Abstract
Essentials.
Uncontrolled clot breakdown with active bleeding can be seen in advanced cirrhosis.
A literature review found little information on optimal management.
We report a case of successful treatment with tranexamic acid for persistent subdural hematoma in this setting.
A 50‐year‐old woman with advanced cirrhosis presented with spontaneous subdural hematoma. She had a worsening clinical course following craniotomy despite administration of multiple blood products. With elevation in D‐dimer, persistently low fibrinogen and poor response to factor/fibrinogen replacement therapies, we had a suspicion for uncontrolled fibrinolysis. A literature review was conducted on treatment of hyperfibrinolysis in cirrhosis, finding 4 reports in which antifibrinolytics were used to control bleeding with different outcomes. The dose of tranexamic acid used in our patient was employed from previous experience in trauma patients. We transitioned from intravenous to oral administration based on expected pharmacokinetics. Our patient had a successful outcome with resolution of bleeding.
Keywords: liver cirrhosis, D‐dimer, fibrinolysis, spontaneous subdural hematoma, tranexamic acid
1. INTRODUCTION
Patients with cirrhosis have rebalanced hemostasis with simultaneous decreases in pro‐ and anticoagulants. However, the coagulation system in these patients is very tenuous and can be tipped, resulting in hemorrhage or thrombosis.1 Alteration in fibrinolytic pathways is reported in 30%‐46% of patients with end‐stage liver disease, although the exact incidence is unknown due to difficulty in diagnosis.2 Here, we report a patient with end‐stage liver disease who had a spontaneous subdural hematoma and responded to antifibrinolytic treatment with tranexamic acid (TXA). The Key Clinical Question addressed here is, “Does TXA help to control bleeding which is refractory to factor replacement therapy in a patient with end‐stage liver disease?”
2. CASE REPORT
A 50‐year‐old woman awaiting liver transplantation for Child‐Pugh's Class C cirrhosis due to nonalcoholic steatohepatitis presented to the emergency department with 5 days of worsening headaches, one episode of vomiting, and lethargy. A year prior, the patient had transarterial embolization of a right lobe hepatocellular carcinoma. On physical examination, she was minimally alert and jaundiced with a fixed right pupil. There was no overt bleeding. Brain computed tomography (CT) showed a large 2.6‐cm‐diameter subdural hematoma with 2 cm of leftward midline shift (Figure 1A). Laboratory studies revealed hemoglobin 7.0 g/dL, platelet count 61 000/cmm, prothrombin time 18.1 seconds, partial thromboplastin time 42 seconds, and fibrinogen 87 mg/dL. She was given 10 mg of intravenous vitamin K, 2650 units of prothrombin complex concentrate, 3 units of red blood cells (RBCs), 2 units of apheresis platelets, and 1 pool of cryoprecipitate and was taken to the operating room for emergency decompression. Intraoperatively, she received 2 units of RBCs, 2 pools of cryoprecipitate, and 1 unit of apheresis platelets plus an additional 2 units of plasma postoperatively for ongoing bleeding. As shown in Figure 2, multiple blood products were administered during the first week of hospitalization for ongoing bleeding, totaling 16 units of RBCs, 18 units of apheresis platelets, 20 pools of cryoprecipitate, and 6 units of plasma.
Figure 1.
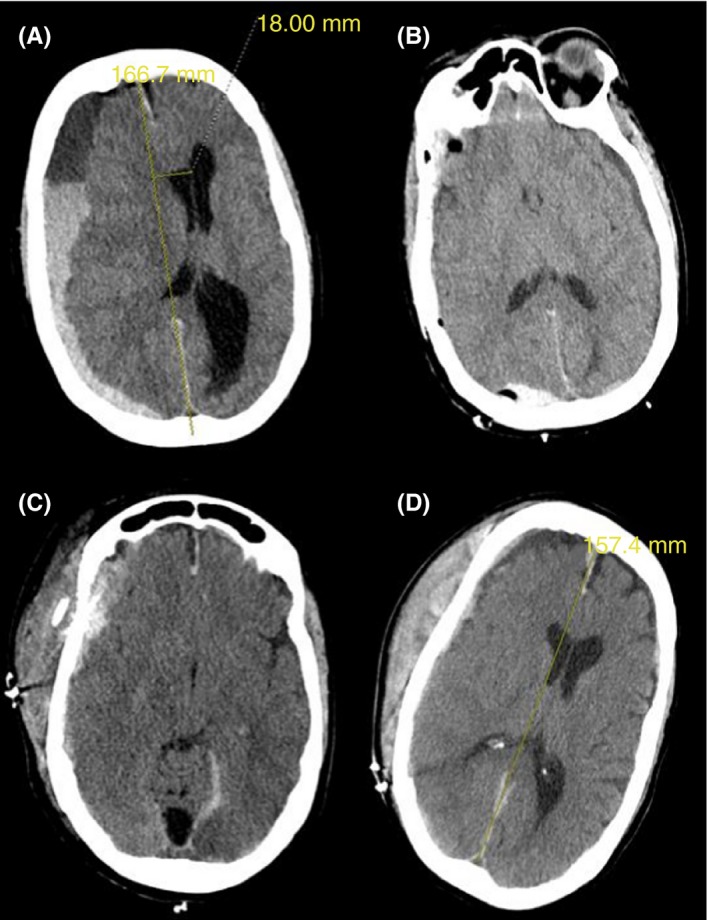
Serial cranial computed tomography. (A: D1) Large subdural hematoma, leftward midline shift. (B: D2) Improvement in subdural hematoma after craniotomy. (C: D4) New scalp hematoma, worsening subdural hematoma and worsening midline shift. (D: D9) Stable scalp hematoma, decrease in subdural hematoma, decreased midline shift. D represents day of hospitalization
Figure 2.
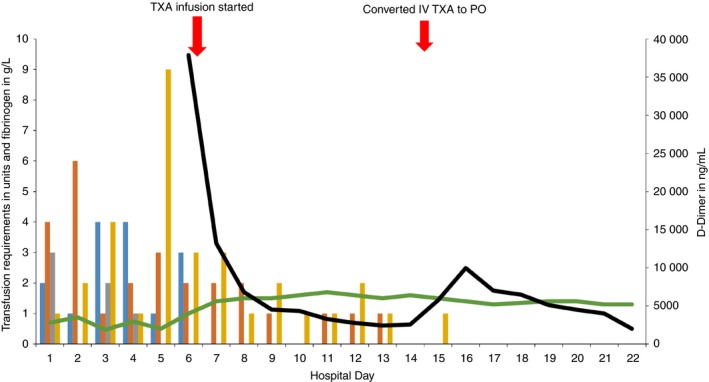
Day of hospitalization (x‐axis). Total transfusion requirements, fibrinogen and D‐dimer (y‐axis).  , PRBC;
, PRBC;  , platelets;
, platelets;  , FFP;
, FFP;  , Cryo;
, Cryo;  , fibrinogen;
, fibrinogen;  , D‐dimer
, D‐dimer
She continued to be minimally responsive, with persistent oozing at the surgical site. Repeat head CT showed an increase in the size of the subdural hematoma. Plasma fibrinogen remained low, at 100 mg/dL, despite appropriate transfusion support. On hospital day 6, D‐dimer was checked due to concern for hyperfibrinolysis, and was extremely elevated, at 37 890 ng/mL D‐dimer units (DDUs). Kaolin‐activated thromboelastography showed normal R time (reaction time: time from start of the test to initial fibrin formation), normal K time (time from initial clot formation to an amplitude of 20 mm), normal alpha angle (slope between R and K, indicating rate of clot formation and fibrin crosslinking), normal Ly‐30 (amplitude at 30 minutes measuring fibrinolysis), and a decrease in maximum amplitude (representing overall stability of the clot) at 27.30 mm. The thromboelastography assay was performed following aggressive transfusion support, possibly reducing the sensitivity of the assay to detect a hemostatic or fibrinolytic abnormality. In response, on hospital day 7, TXA was started with a 2 mg/kg bolus intravenously, and continuous infusion at 200 mg/h. D‐dimer decreased to 13 221 ng/mL DDU within 24 hours, and transfusion requirements decreased as depicted in Figure 2. On hospital day 8, the patient was extubated and transferred to the medical floor. TXA was decreased to 100 mg/h continuously on day 14. As imaging and D‐dimer stabilized, neurologic status also improved. On hospital day 15, intravenous TXA was converted to oral dosing by starting 1300 mg of oral TXA and discontinuing the infusion 2 hours later. This was followed by administration of 1300 mg of TXA orally every 6 hours with intent to taper the dose following D‐dimer. On hospital day 16, the dosing frequency was reduced to every 8 hours, but D‐dimer increased from 5000 to 9900 ng/mL DDU within 12 hours, and the fibrinogen level decreased from 145 mg/dL to 120 mg/dL. As the decrease in D‐dimer correlated with improvement in her neurological status and head CT findings, we were concerned that her clinical status could deteriorate with increasing D‐dimer level. In response, TXA dosing frequency was empirically increased to every 6 hours, and the D‐dimer remained stable. Throughout the hospital stay there was no clinical or radiologic evidence of thrombosis. On hospital day 24, the patient was transferred to a transplant center and is currently being evaluated for liver transplant.
3. DISCUSSION
This case illustrates the potential role of antifibrinolytic medication for life‐threatening bleeding in decompensated cirrhosis. Patients with liver disease have alterations in primary hemostasis, with decreases in platelet number and function; decreases in production of all procoagulant factors except factor VIII; decreases in anticoagulant proteins including antithrombin, protein C, protein S; and variable alterations in fibrinolytic proteins. However, the hemostatic milieu is rebalanced in cirrhosis.3
In cirrhosis, comorbidities including bacterial infection, renal disease, and portal hypertension can tip the hemostatic balance leading to bleeding.1, 3 Management of active bleeding in cirrhosis is aimed at RBC transfusions targeting hemoglobin of 7‐8 g/dL, platelet transfusions for thrombocytopenia to maintain platelet counts >50 000/cmm, and transfusion of cryoprecipitate to maintain a fibrinogen level >100 mg/dL.3 Our patient received multiple hemostatic products including RBCs, apheresis platelets, frozen plasma, and cryoprecipitate, and the clinical course worsened due to an increasing subdural hematoma and persistent hypofibrinogenemia. This, along with an extremely elevated D‐dimer on hospital day 6, raised the suspicion for uncontrolled fibrinolysis, and she was treated with TXA, with rapid improvement. There could also have been a component of secondary hyperfibrinolysis from disseminated intravascular coagulation (DIC). However, the fibrinogen level, which was dropping despite cryoprecipitate infusions, stabilized on day 6 with TXA, suggesting a component of primary hyperfibrinolysis. The exact incidence of hyperfibrinolysis is unknown, partly due to a lack of appropriately sensitive laboratory tests for evaluation, although it is reported to occur in 30%‐50% of patients with end‐stage liver disease.2, 4, 5 Both primary and secondary hyperfibrinolysis can be seen in patients with cirrhosis. Primary hyperfibrinolysis is due to impaired synthesis of α2 antiplasmin and thrombin‐activatable fibrinolysis inhibitor leading to accelerated fibrinolysis (Figure 3). Secondary hyperfibrinolysis in liver disease occurs in the setting of DIC.5 Several assays have been developed to detect hyperfibrinolysis, including the euglobulin clot lysis time, dilute whole blood clot lysis time, turbidimetric plasma clot lysis assay, global fibrinolytic capacity in the whole blood, and thromboelastography. However, none of these assays are sensitive enough to detect hyperfibrinolysis in cirrhosis due to the complex interactions between pro‐ and antifibrinolytic pathways.4, 6, 7, 8 Hugenholtz et al9 similarly found normal thromboelastography lysis times for cirrhotic patients, although median Ly‐30 time decreased with advanced cirrhosis.
Figure 3.
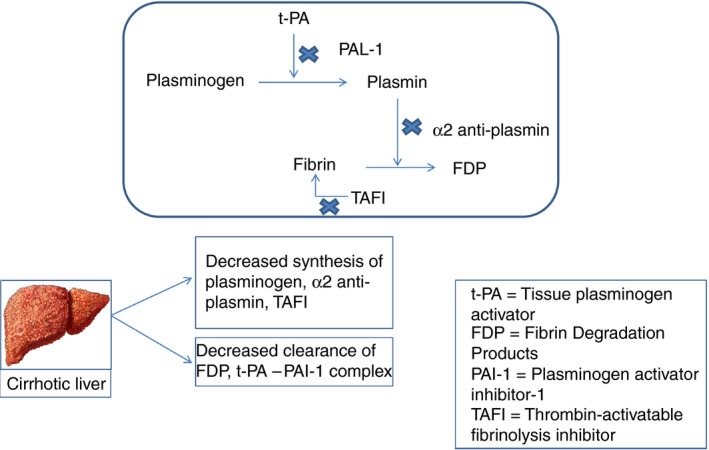
Pathophysiology of hyperfibrinolysis in cirrhosis
It was difficult to prove hyperfibrinolysis in our patient with conventional laboratory studies, but there was a high index of suspicion due to persistent hypofibrinogenemia, extremely elevated D‐dimer levels, and ultimately the response to TXA treatment. Unfortunately, frozen plasma was not available to perform any of the assays developed to detect hyperfibrinolysis as described above. Review of the literature on treatment of hyperfibrinolysis in liver disease was conducted using PubMed from 1980 to 2017. There were no randomized controlled trials demonstrating the role of antifibrinolytics for bleeding in patients with end‐stage liver disease. Antifibrinolytics have been studied primarily in the control of bleeding in trauma patients.7, 10, 11, 12 They have also been studied to decrease transfusion requirements during liver transplantation.13, 14 Gunawan et al15 reported the benefit of ε‐aminocaproic acid (EACA) to treat hyperfibrinolysis in a case series of 52 patients with cirrhosis. Of the 52 patients, 37 patients received EACA due to bleeding episodes, with subcutaneous/soft tissue bleeding being the most common type. Fifteen of 52 patients received EACA due to shortened euglobulin lysis time or prior to an invasive procedure but had no signs of bleeding. Nair et al16 published a case report in which EACA was successfully employed to treat hyperfibrinolysis with spontaneous intramuscular hematoma in cirrhosis. TXA was used by Laskiewicz et al17 to control refractory bleeding that manifested as persistent oozing at the catheter sites in a patient with cirrhosis, but the patient died of sepsis. Louro et al18 used TXA and factor VIIa to correct coagulopathy and hyperfibrinolysis in a patient with cirrhosis who sustained a blunt abdominal injury from a motor vehicle collision, but the patient died from multiorgan failure. Table 1 shows a summary of reports in which antifibrinolytics were used in cirrhosis‐related bleeding.
Table 1.
Summary of case reports with antifibrinolytics in cirrhosis‐related bleeding
| Authors | Number of patients | Age/sex | Presentation/indication | Treatment type/dosing | Outcome |
|---|---|---|---|---|---|
| Gunawan et al15 | 52 | Mean age 49.6 y (32‐67) Males, 73% | Study included 37 bleeding patients and 15 patients with no bleeding but shortened euglobulin clot lysis time | EACA‐1 g q6h | 37 bleeding patients—34 had resolution of bleeding, 2 patients with no improvement, 1 patient died 15 patients without bleeding—14 did well with no bleeding episodes, 1 had melena and died from liver failure complications |
| Nair et al16 | 1 | 65/male | Cirrhosis with spontaneous intramuscular hematoma | EACA 150 mg/kg loading dose followed by 1 g q4h × 2 | Resolution of bleeding |
| Laskiewicz et al17 | 1 | 72/male | Advanced cirrhosis with bleeding | TXA 1000 mg intravenous bolus followed by 1000 mg IV over 8 h on days 16, 18, and 23 | Expired on day 23 from pulseless electrical activity |
| Louro et al18 | 1 | 69/male | Bleeding from blunt abdominal trauma | Factor VIIa 1 mg and TXA 1 g i.v. bolus | Expired from multiorgan failure |
Abbreviations: EACA: ε‐aminocaproic acid; TXA; tranexamic acid.
Tranexamic acid is a synthetic lysine analogue and mimics lysine residues on fibrin, to which plasminogen normally binds.19, 20 It prevents fibrinolysis by binding to plasminogen, preventing its conversion to plasmin. It can directly inhibit plasmin activity at higher doses.10 TXA was also shown to attenuate the inflammatory response in trauma patients with survival benefit.12 There is no general consensus on the dosing of TXA for hyperfibrinolysis in cirrhosis. It can be given as an intravenous (i.v.) bolus (20‐25 mg/kg) followed by continuous infusion at 1‐2 mg/kg/h or 1 g over 10 minutes followed by 1 g over 8 hours.10 We chose to give an i.v. bolus, then a continuous infusion at 2 mg/kg/h. The D‐dimer markedly decreased within a day of infusion, there were no thrombosis complications, and the clinical course improved (Figure 2). Risk of thrombosis in patients with DIC and hyperfibrinolysis receiving TXA should be considered, as enhanced coagulation activity from DIC compounded by shutdown of the fibrinolytic pathway from TXA can increase the risk for thrombus formation.21 However, there are very limited data on TXA and increased risk for thrombosis in patients with hematologic disorders.20 Thus, it is reasonable to consider administration of TXA in a patient with life‐threatening bleeding.22
4. CONCLUSION
This case report is the first that we are aware of in which TXA was successfully employed to control life‐threatening bleeding in a patient with end‐stage cirrhosis. Our patient received substantial transfusion support aiming to correct primary hemostatic and coagulation abnormalities, but her bleeding persisted. This raised a suspicion for hyperfibrinolysis, although it was difficult to prove with conventional laboratory studies. It is possible that bleeding was due to primary hyperfibrinolysis or secondary hyperfibrinolysis from DIC. Recognition of uncontrolled fibrinolysis may prevent morbidity and mortality in similar cases. Earlier identification in this case might have shortened the hospitalization.
RELATIONSHIP DISCLOSURE
The authors have no relationships to disclose.
Kodali S, Holmes CE, Tipirneni E, Cahill CR, Goodwin AJ, Cushman M. Successful management of refractory bleeding in liver failure with tranexamic acid: Case report and literature review. Res Pract Thromb Haemost. 2019;3:424–428. 10.1002/rth2.12203
REFERENCES
- 1. Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med. 2011;365:147–56. [DOI] [PubMed] [Google Scholar]
- 2. Hu KQ, Yu AS, Tiyyagura L, Redeker AG, Reynolds TB. Hyperfibrinolytic activity in hospitalized cirrhotic patients in a referral liver unit. Am J Gastroenterol. 2001;96:1581–6. [DOI] [PubMed] [Google Scholar]
- 3. Kujovich JL. Coagulopathy in liver disease: a balancing act. Hematology Am Soc Hematol Educ Program. 2015;2015:243–9. [DOI] [PubMed] [Google Scholar]
- 4. Leebeek FW, Rijken DC. The fibrinolytic status in liver diseases. Semin Thromb Hemost. 2015;41:474–80. [DOI] [PubMed] [Google Scholar]
- 5. Ferro D, Celestini A, Violi F. Hyperfibrinolysis in liver disease. Clin Liver Dis. 2009;13:21–31. [DOI] [PubMed] [Google Scholar]
- 6. Bennani‐Baiti N, Daw HA. Primary hyperfibrinolysis in liver disease: a critical review. Clin Adv Hematol Oncol. 2011;9:250–2. [PubMed] [Google Scholar]
- 7. Gall LS, Brohi K, Davenport RA. Diagnosis and treatment of hyperfibrinolysis in trauma (a European perspective). Semin Thromb Hemost. 2017;43:224–34. [DOI] [PubMed] [Google Scholar]
- 8. Blasi A. Coagulopathy in liver disease: lack of an assessment tool. World J Gastroenterol. 2015;21:10062–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hugenholtz GCG, Lisman T, Stravitz RT. Thromboelastography does not predict outcome in different etiologies of cirrhosis. Res Pract Thromb Haemost. 2017;1:275–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pabinger I, Fries D, Schochl H, Streif W, Toller W. Tranexamic acid for treatment and prophylaxis of bleeding and hyperfibrinolysis. Wien Klin Wochenschr. 2017;129:303–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Roberts I, Shakur H, Coats T, Hunt B, Balogun E, Barnetson L, et al. The CRASH‐2 trial: a randomised controlled trial and economic evaluation of the effects of tranexamic acid on death, vascular occlusive events and transfusion requirement in bleeding trauma patients. Health Technol Assess. 2013;17:1–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Morrison JJ, Dubose JJ, Rasmussen TE, Midwinter MJ. Military Application of Tranexamic Acid in Trauma Emergency Resuscitation (MATTERs) Study. Arch Surg. 2012;147:113–9. [DOI] [PubMed] [Google Scholar]
- 13. Molenaar IQ, Warnaar N, Groen H, Tenvergert EM, Slooff MJ, Porte RJ. Efficacy and safety of antifibrinolytic drugs in liver transplantation: a systematic review and meta‐analysis. Am J Transplant. 2007;7:185–94. [DOI] [PubMed] [Google Scholar]
- 14. Porte RJ, Molenaar IQ, Begliomini B, Groenland TH, Januszkiewicz A, Lindgren L, et al. Aprotinin and transfusion requirements in orthotopic liver transplantation: a multicentre randomised double‐blind study. EMSALT Study Group. Lancet. 2000;355:1303–9. [DOI] [PubMed] [Google Scholar]
- 15. Gunawan B, Runyon B. The efficacy and safety of epsilon‐aminocaproic acid treatment in patients with cirrhosis and hyperfibrinolysis. Aliment Pharmacol Ther. 2006;23:115–20. [DOI] [PubMed] [Google Scholar]
- 16. Nair GB, Lajin M, Muslimani A. A cirrhotic patient with spontaneous intramuscular hematoma due to primary hyperfibrinolysis. Clin Adv Hematol Oncol. 2011;9:249–52. [PubMed] [Google Scholar]
- 17. Laskiewicz L, Fabricius W, Weinstein R. The clinical utility of tranexamic acid in the management of refractory bleeding in a patient with advanced liver disease. J Hematol. 2014;3:46–9. [Google Scholar]
- 18. Louro J, Andersen K, Dudaryk R. Correction of severe coagulopathy and hyperfibrinolysis by tranexamic acid and recombinant factor VIIa in a cirrhotic patient after trauma: a case report. A A Case Rep. 2017;9:144–7. [DOI] [PubMed] [Google Scholar]
- 19. de Leede‐van der Maarl MG, Hilkens P, Bosch F. The epileptogenic effect of tranexamic acid. J Neurol. 1999;246:843. [DOI] [PubMed] [Google Scholar]
- 20. Estcourt LJ, Desborough M, Brunskill SJ, Doree C, Hopewell S, Murphy MF, et al. Antifibrinolytics (lysine analogues) for the prevention of bleeding in people with haematological disorders. Cochrane Database Syst Rev. 2016;3:CD009733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Levi M, Toh CH, Thachil J, Watson HG. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol. 2009;145:24–33. [DOI] [PubMed] [Google Scholar]
- 22. Prokopchuk‐Gauk O, Brose K. Tranexamic acid to treat life‐threatening hemorrhage in prostate cancer associated disseminated intravascular coagulation with excessive fibrinolysis. Cureus. 2015;7:e428. [DOI] [PMC free article] [PubMed] [Google Scholar]


