's disease-like lesions. Int J Mol Sci. 2014;15:14396–410. doi: 10.3390/ijms150814396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park HM, Kim JA, Kwak MK. Protection against amyloid beta cytotoxicity by sulforaphane: Role of the proteasome. Arch Pharm Res. 2009;32:109–15. doi: 10.1007/s12272-009-1124-2. [DOI] [PubMed] [Google Scholar]
- 50.Sunkaria A, Bhardwaj S, Yadav A, Halder A, Sandhir R. Sulforaphane attenuates postnatal proteasome inhibition and improves spatial learning in adult mice. J Nutr Biochem. 2018;51:69–79. doi: 10.1016/j.jnutbio.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 51.Lee S, Kim J, Seo SG, Choi BR, Han JS, Lee KW, et al. Sulforaphane alleviates scopolamine-induced memory impairment in mice. Pharmacol Res. 2014;85:23–32. doi: 10.1016/j.phrs.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 52.Deng C, Tao R, Yu SZ, Jin H. Sulforaphane protects against 6-hydroxydopamine-induced cytotoxicity by increasing expression of heme oxygenase-1 in a PI3K/Akt-dependent manner. Mol Med Rep. 2012;5:847–51. doi: 10.3892/mmr.2011.731. [DOI] [PubMed] [Google Scholar]
- 53.Vauzour D, Buonfiglio M, Corona G, Chirafisi J, Vafeiadou K, Angeloni C, et al. Sulforaphane protects cortical neurons against 5-S-cysteinyl-dopamine-induced toxicity through the activation of ERK1/2, Nrf-2 and the upregulation of detoxification enzymes. Mol Nutr Food Res. 2010;54:532–42. doi: 10.1002/mnfr.200900197. [DOI] [PubMed] [Google Scholar]
- 54.Siebert A, Desai V, Chandrasekaran K, Fiskum G, Jafri MS. Nrf2 activators provide neuroprotection against 6-hydroxydopamine toxicity in rat organotypic nigrostriatal cocultures. J Neurosci Res. 2009;87:1659–69. doi: 10.1002/jnr.21975. [DOI] [PubMed] [Google Scholar]
- 55.Han JM, Lee YJ, Lee SY, Kim EM, Moon Y, Kim HW, et al. Protective effect of sulforaphane against dopaminergic cell death. J Pharmacol Exp Ther. 2007;321:249–56. doi: 10.1124/jpet.106.110866. [DOI] [PubMed] [Google Scholar]
- 56.Soane L, Li Dai W, Fiskum G, Bambrick LL. Sulforaphane protects immature hippocampal neurons against death caused by exposure to hemin or to oxygen and glucose deprivation. J Neurosci Res. 2010;88:1355–63. doi: 10.1002/jnr.22307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Black AM, Armstrong EA, Scott O, Juurlink BJ, Yager JY. Broccoli sprout supplementation during pregnancy prevents brain injury in the newborn rat following placental insufficiency. Behav Brain Res. 2015;291:289–98. doi: 10.1016/j.bbr.2015.05.033. [DOI] [PubMed] [Google Scholar]
- 58.Ping Z, Liu W, Kang Z, Cai J, Wang Q, Cheng N, et al. Sulforaphane protects brains against hypoxic-ischemic injury through induction of Nrf2-dependent phase 2 enzyme. Brain Res. 2010;1343:178–85. doi: 10.1016/j.brainres.2010.04.036. [DOI] [PubMed] [Google Scholar]
- 59.Zhao X, Song S, Sun G, Strong R, Zhang J, Grotta JC, et al. Neuroprotective role of haptoglobin after intracerebral hemorrhage. J Neurosci. 2009;29:15819–27. doi: 10.1523/JNEUROSCI.3776-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang X, de Rivero Vaccari JP, Wang H, Diaz P, German R, Marcillo AE, et al. Activation of the nuclear factor E2-related factor 2/antioxidant response element pathway is neuroprotective after spinal cord injury. J Neurotrauma. 2012;29:936–45. doi: 10.1089/neu.2011.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Benedict AL, Mountney A, Hurtado A, Bryan KE, Schnaar RL, Dinkova-Kostova AT, et al. Neuroprotective effects of sulforaphane after contusive spinal cord injury. J Neurotrauma. 2012;29:2576–86. doi: 10.1089/neu.2012.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Socała K, Nieoczym D, Kowalczuk-Vasilev E, Wyska E, Wlaź P. Increased seizure susceptibility and other toxicity symptoms following acute sulforaphane treatment in mice. Toxicol Appl Pharmacol. 2017;326:43–53. doi: 10.1016/j.taap.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 63.Ren X, Wang NN, Qi H, Qiu YY, Zhang CH, Brown E, et al. Up-regulation thioredoxin inhibits advanced glycation end products-induced neurodegeneration. Cell Physiol Biochem. 2018;50:1673–86. doi: 10.1159/000494787. [DOI] [PubMed] [Google Scholar]
- 64.Di W, Shi X, Lv H, Liu J, Zhang H, Li Z, et al. Activation of the nuclear factor E2-related factor 2/anitioxidant response element alleviates the nitroglycerin-induced hyperalgesia in rats. J Headache Pain. 2016;17:99. doi: 10.1186/s10194-016-0694-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu YW, Cheng YQ, Liu XL, Hao YC, Li Y, Zhu X, et al. Mangiferin upregulates glyoxalase 1 through activation of Nrf2/ARE signaling in central neurons cultured with high glucose. Mol Neurobiol. 2017;54:4060–70. doi: 10.1007/s12035-016-9978-z. [DOI] [PubMed] [Google Scholar]
- 66.Shirai Y, Fujita Y, Hashimoto R, Ohi K, Yamamori H, Yasuda Y, et al. Dietary intake of sulforaphane-rich broccoli sprout extracts during juvenile and adolescence can prevent phencyclidine-induced cognitive deficits at adulthood. PLoS One. 2015;10:e0127244. doi: 10.1371/journal.pone.0127244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Singh K, Connors SL, Macklin EA, Smith KD, Fahey JW, Talalay P, et al. Sulforaphane treatment of autism spectrum disorder (ASD) Proc Natl Acad Sci U S A. 2014;111:15550–5. doi: 10.1073/pnas.1416940111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bent S, Lawton B, Warren T, Widjaja F, Dang K, Fahey JW, et al. Identification of urinary metabolites that correlate with clinical improvements in children with autism treated with sulforaphane from broccoli. Mol Autism. 2018;9:35. doi: 10.1186/s13229-018-0218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang L, Gallagher EP. Role of Nrf2 antioxidant defense in mitigating cadmium-induced oxidative stress in the olfactory system of zebrafish. Toxicol Appl Pharmacol. 2013;266:177–86. doi: 10.1016/j.taap.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 70.Chang G, Guo Y, Jia Y, Duan W, Li B, Yu J, et al. Protective effect of combination of sulforaphane and riluzole on glutamate-mediated excitotoxicity. Biol Pharm Bull. 2010;33:1477–83. doi: 10.1248/bpb.33.1477. [DOI] [PubMed] [Google Scholar]
- 71.Pearson BL, Simon JM, McCoy ES, Salazar G, Fragola G, Zylka MJ, et al. Identification of chemicals that mimic transcriptional changes associated with autism, brain aging and neurodegeneration. Nat Commun. 2016;7:11173. doi: 10.1038/ncomms11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Egea J, Buendia I, Parada E, Navarro E, Rada P, Cuadrado A, et al. Melatonin-sulforaphane hybrid ITH12674 induces neuroprotection in oxidative stress conditions by a ‘drug-prodrug’ mechanism of action. Br J Pharmacol. 2015;172:1807–21. doi: 10.1111/bph.13025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.U.S. National Library of Medicine. Search Results for “sulforaphane.”. National Institute of Health. U.S. Department of Health and Human Services. [Last accessed on 2019 Feb 12]. Available from: http://Clinicaltrials. gov .