Abstract
Purpose:
The aim of the study is to identify risk factors, clinical characteristics, causative fungi, and treatment outcome of dematiaceous fungal keratitis in North India.
Methods:
Consecutive cases of culture-proven dematiaceous fungal keratitis between January 2012 and June 2017 were retrieved from the medical record department. Risk factors, clinical signs, and outcome were registered.
Results:
Eighty-three patients were included. Identified dematiaceous fungal organism were Curvularia sp. (n = 55/83; 66.3%), Alternaria sp. (n = 12/83; 14.5%), Ulocladium sp. (n = 5/83; 6%), Bipolaris sp. (n = 5/83; 6.1%), Scedosporium sp. (n = 3/83; 3.6%), Acremonium sp. (n = 2/83; 2.4%), and Epicoccum sp. (n = 1/83; 1.2%). Male preponderance was reported. The most common predisposing factor was corneal trauma (67.4%). In cases associated with corneal trauma due to vegetative matter, sugarcane was the most common cause. In all, 89% of the patients were more than 30 years of age. The median infiltrate size was 8 mm2. The median time of antifungal therapy was 4.2 weeks (interquartile range [IQR]: 1-25 weeks). Complications were seen in 14 (n = 14/65; 21.5%) patients. Complete resolution of dematiaceous fungal keratitis was present in 27 (n = 27/65; 41.5%) eyes.
Conclusion:
Curvularia sp. and Alternaria sp. were the predominant pathogenic genera causing dematiaceous fungal keratitis. Among the causative fungi, infections due to Scedosporium sp. were associated with the worst outcomes. Ulocladium sp. and Epicoccum sp. were also identified. Both the species are not reported previously as a causal organism of dematiaceous fungal keratitis from North India.
Keywords: Alternaria, Curvularia, dematiaceous fungi, Epicoccum, keratitis, Ulocladium, Uttar Pradesh
Fungal keratitis is more virulent and damaging than bacterial keratitis.[1] Ocular trauma by vegetative matter, topical steroid use, and use of contact lens are the associated risk factors of fungal keratitis. It is more common in males, compared with females. Previous studies from India reported that 34% to 44% of all keratitis were caused by fungi.[2,3,4,5,6,7] In tropical countries, 8% to 17% of the keratitis are caused by dematiaceous fungi.[8] Dematiaceous fungi are uncommon but important cause of human disease. Over 100 species of dematiaceous fungi have been identified causing infections in human beings.[9] Alternaria sp., Bipolaris sp., Curvularia sp., Exophiala sp., Madurella sp., Phialophora sp., Scedosporium prolificans, Fonsecaea pedrosoi, Cladophialophora bantiana, Scytalidium dimidiatum, and Wangiella dermatitidis are the commonest pigmented fungal species causing infections in humans.[10] These fungi are distributed worldwide especially in tropics including India.[11,12,13,14] Dematiaceous fungi are found in soil and decomposing plant material. The characteristic dark color of their spores and hyphae is because of presence of melanin in their cell wall. Macroscopic and microscopic pigmentation of corneal infiltrates in the form of raised plaques is a characteristic of dematiaceous fungal keratitis.[15]
The outcome of non-pigmented fungal keratitis has been extensively reported.[2,3,4,5,6,7] However, the outcome of dematiaceous fungal keratitis has been reported mostly in case report. There are few case series published from India,[11,16] and United States[17] on outcome of dematiaceous fungal keratitis. These studies were published more than a decade ago. There were no reports available about recent pattern and outcome of dematiaceous fungal keratitis during last 5 years. The epidemiological pattern and causative agents for dematiaceous fungal keratitis can vary significantly from region to region within a country. Knowledge of common fungal isolates in a region is important for management of fungal keratitis. A regular reporting of fungal isolates is necessary for identification of any new pattern. The objective of this study was to report clinical characteristics, microbiological characteristics, and treatment outcome of dematiaceous fungal keratitis in North India.
Methods
This is a retrospective, noncomparative, observational case series. The study was approved by institutional ethics committee and adhered to the principles of the Declaration of Helsinki. Clinical records of all consecutive patients with culture proven diagnosis of dematiaceous fungal keratitis, who presented to the cornea services, from January 2012 to June 2017 were included. Diagnosis of dematiaceous fungal keratitis was made on the basis of microbiological investigations and clinical findings such as corneal epithelial defect, stromal infiltrate, and raised pigmented plaque. Patients with evidence of keratitis due to non-pigmented fungi, bacteria, Herpes simplex virus, and Acanthamoeba species were excluded from this study.
A detailed examination of both eyes was performed using the slit-lamp biomicroscope. Standard case report form was developed to capture details of each patient including socio-demographic information clinical finding, predisposing factors, history of corneal trauma, nature of agent causing trauma, associated ocular conditions, other systemic diseases, use of steroid eye drop, therapy received prior to presentation, and visual acuity at the time of presentation. Symptoms and size of epithelial defect, with or without hypopyon, and infiltrate as measured by the variable slit on the biomicroscope were also recorded.
Corneal ulceration was defined as a loss of the corneal epithelium with underlying stromal infiltration and suppuration associated with signs of inflammation with or without hypopyon.[2] Corneal scrapings were obtained from the base and edge of the ulcer using a sterile surgical blade (# 15 on a Bard Parker handle) under topical anesthesia (0.5% proparacaine hydrochloride) and slit-lamp magnification. Gram stain and 10% potassium hydroxide mount were included as a part of the standard protocol for microscopic evaluation of corneal smears. Gram-stained smears were examined at ×400 and ×1000 magnification, and the potassium hydroxide (KOH) preparations were examined at ×200 and ×400 magnification under light microscope. Scrapings for smears were collected prior to those for culture.
For culture, the material was inoculated on to chocolate agar, blood agar, sabouraud dextrose agar (SDA), brain heart infusion, and thioglycolate and incubated at 25°C and 37°C. Cultures were examined daily during first week, twice weekly for next 3 weeks, and discarded after 3 to 4 weeks if there was no growth. Dematiaceous fungi were identified by their colony characteristics on SDA and by the morphological appearance of the spores in lactophenol cotton blue stain and, in some cases, by slide culture method. All laboratory methods were performed under standard protocol, which have been discussed in detail in the previous studies.[1,2,11,12,13,14] An isolate was considered dematiaceous if fungal colonies revealed black or brown pigmentation, and lactophenol cotton blue mount from the culture revealed black or brown pigmented hyphae, conidia, or both.
The eyes were treated initially based on the clinical evaluation and microbiological smear examinations. The eyes were treated with 5% natamycin suspension on an hourly basis. Topical voriconazole 1% (Vozole, Aurolab, India) was supplemented for larger and deeper fungal ulcers.
Statistical analysis
The statistical analysis was performed with SPSS 17.0 software (SPSS Inc, Chicago, IL, USA). Descriptive statistics were obtained to determine the frequency and proportions. Mean and standard deviation were calculated for continuous variables.
Results
A total of 83 patients were found eligible for the study. In all, 57.8% (n = 48) of them were male, and 42.2% (n = 35) of them were female. The median age of all patients was 45 years (interquartile range [IQR]: 35-55 years). Average age of male patients was 45.3 ± 15.1 years, and of female patients was 44.7 ± 12.8 years (P = 0.84, independent t test). Left eye was involved in 43 (n = 43/83; 51.8%) patients, and right eye was involved in 40 (n = 40/83; 48.2%) patients. In all, 66.2% (n = 55/83) patients with dematiaceous fungal keratitis were presented from September to December [Fig. 1]. Monthly distribution of culture proven dematiaceous fungal keratitis patients reported to our cornea clinic was presented in Table 1.
Figure 1.
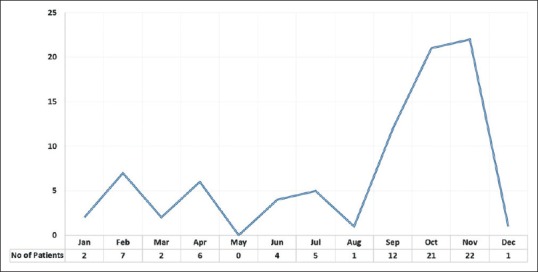
Monthly trend of culture proven dematiaceous keratitis patients presented to cornea clinic
Table 1.
Month wise distribution of dematiaceous fungal keratitis
| ORGANISM | Month | Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| JAN | FEB | MAR | APR | MAY | JUN | JUL | AUG | SEP | OCT | NOV | DEC | ||
| Curvularia sp. | 1 | 3 | 2 | 1 | × | × | 2 | 1 | 12 | 19 | 14 | × | 55 |
| Alternaria sp. | × | 2 | × | 2 | × | 1 | 1 | × | × | × | 5 | 1 | 12 |
| Ulocladium sp. | × | 1 | × | × | × | 1 | 2 | × | × | × | 1 | × | 5 |
| Bipolaris sp. | 1 | × | × | 2 | × | 1 | × | × | × | × | 1 | × | 5 |
| Scedosporium sp. | × | 1 | × | × | × | 1 | × | × | × | × | 1 | × | 3 |
| Acremonium sp. | × | × | × | × | × | × | × | × | × | 2 | × | × | 2 |
| Epicoccum sp. | × | × | × | 1 | × | × | × | × | × | × | × | × | 1 |
| Total | 2 | 7 | 2 | 6 | × | 4 | 5 | 1 | 12 | 21 | 22 | 1 | 83 |
Corneal trauma was sustained in 67.4% (n = 56/83) of participants prior to onset of the ulcer. The most common cause of trauma was vegetative matter in 32 of these 56 (56.1%) patients. In all, 53.1% (n = 17/32) of the injuries due to vegetative matter were caused by sugarcane. Wood stick injury (n = 8/24; 33.3%) and insects (n = 4/24; 16.4%) were the main non-vegetative cause of trauma [Table 2]. Three patients (n = 3/83; 3.6%) were using topical steroids at the time of presentation. Systemic illness was present in 12 (n = 12/83; 14.5%) patients. Concurrent systemic diseases were hypertension (n = 5/83; 6%), diabetes (n = 3/83; 3.6%), asthma (n = 2/83; 2.4%), coronary artery disease (n = 1/83; 1.2%), thyroid (n = 1/83; 1.2%), and arteritis (n = 1/83; 1.2%). History of prior medication was present in 60 (n = 60/83; 72.3%) patients; 32 (n = 32/83; 38.6%) of them were using topical antibiotics. Remaining 28 (n = 28/60; 46.7%) patients did not remember the name of medication they were using.
Table 2.
Nature of trauma
| Nature of trauma | Number | Frequency |
|---|---|---|
| Sugarcane | 17 | 30.4% |
| Plant leaf | 13 | 23.2% |
| Wood | 8 | 14.3% |
| Dust | 5 | 8.9% |
| Insect | 4 | 7.1% |
| Paddy | 2 | 3.6% |
| Bed sheet | 1 | 1.8% |
| Buffalo tail | 1 | 1.8% |
| Finger | 1 | 1.8% |
| Hand | 1 | 1.8% |
| Powder | 1 | 1.8% |
| Soap | 1 | 1.8% |
| Stone | 1 | 1.8% |
Ocular comorbidities were present in four (4.8%) eyes. The cornea was totally melted in seven (8.4%) patients. In the remaining 76 patients, the median infiltrate size was 8 mm2 (IQR: 2.0–17 mm2). Hypopyon was present in 14 (n = 14/83; 16.4%) patients. The mean infiltrate size in eyes with hypopyon was 15.2 ± 7.5 mm2, as compared to 8.8 ± 8.7 mm2 in eyes without hypopyon (P = 0.012, independent t test). Fifteen (n = 15/83; 18.1%) patients were presented with pigmented, raised, plaque-like infiltrate. The presenting visual acuity in affected eye was more than 20/30 in 20 (n = 20/83; 24.1%) eyes, from 20/30 to 20/60 in 12 (n = 12/83; 14.5%) eyes, less than 20/60 to 20/200 in 14 (n = 14/83; 16.8%) eyes, and less than 20/200 in 37 (n = 37/83; 44.5%) eyes.
Only fungal infection was reported in 73 (n = 73/83; 87.9%) patients, and mixed bacterial/fungal infection was reported in 10 (n = 10/83; 12.1%) patients. Among fungal infections, the most frequently identified fungi was Curvularia sp. (n = 55/83; 66.3%). Other fungal organisms were Alternaria sp. (n = 12/83; 14.5%), Ulocladium sp. (n = 5/83; 6%), Bipolaris sp. (n = 5/83; 6%), Scedosporium sp. (n = 3/83; 3.6%), Acremonium sp. (n = 2/83; 2.4%), and Epicoccum sp. (n = 1/83; 1.2%) [Table 3]. In 10 patients with mixed infection, fungal isolates were Curvularia sp. (n = 4), Alternaria sp. (n = 3), Bipolaris sp. (n = 2), and Ulocladium sp. (n = 1). Bacterial isolates in these patients were Staphylococcus sp. (n = 5), Streptococcus sp. (n = 1), Acinetobacter (n = 1), Corynebacterium (n = 1), and Pseudomonas sp. (n = 2).
Table 3.
Comparison of previous studies on dematiaceous fungal keratitis with the present study
| Dematiaceous fungal isolate | Sengupta et al., 2010 | Garg et al., 1999 | Kumar et al., 2018 Present Study |
|---|---|---|---|
| Curvularia | 84 (72%) | 20 (22.7%) | 55 (66.3%) |
| Bipolaris and Exserohilum | 8 (6.8%) | 13 (14.7%) | 5 (6.1%) |
| Lasiodiplodia | 0 (0%) | 3 (3.4%) | 0 (0%) |
| Torula | 0 (0%) | 3 (3.4%) | 0 (0%) |
| Alternaria | 4 (3.4%) | 2 (2.2%) | 12 (14.5%) |
| Humicola | 0 (0%) | 2 (2.2%) | 0 (0%) |
| Aureobasidium | 0 (0%) | 2 (2.2%) | 0 (0%) |
| Ulocladium | 0 (0%) | 0 (0%) | 5 (6%) |
| Scedosporium | 0 (0%) | 0 (0%) | 3 (3.6%) |
| Acremonium | 0 (0%) | 0 (0%) | 2 (2.4%) |
| Epicoccum | 0 (0%) | 0 (0%) | 1 (1.2%) |
Eighteen (n = 18/83; 21.7%) patients did not present for follow-up after initiation of antifungal therapy. For the rest (n = 65), median time of antifungal therapy was 4.2 weeks (IQR: 1-25 weeks). Complications were seen in 14 (14/65; 21.5%) patients. Corneal thinning was present in eight (8/65; 12.3%) eyes, corneal perforation in four (4/65; 6.1%) eyes, descemetocele in one (1/65; 1.5%) eye, and iris prolapse in one (1/65; 1.5%) eye. Tissue adhesive and bandage contact lens (TA + BCL) was applied in four (4/65; 6.1%) eyes, therapeutic penetrating keratoplasty was performed in four (4/65; 6.1%) eyes, and evisceration was done in two (2/65; 3.1%) eyes. Resolution of stromal infiltrate with corneal scarring was seen in 52 (52/65; 80%) patients. The median time of scar appearance was 7 days (IQR: 6-10.5 days). Complete resolution of dematiaceous fungal keratitis was present in 27 (n = 27/65; 41.5%) eyes.
Visual acuity at last follow-up was recorded in 53 (n = 53/65; 81.5%) patients. Of them, visual acuity was improved or remain unchanged in 44 (n = 44/53; 83%) patients, and decreased in 9 (n = 9/53; 17%) patients. As shown in Table 4, there was a statistically significant difference (P = 0.00; Fisher's exact test) between presenting and final visual acuity. Final visual acuity was recorded in six patients (out of 10) with mixed infection. Of them, four (n = 4/6; 66.7%) had final visual acuity between 20/30 and 20/60, and two (n = 2/6; 33.3%) had final visual acuity worse than 20/200. There was no statistically significant difference (P = 0.083; Fisher's exact test) in final visual acuity between mono and mixed infection. The results are summarized in Table 5.
Table 4.
Cross-tabulation of presenting VA and final VA (n=53)
| Presenting VA | VA at last follow-up | Total | |||
|---|---|---|---|---|---|
| >20/30 | <20/30 to 20/60 | <20/60 to 20/200 | <20/200 | ||
| >20/30 | 14 | 0 | 0 | 0 | 14 |
| <20/30 to 20/60 | 3 | 2 | 1 | 1 | 7 |
| <20/60 to 20/200 | 2 | 5 | 1 | 3 | 11 |
| <20/200 | 1 | 4 | 5 | 11 | 21 |
| Total | 20 | 11 | 7 | 15 | 53 |
| Value | Degree of freedom | Significance (2-sided) | |||
| Pearson Chi-square | 40.510a | 9 | 0.00 | ||
| Likelihood ratio | 46.679 | 9 | 0.00 | ||
| Fisher's exact test | 38.561 | 0.00 | |||
a13 cells (81.2%) have expected count <5
Table 5.
Distribution of final visual acuity achieved in patients with mono and mixed infections
| Infection Type | Final Visual Acuity | Total | |||
|---|---|---|---|---|---|
| >20/30 | 20/30-20/60 | 20/60-20/200 | <20/200 | ||
| Mixed infection | 0 | 4 | 0 | 2 | 6 |
| Mono infection | 16 | 11 | 7 | 13 | 47 |
| Total | 16 | 15 | 7 | 15 | 53 |
| Value | Degree of freedom | Significance (2-sided) | |||
| Pearson Chi-square | 6.515a | 3 | 0.060b | ||
| Likelihood ratio | 8.258 | 3 | 0.053b | ||
| Fisher's exact test | 5.371 | 0.083b | |||
a4 cells (50.0%) have expected count <5. The minimum expected count is 0.79. bBased on 10,000 sampled tables
Discussion
The prevalence of fungal keratitis among all corneal ulcer has been increasing in India. Srinivasan et al.[2] in 1997 reported 32% fungal keratitis, and Tilak et al.[7] in 2010 reported 45.5% fungal keratitis among all microbial keratitis. Most of the patients with dematiaceous fungal keratitis were presented in the month of September, October, and November. These months correspond to the harvesting season in the study area. The average age of patients (45.1 years) in this study was consistent with multiple reported from South India.[11,16,18,19] Age of patients in this study ranged from 11 to 80 years; however, in a study by Garg et al.,[11] patients age ranged from 1 to 75 years. In this study, none of the patients belong to newborns, infants, and preschool age group. Majority of patients were more than 30 years of age. This suggests that working individuals are more affected from dematiaceous fungal keratitis in the study area. In this study, male preponderance was reported, which is consistent with the previous studies.[11,16,18]
In this study, corneal injury with vegetative matter predisposing to corneal infection was found to be similar with previous studies.[2,3,4,11] The vegetative nature of trauma was the most frequent risk factor identified. Among vegetative matter, sugarcane was the most frequent cause of corneal trauma. This is due to the geographic location of the patient population, which is Uttar Pradesh, the highest sugarcane producing state of India. The tall leaves of sugarcane plant being closure to eye level is the main reason of corneal injury due to sugarcane plant. Wearing contact lens was not identified as a predisposing factor in this series. This result is consistent with other studies reported from India.[2,3,4,11] This may be explained by less popularity of contact lens due to its cost, or unavailability of contact lens services in rural North India.
Hypopyon is common in eyes with fungal keratitis.[20] Presence of hypopyon was found associated with large infiltrate size. In this study, 44.5% of patients had presenting visual acuity of less than 20/400. This frequency was significantly lower than reported by Garg et al. (71.6%).[11] Average duration of healing in this study was 4.8 weeks. In all, 57% patients had final visual acuity of less than 20/200. The final visual acuity was significantly better than the presenting visual acuity. The final visual acuity was decreased in 17% eyes. In mixed infection group, none of the patients achieved final visual acuity better than 20/30. Use of topical steroids was found in three (n = 3/83; 3.6%) patients, as compared to 1.19% by Bharathi et al.,[4] 7.8% Chander et al.,[5] 14.1% by Garg et al.,[11] and 21% by Chaudhary et al.[14]
In this series, Curvularia sp. was the most frequent species identified in 66.3% of patients. In all, 61.8% of them were males, and ocular trauma was present in 69% of eyes. Thirteen (n = 13/55; 23.6%) patients of Curvularia keratitis were presented with pigmented plaque-like infiltrate. Curvularia sp. has been identified in almost every previously published studies,[2,5,7,13,14] except study by Saha et al.[6] from West Bengal. They did not identify Curvularia sp. in their series. In series by Chander et al.[5] and Chaudhary et al.[14] from North India (Chandigarh, Delhi), Alternaria sp., and Bipolaris sp. were more frequent than Curvularia sp. In another series by Bharathi et al.,[4] Cladosporium sp. and Botryodiplodia sp. were more frequent than Curvularia sp. However, studies by Garg et al.[11] and Sengupta et al.[16] reported Curvularia sp. as the most frequently isolated fungi among dematiaceous fungal keratitis.
In this series, Ulocladium was identified in five cases of dematiaceous fungal keratitis. Of them, four were females. Three of them had history of trauma from sugarcane leaves during harvesting season. There was total corneal melt in two cases. Final visual acuity was counting finger in three patients, and two patients were lost to follow-up before final resolution. Badenoch et al. in 2006 reported a case of Ulocladium atrum keratitis in a 43-year-old man from Australia.[21] The identification of Ulocladium in five patients was unusual. To best of our knowledge, fungal keratitis caused by Ulocladium species is not reported from India.
In this series, Scedosporium sp. was identified in three patients. Out of them, sclera was involved in two patients. Rathi et al. in 2016 reported the outcome of Scedosporium keratitis in a series of 10 patients.[18] In their series, 9 out of 10 patients responded to medical therapy (topical Natamycin 5%), and one patient required penetrating keratoplasty. However, in this study, out of three eyes of Scedosporium keratitis, TA + BCL was applied on one eye, one eye was eviscerated, and one patient was lost to follow-up. Ramakrishnan et al.[19] also reported a series of Scedosporium apiospermum ocular infection in 13 patients (11 responded to topical natamycin 5%, one requires therapeutic keratoplasty).
Acremonium species was identified in two cases of dematiaceous fungal keratitis. One of them had history of trauma from paddy field. Verghese S also reported a case of post-traumatic fungal keratitis in a 33-year-old male from India.[22] Acremonium is a filamentous fungus found in plant debris and soil.[23] Epicoccum was also identified in a 45-year-old female, with history of corneal trauma with insect. It is a plant pathogen and was found associated with onychomycosis in humans. Dudeja et al. reported a case of Epicoccum keratitis in a 40-year-old male, who has no history of corneal trauma.[24] Ho et al.[25] and Paul et al.[26] also reported one case of Epicoccum keratitis.
Therapeutic penetrating keratoplasty was performed in four (3.6%) eyes. Three of them were male. All of them had history of ocular trauma caused by vegetative matter. Curvularia sp. was isolated in all these eyes. All of them were using topical antibiotics (Natamycin, Tobramycin + Moxifloxacin, Natamycin + Moxifloxacin + Voriconazole) at the time of presentation. Final visual acuity was hand movement in two patients, and light perception in two patients. In the literature, therapeutic corneal transplant has been reported to range in 12% to 38% of fungal keratitis.[11,27,28] TPK is an effective treatment for uncontrolled, refractory fungal keratitis cases to save the affected eye.[29,30]
Two eyes needed evisceration because of unsalvageable corneal melt. Of them, one eye was infected by Curvularia sp., and another was infected by Scedosporium sp. Evisceration after dematiaceous fungal keratitis was also reported by Garg et al.[11] (in 6/83 cases) and Sengupta et al.[16] (in 9/81). However, all patients were managed successfully in a series of 10 cases of Scedosporium keratitis by Rathi et al.[18]
Conclusion
In conclusion, Dematiaceous fungal infections of the ocular surface are most commonly caused by Curvularia sp. and Alternaria sp. in North India. Scedosporium sp. is associated with the worst outcomes. Two new genera were identified in this series. These are Ulocladium and Epicoccum species, and both have not been reported previously as a causal organism of fungal keratitis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Wong TY, Ng TP, Fong KS, Tan DT. Risk factors and clinical outcomes between fungal and bacterial keratitis: A comparative study. CLAO J. 1997;23:275–81. [PubMed] [Google Scholar]
- 2.Srinivasan M, Gonzales CA, George C. Epidemiology and aetiological diagnosis of corneal ulceration in Madurai, South India. Br J Ophthalmol. 1997;81:965–71. doi: 10.1136/bjo.81.11.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basak SK, Basak S, Mohanta A, Bhowmick A. Epidemiological and microbiological diagnosis of suppurative keratitis in Gangetic West Bengal, eastern India. Indian J Ophthalmol. 2005;53:17–22. doi: 10.4103/0301-4738.15280. [DOI] [PubMed] [Google Scholar]
- 4.Bharathi MJ, Ramakrishnan R, Meenakshi R, Padmavathy S, Shivakumar C, Srinivasan M. Microbial keratitis in South India: Influence of risk factors, climate, and geographical variation. Ophthalmic Epidemiol. 2007;14:61–9. doi: 10.1080/09286580601001347. [DOI] [PubMed] [Google Scholar]
- 5.Chander J, Singla N, Agnihotri N, Arya SK, Deep A. Keratomycosis in and around Chandigarh: A five-year study from a north Indian tertiary care hospital. Indian J Pathol Microbiol. 2008;51:304–6. doi: 10.4103/0377-4929.41700. [DOI] [PubMed] [Google Scholar]
- 6.Saha S, Banerjee D, Khetan A, Sengupta J. Epidemiological profile of fungal keratitis in urban population of West Bengal, India. Oman J Ophthalmol. 2009;2:114. doi: 10.4103/0974-620X.57310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tilak R, Singh A, Maurya OP, Chandra A, Tilak V, Gulati AK. Mycotic keratitis in India: A five-year retrospective study. J Infect Dev Ctries. 2010;4:171–4. doi: 10.3855/jidc.309. [DOI] [PubMed] [Google Scholar]
- 8.Srinivasan M. Fungal keratitis. Curr Opin Ophthalmol. 2004;15:321–7. doi: 10.1097/00055735-200408000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto T, Ajello L, Matsuda T, Szaniszlo PJ, Walsh TJ. Developments in hyalohyphomycosis and phaeohyphomycosis. J Med Vet Mycol. 1994;32:329–49. doi: 10.1080/02681219480000951. [DOI] [PubMed] [Google Scholar]
- 10.Brandt ME, Warnock DW. Epidemiology, clinical manifestations, and therapy of infections caused by dematiaceous fungi. J Chemother. 2003;15:36–47. doi: 10.1179/joc.2003.15.Supplement-2.36. [DOI] [PubMed] [Google Scholar]
- 11.Garg P, Gopinathan U, Choudhary K, Rao GN. Keratomycosis: Clinical and microbiologic experience with dematiaceous fungi. Ophthalmology. 2000;107:574–80. doi: 10.1016/s0161-6420(99)00079-2. [DOI] [PubMed] [Google Scholar]
- 12.Gopinathan U, Garg P, Fernandes M, Sharma S, Athmanathan S, Rao GN. The epidemiological features and laboratory results of fungal keratitis: A 10-year review at a referral eye care center in South India. Cornea. 2002;21:555–9. doi: 10.1097/00003226-200208000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Bharathi MJ, Ramakrishnan R, Vasu S, Meenakshi R, Palaniappan R. Epidemiological characteristics and laboratory diagnosis of fungal keratitis. A three-year study. Indian J Ophthalmol. 2003;51:315–21. [PubMed] [Google Scholar]
- 14.Chowdhary A, Singh K. Spectrum of fungal keratitis in North India. Cornea. 2005;24:8–15. doi: 10.1097/01.ico.0000126435.25751.20. [DOI] [PubMed] [Google Scholar]
- 15.Oldenburg CE, Prajna VN, Prajna L, Krishnan T, Mascarenhas J, Vaitilingam CM, et al. Clinical signs in dematiaceous and hyaline fungal keratitis. Br J Ophthalmol. 2011;95:750–1. doi: 10.1136/bjo.2010.198648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sengupta S, Rajan S, Reddy PR, Thiruvengadakrishnan K, Ravindran RD, Lalitha P, et al. Comparative study on the incidence and outcomes of pigmented versus non pigmented keratomycosis. Indian J Ophthalmol. 2011;59:291–6. doi: 10.4103/0301-4738.81997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilhelmus KR, Jones DB. Curvularia Keratitis. Trans Am Ophthalmol Soc. 2001;99:111–32. [PMC free article] [PubMed] [Google Scholar]
- 18.Rathi HS, Venugopal A, Rengappa R, Ravindran M. Scedosporium keratitis: An experience from a tertiary eye hospital in South India. Cornea. 2016;35:1575–7. doi: 10.1097/ICO.0000000000000969. [DOI] [PubMed] [Google Scholar]
- 19.Ramakrishnan S, Mandlik K, Sathe TS, Gubert J, Krishnan T, Baskaran P. Ocular infections caused by Scedosporium apiospermum: A case series. Indian J Ophthalmol. 2018;66:137–40. doi: 10.4103/ijo.IJO_524_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu LJ, Song XS, Zhao J, Sun SY, Xie LX. Hypopyon in patients with fungal keratitis. Chinese Med J. 2012;125:470–5. [PubMed] [Google Scholar]
- 21.Badenoch PR, Halliday CL, Ellis DH, Billing KJ, Mills RA. Ulocladium atrum keratitis. J Clin Microbiol. 2006;44:1190–3. doi: 10.1128/JCM.44.3.1190-1193.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verghese S. Post traumatic fungal keratitis caused by Acremonium recifei. Indian J Pathol Microbiol. 2010;53:587–8. doi: 10.4103/0377-4929.68263. [DOI] [PubMed] [Google Scholar]
- 23.Fincher RM, Fisher JF, Lovell RD, Newman CL, Espinel-Ingroff A, Shadomy HJ. Infection due to the fungus acremonium (cephalosporium) Med (Baltimore) 1991;70:398–409. doi: 10.1097/00005792-199111000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Dudeja L, Lakshmipriya J, Karpagam R, Prajna NV, Prajna L. Fungal Keratitis Caused By Epicoccum Sorghi–A Case Report. Delhi J Ophthalmol. 2016;27:121–3. [Google Scholar]
- 25.Ho JW, Fernandez MM, Rebong RA, Carlson AN, Kim T, Afshari NA. Microbiological profiles of fungal keratitis: A 10-year study at a tertiary referral center. J Ophthalmic Inflamm Infect. 2016;6:5. doi: 10.1186/s12348-016-0071-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paul B, Girija Devi PS. Microbial profile of suppurative keratitis a prospective study. J Acad Clin Microbiol. 2014;16:8–10. [Google Scholar]
- 27.Ti SE, Scott JA, Janardhanan P, Tan DT. Therapeutic keratoplasty for advanced suppurative keratitis. Am J Ophthalmol. 2007;143:755–62. doi: 10.1016/j.ajo.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 28.Thew MR, Todd B. Fungal keratitis in far north Queensland, Australia. Clin Experiment Ophthalmol. 2008;36:721–4. doi: 10.1111/j.1442-9071.2008.01879.x. [DOI] [PubMed] [Google Scholar]
- 29.Xie L, Dong X, Shi W. Treatment of fungal keratitis by penetrating keratoplasty. Br J Ophthalmol. 2001;85:1070–4. doi: 10.1136/bjo.85.9.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prakash G, Sharma N, Goel M, Titiyal JS, Vajpayee RB. Evaluation of intrastromal injection of voriconazole as a therapeutic adjunctive for the management of deep recalcitrant fungal keratitis. Am J Ophthalmol. 2008;146:56–9. doi: 10.1016/j.ajo.2008.02.023. [DOI] [PubMed] [Google Scholar]


