Abstract
Essentials.
Acquired hemophilia A is a rare bleeding disorder often accompanied by other comorbidities.
We describe emicizumab use in acquired hemophilia A complicated by acute coronary syndrome.
Emicizumab proved safe and effective in a patient with acquired hemophilia A.
Emicizumab facilitated successful administration of dual antiplatelet therapy.
Abstract
We report a patient with a high‐titer factor VIII inhibitor refractory to immunosuppression. He initially presented with myocardial infarction requiring percutaneous coronary intervention (PCI) with bare metal stent placement. Despite Feiba prophylaxis, inadequate hemostasis prompted premature discontinuation of dual antiplatelet therapy (DAPT). Fifteen weeks later, the patient presented with a left anterior descending artery in‐stent restenosis. This case report examines the Key Clinical Question of how to manage in‐stent restenosis in a patient with acquired hemophilia A (AHA). After multidisciplinary discussions including hematology, cardiology, cardiac surgery, laboratory medicine, and pharmacy, emicizumab was initiated to facilitate PCI. Four weeks after emicizumab initiation, the patient underwent successful PCI with drug‐eluting stent placement. Five months after discharge, he remains without signs or symptoms of cardiac disease or bleeding on DAPT and emicizumab. This case provides evidence of the potential of emicizumab for bleeding prophylaxis in AHA.
Keywords: acute coronary syndrome, blood coagulation factor inhibitors, coronary artery disease, hemophilia A, platelet aggregation inhibitors
1. BACKGROUND
Acquired hemophilia A (AHA) results from the development of autoantibodies against factor VIII (FVIII). Although rare, AHA is the most common acquired coagulation factor inhibitor. AHA primarily arises in patients 65 years of age or older, and can be seen in association with autoimmune disease or malignancies.1, 2, 3, 4 The United Kingdom Hemophilia Centre Doctor's Organization prospective surveillance study estimated the incidence of AHA to be 1.48 cases per million per year.2 Compared to patients with congenital hemophilia A, achievement of hemostasis in AHA is difficult because of the reduced efficacy of standard FVIII replacement products and need for bypassing agent administration in many patients. The management of AHA‐related bleeding is particularly challenging in patients with coronary artery disease (CAD) because of the increased risk of thromboembolism associated with the administration of bypassing agents.5, 6 This case report examines the Key Clinical Question of how to manage in‐stent restenosis in a patient with refractory AHA.
2. CASE
Our patient is a 72‐year‐old male with a history of bullous pemphigoid–associated AHA with a high‐titer FVIII inhibitor who developed symptomatic CAD. He developed AHA in August 2012. His FVIII inhibitor was refractory to multiple immunosuppression therapies, including corticosteroids, rituximab, cyclophosphamide, cyclosporine, azathioprine, bortezomib, mycophenolate, cladribine, and tacrolimus (Figure 1). For 2 years before presentation, he was managed solely with FVIII inhibitor bypassing activity (Feiba, Shire US, Inc.) prophylaxis dosed at 50 units/kg 3 times weekly. In the year before presentation, he required treatment with Feiba only occasionally. His only risk factor for CAD was his age. Although long‐term exposure to Feiba may have also contributed, no data exist describing the risk of CAD development in patients with AHA receiving long‐term prophylactic Feiba. In March 2018 he developed chest pain and was diagnosed at an outside hospital with non–ST‐segment elevation myocardial infarction. Feiba was held, and loading doses of aspirin (325 mg) and clopidogrel (600 mg) were administered, resulting in large bilateral forearm hematomas. At the time of transfer to The Johns Hopkins Hospital, his FVIII inhibitor titer was 409 Bethesda Units (BU)/mL, and his most recent FVIII activity was undetectable. He underwent percutaneous coronary intervention (PCI) via radial approach upon arrival to repair critical mid–left anterior descending (LAD) artery stenosis. A bare metal stent (BMS) was placed to minimize dual antiplatelet therapy duration. Feiba 75 units/kg was administered 30 minutes before PCI and 12 hours after the preprocedure dose. Due to soft tissue bleeding in the setting of dual antiplatelet therapy (DAPT) administration, aspirin 81 mg and clopidogrel 75 mg were administered daily for 2 weeks with concomitant Feiba 50 units/kg every 12 hours, initiated 24 hours after the preprocedural dose. This regimen was tolerated without recurrent chest pain or bleeding, and the patient was discharged on his preadmission Feiba prophylaxis regimen with aspirin 81 mg daily. Additional inhibitor eradication therapy was not attempted due to prior nonresponse to multiple immunosuppressive regimens.
Figure 1.
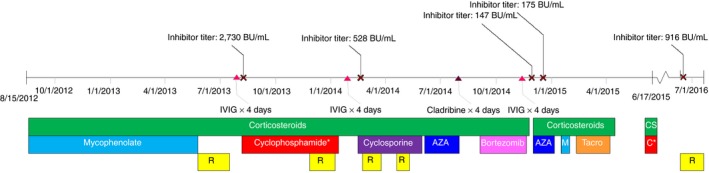
Immunosuppression course. R, rituximab; IVIG, intravenous immune globulin; AZA, azathioprine; M, mycophenolate; CS, corticosteroids; C, cyclophosphamide; tacro, tacrolimus; BU, Bethesda unit; cyclophosphamide: patient received oral low‐dose 8/7/2013‐11/27/2013 and 5/28/2015‐6/17/2015; intravenous high‐dose administration occurred 12/3/2013‐1/14/2014
The patient returned to our institution 15 weeks after discharge with severe angina. Feiba 75 units/kg was administered 30 minutes before diagnostic left heart catheterization via radial approach, which revealed 99% in‐stent restenosis of the distal portion of the mid‐LAD BMS. Because of previous Feiba failure in the setting of DAPT and concern regarding the use of other prothrombotic agents, we explored alternative approaches to promote adequate hemostasis during coronary artery bypass graft (CABG) surgery or drug‐eluting stent (DES) placement.
Multidisciplinary discussions were held involving hematology, cardiology, cardiac surgery, pharmacy, and laboratory medicine to evaluate potential treatment options. Although recombinant FVIIa (NovoSeven; Novo Nordisk Inc., Plainsboro, NJ) has demonstrated efficacy in patients refractory to Feiba, there were significant concerns about the thrombogenicity of this agent and the possibility of stent occlusion given the tenuous status of his BMS.7 In this setting, emicizumab (Hemlibra; Genetech USA, Inc., San Francisco, CA) was considered a safer option to facilitate either PCI with DES placement or CABG surgery. However, timely emicizumab initiation was not possible because of delays in insurance approval for off‐label administration. Therefore, recombinant porcine FVIII (Obizur; Shire US, Inc.) was initiated. One hour after administration of 300 units/kg, peak FVIII activity was 0.25 IU/dL, which declined to 0.21 IU/dL 3.5 hours after administration. In light of these results, porcine FVIII therapy did not appear to be a viable management option for achieving periprocedural hemostasis. This patient's suboptimal response was likely secondary to a high antiporcine FVIII inhibitor (titer of 18 BU/mL measured before porcine FVIII initiation).
With porcine FVIII no longer an option, we were able to obtain insurance approval for emicizumab administration. Because emicizumab administration results in a false reduction of the activated clotting time, intraoperative titration of unfractionated heparin during CABG surgery was no longer considered feasible.8 Although intraoperative heparin monitoring could occur utilizing anti‐Xa levels, this was not a viable strategy because of the prolonged turnaround time for this assay at our institution. Therefore, DES placement with 6‐12 months of DAPT was pursued instead of CABG surgery. Emicizumab was initiated at 3 mg/kg (255 mg) once weekly 3 days after discontinuation of Feiba prophylaxis. Twenty‐two days after emicizumab initiation, the patient was loaded with aspirin 325 mg and clopidogrel 600 mg, followed by administration of aspirin 81 mg and clopidogrel 75 mg daily. In the absence of clinically significant bleeding with DAPT exposure, the patient was taken for PCI with successful placement of 2 mid‐LAD DESs 27 days after emicizumab initiation. To avoid the challenges of monitoring intraprocedural unfractionated heparin anticoagulation in the presence of emicizumab, a bivalirudin 0.75 mg/kg bolus was administered intraprocedurally, followed by an infusion of 1.75 mg/kg/h for the duration of the procedure without activated clotting time monitoring.8 The patient tolerated the procedure and continued DAPT without breakthrough bleeding or the need for bypassing agent administration. The patient was transitioned to emicizumab 1.5 mg/kg (120 mg) once weekly 28 days after emicizumab initiation. Two days after PCI, he was discharged on emicizumab 120 mg once weekly and aspirin 81 mg daily to be administered indefinitely, with clopidogrel 75 mg daily for 6‐12 months after DES placement. Five months after discharge, the patient remains free from bleeding and recurrent cardiac complications.
3. DISCUSSION
AHA is a rare coagulopathy that preferentially affects older individuals who are also at greater risk for cardiovascular disease. Immunosuppression is first‐line therapy for AHA, and although the majority of patients with AHA achieve a complete remission with immunosuppressive therapy (71% in the United Kingdom Hemophilia Centre Doctor's Organization registry), a significant proportion relapse or are refractory despite multiple attempts at inhibitor eradication.2 Therefore, it is likely that hematologists will care for patients with refractory FVIII inhibitors and age‐related cardiovascular events.
Several methods should be employed in this population to reduce procedural‐related bleeding. The use of radial access over femoral access has proven to decrease procedural bleeding by 30% in the general population and has increasingly become the access of choice for PCI.9, 10, 11, 12, 13 The use of a BMS over DES is also recommended to limit the duration of DAPT.12, 13 However, BMSs are associated with significantly higher rates of in‐stent restenosis compared to DES—a complication that led to our patient's second presentation and need for further intervention to restore patency to the mid‐LAD stent.14 Additionally, when P2Y12 inhibitor therapy is indicated, relative platelet inhibition potency among available agents should be considered to minimize bleeding.12, 15, 16 For our patient, radial access was used, and clopidogrel was chosen over more potent P2Y12 inhibitors associated with higher bleeding risk. However, a DES was placed due to the location of the coronary lesion and the prior failure of a BMS.
Although bypassing therapies are effective for management of most acute bleeding events,17 Feiba prophylaxis was unable to provide adequate hemostasis during DAPT in our patient. Our case report demonstrates that emicizumab may represent an attractive option for patients with AHA and may facilitate safe DAPT administration. However, further investigation is warranted to confirm the safety and efficacy of emicizumab in AHA.
Two open‐label, randomized trials have demonstrated that emicizumab is an effective therapy for bleeding prevention in patients with hemophilia with and without inhibitors.18, 19 However, concomitant administration of Feiba in doses of 100 units/kg/day or higher for 24 hours or more was associated with a significant increase in the risk of thrombotic complications. Therefore, recombinant FVIIa should be used preferentially for the treatment of bleeding events in patients receiving emicizumab. Early experience with emicizumab in 22 patients with congenital hemophilia with inhibitors who underwent 29 invasive procedures was reported by Kruse‐Jarres et al20 at the American Society of Hemophilia meeting in December 2017. Twenty procedures were performed without administration of bypassing agents, and adequate hemostasis was achieved in 70%. Of the 6 procedures complicated by postoperative bleeding, only 2 (a dental extraction and an arthroscopic knee surgery) required administration of bypassing agents. Similarly, our patient underwent PCI with DES placement without preprocedural bypassing agents and experienced no hemostatic difficulties despite periprocedural bivalirudin and DAPT administration.
Although emicizumab administration was not associated with adverse effects in our patient, the thrombotic risk of emicizumab in AHA remains unknown. One published report to date described the development of thromboembolism in an 80‐year‐old patient with AHA and several medical comorbidities receiving emicizumab postoperatively following abdominal surgery21. This patient experienced a minor thromboembolic stroke on day 16 of emicizumab treatment, at which time she was receiving concomitant recombinant FVIIa. This event occurred in the setting of FVIII activity of 10%. It is unclear whether this event was precipitated by emicizumab administration or if this patient was predisposed to thromboembolism due to comorbidities, postsurgical risk, and concomitant recombinant FVIIa administration. In our patient, the risk of emicizumab‐induced thromboembolism may have been mitigated by concomitant DAPT administration. Additionally, our patient's FVIII inhibitor was deemed permanent after failure of multiple attempts at eradication. In patients with AHA successfully undergoing inhibitor eradication, thromboembolic risk may increase as FVIII inhibitor titers decline and FVIII levels normalize.
In conclusion, we present a patient with AHA refractory to multiple immunosuppressive regimens presenting with ACS requiring PCI who was successfully managed with emicizumab. We believe this case supports further investigation of emicizumab in patients with AHA.
RELATIONSHIP DISCLOSURE
KED, JPL, ARM and MKK have no conflicts of interest to disclose. SS has received institutional grant support from Daiichi‐Sankyo outside the purview of this work. MBS reports grants from Boehringer‐Ingelheim, Roche, personal fees from Daiichi‐Sankyo, Bayer, grants and personal fees from Janssen, Portola, personal fees from Pfizer and CSL Behring, all outside the submitted work. MBS also provides expert testimony for various legal cases involving venous thromboembolism management.
AUTHOR CONTRIBUTION
KD, JL, MS, and SS drafted and critically revised the manuscript. AM and MK critically revised the manuscript.
Dane KE, Lindsley JP, Streiff MB, Moliterno AR, Khalid MK, Shanbhag S. Successful use of emicizumab in a patient with refractory acquired hemophilia A and acute coronary syndrome requiring percutaneous coronary intervention. Res Pract Thromb Haemost. 2019;3:420–423. 10.1002/rth2.12201
Funding information
This work was funded by the Raymond Stralka Fund for Research.
REFERENCES
- 1. Knoebl P, Marco P, Baudo F, Collins P, Huth‐Kuhne A, Nemes L, et al. Demographic and clinical data in acquired hemophilia A: results from the European Acquired Haemophilia Registry (EACH2). J Thromb Haemost. 2012;10:622–31. [DOI] [PubMed] [Google Scholar]
- 2. Collins PW, Hirsch S, Baglin TP, Dolan G, Hanley J, Makris M, et al. Acquired hemophilia A in the United Kingdom: a 2‐year national surveillance study by the United Kingdom Haemophilia Centre Doctors’ Organisation. Blood. 2007;109:1870–7. [DOI] [PubMed] [Google Scholar]
- 3. Collins P, Macartney N, Davies R, Lees S, Giddings J, Majer R. A population based, unselected, consecutive cohort of patients with acquired haemophilia A. Br J Haematol. 2004;124:86–90. [DOI] [PubMed] [Google Scholar]
- 4. Sharathkumar AA, Soucie JM, Trawinski B, Greist A, Shapiro AD. Prevalence and risk factors of cardiovascular disease (CVD) events among patients with haemophilia: experience of a single haemophilia treatment centre in the United States (US). Haemophilia. 2011;17:597–604. [DOI] [PubMed] [Google Scholar]
- 5. Anti‐inhibitor coagulant complex (FEIBA®) [package insert]. Lexington, MA: Baxalta US, Inc.; 2018. [Google Scholar]
- 6. Coagulation factor VIIa (recombinant) (NovoSeven RT®) [package insert]. Plainsboro, NJ: Novo Nordisk; 2017. [Google Scholar]
- 7. Aledort LM. Comparative thrombotic event incidence after infusion of recombinant factor VIIa versus factor VIII inhibitor bypass activity. J Thromb Haemost. 2004;2:1700–8. [DOI] [PubMed] [Google Scholar]
- 8. Emicizumab (HEMLIBRA®) [package insert]. San Francisco, CA: Genetech, Inc.; 2017. [Google Scholar]
- 9. Reilley MJ, Blair A, Matthai WH, Vega R, Buckley M, Gimotty PA, et al. Revascularization strategies and in‐hospital management in acute coronary syndromes complicated by hemophilia A or hemophilia B. Blood Coagul Fibrinolysis. 2017;28:650–7. [DOI] [PubMed] [Google Scholar]
- 10. Valgimigli M, Gagnor A, Calabro P, Frigoli E, Leonardi S, Zaro T, et al. Radial versus femoral access in patients with acute coronary syndromes undergoing invasive management: a randomised multicentre trial. Lancet. 2015;385:2465–76. [DOI] [PubMed] [Google Scholar]
- 11. Mannucci PM, Schutgens RE, Santagostino E, Mauser‐Bunschoten EP. How I treat age‐related morbidities in elderly persons with hemophilia. Blood. 2009;114:5256–63. [DOI] [PubMed] [Google Scholar]
- 12. Martin K, Key NS. How I treat patients with inherited bleeding disorders who need anticoagulant therapy. Blood. 2016;128:178–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Srivastava A, Brewer AK, Mauser‐Bunschoten EP, Key NS, Kitchen S, Llinas A, et al. Guidelines for the management of hemophilia. Haemophilia. 2013;19:e1–47. [DOI] [PubMed] [Google Scholar]
- 14. Cassese S, Byrne RA, Tada T, Pinieck S, Joner M, Ibrahim T, et al. Incidence and predictors of restenosis after coronary stenting in 10 004 patients with surveillance angiography. Heart. 2014;100:153–9. [DOI] [PubMed] [Google Scholar]
- 15. Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–15. [DOI] [PubMed] [Google Scholar]
- 16. Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–57. [DOI] [PubMed] [Google Scholar]
- 17. Baudo F, Collins P, Huth‐Kuhne A, Levesque H, Marco P, Nemes L, et al. Management of bleeding in acquired hemophilia A: results from the European Acquired Haemophilia (EACH2) Registry. Blood. 2012;120:39–46. [DOI] [PubMed] [Google Scholar]
- 18. Oldenburg J, Mahlangu JN, Kim B, Schmitt C, Callaghan MU, Young G, et al. Emicizumab prophylaxis in hemophilia A with inhibitors. N Engl J Med. 2017;377:809–18. [DOI] [PubMed] [Google Scholar]
- 19. Mahlangu J, Oldenburg J, Paz‐Priel I, Negrier C, Niggli M, Mancuso ME, et al. Emicizumab prophylaxis in patients who have hemophilia A without inhibitors. N Engl J Med. 2018;379:811–22. [DOI] [PubMed] [Google Scholar]
- 20. Kruse‐Jarres R, Callaghan MU, Croteau SE, Jimenez‐Yuste V, Khoo L, Liesner R, et al. Surgical experience in two multicenter, open‐label phase 3 studies of emicizumab in persons with hemophilia A with inhibitors (HAVEN 1 and HAVEN 2). [abstract] Blood. 2017;130(suppl 1):89. [Google Scholar]
- 21. Knoebl P, Sperr W, Schellongowski P, Staudinger T, Jilma‐Stohlawetz P, Quehenberger P, et al. Emicizumab for the treatment of acquired hemophilia A: lessons learned from 4 very different cases [abstract] Blood. 2018;132(suppl 1):2476. [Google Scholar]


