Abstract
Purpose:
To compare clinical outcomes following implantation of two types of posterior chamber phakic intraocular lenses: Visian™ Implantable Collamer Lens with Centraflow (ICL, V4C Staar Surgical, Nidau, Switzerland) and Implantable Phakic Contact Lens (IPCL, V1, Caregroup Sight Solution, India) for the correction of myopia and myopic astigmatism.
Methods:
This retrospective case series included eyes which underwent phakic intraocular lens implantation with a minimum follow-up period of 1 year. Visual outcomes including safety, efficacy, refractive predictability, and stability were compared at 1 week and at 1, 6, and 12 months’ postoperative visit. Complications and adverse events were analyzed.
Results:
The study included 119 and 203 eyes in the IPCL and ICL groups, respectively. At 1-year postoperative visit, median corrected distance visual acuity was 0.10 (interquartile range [IQR] 0,0.10) and 0 (IQR 0,0) in the IPCL and ICL cohorts, respectively (P = 0.066). An uncorrected visual acuity of 20/32 or better was achieved in 86.5% and 88.67% of the eyes, respectively (P = 0.574). Ninety and 94% of the eyes achieved a postoperative manifest spherical equivalent within ± 0.5D (P = 0.169, χ2 test). Three eyes (2.52%) in the IPCL group versus one eye (0.49%) in the ICL group developed visually significant cataract requiring surgical intervention (P = 0.113). No vision-threatening complications were noted in either cohort. The mean follow-up period was 94.69 ± 32.45 and 102.67 ± 61.82 weeks, respectively.
Conclusion:
Both groups demonstrated similar efficacy and safety profile. The IPCL is an effective and economically viable option for the correction of myopia.
Keywords: ICL, IPCL, implantable collamer lens, implantable phakic contact lens, myopia, phakic intraocular lens
Phakic intraocular lens implantation demonstrates several advantages over keratorefractive procedures, especially for the treatment of high ametropia. Relatively lower induction of higher order aberrations, retinal image magnification, and higher contrast sensitivity has been demonstrated in comparison with laser in situ keratomileusis, for both low and high myopia.[1,2,3] Preservation of accommodation, advantages of reversibility, and the ability to offer refractive correction in cases wherein keratorefractive procedures are contraindicated are additional benefits.[4]
Safety and efficacy of the Visian implantable collamer lens (ICL) (Staar surgical, Nidau, Switzerland) has been demonstrated for moderate and high ametropia over long-term follow-up.[5,6,7,8] A limitation, however, is the economic burden of treatment, especially in developing nations. The implantable phakic contact lens (IPCL V1, Caregroup Sight Solution, India) has been developed as an alternative treatment option, at a distinct economic advantage, wherein the cost of the ICL implant is 2.5 times the IPCL impant.
This study aimed to compare the efficacy and safety profiles over a minimum follow-up period of 1 year. To the best of our knowledge, no similar study has been reported in literature thus far.
Methods
Study population
This retrospective, interventional case series was conducted at a tertiary eye care hospital in South India. The protocol was registered with the Ethics Committee of our Institute and adhered to the tenets of the Declaration of Helsinki. One hundred twenty-one (121) eyes and 203 eyes underwent implantation with IPCL and ICL, respectively, for correction of myopia and myopic astigmatism.
Inclusion criteria were as follows: Stable refraction (change in mean spherical equivalent of -0.25 D or less) for a minimum period of 1 year, age ≥21 years, eyes with borderline corneal tomography or inadequate pachymetry for keratorefractive procedures, endothelial cell count ≥2,500 cells/mm2 and anterior chamber depth ≥2.8 mm. Eyes with prior ocular surgery, comorbidities including cataract, glaucoma, or uveitis, and corneal ectasia were excluded from the study. Following parameters were compared preoperatively and at 1, 6, and 12 months’ postoperative visit: Logarithm of the minimal angle of resolution (logMAR) of uncorrected distance visual acuity (UDVA), logMAR of corrected distance visual acuity (CDVA), manifest refractive error (spherical equivalent), intraocular pressure (IOP) using Goldmann applanation tonometry, and endothelial cell density (ECD) using specular microscopy (Topcon SP-1P).
All eyes underwent a slit-lamp biomicroscopic and dilated fundus evaluation. Preoperatively, the horizontal white-to-white was measured using the laser interferometry biometer (Lenstar, Haag Streit, USA) and hand-held digital calipers. Anterior chamber depth (distance from corneal endothelium to anterior lens capsule) and keratometric values were obtained using Scheimpflung corneal tomography (Pentacam HR, Oculus Optikgerate).
Eyes wherein the manifest cylinder was –1.0 D or less underwent non-toric IPCL and ICL implantation (31 and 118 eyes, respectively). Remaining 88 and 85 eyes in the IPCL and ICL group respectively received toric implantation.
The phakic intraocular lens power was calculated using a modified vertex formula as per the manufacturer's recommendation, with target refraction of emmetropia. The implant size was selected based on the anterior chamber depth and the horizontal white-to-white.
Preoperative peripheral iridotomies (at 10 or 2 o′clock) were done using the neodymium-yttrium aluminum garnet (Nd: YAG) laser in the IPCL group. A central 360 μm artificial hole in the ICL optic obviated the need for peripheral iridotomy in the cohort.[9]
IPCL is a hydrophilic hybrid acrylic implant, with six haptic pads for better stability in the ciliary sulcus. Customization of the implant allows treatment for a wide range of ametropia (+15 D to –30 D in 0.5 D increments) and astigmatism (up to –10 D in 0.5 D increments). The lens design includes eight holes: two in the haptics, four along the optic-haptic transition zone, and two along the optic periphery to determine correct orientation. The IPCL (version 1) was commercially introduced in 2013, and at present, it is distributed worldwide in 20 countries. The IPCL is afforded the DGCA India and CE approval.
Surgical procedure
Written informed consent was obtained from all patients. All surgeries were performed by a single experienced surgeon (DR). Topical anesthetic and mydriatic agents were instilled before the procedure. The phakic posterior chamber intraocular lens was implanted into the anterior chamber through a 3-mm clear corneal incision. The footplates were subsequently tucked behind the iris, followed by a thorough ophthalmic viscoelastic device (HEALON OVD, sodium hyaluronate, Johnson and Johnson Vision, Santa Ana, CA, USA) wash. Digital image-guided system (Verion Image guided system, Alcon, Novartis) allowed measurement of intraoperative cyclotorsion and correct placement of the toric implants. The placement of the ICL entails rotation of up to 22.5° from the horizontal axis. Customization of the IPCL on the other hand allows placement of the implant at the 0–180° horizontal axis in all cases.
Postoperative treatment regimen included steroids (L-Pred, loteprednol 0.5%, Allergan) in tapering doses and antibiotic drops (Vigamox, moxifloxacin ophthalmic solution 0.5%, Alcon, Novartis AG) for 2 weeks.
Statistical analysis
IBM SPSS version 22 was used for statistical analysis. Snellen visual acuity measurements were converted to logarithm of the minimum angle of resolution (logMAR) equivalents for the purpose of data analysis. Data were checked for a normal distribution within each category of study group by using visual inspection of histograms and normality Q-Q plots. Shapiro–Wilk test was also conducted to assess normal distribution, wherein a test P value of > 0.05 was considered as a normal distribution. For non-normally distributed data, median and interquartile range (IQR) were compared between study groups using Mann–Whitney U test (two groups). Categorical outcomes were compared between study groups using χ2 test. A P value <0.05 was considered statistically significant.
Results
Preoperative demographics of the study population are summarized in Table 1. Preoperative manifest refractive sphere and cylinder were significantly higher in the IPCL cohort. This could be attributed to the availability of a wider range of correction with the IPCL in comparison with ICL implants.
Table 1.
Preoperative patient demographics
| Parameters | IPCL (n=119) Median (IQR) | ICL (n=203) Median (IQR) | P (Mann-Whitney U test) |
|---|---|---|---|
| Age (years) | 23 (21, 27) | 24 (22, 28) | 0.083 |
| Manifest sphere (D) | -9.25 (-12, -7.25) | -7.75 (-12, -6) | 0.053 |
| Manifest cylinder (D) | -1.5 (-2.25, -0.75) | -0.75 (-1.5, -0.5) | < 0.001 |
| Manifest refractive spherical equivalent (D) | -10.37 (-12.87, -7.75) | -8.37 (-12.37, -6.37) | 0.013 |
| Flat keratometry (D) | 43.25 (42.75, 44.8) | 44 (43.2, 44.8) | 0.008 |
| Steep keratometry (D) | 45.10 (44, 46) | 45.3 (44.2, 46) | 0.763 |
| Anterior chamber depth (mm) | 3.21 (3.01, 3.34) | 3.19 (3, 3.33) | 0.410 |
| Thinnest pachymetry (μm) | 505 (478, 545) | 486 (469, 508) | <0.001 |
| White-to-white (mm) | 11.73 (11.56, 11.89) | 11.70 (11.5, 11.95) | 0.894 |
| Axial length (mm) | 26.72 (25.66, 28.35) | 26.2 (25.22, 28.11) | 0.083 |
IPCL: implantable phakic contact lens, IQR: interquartile range
Safety outcomes
Median LogMAR CDVA at 1-year postoperative visit was 0 (IQR 0.0,0.1) and 0 (IQR 0,0.10) in the IPCL and ICL groups (P = 0.038). This was attributed to lower preoperative corrected visual acuity in the IPCL group (median 0, IQR 0,0.18) as compared to ICL (median 0, IQR 0,0.10) (P = 0.023).
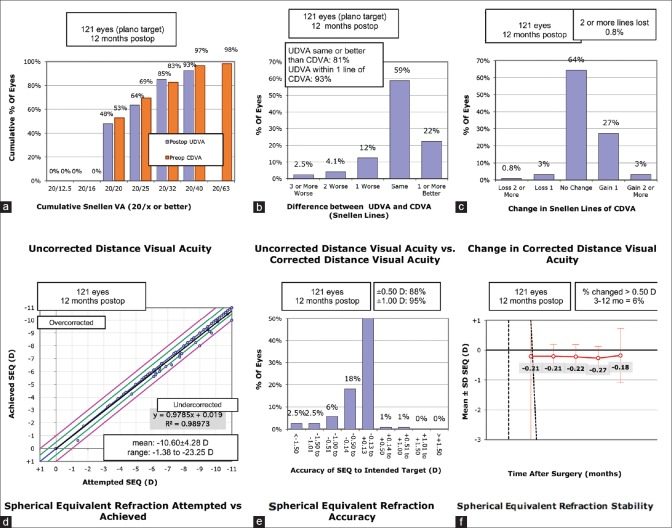
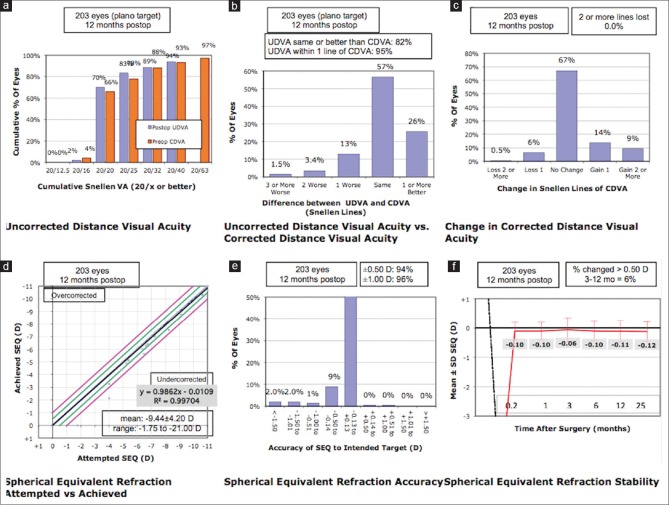
At 1-year postoperative visit, 79 eyes (66.38%) showed no change in CDVA, 37 eyes (31.09%) gained one or more lines while 3 eyes (2.52%) lost one or more lines in the IPCL group [Fig. 1c]. The ICL group demonstrated no change in CDVA in 136 eyes (67%), gain of one or more lines in 53 eyes (26.1%), and loss of one or more lines in 14 eyes (6.89%) [Fig. 2c] (P = 0.183).
Figure 1.
Standard graphs for reporting surgical outcomes following IPCL implantation. CDVA: corrected distance visual acuity, IPCL: implantable phakic contact lens, UDVA: uncorrected distance visual acuity. (a) Cumulative visual acuity (b) Efficacy (c) Safety d) Predictability e) Accuracy (f) Stability of visual outcomes
Figure 2.
Standard graphs for reporting surgical outcomes following ICL implantation. CDVA: corrected distance visual acuity, ICL: implantable collamer lens, UDVA: uncorrected distance visual acuiy. (a) Cumulative visual acuity (b) Efficacy (c) Safety d) Predictability e) Accuracy (f) Stability of visual outcomes
Efficacy outcomes
At 1 year postoperatively, median logMAR UDVA was 0.10 (IQR 0,0.18) and 0 (IQR 0,0.10) in the IPCL and ICL groups (P = 0.051). At the 1-year postoperative visit, 86.55% and 88.67% of the eyes demonstrated an uncorrected visual acuity of 20/32 or better (P = 0.574) [Figs. 1a and 2a]. The postoperative UDVA was within one line of preoperative CDVA in 90% and 95% of the eyes, respectively (P = 0.231) [Figs. 1b and 2b].
Predictability
Figs. 1d and 2d show attempted versus the achieved spherical equivalent correction. At the 1-year postoperative visit, 89.92% and 95.8% of eyes were within 0.5 D and 1.0 D of the attempted correction following IPCL implantation [Fig. 1e]. The ICL group demonstrated 94.09% and 96.06% of eyes within 0.5 D and 1.0 D of the attempted correction [Fig. 2e] (P = 0.169 and 0.909).
Stability
Figs. 1f and 2f demonstrate the time-course changes in manifest refraction. An improvement of the manifest refraction in the IPCL group at 1 year was noted, subsequent to phacoemulsification and intraocular lens implantation in eyes with visually significant cataracts.
Secondary surgeries/adverse events
Three eyes (2.52%) in the IPCL group versus one eye (0.49%) in the ICL group developed visually significant cataract (P = 0.113). A higher mean age (39.66 ± 0.58 years) and manifest refraction (–17.29 ± 3.38) was noted in the IPCL group. In all eyes, the cataract developed after a minimum duration of 6 months following implantation. None of the eyes demonstrated a low vault (<250 μm) with a mean vault of 311.45 ± 180.93 μm.
Three eyes (2.52%) versus two eyes (0.98%) in the IPCL and ICL groups developed moderately high IOP rise (mean 30.02 ± 2.45 mm Hg) secondary to steroid response (P = 0.22). They were managed conservatively with antiglaucoma medications, following which IOP normalized. One eye (0.84%) in the IPCL group required a repeat Nd: YAG peripheral iridotomy to enlarge the pre-existing inadequate opening and relieve the pupillary block.
There were no statistically significant changes in ECD in the IPCL group (P = 0.68) or in the ICL group (P = 0.72) between preoperative and 1-year postoperative visits.
No cases of retinal detachment or endophthalmitis were noted in either group.
Discussion
Posterior chamber phakic intraocular lens implantation demonstrates several advantages over keratorefractive procedures and is a viable option for myopic correction. Numerous studies demonstrate promising visual outcomes following ICL implantation. US Food and Drug Administration clinical trial, in a 3-year follow-up of 526 eyes, demonstrated a UDVA of 20/40 or better in 94.7% eyes.[5] We demonstrated similar outcomes wherein 94% of the eyes achieved a UDVA of 20/40 or better. Additionally, high levels of refractive predictability achieved were similar to earlier cohorts. Alfonso et al. in a study of 182 eyes demonstrated 96.8% eyes within ± 1.0 D of attempted correction at the 1-month follow-up.[8] Our results were similar, with 96.06% of the eyes achieving a manifest refractive error within ± 1.0 D of intended correction.
A limitation of the ICL, however, is the increased economic burden of treatment, especially in developing nations. The IPCL is a more economically viable option for refractive correction. This study compared the refractive outcomes between the two implants and demonstrated equivalent results for myopic correction, in terms of safety, predictability, and stability of refractive outcomes. No similar studies have been published in the literature thus far, to the best of our knowledge.
Increased risk of cataract, raised intraocular pressure (IOP), and infections are concerns following intraocular procedures. Postoperative complications were similar in both cohorts, including IOP rise and cataract formation. This was in accordance with data published thus far.[9,10]
The lack of a central hole in the IPCL model (V1) mandates the construction of a peripheral iridectomy, preoperative or intraoperative. Pupillary block glaucoma secondary to inadequate iridectomy can result in the rise of IOP. Additionally, visually significant cataracts were noted in three eyes following IPCL during a 1-year follow-up period. As the vault sizing was adequate in all eyes (311.45 ± 180.93 μm), the anterior subcapsular opacification was most likely secondary to metabolic changes resulting in fibrous metaplasia of the lens epithelial cells.[11]
Kawamorita and co-workers, using computational analysis, demonstrated an increase in the velocity of aqueous humor in the central hole ICL model.[12] The lack of a central optic hole in the IPCL could potentially cause a greater disturbance in the aqueous circulation, resulting in an increased incidence of cataract. The new version of the IPCL (IPCL V2) containing a 350-μm central artificial hole has been made commercially available recently. This would obviate the need for a peripheral iridectomy and possibly bring down the incidence of cataract and pupillary block glaucoma. Studies comparing the outcomes of the hole and non-hole IPCL are needed to demonstrate the same. Additionally, the mean age and preoperative manifest refraction were higher in the IPCL cohort which is associated with a greater incidence of cataract formation.[13,14]
This study has certain limitations. First, the construct of the study was a retrospective analysis. A prospective, randomized study would be ideal for confirming our results. Second, the ECD measurements were obtained for the central cornea only. Goukon Hiroyas compared the endothelial cell loss and morphology following implantation of two different models of ICL and concluded significantly lower ECD in the superior cornea, in the non-hole cohort.[15] This was attributed to the effect of the preoperative peripheral iridectomy performed in the conventional ICL group. No such comparisons were made in our cohort. For analysis, patients who completed a 1-year follow-up were only included in this study. As the patients who are satisfied with their visual performance after refractive surgery tended to be lost to follow-up, our longitudinal data may have a possible source of selection bias.
Conclusion
In conclusion, the results of IPCL are equivalent to ICL implants for myopic correction, in terms of safety, efficacy, predictability, and stability. IPCL is an economically viable option for the treatment of myopia and myopic astigmatism.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Igarashi A, Kamiya K, Shimizu K, Komatsu M. Visual performance after implantable collamer lens implantation and wavefront-guided laser in situ keratomileusis for high myopia. Am J Ophthalmol. 2009;148:16–70. doi: 10.1016/j.ajo.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Kamiya K, Igarashi A, Shimizu K, Matsumura K, Komatsu M. Visual performance after posterior chamber phakic intraocular lens implantation and wavefront-guided laser in situ keratomileusis for low to moderate myopia. Am J Ophthalmol. 2012;153:1178–86. doi: 10.1016/j.ajo.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Sanders D, Vukich JA. Comparison of implantable collamer lens (ICL) and laser assisted in situ keratomileusis (LASIK) for low myopia. Cornea. 2006;25:1139–46. doi: 10.1097/ICO.0b013e31802cbf3c. [DOI] [PubMed] [Google Scholar]
- 4.Pineda- Fernandez A, Jaramillo M, Vargas J, Jaramillo M, Jaramillo J, Galindez A. Phakic posterior chamber intraocular lens for high myopia. J Cataract Refract Surg. 2004;30:2277–83. doi: 10.1016/j.jcrs.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 5.Sander DR, Doney K, Poco M ICL in treatment of Myopia Study Group. United States Food and Drug Administration clinical trial of the implantable collamer lens (ICL) for moderate to high myopia: Three-year follow-up. Ophthalmology. 2004;111:1683–92. doi: 10.1016/j.ophtha.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 6.Igarashi A, Shimizu K, Kamiya K. Eight-year follow-up of posterior chamber phakic intraocular lens implantation for moderate to high myopia. Am J Ophthalmol. 2014;157:532–9. doi: 10.1016/j.ajo.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Kamiya K, Shimizu K, Igarashi A, Hikita F, Komatsu M. Four-year follow-up of posterior chamber phakic intraocular lens implantation for moderate to high myopia. Arch Ophthalmol. 2009;127:854–50. doi: 10.1001/archophthalmol.2009.67. [DOI] [PubMed] [Google Scholar]
- 8.Alfonso JF, Baamonde B, Fernandez-Vega L, Fernandes P, Gonzalez-Meijome JM, Montes-Mico R. Posterior chamber collagen copolymer phakic intraocular lenses to correct myopia: Five-year follow-up. J Cataract Refract Surg. 2011;37:873–80. doi: 10.1016/j.jcrs.2010.11.040. [DOI] [PubMed] [Google Scholar]
- 9.Fernandes P, Gonzalez-Meijome JM, Madrid-Costa D, Ferrer- Blasco T, Jorge J, Montes-Mico R. Implantable collamer posterior chamber intraocular lenses: A review of potential complications. J Refract Surg. 2011;27:765–76. doi: 10.3928/1081597X-20110617-01. [DOI] [PubMed] [Google Scholar]
- 10.Sanders DR. Anterior subcapsular opacities and cataracts 5 years after surgery in visian implantable collamer lens FDA trial. J Refract Surg. 2008;24:566–70. doi: 10.3928/1081597X-20080601-04. [DOI] [PubMed] [Google Scholar]
- 11.Khalifa YM, Moshirfar M, Mifflin D, Kamae K, Mamalis N, Werner L. Cataract development associated with collagen copolymer posterior chamber phakic intraocular lenses: Clinicopathological correlation. J Cataract Refract Surg. 2010;36:1768–74. doi: 10.1016/j.jcrs.2010.04.039. [DOI] [PubMed] [Google Scholar]
- 12.Kawamorita T, Shimizu K, Shoji N. Effect of hole size on fluid dynamics of a posterior- chamber phakic intraocular lens with a central perforation by using computational fluid dynamics. Graefes Arch Clin Exp Ophthalmol. 2016;254:739–44. doi: 10.1007/s00417-016-3304-3. [DOI] [PubMed] [Google Scholar]
- 13.Alfonso JF, Lisa C, Abdelhamid A, Fernandes P, Jorge J, Montes-Mico R. Three-year follow-up of subjective vault following myopic implantable collamer lens implantation. Graefes Arch Clin Exp Ophthalmology. 2010;248:1827–35. doi: 10.1007/s00417-010-1322-0. [DOI] [PubMed] [Google Scholar]
- 14.Gonvers N, Bornet C, Othenin-Girard P. Implantable contact lens for moderate to high myopia: Relationship of vaulting to cataract formation. J Cataract Refract Surgery. 2003;29:918–24. doi: 10.1016/s0886-3350(03)00065-8. [DOI] [PubMed] [Google Scholar]
- 15.Goukon H, Kamiya K, Shimizu K, Igarashi A. Comparison of corneal endothelial cell density and morphology after posterior chamber phakic intraocular lens implantation with and without central hole. Br J Ophthalmol. 2017;101:1461–5. doi: 10.1136/bjophthalmol-2016-309363. [DOI] [PubMed] [Google Scholar]