Abstract
Walking can be exceedingly complex to analyze due to highly nonlinear multibody dynamics, nonlinear relationships between muscle excitations and resulting muscle forces, dynamic coupling that allows muscles to accelerate joints and segments they do not span, and redundant muscle control. Walking requires the successful execution of a number of biomechanical functions such as providing body support, forward propulsion, and balance control, with specific muscle groups contributing to their execution. Thus, muscle injury or neurological impairment that affects muscle output can alter the successful execution of these functions and impair walking performance. The loss of balance control in particular can result in falls and subsequent injuries that lead to the loss of mobility and functional independence. Thus, it is important to assess the mechanisms used to control balance in clinical populations using reliable methods with the ultimate goal of improving rehabilitation outcomes. In this review, we highlight common clinical and laboratory-based measures used to assess balance control and their potential limitations, show how these measures have been used to analyze balance in several clinical populations, and consider the translation of specific laboratory-based measures from the research laboratory to the clinic.
Keywords: gait, biomechanics, stability, amputee, poststroke, rehabilitation
Introduction
Human movement can be exceedingly complex to analyze due to highly nonlinear multibody dynamics, nonlinear relationships between muscle excitations and resulting muscle forces, dynamic coupling that allows muscles to accelerate joints and segments they do not span, and redundancy in muscle control [1,2]. Walking involves the execution of critical biomechanical functions such as providing body support, forward propulsion, leg swing, foot clearance/placement, and balance control that often requires synergistic activity between multiple muscle groups. A number of studies have used musculoskeletal modeling and simulation to analyze in detail individual muscle contributions to these biomechanical functions (e.g., Refs. [3–5]). From these studies, specific muscle groups have been identified as essential to the successful execution of these functions. For example, the ankle plantarflexors have been shown to provide body support and forward propulsion, accelerate the leg into swing [3–5], and provide frontal and sagittal plane balance control [6–8]. Thus, ankle muscle injury or impairment can dramatically hinder the walking ability in various populations such as those with traumatic injuries (e.g., lower-limb amputees) or impaired neural control (e.g., individuals poststroke). Of particular importance is the successful execution of maintaining dynamic balance, with the loss of balance potentially leading to falls and subsequent long-term injuries that can result in the loss of mobility and functional independence. As a result, significant research has been devoted to developing effective methods to assess balance control. In this review, we highlight common clinical and laboratory-based measures used to assess balance control and their potential limitations, show how these measures have been used to analyze balance in several clinical populations, and consider the translation of specific laboratory-based measures from the research laboratory to the clinic.
Methods to Assess Balance Performance
A number of methods have been used to evaluate balance control during human movement. These methods range from simple ordinal scale clinical balance measures to more comprehensive kinematic and kinetic-based measures derived in research laboratories.
Clinical Balance Measures.
Clinical balance measures are often based on discrete score assignments while completing a series of movement tasks. The Tinetti Performance Oriented Mobility Assessment (POMA) is designed to measure balance and gait function in elderly [9]. The assessment consists of nine balance items involving sit to stand tasks and eight gait items focused on spatiotemporal gait characteristics. The Tinetti POMA measure uses a cutoff score (<20) to predict fall risk in individuals poststroke [10] and those with Parkinson disease [11]. One limitation of this measure is it has shown ceiling effects (e.g., Ref. [12]).
Berg balance scale (BBS) is used to assess functional balance and consists of 14 tasks ranging from sitting to standing and turning [13]. BBS is perhaps the most commonly used balance measure in stroke rehabilitation [14], although it is not a measure of dynamic balance, but uses a cutoff score (<42) that relates to a higher risk of falls (e.g., Ref. [15]). BBS has been shown to be an effective method for balance assessment in individuals poststroke, although some studies have observed floor and ceiling effects, and thus, combining it with other balance measures has been recommended [16]. BBS has also been shown to be a valid measure of balance in lower-limb amputees, although it has not shown promise to discriminate those with higher versus lower risk of falls [17].
Dynamic gait index (DGI) is widely used to assess dynamic balance during gait activities, which was developed by Shumway-Cook and Woollacott to evaluate functional stability and risk of falling in older adults [18]. Similar to BBS, DGI utilizes a cutoff score (<19) to indicate increased risk of falls [19,20]. In addition to older adults, DGI has been used to assess balance in individuals with vestibular dysfunction (e.g., Ref. [21]), chronic stroke (e.g., Ref. [22]), Parkinson disease (e.g., Ref. [23]), and multiple sclerosis (e.g., Ref. [24]).
Like the Tinetti POMA and BBS, DGI has shown ceiling effects in both individuals with vestibular dysfunction (e.g., Ref. [21]) and poststroke hemiparesis [25]. Moreover, in higher functioning elderly, all three measures have shown low sensitivity to responsiveness and change in scores [26]. Thus, Wrisley et al. [27] designed the functional gait assessment to address the ceiling effects. Importantly, this was done by evaluating the ceiling effects of the DGI and including more challenging tasks such as walking with a narrow base of support, walking with closed eyes and backward walking. Functional gait assessment has shown the least ceiling effect among the clinical balance scores and has been recommended for assessments in high level individuals poststroke [25]. In addition, the community balance and mobility scale was developed to assess high level deficit in balance and mobility [28]. Community balance and mobility includes tasks such as forward and backward walking, running with controlled stop, descending stairs, stepping up and dual tasking and did not show floor or ceiling effects in individuals poststroke with mild to moderate impairment [29].
In addition to potential floor and ceiling effects, an important limitation of the clinical measures is that they are global in nature and limited in their ability to provide insight into the underlying mechanisms for balance control and the biomechanical deficits linked to balance impairments. Understanding such deficits and mechanisms is needed to inform rehabilitation interventions.
Laboratory-Based Balance Measures.
Laboratory-based measures can provide continuous measurements obtained using kinematic and kinetic data during walking both overground and/or on a treadmill. One commonly used measure is margin-of-stability (MoS), which is defined as the minimum distance between the base of support and the extrapolated center-of-mass (CoM) [30]. MoS is based on foot placement while accounting for body CoM position and velocity and has been used to assess dynamic balance in various populations such as in young healthy individuals in destabilizing environments (e.g., Ref. [31]), older adults while stepping to targets [32], amputees during various movement activities (e.g., Refs. [33–35]), and individuals poststroke during walking (e.g., Refs. [36] and [37]). One limitation of MoS is that it is a global measure of whole-body movement, and similar to clinical measures, does not provide insight into the biomechanical mechanisms used to control balance.
Another laboratory-based measure is whole-body angular momentum (H), which is a mechanics-based measure defined with respect to the body CoM as
where and are the position and velocity vectors of the ith segment's CoM, respectively. and are the position and velocity vectors of the whole-body CoM, respectively, , and are the mass, moment of inertia, and the angular velocity of each segment, respectively, and n is the number of body segments. We have performed a number of experimental and simulation studies using whole-body angular momentum to investigate the control of dynamic balance over a range of walking tasks including steady-state walking [6,7], walking at increasing speeds [38], walking with a unilateral solid ankle-foot orthosis [39], stepping on uneven terrain [40], incline/decline walking [41], and stair ascent/descent [42] and in different subject populations including amputees [38] and individuals poststroke during steady-state walking [43] and during walking adaptability tasks [44]. Others have used H to investigate balance control in younger and older adults recovering from a trip [45], amputees using powered ankle-foot prostheses during stair ascent [46] and walking at different speeds [47], amputees during perturbed walking [48], healthy adults during multidirectional perturbed walking [49], and children with cerebral palsy walking overground [50].
The regulation of H is essential for maintaining dynamic balance during walking (e.g., Ref. [51]) and can be quantified by analyzing the time rate of change of angular momentum about the body's CoM, which is equivalent to the net external moment (i.e., the cross-product of the moment arm vector () and ground reaction force (GRF) vector) as
where r is the moment arm vector from the body CoM to the center-of pressure and GRF is the vector of GRFs (Fig. 1). For example, adjustments in the GRFs and foot placement in the vertical and mediolateral directions influence the net external moment in the frontal plane (Fig. 1). A higher external moment or time rate of change of H results in a higher peak-to-peak (max–min) range of H (HR) during that time interval, which may impose a challenge to balance control.
Fig. 1.
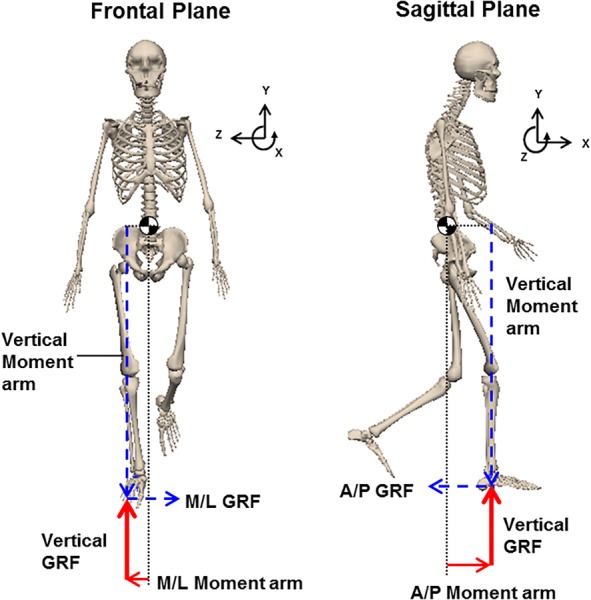
The components of net external moment in the frontal and sagittal planes during single-leg stance. Whole-body CoM is shown with “ ” The GRF vectors and their corresponding moment arms appear in the same line type.
” The GRF vectors and their corresponding moment arms appear in the same line type.
Comparison Between Balance Measures.
We previously investigated the associations between the time rate of change of frontal plane H in six regions of the gait cycle during steady-state walking with the DGI and BBS measures in individuals poststroke [43]. We found a correlation between higher clinical scores (i.e., better balance control) and lower rate of change of frontal plane angular momentum during the paretic single-leg stance. In addition, we classified the subjects as fallers or nonfallers based on their BBS and DGI scores and compared their rate of change of H between the two groups. We found that during the paretic leg single stance, fallers (based on BBS) had a significantly higher rate of change of H than nonfallers (Fig. 2). The higher rate of change of H during steady-state walking can be attributed to nonoptimal cancellation of external moment components in the fallers due to altered foot placement and/or impaired GRFs. The higher rate of change of H and corresponding HR can be more challenging to control, particularly in impaired populations.
Fig. 2.
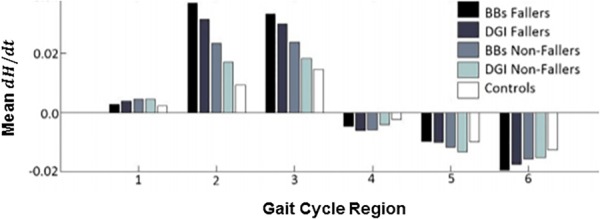
The mean, time rate of change of H () in the frontal plane during the six regions of the gait cycle during steady-state walking. Each bar depicts the mean values across the subjects grouped as fallers and nonfallers based on their BBS and DGI scores. There is a significant difference in between the BBS fallers and nonfallers during the paretic leg single stance (regions 2 and 3). Region 1 is the first double support phase, regions 2 and 3 are the first and second halves of single-leg stance, respectively, region 4 is the second double support phase, and regions 5 and 6 are the first and second halves of swing, respectively. Figure adopted from Ref. [43].
In a subsequent study, we built upon these findings and assessed dynamic balance using the BBS, DGI, MoS, and frontal plane HR to determine whether these measures provided consistent assessments of balance control [52]. Correlation analyses revealed moderate associations between all measures. Overall, a higher HR was associated with a higher MoS, wider step width, and lower BBS and DGI scores, which indicate poor balance control.
Given that balance is multidimensional, each measure can assess different constructs of dynamic balance. The advantage of clinical balance scores is that they provide a simple global assessment, which does not require the collection and processing of more complex body-segment kinematic and kinetic data. However, contrary to the laboratory-based measures, the clinical balance scores are limited in their ability to provide insight into the biomechanical mechanisms for maintaining dynamic balance or the biomechanical mechanisms leading to the loss of balance. Similarly, MoS can provide some insight into foot placement, but not the GRFs, which are generated primarily by muscle forces and are critical to the regulation of dynamic balance. Although the analysis is more complex to perform, H has a number of advantages such as it can provide a more comprehensive assessment of dynamic balance since it accounts for the motion and inertia of all the body segments, which collectively generate the whole-body angular momentum about the center-of-mass. Further, the analysis of the rate of change of H or net external moment can provide insight into the influence of foot placement and GRF generation on maintaining dynamic balance. Thus, the analysis of H reflects not only direct balance control strategies such as from muscle force generation, but also indirect methods such as counter rotation strategies using arm swing and trunk motion. Thus, the relationships between the H trajectories and corresponding GRFs and moment arms (i.e., foot placements) (Fig. 3) can be analyzed to gain insight into the biomechanical mechanisms of balance control, which reflect the whole-body response used to maintain balance. Finally, the analysis of H can be performed during specific movement tasks, in each anatomical plane independently and across a wide range of participants with no reported ceiling effects. Thus, H can provide an objective method for monitoring the biomechanical changes in balance control and assessing the effectiveness of specific balance training programs.
Fig. 3.
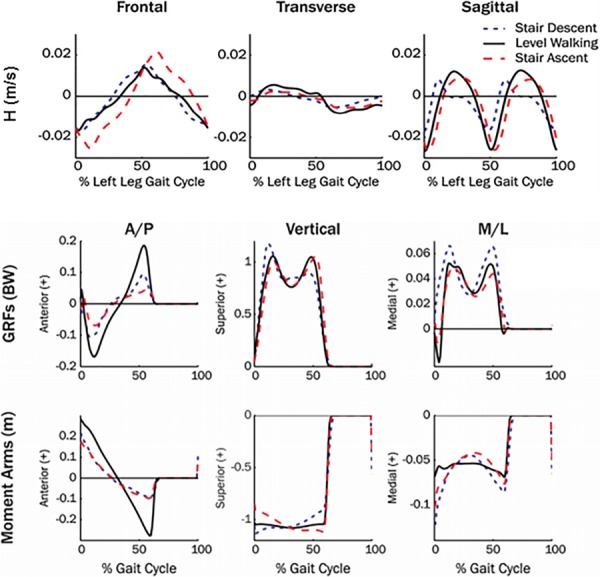
Mean three-dimensional (3D) H trajectories, normalized by height and weight during healthy adult level walking, and stair descent and ascent. Mean GRFs and moment arms during each walking condition are shown in the anterior-posterior (A/P), vertical and mediolateral (M/L) directions. Figure adapted from Ref. [42].
Muscle Contributions to Dynamic Balance
Muscles play a critical role in maintaining dynamic balance during human walking. Thus, understanding which muscles contribute to balance control has the potential to help diagnose and treat balance disorders. We previously used musculoskeletal modeling and simulation analyses to quantify individual muscle contributions to the regulation of H in the sagittal [6] and frontal [7] planes. Modeling and simulation is a powerful tool that allows one to identify the causal relationships between individual muscle excitations and the performance of specific biomechanical functions. The simulation results revealed that the regulation of H was provided by a few dominant muscle groups. In the sagittal plane, in early stance, the gluteus maximus, biarticular hamstrings, ankle dorsiflexors, and gravity contributed to the backward external moment (i.e., acted to rotate the body backward), while the soleus, gastrocnemius, and rectus femoris contributed to the forward external moment (i.e., acted to rotate the body forward). In late stance, the soleus and gastrocnemius generated angular momentum in opposite directions due to differences in their relative contributions to the horizontal and vertical ground reaction forces. The soleus generated primarily forward momentum, while the gastrocnemius generated backward momentum.
In the frontal plane, previous simulation studies have investigated mediolateral body control by analyzing muscle contributions to the linear accelerations of the whole-body center-of-mass [8,53,54] or frontal plane trunk angular accelerations [55]. We extended this work by analyzing muscle contributions to the regulation of H and found in early stance the vasti, adductor magnus, and gravity acted to rotate the body toward the contralateral leg, while the gluteus medius acted to rotate the body toward the ipsilateral leg (Fig. 4). In late stance, the gluteus medius continued to rotate the body toward the ipsilateral leg, while the soleus and gastrocnemius acted to rotate the body toward contralateral leg (Fig. 4).
Fig. 4.
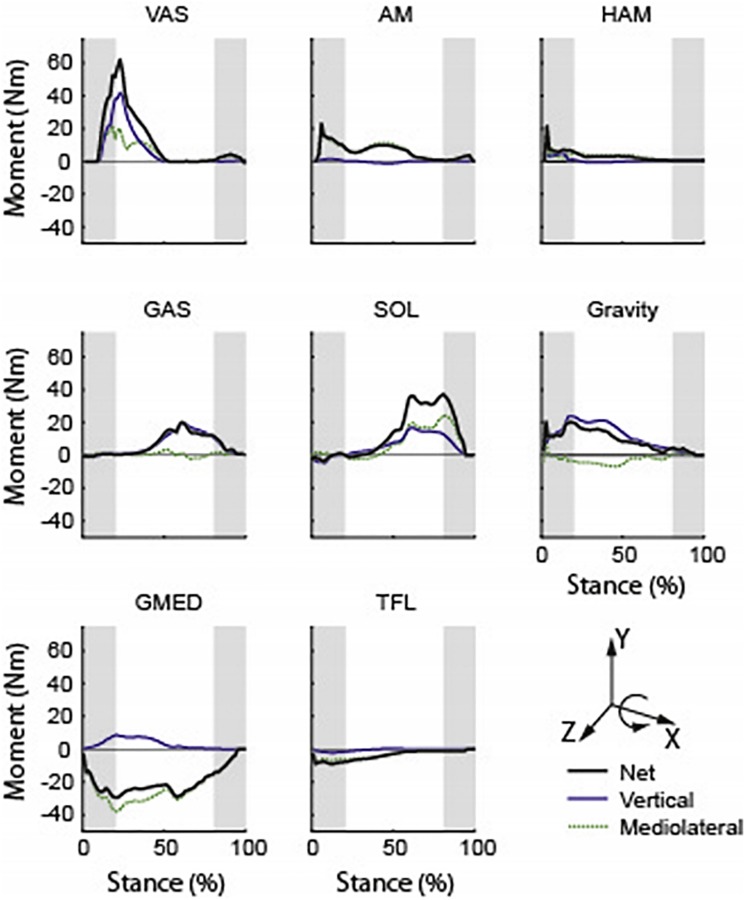
Individual muscle contributions to the time rate of change of frontal plane H (i.e., external moment). “vertical” and “mediolateral” are contributions from the vertical and mediolateral GRFs, respectively. Positive (negative) values indicate the moment contribution from that muscle acts to rotate the body toward the contralateral (ipsilateral) leg. Shaded regions indicate the double support phases. Abbreviations: VAS, vasti; AM, adductor magnus; HAM, hamstrings; GAS, gastrocnemii; SOL, soleus; GMED, gluteus medius; and TFL, tensor fasciae latae. Figure adapted from Ref. [7].
An important finding in these studies was the critical role the ankle plantarflexors play in maintaining dynamic balance during walking in both the frontal and sagittal planes. Others have also shown that the plantarflexors are important in balance recovery during walking [45] and standing [56] perturbations and that individuals with a history of falls have reduced ankle plantarflexor output [57]. The critical role of the plantarflexors in maintaining dynamic balance has important implications for the diagnosis and treatment of movement and balance disorders, and also in the design and prescription of ankle-foot orthotic (AFO) devices.
What is the Role of Step Width in Balance Control?
Previous studies have suggested that wider steps are used to increase lateral stability [58–61] and some have observed that elderly fallers take narrower steps [62]. Others have associated wider steps with increased step width variability and increased instability [63] and some have observed a higher rate of falls in subjects with wider steps [64–67]. Lower-limb amputees often walk with wider steps and a more variable base of support [35,68] and are more likely to fall compared to nonamputees [69]. We found that in individuals poststroke, a wider step width was associated with a greater HR, a higher MoS, and lower BBS and DGI scores, which indicates poor balance control [52]. Wider step widths during single limb-stance create a greater moment-arm from the body's center-of-mass to the center-of-pressure that along with the vertical GRF act to rotate the body toward the contralateral limb and increase HR. A higher HR most likely makes the individual more susceptible to falling when they are near their peak H and a perturbation occurs in the same direction. This presents a challenge for individuals with muscle weakness or neuromotor impairments who do not have the neuromuscular capacity to provide a timely response to counteract the perturbation through proper foot placement and/or generation of appropriate GRFs. Perturbation studies are needed to assess the relationships between HR, neuromuscular capacity, and the ability to recover balance in various clinical populations.
MoS can provide insight into foot placement while accounting for center-of-mass position and velocity (see Methods to Assess Balance Performance section). Prior studies (e.g., Ref. [32]) have interpreted higher MoS in older adults as indicative of better dynamic stability compared to younger adults, and a similar interpretation of MoS was made in individuals poststroke (e.g., Ref. [70]). However, based on the correlations between larger MoS and lower BBS and DGI scores [52] and the higher rate of falls in both older adults [71] and individuals poststroke [72], an alternative interpretation is that individuals with higher MoS may be at a higher risk of falling. It is not clear if the higher MoS is adopted as a compensatory mechanism for those with poor balance control or if an increased level of MoS may paradoxically represent decreased stability during walking. When we examined MoS for each foot, we found that only the MoS corresponding to the paretic foot placement was correlated with other balance measures [52]. This highlights the importance of the paretic mediolateral foot placement in dynamic balance control, suggesting that in individuals poststroke it may be more suitable to examine the MoS for each foot individually rather than the average sum of both legs (e.g., Refs. [36] and [70]).
Previous research has shown that mediolateral foot placement requires active recruitment of the sensory-motor processes [73] and that overall frontal plane movements require greater active control than sagittal plane movements [58]. In young healthy adults, changes in the lateral foot placement were correlated with gluteus medius muscle activity [74]. Others have shown that poststroke nonfallers and healthy adults used a similar neuromechanical strategy to control their mediolateral foot placement, which was influenced by the swing phase gluteus medius activation and associated with the state of the contralateral stance limb. However, in poststroke fallers, this strategy was disrupted, especially when taking a step with the paretic leg [75].
Individuals poststroke have shown significantly lower levels of accuracy and precision in their mediolateral foot placement during walking compared to healthy controls. The lowest accuracy was observed during extreme (i.e., narrowest and widest) targeted values [76]. Others have observed that individuals poststroke had difficulty controlling their step width and foot placement variability during a gait tracking task. Specifically, the variability in their step width and paretic foot placement increased as the targeted step width decreased, highlighting task-dependent inter-limb differences in frontal plane motor control variability [77]. These studies collectively highlight the importance of mediolateral foot placement in dynamic balance control, and deficits in foot placement should be a focus in balance training programs.
Given the importance of mediolateral foot placement and step width in dynamic balance control, it would be extremely insightful to be able to measure these quantities in the clinic. In contrast to individual foot placement (i.e., moment arm) which involves the more complex calculation of body center-of-mass location, step width has been measured in the clinic using various methods. Less expensive methods involve specially designed color coded walkway grids, which register the footfalls using ink marks [78], and pressure-sensitive papers [79]. More expensive methods involve instrumented walkways registering footfalls using pressure sensors (e.g., gaitrite) [80]. Future work is needed to further understand the role of foot placement in balance control and how simple, clinic-based methods can be used to inform treatment decisions.
Balance Control in Lower-Limb Amputees
Below-knee amputees have an increased risk of falling relative to nonamputees [69]. To begin understanding the balance control deficits in amputees, we examined H between 12 amputees and 10 nonamputees at four walking speeds ranging from 0.6 to 1.5 m/s with 0.3m/s increments [38]. In the frontal plane, HR over the entire gait cycle was found to be greater in amputees compared to nonamputees at the first three walking speeds (Fig. 5), which was correlated with a reduced vertical GRF peak during late stance in both the sound and residual legs. In the sagittal plane, the amputee HR in the first half of the residual leg gait cycle was significantly larger than in the nonamputees at the three highest speeds. In the second half of the gait cycle, the sagittal plane HR was significantly smaller in amputees compared to nonamputees at all speeds. Correlation analyses suggested that the greater HR in the first half of the amputee gait cycle is associated with reduced residual leg braking GRF peak and that the smaller HR in the second half of the gait cycle is associated with reduced residual leg propulsive GRF peak. Thus, reducing residual leg braking appears to be an important compensatory mechanism to help regulate the sagittal plane angular momentum over the gait cycle, but the increased H in the first half of the gait cycle may lead to an increased risk of falling.
Fig. 5.
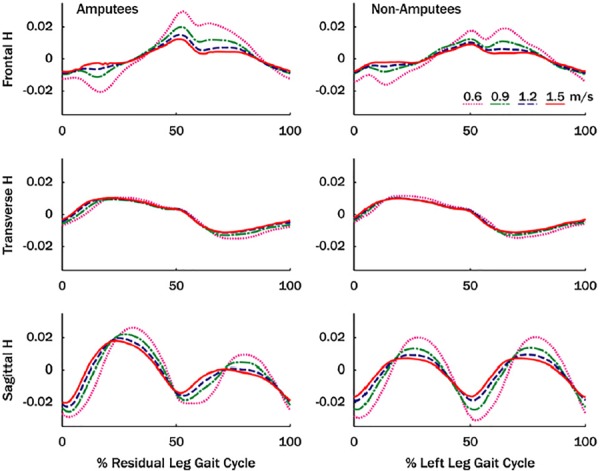
Mean normalized three-dimensional angular momentum (H) for amputee and nonamputee subjects over the gait cycle. Angular momentum was normalized by body mass, body height, and walking speed. Note, in this study, H was normalized by walking speed which can influence the magnitude of H across the walking speeds. Figure adapted from Ref. [38].
In a subsequent study, we compared H during stair ascent and descent using passive and powered lower-limb prostheses with the expectation that the powered prosthesis would eliminate the propulsion deficit and improve balance control [46]. Similar to our steady-state walking study, amputees had a larger HR in the sagittal plane during prosthetic limb stance compared to nonamputees during stair ascent. However, there were no differences in HR between the passive and powered prostheses in the frontal, transverse, or sagittal planes during stair ascent or descent. These results suggest that amputees have altered angular momentum trajectories during stair walking compared to able-bodied individuals, which may contribute to their increased fall risk. The results also suggest that powered prostheses provide no distinct advantage over passive prostheses in maintaining dynamic balance during stair walking. This may be due to the inability of current ankle-foot prosthetic devices to replicate the functions of both the uniarticular soleus and the biarticular gastrocnemii, which often have distinctly different biomechanical functions [81,82]. In contrast to these results, others found that amputees more effectively regulate HR at some walking speeds when using a powered prosthesis compared with passive-elastic prostheses [47]. This finding is likely due to forward propulsion generation being dominated by the uniarticular soleus, which can be effectively replaced by a powered ankle-foot prosthesis.
We further analyzed the contributions of a passive prosthesis and individual muscles to balance control during amputee and nonamputee stair ascent [83] and found that the passive prosthesis replicated the role of nonamputee plantarflexors in the sagittal plane but caused a larger change in angular momentum in the transverse plane. In the frontal plane, nonamputee plantarflexors contributed minimally, while the prosthesis was a critical contributor to angular momentum that acted to rotate the body toward the contralateral leg. This resulted in altered muscle contributions from the vasti, hamstrings, and hip abductors. These results suggest that improved prostheses with reduced contributions to transverse and frontal plane angular momentum could improve dynamic balance during amputee stair ascent and minimize necessary muscle compensations.
Most studies have analyzed balance control during steady-state conditions. However, perturbations frequently occur during walking and compromise balance. Studies have shown that the majority of falls occur due to trips or unexpected perturbations [84]. Perturbations are particularly challenging for lower limb amputees who lack active ankle control to help recover their balance. Thus, understanding the neuromuscular balance recovery mechanisms used by amputees to recover from such perturbations could provide insight into developing interventions aimed at decreasing their risk for falls and injuries. Sheehan et al. [48] assessed the effects of surface perturbations on balance control in able-bodied controls and individuals with unilateral transtibial amputation using mediolateral platform oscillations. Amputees and nonamputees walked at a fixed speed with no perturbations and continuous, pseudo-random, mediolateral platform oscillations. Amputees were significantly more affected by the perturbations and had a greater HR in the frontal plane. They noted that their findings support the use of angular momentum in relation to dynamic stability and that increased HR is associated with greater fall risk.
Segal and Klute [85] used a novel mediolateral foot perturbation protocol that enabled them to study the effect of medial and lateral step width disturbances on balance control in both amputees (n = 10) and nonamputees (n = 12). They used a pneumatic device attached to the foot to release a medial or lateral burst of air just before heel strike that imposed a repeatable medial or lateral disturbance in foot placement. They found amputees required five steps to return to undisturbed step width after a prosthetic limb medial disturbance versus two steps for the sound limb and for nonamputees. Following a lateral disturbance, amputees returned to their undisturbed step width within three steps, which was similar to the sound and nonamputee limbs. Thus, for amputees, a medial perturbation was much more challenging than a lateral perturbation in terms of the number of steps needed to recover their balance.
In a follow-up study, we analyzed the same dataset to further understand the balance recovery mechanisms used by lower limb amputees in response to the perturbations by examining changes to frontal plane H and hip joint work [86]. The lateral perturbations of the residual, sound, and nonamputee limbs resulted in a reduced HR and an increased positive frontal plane hip work in the first half of single limb support. Medial perturbations for all limbs resulted in increased HR and decreased positive frontal plane hip work, also in the first half of single limb support. These results further support the important role hip strategies play in balance control. Thus, rehabilitation interventions that focus on hip muscles that regulate mediolateral balance, particularly the hip abductors, and the use of prostheses with active ankle control, may reduce the risk of falls in lower-limb amputees.
Another common gait disturbance that can lead to the loss of balance and falls is stepping on unpredictable terrain. We previously identified the biomechanical response to a step on coronally uneven and unpredictable terrain [40]. Able-bodied subjects traversed a walkway with a middle step that was blinded to participants, and positioned either 15 deg inverted, 15 deg everted or flush. The analysis of HR in the frontal plane showed that it increased during blinded eversion and decreased during blinded inversion (Fig. 6). In the frontal plane, the analysis of external moments applied to the body about the center-of-mass by the disturbed and recovery legs suggested that the disturbed leg contributed more to differences in HR, and thus, to balance recovery. During the disturbed step, distinct differences between blinded inversion and eversion in the frontal plane moments of the hip and ankle suggested that the hip and ankle joint moment strategies were important for adapting to the terrain angle. Thus, amputees would most likely have difficulty adapting to such balance perturbations without an active ankle strategy.
Fig. 6.
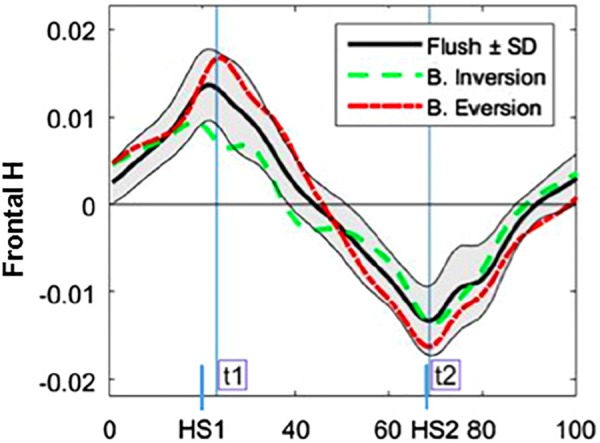
Mean normalized H in the frontal plane for each perturbation condition. The everted perturbations resulted in greater HR. Figure adapted from Ref. [40].
These studies of individuals with below-knee amputations and balance perturbations have highlighted the usefulness of H in gaining insight into balance control mechanisms and provided the basis for targeted rehabilitation programs to strengthen specific muscle groups and help improve dynamic balance and subsequently reduce the risk of falls and injuries.
Balance in Individuals Poststroke
More than 50% of stroke survivors experience falls within one year poststroke (e.g., Ref. [87]), which can lead to physical injuries and long-term disabilities. Although 85% of individuals poststroke regain some level of steady-state walking function, over one-third of these individuals reportedly do not walk in the community (e.g., Ref. [88]). Community walking involves the performance of adaptability tasks such as obstacle negotiation, stepping up a curb, and changing the walking direction [89]. Successful performance of these tasks requires precise balance control, which is a major challenge poststroke.
During steady-state walking, Nott et al. [43] showed that healthy adults demonstrated timely regulation of frontal plane H during the first half of single-leg stance, with the level of regulation depending on the initial magnitude. In contrast, individuals poststroke who poorly regulated their frontal plane H during initial paretic leg single stance exhibited lower DGI and BBS scores and some were categorized as fallers.
To gain insight into balance control impairments during community ambulation, we have recently studied the regulation of H in all three anatomical planes in 15 individuals poststroke and 10 healthy adults during a variety of walking tasks including steady-state self-selected and fastest-comfortable walking, backward walking, obstacle negotiation, and stepping up a box (Step) [44]. We observed that individuals poststroke had significant deficits regulating their H in the frontal plane, manifested in significantly higher HR than the healthy adults (Fig. 7). Further, the obstacle negotiation task was associated with a higher HR compared to the rest of the tasks and imposed a higher demand for balance control particularly in individuals poststroke. In addition, in contrast to healthy adults who had higher soleus activation levels during the adaptability tasks compared to steady-state walking, minimal changes in the soleus activation levels were evident in individuals poststroke [44]. Thus, the inability to recruit the plantarflexors, which are critical in controlling dynamic balance in the frontal plane (see Muscle contributions to Dynamic Balance section), may compromise dynamic balance during these more challenging adaptability tasks.
Fig. 7.
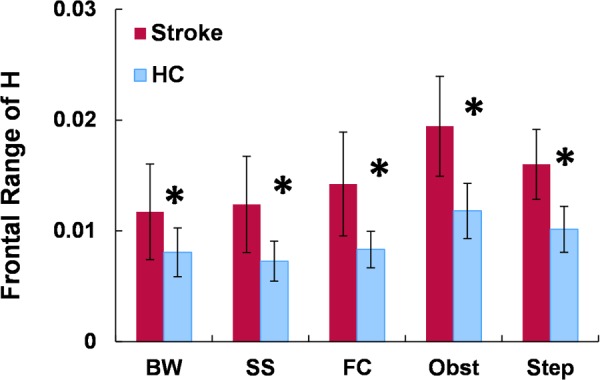
Mean normalized range of H (HR) in the frontal plane during backward, self-selected, and fastest-comfortable forward walking as well as obstacle negotiation and stepping up (Step). A significant difference between poststroke and healthy controls is indicated with *(P < 0.05).
Influence of Ankle-Foot Orthoses on Balance Control
Ankle-foot orthoses are widely prescribed to improve walking ability for those with various neurological deficits by assisting with foot clearance during swing while stabilizing the ankle during stance and keeping it in a near neutral position. As a result, ankle motion and plantarflexor function during stance is limited and may hinder the ability of those with volitional plantarflexor activity to regulate angular momentum in response to dynamic perturbations, and therefore, compromise their dynamic balance.
We examined the influence of a common clinically prescribed solid unilateral polypropylene AFO on dynamic balance in healthy adults during steady-state slow (0.6 m/s) and moderate (1.2 m/s) speeds, and during accelerated (0–1.8 m/s at 0.06 m/s2) and decelerated (1.8–0m/s at −0.06 m/s2) walking [39]. We again used HR to quantify dynamic balance. We found AFO use resulted in a greater HR in both the frontal and sagittal planes (Fig. 8), which were correlated with the reduced peak hip abduction and reduced ankle plantarflexor moments, respectively. In addition, walking with the AFO decreased body forward propulsion and ankle power generation (Fig. 9). These results suggested that AFOs may hinder the successful execution of important biomechanical subtasks and ankle function in healthy adults. Clearly, for those with ankle eversion or low plantarflexor activity, AFO prescription is a useful component of their rehabilitation to provide needed foot clearance and stability. However, for those that have volitional plantarflexor activity, AFOs may limit the successful execution of important mobility subtasks.
Fig. 8.
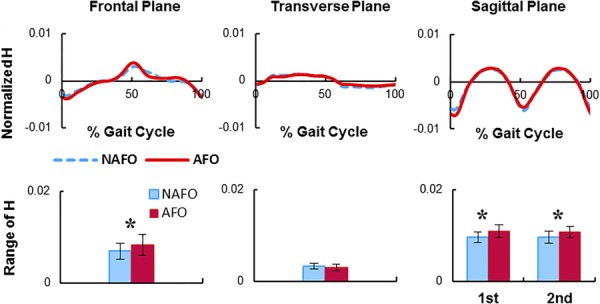
Normalized, mean three-dimensional whole-body angular momentum (H) during steady-state walking with (AFO) and without (NAFO) a solid unilateral AFO. Figures are in the AFO leg reference frame. The HR mean (SD) is shown in the bottom row. A significant difference between AFO and NAFO is indicated with “*” (P < 0.05). Figure adapted from Ref. [39].
Fig. 9.
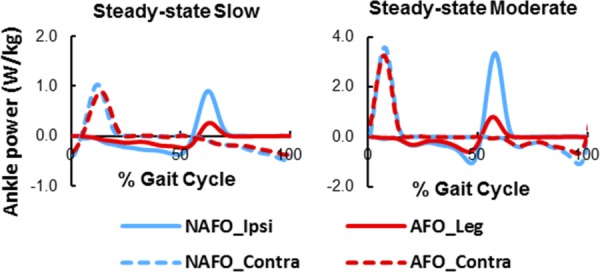
Mean normalized ankle power when walking with (AFO) and without (NAFO) a solid unilateral AFO at slow (0.6 m/s) and moderate (1.2 m/s) speeds. Figure adapted from Ref. [39].
Considering that improving the execution of walking subtasks such as balance control is an important element in rehabilitation, future research is needed to assess the tradeoffs of AFO use. For instance, in addition to examining the mechanical influence of AFOs on walking biomechanics, it is critical to identify the consequences of AFO use on muscle activity and neural plasticity during rehabilitation. We expect that AFOs will hinder the contribution of the plantarflexors and potentially require compensatory actions by other muscles to achieve balance control in individuals poststroke. This is especially critical during the acute recovery phase when neural plasticity is highest and poor muscle coordination patterns can be learned. Thus, future work is needed to critically assess the ramifications of long-term AFO use on rehabilitation outcomes.
Translation of Laboratory-Based Measures of H to the Clinic
One limitation of whole-body angular momentum is that the acquisition and analysis of H requires elaborate and expensive motion capture equipment that is not readily available in the clinic. Eventually, technologies such as inertial measurement unit sensors may allow for such assessments in the clinic, but currently they are limited in their capabilities. An important use of H analyses is that they can be used to gain insight into the effectiveness of common or new interventions that are considered in the clinic. For example, the analysis of H has been used to assess the effectiveness of a locomotor training program on balance control during steady-state walking in individuals poststroke [90]. Further, this analysis identified the underlying mechanisms (i.e., foot placement and GRF modifications) used from pre- to posttraining that were associated with observed improvements in dynamic balance. In addition, relationships between improvements in H postintervention and simple biomechanical markers pretraining (e.g., walking speed) were identified to distinguish those who are likely to show improved balance control from the training program [90].
Aside from assessing the effectiveness of locomotor training interventions and specific balance training programs, the analysis of H can also be used to objectively identify movement tasks that challenge individuals' balance control and may provide a basis for improving their mobility in the community. For instance, we have used the analysis of H during a variety of walking adaptability tasks and identified that individuals poststroke were most challenged in controlling their angular momentum during obstacle negotiation [44]. The most pronounced deficits in controlling H occurred in the mediolateral direction and during single-limb-stance when the trailing limb was behind the obstacle. These deficits were associated with lack of soleus activation and a wider separation between the body center-of-mass and the paretic foot center-of-pressure (i.e., deficits in weight shifting toward the paretic limb). Thus, practicing obstacle negotiation tasks in the clinic with an emphasis on the trailing limb single-stance phase may be an effective way to improve community ambulation poststroke.
Summary
Maintaining dynamic balance during human movement is critical to preventing falls and injuries that can lead to loss of mobility and functional independence. Various clinical and laboratory-based measures can be used to assess balance control with each having their own strengths and weaknesses. We have found the analysis of whole-body angular momentum to be a powerful tool to gain insight into the underlying mechanisms for maintaining dynamic balance in healthy adults and individuals with mobility impairments. A challenge for future work is to benchmark whole-body angular momentum against fall rates and determine a threshold for the range of H that is associated with falls in specific patient populations. Another avenue for future work is to identify associations between H and other measures that are readily available in the clinic (e.g., walking speed, spatiotemporal gait characteristics, or other simple clinical measures) and investigate if these measures can help predict the effectiveness of therapeutic interventions.
Acknowledgment
The authors would like to thank our many outstanding collaborators who contributed to the studies highlighted in this work and especially Steve Kautz, Ph.D., Mark Bowden, Ph.D., PT, Christy Conroy, MSPT, NCS, and Emily Fox, Ph.D., DPT, NCS who provided very helpful feedback on the manuscript.
Contributor Information
Richard R. Neptune, Walker Department of Mechanical Engineering, , The University of Texas at Austin, , Austin 204 E. Dean Keeton Street, , Stop C2200, , Austin, TX 78712 , e-mail: rneptune@mail.utexas.edu.
Arian Vistamehr, Brooks Rehabilitation Motion Analysis Center, , Jacksonville, FL 32216.
Funding Data
National Institutes of Health (R21 HD83964, Funder ID. 10.13039/100000002).
References
- [1]. Zajac, F. E. , Neptune, R. R. , and Kautz, S. A. , 2002, “ Biomechanics and Muscle Coordination of Human Walking—Part I: Introduction to Concepts, Power Transfer, Dynamics and Simulations,” Gait Posture, 16(3), pp. 215–232. 10.1016/S0966-6362(02)00068-1 [DOI] [PubMed] [Google Scholar]
- [2]. Zajac, F. E. , Neptune, R. R. , and Kautz, S. A. , 2003, “ Biomechanics and Muscle Coordination of Human Walking—Part II: Lessons From Dynamical Simulations and Clinical Implications,” Gait Posture, 17(1), pp. 1–17. 10.1016/S0966-6362(02)00069-3 [DOI] [PubMed] [Google Scholar]
- [3]. Anderson, F. C. , and Pandy, M. G. , 2003, “ Individual Muscle Contributions to Support in Normal Walking,” Gait Posture, 17(2), pp. 159–169. 10.1016/S0966-6362(02)00073-5 [DOI] [PubMed] [Google Scholar]
- [4]. Liu, M. Q. , Anderson, F. C. , Pandy, M. G. , and Delp, S. L. , 2006, “ Muscles That Support the Body Also Modulate Forward Progression During Walking,” J. Biomech., 39(14), pp. 2623–2630. 10.1016/j.jbiomech.2005.08.017 [DOI] [PubMed] [Google Scholar]
- [5]. Neptune, R. R. , Kautz, S. A. , and Zajac, F. E. , 2001, “ Contributions of the Individual Ankle Plantar Flexors to Support, Forward Progression and Swing Initiation During Walking,” J. Biomech., 34(11), pp. 1387–1398. 10.1016/S0021-9290(01)00105-1 [DOI] [PubMed] [Google Scholar]
- [6]. Neptune, R. R. , and McGowan, C. P. , 2011, “ Muscle Contributions to Whole-Body Sagittal Plane Angular Momentum During Walking,” J. Biomech., 44(1), pp. 6–12. 10.1016/j.jbiomech.2010.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Neptune, R. R. , and McGowan, C. P. , 2016, “ Muscle Contributions to Frontal Plane Angular Momentum During Walking,” J. Biomech., 49(13), pp. 2975–2981. 10.1016/j.jbiomech.2016.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Pandy, M. G. , Lin, Y. C. , and Kim, H. J. , 2010, “ Muscle Coordination of Mediolateral Balance in Normal Walking,” J. Biomech., 43(11), pp. 2055–2064. 10.1016/j.jbiomech.2010.04.010 [DOI] [PubMed] [Google Scholar]
- [9]. Tinetti, M. E. , 1986, “ Performance-Oriented Assessment of Mobility Problems in Elderly Patients,” J. Am. Geriatr. Soc., 34(2), pp. 119–126. 10.1111/j.1532-5415.1986.tb05480.x [DOI] [PubMed] [Google Scholar]
- [10]. Soyuer, F. , and Ozturk, A. , 2007, “ The Effect of Spasticity, Sense and Walking Aids in Falls of People After Chronic Stroke,” Disabil. Rehabil., 29(9), pp. 679–687. 10.1080/09638280600925860 [DOI] [PubMed] [Google Scholar]
- [11]. Kegelmeyer, D. A. , Kloos, A. D. , Thomas, K. M. , and Kostyk, S. K. , 2007, “ Reliability and Validity of the Tinetti Mobility Test for Individuals With Parkinson Disease,” Phys. Ther., 87(10), pp. 1369–1378. 10.2522/ptj.20070007 [DOI] [PubMed] [Google Scholar]
- [12]. Behrman, A. L. , Light, K. E. , and Miller, G. M. , 2002, “ Sensitivity of the Tinetti Gait Assessment for Detecting Change in Individuals With Parkinson's Disease,” Clin. Rehabil., 16(4), pp. 399–405. 10.1191/0269215502cr494oa [DOI] [PubMed] [Google Scholar]
- [13]. Berg, K. O. , Wood-Dauphinee, S. L. , Williams, J. I. , and Maki, B. , 1992, “ Measuring Balance in the Elderly: Validation of an Instrument,” Can. J. Public Health, 83(Suppl. 2), pp. S7–S11.https://www.ncbi.nlm.nih.gov/pubmed/1468055 [PubMed] [Google Scholar]
- [14]. Korner-Bitensky, N. , Wood-Dauphinee, S. , Teasell, R. , Desroisers, J. , Malouin, F. , Thomas, A. , Harrison, M. , Hanley, J. , Kaizer, F. , Kehayia, E. , Menon-Nair, A. , Rochette, A. , and Dumoulin, C. , 2006, “ Best Versus Actual Practices in Stroke Rehabilitation: Results of the Canadian National Survey,” Stroke, 37(2), p. 631. [Google Scholar]
- [15]. Tilson, J. K. , Wu, S. S. , Cen, S. Y. , Feng, Q. , Rose, D. R. , Behrman, A. L. , Azen, S. P. , and Duncan, P. W. , 2012, “ Characterizing and Identifying Risk for Falls in the LEAPS Study: A Randomized Clinical Trial of Interventions to Improve Walking Poststroke,” Stroke, 43(2), pp. 446–452. 10.1161/STROKEAHA.111.636258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Blum, L. , and Korner-Bitensky, N. , 2008, “ Usefulness of the Berg Balance Scale in Stroke Rehabilitation: A Systematic Review,” Phys. Ther., 88(5), pp. 559–566. 10.2522/ptj.20070205 [DOI] [PubMed] [Google Scholar]
- [17]. Major, M. J. , Fatone, S. , and Roth, E. J. , 2013, “ Validity and Reliability of the Berg Balance Scale for Community-Dwelling Persons With Lower-Limb Amputation,” Arch. Phys. Med. Rehabil., 94(11), pp. 2194–2202. 10.1016/j.apmr.2013.07.002 [DOI] [PubMed] [Google Scholar]
- [18]. Shumway-Cook, A. , and Woollacott, M. , 1995, Motor Control: Theory and Practical Applications, Williams & Wilkins, Baltimore, MD, pp. 323–324. [Google Scholar]
- [19]. Shumway-Cook, A. , Baldwin, M. , Polissar, N. L. , and Gruber, W. , 1997, “ Predicting the Probability for Falls in Community-Dwelling Older Adults,” Phys. Ther., 77(8), pp. 812–819. 10.1093/ptj/77.8.812 [DOI] [PubMed] [Google Scholar]
- [20]. Wrisley, D. M. , and Kumar, N. A. , 2010, “ Functional Gait Assessment: Concurrent, Discriminative, and Predictive Validity in Community-Dwelling Older Adults,” Phys. Ther., 90(5), pp. 761–773. 10.2522/ptj.20090069 [DOI] [PubMed] [Google Scholar]
- [21]. Whitney, S. L. , Hudak, M. T. , and Marchetti, G. F. , 2000, “ The Dynamic Gait Index Relates to Self-Reported Fall History in Individuals With Vestibular Dysfunction,” J. Vestib. Res., 10(2), pp. 99–105.https://content.iospress.com/articles/journal-of-vestibular-research/ves00059 [PubMed] [Google Scholar]
- [22]. Fritz, S. L. , Pittman, A. L. , Robinson, A. C. , Orton, S. C. , and Rivers, E. D. , 2007, “ An Intense Intervention for Improving Gait, Balance, and Mobility for Individuals With Chronic Stroke: A Pilot Study,” J. Neurol. Phys. Ther., 31(2), pp. 71–76. 10.1097/NPT.0b013e3180674a3c [DOI] [PubMed] [Google Scholar]
- [23]. Dibble, L. E. , and Lange, M. , 2006, “ Predicting Falls in Individuals With Parkinson Disease: A Reconsideration of Clinical Balance Measures,” J. Neurol. Phys. Ther., 30(2), pp. 60–67. 10.1097/01.NPT.0000282569.70920.dc [DOI] [PubMed] [Google Scholar]
- [24]. Cattaneo, D. , Regola, A. , and Meotti, M. , 2006, “ Validity of Six Balance Disorders Scales in Persons With Multiple Sclerosis,” Disabil. Rehabil., 28(12), pp. 789–795. 10.1080/09638280500404289 [DOI] [PubMed] [Google Scholar]
- [25]. Lin, J. H. , Hsu, M. J. , Hsu, H. W. , Wu, H. C. , and Hsieh, C. L. , 2010, “ Psychometric Comparisons of 3 Functional Ambulation Measures for Patients With Stroke,” Stroke, 41(9), pp. 2021–2025. 10.1161/STROKEAHA.110.589739 [DOI] [PubMed] [Google Scholar]
- [26]. Pardasaney, P. K. , Latham, N. K. , Jette, A. M. , Wagenaar, R. C. , Ni, P. , Slavin, M. D. , and Bean, J. F. , 2012, “ Sensitivity to Change and Responsiveness of Four Balance Measures for Community-Dwelling Older Adults,” Phys. Ther., 92(3), pp. 388–397. 10.2522/ptj.20100398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Wrisley, D. M. , Marchetti, G. F. , Kuharsky, D. K. , and Whitney, S. L. , 2004, “ Reliability, Internal Consistency, and Validity of Data Obtained With the Functional Gait Assessment,” Phys. Ther., 84(10), pp. 906–918.https://www.ncbi.nlm.nih.gov/pubmed/15449976 [PubMed] [Google Scholar]
- [28]. Howe, J. A. , Inness, E. L. , Venturini, A. , Williams, J. I. , and Verrier, M. C. , 2006, “ The Community Balance and Mobility Scale—A Balance Measure for Individuals With Traumatic Brain Injury,” Clin. Rehabil., 20(10), pp. 885–895. 10.1177/0269215506072183 [DOI] [PubMed] [Google Scholar]
- [29]. Knorr, S. , Brouwer, B. , and Garland, S. J. , 2010, “ Validity of the Community Balance and Mobility Scale in Community-Dwelling Persons After Stroke,” Arch. Phys. Med. Rehabil., 91(6), pp. 890–896. 10.1016/j.apmr.2010.02.010 [DOI] [PubMed] [Google Scholar]
- [30]. Hof, A. L. , 2008, “ The ‘extrapolated Center of Mass’ Concept Suggests a Simple Control of Balance in Walking,” Hum. Mov. Sci., 27(1), pp. 112–125. 10.1016/j.humov.2007.08.003 [DOI] [PubMed] [Google Scholar]
- [31]. McAndrew Young, P. M. , Wilken, J. M. , and Dingwell, J. B. , 2012, “ Dynamic Margins of Stability During Human Walking in Destabilizing Environments,” J. Biomech., 45(6), pp. 1053–1059. 10.1016/j.jbiomech.2011.12.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32]. Hurt, C. P. , and Grabiner, M. D. , 2015, “ Age-Related Differences in the Maintenance of Frontal Plane Dynamic Stability While Stepping to Targets,” J. Biomech., 48(4), pp. 592–597. 10.1016/j.jbiomech.2015.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Bolger, D. , Ting, L. H. , and Sawers, A. , 2014, “ Individuals With Transtibial Limb Loss Use Interlimb Force Asymmetries to Maintain Multi-Directional Reactive Balance Control,” Clin. Biomech., 29(9), pp. 1039–1047. 10.1016/j.clinbiomech.2014.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34]. Gates, D. H. , Scott, S. J. , Wilken, J. M. , and Dingwell, J. B. , 2013, “ Frontal Plane Dynamic Margins of Stability in Individuals With and Without Transtibial Amputation Walking on a Loose Rock Surface,” Gait Posture, 38(4), pp. 570–575. 10.1016/j.gaitpost.2013.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35]. Hof, A. L. , van Bockel, R. M. , Schoppen, T. , and Postema, K. , 2007, “ Control of Lateral Balance in Walking. Experimental Findings in Normal Subjects and Above-Knee Amputees,” Gait Posture, 25(2), pp. 250–258. 10.1016/j.gaitpost.2006.04.013 [DOI] [PubMed] [Google Scholar]
- [36]. Hak, L. , Houdijk, H. , van der Wurff, P. , Prins, M. R. , Mert, A. , Beek, P. J. , and van Dieen, J. H. , 2013, “ Stepping Strategies Used by Post-Stroke Individuals to Maintain Margins of Stability During Walking,” Clin. Biomech., 28(9–10), pp. 1041–1048. 10.1016/j.clinbiomech.2013.10.010 [DOI] [PubMed] [Google Scholar]
- [37]. Kao, P. C. , Dingwell, J. B. , Higginson, J. S. , and Binder-Macleod, S. , 2014, “ Dynamic Instability During Post-Stroke Hemiparetic Walking,” Gait Posture, 40(3), pp. 457–463. 10.1016/j.gaitpost.2014.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38]. Silverman, A. K. , and Neptune, R. R. , 2011, “ Differences in Whole-Body Angular Momentum Between Below-Knee Amputees and Non-Amputees Across Walking Speeds,” J. Biomech., 44(3), pp. 379–385. 10.1016/j.jbiomech.2010.10.027 [DOI] [PubMed] [Google Scholar]
- [39]. Vistamehr, A. , Kautz, S. A. , and Neptune, R. R. , 2014, “ The Influence of Solid Ankle-Foot-Orthoses on Forward Propulsion and Dynamic Balance in Healthy Adults During Walking,” Clin. Biomech., 29(5), pp. 583–589. 10.1016/j.clinbiomech.2014.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40]. Yeates, K. H. , Segal, A. D. , Neptune, R. R. , and Klute, G. K. , 2016, “ Balance and Recovery on Coronally-Uneven and Unpredictable Terrain,” J. Biomech., 49(13), pp. 2734–2740. 10.1016/j.jbiomech.2016.06.014 [DOI] [PubMed] [Google Scholar]
- [41]. Silverman, A. K. , Wilken, J. M. , Sinitski, E. H. , and Neptune, R. R. , 2012, “ Whole-Body Angular Momentum in Incline and Decline Walking,” J. Biomech., 45(6), pp. 965–971. 10.1016/j.jbiomech.2012.01.012 [DOI] [PubMed] [Google Scholar]
- [42]. Silverman, A. K. , Neptune, R. R. , Sinitski, E. H. , and Wilken, J. M. , 2014, “ Whole-Body Angular Momentum During Stair Ascent and Descent,” Gait Posture, 39(4), pp. 1109–1114. 10.1016/j.gaitpost.2014.01.025 [DOI] [PubMed] [Google Scholar]
- [43]. Nott, C. R. , Neptune, R. R. , and Kautz, S. A. , 2014, “ Relationships Between Frontal-Plane Angular Momentum and Clinical Balance Measures During Post-Stroke Hemiparetic Walking,” Gait Posture, 39(1), pp. 129–134. 10.1016/j.gaitpost.2013.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44]. Vistamehr, A. , Balasubramanian, C. K. , Clark, D. J. , Neptune, R. R. , and Fox, E. J. , 2018, “ Dynamic Balance During Walking Adaptability Tasks in Individuals Post-Stroke,” J. Biomech., 74, pp. 106–115. 10.1016/j.jbiomech.2018.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45]. Pijnappels, M. , Bobbert, M. F. , and van Dieen, J. H. , 2005, “ Push-Off Reactions in Recovery After Tripping Discriminate Young Subjects, Older Non-Fallers and Older Fallers,” Gait Posture, 21(4), pp. 388–394. 10.1016/j.gaitpost.2004.04.009 [DOI] [PubMed] [Google Scholar]
- [46]. Pickle, N. T. , Wilken, J. M. , Aldridge, J. M. , Neptune, R. R. , and Silverman, A. K. , 2014, “ Whole-Body Angular Momentum During Stair Walking Using Passive and Powered Lower-Limb Prostheses,” J. Biomech., 47(13), pp. 3380–3389. 10.1016/j.jbiomech.2014.08.001 [DOI] [PubMed] [Google Scholar]
- [47]. D'Andrea, S. , Wilhelm, N. , Silverman, A. K. , and Grabowski, A. M. , 2014, “ Does Use of a Powered Ankle-Foot Prosthesis Restore Whole-Body Angular Momentum During Walking at Different Speeds?,” Clin. Orthop. Relat. Res., 472(10), pp. 3044–3054. 10.1007/s11999-014-3647-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48]. Sheehan, R. C. , Beltran, E. J. , Dingwell, J. B. , and Wilken, J. M. , 2015, “ Mediolateral Angular Momentum Changes in Persons With Amputation During Perturbed Walking,” Gait Posture, 41(3), pp. 795–800. 10.1016/j.gaitpost.2015.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49]. Martelli, D. , Monaco, V. , Bassi Luciani, L. , and Micera, S. , 2013, “ Angular Momentum During Unexpected Multidirectional Perturbations Delivered While Walking,” IEEE Trans. Biomed. Eng., 60(7), pp. 1785–1795. 10.1109/TBME.2013.2241434 [DOI] [PubMed] [Google Scholar]
- [50]. Bruijn, S. M. , Meyns, P. , Jonkers, I. , Kaat, D. , and Duysens, J. , 2011, “ Control of Angular Momentum During Walking in Children With Cerebral Palsy,” Res. Dev. Disabil., 32(6), pp. 2860–2866. 10.1016/j.ridd.2011.05.019 [DOI] [PubMed] [Google Scholar]
- [51]. Herr, H. , and Popovic, M. , 2008, “ Angular Momentum in Human Walking,” J. Exp. Biol., 211(Pt. 4), pp. 467–481. 10.1242/jeb.008573 [DOI] [PubMed] [Google Scholar]
- [52]. Vistamehr, A. , Kautz, S. A. , Bowden, M. G. , and Neptune, R. R. , 2016, “ Correlations Between Measures of Dynamic Balance in Individuals With Post-Stroke Hemiparesis,” J. Biomech., 49(3), pp. 396–400. 10.1016/j.jbiomech.2015.12.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53]. Jansen, K. , De Groote, F. , Duysens, J. , and Jonkers, I. , 2014, “ How Gravity and Muscle Action Control Mediolateral Center of Mass Excursion During Slow Walking: A Simulation Study,” Gait Posture, 39(1), pp. 91–97. 10.1016/j.gaitpost.2013.06.004 [DOI] [PubMed] [Google Scholar]
- [54]. John, C. T. , Anderson, F. C. , Higginson, J. S. , and Delp, S. L. , 2013, “ Stabilisation of Walking by Intrinsic Muscle Properties Revealed in a Three-Dimensional Muscle-Driven Simulation,” Comput. Methods Biomech. Biomed. Eng., 16(4), pp. 451–462. 10.1080/10255842.2011.627560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55]. Klemetti, R. , Steele, K. M. , Moilanen, P. , Avela, J. , and Timonen, J. , 2014, “ Contributions of Individual Muscles to the Sagittal- and Frontal-Plane Angular Accelerations of the Trunk in Walking,” J. Biomech., 47(10), pp. 2263–2268. 10.1016/j.jbiomech.2014.04.052 [DOI] [PubMed] [Google Scholar]
- [56]. Runge, C. F. , Shupert, C. L. , Horak, F. B. , and Zajac, F. E. , 1999, “ Ankle and Hip Postural Strategies Defined by Joint Torques,” Gait Posture, 10(2), pp. 161–170. 10.1016/S0966-6362(99)00032-6 [DOI] [PubMed] [Google Scholar]
- [57]. LaRoche, D. P. , Cremin, K. A. , Greenleaf, B. , and Croce, R. V. , 2010, “ Rapid Torque Development in Older Female Fallers and Nonfallers: A Comparison Across Lower-Extremity Muscles,” J. Electromyogr. Kinesiol., 20(3), pp. 482–488. 10.1016/j.jelekin.2009.08.004 [DOI] [PubMed] [Google Scholar]
- [58]. Bauby, C. E. , and Kuo, A. D. , 2000, “ Active Control of Lateral Balance in Human Walking,” J. Biomech., 33(11), pp. 1433–1440. 10.1016/S0021-9290(00)00101-9 [DOI] [PubMed] [Google Scholar]
- [59]. Dean, J. C. , Alexander, N. B. , and Kuo, A. D. , 2007, “ The Effect of Lateral Stabilization on Walking in Young and Old Adults,” IEEE Trans. Biomed. Eng., 54(11), pp. 1919–1926. 10.1109/TBME.2007.901031 [DOI] [PubMed] [Google Scholar]
- [60]. Donelan, J. M. , Shipman, D. W. , Kram, R. , and Kuo, A. D. , 2004, “ Mechanical and Metabolic Requirements for Active Lateral Stabilization in Human Walking,” J. Biomech., 37(6), pp. 827–835. 10.1016/j.jbiomech.2003.06.002 [DOI] [PubMed] [Google Scholar]
- [61]. Gabell, A. , and Nayak, U. S. , 1984, “ The Effect of Age on Variability in Gait,” J. Gerontol., 39(6), pp. 662–666. 10.1093/geronj/39.6.662 [DOI] [PubMed] [Google Scholar]
- [62]. Guimaraes, R. M. , and Isaacs, B. , 1980, “ Characteristics of the Gait in Old People Who Fall,” Int. Rehabil. Med., 2(4), pp. 177–180. 10.3109/09638288009163984 [DOI] [PubMed] [Google Scholar]
- [63]. McAndrew Young, P. M. , and Dingwell, J. B. , 2012, “ Voluntarily Changing Step Length or Step Width Affects Dynamic Stability of Human Walking,” Gait Posture, 35(3), pp. 472–477. 10.1016/j.gaitpost.2011.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64]. Gehlsen, G. M. , and Whaley, M. H. , 1990, “ Falls in the Elderly—Part I: Gait,” Arch. Phys. Med. Rehabil., 71(10), pp. 735–738.https://www.ncbi.nlm.nih.gov/pubmed/2403278 [PubMed] [Google Scholar]
- [65]. Maki, B. E. , 1997, “ Gait Changes in Older Adults: Predictors of Falls or Indicators of Fear,” J. Am. Geriatr. Soc., 45(3), pp. 313–320. 10.1111/j.1532-5415.1997.tb00946.x [DOI] [PubMed] [Google Scholar]
- [66]. Moe-Nilssen, R. , and Helbostad, J. L. , 2005, “ Interstride Trunk Acceleration Variability But Not Step Width Variability Can Differentiate Between Fit and Frail Older Adults,” Gait Posture, 21(2), pp. 164–170. 10.1016/j.gaitpost.2004.01.013 [DOI] [PubMed] [Google Scholar]
- [67]. Nelson, A. J. , Certo, L. J. , Lembo, L. S. , Lopez, D. A. , Manfredonia, E. F. , Vanichpong, S. K. , and Zwick, D. , 1999, “ The Functional Ambulation Performance of Elderly Fallers and Non-Fallers Walking at Their Preferred Velocity,” Neurorehabilitation, 13(3), pp. 141–146.http://psycnet.apa.org/record/2001-09363-001 [Google Scholar]
- [68]. Su, P. F. , Gard, S. A. , Lipschutz, R. D. , and Kuiken, T. A. , 2007, “ Gait Characteristics of Persons With Bilateral Transtibial Amputations,” J. Rehabil. Res. Dev., 44(4), pp. 491–501. 10.1682/JRRD.2006.10.0135 [DOI] [PubMed] [Google Scholar]
- [69]. Miller, W. C. , Speechley, M. , and Deathe, B. , 2001, “ The Prevalence and Risk Factors of Falling and Fear of Falling Among Lower Extremity Amputees,” Arch. Phys. Med. Rehabil., 82(8), pp. 1031–1037. 10.1053/apmr.2001.24295 [DOI] [PubMed] [Google Scholar]
- [70]. Hak, L. , Houdijk, H. , van der Wurff, P. , Prins, M. R. , Beek, P. J. , and van Dieen, J. H. , 2015, “ Stride Frequency and Length Adjustment in Post-Stroke Individuals: Influence on the Margins of Stability,” J. Rehabil. Med., 47(2), pp. 126–132. 10.2340/16501977-1903 [DOI] [PubMed] [Google Scholar]
- [71]. Woollacott, M. H. , and Tang, P. F. , 1997, “ Balance Control During Walking in the Older Adult: Research and Its Implications,” Phys. Ther., 77(6), pp. 646–660. 10.1093/ptj/77.6.646 [DOI] [PubMed] [Google Scholar]
- [72]. Forster, A. , and Young, J. , 1995, “ Incidence and Consequences of Falls Due to Stroke—A Systematic Inquiry,” Brit. Med. J., 311(6997), pp. 83–86. 10.1136/bmj.311.6997.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73]. Hof, A. L. , Vermerris, S. M. , and Gjaltema, W. A. , 2010, “ Balance Responses to Lateral Perturbations in Human Treadmill Walking,” J. Exp. Biol., 213(15), pp. 2655–2664. 10.1242/jeb.042572 [DOI] [PubMed] [Google Scholar]
- [74]. Stokes, H. E. , Thompson, J. D. , and Franz, J. R. , 2017, “ The Neuromuscular Origins of Kinematic Variability During Perturbed Walking,” Sci. Rep., 7(1), p. 808. 10.1038/s41598-017-00942-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75]. Dean, J. C. , and Kautz, S. A. , 2015, “ Foot Placement Control and Gait Instability Among People With Stroke,” J. Rehabil. Res. Dev., 52(5), pp. 577–590. 10.1682/JRRD.2014.09.0207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76]. Zissimopoulos, A. , Stine, R. , Fatone, S. , and Gard, S. , 2014, “ Mediolateral Foot Placement Ability During Ambulation in Individuals With Chronic Post-Stroke Hemiplegia,” Gait Posture, 39(4), pp. 1097–1102. 10.1016/j.gaitpost.2014.01.015 [DOI] [PubMed] [Google Scholar]
- [77]. Reissman, M. E. , and Dhaher, Y. Y. , 2015, “ A Functional Tracking Task to Assess Frontal Plane Motor Control in Post Stroke Gait,” J. Biomech., 48(10), pp. 1782–1788. 10.1016/j.jbiomech.2015.05.008 [DOI] [PubMed] [Google Scholar]
- [78]. Wall, J. C. , Devlin, J. , Khirchof, R. , and Lackey, B. , 2000, “ Measurement of Step Widths and Step Lengths: A Comparison of Measurements Made Directly From a Grid With Those Made From a Video Recording,” J. Orthop. Sports Phys. Ther., 30(7), pp. 410–417. 10.2519/jospt.2000.30.7.410 [DOI] [PubMed] [Google Scholar]
- [79]. Falconer, J. , and Hayes, K. W. , 1991, “ A Simple Method to Measure Gait for Use in Arthritis Clinical Research,” Arthritis Care Res., 4(1), pp. 52–57. 10.1002/art.1790040110 [DOI] [PubMed] [Google Scholar]
- [80]. Hollman, J. H. , McDade, E. M. , and Petersen, R. C. , 2011, “ Normative Spatiotemporal Gait Parameters in Older Adults,” Gait Posture, 34(1), pp. 111–118. 10.1016/j.gaitpost.2011.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81]. McGowan, C. P. , Kram, R. , and Neptune, R. R. , 2009, “ Modulation of Leg Muscle Function in Response to Altered Demand for Body Support and Forward Propulsion During Walking,” J. Biomech., 42(7), pp. 850–856. 10.1016/j.jbiomech.2009.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82]. McGowan, C. P. , Neptune, R. R. , and Kram, R. , 2008, “ Independent Effects of Weight and Mass on Plantar Flexor Activity During Walking: Implications for Their Contributions to Body Support and Forward Propulsion,” J. Appl. Physiol., 105(2), pp. 486–494. 10.1152/japplphysiol.90448.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83]. Harper, N. G. , 2015, “ Muscle Function and Coordination of Amputee and Non-Amputee Stair Ascent,” Ph.D. dissertation, The University of Texas at Austin, TX.https://repositories.lib.utexas.edu/handle/2152/31547
- [84]. Chang, W. R. , Leclercq, S. , Lockhart, T. E. , and Haslam, R. , 2016, “ State of Science: Occupational Slips, Trips and Falls on the Same Level,” Ergonomics, 59(7), pp. 861–883. 10.1080/00140139.2016.1157214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85]. Segal, A. D. , and Klute, G. K. , 2014, “ Lower-Limb Amputee Recovery Response to an Imposed Error in Mediolateral Foot Placement,” J. Biomech., 47(12), pp. 2911–2918. 10.1016/j.jbiomech.2014.07.008 [DOI] [PubMed] [Google Scholar]
- [86]. Miller, S. E. , Segal, A. D. , Klute, G. K. , and Neptune, R. R. , 2018, “ Hip Recovery Strategy Used by Below-Knee Amputees Following Mediolateral Foot Perturbations,” J. Biomech., 76, pp. 61–67. 10.1016/j.jbiomech.2018.05.023 [DOI] [PubMed] [Google Scholar]
- [87]. Ashburn, A. , Hyndman, D. , Pickering, R. , Yardley, L. , and Harris, S. , 2008, “ Predicting People With Stroke at Risk of Falls,” Age Ageing, 37(3), pp. 270–276. 10.1093/ageing/afn066 [DOI] [PubMed] [Google Scholar]
- [88]. Lord, S. E. , McPherson, K. , McNaughton, H. K. , Rochester, L. , and Weatherall, M. , 2004, “ Community Ambulation After Stroke: How Important and Obtainable is it and What Measures Appear Predictive?,” Arch. Phys. Med. Rehabil., 85(2), pp. 234–239. 10.1016/j.apmr.2003.05.002 [DOI] [PubMed] [Google Scholar]
- [89]. Balasubramanian, C. K. , Clark, D. J. , and Fox, E. J. , 2014, “ Walking Adaptability After a Stroke and Its Assessment in Clinical Settings,” Stroke Res. Treat., 2014, p. 591013. 10.1155/2014/591013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90]. Vistamehr, A. , Kautz, S. A. , Bowden, M. G. , and Neptune, R. R. , 2018, “ The Influence of Locomotor Training on Dynamic Balance During Steady-State Walking Post-Stroke,” J. Biomech. (under review). [DOI] [PMC free article] [PubMed] [Google Scholar]


