Abstract
OBJECTIVE:
Confirming the histologic diagnosis of small pulmonary nodules or Ground-glass opacity nodules (GGNs) of unknown origin is difficult. These nodules are not always appropriate for percutaneous transthoracic needle biopsy. Preoperative localization of pulmonary lesions provides more precise target points to ensure complete surgical excision. The goal of the present study was to evaluate the validity and effectiveness of computed tomography-guided preoperative hook wire localization with our technique for video-assisted thoracoscopic surgery (VATS).
METHODS:
We retrospectively investigated 113 patients who had undergone preoperative hook wire localization before VATS resection for newly present or growing pulmonary nodular lesions between May 2007 and December 2016. Procedural and perioperative outcomes were assessed to evaluate the safety and efficacy of preoperative localization technique.
RESULTS:
A total of 113 pulmonary nodules were localized and successfully resected in all 113 patients. The mean diameter of nodules was 10.8 ± 6.1 mm (range, 3–28). The mean distance from the pleural surface was 20.2 ± 12.4 mm (range, 5–55). The mean procedure time of localization was 23.7 ± 6.3 min. Asymptomatic minimal pneumothorax and mild parenchymal hemorrhage occurred in 26 (23.0%) and 8 (7.1%) patients, respectively. There were 32 (28.3%) deep lung nodules, in which the distance to pleural surface was more than 25 mm. Wire dislodgement occurred in 4 (3.5%) patients. Complete resection of all lung lesions was achieved, and definite histological diagnosis was obtained in all patients. Pathologic examination revealed 42 (37.2%) primary lung cancers, 2 (1.8%) lymphomas, 53 (46.9%) metastases, 16 (14.1%) benign lesions.
CONCLUSIONS:
Preoperative percutaneous hook wire localization is a dependable and useful technique to facilitate positioning small and deep pulmonary nodules for thoracoscopic complete excision and accurate diagnosis.
Keywords: Complications, hook wire localization, lung cancer, pulmonary nodules, video-assisted thoracoscopic surgery
The solitary pulmonary nodule (SPN) represents a single, round-shaped lesion in the lung completely surrounded by lung parenchyma with a diameter <3 cm and without associated pneumonia, atelectasis, or lymphadenopathies. As high-resolution computed tomography (HRCT) imaging technique becomes more advanced, the frequency at which subcentimeter pulmonary nodular lesions including ground-grass opacity nodules (GGNs) are detected is increasing. The precise histological diagnosis for pulmonary nodules is promptly required because they carry a more than 50% risk of malignancy.[1] Conventional procedures such as transthoracic fine-needle biopsy and transbronchial biopsy have been usually performed, but they are highly associated with a risk of pneumothorax and are liable to sampling errors.[2] When the diagnostic confirmation was not achieved with these nonsurgical ways, video-assisted thoracoscopic surgery (VATS) is commonly recommended for accurate diagnosis and therapeutic resection of nodular lesions. Nowadays, thoracoscopic surgery is routinely used for both diagnosis and treatment with the advantages of decreasing postoperative morbidities and shortening the hospitalization period.[3,4] Nevertheless, exact intraoperative identification of the lung lesion is not simple because of difficulty in palpating the lung directly during VATS. Particularly, in the case of deeply located small lung lesions, ground-glass opacity (GGO) nodules (GGNs), or lesions accompanied by diffuse emphysematous lung or thickened pleural adhesions, it becomes much more vulnerable to failure of location. Suzuki et al. reported that the conversion rate to thoracotomy increase up to 63% when the nodules are < 10 mm in size or deeper than 5 mm from the closest pleural surface.[5] Therefore, numerous perioperative or intraoperative techniques of localization have been introduced to decrease the probability of conversion to thoracotomy. They described various alternative ways such as finger palpation;[5] intraoperative ultrasound;[6,7,8] CT-guided localization using microcoils,[9] methylene blue,[10] lipiodol,[11] or radionuclides;[12] and hook wire.[13,14,15,16,17] Among them, preoperative hook wire localization method showed 58%–97% diverse success rates with relatively higher failure rate up to 47% due to wire dislodgement or migration.[18] There are some reports associated mild complications with asymptomatic minimal pneumothorax or lung parenchymal hemorrhages, while major adverse events are not reported often. We initially reported our preliminary results of preoperative localization technique with hook wire for lung nodules of 31 patients in 2007.[19] Based on our updated experience of 10 years, we herein present a retrospective study to reevaluate the efficacy and availability of our own localization technique. In addition, we tried to focus on the detailed technique aspects of hook wire localization procedure in our medical center.
Methods
Patients and study design
From May 2007 to December 2016, a total of 113 patients carried out percutaneous localization procedure using hook wire, and subsequently, thoracoscopic wedge resection was performed in operation room. It consists of 63 males and 50 females, with an average age of 62.8 ± 11.1 years (range, 20–83). Eighty-one patients had a previous malignancy history of the 113 patients. Patient characteristics are shown in Table 1. In our institution, the selection criteria for wire localization were decided on the basis of one of the following CT findings: patients with unsettled lung nodules of 10 mm or less in size and more than 10 mm deep to the adjacent pleural surface, pure GGN, or a lesion mostly consisted of GGO compartments. On some occasions, the operator may determine the preoperative localization procedure if the target nodular lesions are assumed to be invisible during thoracoscopic surgery. The distance from the nodule to pleural surface, the largest diameters of nodules, and consolidation/tumor (C/T) ratio were measured on HRCT. The pure GGNs, part-solid GGNs, and characteristics of nodular lesions are shown in Tables 1 and 2. The Institutional Review Board of Konkuk University Medical Center had approved this study (No. 1080028). Informed consent for preoperative procedure and operation was naturally obtained from all patients.
Table 1.
Characteristics of patients and pulmonary nodules
| Characteristic | Value |
|---|---|
| Total patients | 113 |
| Total nodules of localization | 113 |
| Gender | |
| Male | 63 |
| Female | 50 |
| Mean age (years) | 62.8±11.1 (20-83) |
| Previous extrathoracic malignancy (%) | 81 (71.7) |
| Smoking status (%) | |
| Current | 31 (27.4) |
| Former, quit | 20 (17.7) |
| Never smoked | 62 (54.9) |
| Pulmonary function | |
| FEV1 (percentage predicted) | 86.2±14.3 (46-124) |
| DLCO (percentage predicted) | 58.5±11.9 (35-87) |
| Nodule location (%) | |
| Right upper lobe | 30 (26.5) |
| Right middle lobe | 10 (8.9) |
| Right lower lobe | 25 (22.1) |
| Left upper lobe | 28 (24.8) |
| Left lower lobe | 20 (17.7) |
| Radiologic nodule features (%) | |
| Pure GGN | 35 (30.9) |
| Part-solid GGN | 50 (44.2) |
| Solid nodule | 28 (24.9) |
Values are presented as mean±SD (range) or n (%). FEV1=Forced expiratory volume in 1 s, DLCO=Diffusion capacity of the lung for carbon monoxide, GGN=Ground glass opacity nodule, SD=Standard deviation
Table 2.
Outcomes of localization procedure
| Characteristic | Value |
|---|---|
| Success rate of localization (%) | 109 (96.5) |
| Nodule diameter (mm) | 10.8±6.1 (3-28) |
| Depth from the pleural surface (mm) | 20.2±12.4 (5-55) |
| Deep lung nodules (>25 mm) (%) | 32 (28.3) |
| Duration of hook wire localization procedure (min) | 23.7±6.3 (14-40) |
| Duration from localization to surgery (min) | 34.6±19.9 (15-100) |
| Position of the patient during localization procedure (%) | |
| Supine | 61 (54) |
| Prone | 43 (38) |
| Lateral | 9 (8) |
| Localization-related complications (%) | |
| Pneumothorax | 26 (23.0) |
| Hemorrhage | 8 (7.1) |
| Dislodgement | 4 (3.5) |
| Air embolism | 1 (0.8) |
Values are presented as mean±SD (range) or n (%). SD=Standard deviation
Computed tomography-guided hook wire localization
Immediately before the surgery, patients were transferred to the radiology department and placed on the CT scan table in a prone, supine, or lateral decubitus position by the location of nodular lesion. CT was examined to distinguish the shape, size, and location of the lesion, including the interaction with the adjacent tissues and vessels with the assistance of a radio-opaque grid mesh over the chest wall. Then, we measured the distance between the edge of the lesion and the skin layer to obtain the ideal route and marked the point of puncture. A 2% lidocaine was injected into the chest wall of needle insertion site after administration of sterilization and draping of the patient. A 10-cm-long, 21-gauge-needle with hook wire with a sharp thorn (Argon Medical Devices, Inc., Athens, Texas, USA) was used for nodule localization. The cannula needle was slowly inserted into the chest wall and lung parenchyma layer, and located as close to the target nodular lesion as possible, which is generally <15 mm from the nodule [Figure 1]. The hook wire was released after identifying the adequate placement of the introducer needle under the guiding CT scan, and then the introducer needle was cautiously pulled out. The examination of CT scan was repeated to ensure the last location of hook wire and to assess the presence of following complications such as pneumothorax [Figure 2a], hemorrhage [Figure 2b], or subcutaneous emphysema. In general, the part of the wire which is protruding from the chest wall is bolstered with a couple of gauzes, and taped to the skin to secure the segment of wire is not bent or curved. However, we directly trimmed off the protruding tips of hook wire as close as possible from the skin in our own manner [Figure 3]. It allows the wire to leave free to move toward the collapse of the lung in the middle of VATS operation under the single-lung ventilation system. On finishing localization procedure, patients were immediately transferred to the operation room.
Figure 1.
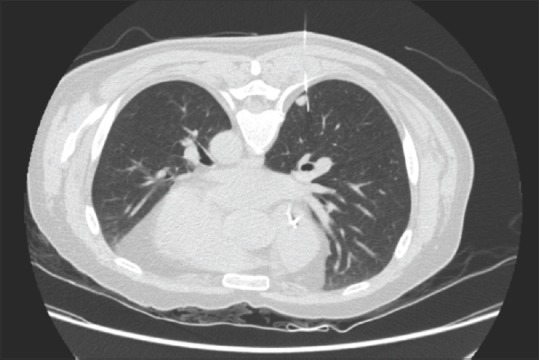
Computed tomography scan identifies a hook wire positioned on the edge of the nodular lesion. CT: Computed tomography
Figure 2.
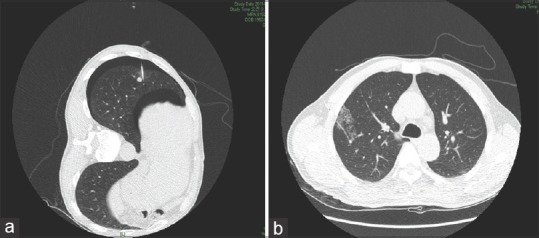
(a) Computed tomography scan shows new appearance of minimal pneumothorax after hook wire localization. (b) Computed tomography image of asymptomatic hemorrhage around the nodular lesion
Figure 3.

A procedure of the trimming technique of cutting off the wire sticking out of the skin
Surgical procedure (video-assisted thoracoscopic surgery)
On arriving operation room, all patients were intubated with a double-lumen endobronchial tube or single-lumen tube with bronchial blockade to achieve single-lung ventilation under general anesthesia. After positioning of patients in either the right or left lateral decubitus according to the location of nodule, skin preparation and draping were done. Intrathoracic access for exploration of the lung was usually obtained at a level between the fifth and seventh intercostal space at the anterior midaxillary line. A 10-mm, 30° operating thoracoscope (Karl Storz, Tuttlingen, Germany) was routinely used to explore the pleural space. Two additional access ports were established for grasping the lung, switching the port for the thoracoscope, and insertion of an endoscopic liner stapler (Echelon Endostapler, Ethicon, Cincinnati, OH, USA). Wedge resection ensuring a sufficient resection margin was performed after identifying the localized nodule lesion with hook wire [Figure 4]. In case of difficulty to visualize the location of localized nodule or tip of hook wire, we first tried to find the parenchymal hemorrhage point by previous needle pricking, and subsequent blind wedge resection was accomplished. Intraoperative frozen section pathology was performed. If the pathologic result of the lesion was benign or metastasis from an extrathoracic tumor, we decided to terminate the operation. If the pulmonary nodular lesion was a Non small cell lung cancer (NSCLC), the patient subsequently underwent complete therapeutic lobectomy with mediastinal lymph nodes dissection, unless the patient had insufficient and poor pulmonary function to stand a lobectomy, hard to endure the one-lung ventilation intraoperatively, or the patient had a previous lobectomy surgery due to primary lung cancer.
Figure 4.
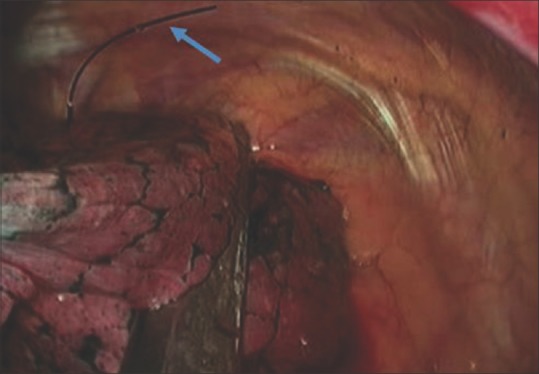
Intraoperative thoracoscopic image of a wedge resection of a pulmonary nodule which was preoperatively localized by hook wire (arrow)
Pathologic analysis
The specimen was sent to the department of pathology for frozen section pathologic examination. Both the assessment of histology in nodular lesion (malignancy or benign) and the adequacy of the surgical resection margins were immediately required to know during operation. Dissection of lymph nodes and staging process are done together as well. The final histological features of the lung cancer and staging were determined in accordance with the World Health Organization and the International Association for the Study of Lung Cancer histologic classification.[20,21]
Assessment of the clinical outcomes
We retrospectively reviewed the medical records and obtained data such as patient's age, gender, smoking status, pulmonary function test results, location, radiological characteristics of nodule, nodule diameter, depth from pleural surface, duration of CT localization procedure, time from the end of the localization to the induction of general anesthesia, complications, type of operation, histopathologic findings, conversion rate to thoracotomy, postoperative complications and hospitalization period, and 30-day mortality.
Statistical analysis
The descriptive analysis results data were presented as mean ± standard deviation with range for continuous variables, unless otherwise indicated. Categorical variables are expressed as percentages or numbers.
Results
Of 113 patients who underwent CT-guided localization procedure, 63 (55.8%) patients were male, with a mean age of 62.8 ± 11.1 (range, 20–83) years. There were 51 patients who had a smoking history (current and former). Eighty-one patients had a previous cancer history. There were 30 nodules located in the right upper lobe, 10 in the right middle lobe, 25 in the right lower lobe, 28 in the left upper lobe, and 20 in the left lower lobe. Based on CT scanning, pulmonary nodules could be categorized as pure GGNs (n = 35, 30.9%), part-solid GGNs (n = 50, 44.2%), and solid nodules (n = 28, 24.9%). Other characteristics of patients and nodules are summarized in Table 1. The average nodule size was 10.8 ± 6.1 mm (range, 3–28). The mean distance from nodular lesion to the nearest pleural surface was 20.2 ± 12.4 mm (range, 5–55). The mean localization procedural time was 23.7 ± 6.3 mm (range, 14–40). The mean duration from localization to surgery was 34.6 ± 19.9 mm (range, 15–100). Hook wire localization was successful in 109 (96.4%) of 113 nodules. The hook wire was dislodged or fallen out before VATS resection in four targeted nodular lesions, but two of them were successfully resected with the assistance of remnant hemorrhagic marks on the lung parenchymal surface arising from the procedure of localization. The other two patients are required to switch from VATS to mini-thoracotomy via extended incision during the operation because it was very difficult to palpate and find the targeted nodular lesion. The most common complication related to hook wire localization was pneumothorax (n = 26, 23.0%), diagnosed by CT scanning right after localization procedure. However, all patients with pneumothorax were asymptomatic, and no further interventional procedure was required before operation. Other localization-related lung parenchymal hemorrhage (n = 8, 7.1%) and systemic air embolism (n = 1, 0.8%) were also observed. No patients developed hemodynamic instability until general anesthesia was induced, except only one case of air embolism. However, the patient was fortunate to recover rapidly to normal hemodynamic stabilization within approximately 1 min after immediate cardiopulmonary resuscitation management. Other details of hook wire localization procedure are shown in Table 2. Histologic diagnosis included primary lung malignancy (n = 42), metastases (n = 53), lymphoma (n = 2), and benign lesions (n = 16). Primary lung cancer consisted of invasive adenocarcinoma (n = 38), minimally invasive adenocarcinoma (n = 3), and squamous cell carcinoma (n = 1). The benign lesions comprised chronic granulomatous inflammation (n = 7), anthracofibrotic nodule (n = 4), Aspergillus (n = 1), epithelioid hemangioendothelioma (n = 1), acute fibrinoid suppurative inflammation (n = 1), foreign body giant cells with fibrin coagulum (n = 1), and pneumonia (n = 1). Table 3 represents the details of pathologic results. For patients with nonsmall cell lung carcinoma (NSCLC) (n = 42, 37.2%), complete lobectomy was performed in 39 individuals by VATS approach. Two patients underwent widely wedge resection via thoracoscopic surgery because of difficulty in tolerating single-lung ventilation maneuver due to the poor lung capacity. The other one patient also had wide wedge resection of the lung because he had previous surgery history of ipsilateral lobectomy operation. The average length of hospital stay was 5.2 ± 2.1 days (range, 3–12) for VATS wedge resection and 8.9 ± 2.6 days (range, 5–14) for VATS lobectomy. There were two forms of morbidities postoperatively. One is acute pulmonary thromboembolism with exacerbation of pneumonia and pulmonary fibrosis which is not related to the surgery (n = 1, 0.8%) and the other is persistent air leakage more than 4 days (n = 10, 8.8%) [Table 4].
Table 3.
Histologic diagnosis of pulmonary nodules
| Characteristic | n (%) |
|---|---|
| Primary lung malignancy | 42 (37.2) |
| Invasive adenocarcinoma | 38 (33.6) |
| Minimally invasive adenocarcinoma | 3 (2.6) |
| Squamous cell carcinoma | 1 (0.9) |
| Metastasis | 53 (46.9) |
| Colon | 43 (38.1) |
| Breast | 4 (3.5) |
| Thyroid | 3 (2.6) |
| Kidney | 1 (0.9) |
| Liver | 1 (0.9) |
| Gall bladder | 1 (0.9) |
| Benign | 16 (14.1) |
| Chronic granulomatous inflammation | 7 (6.2) |
| Anthracofibrotic nodule | 4 (3.5) |
| Aspergillus | 1 (0.9) |
| Epithelioid hemangioendothelioma | 1 (0.9) |
| Fibrinoid suppurative inflammation | 1 (0.9) |
| Foreign body giant cells with fibrin coagulum | 1 (0.9) |
| Pneumonia | 1 (0.9) |
| Lymphoma | 2 (1.8) |
Data expressed as n (%), unless noted otherwise
Table 4.
Perioperative outcomes
| Characteristic | Value |
|---|---|
| Type of operation (%) | |
| Primary lung cancer | 42 (37.2) |
| Lobectomy-VATS | 39 (34.5) |
| Wide wedge resection-VATS | 3 (2.7) |
| Metastasis (%) | |
| Wedge resection-VATS | 53 (46.9) |
| Benign lesions (%) | |
| Wedge resection-VATS | 16 (14.1) |
| Lymphoma | 2 (1.8) |
| Mean postoperative hospitalization period (day) | |
| Wedge resection-VATS | 5.2±2.1 (3-12) |
| Lobectomy-VATS | 8.9±2.6 (5-14) |
| Postoperative morbidity (%) | |
| Pulmonary thromboembolism | 1 (0.8) |
| Persistent air leak (>4 day) | 10 (8.8) |
Values are presented as mean±SD (range) or n (%). SD=Standard deviation, VATS=Video-assisted thoracoscopic surgery
Discussion
Nowadays, not only radiologists but also thoracic surgeons often come across an increasing number of patients of SPNs during follow-up screening in patients with lung cancer or even extrathoracic cancer. When pulmonary nodules are encountered, especially in patients with previous malignancy history, an accurate histological analysis is always mandatory because they are highly suspicious for malignancy. Early detection and adequate surgical resection is the most optimal scheme for improving the survival of lung cancer. Most of pulmonary nodular lesions have no typical radiologic features; if lung nodule more than 1 cm in diameter, we first try to diagnose with relatively simple methods such as transbronchial biopsy or percutaneous transthoracic needle biopsy. However, the efficacy of these conventional methods can be limited by unfavorable location or the small sized nodule, and moreover, inadequate tissue sampling or biopsy failure sometimes happens.[2] Thanks to the development of thoracoscopic surgery and the precision of surgical instrument, VATS has been broadly applied for the excision of pulmonary nodular lesions with minimal postoperative morbidity. Furthermore, VATS is now indicated for definite surgical treatment in early stage of nonsmall cell lung cancer (NSCLC) proceeding with lobectomy including lymphadenectomy.[4] However, VATS resection of low-attenuation or subcentimeter lung nodules can be quite demanding because it is not simple to distinguish GGNs with normal lung tissue and is hard to touch nodular lesion far from the pleural surface after lung deflation. Actually, Ichinose et al. described that nodules <10 mm in size and located more than 5 mm deep from the pleural surface may be associated with a 63% chance of failure, besides the surgery may be prolonged as it took longer to find tiny lung nodules, and VATS approach procedure is converted to (mini-) thoracotomy in more than 50%.[15] Therefore, various targeting localization techniques have been introduced and discussed their strengths and weaknesses to facilitate the resection of nodular lesions during VATS. One of the most conventional ways is simple palpation by fingers introduced in the trocar or utility incision. Finger localization can also be difficult depending on the characteristics of the nodule (GGNs, solidity, or cavitation) and on the lung parenchymal features (fibrosis, emphysema, and strong adhesion).[16] CT-guided dye injection is a sort of convenient method. However, the colorant can quickly proceed into pleural surface and surroundings, and location of the nodule becomes much more difficult when broad anthracotic pigmentation is present.[5] This technique has been associated with a rate of failure up to 13%. Although intraoperative ultrasound detection technique is a noninvasive procedure, specific flexible ultrasound probe is essentially required and is somewhat variable depending on the operators. Besides, it is restricted by the presence of air, which can be difficult to obtain in noncollapsed lung or in patients with underlying emphysema.[6,7] There is other new technique for localization as electromagnetic navigation bronchoscopy-guided dye marking. However, it also requires special equipment and experience like the ultrasound localization detecting technique.[22,23]
In our institute, we adopted CT-guided hook wire localization technique before VATS. Currently, CT-guided hook wire localization is the most commonly used localization method. In the past, hook wire localization was initially used to biopsy in mammary glands. Since then, it was increasingly applied to detect pulmonary nodules with the development of VATS and employed to find lesions in the intra-abdominal space, retroperitoneum, and even muscles.[24,25] We previously reported our preliminary experience of preoperative hook wire localization and concluded that this technique provided efficient and fast procedure with a low rate of morbidities and complications.[19] In this study, hook wire dislodgement was observed in 4 (3.5%) patients. The rate of dislodgement in our study is acceptable and consistent with a rate of 5%–8% reported in the previous literatures.[13,15,16] In two of them, thoracoscopic wedge resection was possible in spite of wire dislodgement with the assistance of visualization of the puncture site on the pleural surface. The reason why this study showed a relatively lower rate of dislodgement is thought to be our own trimming technique of cutting off the wire sticking out of skin as closely as possible. It may allow the wire to move toward the collapsed lung during single-lung ventilation or in case of pneumothorax. One of the most common complications of hook wire localization procedure is pneumothorax. Pneumothorax occurred in 26 (23.0%) patients after localization procedure in our study. This proportion is actually agreeable with other prior studies (7.5%–56.2%).[13,15,26,27] However, all patients with pneumothorax did not complain other specific symptom, and none of them required further clinical intervention or chest tube insertion. Although other localization-related lung parenchymal hemorrhage was observed in 8 (7.1%) patients, it was also successfully managed by conservative treatment.
Meanwhile, systemic air embolism as a complication of transthoracic needle penetration procedure such as hook wire localization is extremely rare, but potentially life-threatening with a rate of 0.02%–0.06%.[28] However, air embolism incidence rate is considered to be underestimated because of undiagnosed asymptomatic patients.[29,30] Unfortunately, we encountered one patient who had abruptly no response and no consciousness while waiting to move to the operating room after the localization procedure was finished. Immediate intubation with 100% oxygen administration was accomplished. Within a minute or so, the patient, fortunately, came back to normal respiratory and hemodynamic status. A review of CT scanning revealed moderate amount of intracardiac air. The mechanism of air embolism complicating transthoracic lung needle biopsy remains unclear, though a couple of theories have been presented considering possible pathways whereby air is introduced into the systemic circulation during a fine-needle biopsy.[31,32] Our experiences with our own way of preoperative hook wire localization were made possible by the support of constant multidisciplinary efforts for years. In the present study, we are willing to highlight the following aspects. First, we have stopped ensuring the stability with fixation the hook wire at the skin with exclusive skin retention clip or taping, because we have noticed more often dislodgement of wire in case the wire is firmly fixed to the skin. This technique enables the wire to go toward collapsed lung during single-lung ventilation or even in the event of pneumothorax. Second, it is recommended to avoid direct needle and penetration through the targeted nodular lesion. Direct penetration through the lesion is feared to damage the intact capsula of the lesion and may also precipitate potential spread of the malignant cells. Hence, we practically place the hook wire just adjacent to the target lesion under the guidance of CT scan. Finally, it is significant that the patient should be moved to the operating room as soon as localization procedure ended, consecutively. The longer the waiting time before the surgery begins, the higher the probability of the wire dislodgement. In this respect, this sequential procedural technique requires intercommunication and multidisciplinary efforts between surgeons and radiologist. A high degree of close cooperation between both departments can drive the best coordination. We acknowledge that this study has some limitations of nonrandomized and retrospective analysis in a single institution experience, which may involve selection bias. Besides, there is no control group as similar comparative patients who were not preoperatively localized before VATS. A large prospective randomized trial is warranted to assess and confirm our findings for the avoidance of selection bias.
Conclusions
CT-guided preoperative hook wire localization appears to be a feasible, convenient, and secure strategy to localize small and deep lung nodules for thoracoscopic wedge resection and accurate diagnosis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Shah PL, Singh S, Bower M, Livni N, Padley S, Nicholson AG. The role of transbronchial fine needle aspiration in an integrated care pathway for the assessment of patients with suspected lung cancer. J Thorac Oncol. 2006;1:324–7. [PubMed] [Google Scholar]
- 2.Gould MK, Donington J, Lynch WR, Mazzone PJ, Midthun DE, Naidich DP, et al. Evaluation of individuals with pulmonary nodules: When is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e93S–120S. doi: 10.1378/chest.12-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e142S–65S. doi: 10.1378/chest.12-2353. [DOI] [PubMed] [Google Scholar]
- 4.Howington JA, Blum MG, Chang AC, Balekian AA, Murthy SC. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e278S–313S. doi: 10.1378/chest.12-2359. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki K, Nagai K, Yoshida J, Ohmatsu H, Takahashi K, Nishimura M, et al. Video-assisted thoracoscopic surgery for small indeterminate pulmonary nodules: Indications for preoperative marking. Chest. 1999;115:563–8. doi: 10.1378/chest.115.2.563. [DOI] [PubMed] [Google Scholar]
- 6.Greenfield AL, Steiner RM, Liu JB, Cohn HE, Goldberg BB, Rawool NM, et al. Sonographic guidance for the localization of peripheral pulmonary nodules during thoracoscopy. AJR Am J Roentgenol. 1997;168:1057–60. doi: 10.2214/ajr.168.4.9124115. [DOI] [PubMed] [Google Scholar]
- 7.Santambrogio R, Montorsi M, Bianchi P, Mantovani A, Ghelma F, Mezzetti M. Intraoperative ultrasound during thoracoscopic procedures for solitary pulmonary nodules. Ann Thorac Surg. 1999;68:218–22. doi: 10.1016/s0003-4975(99)00459-2. [DOI] [PubMed] [Google Scholar]
- 8.Kondo R, Yoshida K, Hamanaka K, Hashizume M, Ushiyama T, Hyogotani A, et al. Intraoperative ultrasonographic localization of pulmonary ground-glass opacities. J Thorac Cardiovasc Surg. 2009;138:837–42. doi: 10.1016/j.jtcvs.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Mayo JR, Clifton JC, Powell TI, English JC, Evans KG, Yee J, et al. Lung nodules: CT-guided placement of microcoils to direct video-assisted thoracoscopic surgical resection. Radiology. 2009;250:576–85. doi: 10.1148/radiol.2502080442. [DOI] [PubMed] [Google Scholar]
- 10.Lenglinger FX, Schwarz CD, Artmann W. Localization of pulmonary nodules before thoracoscopic surgery: Value of percutaneous staining with methylene blue. AJR Am J Roentgenol. 1994;163:297–300. doi: 10.2214/ajr.163.2.7518642. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe K, Nomori H, Ohtsuka T, Kaji M, Naruke T, Suemasu K, et al. Usefulness and complications of computed tomography-guided lipiodol marking for fluoroscopy-assisted thoracoscopic resection of small pulmonary nodules: Experience with 174 nodules. J Thorac Cardiovasc Surg. 2006;132:320–4. doi: 10.1016/j.jtcvs.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 12.Chella A, Lucchi M, Ambrogi MC, Menconi G, Melfi FM, Gonfiotti A, et al. A pilot study of the role of TC-99 radionuclide in localization of pulmonary nodular lesions for thoracoscopic resection. Eur J Cardiothorac Surg. 2000;18:17–21. doi: 10.1016/s1010-7940(00)00411-5. [DOI] [PubMed] [Google Scholar]
- 13.Ciriaco P, Negri G, Puglisi A, Nicoletti R, Del Maschio A, Zannini P, et al. Video-assisted thoracoscopic surgery for pulmonary nodules: Rationale for preoperative computed tomography-guided hookwire localization. Eur J Cardiothorac Surg. 2004;25:429–33. doi: 10.1016/j.ejcts.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 14.Molins L, Mauri E, Sánchez M, Fibla JJ, Gimferrer JM, Arguis P, et al. Locating pulmonary nodules with a computed axial tomography-guided harpoon prior to videothoracoscopic resection. Experience with 52 cases. Cir Esp. 2013;91:184–8. doi: 10.1016/j.ciresp.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Ichinose J, Kohno T, Fujimori S, Harano T, Suzuki S. Efficacy and complications of computed tomography-guided hook wire localization. Ann Thorac Surg. 2013;96:1203–8. doi: 10.1016/j.athoracsur.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 16.Dendo S, Kanazawa S, Ando A, Hyodo T, Kouno Y, Yasui K, et al. Preoperative localization of small pulmonary lesions with a short hook wire and suture system: Experience with 168 procedures. Radiology. 2002;225:511–8. doi: 10.1148/radiol.2252011025. [DOI] [PubMed] [Google Scholar]
- 17.Chen YR, Yeow KM, Lee JY, Su IH, Chu SY, Lee CH, et al. CT-guided hook wire localization of subpleural lung lesions for video-assisted thoracoscopic surgery (VATS) J Formos Med Assoc. 2007;106:911–8. doi: 10.1016/S0929-6646(08)60061-3. [DOI] [PubMed] [Google Scholar]
- 18.Bernard A. Resection of pulmonary nodules using video-assisted thoracic surgery. The thorax group. Ann Thorac Surg. 1996;61:202–4. doi: 10.1016/0003-4975(95)01014-9. [DOI] [PubMed] [Google Scholar]
- 19.Kim J, Hwang JJ, Lee SA, Lee WS, Kim YH, Kim JS, et al. Lung biopsy after localization of pulmonary nodules with hook wire. Korean J Thorac Cardiovasc Surg. 2010;43:681–6. [Google Scholar]
- 20.Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. Introduction to the 2015 World Health Organization classification of tumors of the laung, pleura, thymus, and heart. J Thorac Oncol. 2015;10:1240–2. doi: 10.1097/JTO.0000000000000663. [DOI] [PubMed] [Google Scholar]
- 21.Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of Lung Adenocarcinoma. J Thorac Oncol. 2011;6:244–85. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Awais O, Reidy MR, Mehta K, Bianco V, Gooding WE, Schuchert MJ, et al. Electromagnetic navigation bronchoscopy-guided dye marking for thoracoscopic resection of pulmonary nodules. Ann Thorac Surg. 2016;102:223–9. doi: 10.1016/j.athoracsur.2016.02.040. [DOI] [PubMed] [Google Scholar]
- 23.Luo K, Lin Y, Lin X, Yu X, Wen J, Xi K, et al. Localization of peripheral pulmonary lesions to aid surgical resection: A novel approach for electromagnetic navigation bronchoscopic dye marking. Eur J Cardiothorac Surg. 2017;52:516–21. doi: 10.1093/ejcts/ezx114. [DOI] [PubMed] [Google Scholar]
- 24.Pittet O, Christodoulou M, Pezzetta E, Schmidt S, Schnyder P, Ris HB, et al. Video-assisted thoracoscopic resection of a small pulmonary nodule after computed tomography-guided localization with a hook-wire system. Experience in 45 consecutive patients. World J Surg. 2007;31:575–8. doi: 10.1007/s00268-006-0343-7. [DOI] [PubMed] [Google Scholar]
- 25.Morrison WB, Sanders TG, Parsons TW, Penrod BJ. Preoperative CT-guided hookwire needle localization of musculoskeletal lesions. AJR Am J Roentgenol. 2001;176:1531–3. doi: 10.2214/ajr.176.6.1761531. [DOI] [PubMed] [Google Scholar]
- 26.Iguchi T, Hiraki T, Gobara H, Fujiwara H, Matsui Y, Miyoshi S, et al. CT fluoroscopy-guided preoperative short hook wire placement for small pulmonary lesions: Evaluation of safety and identification of risk factors for pneumothorax. Eur Radiol. 2016;26:114–21. doi: 10.1007/s00330-015-3815-z. [DOI] [PubMed] [Google Scholar]
- 27.Klinkenberg TJ, Dinjens L, Wolf RF, van der Wekken AJ, van de Wauwer C, de Bock GH, et al. CT-guided percutaneous hookwire localization increases the efficacy and safety of VATS for pulmonary nodules. J Surg Oncol. 2017;115:898–904. doi: 10.1002/jso.24589. [DOI] [PubMed] [Google Scholar]
- 28.Tomiyama N, Yasuhara Y, Nakajima Y, Adachi S, Arai Y, Kusumoto M, et al. CT-guided needle biopsy of lung lesions: A survey of severe complication based on 9783 biopsies in Japan. Eur J Radiol. 2006;59:60–4. doi: 10.1016/j.ejrad.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Hiraki T, Fujiwara H, Sakurai J, Iguchi T, Gobara H, Tajiri N, et al. Nonfatal systemic air embolism complicating percutaneous CT-guided transthoracic needle biopsy: Four cases from a single institution. Chest. 2007;132:684–90. doi: 10.1378/chest.06-3030. [DOI] [PubMed] [Google Scholar]
- 30.Freund MC, Petersen J, Goder KC, Bunse T, Wiedermann F, Glodny B, et al. Systemic air embolism during percutaneous core needle biopsy of the lung: Frequency and risk factors. BMC Pulm Med. 2012;12:2. doi: 10.1186/1471-2466-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mansour A, AbdelRaouf S, Qandeel M, Swaidan M. Acute coronary artery air embolism following CT-guided lung biopsy. Cardiovasc Intervent Radiol. 2005;28:131–4. doi: 10.1007/s00270-004-0118-1. [DOI] [PubMed] [Google Scholar]
- 32.Cheng HM, Chiang KH, Chang PY, Chou YF, Huang HW, Chou AS, et al. Coronary artery air embolism: A potentially fatal complication of CT-guided percutaneous lung biopsy. Br J Radiol. 2010;83:e83–5. doi: 10.1259/bjr/39096533. [DOI] [PMC free article] [PubMed] [Google Scholar]


